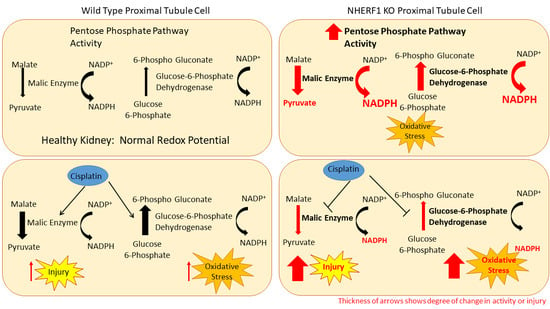
NHERF1 Loss Upregulates Enzymes of the Pentose Phosphate Pathway in Kidney Cortex
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Treatments
2.2. Fructose-1,6-Bisphosphatase (FBPase) Activity Assay
2.3. Glucose-6-Phosphatase (G6Pase) Activity Assay
2.4. Lactate Dehydrogenase (LDH) Activity Assay
2.5. Malate Dehydrogenase (MDH) Activity Assay
2.6. Malic Enzyme (ME) Activity Assay
2.7. Glucose-6-Phosphate Dehydrogenase (G6PD) Activity Assay
2.8. Liquid Chromatography-Mass Spectrometry of Kidney Cortex for ATP Quantification
2.9. Perfusion Fixation of Total Kidney In Situ for Electron Microscopy
2.10. Seahorse XF24 Mitochondrial Respiration Analysis
2.11. Brush Border Membrane Isolation
2.12. Label-Free Quantitative Liquid Chromatography-Mass Spectrometry
2.13. Interpretation of Protein Assignment Results
2.14. Statistical Analysis
3. Results
3.1. Absence of NHERF1 Results in Extensive Changes in Kidney BBM Protein Expression
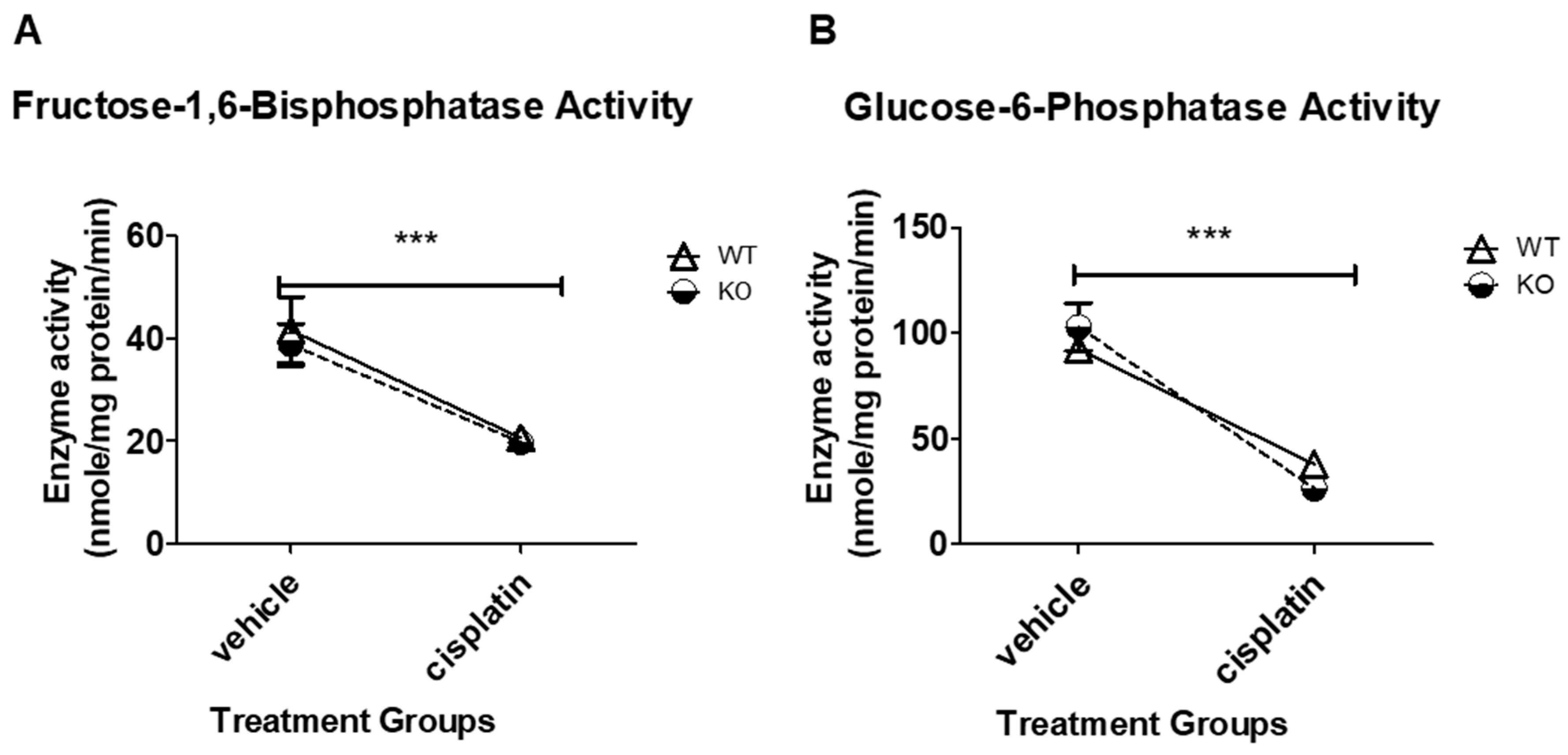
3.2. Cisplatin Treatment Significantly Decreases FBPase and G6Pase Enzyme Activity in Both WT and NHERF1 KO Mice
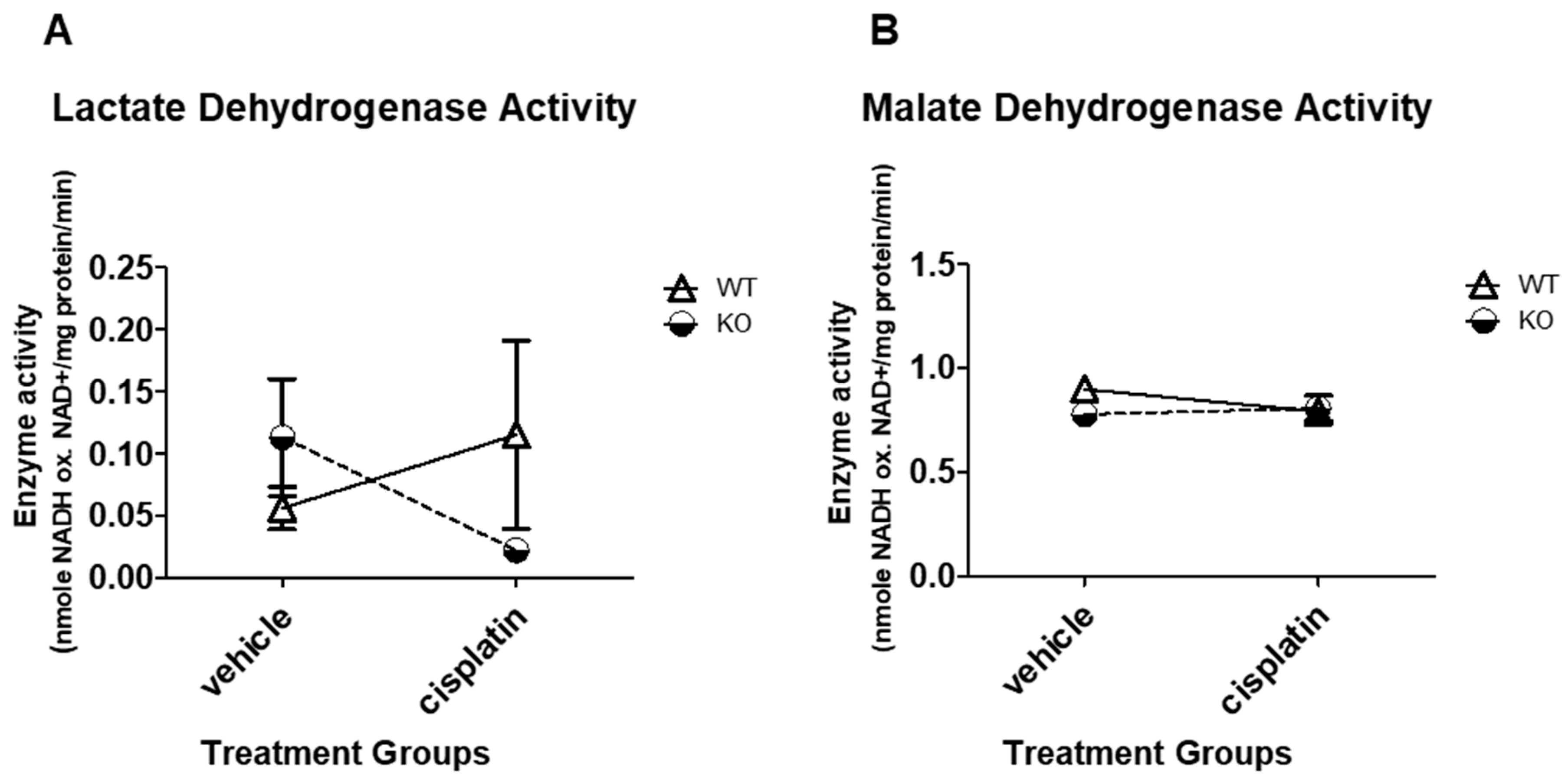
3.3. NHERF1 Deficiency or Cisplatin Treatment Does Not Significantly Affect LDH or MDH Enzyme Activity in Mice
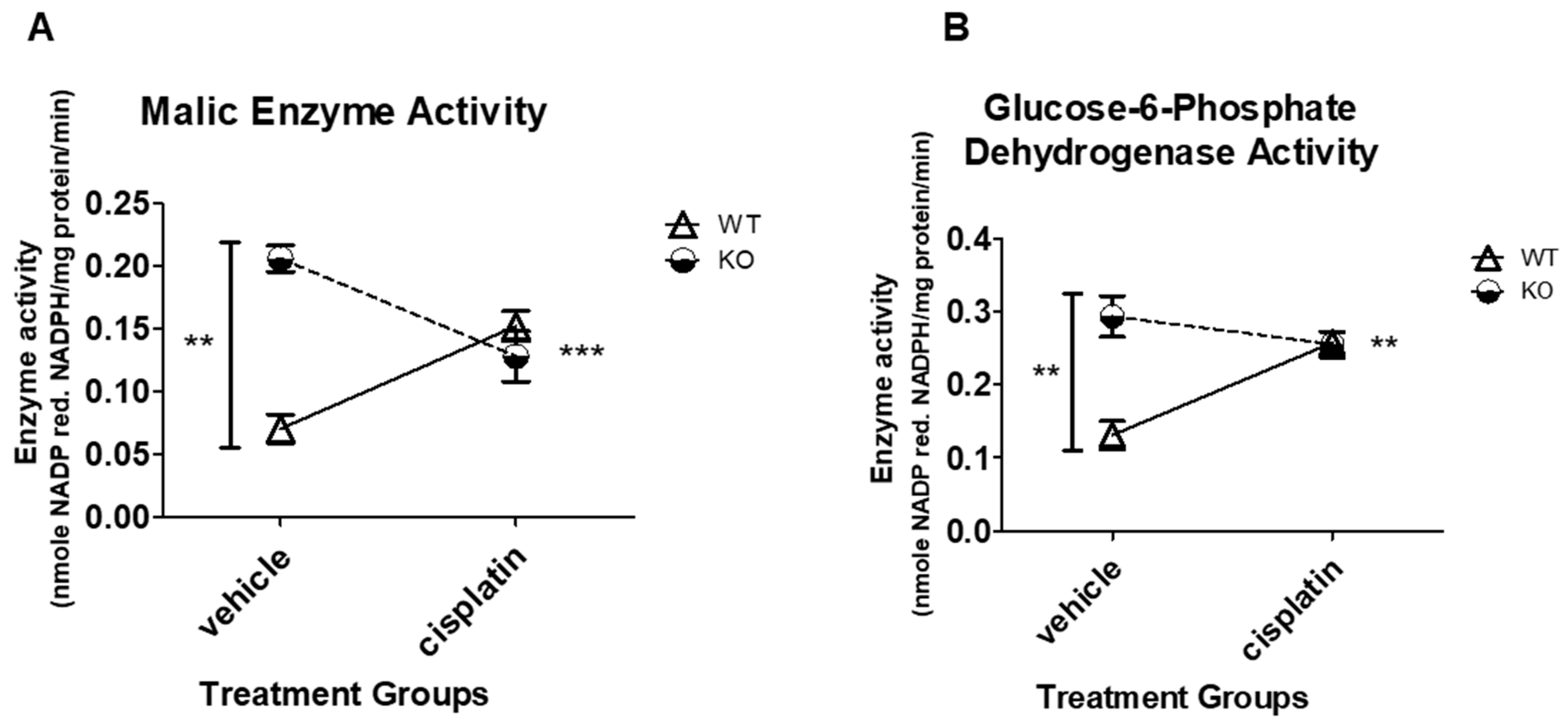
3.4. NHERF1 Deficiency Upregulates ME and G6PD Activity
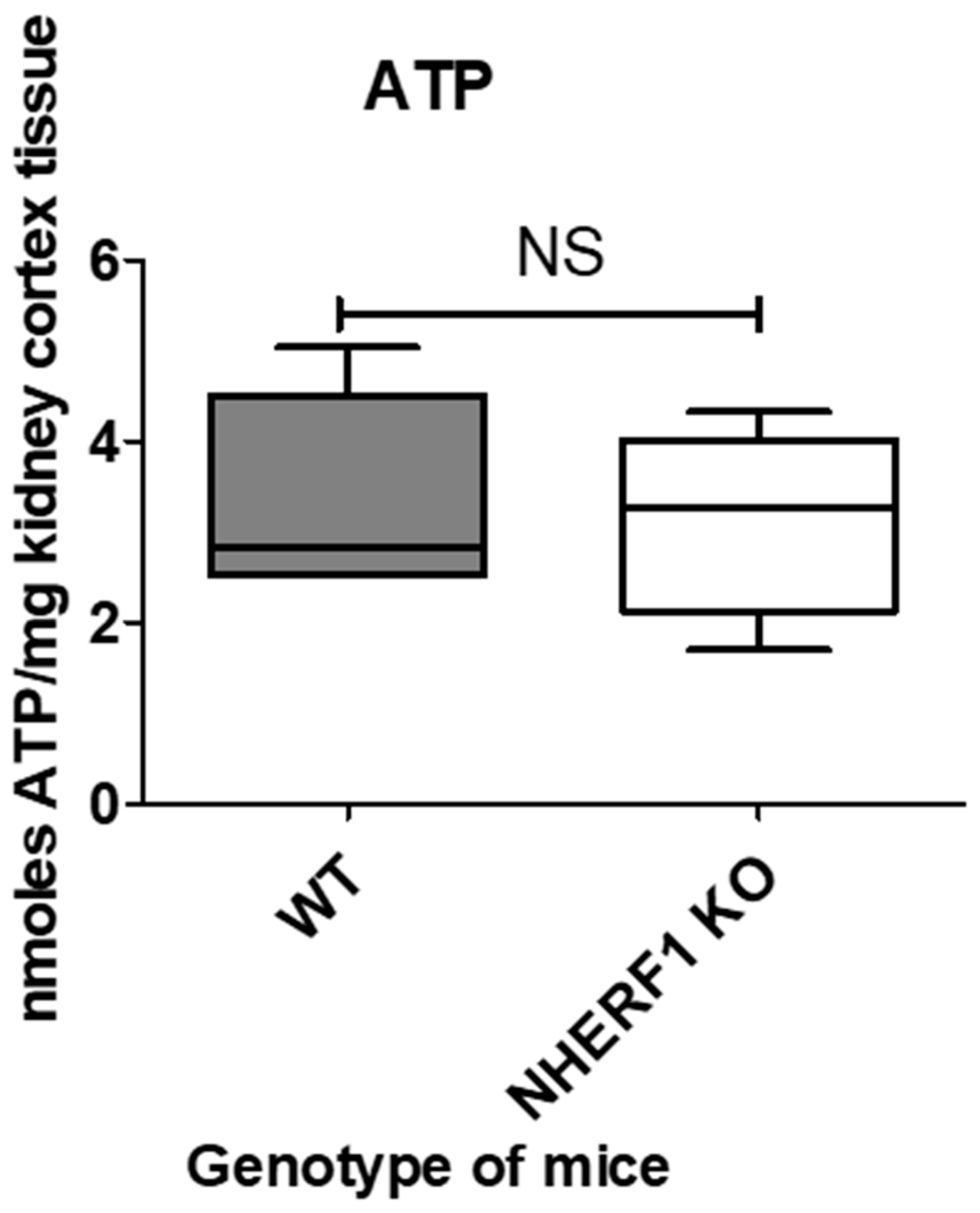
3.5. NHERF1 Deficiency Does Not Affect ATP Abundance in Mouse Kidneys
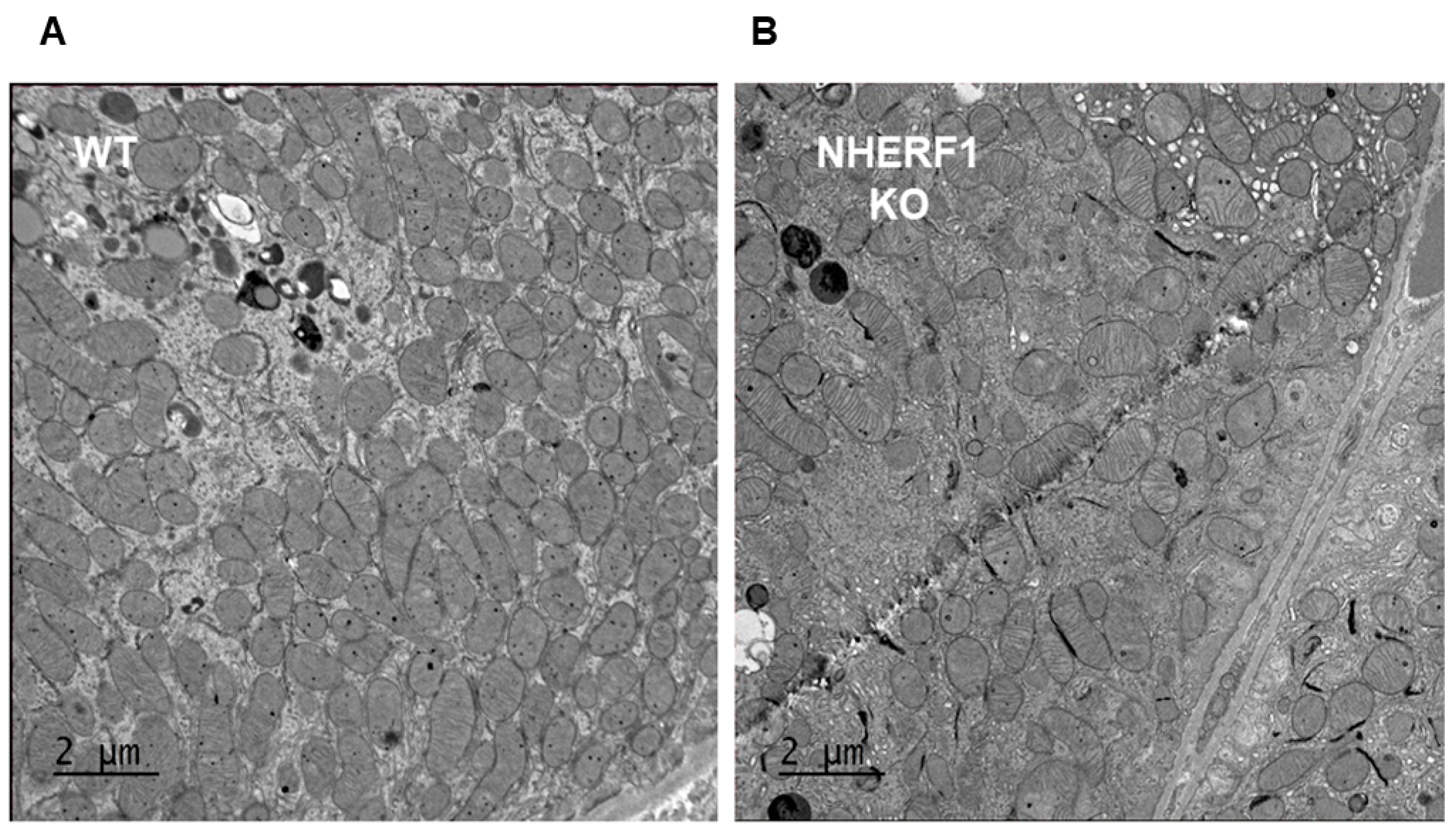
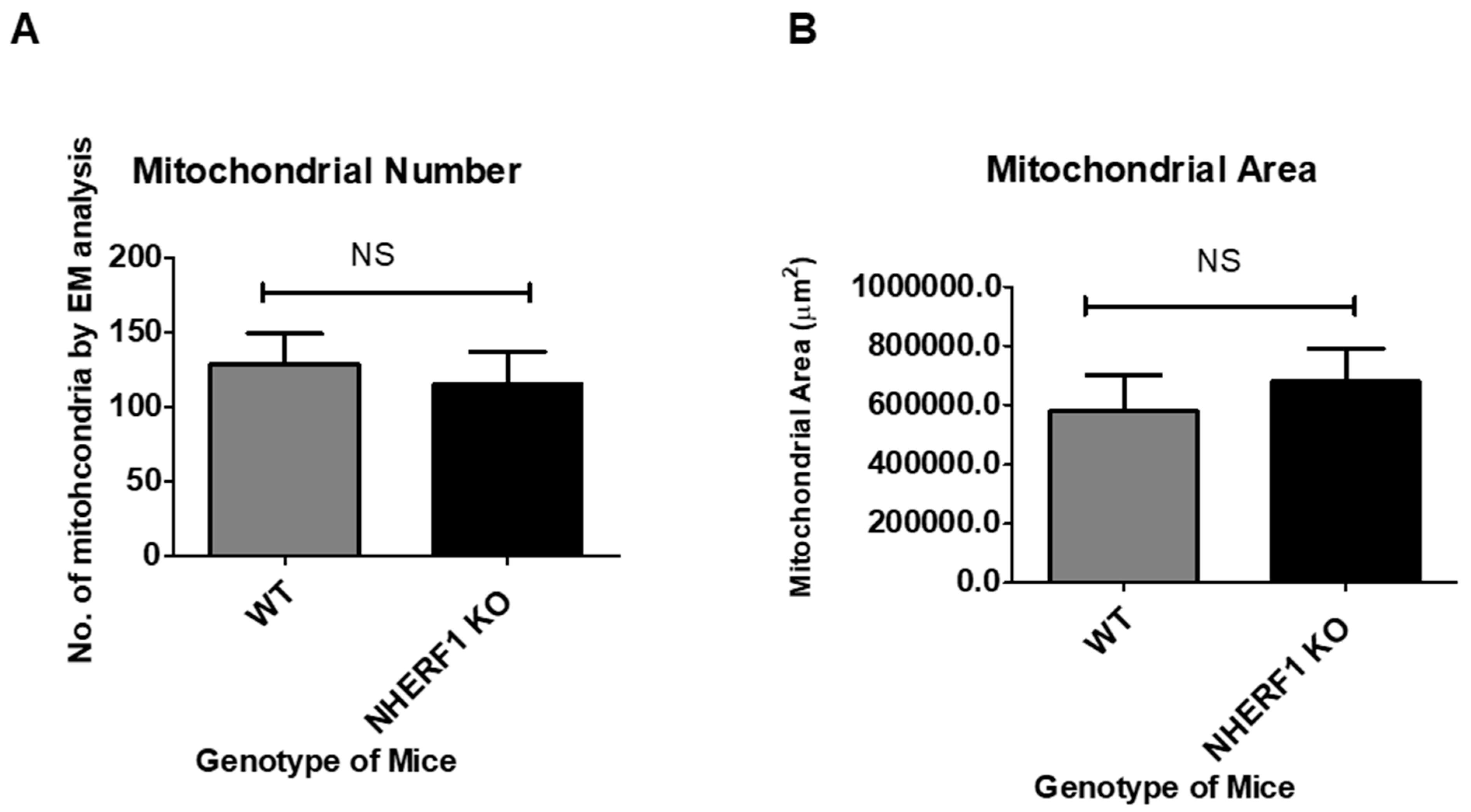
3.6. NHERF1 Deficiency Does Not Affect Kidney Proximal Tubule Mitochondria Morphology, Number, or Area
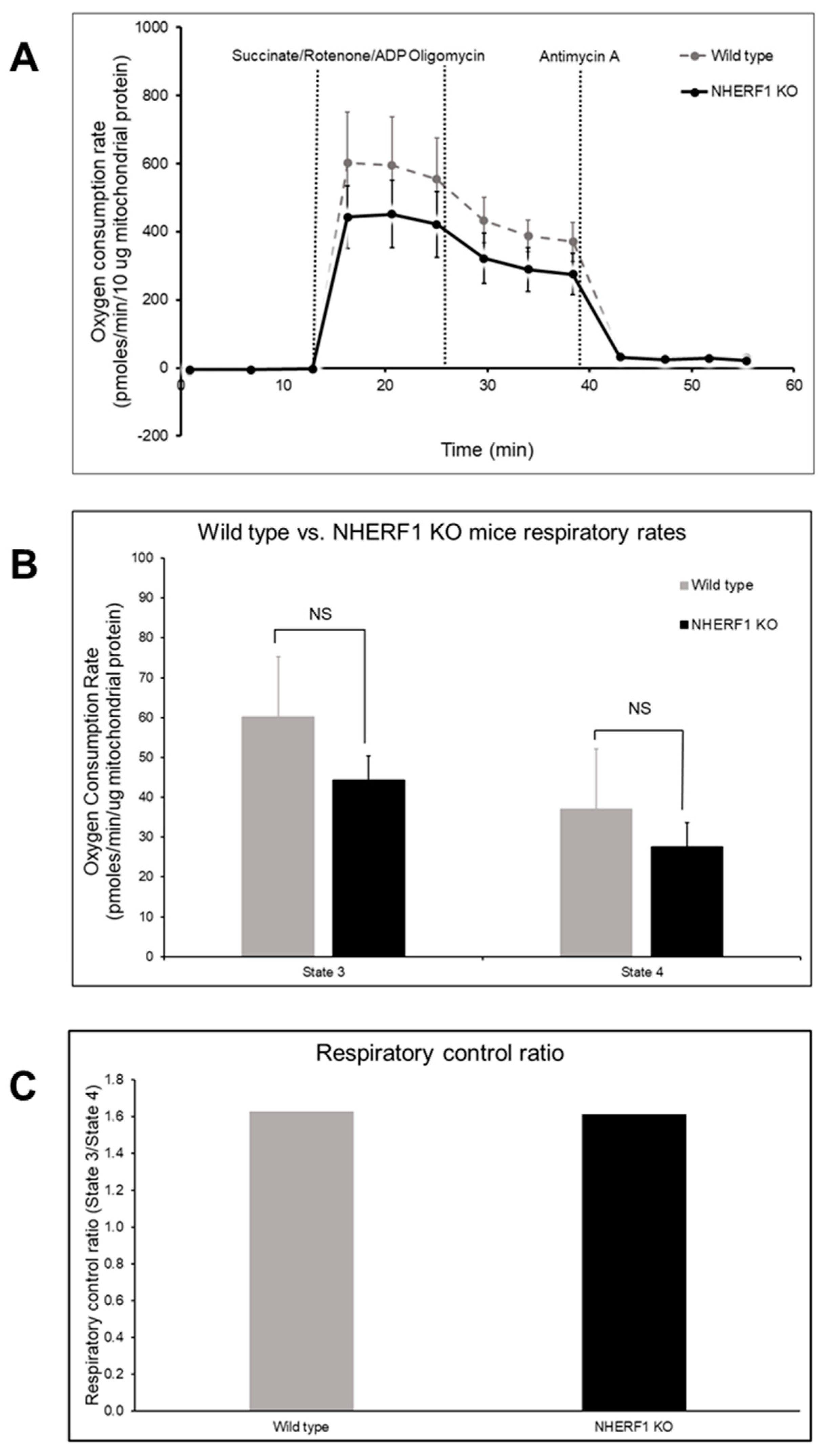
3.7. WT and NHERF1 KO Mouse Kidney Mitochondria Have Similar Oxidative Capacities
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Data Sharing
References
- Hanigan, M.H.; Devarajan, P. Cisplatin nephrotoxicity: Molecular mechanisms. Cancer Ther. 2003, 1, 47–61. [Google Scholar] [PubMed]
- Lagies, S.; Pichler, R.; Kaminski, M.M.; Schlimpert, M.; Walz, G.; Lienkamp, S.S.; Kammerer, B. Metabolic characterization of directly reprogrammed renal tubular epithelial cells (iRECs). Sci. Rep. 2018, 8, 3878. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Chen, J.-Q.; Huang, W.-Q.; Li, W.; Huang, Y.; Zhang, Z.-J.; Xu, F.-G. Renal Medulla is More Sensitive to Cisplatin than Cortex Revealed by Untargeted Mass Spectrometry-Based Metabolomics in Rats. Sci. Rep. 2017, 7, 44804. [Google Scholar] [CrossRef] [PubMed]
- Boudonck, K.J.; Mitchell, M.W.; Német, L.; Keresztes, L.; Nyska, A.; Shinar, D.; Rosenstock, M. Discovery of Metabolomics Biomarkers for Early Detection of Nephrotoxicity. Toxicol. Pathol. 2009, 37, 280–292. [Google Scholar] [CrossRef]
- Portilla, D.; Li, S.; Nagothu, K.K.; Megyesi, J.; Kaissling, B.; Schnackenberg, L.; Safirstein, R.L.; Beger, R.D. Metabolomic study of cisplatin-induced nephrotoxicity. Kidney Int. 2006, 69, 2194–2204. [Google Scholar] [CrossRef]
- Wilmes, A.; Bielow, C.; Ranninger, C.; Bellwon, P.; Aschauer, L.; Limonciel, A.; Chassaigne, H.; Kristl, T.; Aiche, S.; Huber, C.G.; et al. Mechanism of cisplatin proximal tubule toxicity revealed by integrating transcriptomics, proteomics, metabolomics and biokinetics. Toxicology In Vitro 2015, 30, 117–127. [Google Scholar] [CrossRef]
- Choi, Y.M.; Kim, H.K.; Shim, W.; Anwar, M.A.; Kwon, J.W.; Kwon, H.K.; Kim, H.J.; Jeong, H.; Kim, H.M.; Hwang, D.; et al. Mechanism of Cisplatin-Induced Cytotoxicity Is Correlated to Impaired Metabolism Due to Mitochondrial ROS Generation. PLoS ONE 2015, 10, e0135083. [Google Scholar] [CrossRef]
- Ueda, N.; Kaushal, G.P.; Shah, S.V. Apoptotic mechanisms in acute renal failure. Am. J. Med. 2000, 108, 403–415. [Google Scholar] [CrossRef]
- Richter, C.; Gogvadze, V.; Laffranchi, R.; Schlapbach, R.; Schweizer, M.; Suter, M.; Walter, P.; Yaffee, M. Oxidants in mitochondria: From physiology to diseases. Biochim. Et Biophys. Acta 1995, 1271, 67–74. [Google Scholar] [CrossRef]
- Singh, G. A possible cellular mechanism of cisplatin-induced nephrotoxicity. Toxicology 1989, 58, 71–80. [Google Scholar] [CrossRef]
- Gemba, M.; Fukuishi, N. Amelioration by ascorbic acid of cisplatin-induced injury in cultured renal epithelial cells. Contrib. Nephrol. 1991, 95, 138–142. [Google Scholar] [CrossRef] [PubMed]
- Oh, G.-S.; Kim, H.-J.; Shen, A.; Lee, S.-B.; Yang, S.-H.; Shim, H.; Cho, E.-Y.; Kwon, K.-B.; Kwak, T.H.; So, H.-S. New Therapeutic Concept of NAD Redox Balance for Cisplatin Nephrotoxicity. Biomed. Res. Int. 2016, 2016, 4048390. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Liu, H.; Liu, F.; Dong, Z. Mitochondrial dysregulation and protection in cisplatin nephrotoxicity. Arch. Toxicol. 2014, 88, 1249–1256. [Google Scholar] [CrossRef] [PubMed]
- Zsengellér, Z.K.; Ellezian, L.; Brown, D.; Horváth, B.; Mukhopadhyay, P.; Kalyanaraman, B.; Parikh, S.M.; Karumanchi, S.A.; Stillman, I.E.; Pacher, P. Cisplatin nephrotoxicity involves mitochondrial injury with impaired tubular mitochondrial enzyme activity. J. Histochem. Cytochem. 2012, 60, 521–529. [Google Scholar] [CrossRef] [PubMed]
- Bushau-Sprinkle, A.; Barati, M.; Conklin, C.; Dupre, T.; Gagnon, K.B.; Khundmiri, S.J.; Clark, B.; Siskind, L.; Doll, M.A.; Rane, M.; et al. Loss of the Na(+)/H(+) Exchange Regulatory Factor 1 Increases Susceptibility to Cisplatin-Induced Acute Kidney Injury. Am. J. Pathol. 2019, 189, 1190–1200. [Google Scholar] [CrossRef]
- Khundmiri, S.J.; Weinman, E.J.; Steplock, D.; Cole, J.; Ahmad, A.; Baumann, P.D.; Barati, M.; Rane, M.J.; Lederer, E. Parathyroid hormone regulation of NA+,K+-ATPase requires the PDZ 1 domain of sodium hydrogen exchanger regulatory factor-1 in opossum kidney cells. J. Am. Soc. Nephrol. Jasn 2005, 16, 2598–2607. [Google Scholar] [CrossRef]
- Dai, J.L.; Wang, L.; Sahin, A.A.; Broemeling, L.D.; Schutte, M.; Pan, Y. NHERF (Na+/H+ exchanger regulatory factor) gene mutations in human breast cancer. Oncogene 2004, 23, 8681–8687. [Google Scholar] [CrossRef]
- Georgescu, M.M.; Morales, F.C.; Molina, J.R.; Hayashi, Y. Roles of NHERF1/EBP50 in cancer. Curr. Mol. Med. 2008, 8, 459–468. [Google Scholar] [CrossRef]
- Bushau-Sprinkle, A.M.; Lederer, E.D. New roles of the Na(+)/H(+) exchange regulatory factor 1 scaffolding protein: A review. Am. J. Physiology. Ren. Physiol. 2020, 318, F804–F808. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Zheng, J.; Wang, Q.; Song, R.; Liu, H.; Meng, R.; Tao, T.; Si, Y.; Jiang, W.; He, J. NHERF1 regulates actin cytoskeleton organization through modulation of alpha-actinin-4 stability. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2016, 30, 578–589. [Google Scholar] [CrossRef]
- Ketchem, C.J.; Khundmiri, S.J.; Gaweda, A.E.; Murray, R.; Clark, B.J.; Weinman, E.J.; Lederer, E.D. Role of Na+/H+ exchanger regulatory factor 1 in forward trafficking of the type IIa Na+-Pi cotransporter. Am. J. Physiology. Ren. Physiol. 2015, 309, F109-119. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Lu, G.; Trescott, L.R.; Hou, Y.; Guan, X.; Wang, S.; Stamenkovich, A.; Brunzelle, J.; Sirinupong, N.; Li, C.; et al. New conformational state of NHERF1-CXCR2 signaling complex captured by crystal lattice trapping. PLoS ONE 2013, 8, e81904. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Mennone, A.; Soroka, C.J.; Hagey, L.R.; Ouyang, X.; Weinman, E.J.; Boyer, J.L. Na(+) /H(+) exchanger regulatory factor 1 knockout mice have an attenuated hepatic inflammatory response and are protected from cholestatic liver injury. Hepatology 2015, 62, 1227–1236. [Google Scholar] [CrossRef] [PubMed]
- Leslie, K.L.; Song, G.J.; Barrick, S.; Wehbi, V.L.; Vilardaga, J.P.; Bauer, P.M.; Bisello, A. Ezrin-radixin-moesin-binding phosphoprotein 50 (EBP50) and nuclear factor-kappaB (NF-kappaB): A feed-forward loop for systemic and vascular inflammation. J. Biol. Chem. 2013, 288, 36426–36436. [Google Scholar] [CrossRef] [PubMed]
- Khundmiri, S.J.; Ahmad, A.; Bennett, R.E.; Weinman, E.J.; Steplock, D.; Cole, J.; Baumann, P.D.; Lewis, J.; Singh, S.; Clark, B.J.; et al. Novel regulatory function for NHERF-1 in Npt2a transcription. Am. J. Physiology. Ren. Physiol. 2008, 294, F840-849. [Google Scholar] [CrossRef]
- Weinman, E.J.; Mohanlal, V.; Stoycheff, N.; Wang, F.; Steplock, D.; Shenolikar, S.; Cunningham, R. Longitudinal study of urinary excretion of phosphate, calcium, and uric acid in mutant NHERF-1 null mice. Am. J. Physiology. Ren. Physiol. 2006, 290, F838-843. [Google Scholar] [CrossRef]
- Donowitz, M.; Singh, S.; Singh, P.; Salahuddin, F.F.; Chen, Y.; Chakraborty, M.; Murtazina, R.; Gucek, M.; Cole, R.N.; Zachos, N.C.; et al. Alterations in the proteome of the NHERF1 knockout mouse jejunal brush border membrane vesicles. Physiol. Genom. 2010, 42, 200–210. [Google Scholar] [CrossRef][Green Version]
- Shenolikar, S.; Voltz, J.W.; Minkoff, C.M.; Wade, J.B.; Weinman, E.J. Targeted disruption of the mouse NHERF-1 gene promotes internalization of proximal tubule sodium-phosphate cotransporter type IIa and renal phosphate wasting. Proc. Natl. Acad. Sci. USA 2002, 99, 11470–11475. [Google Scholar] [CrossRef]
- Khundmiri, S.J.; Asghar, M.; Khan, F.; Salim, S.; Yusufi, A.N. Effect of ischemia and reperfusion on enzymes of carbohydrate metabolism in rat kidney. J. Nephrol. 2004, 17, 377–383. [Google Scholar]
- Khundmiri, S.J.; Rane, M.J.; Lederer, E.D. Parathyroid hormone regulation of type II sodium-phosphate cotransporters is dependent on an A kinase anchoring protein. J. Biol. Chem. 2003, 278, 10134–10141. [Google Scholar] [CrossRef] [PubMed]
- Merchant, M.L.; Powell, D.W.; Wilkey, D.W.; Cummins, T.D.; Deegens, J.K.; Rood, I.M.; McAfee, K.J.; Fleischer, C.; Klein, E.; Klein, J.B. Microfiltration isolation of human urinary exosomes for characterization by MS. Proteom. Clin. Appl. 2010, 4, 84–96. [Google Scholar] [CrossRef]
- Luerman, G.C.; Powell, D.W.; Uriarte, S.M.; Cummins, T.D.; Merchant, M.L.; Ward, R.A.; McLeish, K.R. Identification of phosphoproteins associated with human neutrophil granules following chemotactic peptide stimulation. Mol Cell Proteom. 2011, 10. [Google Scholar] [CrossRef]
- Merchant, M.L.; Cummins, T.D.; Wilkey, D.W.; Salyer, S.A.; Powell, D.W.; Klein, J.B.; Lederer, E.D. Proteomic analysis of renal calculi indicates an important role for inflammatory processes in calcium stone formation. Am. J. Physiol. Ren. Physiol. 2008, 295, F1254–F1258. [Google Scholar] [CrossRef]
- Uriarte, S.M.; Powell, D.W.; Luerman, G.C.; Merchant, M.L.; Cummins, T.D.; Jog, N.R.; Ward, R.A.; McLeish, K.R. Comparison of proteins expressed on secretory vesicle membranes and plasma membranes of human neutrophils. J. Immunol. 2008, 180, 5575–5581. [Google Scholar] [CrossRef] [PubMed]
- Mandel, L.J. Metabolic substrates, cellular energy production, and the regulation of proximal tubular transport. Annu. Rev. Physiol. 1985, 47, 85–101. [Google Scholar] [CrossRef] [PubMed]
- Zhou, R.; Vander Heiden, M.G.; Rudin, C.M. Genotoxic Exposure Is Associated with Alterations in Glucose Uptake and Metabolism. Cancer Res. 2002, 62, 3515–3520. [Google Scholar] [PubMed]
- Hannemann, J.; Baumann, K. Cisplatin-induced lipid peroxidation and decrease of gluconeogenesis in rat kidney cortex: Different effects of antioxidants and radical scavengers. Toxicology 1988, 51, 119–132. [Google Scholar] [CrossRef]
- Timson, D.J. Fructose 1,6-bisphosphatase: Getting the message across. Biosci. Rep. 2019, 39, BSR20190124. [Google Scholar] [CrossRef] [PubMed]
- Van Schaftingen, E.; Gerin, I. The glucose-6-phosphatase system. Biochem. J. 2002, 362, 513–532. [Google Scholar] [CrossRef]
- Valvona, C.J.; Fillmore, H.L.; Nunn, P.B.; Pilkington, G.J. The Regulation and Function of Lactate Dehydrogenase A: Therapeutic Potential in Brain Tumor. Brain Pathol. 2016, 26, 3–17. [Google Scholar] [CrossRef]
- Gietl, C. Malate dehydrogenase isoenzymes: Cellular locations and role in the flow of metabolites between the cytoplasm and cell organelles. Biochim. Et Biophys. Acta (BBA) Bioenerg. 1992, 1100, 217–234. [Google Scholar] [CrossRef]
- Frenkel, R. Regulation and Physiological Functions of Malic Enzymes. In Current Topics in Cellular Regulation; Horecker, B.L., Stadtman, E.R., Eds.; Academic Press: Cambridge, MA, USA, 1975; Volume 9, pp. 157–181. [Google Scholar]
- Efferth, T.; Schwarzl, S.M.; Smith, J.; Osieka, R. Role of glucose-6-phosphate dehydrogenase for oxidative stress and apoptosis. Cell Death Differ. 2006, 13, 527–528. [Google Scholar] [CrossRef] [PubMed]
- Bonora, M.; Patergnani, S.; Rimessi, A.; De Marchi, E.; Suski, J.M.; Bononi, A.; Giorgi, C.; Marchi, S.; Missiroli, S.; Poletti, F.; et al. ATP synthesis and storage. Purinergic Signal. 2012, 8, 343–357. [Google Scholar] [CrossRef]
- Chistiakov, D.A.; Sobenin, I.A.; Revin, V.V.; Orekhov, A.N.; Bobryshev, Y.V. Mitochondrial aging and age-related dysfunction of mitochondria. Biomed. Res. Int. 2014, 2014, 238463. [Google Scholar] [CrossRef] [PubMed]
- Brand, M.D.; Nicholls, D.G. Assessing mitochondrial dysfunction in cells. Biochem. J. 2011, 435, 297–312. [Google Scholar] [CrossRef] [PubMed]
- Yao, P.; Sun, H.; Xu, C.; Chen, T.; Zou, B.; Jiang, P.; Du, W. Evidence for a direct cross-talk between malic enzyme and the pentose phosphate pathway via structural interactions. J. Biol. Chem. 2017, 292, 17113–17120. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Yang, Z.; Zhu, B.; Hu, J.; Liew, C.W.; Zhang, Y.; Leopold, J.A.; Handy, D.E.; Loscalzo, J.; Stanton, R.C. Increasing glucose 6-phosphate dehydrogenase activity restores redox balance in vascular endothelial cells exposed to high glucose. PLoS ONE 2012, 7, e49128. [Google Scholar] [CrossRef] [PubMed]
- Sanz, N.; Díez-Fernández, C.; Valverde, A.M.; Lorenzo, M.; Benito, M.; Cascales, M. Malic enzyme and glucose 6-phosphate dehydrogenase gene expression increases in rat liver cirrhogenesis. Br. J. Cancer 1997, 75, 487–492. [Google Scholar] [CrossRef]
- Zunino, F.; Pratesi, G.; Micheloni, A.; Cavalletti, E.; Sala, F.; Tofanetti, O. Protective effect of reduced glutathione against cisplatin-induced renal and systemic toxicity and its influence on the therapeutic activity of the antitumor drug. Chem. Biol. Interact. 1989, 70, 89–101. [Google Scholar] [CrossRef]
- Shiraishi, F.; Curtis, L.M.; Truong, L.; Poss, K.; Visner, G.A.; Madsen, K.; Nick, H.S.; Agarwal, A. Heme oxygenase-1 gene ablation or expression modulates cisplatin-induced renal tubular apoptosis. Am. J. Physiol. Ren. Physiol. 2000, 278, F726–F736. [Google Scholar] [CrossRef]
- Al Ghouleh, I.; Meijles, D.N.; Mutchler, S.; Zhang, Q.; Sahoo, S.; Gorelova, A.; Henrich Amaral, J.; Rodriguez, A.I.; Mamonova, T.; Song, G.J.; et al. Binding of EBP50 to Nox organizing subunit p47phox is pivotal to cellular reactive species generation and altered vascular phenotype. Proc. Natl. Acad. Sci. USA 2016, 113, E5308–E5317. [Google Scholar] [CrossRef] [PubMed]
- Pera, T.; Tompkins, E.; Katz, M.; Wang, B.; Deshpande, D.A.; Weinman, E.J.; Penn, R.B. Specificity of NHERF1 regulation of GPCR signaling and function in human airway smooth muscle. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2019, 33, 9008–9016. [Google Scholar] [CrossRef] [PubMed]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bushau-Sprinkle, A.; Barati, M.T.; Gagnon, K.B.; Khundmiri, S.J.; Kitterman, K.; Hill, B.G.; Sherwood, A.; Merchant, M.; Rai, S.N.; Srivastava, S.; et al. NHERF1 Loss Upregulates Enzymes of the Pentose Phosphate Pathway in Kidney Cortex. Antioxidants 2020, 9, 862. https://doi.org/10.3390/antiox9090862
Bushau-Sprinkle A, Barati MT, Gagnon KB, Khundmiri SJ, Kitterman K, Hill BG, Sherwood A, Merchant M, Rai SN, Srivastava S, et al. NHERF1 Loss Upregulates Enzymes of the Pentose Phosphate Pathway in Kidney Cortex. Antioxidants. 2020; 9(9):862. https://doi.org/10.3390/antiox9090862
Chicago/Turabian StyleBushau-Sprinkle, Adrienne, Michelle T. Barati, Kenneth B. Gagnon, Syed Jalal Khundmiri, Kathleen Kitterman, Bradford G. Hill, Amanda Sherwood, Michael Merchant, Shesh N. Rai, Sudhir Srivastava, and et al. 2020. "NHERF1 Loss Upregulates Enzymes of the Pentose Phosphate Pathway in Kidney Cortex" Antioxidants 9, no. 9: 862. https://doi.org/10.3390/antiox9090862
APA StyleBushau-Sprinkle, A., Barati, M. T., Gagnon, K. B., Khundmiri, S. J., Kitterman, K., Hill, B. G., Sherwood, A., Merchant, M., Rai, S. N., Srivastava, S., Clark, B., Siskind, L., Brier, M., Hata, J., & Lederer, E. (2020). NHERF1 Loss Upregulates Enzymes of the Pentose Phosphate Pathway in Kidney Cortex. Antioxidants, 9(9), 862. https://doi.org/10.3390/antiox9090862