Impairment of PGC-1 Alpha Up-Regulation Enhances Nitrosative Stress in the Liver during Acute Pancreatitis in Obese Mice
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Experimental Model of Acute Pancreatitis
2.3. RNA Extraction and RT-qPCR Analysis of Gene Expression
2.4. Western Blot Analysis
2.5. Co-Immunoprecipitation
2.6. Redox Pairs and Protein Nitration and Chlorination by UPLC-MS/MS Analysis
2.7. Energy Charge Determined by UPLC-MS/MS
2.8. Biochemical Assays
2.9. Histological Analysis
2.10. Statistical Analysis
3. Results
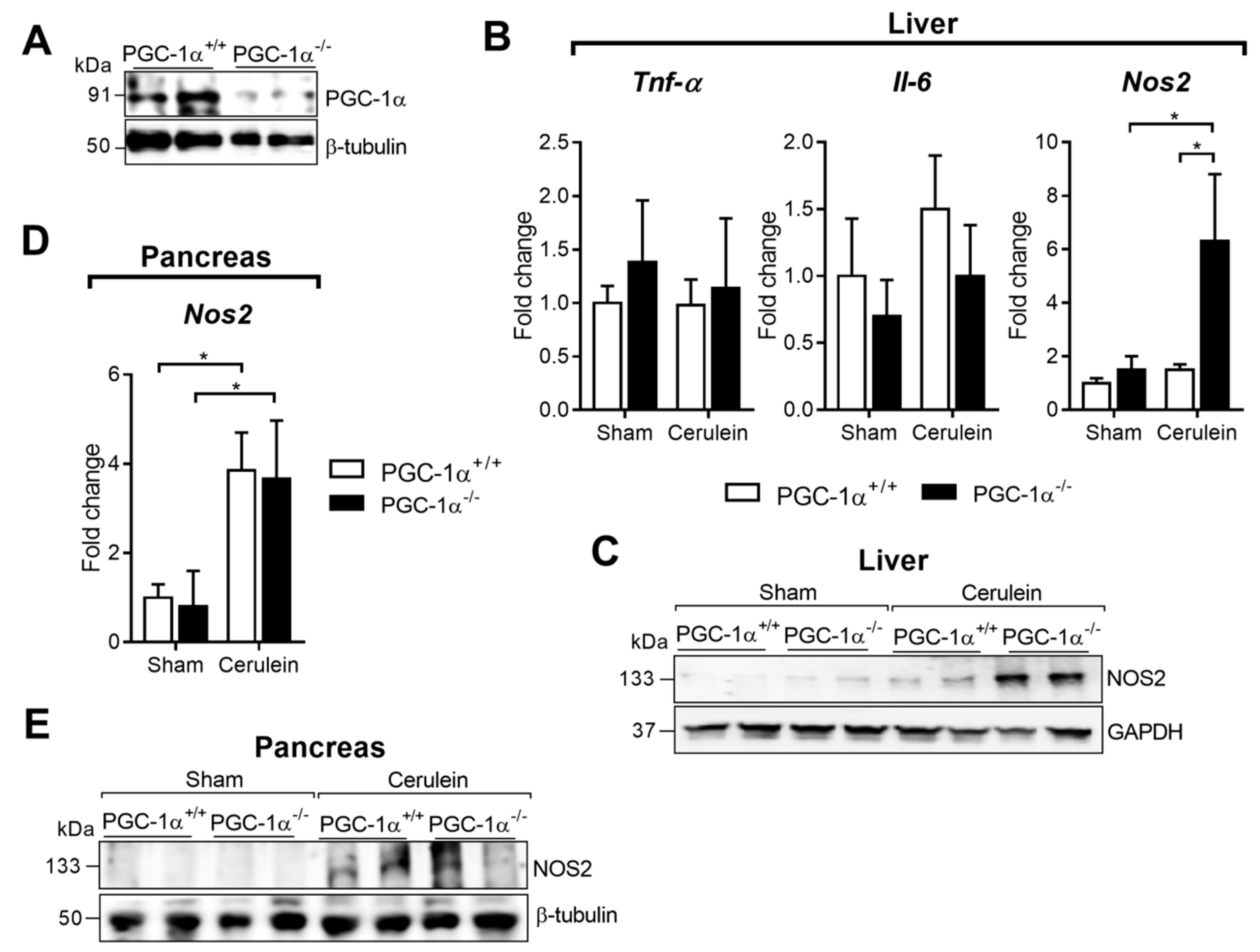
3.1. PGC-1α Levels are Up-Regulated in Mice Livers after Inducing Acute Pancreatitis
3.2. PGC-1α Restrains Nos2 Expression in the Liver after Acute Pancreatitis in Mice
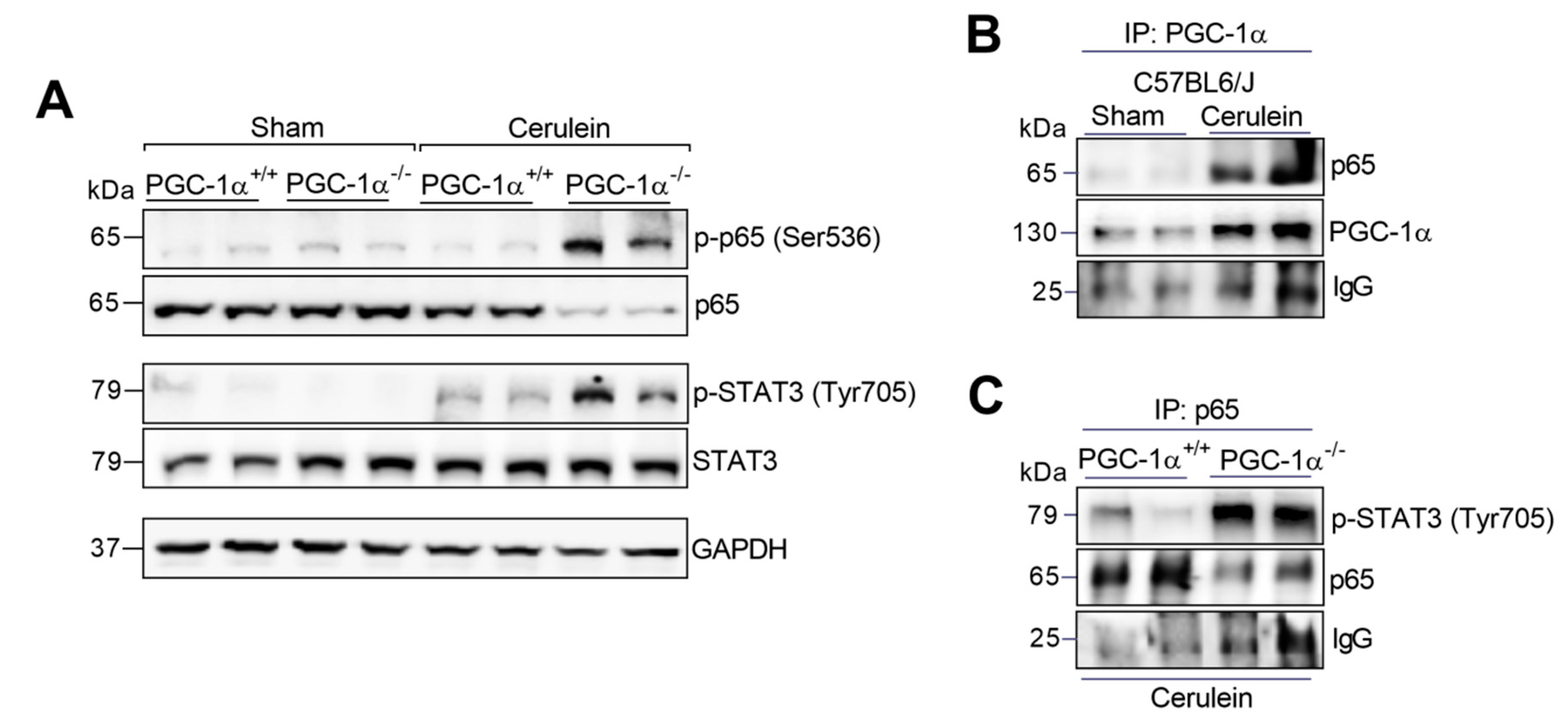
3.3. PGC-1α Avoids the Assembly of the Complex between p65 and Phospho-STAT3 in the Liver during Experimental Acute Pancreatitis
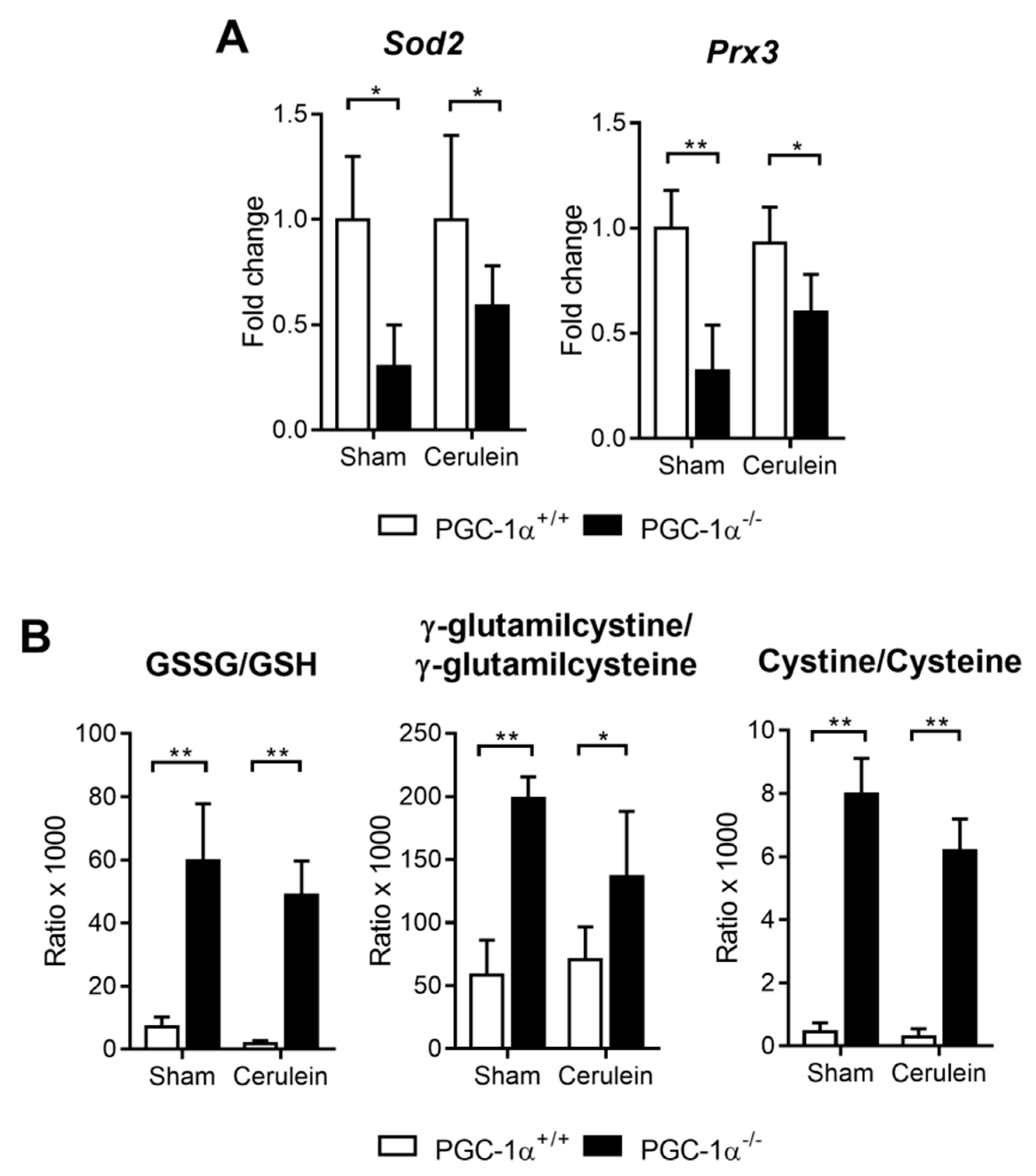
3.4. PGC-1α Deficiency Downregulates Antioxidant Gene Expression and Increases Oxidative Stress in the Liver with Acute Pancreatitis
3.5. PGC-1α Prevents Protein Nitration in the Liver after Inducing Experimental Acute Pancreatitis
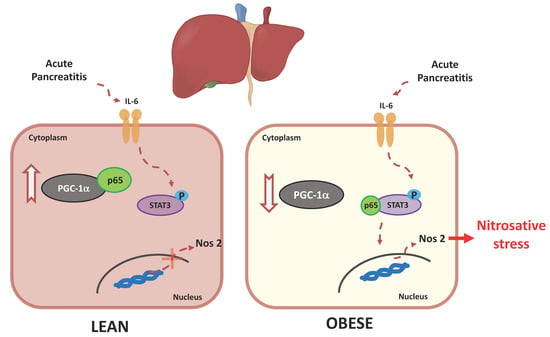
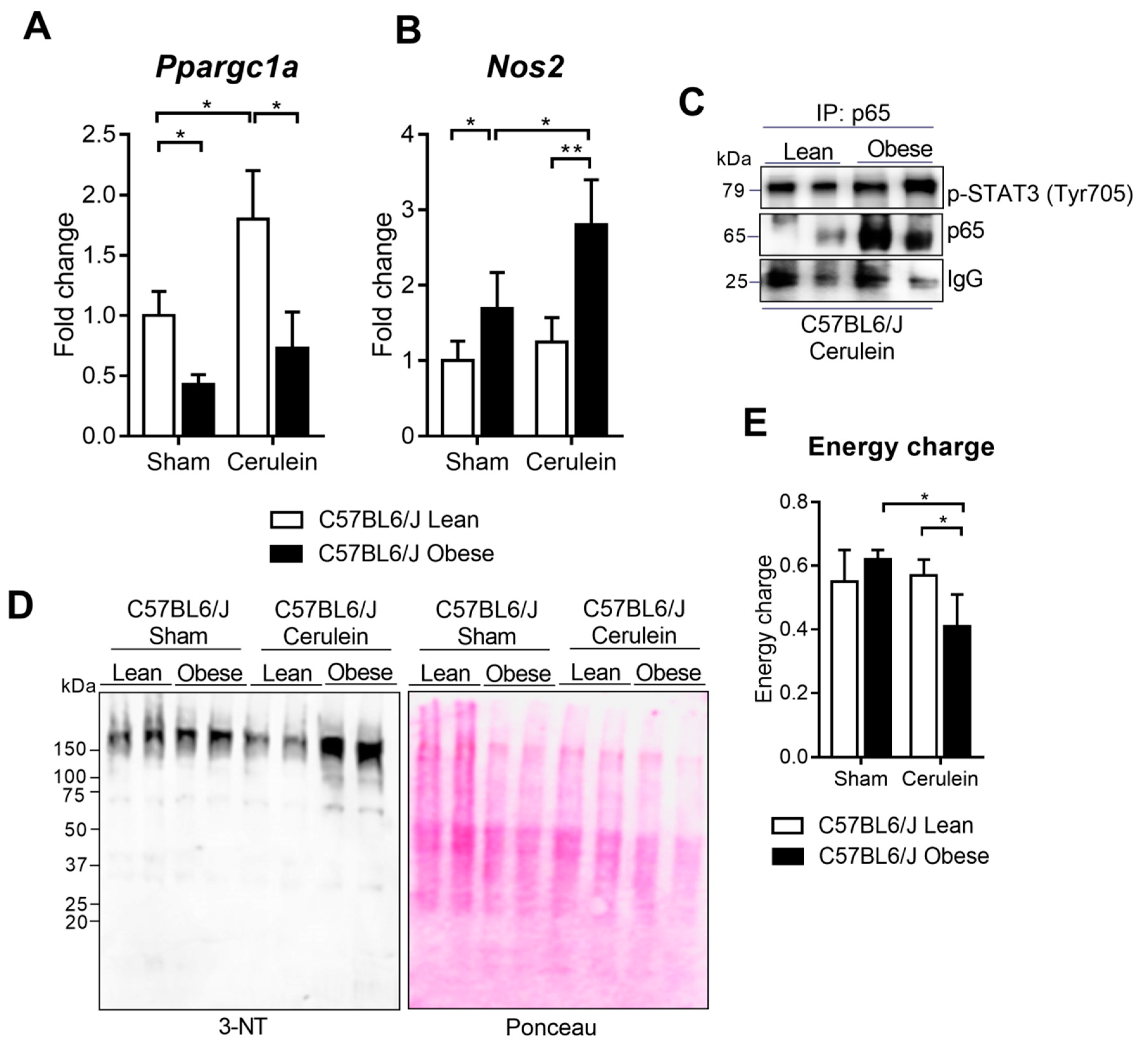
3.6. PGC-1α Levels Lower in the Livers of Obese Mice under Basal Conditions and during Pancreatitis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Crockett, S.D.; Wani, S.; Gardner, T.B.; Falck-Ytter, Y.; Barkun, A.N. American Gastroenterological Association Institute Clinical Guidelines Committee, American Gastroenterological Association Institute Guideline on Initial Management of Acute Pancreatitis. Gastroenterology. 2018, 154, 1096–1101. [Google Scholar] [CrossRef] [PubMed]
- Lankisch, P.G.; Apte, M.; Banks, P.A. Acute pancreatitis. Lancet 2015, 386, 85–96. [Google Scholar] [CrossRef]
- Schepers, N.J.; Bakker, O.J.; Besselink, M.G.; Ali, U.A.; Bollen, T.L.; Gooszen, H.G.; van Santvoort, H.C.; Bruno, M.J. Impact of characteristics of organ failure and infected necrosis on mortality in necrotising pancreatitis. Gut 2019, 68, 1044–1051. [Google Scholar] [CrossRef] [PubMed]
- Pérez, S.; Pereda, J.; Sabater, L.; Sastre, J. Redox signaling in acute pancreatitis. Redox Biol. 2015, 5, 1–14. [Google Scholar] [CrossRef]
- Tsai, K.; Wang, S.; Chen, T.; Kong, C.; Lee, S.; Lu, F. Oxidative stress: An important phenomenon with pathogenetic significance in the progression of acute pancreatitis. Gut 1998, 42, 850–855. [Google Scholar] [CrossRef]
- Sweiry, J.H.; Mann, G.E. Role of oxidative stress in the pathogenesis of acute pancreatitis. Scand. J. Gastroenterol. 1996, 31, 10–15. [Google Scholar] [CrossRef]
- Escobar, J.; Pereda, J.; Arduini, A.; Sandoval, J.; Moreno, M.L.; Pérez, S.; Sabater, L.; Aparici, L.; Cassinello, N.; Hidalgo, J.; et al. Oxidative and nitrosative stress in acute pancreatitis. Modul. Pentoxifylline Oxypurinol. Biochem. Pharmacol. 2011, 83, 122–130. [Google Scholar] [CrossRef]
- Rius-Pérez, S.; Pérez, S.; Torres-Cuevas, I.; Martí-Andrés, P.; Taléns-Visconti, R.; Paradela, A.; Guerrero, L.; Franco, L.; López-Rodas, G.; Torres, L.; et al. Blockade of the trans-sulfuration pathway in acute pancreatitis due to nitration of cystathionine β-synthase. Redox Biol. 2019, 28, 101324. [Google Scholar] [CrossRef]
- Ang, A.D.; Adhikari, S.; Ng, S.W.; Bhatia, M. Expression of Nitric Oxide Synthase Isoforms and Nitric Oxide Production in Acute Pancreatitis and Associated Lung Injury. Pancreatology 2009, 9, 150–159. [Google Scholar] [CrossRef]
- Cuzzocrea, S.; Mazzon, E.; Dugo, L.; Serrino, I.; Centorrino, E.; Ciccolo, A.; Van de Loo, F.; Britti, D.; Caputi, A.; Thiemermann, C. Inducible nitric oxide synthase-deficient mice exhibit resistance to the acute pancreatitis induced by cerulein. Shock 2002, 17, 416–422. [Google Scholar] [CrossRef]
- Yadav, D.; Lowenfels, A.B. The epidemiology of pancreatitis and pancreatic cancer. Gastroenterology 2013, 144, 1252–1261. [Google Scholar] [CrossRef] [PubMed]
- Emerenziani, S.; Pier Luca-Guarino, M.; Trilo-Asensio, L.M.; Altamore, A.; Ribolsi, M.; Balestrieri, P.; Cicala, M. Role of Overweight and Obesity in Gastrointestinal Disease. Nutrients 2020, 12, 111. [Google Scholar] [CrossRef] [PubMed]
- Khatua, B.; El-Kurdi, B.; Singh, V.P. Obesity and pancreatitis. Curr. Opin. Gastroenterol. 2017, 33, 374–382. [Google Scholar] [CrossRef] [PubMed]
- Garg, P.K.; Singh, V.P. Organ Failure due to Systemic Injury in Acute Pancreatitis. Gastroenterology 2019, 156, 2008–2023. [Google Scholar] [CrossRef]
- Folch-Puy, E. Importance of the liver in systemic complications associated with acute pancreatitis: The role of Kupffer cells. J. Pathol. 2007, 211, 383–388. [Google Scholar] [CrossRef]
- Segersvärd, R.; Tsai, J.A.; Herrington, M.K.; Wang, F. Obesity alters cytokine gene expression and promotes liver injury in rats with acute pancreatitis. Obesity (Silver Spring) 2008, 16, 23–28. [Google Scholar] [CrossRef]
- Yoon, S.B.; Lee, I.S.; Choi, M.H.; Lee, K.; Ham, H.; Oh, H.J.; Park, S.H.; Lim, C.-H.; Choi, M.-G. Impact of Fatty Liver on Acute Pancreatitis Severity. Gastroenterol. Res. Pract. 2017, 2017, 4532320. [Google Scholar] [CrossRef]
- Rius-Pérez, S.; Torres-Cuevas, I.; Millán, I.; Ortega, Á.L.; Pérez, S. PGC-1α, Inflammation, and Oxidative Stress: An Integrative View in Metabolism. Oxid. Med. Cell Longev. 2020, 2020, 1452696. [Google Scholar] [CrossRef]
- Valle, I.; Alvarez-Barrientos, A.; Arza, E.; Lamas, S.; Monsalve, M. PGC-1alpha regulates the mitochondrial antioxidant defense system in vascular endothelial cells. Cardiovasc. Res. 2005, 66, 562–573. [Google Scholar] [CrossRef]
- Olmos, Y.; Sánchez-Gómez, F.J.; Wild, B.; García-Quintans, N.; Cabezudo, S.; Lamas, S.; Monsalve, M. SirT1 regulation of antioxidant genes is dependent on the formation of a FoxO3a/PGC-1α complex. Antioxid. Redox Signal. 2013, 19, 1507–1521. [Google Scholar] [CrossRef]
- Eisele, P.S.; Salatino, S.; Sobek, J.; Hottiger, M.O.; Handschin, C. The peroxisome proliferator-activated receptor γ coactivator 1α/β (PGC-1) coactivators repress the transcriptional activity of NF-κB in skeletal muscle cells. J. Biol. Chem. 2013, 288, 2246–2260. [Google Scholar] [CrossRef] [PubMed]
- Yuan, S.; Liu, X.; Zhu, X.; Qu, Z.; Gong, Z.; Li, J.; Xiao, L.; Yang, Y.; Liu, H.; Sun, L.; et al. The Role of TLR4 on PGC-1α-Mediated Oxidative Stress in Tubular Cell in Diabetic Kidney Disease. Oxid. Med. Cell Longev. 2018, 2018, 6296802. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Wu, P.-H.; Tarr, P.T.; Lindenberg, K.S.; St-Pierre, J.; Zhang, C.-Y.; Mootha, V.K.; Jäger, S.; Vianna, C.R.; Reznick, R.M.; et al. Defects in adaptive energy metabolism with CNS-linked hyperactivity in PGC-1alpha null mice. Cell 2004, 119, 121–135. [Google Scholar] [CrossRef] [PubMed]
- Silva-Vaz, P.; Margarida-Abrantes, A.; Castelo-Branco, M.; Gouveia, A.; Botelho, M.F.; Ghiherme-Tralhao, J. Murine Models of Acute Pancreatitis: A Critical Appraisal of Clinical Relevance. Int. J. Mol. Sci. 2019, 20, 2794. [Google Scholar] [CrossRef]
- Monsalve, M.; Wu, Z.; Adelmant, G.; Puigserver, P.; Fan, M.; Spiegelman, B.M. Direct coupling of transcription and mRNA processing through the thermogenic coactivator PGC-1. Mol. Cell 2000, 6, 307–316. [Google Scholar] [CrossRef]
- Escobar, J.; Sánchez-Illana, A.; Kuligowski, J.; Torres-Cuevas, I.; Solberg, R.; Garberg, H.T.; Huun, M.U.; Saugstad, O.D.; Vento, M.; Cháfer-Pericás, C. Development of a reliable method based on ultra-performance liquid chromatography coupled to tandem mass spectrometry to measure thiol-associated oxidative stress in whole blood samples. J. Pharm. Biomed. Anal. 2016, 123, 104–112. [Google Scholar] [CrossRef]
- Henseley, K.; Maidt, M.L.; Pye, Q.N.; Stewart, C.A.; Wack, M.; Tabatabaie, T.; Floyd, R.A. Quantitation of protein-bound 3-nitrotyrosine and 3,4-dihydroxyphenylalanine by high-performance liquid chromatography with electrochemical array detection. Anal. Biochem. 1997, 251, 187–195. [Google Scholar] [CrossRef]
- Torres-Cuevas, I.; Kuligowsky, J.; Cárcel, M.; Cháfer-Pericas, C.; Asensi, M.; Solberg, R.; Cubells, E.; Nuñez, A.; Saugtad, O.D.; Vento, M.; et al. Protein-bound tyrosine oxidation, nitration and chlorination by-products assessed by ultraperformance liquid chromatography coupled to tandem mass spectrometry. Anal. Chem. Acta 2016, 913, 104–110. [Google Scholar] [CrossRef]
- Li, L.; Lu-Nan, Y.; Xiao-Li, C.; Wu-Sheng, L.; Xiao-Dong, X.; Yan-Tao, W. Hepatic adenylate energy charge levels in patients with hepatoma after hepatic artery embolization. World J. Gastroenteroly 1998, 15, 109–111. [Google Scholar] [CrossRef]
- Jiang, Y.; Chengjun, S.; Ding, X.; Yuan, D.; Chen, K.; Gao, B.; Chen, Y.; Sun, A. Simultaneous determination of adenine nucleotides, creatine phosphate and creatine in rat liver by high performance liquid chromatography electrospray ionization-tandem mass spectrometry. J. Pharm. Biomed. Anal. 2012, 66, 258–263. [Google Scholar] [CrossRef]
- Van Laethem, J.L.; Eskinazi, R.; Louis, H.; Rickaert, F.; Robberecht, P.; Deviere, J. Multisystemic production of interleukin 10 limits the severity of acute pancreatitis in mice. Gut 1998, 43, 408–413. [Google Scholar] [CrossRef] [PubMed]
- Pérez, S.; Rius-Pérez, S.; Finamor, I.; Martí-Andrés, P.; Prieto, I.; García, R.; Monsalve, M.; Sastre, J. Obesity causes Pgc-1α deficiency in the pancreas leading to marked Il-6 up-regulation via NF-κB in acute pancreatitis. J. Pathol. 2018, 247, 48–59. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.F.; Sanchez, B.J.; Hall, D.T.; Tremblay, A.K.; Di Marco, S.; Gallouzi, I. STAT3 promotes IFNγ/TNFα-induced muscle wasting in an NF-κB-dependent and IL-6-independent manner. EMBO Mol. Med. 2017, 9, 622–637. [Google Scholar] [CrossRef] [PubMed]
- Nybo, T.; Dieterich, S.; Gamon, L.F.; Chuang, C.Y.; Hammer, A.; Hoefler, G.; Malle, E.; Rogowska-Wrzesinska, A.; Davies, M.J. Chlorination and oxidation of the extracellular matrix protein laminin and basement membrane extracts by hypochlorous acid and myeloperoxidase. Redox Biol. 2019, 20, 496–513. [Google Scholar] [CrossRef]
- Radi, R.; Cassina, A.; Hodara, R. Nitric oxide and peroxynitrite interactions with mitochondria. Biol. Chem. 2002, 383, 401–409. [Google Scholar] [CrossRef] [PubMed]
- Pérez, S.; Finamor, I.; Martí-Andrés, P.; Pereda, J.; Campos, A.; Domingues, R.; Haj, F.; Sabater, L.; de-Madaria, E.; Sastre, J. Role of obesity in the release of extracellular nucleosomes in acute pancreatitis: A clinical and experimental study. Int. J. Obes. (Lond) 2018, 43, 158–168. [Google Scholar] [CrossRef] [PubMed]
- Kleinert, H.; Pautz, A.; Linker, K.; Schwarz, P.M. Regulation of the expression of inducible nitric oxide synthase. Eur. J. Pharmacol. 2004, 500, 255–266. [Google Scholar] [CrossRef]
- Alvarez-Guardia, D.; Palomer, X.; Coll, T.; Davidson, M.M.; Chan, T.O.; Feldman, A.M.; Laguna, J.C.; Vázquez-Carrera, M. The p65 subunit of NF-kappaB binds to PGC-1alpha, linking inflammation and metabolic disturbances in cardiac cells. Cardiovasc. Res. 2010, 87, 449–458. [Google Scholar] [CrossRef]
- Barroso, W.A.; Victorino, V.J.; Jeremias, I.C.; Petroni, R.C.; Ariga, S.K.K.; Salles, T.A.; Barbeiro, D.F.; de Lima, T.M.; de Souza, H.P. High-fat diet inhibits PGC-1α suppressive effect on NFκB signaling in hepatocytes. Eur. J. Nutr. 2018, 57, 1891–1900. [Google Scholar] [CrossRef]
- Reiniers, M.J.; van Golen, R.F.; van Gulik, T.M.; Heger, M. Reactive Oxygen and Nitrogen Species in Steatotic Hepatocytes: A Molecular Perspective on the Pathophysiology of Ischemia-Reperfusion Injury in the Fatty Liver. Antioxid Redox Signal 2014, 21, 1119–1142. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.S.; Yu, H.; Miao, L.; Liu, Z.; He, Y.; Sun, H. Hepatoprotective Effect of the Ethanol Extract of Illicium henryi against Acute Liver Injury in Mice Induced by Lipopolysaccharide. Antioxidants 2019, 8, 446. [Google Scholar] [CrossRef] [PubMed]
- Proniewski, B.; Kij, A.; Sitek, B.; Kelley, E.E.; Chlopicki, S. Multiorgan Development of Oxidative and Nitrosative Stress in LPS-Induced Endotoxemia in C57Bl/6 Mice: DHE-Based In Vivo Approach. Oxid. Med. Cell Longev. 2019, 2019, 7838406. [Google Scholar] [CrossRef] [PubMed]
- Saad, B.; Frei, K.; Scholl, F.A.; Fontana, A.; Maier, P. Hepatocyte-Derived Interleukin-6 and Tumor-Necrosis Factor α Mediate the Lipopolysaccharide-Induced Acute-Phase Response and Nitric Oxide Release by Cultured Rat Hepatocytes. Eur. J. Biochem. 1995, 229, 349–355. [Google Scholar] [CrossRef] [PubMed]
- Dare, A.J.; Phillips, A.R.; Hickey, A.J.; Mittal, A.; Loveday, B.; Thompson, N.; Windsor, J.A. A systematic review of experimental treatments for mitochondrial dysfunction in sepsis and multiple organ dysfunction syndrome. Free Radic. Biol. Med. 2009, 47, 1517–1525. [Google Scholar] [CrossRef]
- Trumbeckaite, S.; Kuliaviene, I.; Deduchovas, O.; Kincius, M.; Baniene, R.; Virketyte, S.; Bukauskas, D.; Jansen, E.; Kupčinskas, L.; Borutaite, V.; et al. Experimental acute pancreatitis induces mitochondrial dysfunction in rat pancreas, kidney and lungs but not in liver. Pancreatology 2013, 13, 216–224. [Google Scholar] [CrossRef]
- Crunkhorn, S.; Dearie, F.; Mantzoros, C.; Gami, H.; da Silva, W.S.; Espinoza, D.; Faucette, R.; Barry, K.; Bianco, A.C.; Patti, M.E. Peroxisome proliferator activator receptor gamma coactivator-1 expression is reduced in obesity: Potential pathogenic role of saturated fatty acids and p38 mitogen-activated protein kinase activation. J. Biol. Chem. 2007, 282, 15439–15450. [Google Scholar] [CrossRef]
- Kaneki, M.; Shimizu, N.; Yamada, D.; Chang, K. Nitrosative Stress and Pathogenesis of Insulin Resistance. Antioxid. Redox Signal. 2007, 9, 319–329. [Google Scholar] [CrossRef]
- García-Ruiz, C.; Rodriguez-Juan, C.; Díaz-Juan, T.; Del Hoyo, P.; Colina, F.; Muñoz-Yagüe, T.; Solís-Herruzo, J.A. Uric acid and anti-TNF antibody improve mitochondrial dysfunction in ob/ob mice. Hepatology 2006, 44, 581–591. [Google Scholar] [CrossRef]
- Fangchao, M.; Jia, Y.; Man, L.; Mingwei, X.; Yupu, H.; Yu, Z.; Yudong, Y.; He, X.; Hongzhong, J.; Weixing, W. Magnesium isoglycyrrhizinate alleviates liver injury in obese rats with acute necrotizing pancreatitis. Pathol. Res. Pract. 2019, 215, 106–114. [Google Scholar] [CrossRef]


| Target Gene (mm) | Direct/Reverse Oligonucleotide |
|---|---|
| Ppargc1a (Gene ID: 19017) | F --> TTAAAGTTCATGGGGCAAGC R --> TAGGAATGGCTGAAGGGATG |
| Sod2 (Gene ID: 20656) | F --> GGCCAAGGGAGATGTTACAA R --> GAACCTTGGACTCCCACAGA |
| Prx3 (Gene ID: 11757) | F --> CAAGAAAGAATGGTGGTTTGG R --> TGCTTGACGACACCATTAGG |
| Nos2 (Gene ID: 18126) | F -->GCATCCCAAGTACGAGTGGGT R -->GAAGTCTCGAACTCCAATC |
| Tbp (Gene ID: 21374) | F -->CAGCCTTCCACCTTATGCTC R --> CCGTAAGGCATCATTGGACT |
| Target Gene (mm) | Sonda TaqMan |
|---|---|
| Tnfα (Gene ID: 21926) | Mm00443258_g1 |
| Il6 (Gene ID: 16193) | Mm00446190_m1 |
| Tbp (Gene ID: 21374) | Mm01277042_m1 |
| Lean Group | Obese Group | |
|---|---|---|
| Body weight (g) | 22.9 ± 1.1 | 29.7 ± 1.8 ** |
| Blood glucose (mg/dl) | 137.8 ± 25.8 | 201.6 ± 50.3 * |
| Liver Triglycerides (mg/g protein) | 86.4 ± 22.4 | 138.8 ± 28.4 ** |
| Liver Total Lipids (mg/g protein) | 665.8 ± 208.4 | 1581.7 ± 437.7 ** |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rius-Pérez, S.; Torres-Cuevas, I.; Monsalve, M.; Miranda, F.J.; Pérez, S. Impairment of PGC-1 Alpha Up-Regulation Enhances Nitrosative Stress in the Liver during Acute Pancreatitis in Obese Mice. Antioxidants 2020, 9, 887. https://doi.org/10.3390/antiox9090887
Rius-Pérez S, Torres-Cuevas I, Monsalve M, Miranda FJ, Pérez S. Impairment of PGC-1 Alpha Up-Regulation Enhances Nitrosative Stress in the Liver during Acute Pancreatitis in Obese Mice. Antioxidants. 2020; 9(9):887. https://doi.org/10.3390/antiox9090887
Chicago/Turabian StyleRius-Pérez, Sergio, Isabel Torres-Cuevas, María Monsalve, Francisco J. Miranda, and Salvador Pérez. 2020. "Impairment of PGC-1 Alpha Up-Regulation Enhances Nitrosative Stress in the Liver during Acute Pancreatitis in Obese Mice" Antioxidants 9, no. 9: 887. https://doi.org/10.3390/antiox9090887
APA StyleRius-Pérez, S., Torres-Cuevas, I., Monsalve, M., Miranda, F. J., & Pérez, S. (2020). Impairment of PGC-1 Alpha Up-Regulation Enhances Nitrosative Stress in the Liver during Acute Pancreatitis in Obese Mice. Antioxidants, 9(9), 887. https://doi.org/10.3390/antiox9090887