Allium Flavonols: Health Benefits, Molecular Targets, and Bioavailability
Abstract
:1. Introduction
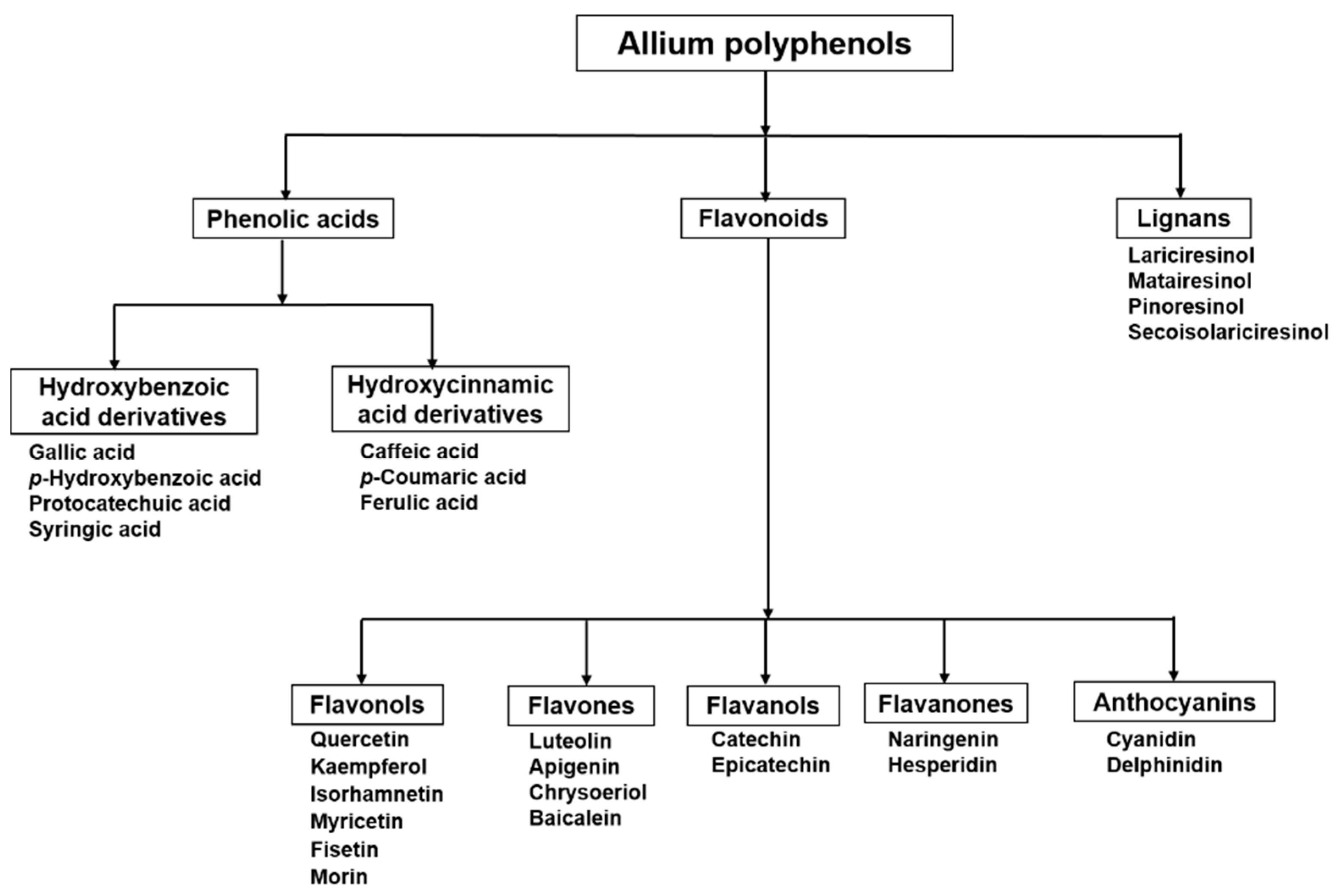
2. Flavonoids in Allium: Structural Properties
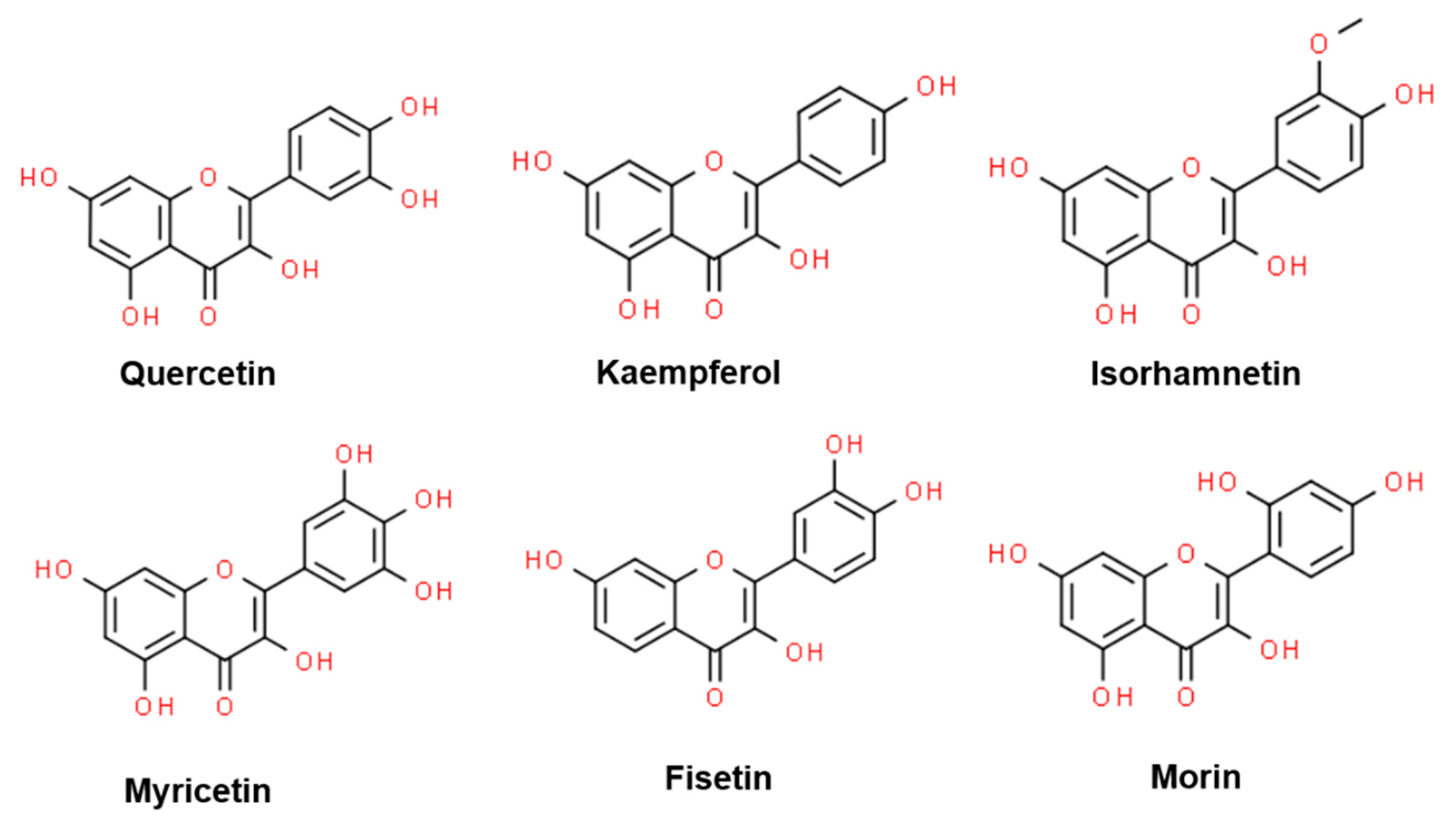
Flavonols
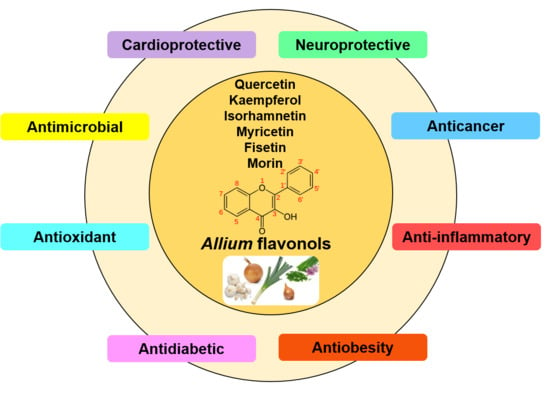
3. Health Benefits of Allium Flavonols
3.1. Anticancer Effects
3.2. Anti-Obesity and Hypolipidemic Effects
3.3. Anti-Diabetic Effects
3.4. Cardio-Protective Effects
3.5. Neuroprotective Effects
3.6. Antimicrobial Effects
3.7. Other Health Benefits
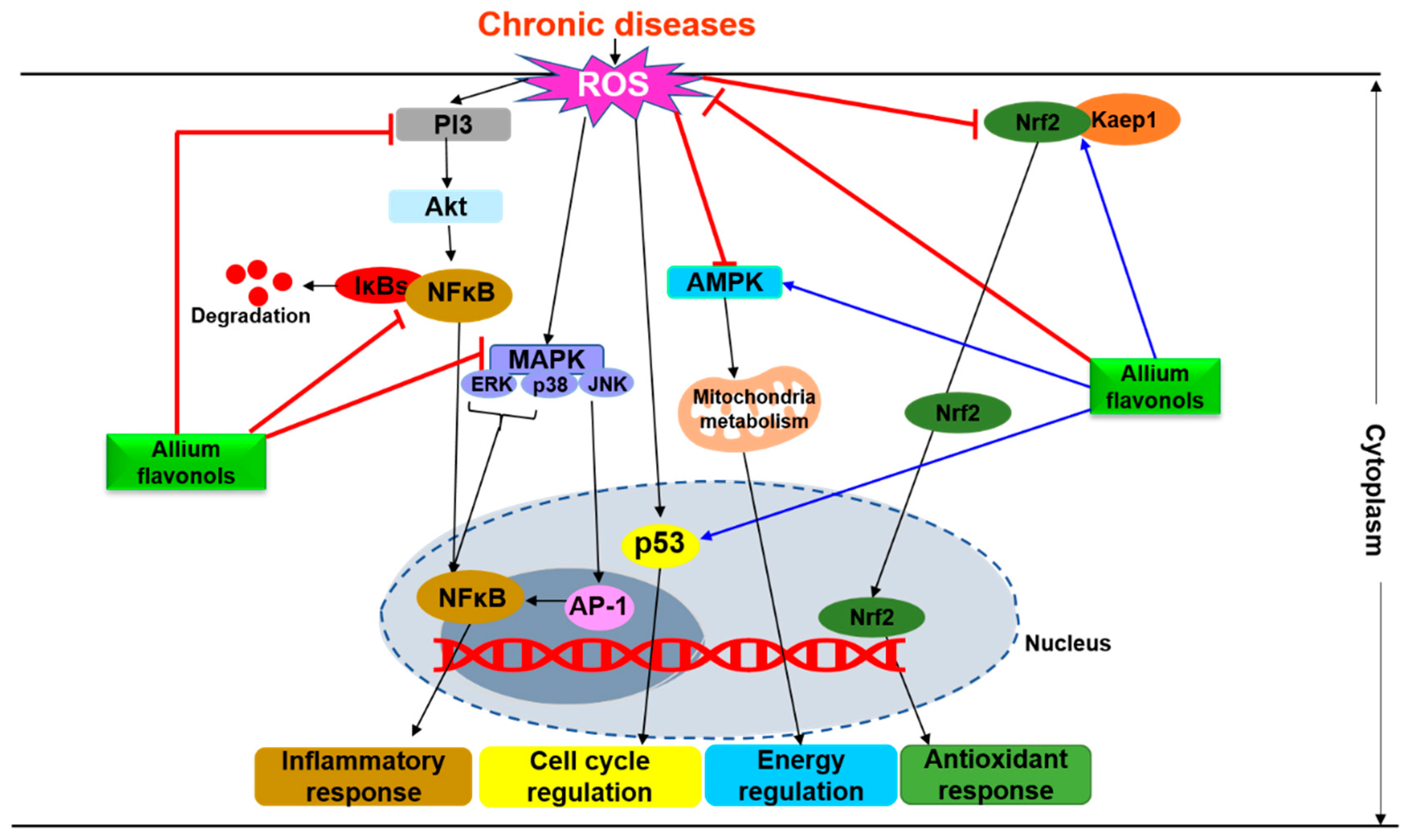
4. Molecular Mechanisms Underlying the Physiological Effects of Flavonols
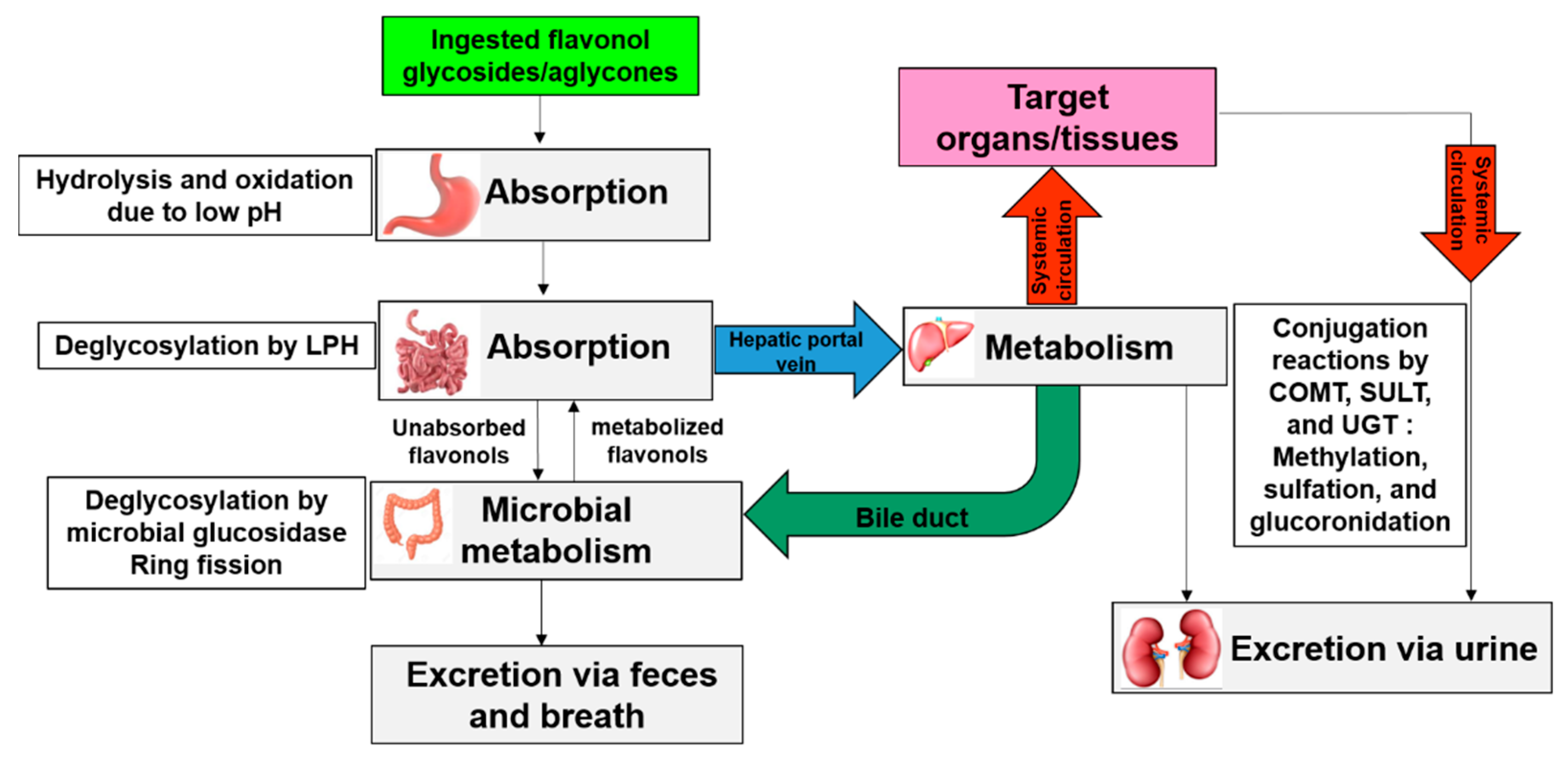
5. Bioavailability of Allium-Derived Flavonols
6. Stability During Domestic and Technological Processing
7. Food Fortification
8. Future Perspectives and Limitations
9. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- World Health Organization. World Health Statistics 2018: Monitoring Health for the SDGs Sustainable Development Goals; WHO: Geneva, Switzerland, 2018. [Google Scholar]
- Miller, V.; Webb, P.; Micha, R.; Mozaffarian, D.; Database, G.D. Defining diet quality: A synthesis of dietary quality metrics and their validity for the double burden of malnutrition. Lancet Planet. Health 2020, 4, e352–e370. [Google Scholar] [CrossRef]
- Bousquet, J.; Anto, J.M.; Iaccarino, G.; Czarlewski, W.; Haahtela, T.; Anto, A.; Akdis, C.A.; Blain, H.; Canonica, G.W.; Cardona, V. Is diet partly responsible for differences in COVID-19 death rates between and within countries? Clin. Transl. Allergy 2020, 10, 16. [Google Scholar] [CrossRef] [PubMed]
- Gupta, R.; Hussain, A.; Misra, A. Diabetes and COVID-19: Evidence, current status and unanswered research questions. Eur. J. Clin. Nutr. 2020, 74, 864–870. [Google Scholar] [CrossRef]
- He, W.; Chen, L.; Chen, L.; Yuan, G.; Fang, Y.; Chen, W.; Wu, D.; Liang, B.; Lu, X.; Ma, Y.; et al. COVID-19 in persons with haematological cancers. Leukemia 2020, 34, 1637–1645. [Google Scholar] [CrossRef]
- Butler, M.J.; Barrientos, R.M. The impact of nutrition on COVID-19 susceptibility and long-term consequences. Brain Behav. Immun. 2020, 87, 53–54. [Google Scholar] [CrossRef]
- Muscogiuri, G.; Barrea, L.; Savastano, S.; Colao, A. Nutritional recommendations for CoVID-19 quarantine. Eur. J. Clin. Nutr. 2020, 74, 850–851. [Google Scholar] [CrossRef]
- Chandra, R.K. Effect of vitamin and trace-element supplementation on immune responses and infection in elderly subjects. Lancet-Lond 1992, 1124. [Google Scholar] [CrossRef]
- Teshika, J.D.; Zakariyyah, A.M.; Zaynab, T.; Zengin, G.; Rengasamy, K.R.; Pandian, S.K.; Fawzi, M.M. Traditional and modern uses of onion bulb (Allium cepa L.): A systematic review. Crit. Rev. Food Sci. Nutr. 2019, 59, S39–S70. [Google Scholar] [CrossRef]
- Putnik, P.; Gabrić, D.; Roohinejad, S.; Barba, F.J.; Granato, D.; Mallikarjunan, K.; Lorenzo, J.M.; Kovačević, D.B. An overview of organosulfur compounds from Allium spp.: From processing and preservation to evaluation of their bioavailability, antimicrobial, and anti-inflammatory properties. Food Chem. 2019, 276, 680–691. [Google Scholar] [CrossRef]
- Hansen, E.A.; Folts, J.D.; Goldman, I.L. Steam-cooking rapidly destroys and reverses onion-induced antiplatelet activity. Nutr. J. 2012, 11, 76. [Google Scholar] [CrossRef] [Green Version]
- Kyung, K.H. Antimicrobial properties of allium species. Curr. Opin. Biotechnol. 2012, 23, 142–147. [Google Scholar] [CrossRef] [PubMed]
- Poojary, M.M.; Putnik, P.; Kovačević, D.B.; Barba, F.J.; Lorenzo, J.M.; Dias, D.A.; Shpigelman, A. Stability and extraction of bioactive sulfur compounds from Allium genus processed by traditional and innovative technologies. J. Food Compos. Anal. 2017, 61, 28–39. [Google Scholar] [CrossRef]
- Elberry, A.A.; Mufti, S.; Al-Maghrabi, J.; Abdel Sattar, E.; Ghareib, S.A.; Mosli, H.A.; Gabr, S.A. Immunomodulatory effect of red onion (Allium cepa Linn) scale extract on experimentally induced atypical prostatic hyperplasia in Wistar rats. Mediat. Inflamm. 2014, 2014, 640746. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, H.-A.; Han, S.-J.; Hong, S.; Kim, D.-W.; Oh, G.-W.; Kim, O. Onion peel water extracts enhance immune status in forced swimming rat model. Lab. Anim. Res. 2014, 30, 161–168. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Han, M.H.; Lee, W.S.; Jung, J.H.; Jeong, J.-H.; Park, C.; Kim, H.J.; Kim, G.; Jung, J.-M.; Kwon, T.K.; Kim, G.-Y. Polyphenols isolated from Allium cepa L. induces apoptosis by suppressing IAP-1 through inhibiting PI3K/Akt signaling pathways in human leukemic cells. Food Chem. Toxicol. 2013, 62, 382–389. [Google Scholar] [CrossRef]
- Neveu, V.; Perez-Jiménez, J.; Vos, F.; Crespy, V.; du Chaffaut, L.; Mennen, L.; Knox, C.; Eisner, R.; Cruz, J.; Wishart, D. Phenol-Explorer: An online comprehensive database on polyphenol contents in foods. Database 2010, 2010, bap024. [Google Scholar] [CrossRef]
- Rauter, A.P.; Ennis, M.; Hellwich, K.-H.; Herold, B.J.; Horton, D.; Moss, G.P.; Schomburg, I. Nomenclature of flavonoids (IUPAC Recommendations 2017). Pure Appl. Chem. 2018, 90, 1429–1486. [Google Scholar] [CrossRef] [Green Version]
- Ververidis, F.; Trantas, E.; Douglas, C.; Vollmer, G.; Kretzschmar, G.; Panopoulos, N. Biotechnology of flavonoids and other phenylpropanoid-derived natural products. Part I: Chemical diversity, impacts on plant biology and human health. Biotechnol. J. Healthc. Nutr. Technol. 2007, 2, 1214–1234. [Google Scholar] [CrossRef]
- Pérez-Gregorio, R.M.; García-Falcón, M.S.; Simal-Gándara, J.; Rodrigues, A.S.; Almeida, D.P. Identification and quantification of flavonoids in traditional cultivars of red and white onions at harvest. J. Food Compos. Anal. 2010, 23, 592–598. [Google Scholar] [CrossRef]
- Robards, K.; Antolovich, M. Analytical chemistry of fruit bioflavonoids: A review. Analyst 1997, 122, 11R–34R. [Google Scholar] [CrossRef]
- Slimestad, R.; Fossen, T.; Vågen, I.M. Onions: A source of unique dietary flavonoids. J. Agric. Food Chem. 2007, 55, 10067–10080. [Google Scholar] [CrossRef] [PubMed]
- Bonaccorsi, P.; Caristi, C.; Gargiulli, C.; Leuzzi, U. Flavonol glucosides in Allium species: A comparative study by means of HPLC–DAD–ESI-MS–MS. Food Chem. 2008, 107, 1668–1673. [Google Scholar] [CrossRef]
- Soininen, T.H.; Jukarainen, N.; Auriola, S.O.; Julkunen-Tiitto, R.; Karjalainen, R.; Vepsäläinen, J.J. Quantitative metabolite profiling of edible onion species by NMR and HPLC–MS. Food Chem. 2014, 165, 499–505. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-S.; Kang, O.-J.; Gweon, O.-C. Comparison of phenolic acids and flavonoids in black garlic at different thermal processing steps. J. Funct. Foods 2013, 5, 80–86. [Google Scholar] [CrossRef]
- Oszmianski, J.; Kolniak-Ostek, J.; Wojdyło, A. Characterization and content of flavonol derivatives of Allium ursinum L. plant. J. Agric. Food Chem. 2013, 61, 176–184. [Google Scholar] [CrossRef] [PubMed]
- Dabeek, W.M.; Kovinich, N.; Walsh, C.; Ventura Marra, M. Characterization and Quantification of Major Flavonol Glycosides in Ramps (Allium tricoccum). Molecules 2019, 24, 3281. [Google Scholar] [CrossRef] [Green Version]
- Miean, K.H.; Mohamed, S. Flavonoid (myricetin, quercetin, kaempferol, luteolin, and apigenin) content of edible tropical plants. J. Agric. Food Chem. 2001, 49, 3106–3112. [Google Scholar] [CrossRef]
- Simin, N.; Orcic, D.; Cetojevic-Simin, D.; Mimica-Dukic, N.; Anackov, G.; Beara, I.; Mitic-Culafic, D.; Bozin, B. Phenolic profile, antioxidant, anti-inflammatory and cytotoxic activities of small yellow onion (Allium flavum L. subsp. flavum, Alliaceae). LWT-Food Sci. Technol. 2013, 54, 139–146. [Google Scholar] [CrossRef]
- Aleksandar, P.; Dragana, M.-Ć.; Nebojša, J.; Biljana, N.; Nataša, S.; Branka, V.; Jelena, K.-V. Wild edible onions—Allium flavum and Allium carinatum—successfully prevent adverse effects of chemotherapeutic drug doxorubicin. Biomed. Pharmacother. 2019, 109, 2482–2491. [Google Scholar] [CrossRef]
- Zheng, Y.-Z.; Deng, G.; Liang, Q.; Chen, D.-F.; Guo, R.; Lai, R.-C. Antioxidant activity of quercetin and its glucosides from propolis: A theoretical study. Sci. Rep. 2017, 7, 7543. [Google Scholar] [CrossRef] [Green Version]
- Rice-Evans, C.A.; Miller, N.J.; Paganga, G. Structure-antioxidant activity relationships of flavonoids and phenolic acids. Free Radic. Biol. Med. 1996, 20, 933–956. [Google Scholar] [CrossRef]
- Rodrigues, A.S.; Pérez-Gregorio, M.R.; García-Falcón, M.S.; Simal-Gándara, J.; Almeida, D.P. Effect of post-harvest practices on flavonoid content of red and white onion cultivars. Food Control 2010, 21, 878–884. [Google Scholar] [CrossRef]
- Rodrigues, A.S.; Pérez-Gregorio, M.R.; García-Falcón, M.S.; Simal-Gándara, J.; Almeida, D.P.F. Effect of meteorological conditions on antioxidant flavonoids in Portuguese cultivars of white and red onions. Food Chem. 2011, 124, 303–308. [Google Scholar] [CrossRef]
- Olsson, M.E.; Gustavsson, K.-E.; Vagen, I.M. Quercetin and isorhamnetin in sweet and red cultivars of onion (Allium cepa L.) at harvest, after field curing, heat treatment, and storage. J. Agric. Food Chem. 2010, 58, 2323–2330. [Google Scholar] [CrossRef]
- Tang, X.; Olatunji, O.J.; Zhou, Y.; Hou, X. Allium tuberosum: Antidiabetic and hepatoprotective activities. Food Res. Int. 2017, 102, 681–689. [Google Scholar] [CrossRef]
- Kim, M.Y.; Kim, Y.C.; Chung, S.K. Identification and in vitro biological activities of flavonols in garlic leaf and shoot: Inhibition of soybean lipoxygenase and hyaluronidase activities and scavenging of free radicals. J. Sci. Food Agric. 2005, 85, 633–640. [Google Scholar] [CrossRef]
- Nakane, R.; Iwashina, T. Flavonol glycosides from the leaves of Allium macrostemon. Nat. Prod. Commun. 2015, 10, 1381–1382. [Google Scholar] [CrossRef] [Green Version]
- Lin, Y.-P.; Lin, L.-Y.; Yeh, H.-Y.; Chuang, C.-H.; Tseng, S.-W.; Yen, Y.-H. Antihyperlipidemic activity of Allium chinense bulbs. J. Food Drug Anal. 2016, 24, 516–526. [Google Scholar] [CrossRef] [Green Version]
- Kothari, D.; Lee, W.-D.; Jung, E.S.; Niu, K.-M.; Lee, C.H.; Kim, S.-K. Controlled Fermentation Using Autochthonous Lactobacillus plantarum improves antimicrobial potential of Chinese chives against poultry pathogens. Antibiotics 2020, 9, 386. [Google Scholar] [CrossRef]
- Arai, Y.; Watanabe, S.; Kimira, M.; Shimoi, K.; Mochizuki, R.; Kinae, N. Dietary intakes of flavonols, flavones and isoflavones by Japanese women and the inverse correlation between quercetin intake and plasma LDL cholesterol concentration. J. Nutr. 2000, 130, 2243–2250. [Google Scholar] [CrossRef] [Green Version]
- Olayeriju, O.S.; Olaleye, M.T.; Crown, O.O.; Komolafe, K.; Boligon, A.A.; Athayde, M.L.; Akindahunsi, A.A. Ethylacetate extract of red onion (Allium cepa L.) tunic affects hemodynamic parameters in rats. Food Sci. Hum. Wellness 2015, 4, 115–122. [Google Scholar] [CrossRef] [Green Version]
- Carotenuto, A.; De Feo, V.; Fattorusso, E.; Lanzotti, V.; Magno, S.; Cicala, C. The flavonoids of Allium ursinum. Phytochemistry 1996, 41, 531–536. [Google Scholar] [CrossRef]
- Corea, G.; Fattorusso, E.; Lanzotti, V. Saponins and flavonoids of Allium triquetrum. J. Nat. Prod. 2003, 66, 1405–1411. [Google Scholar] [CrossRef] [PubMed]
- Dziri, S.; Hassen, I.; Fatnassi, S.; Mrabet, Y.; Casabianca, H.; Hanchi, B.; Hosni, K. Phenolic constituents, antioxidant and antimicrobial activities of rosy garlic (Allium roseum var. odoratissimum). J. Funct. Foods 2012, 4, 423–432. [Google Scholar] [CrossRef]
- Snoussi, M.; Trabelsi, N.; Dehmeni, A.; Benzekri, R.; Bouslama, L.; Hajlaoui, B.; Al-sieni, A.; Papetti, A. Phytochemical analysis, antimicrobial and antioxidant activities of Allium roseum var. odoratissimum (Desf.) Coss extracts. Ind. Crop. Prod. 2016, 89, 533–542. [Google Scholar] [CrossRef]
- Fattorusso, E.; Lanzotti, V.; Taglialatela-Scafati, O.; Cicala, C. The flavonoids of leek, Allium porrum. Phytochemistry 2001, 57, 565–569. [Google Scholar] [CrossRef]
- Shi, G.-Q.; Yang, J.; Liu, J.; Liu, S.-N.; Song, H.-X.; Zhao, W.-E.; Liu, Y.-Q. Isolation of flavonoids from onion skins and their effects on K562 cell viability. Bangladesh J. Pharmacol. 2016, 11, S18–S25. [Google Scholar] [CrossRef] [Green Version]
- Demirtas, I.; Erenler, R.; Elmastas, M.; Goktasoglu, A. Studies on the antioxidant potential of flavones of Allium vineale isolated from its water-soluble fraction. Food Chem. 2013, 136, 34–40. [Google Scholar] [CrossRef]
- Kim, J.; Kim, J.-S.; Park, E. Cytotoxic and anti-inflammatory effects of onion peel extract on lipopolysaccharide stimulated human colon carcinoma cells. Food Chem. Toxicol. 2013, 62, 199–204. [Google Scholar] [CrossRef]
- Bondonno, N.P.; Dalgaard, F.; Kyrø, C.; Murray, K.; Bondonno, C.P.; Lewis, J.R.; Croft, K.D.; Gislason, G.; Scalbert, A.; Cassidy, A. Flavonoid intake is associated with lower mortality in the Danish Diet Cancer and Health Cohort. Nat. Commun. 2019, 10, 3651. [Google Scholar] [CrossRef] [Green Version]
- Rodríguez-García, C.; Sánchez-Quesada, C.; Gaforio, J.J. Dietary flavonoids as cancer chemopreventive agents: An updated review of human studies. Antioxidants 2019, 8, 137. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Theodoratou, E.; Kyle, J.; Cetnarskyj, R.; Farrington, S.M.; Tenesa, A.; Barnetson, R.; Porteous, M.; Dunlop, M.; Campbell, H. Dietary flavonoids and the risk of colorectal cancer. Cancer Epidemiol. Prev. Biomark. 2007, 16, 684–693. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, W.S.; Yi, S.M.; Yun, J.W.; Jung, J.H.; Kim, D.H.; Kim, H.J.; Chang, S.-H.; Kim, G.; Ryu, C.H.; Shin, S.C. Polyphenols isolated from Allium cepa L. induces apoptosis by induction of p53 and suppression of Bcl-2 through inhibiting PI3K/Akt signaling pathway in AGS human cancer cells. J. Cancer Prev. 2014, 19, 14. [Google Scholar] [CrossRef] [Green Version]
- Pan, Y.; Zheng, Y.M.; Ho, W.S. Effect of quercetin glucosides from Allium extracts on HepG2, PC-3 and HT-29 cancer cell lines. Oncol. Lett. 2018, 15, 4657–4661. [Google Scholar] [CrossRef]
- Myint, A.A.; Aregay, M.G.; Kang, M.; Kim, B.-S.; Lee, Y.-W.; Kim, J. Comprehensive study on the formation mechanism of highly bioactive compounds from Allium hookeri root using subcritical water and their antioxidant and anticancer effects. J. Supercrit. Fluids 2020, 157, 104709. [Google Scholar] [CrossRef]
- Grivennikov, S.I.; Greten, F.R.; Karin, M. Immunity, inflammation, and cancer. Cell 2010, 140, 883–899. [Google Scholar] [CrossRef] [Green Version]
- Carmeliet, P.; Jain, R.K. 2000. Angiogenesis in cancer and other diseases. Nature 2000, 407, 249–257. [Google Scholar] [CrossRef]
- Seyfi, P.; Mostafaie, A.; Mansouri, K.; Arshadi, D.; Mohammadi-Motlagh, H.-R.; Kiani, A. In vitro and in vivo anti-angiogenesis effect of shallot (Allium ascalonicum): A heat-stable and flavonoid-rich fraction of shallot extract potently inhibits angiogenesis. Toxicol. In Vitro 2010, 24, 1655–1661. [Google Scholar] [CrossRef]
- Pi-Sunyer, F.X. Obesity: Criteria and classification. Proc. Nutr. Soc. 2000, 59, 505–509. [Google Scholar] [CrossRef] [Green Version]
- Kim, O.Y.; Lee, S.M.; Do, H.; Moon, J.; Lee, K.H.; Cha, Y.J.; Shin, M.J. Influence of quercetin-rich onion peel extracts on adipokine expression in the visceral adipose tissue of rats. Phytother. Res. 2012, 26, 432–437. [Google Scholar] [CrossRef]
- Moon, J.; Do, H.-J.; Kim, O.Y.; Shin, M.-J. Antiobesity effects of quercetin-rich onion peel extract on the differentiation of 3T3-L1 preadipocytes and the adipogenesis in high fat-fed rats. Food Chem. Toxicol. 2013, 58, 347–354. [Google Scholar] [CrossRef]
- Bae, C.R.; Park, Y.K.; Cha, Y.S. Quercetin-rich onion peel extract suppresses adipogenesis by down-regulating adipogenic transcription factors and gene expression in 3T3-L1 adipocytes. J. Sci. Food Agric. 2014, 94, 2655–2660. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.G.; Parks, J.S.; Kang, H.W. Quercetin, a functional compound of onion peel, remodels white adipocytes to brown-like adipocytes. J. Nutr. Biochem. 2017, 42, 62–71. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sung, Y.-Y.; Kim, D.-S.; Kim, S.-H.; Kim, H.K. Aqueous and ethanolic extracts of welsh onion, Allium fistulosum, attenuate high-fat diet-induced obesity. BMC Complement. Altern. Med. 2018, 18, 105. [Google Scholar] [CrossRef] [Green Version]
- Desjardins, E.M.; Steinberg, G.R. Emerging role of AMPK in Brown and Beige adipose tissue (BAT): Implications for obesity, insulin resistance, and type 2 diabetes. Curr. Diabetes Rep. 2018, 18, 80. [Google Scholar] [CrossRef] [PubMed]
- Nile, A.; Nile, S.H.; Kim, D.H.; Keum, Y.S.; Seok, P.G.; Sharma, K. Valorization of onion solid waste and their flavonols for assessment of cytotoxicity, enzyme inhibitory and antioxidant activities. Food Chem. Toxicol. 2018, 119, 281–289. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Li, C.; Feng, B.; Chen, B.; Jin, L.; Shen, Y. UPLC-ESI-MS/MS based identification and antioxidant, antibacterial, cytotoxic activities of aqueous extracts from storey onion (Allium cepa L. var. proliferum Regel). Food Res. Int. 2020, 130, 108969. [Google Scholar] [CrossRef]
- He, Y.; Jin, H.; Gong, W.; Zhang, C.; Zhou, A. Effect of onion flavonoids on colorectal cancer with hyperlipidemia: An in vivo study. Oncotargets Ther. 2014, 7, 101. [Google Scholar] [CrossRef] [Green Version]
- Kim, K.-A.; Yim, J.-E. Antioxidative activity of onion peel extract in obese women: A randomized, double-blind, placebo-controlled study. J. Cancer Prev. 2015, 20, 202. [Google Scholar] [CrossRef] [Green Version]
- Grzelak-Błaszczyk, K.; Milala, J.; Kosmala, M.; Kołodziejczyk, K.; Sójka, M.; Czarnecki, A.; Klewicki, R.; Juśkiewicz, J.; Fotschki, B.; Jurgoński, A. Onion quercetin monoglycosides alter microbial activity and increase antioxidant capacity. J. Nutr. Biochem. 2018, 56, 81–88. [Google Scholar] [CrossRef]
- Kim, S.-H.; Jo, S.-H.; Kwon, Y.-I.; Hwang, J.-K. Effects of onion (Allium cepa L.) extract administration on intestinal α-glucosidases activities and spikes in postprandial blood glucose levels in SD rats model. Int. J. Mol. Sci. 2011, 12, 3757–3769. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jung, J.Y.; Lim, Y.; Moon, M.S.; Kim, J.Y.; Kwon, O. Onion peel extracts ameliorate hyperglycemia and insulin resistance in high fat diet/streptozotocin-induced diabetic rats. Nutr. Metab. 2011, 8, 18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ikechukwu, O.J.; Ifeanyi, O.S. The antidiabetic effects of the bioactive flavonoid (kaempferol-3-O-β-d-6 {P-Coumaroyl} glucopyranoside) isolated from Allium cepa. Recent Pat. Anti-Infect. Drug Discov. 2016, 11, 44–52. [Google Scholar] [CrossRef] [PubMed]
- Schulze, C.; Bangert, A.; Schwanck, B.; Vollert, H.; Blaschek, W.; Daniel, H. Extracts and flavonoids from onion inhibit the intestinal sodium-coupled glucose transporter 1 (SGLT1) in vitro but show no anti-hyperglycaemic effects in vivo in normoglycaemic mice and human volunteers. J. Funct. Foods 2015, 18, 117–128. [Google Scholar] [CrossRef]
- Ni, Z.; Guo, L.; Liu, F.; Olatunji, O.J.; Yin, M. Allium tuberosum alleviates diabetic nephropathy by supressing hyperglycemia-induced oxidative stress and inflammation in high fat diet/streptozotocin treated rats. Biomed. Pharmacother. 2019, 112, 108678. [Google Scholar] [CrossRef]
- Lee, K.-H.; Park, E.; Lee, H.-J.; Kim, M.-O.; Cha, Y.-J.; Kim, J.-M.; Lee, H.; Shin, M.-J. Effects of daily quercetin-rich supplementation on cardiometabolic risks in male smokers. Nutr. Res. Pract. 2011, 5, 28–33. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hamauzu, Y.; Nosaka, T.; Ito, F.; Suzuki, T.; Torisu, S.; Hashida, M.; Fukuzawa, A.; Ohguchi, M.; Yamanaka, S. Physicochemical characteristics of rapidly dried onion powder and its anti-atherogenic effect on rats fed high-fat diet. Food Chem. 2011, 129, 810–815. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.-M.; Moon, J.; Chung, J.H.; Cha, Y.-J.; Shin, M.-J. Effect of quercetin-rich onion peel extracts on arterial thrombosis in rats. Food Chem. Toxicol. 2013, 57, 99–105. [Google Scholar] [CrossRef]
- Nakayama, H.; Tsuge, N.; Sawada, H.; Higashi, Y. Chronic intake of onion extract containing quercetin improved postprandial endothelial dysfunction in healthy men. J. Am. Coll. Nutr. 2013, 32, 160–164. [Google Scholar] [CrossRef]
- Choi, E.-Y.; Lee, H.; Woo, J.S.; Jang, H.H.; Hwang, S.J.; Kim, H.S.; Kim, W.-S.; Kim, Y.-S.; Choue, R.; Cha, Y.-J. Effect of onion peel extract on endothelial function and endothelial progenitor cells in overweight and obese individuals. Nutrition 2015, 31, 1131–1135. [Google Scholar] [CrossRef]
- Brüll, V.; Burak, C.; Stoffel-Wagner, B.; Wolffram, S.; Nickenig, G.; Müller, C.; Langguth, P.; Alteheld, B.; Fimmers, R.; Naaf, S. Effects of a quercetin-rich onion skin extract on 24 h ambulatory blood pressure and endothelial function in overweight-to-obese patients with (pre-) hypertension: A randomised double-blinded placebo-controlled cross-over trial. Br. J. Nutr. 2015, 114, 1263–1277. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ro, J.-Y.; Ryu, J.-H.; Park, H.-J.; Cho, H.-J. Onion (Allium cepa L.) peel extract has anti-platelet effects in rat platelets. Springerplus 2015, 4, 17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ko, E.Y.; Nile, S.H.; Jung, Y.-S.; Keum, Y.S. Antioxidant and antiplatelet potential of different methanol fractions and flavonols extracted from onion (Allium cepa L.). 3 Biotech 2018, 8, 155. [Google Scholar] [CrossRef] [PubMed]
- Hwang, I.K.; Lee, C.H.; Yoo, K.-Y.; Choi, J.H.; Park, O.K.; Lim, S.S.; Kang, I.-J.; Kwon, D.Y.; Park, J.; Yi, J.-S. Neuroprotective effects of onion extract and quercetin against ischemic neuronal damage in the gerbil hippocampus. J. Med. Food 2009, 12, 990–995. [Google Scholar] [CrossRef]
- Woo, K.W.; Moon, E.; Park, S.Y.; Kim, S.Y.; Lee, K.R. Flavonoid glycosides from the leaves of Allium victorialis var. platyphyllum and their anti-neuroinflammatory effects. Bioorg. Med. Chem. Lett. 2012, 22, 7465–7470. [Google Scholar] [CrossRef]
- Yang, E.-J.; Kim, G.-S.; Kim, J.A.; Song, K.-S. Protective effects of onion-derived quercetin on glutamate-mediated hippocampal neuronal cell death. Pharmacogn. Mag. 2013, 9, 302. [Google Scholar]
- Lee, B.K.; Jung, Y.-S. Allium cepa extract and quercetin protect neuronal cells from oxidative stress via PKC-ε inactivation/ERK1/2 activation. Oxidative Med. Cell. Longev. 2016, 2016. [Google Scholar]
- Singh, T.; Goel, R.K. Neuroprotective effect of Allium cepa L. in aluminium chloride induced neurotoxicity. Neurotoxicology 2015, 49, 1–7. [Google Scholar] [CrossRef]
- Sarchielli, E.; Morelli, A.; Guarnieri, G.; Iorizzi, M.; Sgambati, E. Neuroprotective effects of quercetin 4′-O-β-d-diglucoside on human striatal precursor cells in nutrient deprivation condition. Acta Histochem. 2018, 120, 122–128. [Google Scholar] [CrossRef]
- Singh, V.; Krishan, P.; Shri, R. Amelioration of ischaemia reperfusion-induced cerebral injury in mice by liposomes containing Allium cepa fraction administered intranasally. Artif. Cells Nanomed. Biotechnol. 2018, 46, S982–S992. [Google Scholar] [CrossRef] [Green Version]
- Kusminski, C.M.; Scherer, P.E. Mitochondrial dysfunction in white adipose tissue. Trends Endocrinol. Metab. 2012, 23, 435–443. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Henagan, T.; Cefalu, W.; Ribnicky, D.; Noland, R.; Dunville, K.; Campbell, W.; Stewart, L.; Forney, L.; Gettys, T.; Chang, J. In vivo effects of dietary quercetin and quercetin-rich red onion extract on skeletal muscle mitochondria, metabolism, and insulin sensitivity. Genes Nutr. 2015, 10, 2. [Google Scholar] [CrossRef] [PubMed]
- Grzelak-Błaszczyk, K.; Milala, J.; Kołodziejczyk, K.; Sójka, M.; Czarnecki, A.; Kosmala, M.; Klewicki, R.; Fotschki, B.; Jurgoński, A.; Juśkiewicz, J. Protocatechuic acid and quercetin glucosides in onions attenuate changes induced by high fat diet in rats. Food Funct. 2020, 11, 3585–3597. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.-A.; Yim, J.-E. The effect of onion peel extract on inflammatory mediators in korean overweight and obese women. Clin. Nutr. Res. 2016, 5, 261–269. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Global Report on Diabetes: World Health Organization; Report No.: 9789241565257; WHO: Geneva, Switzerland, 2016. [Google Scholar]
- Pantalone, K.M.; Hobbs, T.M.; Wells, B.J.; Kong, S.X.; Kattan, M.W.; Bouchard, J.; Yu, C.; Sakurada, B.; Milinovich, A.; Weng, W.; et al. Clinical characteristics, complications, comorbidities and treatment patterns among patients with type 2 diabetes mellitus in a large integrated health system. BMJ Open Diabetes Res. Care 2015, 3, e000093. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nickerson, H.D.; Dutta, S. Diabetic complications: Current challenges and opportunities. J. Cardiovasc. Transl. Res. 2012, 5, 375–379. [Google Scholar] [CrossRef] [Green Version]
- Stolar, M. Glycemic control and complications in type 2 diabetes mellitus. Am. J. Med. 2010, 123, S3–S11. [Google Scholar] [CrossRef]
- Shi, G.-J.; Li, Y.; Cao, Q.-H.; Wu, H.-X.; Tang, X.-Y.; Gao, X.-H.; Yu, J.-Q.; Chen, Z.; Yang, Y. In vitro and in vivo evidence that quercetin protects against diabetes and its complications: A systematic review of the literature. Biomed. Pharmacother. 2019, 109, 1085–1099. [Google Scholar] [CrossRef]
- Domingueti, C.P.; Dusse, L.M.S.A.; das Graças Carvalho, M.; de Sousa, L.P.; Gomes, K.B.; Fernandes, A.P. Diabetes mellitus: The linkage between oxidative stress, inflammation, hypercoagulability and vascular complications. J. Diabetes Complicat. 2016, 30, 738–745. [Google Scholar] [CrossRef]
- Elmarakby, A.A.; Sullivan, J.C. Relationship between oxidative stress and inflammatory cytokines in diabetic nephropathy. Cardiovasc. Ther. 2012, 30, 49–59. [Google Scholar] [CrossRef]
- Oguntibeju, O.O. Type 2 diabetes mellitus, oxidative stress and inflammation: Examining the links. Int. J. Physiol. Pathophysiol. Pharmacol. 2019, 11, 45. [Google Scholar]
- Priya Rani, M.; Padmakumari, K.P.; Sankarikutty, B.; Lijo Cherian, O.; Nisha, V.M.; Raghu, K.G. Inhibitory potential of ginger extracts against enzymes linked to type 2 diabetes, inflammation and induced oxidative stress. Int. J. Food Sci. Nutr. 2011, 62, 106–110. [Google Scholar] [CrossRef]
- Song, Y.; Manson, J.E.; Buring, J.E.; Sesso, H.D.; Liu, S. Associations of dietary flavonoids with risk of type 2 diabetes, and markers of insulin resistance and systemic inflammation in women: A prospective study and cross-sectional analysis. J. Am. Coll. Nutr. 2005, 24, 376–384. [Google Scholar] [CrossRef] [PubMed]
- Moreira, A.J.; Fraga, C.; Alonso, M.; Collado, P.S.; Zetller, C.; Marroni, C.; Marroni, N.; González-Gallego, J. Quercetin prevents oxidative stress and NF-κB activation in gastric mucosa of portal hypertensive rats. Biochem. Pharmacol. 2004, 68, 1939–1946. [Google Scholar] [CrossRef] [PubMed]
- Alkhalidy, H.; Moore, W.; Zhang, Y.; McMillan, R.; Wang, A.; Ali, M.; Suh, K.-S.; Zhen, W.; Cheng, Z.; Jia, Z. Small molecule kaempferol promotes insulin sensitivity and preserved pancreatic β-cell mass in middle-aged obese diabetic mice. J. Diabetes Res. 2015, 2015, 1–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Al-Numair, K.S.; Chandramohan, G.; Veeramani, C.; Alsaif, M.A. Ameliorative effect of kaempferol, a flavonoid, on oxidative stress in streptozotocin-induced diabetic rats. Redox Rep. 2015, 20, 198–209. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Liu, D. Flavonol kaempferol improves chronic hyperglycemia-impaired pancreatic beta-cell viability and insulin secretory function. Eur. J. Pharmacol. 2011, 670, 325–332. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.J.; Suh, K.S.; Choi, M.C.; Chon, S.; Oh, S.; Woo, J.T.; Kim, S.W.; Kim, J.W.; Kim, Y.S. Kaempferol protects HIT-T15 pancreatic beta cells from 2-deoxy-d-ribose-induced oxidative damage. Phytother. Res. Int. J. Devoted Pharmacol. Toxicol. Eval. Nat. Prod. Deriv. 2010, 24, 419–423. [Google Scholar] [CrossRef]
- Chung, M.; Zhao, N.; Wang, D.; Shams-White, M.; Karlsen, M.; Cassidy, A.; Ferruzzi, M.; Jacques, P.F.; Johnson, E.J.; Wallace, T.C. Dose-response relation between tea consumption and risk of cardiovascular disease and all-cause mortality: A systematic review and meta-analysis of population-based studies. Adv. Nutr. 2020, 11, 790–814. [Google Scholar] [CrossRef] [Green Version]
- Raman, G.; Avendano, E.E.; Chen, S.; Wang, J.; Matson, J.; Gayer, B.; Novotny, J.A.; Cassidy, A. Dietary intakes of flavan-3-ols and cardiometabolic health: Systematic review and meta-analysis of randomized trials and prospective cohort studies. Am. J. Clin. Nutr. 2019, 110, 1067–1078. [Google Scholar] [CrossRef] [Green Version]
- Kim, Y.; Je, Y. Flavonoid intake and mortality from cardiovascular disease and all causes: A meta-analysis of prospective cohort studies. Clin. Nutr. ESPEN 2017, 20, 68–77. [Google Scholar] [CrossRef] [PubMed]
- Hubbard, G.P.; Wolffram, S.; de Vos, R.; Bovy, A.; Gibbins, J.M.; Lovegrove, J.A. Ingestion of onion soup high in quercetin inhibits platelet aggregation and essential components of the collagen-stimulated platelet activation pathway in man: A pilot study. Br. J. Nutr. 2006, 96, 482–488. [Google Scholar] [PubMed]
- Brüll, V.; Burak, C.; Stoffel-Wagner, B.; Wolffram, S.; Nickenig, G.; Müller, C.; Langguth, P.; Alteheld, B.; Fimmers, R.; Stehle, P. Acute intake of quercetin from onion skin extract does not influence postprandial blood pressure and endothelial function in overweight-to-obese adults with hypertension: A randomized, double-blind, placebo-controlled, crossover trial. Eur. J. Nutr. 2017, 56, 1347–1357. [Google Scholar] [CrossRef] [PubMed]
- Boland, B.; Yu, W.H.; Corti, O.; Mollereau, B.; Henriques, A.; Bezard, E.; Pastores, G.M.; Rubinsztein, D.C.; Nixon, R.A.; Duchen, M.R. Promoting the clearance of neurotoxic proteins in neurodegenerative disorders of ageing. Nat. Rev. Drug Discov. 2018, 17, 660–688. [Google Scholar] [CrossRef]
- Frandsen, J.; Narayanasamy, P. Flavonoid enhances the glyoxalase pathway in cerebellar neurons to retain cellular functions. Sci. Rep. 2017, 7, 1–10. [Google Scholar] [CrossRef]
- Lindqvist, D.; Dhabhar, F.S.; James, S.J.; Hough, C.M.; Jain, F.A.; Bersani, F.S.; Reus, V.I.; Verhoeven, J.E.; Epel, E.S.; Mahan, L.; et al. Oxidative stress, inflammation and treatment response in major depression. Psychoneuroendocrinology 2017, 76, 197–205. [Google Scholar] [CrossRef] [Green Version]
- Fischer, R.; Maier, O. Interrelation of oxidative stress and inflammation in neurodegenerative disease: Role of TNF. Oxidative Med. Cell. Longev. 2015, 2015, 610813. [Google Scholar] [CrossRef] [Green Version]
- Reale, M.; Costantini, E.; Di Nicola, M.; D’Angelo, C.; Franchi, S.; D’Aurora, M.; Di Bari, M.; Orlando, V.; Galizia, S.; Ruggieri, S.; et al. Butyrylcholinesterase and acetylcholinesterase polymorphisms in multiple sclerosis patients: Implication in peripheral inflammation. Sci. Rep. 2018, 8, 1319. [Google Scholar] [CrossRef]
- Nkpaa, K.W.; Onyeso, G.I. Rutin attenuates neurobehavioral deficits, oxidative stress, neuro-inflammation and apoptosis in fluoride treated rats. Neurosci. Lett. 2018, 682, 92–99. [Google Scholar] [CrossRef]
- Das, U.N. Acetylcholinesterase and butyrylcholinesterase as possible markers of low-grade systemic inflammation. Med Sci. Monit. 2007, 13, RA214–RA221. [Google Scholar]
- Khan, M.T.H.; Orhan, I.; Şenol, F.; Kartal, M.; Şener, B.; Dvorská, M.; Šmejkal, K.; Šlapetová, T. Cholinesterase inhibitory activities of some flavonoid derivatives and chosen xanthone and their molecular docking studies. Chem.-Biol. Interact. 2009, 181, 383–389. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Wang, Y.; Mai, Y.; Li, H.; Wang, Z.; Xu, J.; He, X. Health benefits of the flavonoids from onion: Constituents and their pronounced antioxidant and anti-neuroinflammatory capacities. J. Agric. Food Chem. 2020, 68, 799–807. [Google Scholar] [CrossRef] [PubMed]
- Sharma, D.; Rani, R.; Chaturvedi, M.; Rohilla, P.; Yadav, J. In silico and in vitro approach of Allium cepa and isolated quercetin against MDR bacterial strains and Mycobacterium smegmatis. S. Afr. J. Bot. 2019, 124, 29–35. [Google Scholar] [CrossRef]
- Beceiro, A.; Tomás, M.; Bou, G. Antimicrobial resistance and virulence: A successful or deleterious association in the bacterial world? Clin. Microbiol. Rev. 2013, 26, 185–230. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gerdt, J.P.; Blackwell, H.E. Competition studies confirm two major barriers that can preclude the spread of resistance to quorum-sensing inhibitors in bacteria. ACS Chem. Biol. 2014, 9, 2291–2299. [Google Scholar] [CrossRef] [PubMed]
- Mahomoodally, F.; Ramcharun, S.; Zengin, G. Onion and garlic extracts potentiate the efficacy of conventional antibiotics against standard and clinical bacterial isolates. Curr. Top. Med. Chem. 2018, 18, 787–796. [Google Scholar] [CrossRef]
- Al-Yousef, H.M.; Ahmed, A.F.; Al-Shabib, N.A.; Laeeq, S.; Khan, R.A.; Rehman, M.T.; Alsalme, A.; Al-Ajmi, M.F.; Khan, M.S.; Husain, F.M. Onion peel ethylacetate fraction and its derived constituent Quercetin 4′-O-β-D Glucopyranoside attenuates quorum sensing regulated virulence and biofilm formation. Front. Microbiol. 2017, 8, 1675. [Google Scholar] [CrossRef] [Green Version]
- Quecan, B.X.V.; Santos, J.T.; Rivera, M.L.; Hassimotto, N.M.; Almeida, F.A.; Pinto, U.M. Effect of quercetin rich onion extracts on bacterial quorum sensing. Front. Microbiol. 2019, 10, 867. [Google Scholar] [CrossRef] [Green Version]
- Farhadi, L.; Mohammadi-Motlagh, H.-R.; Seyfi, P.; Mostafaie, A. Low concentrations of flavonoid-rich fraction of shallot extract induce delayed-type hypersensitivity and TH1 cytokine IFNγ expression in Balb/c Mice. Int. J. Mol. Cell. Med. 2014, 3, 16. [Google Scholar]
- Chen, Y.; Ding, Z.; Wu, Y.; Chen, Q.; Liu, M.; Yu, H.; Wang, D.; Zhang, Y.; Wang, T. Effects of Allium mongolicum Regel and its flavonoids on constipation. Biomolecules 2020, 10, 14. [Google Scholar] [CrossRef] [Green Version]
- Cho, Y.H.; Lee, J.W.; Woo, H.D.; Lee, S.; Kim, Y.J.; Lee, Y.; Shin, S.; Joung, H.; Chung, H.W. Protective effect of onion extract on bleomycin-induced cytotoxicity and genotoxicity in human lymphocytes. Int. J. Environ. Res. Public Health 2016, 13, 227. [Google Scholar] [CrossRef] [PubMed]
- Gansukh, E.; Nile, A.; Kim, D.H.; Oh, J.W.; Nile, S.H. New insights into antiviral and cytotoxic potential of quercetin and its derivatives-A biochemical perspective. Food Chem. 2020, 334, 127508. [Google Scholar] [CrossRef] [PubMed]
- Pandey, P.; Rane, J.S.; Chatterjee, A.; Kumar, A.; Khan, R.; Prakash, A.; Ray, S. Targeting SARS-CoV-2 spike protein of COVID-19 with naturally occurring phytochemicals: An in silico study for drug development. J. Biomol. Struct. Dyn. 2020, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, S.; Sauter, D.; Wang, K.; Zhang, R.; Sun, B.; Karioti, A.; Bilia, A.R.; Efferth, T.; Schwarz, W. Kaempferol derivatives as antiviral drugs against the 3a channel protein of coronavirus. Planta Med. 2014, 80, 177. [Google Scholar] [CrossRef] [Green Version]
- Sharifi-Rad, M.; Anil Kumar, N.V.; Zucca, P.; Varoni, E.M.; Dini, L.; Panzarini, E.; Rajkovic, J.; Tsouh Fokou, P.V.; Azzini, E.; Peluso, I.; et al. Lifestyle, oxidative stress, and antioxidants: Back and forth in the pathophysiology of chronic diseases. Front. Physiol. 2020, 11, 694. [Google Scholar] [CrossRef]
- Sies, H.; Jones, D.P. Reactive oxygen species (ROS) as pleiotropic physiological signalling agents. Nat. Rev. Mol. Cell Biol. 2020, 21, 363–383. [Google Scholar] [CrossRef]
- Liguori, I.; Russo, G.; Curcio, F.; Bulli, G.; Aran, L.; Della-Morte, D.; Gargiulo, G.; Testa, G.; Cacciatore, F.; Bonaduce, D.; et al. Oxidative stress, aging, and diseases. Clin. Interv. Aging 2018, 13, 757–772. [Google Scholar] [CrossRef] [Green Version]
- Pizzino, G.; Irrera, N.; Cucinotta, M.; Pallio, G.; Mannino, F.; Arcoraci, V.; Squadrito, F.; Altavilla, D.; Bitto, A. Oxidative Stress: Harms and benefits for human health. Oxidative Med. Cell. Longev. 2017, 8416763. [Google Scholar] [CrossRef]
- Hayes, J.D.; Dinkova-Kostova, A.T. The Nrf2 regulatory network provides an interface between redox and intermediary metabolism. Trends Biochem. Sci. 2014, 39, 199–218. [Google Scholar] [CrossRef]
- Cuadrado, A.; Rojo, A.I.; Wells, G.; Hayes, J.D.; Cousin, S.P.; Rumsey, W.L.; Attucks, O.C.; Franklin, S.; Levonen, A.-L.; Kensler, T.W. Therapeutic targeting of the NRF2 and KEAP1 partnership in chronic diseases. Nat. Rev. Drug Discov. 2019, 18, 295–317. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, T.; Sherratt, P.J.; Nioi, P.; Yang, C.S.; Pickett, C.B. Nrf2 controls constitutive and inducible expression of ARE-driven genes through a dynamic pathway involving nucleocytoplasmic shuttling by Keap1. J. Biol. Chem. 2005, 280, 32485–32492. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahmed, S.M.U.; Luo, L.; Namani, A.; Wang, X.J.; Tang, X. Nrf2 signaling pathway: Pivotal roles in inflammation. Biochim. Biophys. Acta (BBA)-Mol. Basis Dis. 2017, 1863, 585–597. [Google Scholar] [CrossRef] [PubMed]
- Pang, S.; Lynn, D.A.; Lo, J.Y.; Paek, J.; Curran, S.P. SKN-1 and Nrf2 couples proline catabolism with lipid metabolism during nutrient deprivation. Nat. Commun. 2014, 5, 5048. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chowdhry, S.; Zhang, Y.; McMahon, M.; Sutherland, C.; Cuadrado, A.; Hayes, J.D. Nrf2 is controlled by two distinct β-TrCP recognition motifs in its Neh6 domain, one of which can be modulated by GSK-3 activity. Oncogene 2013, 32, 3765–3781. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bryan, H.K.; Olayanju, A.; Goldring, C.E.; Park, B.K. The Nrf2 cell defence pathway: Keap1-dependent and-independent mechanisms of regulation. Biochem. Pharmacol. 2013, 85, 705–717. [Google Scholar] [CrossRef] [Green Version]
- Tanigawa, S.; Fujii, M.; Hou, D.-X. Action of Nrf2 and Keap1 in ARE-mediated NQO1 expression by quercetin. Free Radic. Biol. Med. 2007, 42, 1690–1703. [Google Scholar] [CrossRef]
- Yang, J.H.; Shin, B.Y.; Han, J.Y.; Kim, M.G.; Wi, J.E.; Kim, Y.W.; Cho, I.J.; Kim, S.C.; Shin, S.M.; Ki, S.H. Isorhamnetin protects against oxidative stress by activating Nrf2 and inducing the expression of its target genes. Toxicol. Appl. Pharmacol. 2014, 274, 293–301. [Google Scholar] [CrossRef]
- Pallauf, K.; Duckstein, N.; Hasler, M.; Klotz, L.-O.; Rimbach, G. Flavonoids as putative inducers of the transcription factors Nrf2, FoxO, and PPARγ. Oxidative Med. Cell. Longev. 2017, 2017, 4397340. [Google Scholar] [CrossRef] [Green Version]
- Kashyap, D.; Sharma, A.; Tuli, H.S.; Sak, K.; Punia, S.; Mukherjee, T.K. Kaempferol–A dietary anticancer molecule with multiple mechanisms of action: Recent trends and advancements. J. Funct. Foods 2017, 30, 203–219. [Google Scholar] [CrossRef]
- Hussein, R.M.; Mohamed, W.R.; Omar, H.A. A neuroprotective role of kaempferol against chlorpyrifos-induced oxidative stress and memory deficits in rats via GSK3β-Nrf2 signaling pathway. Pestic. Biochem. Physiol. 2018, 152, 29–37. [Google Scholar] [CrossRef]
- Sun, L.; Xu, G.; Dong, Y.; Li, M.; Yang, L.; Lu, W. Quercetin protects against lipopolysaccharide-induced intestinal oxidative stress in broiler chickens through activation of Nrf2 pathway. Molecules 2020, 25, 1053. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, H.; Sun, S.-C. NF-κB in inflammation and renal diseases. Cell Biosci. 2015, 5, 63. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, T.; Zhang, L.; Joo, D.; Sun, S.-C. NF-κB signaling in inflammation. Signal Transduct. Target. Ther. 2017, 2, 17023. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morgan, M.J.; Liu, Z.-g. Crosstalk of reactive oxygen species and NF-κB signaling. Cell Res. 2011, 21, 103–115. [Google Scholar] [CrossRef] [Green Version]
- Espinosa, L.; Margalef, P.; Bigas, A. Non-conventional functions for NF-κB members: The dark side of NF-κB. Oncogene 2015, 34, 2279–2287. [Google Scholar] [CrossRef]
- Ruiz, P.A.; Braune, A.; Hölzlwimmer, G.; Quintanilla-Fend, L.; Haller, D. Quercetin inhibits TNF-induced NF-κ B transcription factor recruitment to proinflammatory gene promoters in murine intestinal epithelial cells. J. Nutr. 2007, 137, 1208–1215. [Google Scholar] [CrossRef] [Green Version]
- Indra, M.R.; Karyono, S.; Ratnawati, R.; Malik, S.G. Quercetin suppresses inflammation by reducing ERK1/2 phosphorylation and NF kappa B activation in leptin-induced human umbilical vein endothelial cells (HUVECs). BMC Res. Notes 2013, 6, 275. [Google Scholar] [CrossRef] [Green Version]
- Chekalina, N.; Burmak, Y.; Petrov, Y.; Borisova, Z.; Manusha, Y.; Kazakov, Y.; Kaidashev, I. Quercetin reduces the transcriptional activity of NF-kB in stable coronary artery disease. Indian Heart J. 2018, 70, 593–597. [Google Scholar] [CrossRef]
- Lee, C.S. Flavonoid myricetin inhibits TNF-α-stimulated production of inflammatory mediators by suppressing the Akt, mTOR and NF-κB pathways in human keratinocytes. Eur. J. Pharmacol. 2016, 784, 164–172. [Google Scholar] [CrossRef]
- Garg, S.; Malhotra, R.K.; Khan, S.I.; Sarkar, S.; Susrutha, P.; Singh, V.; Goyal, S.; Nag, T.C.; Ray, R.; Bhatia, J. Fisetin attenuates isoproterenol-induced cardiac ischemic injury in vivo by suppressing RAGE/NF-κB mediated oxidative stress, apoptosis and inflammation. Phytomedicine 2019, 56, 147–155. [Google Scholar] [CrossRef]
- Qiu, S.; Sun, G.; Zhang, Y.; Li, X.; Wang, R. Involvement of the NF-κB signaling pathway in the renoprotective effects of isorhamnetin in a type 2 diabetic rat model. Biomed. Rep. 2016, 4, 628–634. [Google Scholar] [CrossRef] [Green Version]
- Lacroix, M.; Riscal, R.; Arena, G.; Linares, L.K.; Le Cam, L. Metabolic functions of the tumor suppressor p53: Implications in normal physiology, metabolic disorders, and cancer. Mol. Metab. 2020, 33, 2–22. [Google Scholar] [CrossRef] [PubMed]
- Beyfuss, K.; Hood, D.A. A systematic review of p53 regulation of oxidative stress in skeletal muscle. Redox Rep. 2018, 23, 100–117. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Clemente-Soto, A.F.; Salas-Vidal, E.; Milan-Pacheco, C.; Sánchez-Carranza, J.N.; Peralta-Zaragoza, O.; González-Maya, L. Quercetin induces G2 phase arrest and apoptosis with the activation of p53 in an E6 expression-independent manner in HPV-positive human cervical cancer-derived cells. Mol. Med. Rep. 2019, 19, 2097–2106. [Google Scholar] [CrossRef] [Green Version]
- Luo, H.; Rankin, G.O.; Li, Z.; DePriest, L.; Chen, Y.C. Kaempferol induces apoptosis in ovarian cancer cells through activating p53 in the intrinsic pathway. Food Chem. 2011, 128, 513–519. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Turrens, J.F. Mitochondrial formation of reactive oxygen species. J. Physiol. 2003, 552, 335–344. [Google Scholar] [CrossRef] [PubMed]
- Lagoa, R.; Graziani, I.; Lopez-Sanchez, C.; Garcia-Martinez, V.; Gutierrez-Merino, C. Complex I and cytochrome care molecular targets of flavonoids that inhibit hydrogen peroxide production by mitochondria. Biochim. Biophys. Acta (BBA)-Bioenerg. 2011, 1807, 1562–1572. [Google Scholar] [CrossRef] [Green Version]
- Krauss, S.; Zhang, C.-Y.; Lowell, B.B. The mitochondrial uncoupling-protein homologues. Nat. Rev. Mol. Cell Biol. 2005, 6, 248–261. [Google Scholar] [CrossRef]
- Tarasov, A.I.; Griffiths, E.J.; Rutter, G.A. Regulation of ATP production by mitochondrial Ca2+. Cell Calcium 2012, 52, 28–35. [Google Scholar] [CrossRef] [Green Version]
- Hamilton, S.; Terentyeva, R.; Kim, T.Y.; Bronk, P.; Clements, R.T.; O-Uchi, J.; Csordás, G.; Choi, B.R.; Terentyev, D. Pharmacological modulation of mitochondrial Ca2+ content regulates sarcoplasmic reticulum Ca2+ release via oxidation of the ryanodine receptor by mitochondria-derived reactive oxygen species. Front. Physiol. 2018, 9, 1831. [Google Scholar] [CrossRef] [Green Version]
- Zheng, H.-C. The molecular mechanisms of chemoresistance in cancers. Oncotarget 2017, 8, 59950. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Steinberg, G.R.; Carling, D. AMP-activated protein kinase: The current landscape for drug development. Nat. Rev. Drug Discov. 2019, 18, 527–551. [Google Scholar] [CrossRef]
- Carbonell-Capella, J.M.; Buniowska, M.; Barba, F.J.; Esteve, M.J.; Frígola, A. Analytical methods for determining bioavailability and bioaccessibility of bioactive compounds from fruits and vegetables: A review. Compr. Rev. Food Sci. Food Saf. 2014, 13, 55–171. [Google Scholar] [CrossRef]
- Bohn, T. Dietary factors affecting polyphenol bioavailability. Nutr. Rev. 2014, 72, 429–452. [Google Scholar] [CrossRef] [PubMed]
- Williamson, G.; Kay, C.D.; Crozier, A. The bioavailability, transport, and bioactivity of dietary flavonoids: A review from a historical perspective. Compr. Rev. Food Sci. Food Saf. 2018, 17, 1054–1112. [Google Scholar] [CrossRef] [Green Version]
- Crozier, A.; Del Rio, D.; Clifford, M.N. Bioavailability of dietary flavonoids and phenolic compounds. Mol. Asp. Med. 2010, 31, 446–467. [Google Scholar] [CrossRef]
- Williamson, G.; Manach, C. Bioavailability and bioefficacy of polyphenols in humans. II. Review of 93 intervention studies. Am. J. Clin. Nutr. 2005, 81, 243S–255S. [Google Scholar] [CrossRef]
- Manach, C.; Williamson, G.; Morand, C.; Scalbert, A.; Rémésy, C. Bioavailability and bioefficacy of polyphenols in humans. I. Review of 97 bioavailability studies. Am. J. Clin. Nutr. 2005, 81, 230S–242S. [Google Scholar] [CrossRef] [Green Version]
- DuPont, M.S.; Day, A.J.; Bennett, R.N.; Mellon, F.A.; Kroon, P.A. Absorption of kaempferol from endive, a source of kaempferol-3-glucuronide, in humans. Eur. J. Clin. Nutr. 2004, 58, 947–954. [Google Scholar] [CrossRef] [Green Version]
- Guo, Y.; Bruno, R.S. Endogenous and exogenous mediators of quercetin bioavailability. J. Nutr. Biochem. 2015, 26, 201–210. [Google Scholar] [CrossRef]
- Crespy, V.; Morand, C.; Besson, C.; Manach, C.; Demigne, C.; Remesy, C. Quercetin, but not its glycosides, is absorbed from the rat stomach. J. Agric. Food Chem. 2002, 50, 618–621. [Google Scholar] [CrossRef] [PubMed]
- Hollman, P.C.; de Vries, J.H.; van Leeuwen, S.D.; Mengelers, M.J.; Katan, M.B. Absorption of dietary quercetin glycosides and quercetin in healthy ileostomy volunteers. Am. J. Clin. Nutr. 1995, 62, 1276–1282. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wiczkowski, W.; Romaszko, J.; Bucinski, A.; Szawara-Nowak, D.; Honke, J.; Zielinski, H.; Piskula, M.K. Quercetin from shallots (Allium cepa L. var. aggregatum) is more bioavailable than its glucosides. J. Nutr. 2008, 138, 885–888. [Google Scholar] [CrossRef] [PubMed]
- Day, A.J.; Mellon, F.; Barron, D.; Sarrazin, G.; Morgan, M.R.; Williamson, G. Human metabolism of dietary flavonoids: Identification of plasma metabolites of quercetin. Free Radic. Res. 2001, 35, 941–952. [Google Scholar] [CrossRef] [PubMed]
- Nemeth, K.; Piskula, M. Food content, processing, absorption and metabolism of onion flavonoids. Crit. Rev. Food Sci. Nutr. 2007, 47, 397–409. [Google Scholar] [CrossRef] [PubMed]
- Hollman, P.C.; Bijsman, M.N.; Van Gameren, Y.; Cnossen, E.P.; De Vries, J.H.; Katan, M.B. The sugar moiety is a major determinant of the absorption of dietary flavonoid glycosides in man. Free Radic. Res. 1999, 31, 569–573. [Google Scholar] [CrossRef] [PubMed]
- Olthof, M.R.; Hollman, P.C.; Vree, T.B.; Katan, M.B. Bioavailabilities of quercetin-3-glucoside and quercetin-4′-glucoside do not differ in humans. J. Nutr. 2000, 130, 1200–1203. [Google Scholar] [CrossRef]
- Lee, J.; Mitchell, A.E. Pharmacokinetics of quercetin absorption from apples and onions in healthy humans. J. Agric. Food Chem. 2012, 60, 3874–3881. [Google Scholar] [CrossRef]
- Lesser, S.; Cermak, R.; Wolffram, S. Bioavailability of quercetin in pigs is influenced by the dietary fat content. J. Nutr. 2004, 134, 1508–1511. [Google Scholar] [CrossRef]
- Guo, Y.; Mah, E.; Davis, C.G.; Jalili, T.; Ferruzzi, M.G.; Chun, O.K.; Bruno, R.S. Dietary fat increases quercetin bioavailability in overweight adults. Mol. Nutr. Food Res. 2013, 57, 896–905. [Google Scholar] [CrossRef]
- Mullen, W.; Edwards, C.A.; Crozier, A. Absorption, excretion and metabolite profiling of methyl-, glucuronyl-, glucosyl-and sulpho-conjugates of quercetin in human plasma and urine after ingestion of onions. Br. J. Nutr. 2006, 96, 107–116. [Google Scholar] [CrossRef] [PubMed]
- Gowd, V.; Karim, N.; Shishir, M.R.I.; Xie, L.; Chen, W. Dietary polyphenols to combat the metabolic diseases via altering gut microbiota. Trends Food Sci. Technol. 2019, 93, 81–93. [Google Scholar] [CrossRef]
- Wang, W.; Sun, C.; Mao, L.; Ma, P.; Liu, F.; Yang, J.; Gao, Y. The biological activities, chemical stability, metabolism and delivery systems of quercetin: A review. Trends Food Sci. Technol. 2016, 56, 21–38. [Google Scholar] [CrossRef]
- Cardona, F.; Andrés-Lacueva, C.; Tulipani, S.; Tinahones, F.J.; Queipo-Ortuño, M.I. Benefits of polyphenols on gut microbiota and implications in human health. J. Nutr. Biochem. 2013, 24, 1415–1422. [Google Scholar] [CrossRef] [Green Version]
- Manach, C.; Scalbert, A.; Morand, C.; Rémésy, C.; Jiménez, L. Polyphenols: Food sources and bioavailability. Am. J. Clin. Nutr. 2004, 79, 727–747. [Google Scholar] [CrossRef] [Green Version]
- Zhao, J.; Yang, J.; Xie, Y. Improvement strategies for the oral bioavailability of poorly water-soluble flavonoids: An overview. Int. J. Pharm. 2019, 570, 118642. [Google Scholar] [CrossRef]
- Chen, Z.-p.; Sun, J.; Chen, H.-x.; Xiao, Y.-y.; Liu, D.; Chen, J.; Cai, H.; Cai, B.-c. Comparative pharmacokinetics and bioavailability studies of quercetin, kaempferol and isorhamnetin after oral administration of Ginkgo biloba extracts, Ginkgo biloba extract phospholipid complexes and Ginkgo biloba extract solid dispersions in rats. Fitoterapia 2010, 81, 1045–1052. [Google Scholar] [CrossRef]
- Khalilzadeh, M.A.; Borzoo, M. Green synthesis of silver nanoparticles using onion extract and their application for the preparation of a modified electrode for determination of ascorbic acid. J. Food Drug Anal. 2016, 24, 796–803. [Google Scholar] [CrossRef]
- Peñalva, R.; Esparza, I.; Morales-Gracia, J.; Gonzalez-Navarro, C.J.; Larrañeta, E.; Irache, J.M. Casein nanoparticles in combination with 2-hydroxypropyl-β-cyclodextrin improves the oral bioavailability of quercetin. Int. J. Pharm. 2019, 570, 118652. [Google Scholar] [CrossRef]
- Ahmad, N.; Ahmad, R.; Naqvi, A.A.; Alam, M.A.; Ashafaq, M.; Abdur Rub, R.; Ahmad, F.J. Intranasal delivery of quercetin-loaded mucoadhesive nanoemulsion for treatment of cerebral ischaemia. Artif. Cells Nanomed. Biotechnol. 2018, 46, 717–729. [Google Scholar] [CrossRef] [Green Version]
- Zhao, G.; Duan, J.; Xie, Y.; Lin, G.; Luo, H.; Li, G.; Yuan, X. Effects of solid dispersion and self-emulsifying formulations on the solubility, dissolution, permeability and pharmacokinetics of isorhamnetin, quercetin and kaempferol in total flavones of Hippophae rhamnoides L. Drug Dev. Ind. Pharm. 2013, 39, 1037–1045. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.; Gao, Y.; Pei, Y.; Guo, C.; Li, H.; Cao, F.; Yu, A.; Zhai, G. Development of nanosuspension formulation for oral delivery of quercetin. J. Biomed. Nanotechnol. 2010, 6, 325–332. [Google Scholar] [CrossRef] [PubMed]
- Forte, L.; Torricelli, P.; Boanini, E.; Gazzano, M.; Rubini, K.; Fini, M.; Bigi, A. Antioxidant and bone repair properties of quercetin-functionalized hydroxyapatite: An in vitro osteoblast–osteoclast–endothelial cell co-culture study. Acta Biomater. 2016, 32, 298–308. [Google Scholar] [CrossRef]
- Mulholland, P.; Ferry, D.; Anderson, D.; Hussain, S.; Young, A.; Cook, J.; Hodgkin, E.; Seymour, L.; Kerr, D. Pre-clinical and clinical study of QC12, a water-soluble, pro-drug of quercetin. Ann. Oncol. 2001, 12, 245–248. [Google Scholar] [CrossRef]
- Vissiennon, C.; Nieber, K.; Kelber, O.; Butterweck, V. Route of administration determines the anxiolytic activity of the flavonols kaempferol, quercetin and myricetin—Are they prodrugs? J. Nutr. Biochem. 2012, 23, 733–740. [Google Scholar] [CrossRef] [PubMed]
- Bahram-Parvar, M.; Lim, L.T. Fresh-cut onion: A review on processing, health benefits, and shelf-life. Compr. Rev. Food Sci. Food Saf. 2018, 17, 290–308. [Google Scholar] [CrossRef] [Green Version]
- Rohn, S.; Buchner, N.; Driemel, G.; Rauser, M.; Kroh, L.W. Thermal degradation of onion quercetin glucosides under roasting conditions. J. Agric. Food Chem. 2007, 55, 1568–1573. [Google Scholar] [CrossRef]
- Rodrigues, A.; Pérez-Gregorio, M.; García-Falcón, M.; Simal-Gándara, J. Effect of curing and cooking on flavonols and anthocyanins in traditional varieties of onion bulbs. Food Res. Int. 2009, 42, 1331–1336. [Google Scholar] [CrossRef]
- Makris, D.P.; Rossiter, J.T. Domestic processing of onion bulbs (Allium cepa) and asparagus spears (Asparagus officinalis): Effect on flavonol content and antioxidant status. J. Agric. Food Chem. 2001, 49, 3216–3222. [Google Scholar] [CrossRef]
- Lee, S.U.; Lee, J.H.; Choi, S.H.; Lee, J.S.; Ohnisi-Kameyama, M.; Kozukue, N.; Levin, C.E.; Friedman, M. Flavonoid content in fresh, home-processed, and light-exposed onions and in dehydrated commercial onion products. J. Agric. Food Chem. 2008, 56, 8541–8548. [Google Scholar] [CrossRef]
- Bhatta, S.; Stevanovic Janezic, T.; Ratti, C. Freeze-drying of plant-based foods. Foods 2020, 9, 87. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pérez-Gregorio, M.; Regueiro, J.; González-Barreiro, C.; Rial-Otero, R.; Simal-Gándara, J. Changes in antioxidant flavonoids during freeze-drying of red onions and subsequent storage. Food Control 2011, 22, 1108–1113. [Google Scholar] [CrossRef]
- Bisakowski, B.; Atwal, A.S.; Gardner, N.; Champagne, C.P. Effect of lactic acid fermentation of onions (Allium cepa) on the composition of flavonol glucosides. Int. J. Food Sci. Technol. 2007, 42, 783–789. [Google Scholar] [CrossRef]
- Yang, E.J.; Kim, S.I.; Park, S.Y.; Bang, H.Y.; Jeong, J.H.; So, J.H.; Rhee, I.K.; Song, K.S. Fermentation enhances the in vitro antioxidative effect of onion (Allium cepa) via an increase in quercetin content. Food Chem. Toxicol. 2012, 50, 2042–2048. [Google Scholar] [CrossRef]
- Chung, D.-M.; Chung, Y.-C.; Maeng, P.J.; Chun, H.-K. Regioselective deglycosylation of onion quercetin glucosides by Saccharomyces cerevisiae. Biotechnol. Lett. 2011, 33, 783–786. [Google Scholar] [CrossRef]
- Lee, Y.G.; Cho, J.Y.; Kim, Y.M.; Moon, J.H. Change in flavonoid composition and antioxidative activity during fermentation of onion (Allium cepa L.) by Leuconostoc mesenteroides with different salt concentrations. J. Food Sci. 2016, 81, C1385–C1393. [Google Scholar] [CrossRef]
- Millet, A.S.; Lamy, E.; Jonas, D.; Stintzing, F.; Mersch-Sundermann, V.; Merfort, I. Fermentation enhances the biological activity of Allium cepa bulb extracts. J. Agric. Food Chem. 2012, 60, 2148–2156. [Google Scholar] [CrossRef]
- Świeca, M.; Gawlik-Dziki, U.; Dziki, D.; Baraniak, B.; Czyż, J. The influence of protein–flavonoid interactions on protein digestibility in vitro and the antioxidant quality of breads enriched with onion skin. Food Chem. 2013, 141, 451–458. [Google Scholar] [CrossRef]
- Piechowiak, T.; Grzelak-Błaszczyk, K.; Bonikowski, R.; Balawejder, M. Optimization of extraction process of antioxidant compounds from yellow onion skin and their use in functional bread production. LWT 2020, 117, 108614. [Google Scholar] [CrossRef]
- Świeca, M.; Gawlik-Dziki, U. Nutritional and health-promoting properties of bean paste fortified with onion skin in the light of phenolic–food matrix interactions. Food Funct. 2015, 6, 3560–3566. [Google Scholar]
- Sung, Y.-Y.; Kim, S.-H.; Kim, D.-S.; Park, S.H.; Yoo, B.W.; Kim, H.K. Nutritional composition and anti-obesity effects of cereal bar containing Allium fistulosum (welsh onion) extract. J. Funct. Foods 2014, 6, 428–437. [Google Scholar] [CrossRef]
- Zhao, Y.; Fan, D.; Zheng, Z.P.; Li, E.T.; Chen, F.; Cheng, K.W.; Wang, M. 8-C-(E-phenylethenyl) quercetin from onion/beef soup induces autophagic cell death in colon cancer cells through ERK activation. Mol. Nutr. Food Res. 2017, 61, 1600437. [Google Scholar] [CrossRef] [PubMed]
- Zmora, N.; Suez, J.; Elinav, E. You are what you eat: Diet, health and the gut microbiota. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 35–56. [Google Scholar] [CrossRef] [PubMed]
| Common Name | Scientific Name | Plant Part | Total Flavonol Content | References |
|---|---|---|---|---|
Red Onion | A. cepa | Bulb | 415-1917 mg/kg F.W. | [22] |
Yellow onion | A. cepa | Bulb | 270-1187 mg/kg F.W. | [22] |
White onion | A. cepa | Bulb | 7 mg/kg F.W. | [23] |
Italian shallot | A. ascalonicum | Bulb | 1023 mg/kg F.W. | [23] |
French shallot | A. ascalonicum | Bulb | 1167 mg/kg F.W. | [23] |
Leek | A. porrum | Bulb | 246 mg/kg F.W. | [24] |
Garlic | A. sativum | Cloves | 16.19 mg/kg D.W. | [25] |
Ramson bear’s garlic | A. ursinum | Green leaves | 1856.31 mg/100 g D.W. | [26] |
| Yellow leaves | 2362.96 mg/100 D.W. | |||
| Stalks | 206.07 mg/100 g D.W. | |||
| Seeds | 73.14 mg/100 g D.W. | |||
Ramps | A. tricoccum | Leaves | 11.81 mg/g D.W. | [27] |
| Stem | 0.0382 mg/g D.W. | |||
| Bulb | -- | |||
Chinese chives | A. odorum (A. tuberosum) | Leaves | 160 mg/kg D.W. | [28] |
Welsh onion | A. fistulosum | Leaves | 2329 mg/kg D.W. | [28] |
Yellow flowered garlic | A. flavum subsp. flavum | Aerial parts | 44-264 mg/g D.W. | [29] |
| Bulb | 0.77-832 µg/g D.W. | |||
Keeled garlic | A. carinatum | Whole plant | 11.14 mg/g D.W. | [30] |
| S. No. | Flavonol Aglycones/Glycosides | Plant Species | References |
|---|---|---|---|
| 1 | Quercetin (Que) | A. cepa | [22] |
| 2 | Que-3-O-glucoside | A. cepa, A. sativum, A. flavum, A. macrostemon | [16,22,29,37,38] |
| 3 | Que-4′-O-glucoside | A. cepa | [16] |
| 4 | Que-3,4′-O-diglucoside | A. cepa, A. tuberosum | [16,22,36] |
| 5 | Que-3-O-rutinoside | A. cepa, A. chinense | [22,39] |
| 6 | Que-7-O-glucoside | A. cepa | [22] |
| 7 | Que-7-O-rhamnoside | A. cepa | [22] |
| 8 | Que-7,4′-O-diglucoside | A. cepa | [22] |
| 9 | Que-3,7-O-diglucoside | A. cepa | [22] |
| 10 | Que-3,7,4′-O-triglucoside | A. cepa | [16,22] |
| 11 | Que-3-O-rhamnoside | A. cepa, A. fistulosum | [22] |
| 12 | Que dimer | A. cepa | [22] |
| 13 | 4′-Glucoside of que dimer | A. cepa | [22] |
| 14 | Que trimer | A. cepa | [22] |
| 15 | Quercetin sophoroside glucuronide | A. tricoccum | [27] |
| 16 | Que hexoside glucuronide | A. tricoccum | [27] |
| 17 | Que sophoroside | A. tuberosum | [40] |
| 18 | Que-3-O-β-d-xylopyranoside | A. sativum | [37] |
| 19 | Kaempferol (Kae) | A. cepa, A. tuberosum | [36,41,42] |
| 20 | Kae-3-O-glucoside | A. cepa, A. sativum, A. flavum, A. ursinum, A. macrostemon | [22,29,37,38,43] |
| 21 | Kae-4′-O-glucoside | A. cepa | [22] |
| 22 | Kae-7,4′-O-diglucoside | A. cepa | [22] |
| 23 | Kae-7-O-glucoside | A. triquetrum | [44] |
| 24 | Kae-3,4′-O-diglucoside | A. cepa, A. tuberosum, A. macrostemon | [22,36,38] |
| 25 | Kae-3,7-di-O-rhamnoside | A. roseum | [45] |
| 26 | Kae-3,7-di-O-glucoside | A. macrostemon | [38] |
| 27 | Kae-3-O-glucuronide-7-O-rhamnosylglucoside | A. roseum | [46] |
| 28 | Kae-3-O-rutinoside | A. roseum, A. tuberosum, A. triquetrum | [36,44,46] |
| 29 | Kae-3-O-glucoside-7-O- glucuronide | A. roseum | [46] |
| 30 | Kae-7-O-glucuronide | A. roseum | [46] |
| 31 | Kae-3-O-glucuronide | A. roseum | [46] |
| 32 | Kae-7-O-(6”-malonyl)-glucoside | A. roseum | [46] |
| 33 | Kae-3-O-sophoroside | A. tuberosum, A. tricoccum | [27,36] |
| 34 | Kae-3-O-β-d-glucosyl-(1 2)-O-α-L-xylopyranoside | A. tuberosum | [36] |
| 35 | 3-O-β-d-(2-O-feruloyl)-glucosyl-7,4’-di-O-β-d-glucosylkaempferol | A. tuberosum | [36] |
| 36 | 3-O-β-sophorosyl-7-O-β-d-(2-O-feruloyl)-glucosylkaempferol | A. tuberosum | [36] |
| 37 | Kae-3-O-neohesperidoside | A. ursinum | [43] |
| 38 | Kae-3-O-flneohesperidoside-7-O-[2-O-(trans-p-coumaroyl)]-fl-d-glucopyranoside, | A. ursinum | [43] |
| 39 | Kae-3-O-fl-neohesperidoside-7-O-[2-O-(trans-feruloyl)]-fl-d-glucopyranoside | A. ursinum | [43] |
| 40 | Kae-3-O-fl-neohesperidoside-7-O-[2-O-(trans-p-coumaroyl)-3-O-flD-glucopyranosyl-1-fl-d-glucopyranoside | A. ursinum | [43] |
| 41 | Kae-3-O-[2-O-(trans-p-coumaryl)-β-d-galactopyranosyl]-(1→4)-O-β-d-glucopyranoside | A. porrum | [47] |
| 42 | Kae-3-O-[2-O-(trans-p-coumaryl)-β-d-glucopyranosyl]-(1→6)-O-β-d-glucopyranoside | A. porrum,A. triquetrum | [44,47] |
| 43 | Kae-3-O-(2-O-trans-p-feruloyl)glucoside | A. triquetrum | [44] |
| 44 | 8-hydroxykaempferol 8-O-glucoside | A. triquetrum | [44] |
| 45 | Kae-3-O-[2-O-(trans-p-coumaroyl)-3-O-β-d-glucopyranosyl]-β-d-glucopyranoside | A. triquetrum | [44] |
| 46 | Isorhamnetin (Iso) | A. cepa | [48] |
| 47 | Iso-4′-O-glucoside | A. cepa | [16,22] |
| 48 | Iso-3-O-glucoside | A. cepa, A. vineale, A. macrostemon | [22,38,49] |
| 49 | Iso-3,4′-O-diglucoside | A. cepa; A. tuberosum | [16,22,40] |
| 50 | Iso-4′-O-galactoside | A. cepa | [16] |
| 51 | Myricetin | A. cepa | [41] |
| 52 | Fisetin | A. cepa | [41] |
| 53 | Morin | A. cepa | [50] |
| Plant Species (Part Used) | Major Identified Flavonol(s) | Study System, Dose, and Duration | Activity | Biological Effects | Molecular Targets | References |
|---|---|---|---|---|---|---|
| A. cepa (peel) | Quercetin, quercitrin, kaempferol, and morin | In vitro: HT–29, 50–250 µg/mL for 24 h | Anticancer | - Inhibits proliferation - Reduces oxidative stress - Reduces inflammation | ↑ LDH release; ↓ HO-1; ↓ TNF-α; ↓ GSTs, GSTM1, GSTT1, and GSTP1 | [50] |
| A. cepa (bulb) | Quercetin 3,7,4′-triglucoside, quercetin 7,4′- diglucoside, quercetin 3,4′-diglucoside, isorhamnetin 3,4′-diglucoside, quercetin 3-glucoside, quercetin 4′-glucoside, isorhamnetin 4′-galactoside, and isorhamnetin 4′-glucoside | In vitro: THP-1, K562, and U937, 20–100 µg/mL for 48 h | Anticancer | - Inhibits cell proliferation - Induces apoptosis | ↓ Caspase-3, -8 and -9 activity; ↑ Bid; ↓ Bcl-xL; ↑ DR5 TRAIL; ↓ Survivin; ↓ cIAP-1; ↓ PI3K/Akt | [16] |
| A. cepa (bulb) | -do- | In vitro: AGS, 1–100 µg/mL for 48 h | Anticancer | - Inhibits cell proliferation - Induces apoptosis | ↑ PAPR; ↓ Procaspase-3; ↓ Bcl-2; ↑ Bid; ↑ Bax; ↑ p53; ↓ MMP (Δ Ψm); ↓ PI3K/Akt | [54] |
| A. cepa (scale) | Quercetin and quercetin-4′-β-O-d-glucoside | In vivo: Atypical prostatic hyperplasia model of Wistar rats, 75, 150, or 300 mg/kg/d orally for 30 d | Anticancer | - Inhibits proliferation - Induces apoptosis - Reduces inflammation | ↓ IL-6; ↓ IL-8; ↓ TNF-α; ↓ IGF-1 | [14] |
| A. cepa (solid waste) | Quercetin, quercetin-3,4′-O-diglucoside, and quercetin-4′-O-monoglucoside | In vitro: ACHN, Panc 1, Calu 1, H460, and HCT 116, 1–5 mg/mL for 24 h | Anticancer | - Inhibits proliferation | n.r. | [67] |
| A. cepa var. proliferum (stems) | Isorhamnetin and kaempferol | In vitro: HepG2, 20–100 mg/mL for 72 h | Anticancer | - Inhibits proliferation - Induces apoptosis | n.r. | [68] |
| A. cepa (n.r.) | Rutin | In vivo: Hyperlipidemia colon tumor model of BALB/C nu/nu mice, 100-300 mg/kg/d intragastrically for 3 wk | Antihyperlipidemic and anticancer | - Improves lipid metabolism - Inhibits tumor proliferation | n.r. | [69] |
| A. cepa (peel) | Quercetin | In vivo: HFD-fed SD rats, 0.2% in diet for 8 wk | Antiobesity | - Reduces mesenteric fat | ↑ Adiponectin; ↓ PPAR-γ | [61] |
| A. cepa (peel) | Quercetin | In vitro: 3T3-L1, 25–100 µg/mL for 24 h In vivo: HFD-fed SD rats, 0.36% or 0.72% in diet for 8 wk | Antiobesity | - Attenuates lipid metabolism - Reduces body weight - Reduces adipose tissue - Improves lipid metabolism | ↓ AP-2; ↑ CPT-1α; ↑ FABP4; ↓ PPAR-γ ↓ C/EBP-α; ↓ FAS; ↓ ACC ↑ CPT-1α; ↑ UCP-1 | [62] |
| A. cepa (peel) | Quercetin | In vitro: 3T3-L1, 1–4 μg/mL for 24 h | Antiobesity | - Reduces lipid accumulation - Reduces adipogenesis - Induces lipolysis | ↓ GPDH activity; ↓ PPAR-γ; ↓ C/EBP-α ↓ AP2; ↓ LPL; ↑ ATGL; ↑ HSL | [63] |
| A. cepa (peel) | Quercetin and isoquercetin | In vitro: 3T3-L1, 50–150 μg/mL for 11 d (on day 5, 7, and 9) In vivo: HFD-fed C57BL/6 mice, 0.5% in diet for 8 wk | Antiobesity | - Induces adipocyte browning - Reduces adipogenesis - Reduces lipogensis | ↓ PPAR-γ; ↓ ACC; ↓ FAS; ↑ PRDM16; ↑ UCP1; ↓ FGF21; ↑ TBX1; ↓ CIDEA; ↑ PGC1α; ↑ CPT1-α ↓ ACC; ↑ PRDM16; ↑ UCP1; ↑ FGF21; ↑ CIDEA; ↑ PGC1α | [64] |
| A. cepa (peel) | Quercetin | Randomized, double-blind, placebo-controlled study: Obese women, 100 mg/d (50 mg bis in die) orally for 12 wk | Antiobesity | - Reduces waist and hip circumferences - Reduces oxidative stress | ↓ ROS; ↑ SOD activity | [70] |
| A. fistulosum (bulbs and roots) | Quercetin | In vivo: HFD-obese C57BL/6 J mice, 100 mg/kg/d orally for 6 wk | Antiobesity | - Reduces body weight - Improves lipid and glucose metabolism | ↑ AMPK (AMPKα1 and AMPKα2); ↑ Adiponectin; ↑ UCP2; ↓ PPAR-γ | [65] |
| A. cepa (peel) | Quercetin (Q) and quercetin monoglucoside (Qmg) | In vivo: HFD-fed Wistar rats, 0.21% (Q) or 0.36% (Q+Qmg) in diet for 4 wk | Antiobesity | - Reduces oxidative stress - Improves lipid metabolism - Increases gut microbial enzyme activity | n.r. | [71] |
| A. chinense (bulbs) | Quercetin and rutin | In vivo: HFD-fed Wistar rats, 0.09 or 0.18% per day orally for 12 wk | Anti-hyperlipidemic | - Improves lipid metabolism | n.r. | [39] |
| A. cepa (skin) | Quercetin | In vivo: OGTT in SD rats, 0–500 mg/kg, single oral dose | Antidiabetic | - Reduces post-prandial blood glucose - Inhibits carbohydrate hydrolases (sucrase and maltase) | n.r. | [72] |
| A. cepa (peel) | Quercetin | In vivo: HFD/STZ-diabetic SD rats, 0.5 or 1% in diet for 8 wk | Antidiabetic and antioxidant | - Increases IAUC - Reduces blood glucose - Reduces fasting blood glucose - Increases glycogen levels - Reduces oxidative stress - Reduces inflammation | ↑ INSR and GLUT4 ↑ SOD activity; ↓ MDA level; ↓ IL-6 | [73] |
| A. cepa (bulb) | Kaempferol-3-O-β-d-6{p- coumaroyl} glucopyranoside | In vivo: Alloxan-diabetic Wistar rats, 25 mg/kg single oral dose | Antidiabetic | - Reduces blood glucose | n.r. | [74] |
| A. cepa (n.r.) | Quercetin and quercetin glycosides | In vitro: Xenopus laevis oocytes, 1–15 mg/mL for 30 min In vivo: OGTT in HFD fed C57BL/6N mice, 14 mg single oral dose | Antidiabetic | - Reduces glucose uptake | ↓ SGLT1 | [75] |
| A. tuberosum (leaves) | Kaempferol glycoside derivatives | In vivo: Alloxan-diabetic Wistar rats, 100-400 mg/kg/d orally for 30 d | Antidiabetic | - Reduces oxidative stress - Reduces fasting blood glucose - Improves lipid metabolism | ↑ GSH; ↑ SOD and CAT activities | [36] |
| A. tuberosum (leaves) | Kaempferol glycoside | In vivo: HFD/STZ-diabetic Wistar rats, 100 or 400 mg/kg/d orally for 40 d | Antidiabetic | - Reduces renal oxidative stress - Reduces inflammation - Reduces blood glucose - Improves renal and serum lipid profiles - Reduces serum creatinine - Reduces blood urea nitrogen - Reduces urinary albumin levels | ↑ GSH; ↑ CAT and SOD activities; ↓ TGF-β1; ↓ TNF-α; ↓ IL-6; ↓ IL-1β | [76] |
| A. cepa (peel) | Quercetin | Randomized, double-blind, placebo-controlled parallel design: Healthy smokers, 100 mg/d for 10 wk | Cardioprotective | - Lowers blood pressure - Improves lipid profiles - Lowers blood glucose | ⇔ Inflammatory markers | [77] |
| A. cepa (outer skin) | Quercetin, quercetin 4′-glucoside, and quercetin 3,4′-diglucoside | In vivo: HFD-fed Wistar rats, 5% in diet for 18 wk | Cardioprotective | - Atherogenic index - Lowers incremental elastic modulus | n.r. | [78] |
| A. cepa (peel) | Quercetin | In vitro: HUVEC, 50 and 100 μg/mL for 1 h In vivo: SD rats, 2 or 10 mg/d orally for 6 wk | Cardioprotective | - Delays arterial thrombus formation | ↓ Tissue factor; ↓ JNK and ERK (MAPK) | [79] |
| A. cepa (peel) | Quercetin | Epidemiologic study: Healthy men, 4.3 g/d orally for 30 d | Cardioprotective | - Improves postprandial flow-mediated dilation | n.r. | [80] |
| A. cepa (peel) | Quercetin | Randomized double-blind, placebo-controlled prospective trial: Healthy overweight and obese individuals, 100 mg/d (50 mg twice daily) orally for 12 wk | Cardioprotective | - Improves flow-mediated dilation - Improves circulating endothelial progenitor cell count | n.r. | [81] |
| A. cepa(peel) | Quercetin quercetin hexoside 1, quercetin hexoside 2, quercetin dihexoside, methylquercetin hexoside, kaempferol, and methyl quercetin | Randomized double-blind, placebo-controlled prospective trial: Overweight-to-obese patients with (pre-)hypertension, 162 mg/d for 6 wk | Cardioprotective | - Lowers systolic ambulatory blood pressure | ⇔ Biomarkers of inflammation and endothelial function | [82] |
| A. cepa (peel) | Quercetin | In vitro: SD rat platelets, 50–500 μg/mL for 3 min | Cardioprotective | - Inhibits platelet aggregation - Reduces oxidative stress | ↓ TXA2 production; ↓ TXAS and COX-1 activity; ↓ Intracellular Ca2+; ↑ cAMP | [83] |
| A. cepa (tunic) | Quercetin, quercitrin, isoquercitrin, rutin, and kaempferol | In vivo: Wistar rats, 10 mg/kg/d, orally for 14 d | Cardioprotective | - Lowers blood pressure parameters - Reduces oxidative stress | ↑ SOD and CAT activity; ↑ GSH levels | [42] |
| A. cepa (bulb) | Quercetin, quercetin-3,4′-O-diglucoside, and quercetin-4′-O-monoglucoside | In vitro: SD Rat platelet-rich plasma, 1–5 mg/mL for 5 min | Cardioprotective | - Inhibits platelet aggregation | n.r. | [84] |
| A. flavum and A. carinatum (whole plant) | Quercetin, kaempferol, isorhamnetin, rutin, quercetin 3-O-glucoside, and kaempferol-3-O-glucoside, | In vitro: A549 and HepG215-125 µg/mL for 24 h In vivo: Doxorubicin-induced toxicity in zebrafish embryos, 1–60 μg/mL for 96 h | AnticancerCardioprotective and myeloprotective | - Reduces oxidative stress - Reduces cardiovascular and morphological abnormalities - Anti-angiogenesis | ↑ SOD and CAT activity | [30] |
| A. cepa (bulb) | Quercetin | In vivo: Ischemia/reperfusion induced injury in gerbil hippocampus, 50 or 100 mg/kg/d orally for 15 d | Neuroprotection | - Reduces lipid peroxidation - Attenuates activations of astrocytes and microglia | ↓ 4-hydroxy-2-nonenal | [85] |
| A. victorialis (leaves) | Kaempferol and quercetin glycosides | In vitro: LPS-activated BV-2 cells, 20 μM for 24 h | Neuroprotection | - Anti-inflammatory effects | ↓ NO production | [86] |
| A. cepa (bulb) | Quercetin | In vitro: glutamate-mediated oxidative stress in HT22 cells, 1–25 µM for 12 h | Neuroprotection | - Reduces apoptosis | ↓ ROS; ↓ Ca2+ influx; ↑ MMP (ΔΨm); ↓ Bid and Bax ↓ MAPKs (ERK, JNK, and p38) | [87] |
| A. cepa (bulb) | Quercetin | In vitro: BSO-induced oxidative stress in mouse neocortices, 1–10 mg/mL, for 30 min | Neuroprotection | - Reduces oxidative stress | ↓ ROS; ↓ LDH release; ↑ ERK1/2; ↓ p38MAPK; ↓ PKC-ε | [88] |
| A. cepa (bulb) | Quercetin | In vivo: AlCl3 induced injury in Swiss albino mice, 50, 100 or 200 mg/kg/d orally for 60 d | Neuroprotection | - Improves muscle coordination and memory deficits - Reduces oxidative stress - Reduces inflammation | Acts as PPARγ agonist ↓ ROS; ↑ GSH, CAT ↓ AChE | [89] |
| A. cepa (bulb) | Quercetin 3,4′-O-β-d-diglucoside | In vitro: HSP cells under nutrient deprived condition, 0.1–500 µM for 20 h | Neuroprotection | - Reduces apoptosis - Alters cell morphology | ↑ Ki-67; ↓ Bax/Bcl-2; ↑ Adhesion molecules (pan-cadherin and focal adhesion kinase) | [90] |
| A. cepa (outer scale) | Quercetin | In vivo: Cerebral ischemia/reperfusion-induced injury in Swiss Albino mice, 85 mg/kg/d for 7 d | Neuroprotection | - Improves cognitive/sensorimotor functions - Reduces cerebral infarct size - Reduces brain oxidative stress | ↑ GSH; ↑ SOD activity ↑ TBARS | [91] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kothari, D.; Lee, W.-D.; Kim, S.-K. Allium Flavonols: Health Benefits, Molecular Targets, and Bioavailability. Antioxidants 2020, 9, 888. https://doi.org/10.3390/antiox9090888
Kothari D, Lee W-D, Kim S-K. Allium Flavonols: Health Benefits, Molecular Targets, and Bioavailability. Antioxidants. 2020; 9(9):888. https://doi.org/10.3390/antiox9090888
Chicago/Turabian StyleKothari, Damini, Woo-Do Lee, and Soo-Ki Kim. 2020. "Allium Flavonols: Health Benefits, Molecular Targets, and Bioavailability" Antioxidants 9, no. 9: 888. https://doi.org/10.3390/antiox9090888
APA StyleKothari, D., Lee, W.-D., & Kim, S.-K. (2020). Allium Flavonols: Health Benefits, Molecular Targets, and Bioavailability. Antioxidants, 9(9), 888. https://doi.org/10.3390/antiox9090888