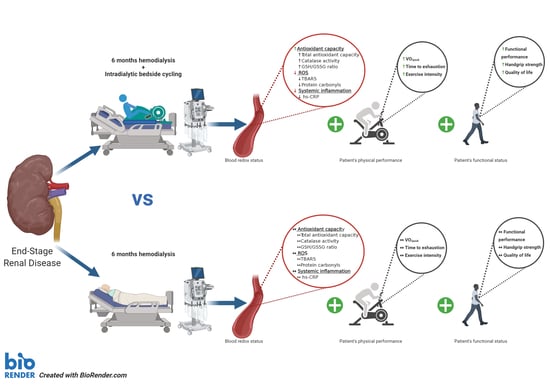
Intradialytic Cardiovascular Exercise Training Alters Redox Status, Reduces Inflammation and Improves Physical Performance in Patients with Chronic Kidney Disease
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants and Experimental Design
2.2. Exercise Training Program
2.3. Anthropometric Profile
2.4. Physical Performance
2.5. Functional Capacity
2.6. Quality of Life
2.7. Blood Sampling and Assays
2.8. Statistical Analysis
3. Results
3.1. External and Internal Load during the Training Intervention
3.2. Somatometrics
3.3. Physical Performance and Quality of Life
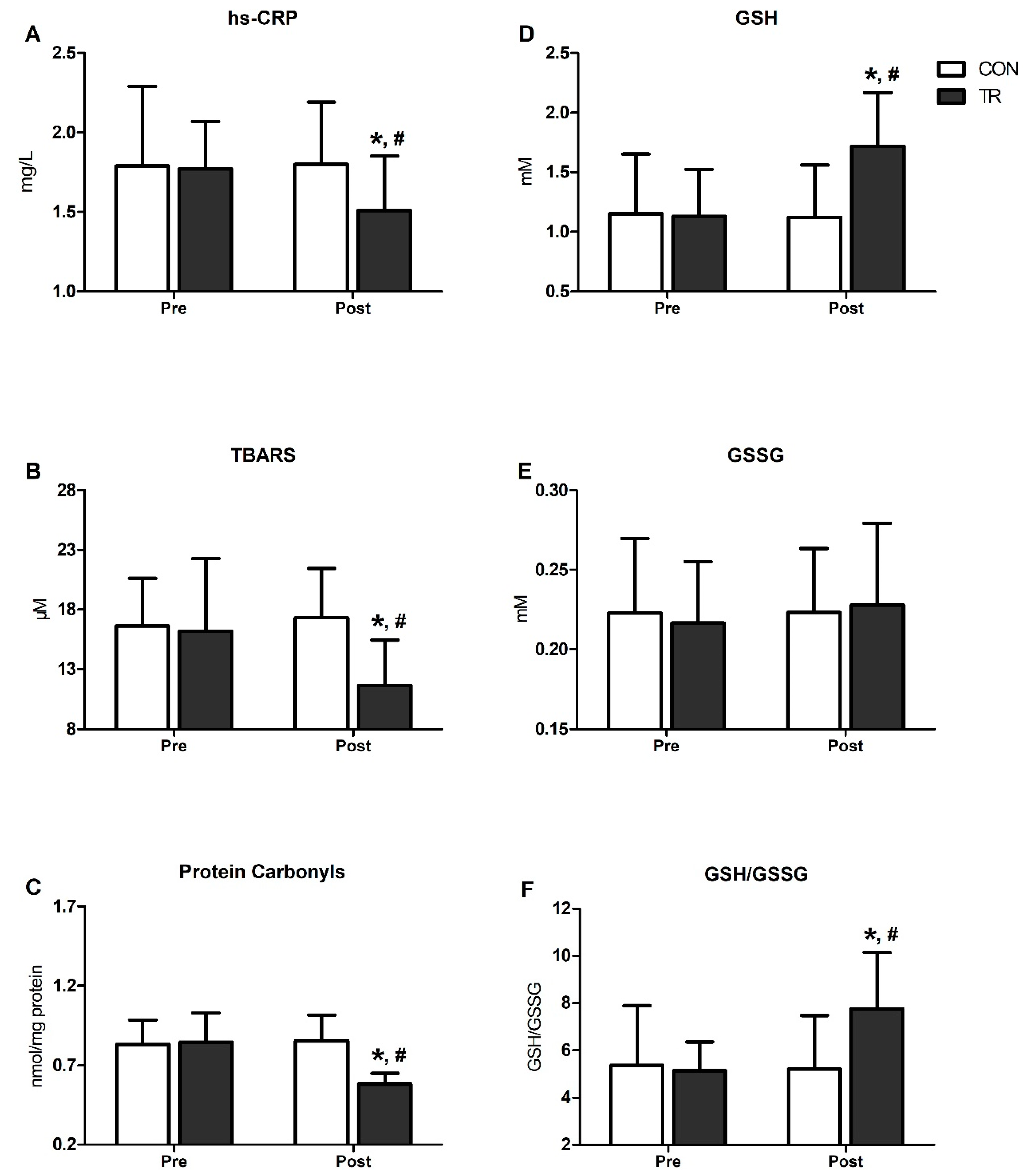
3.4. Inflammation, Oxidative Stress and Antioxidant Status
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| BMI | body mass index |
| CAT | catalase |
| CI | confidence intervals |
| CKD | chronic kidney disease |
| CON | control group |
| CVD | cardiovascular disease |
| DBP | diastolic blood pressure |
| DM | diabetes mellitus |
| EDTA | ethylene diamine tetraacetic acid |
| ES | effect sizes |
| ESRD | end-stage renal disease |
| GFR | glomerular filtration rate |
| GSH | redused glutathione |
| GSSG | oxidized glutathione |
| HD | hemodialysis |
| HR | heart rate |
| hs-CRP | high-sensitivity C-reactive protein |
| NSRI | north staffordshire royal infirmary |
| OS | oxidative stress |
| PC | protein carbonyls |
| PEW | protein wasting energy |
| ROS | reactive oxygen species |
| RPE | rate of perceived exertion |
| RRT | renal replacement therapy |
| RS | redox status |
| SaO2 | oxygen saturation |
| SBP | systolic blood pressure |
| SF-36 | short form-36 |
| STS-60 | sit to stand-60 |
| TAC | total antioxidant capacity |
| TBARS | thiobarbituric acid reactive substances |
| TCA | trichloro acetic acid |
| TR | training group |
| VO2peak | peak oxygen consumption |
References
- Jager, K.J.; Kovesdy, C.; Langham, R.; Rosenberg, M.; Jha, V.; Zoccali, C. A single number for advocacy and communication—Worldwide more than 850 million individuals have kidney diseases. Kidney Int. 2019, 96, 1048–1050. [Google Scholar] [CrossRef]
- Jha, V.; Garcia-Garcia, G.; Iseki, K.; Li, Z.; Naicker, S.; Plattner, B.; Saran, R.; Wang, A.Y.-M.; Yang, C.-W. Chronic kidney disease: Global dimension and perspectives. Lancet 2013, 382, 260–272. [Google Scholar] [CrossRef]
- Foreman, K.J.; Marquez, N.; Dolgert, A.; Fukutaki, K.; Fullman, N.; McGaughey, M.; A Pletcher, M.; E Smith, A.; Tang, K.; Yuan, C.-W.; et al. Forecasting life expectancy, years of life lost, and all-Cause and cause-Specific mortality for 250 causes of death: Reference and alternative scenarios for 2016-40 for 195 countries and territories. Lancet 2018, 392, 2052–2090. [Google Scholar] [CrossRef] [Green Version]
- Chapter 1: Definition and classification of CKD. Kidney Int. Suppl. 2013, 3, 19–62. [CrossRef] [PubMed] [Green Version]
- Saran, R.; Robinson, B.; Abbott, K.C.; Agodoa, L.Y.; Bhave, N.; Bragg-Gresham, J.; Balkrishnan, R.; Dietrich, X.; Eckard, A.; Eggers, P.W.; et al. US Renal Data System 2017 Annual Data Report: Epidemiology of Kidney Disease in the United States. Am. J. Kidney Dis. 2018, 71, A7. [Google Scholar] [CrossRef]
- Duni, A.; Liakopoulos, V.; Roumeliotis, S.; Peschos, D.; Dounousi, E. Oxidative Stress in the Pathogenesis and Evolution of Chronic Kidney Disease: Untangling Ariadne’s Thread. Int. J. Mol. Sci. 2019, 20, 3711. [Google Scholar] [CrossRef] [Green Version]
- Zalba, G.; Fortuño, A.; Díez, J. Oxidative stress and atherosclerosis in early chronic kidney disease. Nephrol. Dial. Transplant. 2006, 21, 2686–2690. [Google Scholar] [CrossRef] [Green Version]
- Dounousi, E.; Papavasiliou, E.; Makedou, A.; Ioannou, K.; Katopodis, K.P.; Tselepis, A.; Siamopoulos, K.C.; Tsakiris, D. Oxidative Stress Is Progressively Enhanced With Advancing Stages of CKD. Am. J. Kidney Dis. 2006, 48, 752–760. [Google Scholar] [CrossRef] [Green Version]
- Liakopoulos, V.; Roumeliotis, S.; Zarogiannis, S.G.; Eleftheriadis, T.; Mertens, P.R. Oxidative stress in hemodialysis: Causative mechanisms, clinical implications, and possible therapeutic interventions. Semin. Dial. 2018, 32, 58–71. [Google Scholar] [CrossRef]
- Liakopoulos, V.; Roumeliotis, S.; Gorny, X.; Dounousi, E.; Mertens, P.R. Oxidative Stress in Hemodialysis Patients: A Review of the Literature. Oxidative Med. Cell. Longev. 2017, 2017, 1–22. [Google Scholar] [CrossRef]
- Locatelli, F.; Canaud, B.; Eckardt, K.-U.; Stenvinkel, P.; Wanner, C.; Zoccali, C. Oxidative stress in end-stage renal disease: An emerging threat to patient outcome. Nephrol. Dial. Transplant. 2003, 18, 1272–1280. [Google Scholar] [CrossRef] [PubMed]
- Podkowińska, A.; Formanowicz, D. Chronic Kidney Disease as Oxidative Stress- and Inflammatory-Mediated Cardiovascular Disease. Antioxidants 2020, 9, 752. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.-C.; Hsu, S.-P.; Wu, M.-S.; Chien, C.-T.; Hsu, S.-M. Effects of vitamin C infusion and vitamin E-coated membrane on hemodialysis-induced oxidative stress. Kidney Int. 2006, 69, 706–714. [Google Scholar] [CrossRef] [Green Version]
- Poulianiti, K.P.; Kaltsatou, A.; Mitrou, G.I.; Jamurtas, A.Z.; Koutedakis, Y.; Maridaki, M.; Stefanidis, I.; Sakkas, G.K.; Karatzaferi, C. Systemic Redox Imbalance in Chronic Kidney Disease: A Systematic Review. Oxidative Med. Cell. Longev. 2016, 2016, 8598253. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tucker, P.S.; Scanlan, A.T.; Dalbo, V.J. Chronic Kidney Disease Influences Multiple Systems: Describing the Relationship between Oxidative Stress, Inflammation, Kidney Damage, and Concomitant Disease. Oxidative Med. Cell. Longev. 2015, 2015, 806358. [Google Scholar] [CrossRef] [PubMed]
- Putri, A.Y.; Thaha, M. Role of oxidative stress on chronic kidney disease progression. Acta Med. Indones. 2014, 46, 244–252. [Google Scholar]
- Daenen, K.; Andries, A.; Mekahli, D.; Van Schepdael, A.; Jouret, F.; Bammens, B. Oxidative stress in chronic kidney disease. Pediatr. Nephrol. 2018, 34, 975–991. [Google Scholar] [CrossRef] [Green Version]
- Mihai, S.; Codrici, E.; Popescu, I.D.; Enciu, A.-M.; Albulescu, L.; Necula, L.G.; Mambet, C.; Anton, G.; Tanase, C. Inflammation-Related Mechanisms in Chronic Kidney Disease Prediction, Progression, and Outcome. J. Immunol. Res. 2018, 2018, 2180373. [Google Scholar] [CrossRef]
- Genís, B.B.; Pastor, M.C.; Bonal, J.; Foraster, A.; Romero, R. Oxidative stress, inflammation and cardiovascular mortality in haemodialysis—Role of seniority and intravenous ferrotherapy: Analysis at 4 years of follow-up. Nephrol. Dial. Transplant. 2005, 21, 984–990. [Google Scholar] [CrossRef] [Green Version]
- Fatouros, I.G.; Jamurtas, A.Z. Insights into the molecular etiology of exercise-induced inflammation: Opportunities for optimizing performance. J. Inflamm. Res. 2016, 9, 175–186. [Google Scholar] [CrossRef] [Green Version]
- Michailidis, Y.; Karagounis, L.G.; Terzis, G.; Jamurtas, A.Z.; Spengos, K.; Tsoukas, D.; Chatzinikolaou, A.; Mandalidis, D.; Stefanetti, R.J.; Papassotiriou, I.; et al. Thiol-based antioxidant supplementation alters human skeletal muscle signaling and attenuates its inflammatory response and recovery after intense eccentric exercise. Am. J. Clin. Nutr. 2013, 98, 233–245. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fatouros, I.G.; Jamurtas, A.Z.; Villiotou, V.; Pouliopoulou, S.; Fotinakis, P.; Taxildaris, K.; Deliconstantinos, G. Oxidative Stress Responses in Older Men during Endurance Training and Detraining. Med. Sci. Sports Exerc. 2004, 36, 2065–2072. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nikolaidis, M.G.; Paschalis, V.; Giakas, G.; Fatouros, I.G.; Sakellariou, G.K.; Theodorou, A.A.; Koutedakis, Y.; Jamurtas, A.Z. Favorable and Prolonged Changes in Blood Lipid Profile after Muscle-Damaging Exercise. Med. Sci. Sports Exerc. 2008, 40, 1483–1489. [Google Scholar] [CrossRef] [PubMed]
- Moinuddin, I.K.; Leehey, D.J. A Comparison of Aerobic Exercise and Resistance Training in Patients With and Without Chronic Kidney Disease. Adv. Chronic Kidney Dis. 2008, 15, 83–96. [Google Scholar] [CrossRef]
- Sousa, C.V.; Sales, M.M.; Rosa, T.S.; Lewis, J.E.; De Andrade, R.V.; Simoes, H.G. The Antioxidant Effect of Exercise: A Systematic Review and Meta-Analysis. Sports Med. 2016, 47, 277–293. [Google Scholar] [CrossRef]
- Smart, N.A.; McFarlane, J.; Cornelissen, V. The Effect of Exercise Therapy on Physical Function, Biochemistry and Dialysis Adequacy in Haemodialysis Patients: A Systematic Review and Meta-Analysis. Open J. Nephrol. 2013, 3, 25–36. [Google Scholar] [CrossRef] [Green Version]
- Storer, T.W.; Casaburi, R.; Sawelson, S.; Kopple, J.D. Endurance exercise training during haemodialysis improves strength, power, fatigability and physical performance in maintenance haemodialysis patients. Nephrol. Dial. Transplant. 2005, 20, 1429–1437. [Google Scholar] [CrossRef] [Green Version]
- Ouzouni, S.; Kouidi, E.; Sioulis, A.; Grekas, D.; Deligiannis, A. Effects of intradialytic exercise training on health-related quality of life indices in haemodialysis patients. Clin. Rehabil. 2009, 23, 53–63. [Google Scholar] [CrossRef]
- Chang, Y.; Cheng, S.-Y.; Lin, M.; Gau, F.-Y.; Chao, Y.-F.C. The effectiveness of intradialytic leg ergometry exercise for improving sedentary life style and fatigue among patients with chronic kidney disease: A randomized clinical trial. Int. J. Nurs. Stud. 2010, 47, 1383–1388. [Google Scholar] [CrossRef]
- Böhm, J.; Monteiro, M.B.; Andrade, F.P.; Veronese, F.V.; Thomé, F.S. Acute effects of intradialytic aerobic exercise on solute removal, blood gases and oxidative stress in patients with chronic kidney disease. Braz. J. Nephrol. 2017, 39, 172–180. [Google Scholar] [CrossRef]
- Levendoglu, F.; Altintepe, L.; Okudan, N.; Uğurlu, H.; Gökbel, H.; Tonbul, Z.; Guney, I.; Turk, S. A twelve week exercise program improves the psychological status, quality of life and work capacity in hemodialysis patients. J. Nephrol. 2004, 17, 826–832. [Google Scholar] [PubMed]
- Wilund, K.R.; Tomayko, E.J.; Wu, P.-T.; Chung, H.R.; Vallurupalli, S.; Lakshminarayanan, B.; Fernhall, B. Intradialytic exercise training reduces oxidative stress and epicardial fat: A pilot study. Nephrol. Dial. Transplant. 2010, 25, 2695–2701. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Groussard, C.; Rouchon-Isnard, M.; Coutard, C.; Romain, F.; Malardé, L.; Lemoine-Morel, S.; Martin, B.; Pereira, B.; Boisseau, N. Beneficial effects of an intradialytic cycling training program in patients with end-stage kidney disease. Appl. Physiol. Nutr. Metab. 2015, 40, 550–556. [Google Scholar] [CrossRef] [PubMed]
- Pechter, U.; Ots, M.; Mesikepp, S.; Zilmer, K.; Kullissaar, T.; Vihalemm, T.; Zilmer, M.; Maaroos, J. Beneficial effects of water-based exercise in patients with chronic kidney disease. Int. J. Rehabil. Res. 2003, 26, 153–156. [Google Scholar] [CrossRef]
- Esgalhado, M.; Stockler-Pinto, M.B.; Cardozo, L.F.M.D.F.; Costa, C.; Barboza, J.E.; Mafra, D. Effect of acute intradialytic strength physical exercise on oxidative stress and inflammatory responses in hemodialysis patients. Kidney Res. Clin. Pr. 2015, 34, 35–40. [Google Scholar] [CrossRef] [Green Version]
- Fatouros, I.G.; Douroudos, I.; Panagoutsos, S.; Pasadakis, P.; Nikolaidis, M.G.; Chatzinikolaou, A.; Sovatzidis, A.; Michailidis, Y.; Jamurtas, A.Z.; Mandalidis, D.; et al. Effects of l-Carnitine on Oxidative Stress Responses in Patients with Renal Disease. Med. Sci. Sports Exerc. 2010, 42, 1809–1818. [Google Scholar] [CrossRef]
- Giannaki, C.D.; Stefanidis, I.; Karatzaferi, C.; Liakos, N.; Roka, V.; Ntente, I.; Sakkas, G.K. The Effect of Prolonged Intradialytic Exercise in Hemodialysis Efficiency Indices. ASAIO J. 2011, 57, 213–218. [Google Scholar] [CrossRef]
- Koufaki, P.; Mercer, T. Assessment and Monitoring of Physical Function for People With CKD. Adv. Chronic Kidney Dis. 2009, 16, 410–419. [Google Scholar] [CrossRef]
- Roberts, H.C.; Denison, H.; Martin, H.J.; Patel, H.P.; Syddall, H.; Cooper, C.; Sayer, A.A. A review of the measurement of grip strength in clinical and epidemiological studies: Towards a standardised approach. Age Ageing 2011, 40, 423–429. [Google Scholar] [CrossRef] [Green Version]
- Kalantar-Zadeh, K.; Kopple, J.D.; Block, G.; Humphreys, M.H. Association among SF36 quality of life measures and nutrition, hospitalization, and mortality in hemodialysis. J. Am. Soc. Nephrol. 2001, 12, 2797–2806. [Google Scholar]
- Theodorou, A.A.; Nikolaidis, M.G.; Paschalis, V.; Sakellariou, G.K.; Fatouros, I.G.; Koutedakis, Y.; Jamurtas, A.Z. Comparison between G6PD-Deficient and Normal Individuals after Eccentric Exercise. Med. Sci. Sports Exerc. 2009, 42, 1. [Google Scholar] [CrossRef] [PubMed]
- Draganidis, D.; Jamurtas, A.Z.; Stampoulis, T.; Laschou, V.C.; Deli, C.K.; Georgakouli, K.; Papanikolaou, K.; Chatzinikolaou, A.; Michalopoulou, M.; Papadopoulos, C.; et al. Disparate Habitual Physical Activity and Dietary Intake Profiles of Elderly Men with Low and Elevated Systemic Inflammation. Nutrients 2018, 10, 566. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Poulios, A.; Fatouros, I.G.; Mohr, M.; Draganidis, D.; Deli, C.K.; Papanikolaou, K.; Sovatzidis, A.; Nakopoulou, T.; Ermidis, G.; Tzatzakis, T.; et al. Post-Game High Protein Intake May Improve Recovery of Football-Specific Performance during a Congested Game Fixture: Results from the PRO-FOOTBALL Study. Nutrients 2018, 10, 494. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fatouros, I.G.; Pasadakis, P.; Sovatzidis, A.; Chatzinikolaou, A.; Panagoutsos, S.; Sivridis, D.; Michailidis, Y.; Douroudos, I.; Taxildaris, K.; Vargemezis, V. Acute Exercise May Exacerbate Oxidative Stress Response in Hemodialysis Patients. Nephron Clin. Pr. 2008, 109, c55–c64. [Google Scholar] [CrossRef]
- Mohr, M.; Draganidis, D.; Chatzinikolaou, A.; Barbero-Alvarez, J.C.; Castagna, C.; Douroudos, I.; Avloniti, A.; Margeli, A.; Papassotiriou, I.; Flouris, A.D.; et al. Muscle damage, inflammatory, immune and performance responses to three football games in 1 week in competitive male players. Graefe’s Arch. Clin. Exp. Ophthalmol. 2015, 116, 179–193. [Google Scholar] [CrossRef]
- Chatzinikolaou, A.; Draganidis, D.; Avloniti, A.; Karipidis, A.; Jamurtas, A.Z.; Skevaki, C.L.; Tsoukas, D.; Sovatzidis, A.; Theodorou, A.; Kambas, A.; et al. The microcycle of inflammation and performance changes after a basketball match. J. Sports Sci. 2014, 32, 870–882. [Google Scholar] [CrossRef]
- Chatzinikolaou, A.; Christoforidis, C.; Avloniti, A.; Draganidis, D.; Jamurtas, A.Z.; Stampoulis, T.; Ermidis, G.; Sovatzidis, A.; Papassotiriou, I.; Kambas, A.; et al. A Microcycle of Inflammation Following a Team Handball Game. J. Strength Cond. Res. 2014, 28, 1981–1994. [Google Scholar] [CrossRef]
- Roshanravan, B.; Gamboa, J.; Wilund, K. Exercise and CKD: Skeletal Muscle Dysfunction and Practical Application of Exercise to Prevent and Treat Physical Impairments in CKD. Am. J. Kidney Dis. 2017, 69, 837–852. [Google Scholar] [CrossRef]
- Rhee, S.Y.; Song, J.K.; Hong, S.C.; Choi, J.W.; Jeon, H.J.; Shin, D.H.; Ji, E.H.; Choi, E.-H.; Lee, J.; Kim, A.; et al. Intradialytic exercise improves physical function and reduces intradialytic hypotension and depression in hemodialysis patients. Korean J. Intern. Med. 2019, 34, 588–598. [Google Scholar] [CrossRef] [Green Version]
- Kouidi, E.; Karagiannis, V.; Grekas, D.; Iakovides, A.; Kaprinis, G.; Tourkantonis, A.; Deligiannis, A. Depression, heart rate variability, and exercise training in dialysis patients. Eur. J. Cardiovasc. Prev. Rehabil. 2010, 17, 160–167. [Google Scholar] [CrossRef]
- Sakkas, G.K.; Hadjigeorgiou, G.M.; Karatzaferi, C.; Maridaki, M.; Giannaki, C.D.; Mertens, P.R.; Rountas, C.; Vlychou, M.; Liakopoulos, V.; Stefanidis, I. Intradialytic Aerobic Exercise Training Ameliorates Symptoms of Restless Legs Syndrome and Improves Functional Capacity in Patients on Hemodialysis. ASAIO J. 2008, 54, 185–190. [Google Scholar] [CrossRef] [PubMed]
- Segura-Ortí, E.; Pérez-Domínguez, B.; De Villar, L.O.-P.; Oliva, E.M.; Gramage, J.M.; Maset, R.G.; Gil, J. Virtual reality exercise intradialysis to improve physical function: A feasibility randomized trial. Scand. J. Med. Sci. Sports 2018, 29, 89–94. [Google Scholar] [CrossRef] [PubMed]
- Koh, K.P.; Fassett, R.G.; E Sharman, J.; Coombes, J.S.; Williams, A.D. Intradialytic versus home based exercise training in hemodialysis patients: A randomised controlled trial. BMC Nephrol. 2009, 10, 2. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matsuzawa, R.; Hoshi, K.; Yoneki, K.; Harada, M.; Watanabe, T.; Shimoda, T.; Yamamoto, S.; Matsunaga, A. Exercise Training in Elderly People Undergoing Hemodialysis: A Systematic Review and Meta-analysis. Kidney Int. Rep. 2017, 2, 1096–1110. [Google Scholar] [CrossRef] [Green Version]
- Aquilano, K.; Baldelli, S.; Ciriolo, M.R. Glutathione: New roles in redox signaling for an old antioxidant. Front. Pharm. 2014, 5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zachara, B.A.; Koterska, D.; Manitius, J.; Sadowski, L.; Dziedziczko, A.; Salak, A.; Wasowicz, W. Selenium Supplementation on Plasma Glutathione Peroxidase Activity in Patients with End-Stage Chronic Renal Failure. Biol. Trace Elem. Res. 2004, 97, 15–30. [Google Scholar] [CrossRef]
- Radák, Z.; Chung, H.Y.; Goto, S. Exercise and hormesis: Oxidative stress-related adaptation for successful aging. Biogerontology 2005, 6, 71–75. [Google Scholar] [CrossRef]
- Yeun, J.Y.; Levine, R.A.; Mantadilok, V.; Kaysen, G.A. C-reactive protein predicts all-cause and cardiovascular mortality in hemodialysis patients. Am. J. Kidney Dis. 2000, 35, 469–476. [Google Scholar] [CrossRef]
- Zimmermann, J.; Herrlinger, S.; Pruy, A.; Metzger, T.; Wanner, C. Inflammation enhances cardiovascular risk and mortality in hemodialysis patients. Kidney Int. 1999, 55, 648–658. [Google Scholar] [CrossRef] [Green Version]
- Fedewa, M.V.; Hathaway, E.D.; Higgins, S.; Forehand, R.L.; Schmidt, M.D.; Evans, E.M. Moderate, but not vigorous, intensity exercise training reduces C-reactive protein. Acta Cardiol. 2017, 73, 283–290. [Google Scholar] [CrossRef]
- Fedewa, M.V.; Hathaway, E.D.; Ward-Ritacco, C.L. Effect of exercise training on C reactive protein: A systematic review and meta-analysis of randomised and non-randomised controlled trials. Br. J. Sports Med. 2016, 51, 670–676. [Google Scholar] [CrossRef] [PubMed]
- Hammonds, T.L.; Gathright, E.C.; Goldstein, C.M.; Penn, M.S.; Hughes, J.W. Effects of exercise on c-reactive protein in healthy patients and in patients with heart disease: A meta-analysis. Hear. Lung 2016, 45, 273–282. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saghebjoo, M.; Nezamdoost, Z.; Ahmadabadi, F.; Saffari, I.; Hamidi, A. The effect of 12 weeks of aerobic training on serum levels high sensitivity C-reactive protein, tumor necrosis factor-alpha, lipid profile and anthropometric characteristics in middle-age women patients with type 2 diabetes. Diabetes Metab. Syndr. Clin. Res. Rev. 2018, 12, 163–168. [Google Scholar] [CrossRef] [PubMed]


| Variables | Exercise Group (n = 10) | Control Group (n = 10) |
|---|---|---|
| Gender (Female/Male) | 2/8 | 1/9 |
| Age (yr) | 52.8 ± 17.1 | 53 ± 7,6 |
| Body Height (m) | 1.71 ± 0.09 | 1.71 ± 0.1 |
| Body Mass (kg) | 72.5 ± 14.6 | 74.6 ± 9.3 |
| BMI (kg/m2) | 24.6 ± 3.54 | 25.5 ± 1.84 |
| Body Fat (%) | 27 ± 2.27 | 27.3 ± 3.54 |
| Dialysis History (months) | 88.8 ± 9.9 | 89.7 ± 10.1 |
| Residual Urea Clearance (ml/min−1) | 1.32 ± 0,2 | 1.28 ± 0.3 |
| Intradialytic Weight Gain (kg) | 2.66 ± 0.6 | 2.55 ± 0.6 |
| Dialyzer Clearance of Urea (Kt/V) | 1.33 ± 0.4 | 1.27 ± 0.3 |
| Variables | 1st Month | 2nd Month | 3rd Month | 4th Month | 5th Month | 6th Month |
|---|---|---|---|---|---|---|
| Duration of exercise (min) | 10.5 ± 0 | 16.5 ± 1 * | 17 ± 2.9 *,# | 19.8 ± 3.8 *,# | 22 ± 3.4 *,# | 24 ± 3.3 *,# |
| Resistance (Watt) | 0 ± 0 | 20 ± 14 * | 39.8 ± 24 *,# | 50.6 ± 28 *,# | 57 ± 24 *,# | 60.6 ± 22 *,# |
| Velocity (Rounds/min) | 35 ± 0 | 35 ± 0 | 35 ± 0 | 35 ± 0 | 35 ± 0 | 41.75 ± 5.4 *,# |
| Mean SBP (mmHg) | 148.64 ± 13.8 | 151.56 ± 12.2 | 152.62 ± 13.1 | 155.08 ± 9.1 | 152.16 ± 11.8 | 149.64 ± 11.1 |
| Mean DBP (mmHg) | 82.48 ± 3.2 | 81.06 ± 7.6 | 86.62 ± 7.5 | 84.98 ± 4.2 | 85.44 ± 5.1 | 81.52 ± 7.1 |
| Mean HR (beats/min) | 102.72 ± 6.1 | 104.78 ± 6.9 | 103.58 ± 6.5 | 102.5 ± 6.1 | 103.44 ± 8.7 | 103.32 ± 8.7 |
| RPE | 12.4 ± 0.7 | 12.1 ± 0.6 | 11.9 ± 0.4 | 12.6 ± 0.7 | 12.2 ± 0.6 | 11.8 ± 0.3 |
| SpO2 (%) | 96.2 ± 1.4 | 97.3 ± 1.3 | 96.9 ± 1.5 | 96.4 ± 1.4 | 97.5 ± 1.6 | 96.6 ± 1.5 |
| Variables | Control Pre | Control Post | Experimental Pre | Experimental Post |
|---|---|---|---|---|
| Body Height (m) | 1.71 ± 0.1 | 1.71 ± 0.09 | ||
| Body Mass (kg) | 74.6 ± 9.3 | 74.9 ± 9.13 | 72.5 ± 14.6 | 71.5 ± 14 * |
| BMI (kg/m2) | 25.5 ± 1.84 | 25.6 ± 3.19 | 24.6 ± 3.54 | 24.2 ± 3.39 |
| Body Fat (%) | 27.3 ± 3.54 | 27.2 ± 2.27 | 27 ± 2.27 | 26.8 ± 3.01 * |
| Variables | Control Pre | Control Post | Experimental Pre | Experimental Post |
|---|---|---|---|---|
| VO2peak (ml/kg/min) | 14.8 ± 3.1 | 14.5 ± 3.03 | 13.81 ± 3.03 | 15.9 ± 2.96 *,# |
| Time to Exhaustion (min) | 9.74 ± 1.7 | 9.64 ± 1.69 | 9.8 ± 2.69 | 11.3 ± 2.16 *,# |
| Resting HR (beats/min) | 79 ± 9.9 | 79.7 ± 6.9 | 78.4 ± 13.2 | 77 ± 11.4 |
| Peak HR (beats/min) | 122.9 ± 17.6 | 122.3 ± 16.9 | 121.5 ± 17.3 | 122.4 ± 16.1 |
| Resting Lactate (mM) | 0.98 ± 0.26 | 1 ± 0.19 | 0.99 ± 0.2 | 0.97 ± 0.17 |
| Peak Lactate (mM) | 6.1 ± 1.1 | 6.02 ± 1.06 | 6.2 ± 1.6 | 7.07 ± 1.35 *,# |
| STS-60 (reps in 60 s) | 33 ± 7.6 | 32.25 ± 7 | 33.83 ± 7.2 | 38.08 ± 6.3 *,# |
| NSRI test (s) | 51 ± 8.78 | 49.9 ± 7.86 | 49.8 ± 10.6 | 53.7 ± 10.5 *,# |
| Handgrip Strength (kg) | 24.7 ± 9.65 | 23 ± 10.2 | 23.4 ± 10.5 | 23.67 ± 10.16 *,# |
| SF-36 | 17.5 ± 3.5 | 17.6 ± 2.6 | 17.2 ± 3.3 | 15.3 ± 3.1 *,# |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sovatzidis, A.; Chatzinikolaou, A.; Fatouros, I.G.; Panagoutsos, S.; Draganidis, D.; Nikolaidou, E.; Avloniti, A.; Michailidis, Y.; Mantzouridis, I.; Batrakoulis, A.; et al. Intradialytic Cardiovascular Exercise Training Alters Redox Status, Reduces Inflammation and Improves Physical Performance in Patients with Chronic Kidney Disease. Antioxidants 2020, 9, 868. https://doi.org/10.3390/antiox9090868
Sovatzidis A, Chatzinikolaou A, Fatouros IG, Panagoutsos S, Draganidis D, Nikolaidou E, Avloniti A, Michailidis Y, Mantzouridis I, Batrakoulis A, et al. Intradialytic Cardiovascular Exercise Training Alters Redox Status, Reduces Inflammation and Improves Physical Performance in Patients with Chronic Kidney Disease. Antioxidants. 2020; 9(9):868. https://doi.org/10.3390/antiox9090868
Chicago/Turabian StyleSovatzidis, Apostolos, Athanasios Chatzinikolaou, Ioannis G. Fatouros, Stylianos Panagoutsos, Dimitrios Draganidis, Eirini Nikolaidou, Alexandra Avloniti, Yiannis Michailidis, Ioannis Mantzouridis, Alexios Batrakoulis, and et al. 2020. "Intradialytic Cardiovascular Exercise Training Alters Redox Status, Reduces Inflammation and Improves Physical Performance in Patients with Chronic Kidney Disease" Antioxidants 9, no. 9: 868. https://doi.org/10.3390/antiox9090868
APA StyleSovatzidis, A., Chatzinikolaou, A., Fatouros, I. G., Panagoutsos, S., Draganidis, D., Nikolaidou, E., Avloniti, A., Michailidis, Y., Mantzouridis, I., Batrakoulis, A., Pasadakis, P., & Vargemezis, V. (2020). Intradialytic Cardiovascular Exercise Training Alters Redox Status, Reduces Inflammation and Improves Physical Performance in Patients with Chronic Kidney Disease. Antioxidants, 9(9), 868. https://doi.org/10.3390/antiox9090868