The Interplay of Oxidative Stress and ROS Scavenging: Antioxidants as a Therapeutic Potential in Sepsis
Abstract
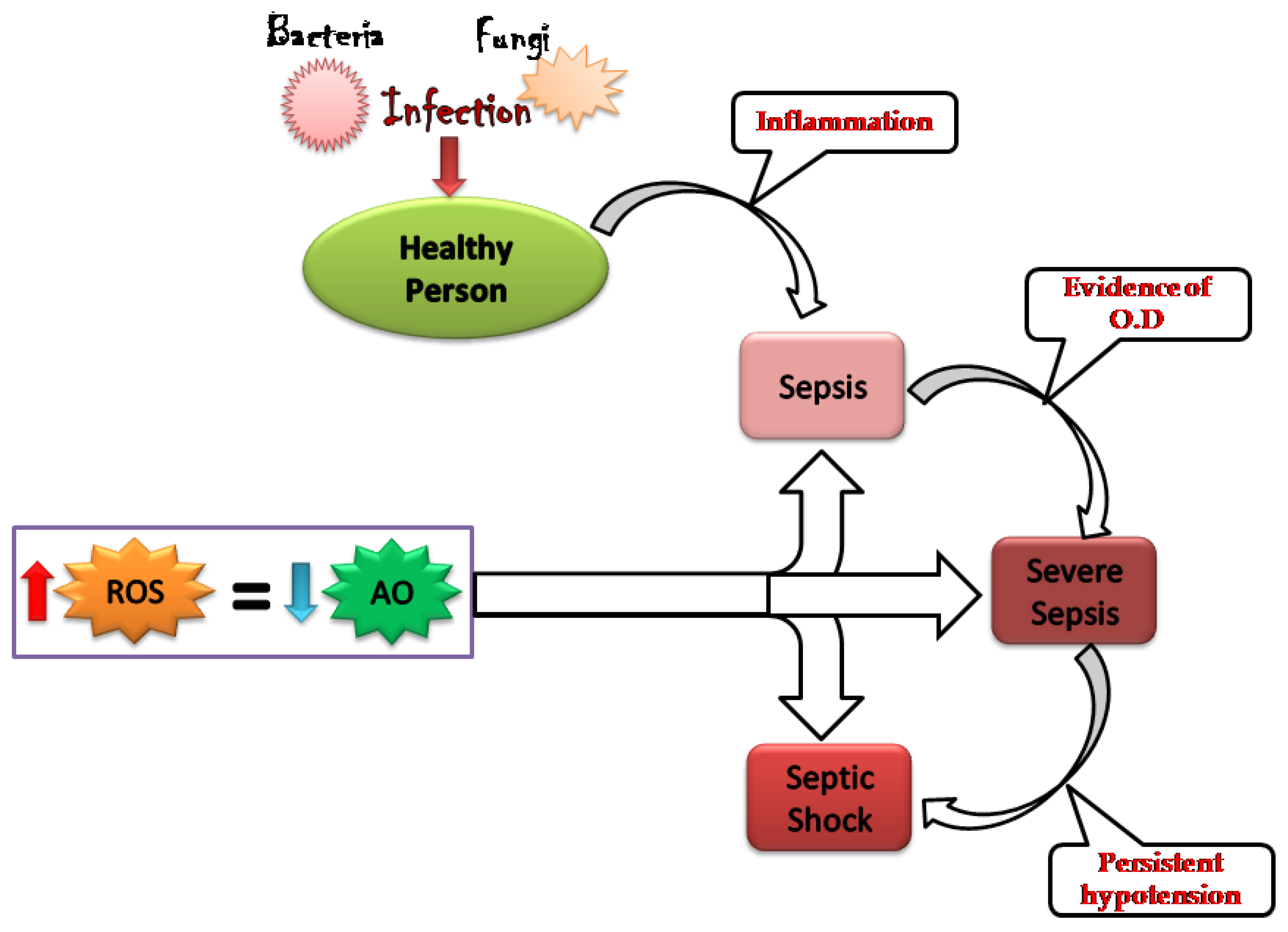
1. Introduction
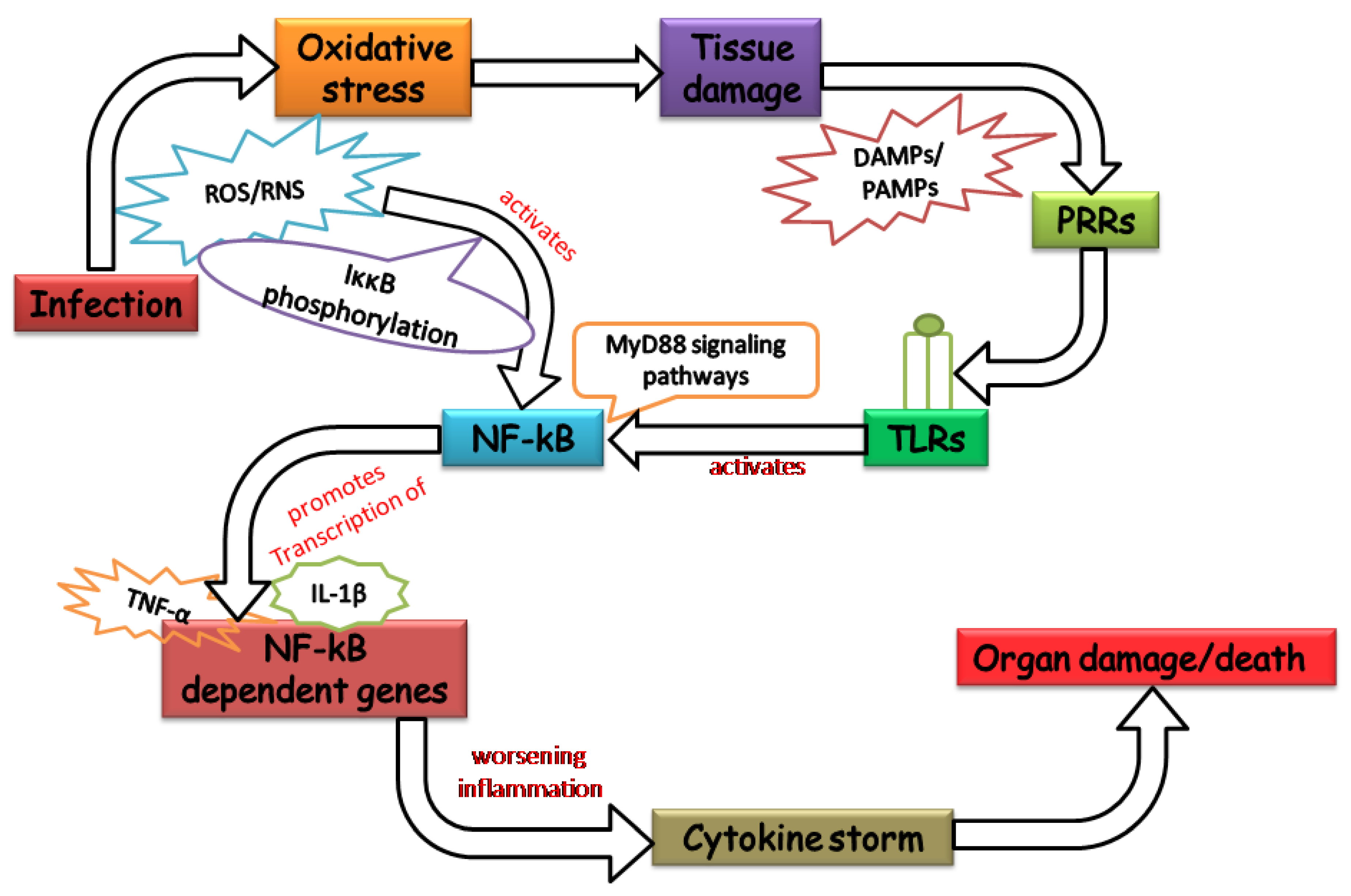
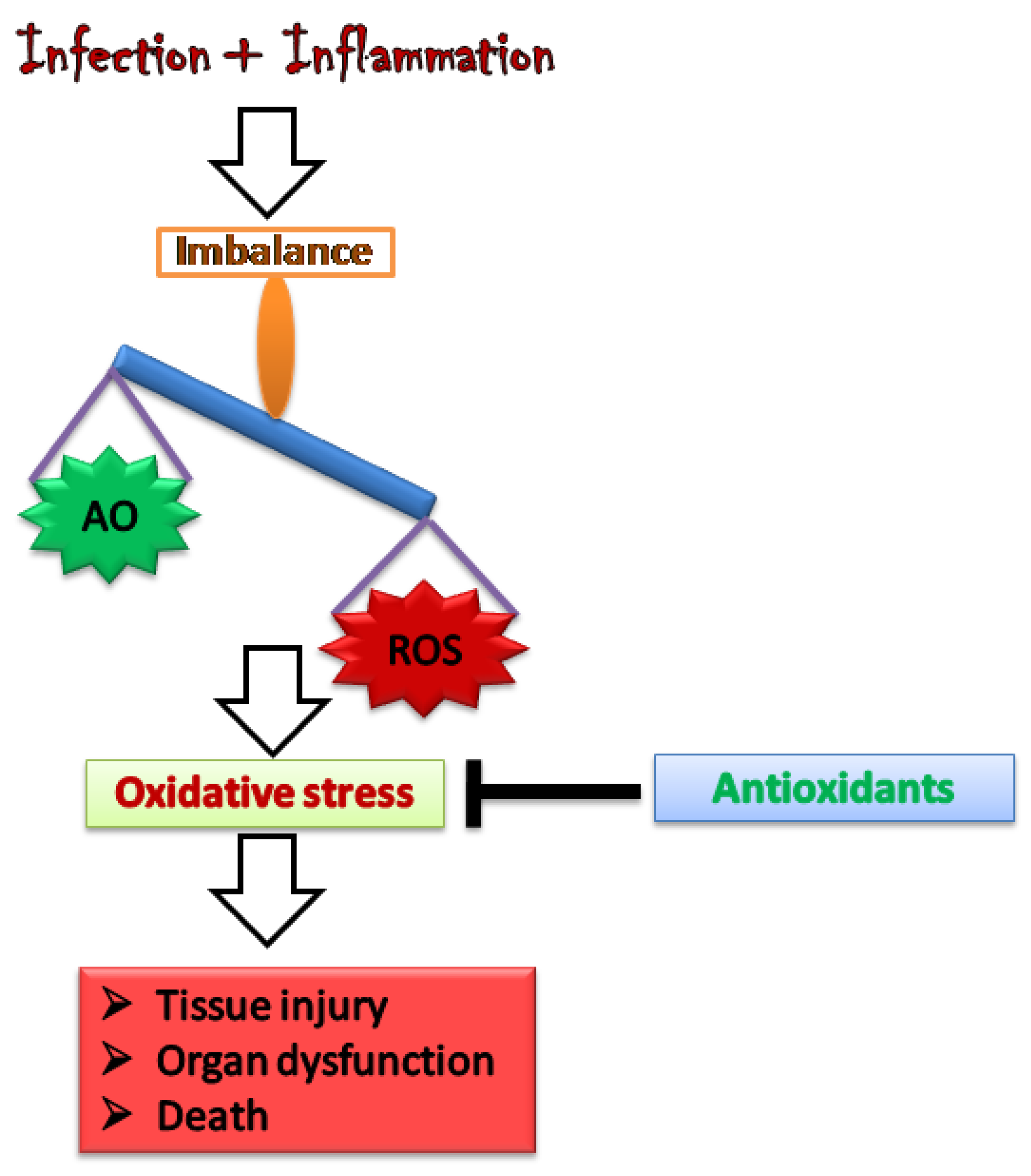
2. Oxidative Stress: Worsening the Pathology of Sepsis
3. Antioxidants as a Potential Therapy for Sepsis
3.1. Superoxide Dismutase (SOD)
3.2. Catalase
3.3. Glutathione
3.4. Vitamin C
3.5. Vitamin E
3.6. Vitamin A
3.7. N-Acetylcysteine (NAC)
3.8. Melatonin
3.9. Antioxidants Protecting Mitochondria
4. Failures and Risks of Antioxidants
5. Future Perspectives
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sherwood, E.R.; Toliver-Kinsky, T. Mechanisms of the inflammatory response. Best Pract. Res. Clin. Anaesthesiol. 2004, 18, 385–405. [Google Scholar] [CrossRef] [PubMed]
- Gotts, J.E.; Matthay, M.A. Sepsis: Pathophysiology and clinical management. BMJ 2016, 353, i1585. [Google Scholar] [CrossRef] [PubMed]
- Jean-Baptiste, E. Cellular Mechanisms in Sepsis. J. Intensive Care Med. 2007, 22, 63–72. [Google Scholar] [CrossRef]
- Cardoso, L.; Kauss, I.; Grion, C.; Cardoso, L.; Anami, E.; Nunes, L.; Ferreira, G.; Matsuo, T.; Bonametti, A. Epidemiology of sepsis in a Brazilian teaching hospital. Crit. Care 2009, 13 (Suppl. 3), P20. [Google Scholar] [CrossRef]
- Adhikari, N.K.J.; Fowler, R.A.; Bhagwanjee, S.; Rubenfeld, G.D. Critical care and the global burden of critical illness in adults. Lancet 2010, 376, 1339–1346. [Google Scholar] [CrossRef]
- Neri, A.; Pezzotti, P.; Fazio, C.; Vacca, P.; D’Ancona, F.P.; Caporali, M.G.; Stefanelli, P. Epidemiological and molecular characterization of invasive meningococcal disease in Italy, 2008/09-2012/13. PLoS ONE 2015, 10, e0139376. [Google Scholar] [CrossRef]
- Schulte, W.; Bernhagen, J.; Bucala, R. Cytokines in sepsis: Potent immunoregulators and potential therapeutic targets—An updated view. Mediat. Inflamm. 2013, 2013, 165974. [Google Scholar] [CrossRef]
- Russell, J.A. Management of sepsis. N. Engl. J. Med. 2006, 355, 1699–1713. [Google Scholar] [CrossRef]
- Cohen, J. The immunopathogenesis of sepsis. Nature 2002, 420, 885–891. [Google Scholar] [CrossRef]
- Kumar, S.; Gupta, E.; Srivastava, V.K.; Kaushik, S.; Saxena, J.; Goyal, L.K.; Mehta, S.; Jyoti, A. Nitrosative stress and cytokines are linked with the severity of sepsis and organ dysfunction. Br. J. Biomed. Sci. 2019, 76, 29–34. [Google Scholar] [CrossRef]
- Azzi, A. Oxidative stress: A dead end or a laboratory hypothesis? Biochem. Biophys. Res. Commun. 2007, 362, 230–232. [Google Scholar] [CrossRef] [PubMed]
- Prauchner, C.A. Oxidative stress in sepsis: Pathophysiological implications justifying antioxidant co-therapy. Burns 2017, 43, 471–485. [Google Scholar] [CrossRef] [PubMed]
- Kehrer, J.P. The Haber-Weiss reaction and mechanisms of toxicity. Toxicology 2000, 149, 43–50. [Google Scholar] [CrossRef]
- Qin, H.; Fan, J.; Mao, S. Exploring the mechanism of the Fe(iii)-activated Fenton-like reaction based on a quantitative study. New J. Chem. 2020, 44, 8952–8959. [Google Scholar] [CrossRef]
- Fridovich, I. Superoxide Radical: An Endogenous Toxicant. Annu. Rev. Pharmacol. Toxicol. 1983, 23, 239–257. [Google Scholar] [CrossRef]
- Lin, S.-S.; Gurol, M.D. Catalytic Decomposition of Hydrogen Peroxide on Iron Oxide: Kinetics, Mechanism, and Implications. Environ. Sci. Technol. 1998, 32, 1417–1423. [Google Scholar] [CrossRef]
- Atkinson, R. Kinetics and mechanisms of the gas-phase reactions of the hydroxyl radical with organic compounds under atmospheric conditions. Chem. Rev. 1986, 86, 69–201. [Google Scholar] [CrossRef]
- Albrich, J.M.; McCarthy, C.A.; Hurst, J.K. Biological reactivity of hypochlorous acid: Implications for microbicidal mechanisms of leukocyte myeloperoxidase. Proc. Natl. Acad. Sci. USA 1981, 78, 210–214. [Google Scholar] [CrossRef]
- Virág, L.; Szabó, É.; Gergely, P.; Szabó, C. Peroxynitrite-induced cytotoxicity: Mechanism and opportunities for intervention. Toxicol. Lett. 2003, 140–141, 113–124. [Google Scholar] [CrossRef]
- Griffith, O.W.; Stuehr, D.J. Nitric Oxide Synthases: Properties and Catalytic Mechanism. Annu. Rev. Physiol. 1995, 57, 707–734. [Google Scholar] [CrossRef]
- Sauer, H.; Wartenberg, M.; Hescheler, J. Reactive Oxygen Species as Intracellular Messengers During Cell Growth and Differentiation. Cell. Physiol. Biochem. 2001, 11, 173–186. [Google Scholar] [CrossRef]
- Di Meo, S.; Reed, T.T.; Venditti, P.; Victor, V.M. Role of ROS and RNS Sources in Physiological and Pathological Conditions. Oxid. Med. Cell. Longev. 2016, 2016, 1245049. [Google Scholar] [CrossRef] [PubMed]
- Andrades, M.; Morina, A.; Spasić, S.; Spasojević, I. Bench-to-bedside review: Sepsis—from the redox point of view. Crit. Care 2011, 15, 230. [Google Scholar] [CrossRef] [PubMed]
- Vanhoutte, P.M. How We Learned to Say NO. Arterioscler. Thromb. Vasc. Biol. 2009, 29, 1156–1160. [Google Scholar] [CrossRef] [PubMed]
- Murphy, M.P. How mitochondria produce reactive oxygen species. Biochem. J. 2009, 417, 1–13. [Google Scholar] [CrossRef]
- Guo, R.-F.; Ward, P.A. Role of Oxidants in Lung Injury during Sepsis. Antioxid. Redox Signal. 2007, 9, 1991–2002. [Google Scholar] [CrossRef]
- Barichello, T.; Fortunato, J.J.; Vitali, Â.M.; Feier, G.; Reinke, A.; Moreira, J.C.F.; Quevedo, J.; Dal-Pizzol, F. Oxidative variables in the rat brain after sepsis induced by cecal ligation and perforation. Crit. Care Med. 2006, 34, 886–889. [Google Scholar] [CrossRef]
- Park, H.S.; Jung, H.Y.; Park, E.Y.; Kim, J.; Lee, W.J.; Bae, Y.S. Cutting edge: Direct interaction of TLR4 with NAD(P)H oxidase 4 isozyme is essential for lipopolysaccharide-induced production of reactive oxygen species and activation of NF-kappa B. J. Immunol. 2004, 173, 3589–3593. [Google Scholar] [CrossRef]
- Gloire, G.; Legrand-Poels, S.; Piette, J. NF-κB activation by reactive oxygen species: Fifteen years later. Biochem. Pharmacol. 2006, 72, 1493–1505. [Google Scholar] [CrossRef]
- Kohchi, C.; Inagawa, H.; Nishizawa, T.; Soma, G.-I. ROS and innate immunity. Anticancer Res. 2009, 29, 817–821. [Google Scholar]
- DeLeo, F.R.; Renee, J.; McCormick, S.; Nakamura, M.; Apicella, M.; Weiss, J.P.; Nauseef, W.M. Neutrophils exposed to bacterial lipopolysaccharide upregulate NADPH oxidase assembly. J. Clin. Investig. 1998, 101, 455–463. [Google Scholar] [CrossRef] [PubMed]
- Hughes, G.; Murphy, M.P.; Ledgerwood, E.C. Mitochondrial reactive oxygen species regulate the temporal activation of nuclear factor kappaB to modulate tumour necrosis factor-induced apoptosis: Evidence from mitochondria-targeted antioxidants. Biochem. J. 2005, 389 Pt 1, 83–89. [Google Scholar] [CrossRef]
- Förstermann, U. Oxidative stress in vascular disease: Causes, defense mechanisms and potential therapies. Nat. Clin. Pract. Cardiovasc. Med. 2008, 5, 338–349. [Google Scholar] [CrossRef] [PubMed]
- Kasahara, S.; Jhingran, A.; Dhingra, S.; Salem, A.; Cramer, R.A.; Hohl, T.M. Role of Granulocyte-Macrophage Colony-Stimulating Factor Signaling in Regulating Neutrophil Antifungal Activity and the Oxidative Burst During Respiratory Fungal Challenge. J. Infect. Dis. 2016, 213, 1289–1298. [Google Scholar] [CrossRef] [PubMed]
- Jyoti, A.; Singh, A.K.; Dubey, M.; Kumar, S.; Saluja, R.; Keshari, R.S.; Verma, A.; Chandra, T.; Kumar, A.; Bajpai, V.K.; et al. Interaction of Inducible Nitric Oxide Synthase with Rac2 Regulates Reactive Oxygen and Nitrogen Species Generation in the Human Neutrophil Phagosomes: Implication in Microbial Killing. Antioxid. Redox Signal. 2014, 20, 417–431. [Google Scholar] [CrossRef] [PubMed]
- Zingarelli, B.; Sheehan, M.; Wong, H.R. Nuclear factor-κB as a therapeutic target in critical care medicine. Crit. Care Med. 2003, 31, S105–S111. [Google Scholar] [CrossRef]
- Sanlioglu, S.; Williams, C.M.; Samavati, L.; Butler, N.S.; Wang, G.; McCray, P.B.; Ritchie, T.C.; Hunninghake, G.W.; Zandi, E.; Engelhardt, J.F. Lipopolysaccharide Induces Rac1-dependent Reactive Oxygen Species Formation and Coordinates Tumor Necrosis Factor-α Secretion through IKK Regulation of NF-κB. J. Biol. Chem. 2001, 276, 30188–30198. [Google Scholar] [CrossRef]
- Blackwell, T.S.; Yull, F.E.; Chen, C.-L.; Venkatakrishnan, A.; Blackwell, T.R.; Hicks, D.J.; Lancaster, L.H.; Christman, J.W.; Kerr, L.D. Multiorgan Nuclear Factor Kappa B Activation in a Transgenic Mouse Model of Systemic Inflammation. Am. J. Respir. Crit. Care Med. 2000, 162, 1095–1101. [Google Scholar] [CrossRef]
- Böhrer, H.; Qiu, F.; Zimmermann, T.; Zhang, Y.; Jllmer, T.; Männel, D.; Böttiger, B.W.; Stern, D.M.; Waldherr, R.; Saeger, H.D.; et al. Role of NFkappaB in the mortality of sepsis. J. Clin. Investig. 1997, 100, 972–985. [Google Scholar] [CrossRef]
- Arnalich, F.; Garcia-Palomero, E.; López, J.; Jiménez, M.; Madero, R.; Renart, J.; Vázquez, J.J.; Montiel, C. Predictive value of nuclear factor kappaB activity and plasma cytokine levels in patients with sepsis. Infect. Immun. 2000, 68, 1942–1945. [Google Scholar] [CrossRef]
- Bertrand, Y.; Pincemail, J.; Hanique, G.; Denis, B.; Leenaerts, L.; Vankeerberghen, L.; Deby, C. Differences in tocopherol-lipid ratios in ARDS and non-ARDS patients. Intensive Care Med. 1989, 15, 87–93. [Google Scholar] [CrossRef]
- Mileva, M.; Dimitrova, A.; Krastev, D.; Alexandrova, A.; Tsvetanova, E.; Georgieva, A.; Galabov, A. Oseltamivir and S-adenosyl-l-methionine combination as effective therapeutic strategy for suppression of oxidative damage in lung caused by influenza virus infection in mice. Drug Res. 2020, 70, 273–279. [Google Scholar] [CrossRef] [PubMed]
- Metnitz, P.G.H.; Bartens, C.; Fischer, M.; Fridrich, P.; Steltzer, H.; Druml, W. Antioxidant status in patients with acute respiratory distress syndrome. Intensive Care Med. 1999, 25, 180–185. [Google Scholar] [CrossRef] [PubMed]
- Sakaguchi, S.; Furusawa, S. Oxidative stress and septic shock: Metabolic aspects of oxygen-derived free radicals generated in the liver during endotoxemia. FEMS Immunol. Med. Microbiol. 2006, 47, 167–177. [Google Scholar] [CrossRef]
- Lu, X.; Xue, L.; Sun, W.; Ye, J.; Zhu, Z.; Mei, H. Identification of key pathogenic genes of sepsis based on the Gene Expression Omnibus database. Mol. Med. Rep. 2018, 17, 3042–3054. [Google Scholar] [CrossRef] [PubMed]
- Galloway, S.P.; McMillan, D.C.; Sattar, N. Effect of the inflammatory response on trace element and vitamin status. Ann. Clin. Biochem. 2000, 37, 289–297. [Google Scholar] [CrossRef] [PubMed]
- Xing, P.-C.; An, P.; Hu, G.-Y.; Wang, D.-L.; Zhou, M.-J. LncRNA MIAT Promotes Inflammation and Oxidative Stress in Sepsis-Induced Cardiac Injury by Targeting miR-330-5p/TRAF6/NF-κB Axis. Biochem. Genet. 2020, 58, 783–800. [Google Scholar] [CrossRef] [PubMed]
- Langenberg, C.; Wan, L.; Egi, M.; May, C.N.; Bellomo, R. Renal blood flow and function during recovery from experimental septic acute kidney injury. Intensive Care Med. 2007, 33, 1614–1618. [Google Scholar] [CrossRef] [PubMed]
- Chand, N.; Sanyal, A.J. Sepsis-induced cholestasis. Hepatology 2007, 45, 230–241. [Google Scholar] [CrossRef] [PubMed]
- Ciencewicki, J.; Trivedi, S.; Kleeberger, S.R. Oxidants and the pathogenesis of lung diseases. J. Allergy Clin. Immunol. 2008, 122, 456–468. [Google Scholar] [CrossRef]
- Wilson, J.X.; Wu, F. Vitamin C in Sepsis. Subcell Biochem. 2012, 56, 67–83. [Google Scholar] [PubMed]
- Okado-Matsumoto, A.; Fridovich, I. Subcellular distribution of superoxide dismutases (SOD) in rat liver: Cu, Zn-SOD in mitochondria. J. Biol. Chem. 2001, 276, 38388–38393. [Google Scholar] [CrossRef] [PubMed]
- Ghio, A.J.; Suliman, H.B.; Carter, J.D.; Abushamaa, A.M.; Folz, R.J. Overexpression of extracellular superoxide dismutase decreases lung injury after exposure to oil fly ash. Am. J. Physiol. Cell. Mol. Physiol. 2002, 283, L211–L218. [Google Scholar] [CrossRef] [PubMed]
- Berger, M.M.; Chioléro, R.L. Antioxidant supplementation in sepsis and systemic inflammatory response syndrome. Crit. Care Med. 2007, 35, S584–S590. [Google Scholar] [CrossRef]
- Cruzat, V.F.; Bittencourt, A.; Scomazzon, S.P.; Leite, J.S.M.; de Bittencourt, P.I.H.; Tirapegui, J. Oral free and dipeptide forms of glutamine supplementation attenuate oxidative stress and inflammation induced by endotoxemia. Nutrition 2014, 30, 602–611. [Google Scholar] [CrossRef] [PubMed]
- Zong, Y.; Zhang, H. Amentoflavone prevents sepsis-associated acute lung injury through Nrf2-GCLc-mediated upregulation of glutathione. Acta Biochim. Pol. 2017, 64, 93–98. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, X.; Zhou, H.; Liu, W.; Li, J. Exogenous S-nitrosoglutathione attenuates inflammatory response and intestinal epithelial barrier injury in endotoxemic rats. J. Trauma Acute Care Surg. 2016, 80, 977–984. [Google Scholar] [CrossRef]
- Bailey, J.D.; Shaw, A.; McNeill, E.; Nicol, T.; Diotallevi, M.; Chuaiphichai, S.; Patel, J.; Hale, A.; Channon, K.M.; Crabtree, M.J. Isolation and culture of murine bone marrow-derived macrophages for nitric oxide and redox biology. Nitric Oxide-Biol. Chem. 2020, 100–101, 17–29. [Google Scholar] [CrossRef]
- Vico, T.A.; Marchini, T.; Ginart, S.; Lorenzetti, M.A.; Areán, J.S.A.; Calabró, V.; Garcés, M.; Ferrero, M.C.; Mazo, T.; D’Annunzio, V.; et al. Mitochondrial bioenergetics links inflammation and cardiac contractility in endotoxemia. Basic Res. Cardiol. 2019, 114, 38. [Google Scholar] [CrossRef]
- Cyr, A.; Chambers, L.; Waltz, P.K.; Whelan, S.P.; Kohut, L.; Carchman, E.; Dyer, M.; Luciano, J.; Kautza, B.; Gomez, H.D.; et al. Endotoxin Engages Mitochondrial Quality Control via an iNOS-Reactive Oxygen Species Signaling Pathway in Hepatocytes. Oxid. Med. Cell. Longev. 2019, 2019, 4745067. [Google Scholar] [CrossRef]
- Yasui, K.; Baba, A. Therapeutic potential of superoxide dismutase (SOD) for resolution of inflammation. Inflamm. Res. 2006, 55, 359–363. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Gupta, E.; Kaushik, S.; Srivastava, V.K.; Mehta, S.K.; Jyoti, A. Evaluation of oxidative stress and antioxidant status: Correlation with the severity of sepsis. Scand. J. Immunol. 2018, 87, e12653. [Google Scholar] [CrossRef] [PubMed]
- Shimozawa, N.; Zhang, Z.; Imamura, A.; Suzuki, Y.; Fujiki, Y.; Tsukamoto, T.; Osumi, T.; Aubourg, P.; Wanders, R.J.; Kondo, N. Molecular Mechanism of Detectable Catalase-Containing Particles, Peroxisomes, in Fibroblasts from a PEX2-Defective Patient. Biochem. Biophys. Res. Commun. 2000, 268, 31–35. [Google Scholar] [CrossRef]
- Kretz-Remy, C.; Mehlen, P.; Mirault, M.E.; Arrigo, A.P. Inhibition of I kappa B-alpha phosphorylation and degradation and subsequent NF-kappa B activation by glutathione peroxidase overexpression. J. Cell Biol. 1996, 133, 1083–1093. [Google Scholar] [CrossRef]
- Hon-Wing, L.; Vang, M.J.; Mavis, R.D. The cooperative interaction between vitamin E and vitamin C in suppression of peroxidation of membrane phospholipids. Biochim. Biophys. Acta-Lipids Lipid Metab. 1981, 664, 266–272. [Google Scholar] [CrossRef]
- Saánchez-Moreno, C.; Dashe, J.F.; Scott, T.; Thaler, D.; Folstein, M.F.; Martin, A. Decreased Levels of Plasma Vitamin C and Increased Concentrations of Inflammatory and Oxidative Stress Markers After Stroke. Stroke 2004, 35, 163–168. [Google Scholar] [CrossRef] [PubMed]
- Austenaa, L.M.; Carlsen, H.; Hollung, K.; Blomhoff, H.K.; Blomhoff, R. Retinoic acid dampens LPS-induced NF-κB activity: Results from human monoblasts and in vivo imaging of NF-κB reporter mice. J. Nutr. Biochem. 2009, 20, 726–734. [Google Scholar] [CrossRef]
- Kim, Y.; Seo, J.H.; Kim, H. β-Carotene and Lutein Inhibit Hydrogen Peroxide-Induced Activation of NF-κB and IL-8 Expression in Gastric Epithelial AGS Cells. J. Nutr. Sci. Vitaminol. 2011, 57, 216–223. [Google Scholar] [CrossRef]
- Zhang, X.; Zhao, W.; Hu, L.; Zhao, L.; Huang, J. Carotenoids inhibit proliferation and regulate expression of peroxisome proliferators-activated receptor gamma (PPARγ) in K562 cancer cells. Arch. Biochem. Biophys. 2011, 512, 96–106. [Google Scholar] [CrossRef]
- Trabetti, E. Homocysteine, MTHFR gene polymorphisms, and cardio-cerebrovascular risk. J. Appl. Genet. 2008, 49, 267–282. [Google Scholar] [CrossRef]
- Griffith, O.W.; Meister, A. Glutathione: Interorgan translocation, turnover, and metabolism. Proc. Natl. Acad. Sci. USA 1979, 76, 5606–5610. [Google Scholar] [CrossRef] [PubMed]
- Escames, G.; Lopez, L.C.; Tapias, V.; Utrilla, P.; Reiter, R.J.; Hitos, A.B.; Leon, J.; Rodriguez, M.I.; Acuna-Castroviejo, D. Melatonin counteracts inducible mitochondrial nitric oxide synthase-dependent mitochondrial dysfunction in skeletal muscle of septic mice. J. Pineal Res. 2006, 40, 71–78. [Google Scholar] [CrossRef] [PubMed]
- Macarthur, H.; Couri, D.M.; Wilken, G.H.; Westfall, T.C.; Lechner, A.J.; Matuschak, G.M.; Chen, Z.; Salvemini, D. Modulation of serum cytokine levels by a novel superoxide dismutase mimetic, M40401, in an Escherichia coli model of septic shock: Correlation with preserved circulating catecholamines. Crit. Care Med. 2003, 31, 237–245. [Google Scholar] [CrossRef] [PubMed]
- Nozik-Grayck, E.; Suliman, H.B.; Piantadosi, C.A. Extracellular superoxide dismutase. Int. J. Biochem. Cell Biol. 2005, 37, 2466–2471. [Google Scholar] [CrossRef] [PubMed]
- van Camp, W.; Inzé, D.; van Montagu, M. The Regulation and Function of Tobacco Superoxide Dismutases. Free Radic. Biol. Med. 1997, 23, 515–520. [Google Scholar] [CrossRef]
- Halliwell, B. Antioxidants in Human Health and Disease. Annu. Rev. Nutr. 1996, 16, 33–50. [Google Scholar] [CrossRef]
- Naito, Y.; Yoshikawa, T.; Ando, T.; Kishi, A.; Ueda, S.; Oyamada, H.; Kondo, M. Changes in superoxide dismutase activity in the gastric mucosa of peptic ulcer patients. J. Clin. Gastroenterol. 1992, 14 (Suppl. 1), S131–S134. [Google Scholar] [CrossRef]
- Kobayashi, S.D.; Voyich, J.M.; Somerville, G.A.; Braughton, K.R.; Malech, H.L.; Musser, J.M.; DeLeo, F.R. An apoptosis-differentiation program in human polymorphonuclear leukocytes facilitates resolution of inflammation. J. Leukoc. Biol. 2003, 73, 315–322. [Google Scholar] [CrossRef]
- Cuzzocrea, S.; Costantino, G.; Caputi, A.P. Protective effect of melatonin on cellular energy depletion mediated by peroxynitrite and poly (ADP-ribose) synthetase activation in a non-septic shock model induced by zymosan in the rat. J. Pineal Res. 1998, 25, 78–85. [Google Scholar] [CrossRef]
- Salvemini, D.; Riley, D.P.; Lennon, P.J.; Wang, Z.-Q.; Currie, M.G.; Macarthur, H.; Misko, T.P. Protective effects of a superoxide dismutase mimetic and peroxynitrite decomposition catalysts in endotoxin-induced intestinal damage. Br. J. Pharmacol. 1999, 127, 685–692. [Google Scholar] [CrossRef]
- Clares, M.P.; Blasco, S.; Inclán, M.; Agudo, L.d.; Verdejo, B.; Soriano, C.; Doménech, A.; Latorre, J.; García-España, E. Manganese(ii) complexes of scorpiand-like azamacrocycles as MnSOD mimics. Chem. Commun. 2011, 47, 5988. [Google Scholar] [CrossRef] [PubMed]
- Clares, M.P.; Serena, C.; Blasco, S.; Nebot, A.; del Castillo, L.; Soriano, C.; Domènech, A.; Sánchez-Sánchez, A.V.; Soler-Calero, L.; Mullor, J.L.; et al. Mn(II) complexes of scorpiand-like ligands. A model for the MnSOD active centre with high in vitro and in vivo activity. J. Inorg. Biochem. 2015, 143, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Serena, C.; Calvo, E.; Clares, M.P.; Diaz, M.L.; Chicote, J.U.; Beltrán-Debon, R.; Fontova, R.; Rodriguez, A.; García-España, E.; García-España, A. Significant In Vivo Anti-Inflammatory Activity of Pytren4Q-Mn a Superoxide Dismutase 2 (SOD2) Mimetic Scorpiand-Like Mn (II) Complex. PLoS ONE 2015, 10, e0119102. [Google Scholar] [CrossRef] [PubMed]
- Coleman, M.; Brouillette, M.; Andresen, N.; Oberley-Deegan, R.; Martin, J.; Coleman, M.C.; Brouillette, M.J.; Andresen, N.S.; Oberley-Deegan, R.E.; Martin, J.M. Differential Effects of Superoxide Dismutase Mimetics after Mechanical Overload of Articular Cartilage. Antioxidants 2017, 6, 98. [Google Scholar] [CrossRef] [PubMed]
- Schrader, M.; Fahimi, H.D. Peroxisomes and oxidative stress. Biochim. Biophys. Acta-Mol. Cell Res. 2006, 1763, 1755–1766. [Google Scholar] [CrossRef]
- Ho, Y.-S.; Xiong, Y.; Ma, W.; Spector, A.; Ho, D.S. Mice lacking catalase develop normally but show differential sensitivity to oxidant tissue injury. J. Biol. Chem. 2004, 279, 32804–32812. [Google Scholar] [CrossRef]
- Siwale, R.C.; Oettinger, C.W.; Addo, R.; Siddig, A.; D’Souza, M.J. The effect of intracellular delivery of catalase and antisense oligonucleotides to NF-κB using albumin microcapsules in the endotoxic shock model. J. Drug Target. 2009, 17, 701–709. [Google Scholar] [CrossRef]
- Dong, H.-P.; Chunag, I.-C.; Wang, D.-C.; Huang, L.-J.; Lee, C.-I.; Tsai, J.-H.; Yang, R.-C. Lipopolysaccharide-stimulated Leukocytes Contribute to Platelet Aggregative Dysfunction, Which is Attenuated by Catalase in Rats. Kaohsiung J. Med. Sci. 2010, 26, 584–592. [Google Scholar] [CrossRef]
- Maksimenko, A.V.; Vavaeva, A.V.; Zvyagintseva, M.A.; Abramov, A.A.; Timoshin, A.A.; Vavaev, A.V.; Lakomkin, V.L. [Protective action figurations for superoxide dismutase—Chondroitin sulfate—Catalase bienzyme conjugate after its medicative administration in endotoxin shock]. Biomed. Khim. 2016, 62, 295–301. [Google Scholar] [CrossRef]
- Kanzok, S.M.; Fechner, A.; Bauer, H.; Ulschmid, J.K.; Müller, H.M.; Botella-Munoz, J.; Schneuwly, S.; Schirmer, R.H.; Becker, K. Substitution of the thioredoxin system for glutathione reductase in Drosophila melanogaster. Science (80-) 2001, 291, 643–646. [Google Scholar] [CrossRef]
- Bashir, A.; Perham, R.N.; Scrutton, N.S.; Berry, A. Altering kinetic mechanism and enzyme stability by mutagenesis of the dimer interface of glutathione reductase. Biochem. J. 1995, 312, 527–533. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.C.; van der Hoeven, L.H.; Haddox, C.H. Glutathione reductase in the red blood cells. Ann. Clin. Lab. Sci. 1978, 8, 23–29. [Google Scholar] [PubMed]
- García, C.; Moragón, C.; López-Fernández, M.E. Frequency of Glutathione Reductase, Pyruvate Kinase and Glucose-6-Phosphate Dehydrogenase Deficiency in a Spanish Population. Hum. Hered. 1979, 29, 310–313. [Google Scholar]
- Redmond, S.M.; Joncourt, F.; Buser, K.; Ziemiecki, A.; Altermatt, H.J.; Fey, M.; Margison, G.; Cerny, T. Assessment of P-glycoprotein, glutathione-based detoxifying enzymes and O6-alkylguanine-DNA alkyltransferase as potential indicators of constitutive drug resistance in human colorectal tumors. Cancer Res. 1991, 51, 2092–2097. [Google Scholar] [PubMed]
- Bounous, G.; Molson, J.H. The antioxidant system. Anticancer Res. 2003, 23B, 1411–1415. [Google Scholar]
- Şener, G.; Toklu, H.; Kapucu, C.; Ercan, F.; Erkanlı, G.; Kaçmaz, A.; Tilki, M.; Yeğen, B.Ç. Melatonin Protects Against Oxidative Organ Injury in a Rat Model of Sepsis. Surg. Today 2004, 35, 52–59. [Google Scholar] [CrossRef]
- Samuvel, D.J.; Shunmugavel, A.; Singh, A.K.; Singh, I.; Khan, M. S-Nitrosoglutathione ameliorates acute renal dysfunction in a rat model of lipopolysaccharide-induced sepsis. J. Pharm. Pharmacol. 2016, 68, 1310–1319. [Google Scholar] [CrossRef]
- Khalili, H. Ascorbic acid in septic shock. J. Res. Pharm. Pract. 2016, 5, 301–302. [Google Scholar] [CrossRef]
- Kuhn, S.-O.; Meissner, K.; Mayes, L.M.; Bartels, K. Vitamin C in sepsis. Curr. Opin. Anaesthesiol. 2018, 31, 55–60. [Google Scholar] [CrossRef]
- Birben, E.; Sahiner, U.M.; Sackesen, C.; Erzurum, S.; Kalayci, O. Oxidative Stress and Antioxidant Defense. World Allergy Organ. J. 2012, 5, 9–19. [Google Scholar] [CrossRef]
- Venarucci, D.; Venarucci, V.; Vallese, A.; Battilà, L.; Casado, A.; de la Torre, R.; Fernandez, M.E.L. Free radicals: Important cause of pathologies refer to ageing. Panminerva Med. 1999, 41, 335–339. [Google Scholar] [PubMed]
- Staal, F.J.T.; Roederer, M.; Raju, P.A.; Anderson, M.T.; Ela, S.W.; Herzenberg, L.A.; Herzenberg, L.A. Antioxidants Inhibit Stimulation of HIV Transcription. AIDS Res. Hum. Retrovir. 1993, 9, 299–306. [Google Scholar] [CrossRef] [PubMed]
- Victor, V.; Guayerbas, N.; Puerto, M.; Medina, S.; de la Fuente, M. Ascorbic acid modulates in vitro the function of macrophages from mice with endotoxic shock. Immunopharmacology 2000, 46, 89–101. [Google Scholar] [CrossRef]
- Armour, J.; Tyml, K.; Lidington, D.; Wilson, J.X. Ascorbate prevents microvascular dysfunction in the skeletal muscle of the septic rat. J. Appl. Physiol. 2001, 90, 795–803. [Google Scholar] [CrossRef]
- Rojas, C.; Cadenas, S.; Herrero, A.; Méndez, J.; Barja, G. Endotoxin depletes ascorbate in the guinea pig heart. Protective effects of vitamins C and E against oxidative stress. Life Sci. 1996, 59, 649–657. [Google Scholar] [CrossRef]
- Galley, H.F.; Howdle, P.D.; Walker, B.E.; Webster, N.R. The Effects of Intravenous Antioxidants in Patients with Septic Shock. Free Radic. Biol. Med. 1997, 23, 768–774. [Google Scholar] [CrossRef]
- Quinlan, G.J.; Margarson, M.P.; Mumby, S.; Evans, T.W.; Gutteridge, J.M. Administration of albumin to patients with sepsis syndrome: A possible beneficial role in plasma thiol repletion. Clin. Sci. 1998, 95, 459–465. [Google Scholar] [CrossRef]
- Cárcamo, J.M.; Pedraza, A.; Bórquez-Ojeda, O.; Golde, D.W. Vitamin C Suppresses TNFα-Induced NFκB Activation by Inhibiting IκBα Phosphorylation. Biochemistry 2002, 41, 12995–13002. [Google Scholar] [CrossRef]
- Wu, F.; Wilson, J.X.; Tyml, K. Ascorbate inhibits iNOS expression and preserves vasoconstrictor responsiveness in skeletal muscle of septic mice. Am. J. Physiol. Integr. Comp. Physiol. 2003, 285, R50–R56. [Google Scholar] [CrossRef]
- Borrelli, E.; Roux-Lombard, P.; Grau, G.E.; Girardin, E.; Ricou, B.; Dayer, J.; Suter, P.M. Plasma concentrations of cytokines, their soluble receptors, and antioxidant vitamins can predict the development of multiple organ failure in patients at risk. Crit. Care Med. 1996, 24, 392–397. [Google Scholar] [CrossRef]
- Long, C.; Maull, K.; Krishnan, R.; Laws, H.; Geiger, J.; Borghesi, L.; Franks, W.; Lawson, T.; Sauberlich, H. Ascorbic acid dynamics in the seriously ill and injured. J. Surg. Res. 2003, 109, 144–148. [Google Scholar] [CrossRef]
- Fisher, B.J.; Kraskauskas, D.; Martin, E.J.; Farkas, D.; Puri, P.; Massey, H.D.; Idowu, M.O.; Brophy, D.F.; Voelkel, N.F.; Fowler, A.A.; et al. Attenuation of Sepsis-Induced Organ Injury in Mice by Vitamin C. J. Parenter. Enter. Nutr. 2014, 38, 825–839. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, H.; Matsuda, T.; Miyagantani, Y.; Yukioka, T.; Matsuda, H.; Shimazaki, S. Reduction of Resuscitation Fluid Volumes in Severely Burned Patients Using Ascorbic Acid Administration. Arch. Surg. 2000, 135, 326. [Google Scholar] [CrossRef] [PubMed]
- Dubick, M.A.; Williams, C.; Elgjo, G.I.; Kramer, G.C. High-dose Vitamin C infusion reduces fluid requirements in the resuscitation of burn-injured sheep. Shock 2005, 24, 139–144. [Google Scholar] [CrossRef]
- Fowler, A.A.; Syed, A.A.; Knowlson, S.; Sculthorpe, R.; Farthing, D.; DeWilde, C.; Farthing, C.A.; Larus, T.L.; Martin, E.; Brophy, D.F.; et al. Phase I safety trial of intravenous ascorbic acid in patients with severe sepsis. J. Transl. Med. 2014, 12, 32. [Google Scholar] [CrossRef]
- Mohammed, B.M.; Fisher, B.J.; Huynh, Q.K.; Wijesinghe, D.S.; Chalfant, C.E.; Brophy, D.F.; Fowler, A.A.; Natarajan, R. Resolution of sterile inflammation: Role for vitamin C. Mediat. Inflamm. 2014, 2014, 173403. [Google Scholar] [CrossRef]
- Traber, M.G.; Atkinson, J. Vitamin E, antioxidant and nothing more. Free Radic. Biol. Med. 2007, 43, 4–15. [Google Scholar] [CrossRef]
- Traber, M.G.; Packer, L. Vitamin E: Beyond antioxidant function. Am. J. Clin. Nutr. 1995, 62, 1501S–1509S. [Google Scholar] [CrossRef]
- Azzi, A.; Boscoboinik, D.; Chatelain, E.; Özer, N.K.; Stäuble, B. d-α-tocopherol control of cell proliferation. Mol. Aspects Med. 1993, 14, 265–271. [Google Scholar] [CrossRef]
- Goode, H.F.; Cowley, H.C.; Walker, B.E.; Howdle, P.D.; Webster, N.R. Decreased antioxidant status and increased lipid peroxidation in patients with septic shock and secondary organ dysfunction. Crit. Care Med. 1995, 23, 646–651. [Google Scholar] [CrossRef]
- Takeda, K.; Shimada, Y.; Amano, M.; Sakai, T.; Okada, T.; Yoshiya, I. Plasma lipid peroxides and alpha-tocopherol in critically ill patients. Crit. Care Med. 1984, 12, 957–959. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, K.; Niki, E. Interaction of α-tocopherol with iron: Antioxidant and prooxidant effects of α-tocopherol in the oxidation of lipids in aqueous dispersions in the presence of iron. Biochim. Biophys. Acta-Lipids Lipid Metab. 1988, 958, 19–23. [Google Scholar] [CrossRef]
- Peck, M.D.; Alexander, J.W. Survival in Septic Guinea Pigs Is Influenced by Vitamin E, but Not by Vitamin C in Enteral Diets. J. Parenter. Enter. Nutr. 1991, 15, 433–436. [Google Scholar] [CrossRef] [PubMed]
- Devaraj, S.; Li, D.; Jialal, I. The effects of alpha tocopherol supplementation on monocyte function. Decreased lipid oxidation, interleukin 1 beta secretion, and monocyte adhesion to endothelium. J. Clin. Investig. 1996, 98, 756–763. [Google Scholar] [CrossRef] [PubMed]
- Bulger, E.M.; Maier, R.V. An argument for Vitamin E supplementation in the management of systemic inflammatory response syndrome. Shock 2003, 19, 99–103. [Google Scholar] [CrossRef]
- Atli, M.; Erikoglu, M.; Kaynak, A.; Esen, H.H.; Kurban, S. The effects of selenium and vitamin E on lung tissue in rats with sepsis. Clin. Investig. Med. 2012, 35, 48. [Google Scholar] [CrossRef]
- Koga, H.; Hagiwara, S.; Inomata, M.; Kono, Y.; Oyama, Y.; Kai, S.; Nishida, T.; Noguchi, T. The New Vitamin E Derivative, ETS-GS, Protects Against Cecal Ligation and Puncture-Induced Systemic Inflammation in Rats. Inflammation 2012, 35, 545–553. [Google Scholar] [CrossRef]
- Gerster, H. The potential role of lycopene for human health. J. Am. Coll. Nutr. 1997, 16, 109–126. [Google Scholar] [CrossRef]
- Rao, A.V.; Rao, L.G. Carotenoids and human health. Pharmacol. Res. 2007, 55, 207–216. [Google Scholar] [CrossRef]
- Iyama, T.; Takasuga, A.; Azuma, M. beta-Carotene accumulation in mouse tissues and a protective role against lipid peroxidation. Int. J. Vitam. Nutr. Res. 1996, 66, 301–305. [Google Scholar]
- Field, C.J.; Johnson, I.R.; Schley, P.D. Nutrients and their role in host resistance to infection. J. Leukoc. Biol. 2002, 71, 16–32. [Google Scholar] [PubMed]
- Cox, S.E.; Arthur, P.; Kirkwood, B.R.; Yeboah-Antwi, K.; Riley, E.M. Vitamin A supplementation increases ratios of proinflammatory to anti-inflammatory cytokine responses in pregnancy and lactation. Clin. Exp. Immunol. 2006, 144, 392–400. [Google Scholar] [CrossRef] [PubMed]
- Bouayed, J.; Bohn, T. Exogenous antioxidants--Double-edged swords in cellular redox state: Health beneficial effects at physiologic doses versus deleterious effects at high doses. Oxid. Med. Cell. Longev. 2010, 3, 228–237. [Google Scholar] [CrossRef] [PubMed]
- Jang, S.H.; Lim, J.W.; Kim, H. Beta-carotene inhibits Helicobacter pylori-induced expression of inducible nitric oxide synthase and cyclooxygenase-2 in human gastric epithelial AGS cells. J. Physiol. Pharmacol. 2009, 60 (Suppl. 7), 131–137. [Google Scholar]
- Gawronski, C.A.; Gawronski, K.M. Vitamin A Supplementation for Prevention of Bronchopulmonary Dysplasia. Ann. Pharmacother. 2016, 50, 680–684. [Google Scholar] [CrossRef]
- Klassert, T.E.; Bräuer, J.; Hölzer, M.; Stock, M.; Riege, K.; Zubiría-Barrera, C.; Müller, M.M.; Rummler, S.; Skerka, C.; Marz, M.; et al. Differential Effects of Vitamins A and D on the Transcriptional Landscape of Human Monocytes during Infection. Sci. Rep. 2017, 7, 40599. [Google Scholar] [CrossRef]
- Atkuri, K.R.; Mantovani, J.J.; Herzenberg, L.A.; Herzenberg, L.A. N-Acetylcysteine—A safe antidote for cysteine/glutathione deficiency. Curr. Opin. Pharmacol. 2007, 7, 355–359. [Google Scholar] [CrossRef]
- Rank, N.; Michel, C.; Haertel, C.; Lenhart, A.; Welte, M.; Meier-Hellmann, A.; Spies, C. N-acetylcysteine increases liver blood flow and improves liver function in septic shock patients: Results of a prospective, randomized, double-blind study. Crit. Care Med. 2000, 28, 3799–3807. [Google Scholar] [CrossRef]
- Zuin, R.; Palamidese, A.; Negrin, R.; Catozzo, L.; Scarda, A.; Balbinot, M. High-Dose N-Acetylcysteine in??Patients with Exacerbations of??Chronic Obstructive Pulmonary Disease. Clin. Drug Investig. 2005, 25, 401–408. [Google Scholar] [CrossRef]
- Pinkus, R.; Weiner, L.M.; Daniel, V. Role of oxidants and antioxidants in the induction of AP-1, NF-kappaB, and glutathione S-transferase gene expression. J. Biol. Chem. 1996, 271, 13422–13429. [Google Scholar] [CrossRef]
- Emet, S.; Memis, D.; Pamukçu, Z. The influence of N-acetyl-L-cystein infusion on cytokine levels and gastric intramucosal pH during severe sepsis. Crit. Care. 2004, 8, R172–R179. [Google Scholar] [CrossRef]
- Spapen, H.D.; Diltoer, M.W.; Nguyen, D.N.; Hendrickx, I.; Huyghens, L.P. Effects of N-acetylcysteine on Microalbuminuria and Organ Failure in Acute Severe Sepsis: Results of a Pilot Study. Chest 2005, 127, 1413–1419. [Google Scholar] [CrossRef]
- Çağlıkülekci, M.; Pata, C.; Apa, D.D.; Dirlik, M.; Tamer, L.; Yaylak, F.; Kanik, A.; Aydin, S. The effect of N-acetylcysteine (NAC) on liver and renal tissue inducible nitric oxide synthase (iNOS) and tissue lipid peroxidation in obstructive jaundice stimulated by lipopolysaccharide (LPS). Pharmacol. Res. 2004, 49, 227–238. [Google Scholar] [CrossRef]
- Najafi, A.; Mojtahedzadeh, M.; Ahmadi, K.; Abdollahi, M.; Mousavi, M.; Chelkeba, L.; Najmeddin, F.; Ahmadi, A. The immunological benefit of higher dose N-acetyl cysteine following mechanical ventilation in critically ill patients. DARU J. Pharm. Sci. 2014, 22, 57. [Google Scholar] [CrossRef]
- Gomes, B.Q.; da Silva, L.; Gomes, A.Q.; Moreira, D.; Dolabela, M.; Santos, R.; Green, M.; Carvalho, E.; Percário, S. N-acetyl cysteine and mushroom Agaricus sylvaticus supplementation decreased parasitaemia and pulmonary oxidative stress in a mice model of malaria. Malar. J. 2015, 14, 202. [Google Scholar] [CrossRef]
- Ergin, B.; Guerci, P.; Zafrani, L.; Nocken, F.; Kandil, A.; Gurel-Gurevin, E.; Demirci-Tansel, C.; Ince, C. Effects of N-acetylcysteine (NAC) supplementation in resuscitation fluids on renal microcirculatory oxygenation, inflammation, and function in a rat model of endotoxemia. Intensive Care Med. Exp. 2016, 4, 29. [Google Scholar] [CrossRef]
- Visvanathan, V. N-acetylcysteine for sepsis and systemic inflammatory response in adults. Crit. Care Nurse. 2013, 33, 76–77. [Google Scholar] [CrossRef]
- Brzezinski, A. Melatonin in Humans. N. Engl. J. Med. 1997, 336, 186–195. [Google Scholar] [CrossRef]
- Tan, D.-X.; Manchester, L.C.; Reiter, R.J.; Plummer, B.F.; Limson, J.; Weintraub, S.T.; Qi, W. Melatonin directly scavenges hydrogen peroxide: A potentially new metabolic pathway of melatonin biotransformation. Free Radic. Biol. Med. 2000, 29, 1177–1185. [Google Scholar] [CrossRef]
- Reiter, R.J.; Tan, D.-X.; Korkmaz, A. The circadian melatonin rhythm and its modulation: Possible impact on hypertension. J. Hypertens. 2009, 27 (Suppl. 6), S17–S20. [Google Scholar] [CrossRef]
- Cuzzocrea, S.; Mazzon, E.; Dugo, L.; Caputi, A.P.; Aston, K.; Riley, D.P.; Salvemini, D. Protective effects of a new stable, highly active SOD mimetic, M40401 in splanchnic artery occlusion and reperfusion. Br. J. Pharmacol. 2001, 132, 19–29. [Google Scholar] [CrossRef]
- López, L.C.; Escames, G.; Tapias, V.; Utrilla, P.; León, J.; Acuña-Castroviejo, D. Identification of an inducible nitric oxide synthase in diaphragm mitochondria from septic mice: Its relation with mitochondrial dysfunction and prevention by melatonin. Int. J. Biochem. Cell Biol. 2006, 38, 267–278. [Google Scholar] [CrossRef]
- Escames, G.; León, J.; Macías, M.; Khaldy, H.; Acuña-Castroviejo, D. Melatonin counteracts lipopolysaccharide-induced expression and activity of mitochondrial nitric oxide synthase in rats. FASEB J. 2003, 17, 932–934. [Google Scholar] [CrossRef]
- Ozkok, E.; Yorulmaz, H.; Ates, G.; Aksu, A.; Balkis, N.; Şahin, Ö.; Tamer, S. Amelioration of energy metabolism by melatonin in skeletal muscle of rats with LPS induced endotoxemia. Physiol. Res. 2016, 65, 833–842. [Google Scholar] [CrossRef]
- Plessis, S.S.d.; Hagenaar, K.; Lampiao, F. The in vitro effects of melatonin on human sperm function and its scavenging activities on NO and ROS. Andrologia 2010, 42, 112–116. [Google Scholar] [CrossRef]
- Zavodnik, I.B.; Domanski, A.V.; Lapshina, E.A.; Bryszewska, M.; Reiter, R.J. Melatonin directly scavenges free radicals generated in red blood cells and a cell-free system: Chemiluminescence measurements and theoretical calculations. Life Sci. 2006, 79, 391–400. [Google Scholar] [CrossRef]
- Hardeland, R.; Cardinali, D.P.; Brown, G.M.; Pandi-Perumal, S.R. Melatonin and brain inflammaging. Prog. Neurobiol. 2015, 127–128, 46–63. [Google Scholar] [CrossRef]
- Hu, W.; Deng, C.; Ma, Z.; Wang, D.; Fan, C.; Li, T.; Di, S.; Gong, B.; Reiter, R.J.; Yang, Y. Utilizing melatonin to combat bacterial infections and septic injury. Br. J. Pharmacol. 2017, 174, 754–768. [Google Scholar] [CrossRef]
- El-Gendy, F.M.; El-Hawy, M.A.; Hassan, M.G. Beneficial effect of melatonin in the treatment of neonatal sepsis. J. Matern. Neonatal Med. 2018, 31, 2299–2303. [Google Scholar] [CrossRef]
- Júnior, J.V.R.; Araújo, G.R.; Pádua, B.D.C.; Magalhães, C.L.d.; Chaves, M.M.; Pedrosa, M.L.; Silva, M.E.; Costa, D.C. Annatto extract and β-carotene enhances antioxidant status and regulate gene expression in neutrophils of diabetic rats. Free Radic. Res. 2012, 46, 329–338. [Google Scholar] [CrossRef]
- Aung, H.H.; Vasu, V.T.; Valacchi, G.; Corbacho, A.M.; Kota, R.S.; Lim, Y.; Obermueller-Jevic, U.C.; Packer, L.; Cross, C.E.; Gohil, K. Effects of dietary carotenoids on mouse lung genomic profiles and their modulatory effects on short-term cigarette smoke exposures. Genes Nutr. 2009, 4, 23. [Google Scholar] [CrossRef]
- Harari, A.; Harats, D.; Marko, D.; Cohen, H.; Barshack, I.; Kamari, Y.; Gonen, A.; Gerber, Y.; Ben-Amotz, A.; Shaish, A. A 9-cis β-Carotene–Enriched Diet Inhibits Atherogenesis and Fatty Liver Formation in LDL Receptor Knockout Mice. J. Nutr. 2008, 138, 1923–1930. [Google Scholar] [CrossRef]
- Milman, U.; Blum, S.; Shapira, C.; Aronson, D.; Miller-Lotan, R.; Anbinder, Y.; Alshiek, J.; Bennett, L.; Kostenko, M.; Landau, M.; et al. Vitamin E Supplementation Reduces Cardiovascular Events in a Subgroup of Middle-Aged Individuals with Both Type 2 Diabetes Mellitus and the Haptoglobin 2-2 Genotype. Arterioscler. Thromb. Vasc. Biol. 2008, 28, 341–347. [Google Scholar] [CrossRef]
- Durant, R.; Klouche, K.; Delbosc, S.; Morena, M.; Amigues, L.; Beraud, J.J.; Canaud, B.; Cristol, J.P. Superoxide anion overproduction in sepsis: Effects of vitamin E and simvastatin. Shock 2004, 22, 34–39. [Google Scholar] [CrossRef]
- Kim, J.Y.; Lee, S.M. Effect of alpha-tocopherol on the expression of hepatic vascular stress genes in response to sepsis. J. Toxicol. Environ. Health-Part A 2005, 68, 2051–2062. [Google Scholar] [CrossRef]
- Aisa-Alvarez, A.; Gamboa, R.; Guarner-Lans, V.; Soto, M. A Randomized clinical trial of antioxidant therapy in patients with septic shock. Reference study to propose adjuvant therapy in patients with critical organic damage by COVID-19. EuropePMC 2020. preprint. [Google Scholar]
- Jun, H.-J.; Kim, S.; Dawson, K.; Choi, D.-W.; Kim, J.-S.; Rodriguez, R.L.; Lee, S.-J. Effects of Acute Oral Administration of Vitamin C on the Mouse Liver Transcriptome. J. Med. Food 2011, 14, 181–194. [Google Scholar] [CrossRef]
- May, J.M.; Harrison, F.E. Role of Vitamin C in the Function of the Vascular Endothelium. Antioxid. Redox Signal. 2013, 19, 2068–2083. [Google Scholar] [CrossRef]
- Ardite, E.; Sans, M.; Panés, J.; Romero, F.J.; Piqué, J.M.; Fernández-Checa, J.C. Replenishment of Glutathione Levels Improves Mucosal Function in Experimental Acute Colitis. Lab. Investig. 2000, 80, 735–744. [Google Scholar] [CrossRef]
- Ortolani, O.; Conti, A.; de Gaudio, A.R.; Moraldi, E.; Cantini, Q.; Novelli, G. The effect of glutathione and N-acetylcysteine on lipoperoxidative damage in patients with early septic shock. Am. J. Respir. Crit. Care Med. 2000, 161, 1907–1911. [Google Scholar] [CrossRef]
- Nieto, N.; Torres, M.I.; Fernández, M.I.; Girón, M.D.; Ríos, A.; Suárez, M.D.; Gil, A. Experimental Ulcerative Colitis Impairs Antioxidant Defense System in Rat Intestine. Dig. Dis. Sci. 2000, 45, 1820–1827. [Google Scholar] [CrossRef]
- Spasojević, I.; Chen, Y.; Noel, T.J.; Yu, Y.; Cole, M.P.; Zhang, L.; Zhao, Y.; Clair, D.K.S.; Batinić-Haberle, I. Mn porphyrin-based superoxide dismutase (SOD) mimic, MnIIITE-2-PyP5+, targets mouse heart mitochondria. Free Radic. Biol. Med. 2007, 42, 1193–1200. [Google Scholar] [CrossRef]
- Clausen, A.; Xu, X.; Bi, X.; Baudry, M. Effects of the Superoxide Dismutase/Catalase Mimetic EUK-207 in a Mouse Model of Alzheimer’s Disease: Protection Against and Interruption of Progression of Amyloid and Tau Pathology and Cognitive Decline. J. Alzheimer’s Dis. 2012, 30, 183–208. [Google Scholar] [CrossRef]
- Nazıroğlu, M.; Şenol, N.; Ghazizadeh, V.; Yürüker, V. Neuroprotection Induced by N-acetylcysteine and Selenium Against Traumatic Brain Injury-Induced Apoptosis and Calcium Entry in Hippocampus of Rat. Cell. Mol. Neurobiol. 2014, 34, 895–903. [Google Scholar] [CrossRef]
- Pandya, J.D.; Readnower, R.D.; Patel, S.P.; Yonutas, H.M.; Pauly, J.R.; Goldstein, G.A.; Rabchevsky, A.G.; Sullivan, P.G. N-acetylcysteine amide confers neuroprotection, improves bioenergetics and behavioral outcome following TBI. Exp. Neurol. 2014, 257, 106–113. [Google Scholar] [CrossRef]
- Hein, O.V.; Ohring, R.; Schilling, A.; Oellerich, M.; Armstrong, V.W.; Kox, W.J.; Spies, C. N-acetylcysteine decreases lactate signal intensities in liver tissue and improves liver function in septic shock patients, as shown by magnetic resonance spectroscopy: Extended case report. Crit. Care 2004, 8, R66–R71. [Google Scholar] [CrossRef]
- Lowes, D.A.; Thottakam, B.M.V.; Webster, N.R.; Murphy, M.P.; Galley, H.F. The mitochondria-targeted antioxidant MitoQ protects against organ damage in a lipopolysaccharide-peptidoglycan model of sepsis. Free Radic. Biol. Med. 2008, 45, 1559–1565. [Google Scholar] [CrossRef]
- Supinski, G.S.; Murphy, M.P.; Callahan, L.A. MitoQ administration prevents endotoxin-induced cardiac dysfunction. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 2009, 297, 1095–1102. [Google Scholar] [CrossRef]
- Macias, C.A.; Chiao, J.W.; Xiao, J.; Arora, D.S.; Tyurina, Y.Y.; Delude, R.L.; Wipf, P.; Kagan, V.E.; Fink, M.P. Treatment with a novel hemigramicidin-TEMPO conjugate prolongs survival in a rat model of lethal hemorrhagic shock. Ann. Surg. 2007, 245, 305–314. [Google Scholar] [CrossRef]
- Plotnikov, E.Y.; Pevzner, I.B.; Zorova, L.D.; Chernikov, V.P.; Prusov, A.N.; Kireev, I.I.; Silachev, D.N.; Skulachev, V.P.; Zorov, D.B. Mitochondrial damage and mitochondria-targeted antioxidant protection in LPS-induced acute kidney injury. Antioxidants 2019, 8, 176. [Google Scholar] [CrossRef]
- Supinski, G.S.; Wang, L.; Schroder, E.A.; Callahan, L.A.P. MitoTEMPOL, a mitochondrial targeted antioxidant, prevents sepsis-induced diaphragm dysfunction. Am. J. Physiol.-Lung Cell. Mol. Physiol. 2020, 319, L228–L238. [Google Scholar] [CrossRef]
- Liu, Y.; Yang, W.; Sun, X.; Xie, L.; Yang, Y.; Sang, M.; Jiao, R. SS31 Ameliorates Sepsis-Induced Heart Injury by Inhibiting Oxidative Stress and Inflammation. Inflammation 2019, 42, 2170–2180. [Google Scholar] [CrossRef]
- Supinski, G.S.; Wang, L.; Schroder, E.A.; Callahan, L.A.P. SS31, a mitochondrially targeted antioxidant, prevents sepsis-induced reductions in diaphragm strength and endurance. J. Appl. Physiol. 2020, 128, 463–472. [Google Scholar] [CrossRef]
- Zhang, S.; Zhou, Q.; Li, Y.; Zhang, Y.; Wu, Y. MitoQ Modulates Lipopolysaccharide-Induced Intestinal Barrier Dysfunction via Regulating Nrf2 Signaling. Mediat. Inflamm. 2020, 2020, 3276148. [Google Scholar] [CrossRef]
- Galkin, I.I.; Pletjushkina, O.Y.; Zinovkin, R.A.; Zakharova, V.V.; Chernyak, B.V.; Popova, E.N. Mitochondria-targeted antioxidant SkQR1 reduces TNF-induced endothelial permeability in vitro. Biochemistry 2016, 81, 1188–1197. [Google Scholar] [CrossRef]
- Shan, B.; Li, J.-Y.; Liu, Y.-J.; Tang, X.-B.; Zhou, Z.; Luo, L.-X. LncRNA H19 Inhibits the Progression of Sepsis-Induced Myocardial Injury via Regulation of the miR-93-5p/SORBS2 Axis. Inflammation 2020, 44, 344–357. [Google Scholar] [CrossRef]
- Liu, Z.-Q. Antioxidants may not always be beneficial to health. Nutrition 2014, 30, 131–133. [Google Scholar] [CrossRef]
- Fang, J.C.; Kinlay, S.; Beltrame, J.; Hikiti, H.; Wainstein, M.; Behrendt, D.; Suh, J.; Frei, B.; Mudge, G.H.; Selwyn, A.; et al. Effect of vitamins C and E on progression of transplant-associated arteriosclerosis: A randomised trial. Lancet 2002, 359, 1108–1113. [Google Scholar] [CrossRef]
- Pironi, L.; Guidetti, M.; Zolezzi, C.; Fasano, M.C.; Paganelli, F.; Merli, C.; Bersani, G.; Pizzoferrato, A.; Miglioli, M. Peroxidation potential of lipid emulsions after compounding in all-in-one solutions. Nutrition 2003, 19, 784–788. [Google Scholar] [CrossRef]
- Dyer, A.; Elliott, P.; Stamler, J.; Chan, Q.; Ueshima, H.; Zhou, B. Dietary intake in male and female smokers, ex-smokers and never smokers: The INTERMAP Study. J. Hum. Hypertens. 2003, 17, 641–654. [Google Scholar] [CrossRef]
- Miller, E.R.; Pastor-Barriuso, R.; Dalal, D.; Riemersma, R.A.; Appel, L.J.; Guallar, E. Meta-Analysis: High-Dosage Vitamin E Supplementation May Increase All-Cause Mortality. Ann. Intern. Med. 2005, 142, 37. [Google Scholar] [CrossRef] [PubMed]
- Mitra, S.; Deshmukh, A.; Sachdeva, R.; Lu, J.; Mehta, J.L. Oxidized Low-Density Lipoprotein and Atherosclerosis Implications in Antioxidant Therapy. Am. J. Med. Sci. 2011, 342, 135–142. [Google Scholar] [CrossRef] [PubMed]
- Ramos, R.; Martínez-Castelao, A. Lipoperoxidation and hemodialysis. Metabolism 2008, 57, 1369–1374. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, A.; Majzoub, A. Laboratory tests for oxidative stress. Indian J. Urol. 2017, 33, 199–206. [Google Scholar] [CrossRef]
- Waldbaum, S.; Patel, M. Mitochondrial dysfunction and oxidative stress: A contributing link to acquired epilepsy? J. Bioenerg. Biomembr. 2010, 42, 449–455. [Google Scholar] [CrossRef]
- Baba, S.; Osakabe, N.; Kato, Y.; Natsume, M.; Yasuda, A.; Kido, T.; Fukuda, K.; Muto, Y.; Kondo, K. Continuous intake of polyphenolic compounds containing cocoa powder reduces LDL oxidative susceptibility and has beneficial effects on plasma HDL-cholesterol concentrations in humans. Am. J. Clin. Nutr. 2007, 85, 709–717. [Google Scholar] [CrossRef]
- Havsteen, B.H. The biochemistry and medical significance of the flavonoids. Pharmacol. Ther. 2002, 96, 67–202. [Google Scholar]
- Procházková, D.; Boušová, I.; Wilhelmová, N. Antioxidant and prooxidant properties of flavonoids. Fitoterapia 2011, 82, 513–523. [Google Scholar] [CrossRef]
- Galati, G.; O’Brien, P.J. Potential toxicity of flavonoids and other dietary phenolics: Significance for their chemopreventive and anticancer properties. Free Radic. Biol. Med. 2004, 37, 287–303. [Google Scholar] [CrossRef]
- Feng, P.; Chen, W.; Lin, H. Identifying Antioxidant Proteins by Using Optimal Dipeptide Compositions. Interdiscip. Sci. Comput. Life Sci. 2016, 8, 186–191. [Google Scholar] [CrossRef]
- Xu, L.; Liang, G.; Shi, S.; Liao, C. SeqSVM: A Sequence-Based Support Vector Machine Method for Identifying Antioxidant Proteins. Int. J. Mol. Sci. 2018, 19, 1773. [Google Scholar]
- Meng, C.; Jin, S.; Wang, L.; Guo, F.; Zou, Q. AOPs-SVM: A Sequence-Based Classifier of Antioxidant Proteins Using a Support Vector Machine. Front. Bioeng. Biotechnol. 2019, 7, 224. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Tang, Q.; Tang, H.; Chen, W. Identifying Antioxidant Proteins by Combining Multiple Methods. Front. Bioeng. Biotechnol. 2020, 8, 858. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, A.; Akbar, S.; Hayat, M.; Ali, F.; Khan, S.; Sohail, M. Identification of antioxidant proteins using a discriminative intelligent model of k-spaced amino acid pairs based descriptors incorporating with ensemble feature selection. Biocybern. Biomed. Eng. 2020, 42, 727–735. [Google Scholar] [CrossRef]
- Olsen, T.H.; Yesiltas, B.; Marin, F.I.; Pertseva, M.; García-Moreno, P.J.; Gregersen, S.; Overgaard, M.T.; Jacobsen, C.; Lund, O.; Hansen, E.B.; et al. AnOxPePred: Using deep learning for the prediction of antioxidative properties of peptides. Sci. Rep. 2020, 10, 21471. [Google Scholar] [CrossRef]
| Oxidants (ROS) | Enzyme/Ion | Mechanism/Reaction | References |
|---|---|---|---|
| Superoxide radical (O2−) | NADPH oxidase | NADPH + 2O2 ➞ 2O2− + NADP+ + H+ | [15] |
| Hydrogen peroxide (H2O2) | SOD | 2O2− + 2H+ ➞ H2O2 + O2 | [16] |
| Hydroxyl radical (OH−) | Fe2+ | H2O2 ➞ OH− + OH. | [17] |
| Hypochlorous acid (HOCl) | Myeloperoxidase | H2O2 + Cl− + H+ ➞ HOCl + H2O | [18] |
| Peroxynitrite (OONO−) | NO. | NO. + O2− ➞ ONOO− | [19] |
| Nitric oxide (NO.) | NOS | L-Arginine + O2 ➞ L-Citrulline + NO | [20] |
| Antioxidant | Location | Mechanism | References | |
|---|---|---|---|---|
| Enzymatic | Superoxide dismutase | Cytoplasm, mitochondria, peroxisome, and chloroplast | O2− to O2 and H2O2 | [61,62] |
| Catalase | Peroxisome and mitochondria | H2O2 to H2O and O2 | [62,63] | |
| Glutathione peroxidase | Cytoplasm, mitochondria, and chloroplast | H2O2 to H2O | [64] | |
| Non-enzymatic | Vitamin E (exogenous) | Cell membrane | Increases plasma NO, protects against oxidative damage induced-impaired vasorelaxation; prevents tumor initiation | [65] |
| Vitamin C (exogenous) | Cytoplasm, mitochondria, peroxisome, and chloroplast | Decreases cellular oxidative damage, prevents tumor initiation; scavenging radical species; decreases pro-inflammatory cytokines; inhibition of NOx and inducible nitric oxide synthase | [49,66] | |
| Vitamin A (exogenous) | Chloroplasts | NF-κβ inhibition; 10% reduction of ROS by β-carotene; upregulation of Nrf2 expression | [67,68,69] | |
| N-Acetyl cysteine (NAC) (endogenous) | Administered orally or topically | Increases glutathione level in the human body; NAC acts as a methyl donor in the conversion of homocysteine to methionine | [70,71] | |
| Melatonin (endogenous) | Pineal gland | Reduces the free radical generation by increasing the activity of the electron transport chain | [72] | |
| Vitamin | Animal Model | Tissue/ Cell | Outcomes | References |
|---|---|---|---|---|
| Vitamin A | Rats | Neutrophils | Reduces ROS generation by upregulating the SOD CAT, p22 and p47. | [159] |
| Vitamin A | Mice | Lung | Regulates expression of cytochrome P450 | [160] |
| Vitamin A | Mice | Liver | Downregulation of NF-κβ associated VCAM-1, IL-1α, MCP-1 and IFN-γ | [161] |
| Vitamin A | Mice | Monoblasts | Decreased NF-κβ activity | [65] |
| Vitamin E | Human | Lung | Reduces the stroke in CVD | [162] |
| Vitamin E | Ex-vivo human samples | Neutrophils | Decreased O2− plasma level | [163] |
| Vitamin E | Rat | Neutrophils | Induces an imbalance in hepatic vasoregulatory gene expression | [164] |
| Vitamin E | Human | Whole Body | Tends to decrease LPO | [165] |
| Vitamin C | Mice | Liver | Gene expression was changed | [166] |
| Vitamin C | Human | Endothelial cell | Improves endothelial function | [167] |
| Vitamin C | Sheep | Skin | Reduces inflammation | [113] |
| Vitamin C | Human | Whole Body | Increases its serum levels, which is associated with decreased levels of CRP, PCT, and NO3−/NO2−. | [165] |
| Glutathione | Rat | Mucosal tissue | Decreases glutathione concentration | [168] |
| Glutathione | Human | Plasma Cells | significantly decreased the peroxidative damage of patients with septic shock | [169] |
| SOD | Rat | Intestine | Increases sod concentration and decreased glutathione | [170] |
| SOD mimetic (M40401) | Rat | E.coli challenged animal, blood cells | Improves the vascular reactivity, reduced cytokine production, and mortality | [72] |
| SOD mimetic (MnIIITE-2-PyP5+) | Rat | Heart | Improves the vascular reactivity, reduced cytokine production, and mortality | [171] |
| CAT mimetic (EUK-207) | Mice | Brain cells | Reduces the level of lipid peroxidation and oxidized nucleic acids in brain cells | [172] |
| NAC | Rat | Hippocampus | Decreases the ROS activity as well as intracellular free Ca2+ | [173] |
| NAC | Rat | Neural cell | Maintains the level of GSH, increases bioenergetics, and decreases oxidative damage | [174] |
| NAC | Human | Whole Body | Reduces LPO and improves antioxidant capacity | [165] |
| NAC | Human | Blood | Improves liver function | [137] |
| NAC | Human | Blood | Decreases hepatic lactate; increases liver perfusion and function | [175] |
| MitoQ | Rat | Lungs, heart, liver, gut, and kidney | Attenuation in the levels of biochemical markers of acute liver and renal dysfunction, maintenance of mitochondrial membrane potential in most organs. | [176] |
| MitoQ | Rat, Mouse | Heart | Restores mitochondrial function and reduces caspase activity | [177] |
| Hemigramicidin-TEMPO conjugates | Rat | Intestine | Improves the survival | [178] |
| Plastoquinone decylrhodamine 19 (SkQR1), | Rat | Kidney | Increases antioxidants and shows a nephroprotective role | [179] |
| MitoTEMPOL | Mouse | Diaphragm | Reduces sepsis-induced diaphragm dysfunction | [180] |
| SS31 | Mouse | Restores myocardial morphological damage and suppresses inflammatory response | [181] | |
| SS31 | Mouse | Diaphragm | Reduces sepsis-induced diaphragm dysfunction, and maintains mitochondrial function | [182] |
| Server | Method | Sensitivity (%) | Specificity (%) | Accuracy (%) | Web-Server | Reference |
|---|---|---|---|---|---|---|
| AodPred | Support vector machine | 75.09 | 74.48 | 74.79 | http://lin.uestc.edu.cn/server/AntioxiPred (accessed on 1 January 2021) | [200] |
| SeqSVM | Support vector machine | --- | --- | 89.46 | --- | [201] |
| AOPs-SVM | Support vector machine | 68 | 98.5 | 94.2 | http://server.malab.cn/AOPs-SVM/index.jsp (accessed on 1 January 2021) | [202] |
| Vote9 | Support vector machine | 65 | 99 | 94.1 | --- | [203] |
| SFS-SVM | Support vector machine | --- | --- | 97.54 | https://github.com/salman-khan-mrd/Antioxident_proteins (accessed on 1 January 2021) | [204] |
| AnOxPePred | Deep convolutional neural network | --- | --- | --- | http://services.bioinformatics.dtu.dk/service.php?AnOxPePred-1.0 (accessed on 1 January 2021) | [205] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kumar, S.; Saxena, J.; Srivastava, V.K.; Kaushik, S.; Singh, H.; Abo-EL-Sooud, K.; Abdel-Daim, M.M.; Jyoti, A.; Saluja, R. The Interplay of Oxidative Stress and ROS Scavenging: Antioxidants as a Therapeutic Potential in Sepsis. Vaccines 2022, 10, 1575. https://doi.org/10.3390/vaccines10101575
Kumar S, Saxena J, Srivastava VK, Kaushik S, Singh H, Abo-EL-Sooud K, Abdel-Daim MM, Jyoti A, Saluja R. The Interplay of Oxidative Stress and ROS Scavenging: Antioxidants as a Therapeutic Potential in Sepsis. Vaccines. 2022; 10(10):1575. https://doi.org/10.3390/vaccines10101575
Chicago/Turabian StyleKumar, Sanni, Juhi Saxena, Vijay Kumar Srivastava, Sanket Kaushik, Himadri Singh, Khaled Abo-EL-Sooud, Mohamed M. Abdel-Daim, Anupam Jyoti, and Rohit Saluja. 2022. "The Interplay of Oxidative Stress and ROS Scavenging: Antioxidants as a Therapeutic Potential in Sepsis" Vaccines 10, no. 10: 1575. https://doi.org/10.3390/vaccines10101575
APA StyleKumar, S., Saxena, J., Srivastava, V. K., Kaushik, S., Singh, H., Abo-EL-Sooud, K., Abdel-Daim, M. M., Jyoti, A., & Saluja, R. (2022). The Interplay of Oxidative Stress and ROS Scavenging: Antioxidants as a Therapeutic Potential in Sepsis. Vaccines, 10(10), 1575. https://doi.org/10.3390/vaccines10101575









