Low Molecular Weight Dextran Sulfate (ILB®) Administration Restores Brain Energy Metabolism Following Severe Traumatic Brain Injury in the Rat
Abstract
:1. Introduction
2. Materials and Methods
2.1. Induction of sTBI, Drug Dosage and Mode of Administration, and Drug Administration Protocol
2.2. Cerebral Tissue Processing for Biochemical Analyses
2.3. HPLC Analysis of Energy Metabolites, Antioxidants, and Oxidative/Nitrosative Stress Biomarkers
2.4. Statistical Analysis
3. Results
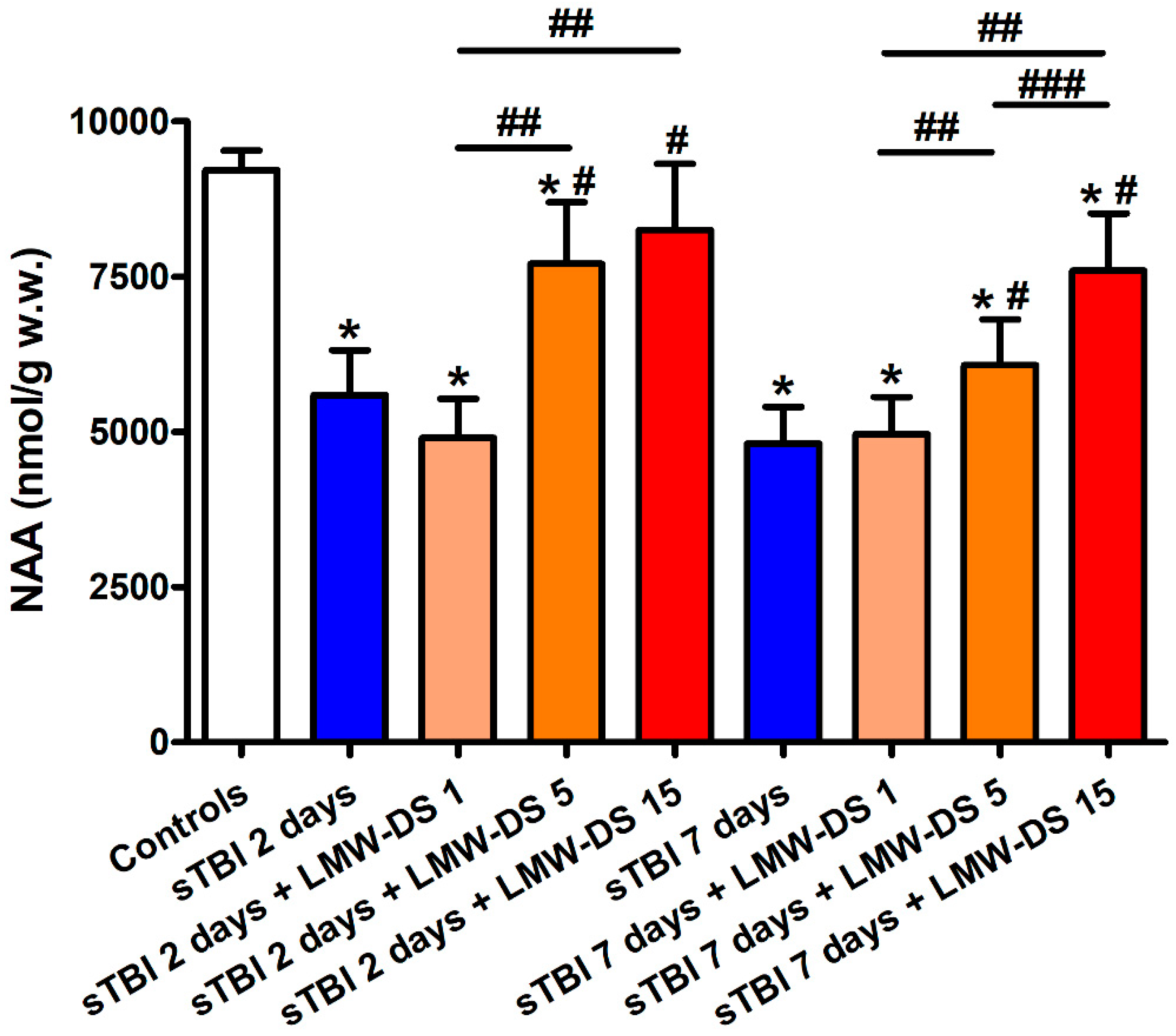
3.1. ILB® Restores Cerebral Mitochondrial-Dependent Energy Metabolism and NAA Following sTBI
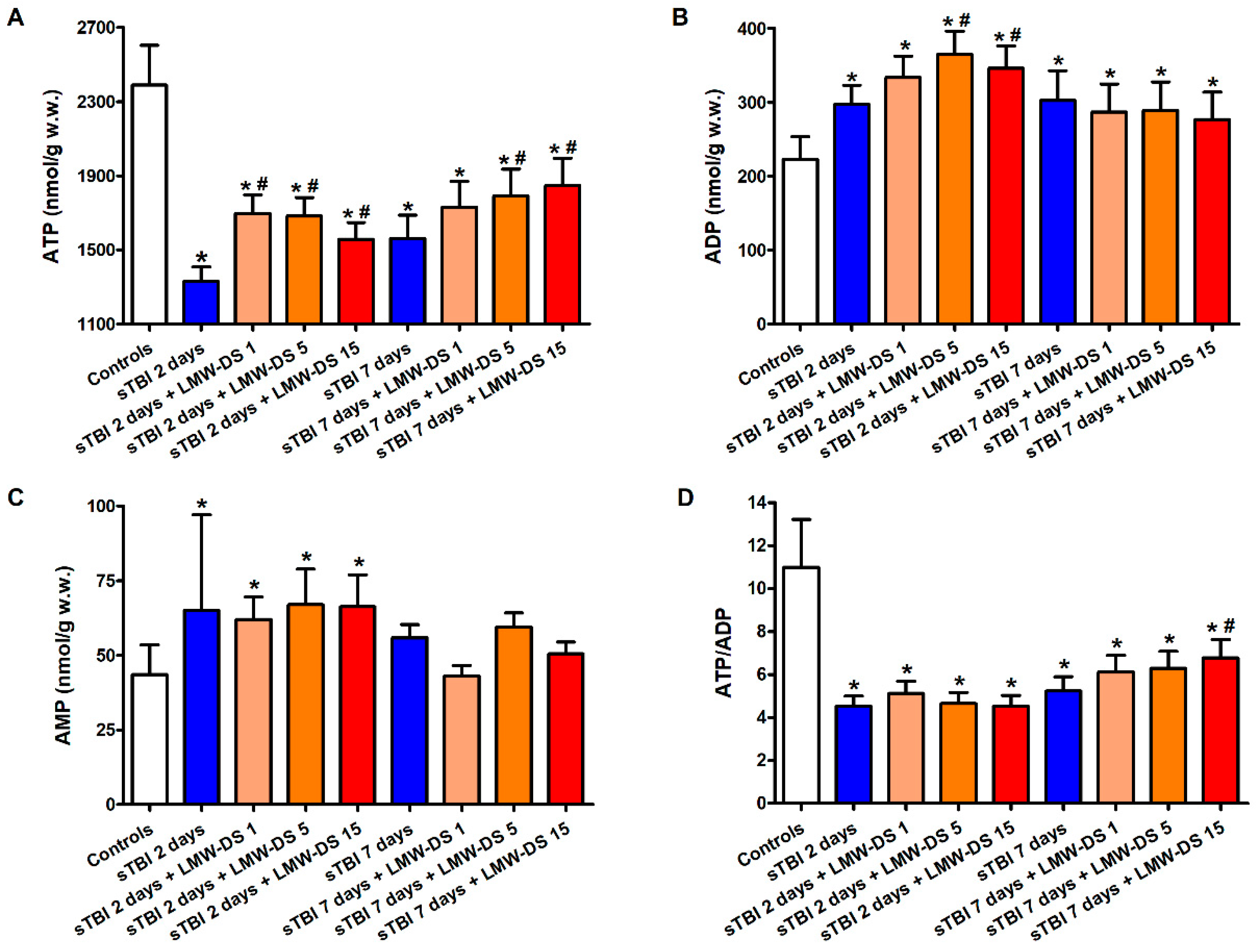
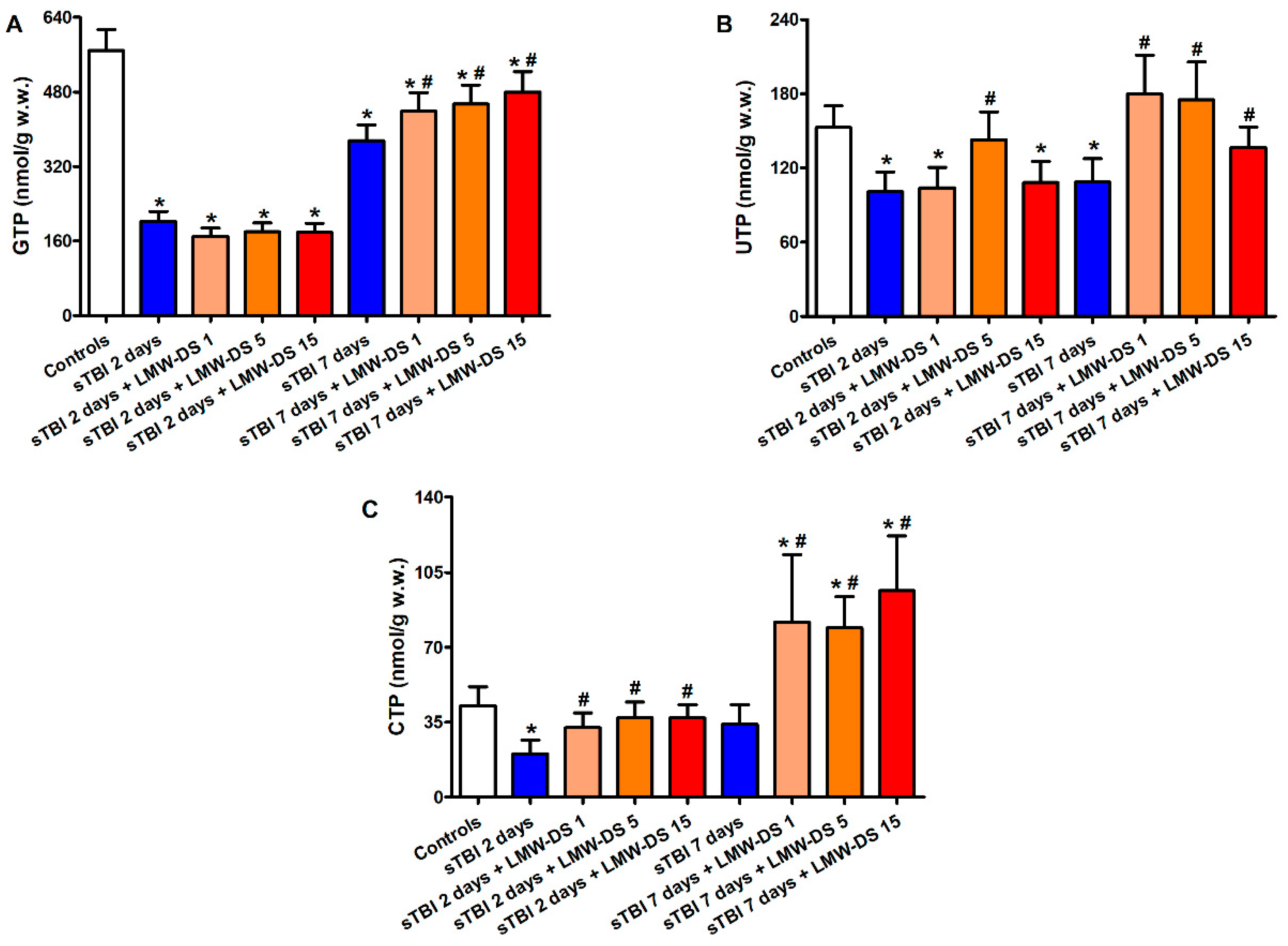
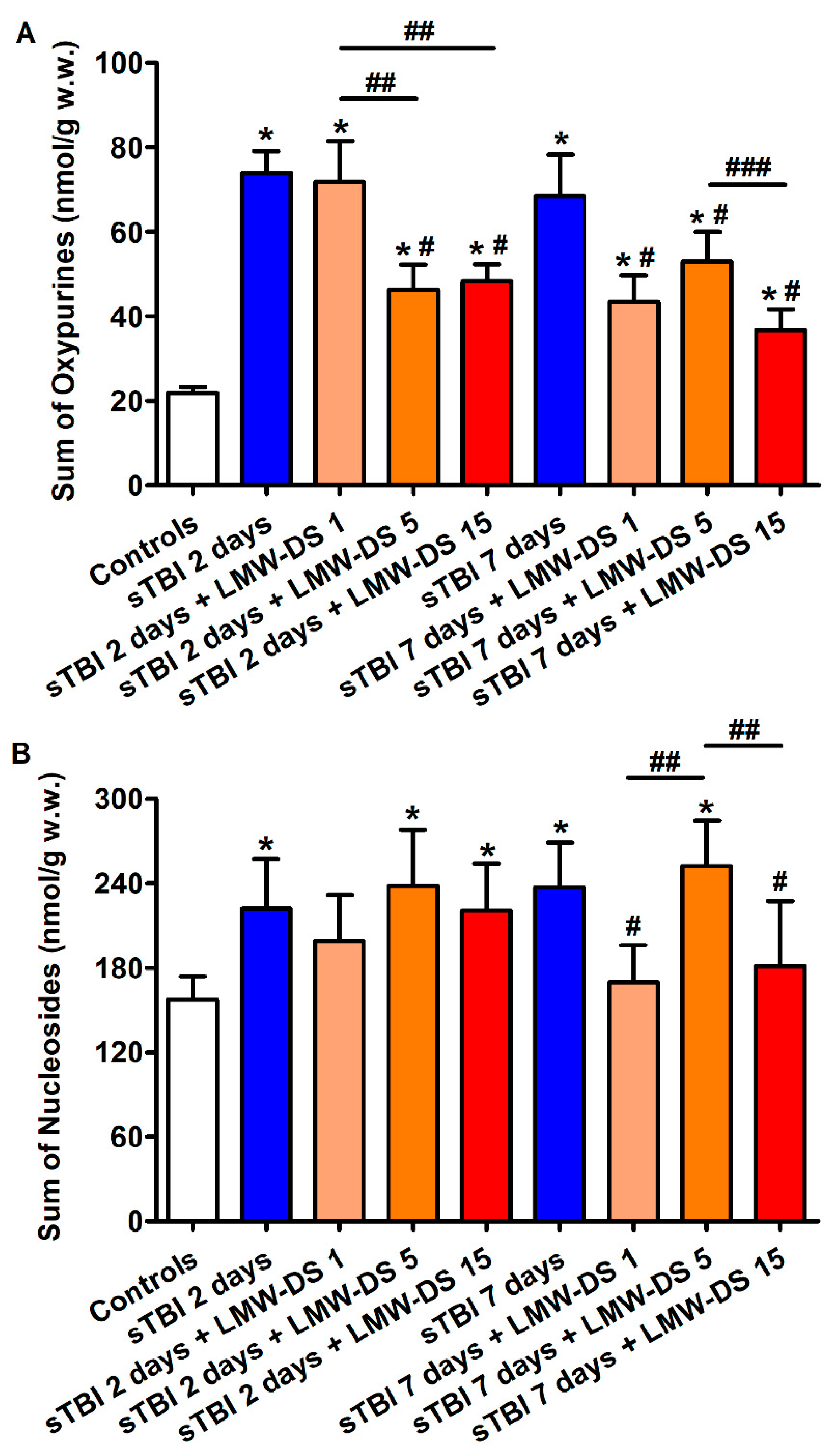
3.2. ILB® Improves Cerebral High Energy Phosphate Concentrations and Reduces ATP Catabolism after sTBI
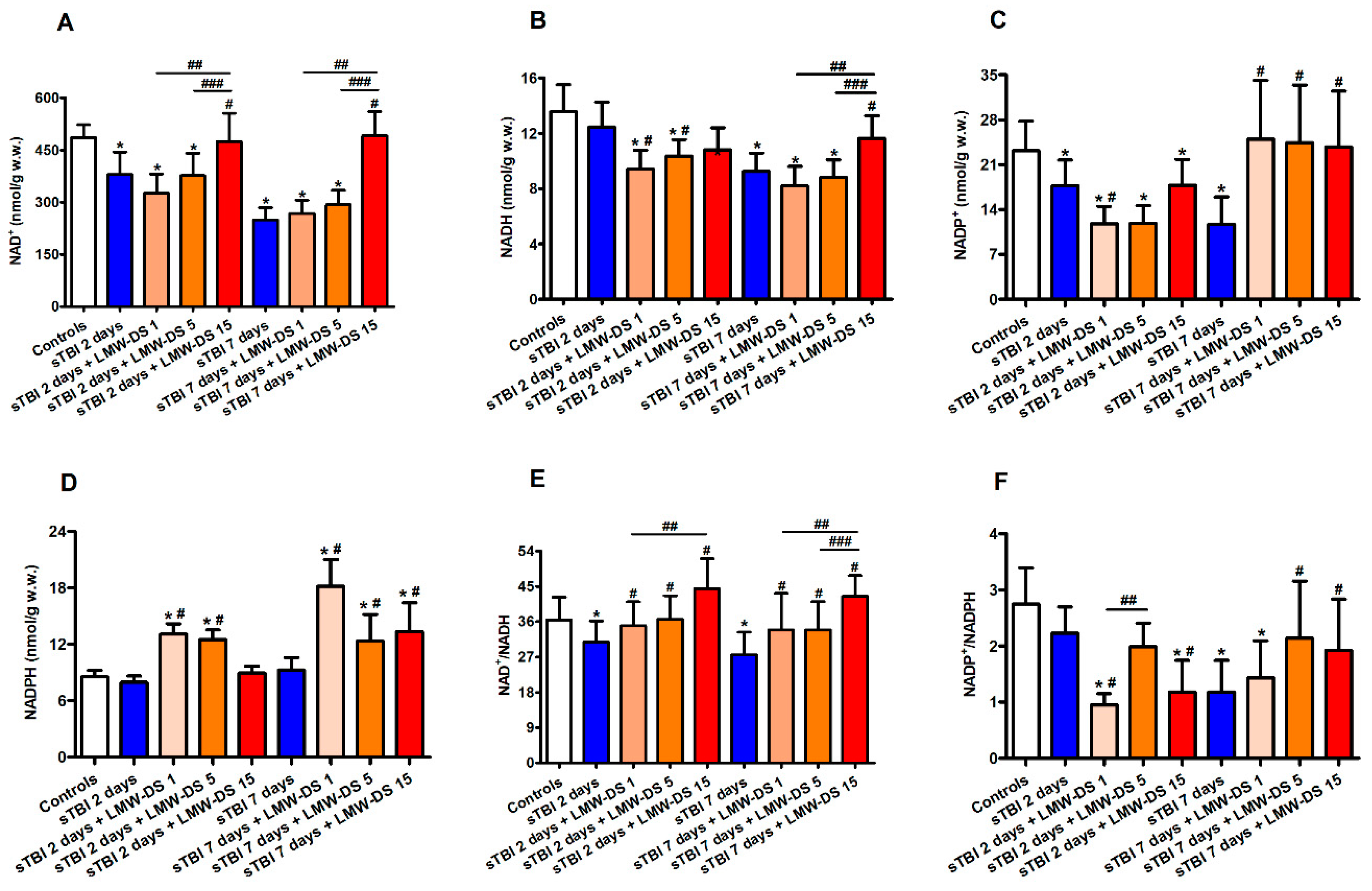
3.3. Redox Metabolism of Cerebral Nicotinic Coenzymes after sTBI Is Improved by ILB®
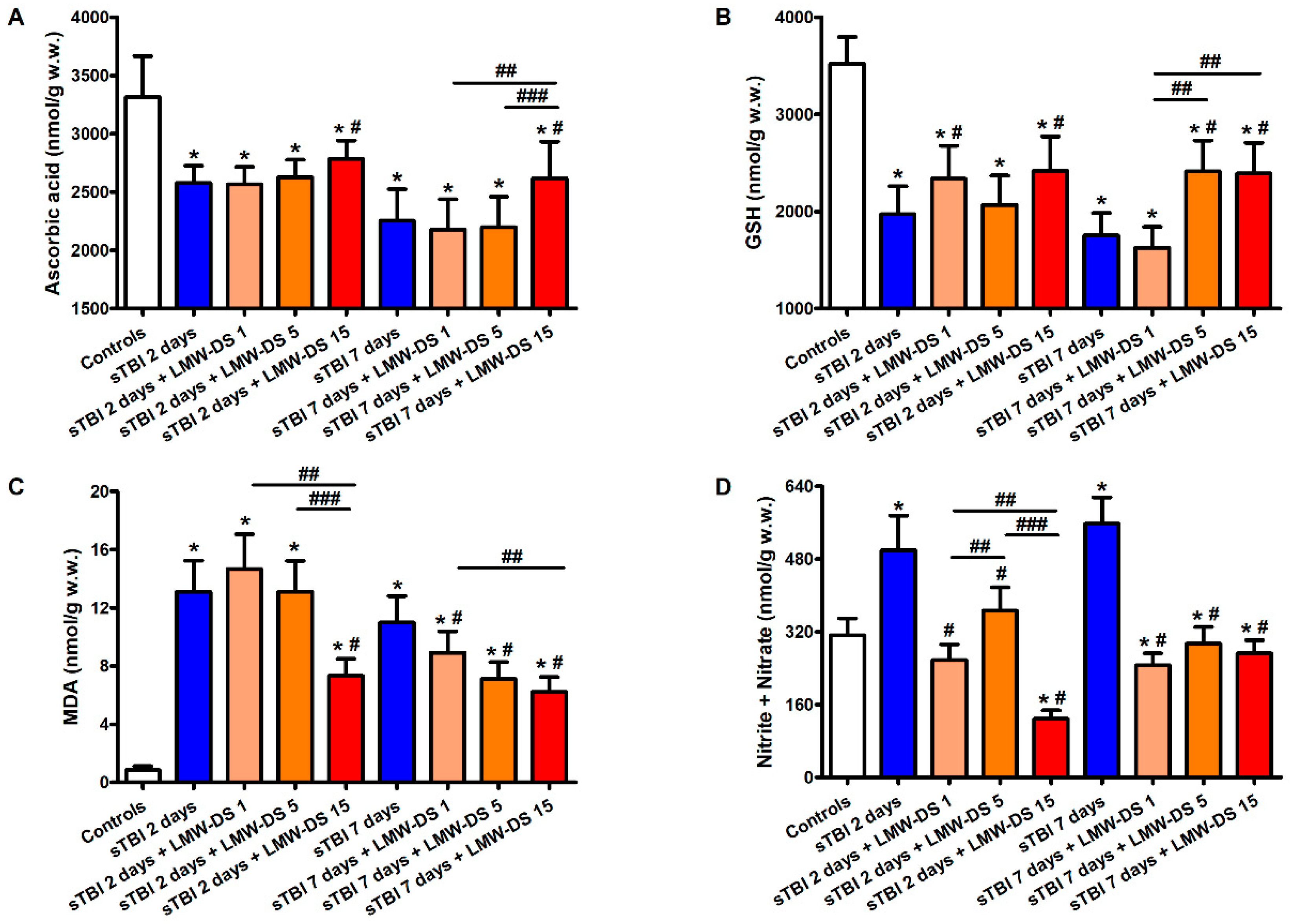
3.4. ILB® Ameliorates Changes in Cerebral Water-Soluble Antioxidants and Decreases Oxidative/Nitrosative Stress after sTBI
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Iaccarino, C.; Carretta, A.; Nicolosi, F.; Morselli, C. Epidemiology of severe traumatic brain injury. J. Neurosurg. Sci. 2018, 62, 535–541. [Google Scholar] [PubMed]
- Abdelmalik, P.A.; Draghic, N.; Ling, G.S.F. Management of moderate and severe traumatic brain injury. Transfusion 2019, 59, 1529–1538. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scerrati, A.; de Rosa, S.; Mongardi, L.; Cavallo, M.A.; Trapella, G.; de Bonis, P. Standard of care, controversies, and innovations in the medical treatment of severe traumatic brain injury. J. Neurosurg. Sci. 2018, 62, 574–583. [Google Scholar] [PubMed]
- McDonald, S.J.; Sun, M.; Agoston, D.V.; Shultz, S.R. The effect of concomitant peripheral injury on traumatic brain injury pathobiology and outcome. J. Neuroinflamm. 2016, 13, 90. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stocchetti, N.; Picetti, E.; Berardino, M.; Buki, A.; Chesnut, R.M.; Fountas, K.N.; Horn, P.; Hutchinson, P.J.; Iaccarino, C.; Kolias, A.G.; et al. Clinical applications of intracranial pressure monitoring in traumatic brain injury: Report of the Milan consensus conference. Acta Neurochir. 2014, 156, 1615–1622. [Google Scholar] [CrossRef] [PubMed]
- Hutchinson, P.J.; Kolias, A.G.; Timofeev, I.S.; Corteen, E.A.; Czosnyka, M.; Timothy, J.; Anderson, I.; Bulters, D.O.; Belli, A.; Eynon, C.A.; et al. Trial of decompressive craniectomy for traumatic intracranial hypertension. N. Engl. J. Med. 2016, 375, 1119–1130. [Google Scholar] [CrossRef] [Green Version]
- Cristofori, L.; Tavazzi, B.; Gambin, R.; Vagnozzi, R.; Vivenza, C.; Amorini, A.M.; Di Pierro, D.; Fazzina, G.; Lazzarino, G. Early onset of lipid peroxidation after human traumatic brain injury: A fatal limitation for the free radical scavenger pharmacological therapy? J. Investig. Med. 2001, 49, 450–458. [Google Scholar] [CrossRef]
- Ladak, A.A.; Enam, S.A.; Ibrahim, M.T. A review of the molecular mechanisms of traumatic brain injury. World Neurosurg. 2019, 131, 126–132. [Google Scholar] [CrossRef]
- Burda, J.E.; Bernstein, A.M.; Sofroniew, M.V. Astrocyte roles in traumatic brain injury. Exp. Neurol. 2016, 275, 305–315. [Google Scholar] [CrossRef] [Green Version]
- Bao, Z.; Fan, L.; Zhao, L.; Xu, X.; Liu, Y.; Chao, H.; Liu, N.; You, Y.; Liu, Y.; Wang, X.; et al. Silencing of A20 aggravates neuronal death and inflammation after traumatic brain injury: A potential trigger of necroptosis. Front. Mol. Neurosci. 2019, 12, 222. [Google Scholar] [CrossRef]
- Lorente, L.; Martín, M.M.; Pérez-Cejas, A.; González-Rivero, A.F.; Argueso, M.; Ramos, L.; Solé-Violán, J.; Cáceres, J.J.; Jiménez, A.; García-Marín, V. Serum caspase-3 levels during the first week of traumatic brain injury. Med. Intensiva 2019. [Google Scholar] [CrossRef] [PubMed]
- Tavazzi, B.; Signoretti, S.; Lazzarino, G.; Amorini, A.M.; Delfini, R.; Cimatti, M.; Marmarou, A.; Vagnozzi, R. Cerebral oxidative stress and depression of energy metabolism correlate with severity of diffuse brain injury in rats. Neurosurgery 2005, 56, 582–589. [Google Scholar] [CrossRef] [PubMed]
- Kumar Sahel, D.; Kaira, M.; Raj, K.; Sharma, S.; Singh, S. Mitochondrial dysfunctioning and neuroinflammation: Recent highlights on the possible mechanisms involved in traumatic brain injury. Neurosci. Lett. 2019, 710. [Google Scholar] [CrossRef]
- Czigler, A.; Toth, L.; Szarka, N.; Berta, G.; Amrein, K.; Czeiter, E.; Lendvai-Emmert, D.; Bodo, K.; Tarantini, S.; Koller, A.; et al. Hypertension exacerbates cerebrovascular oxidative stress induced by mild traumatic brain injury: Protective effects of the mitochondria-targeted antioxidative peptide ss-31. J. Neurotrauma 2019, 36, 3309–3315. [Google Scholar] [CrossRef]
- Carteri, R.B.; Kopczynski, A.; Rodolphi, M.S.; Strogulski, N.R.; Sartor, M.; Feldmann, M.; de Bastiani, M.A.; Duval Wannmacher, C.M.; de Franceschi, I.D.; Hansel, G.; et al. Testosterone administration after traumatic brain injury reduces mitochondrial dysfunction and neurodegeneration. J. Neurotrauma 2019, 36, 2246–2259. [Google Scholar] [CrossRef]
- Darwish, R.S.; Amiridze, N.S. Detectable levels of cytochrome C and activated caspase-9 in cerebrospinal fluid after human traumatic brain injury. Neurocrit. Care 2010, 12, 337–341. [Google Scholar] [CrossRef] [PubMed]
- Tan, D.; Yu, X.; Chen, M.; Chen, J.; Xu, J. Lutein protects against severe traumatic brain injury through anti-inflammation and antioxidative effects via ICAM-1/Nrf-2. Mol. Med. Rep. 2017, 16, 4235–4240. [Google Scholar] [CrossRef] [Green Version]
- Ma, M.W.; Wang, J.; Dhandapani, K.M.; Brann, D.W. Deletion of NADPH oxidase 4 reduces severity of traumatic brain injury. Free Radic. Biol. Med. 2018, 117, 66–75. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Meng, J.; Bi, F.; Li, H.; Chang, C.; Ji, C.; Liu, W. EK7 regulates NLRP3 inflammasome activation and neuroinflammation post-traumatic brain injury. Front. Mol. Neurosci. 2019, 12, 202. [Google Scholar] [CrossRef] [Green Version]
- Kerr, N.; Lee, S.W.; Perez-Barcena, J.; Crespi, C.; Ibañez, J.; Bullock, M.R.; Dietrich, W.D.; Keane, R.W.; de Rivero Vaccari, J.P. Inflammasome proteins as biomarkers of traumatic brain injury. PLoS ONE 2018, 13, e0210128. [Google Scholar] [CrossRef]
- Morganti-Kossmann, M.C.; Semple, B.D.; Hellewell, S.C.; Bye, N.; Ziebell, J.M. The complexity of neuroinflammation consequent to traumatic brain injury: From research evidence to potential treatments. Acta Neuropathol. 2019, 137, 731–755. [Google Scholar] [CrossRef] [PubMed]
- Amorini, A.M.; Lazzarino, G.; di Pietro, V.; Signoretti, S.; Lazzarino, G.; Belli, A.; Tavazzi, B. Metabolic, enzymatic and gene involvement in cerebral glucose dysmetabolism after traumatic brain injury. Biochim. Biophys. Acta 2016, 1862, 679–687. [Google Scholar] [CrossRef]
- Vagnozzi, R.; Tavazzi, B.; Signoretti, S.; Amorini, A.M.; Belli, A.; Cimatti, M.; Delfini, R.; di Pietro, V.; Finocchiaro, A.; Lazzarino, G. Temporal window of metabolic brain vulnerability to concussions: Mitochondrial-related impairment—Part I. Neurosurgery 2007, 61, 379–388. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Di Pietro, V.; Lazzarino, G.; Amorini, A.M.; Signoretti, S.; Hill, L.J.; Porto, E.; Tavazzi, B.; Lazzarino, G.; Belli, A. Fusion or fission: The destiny of mitochondria in traumatic brain injury of different severities. Sci. Rep. 2017, 7, 9189. [Google Scholar] [CrossRef] [PubMed]
- Di Pietro, V.; Lazzarino, G.; Amorini, A.M.; Tavazzi, B.; D’Urso, S.; Longo, S.; Vagnozzi, R.; Signoretti, S.; Clementi, E.; Giardina, B.; et al. Neuroglobin expression and oxidant/antioxidant balance after graded traumatic brain injury in the rat. Free Radic. Biol. Med. 2014, 69, 258–264. [Google Scholar] [CrossRef] [PubMed]
- Tavazzi, B.; Vagnozzi, R.; Signoretti, S.; Amorini, A.M.; Belli, A.; Cimatti, M.; Delfini, R.; di Pietro, V.; Finocchiaro, A.; Lazzarino, G. Temporal window of metabolic brain vulnerability to concussions: Oxidative and nitrosative stresses—Part II. Neurosurgery 2007, 61, 390–395. [Google Scholar] [CrossRef] [Green Version]
- Vagnozzi, R.; Signoretti, S.; Tavazzi, B.; Floris, R.; Ludovici, A.; Marziali, S.; Tarascio, G.; Amorini, A.M.; di Pietro, V.; Delfini, R.; et al. Temporal window of metabolic brain vulnerability to concussion: A pilot 1H-magnetic resonance spectroscopic study in concussed athletes—part III. Neurosurgery 2008, 62, 1286–1295. [Google Scholar] [CrossRef]
- Vagnozzi, R.; Signoretti, S.; Cristofori, L.; Alessandrini, F.; Floris, R.; Isgrò, E.; Ria, A.; Marziale, S.; Zoccatelli, G.; Tavazzi, B.; et al. Assessment of metabolic brain damage and recovery following mild traumatic brain injury: A multicentre, proton magnetic resonance spectroscopic study in concussed patients. Brain 2010, 133, 3232–3242. [Google Scholar] [CrossRef]
- Vagnozzi, R.; Marmarou, A.; Tavazzi, B.; Signoretti, S.; di Pierro, D.; del Bolgia, F.; Amorini, A.M.; Fazzina, G.; Sherkat, S.; Lazzarino, G. Changes of cerebral energy metabolism and lipid peroxidation in rats leading to mitochondrial dysfunction after diffuse brain injury. J. Neurotrauma 1999, 16, 903–913. [Google Scholar] [CrossRef]
- Di Pietro, V.; Amorini, A.M.; Tavazzi, B.; Vagnozzi, R.; Logan, A.; Lazzarino, G.; Signoretti, S.; Lazzarino, G.; Belli, A. The molecular mechanisms affecting N-acetylaspartate homeostasis following experimental graded traumatic brain injury. Mol. Med. 2014, 20, 147–157. [Google Scholar] [CrossRef]
- Tanaka, T. Development of an inflammation-associated colorectal cancer model and its application for research on carcinogenesis and chemoprevention. Int. J. Inflamm. 2012, 2012, 658786. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.J.; Gong, H.F.; Zhao, Q.Q.; Liu, X.S.; Liu, C.; Wang, H. Critical role of toll-like receptor 4 (TLR4) in dextran sulfate sodium (DSS)-Induced intestinal injury and repair. Toxicol. Lett. 2019, 315, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Yassin, M.; Sadowska, Z.; Djurhuus, D.; Nielsen, B.; Tougaard, P.; Olsen, J.; Pedersen, A.E. Upregulation of PD-1 follows tumour development in the AOM/DSS model of inflammation-induced colorectal cancer in mice. Immunology 2019, 158, 35–46. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Johansson, H.; Goto, M.; Dufrane, D.; Siegbahn, A.; Elgue, G.; Gianello, P.; Korsgren, O.; Nilsson, B. Low molecular weight dextran sulfate: A strong candidate drug to block IBMIR in clinical islet transplantation. Am. J. Transplant. 2006, 6, 305–312. [Google Scholar] [CrossRef] [PubMed]
- Von Zur-Mühlen, B.; Lundgren, T.; Bayman, L.; Berne, C.; Bridges, N.; Eggerman, T.; Foss, A.; Goldstein, J.; Jenssen, T.; Jorns, C.; et al. Open randomized multicenter study to evaluate safety and efficacy of low molecular weight sulfated dextran in islet transplantation. Transplantation 2019, 103, 630–637. [Google Scholar] [CrossRef] [Green Version]
- Ley, K.; Allietta, M.; Bullard, D.C.; Morgan, S. Importance of E-selectin for firm leukocyte adhesion in vivo. Circ. Res. 1998, 83, 287–294. [Google Scholar] [CrossRef]
- Matsumiya, A.; Yamaguchi, M.; Nakano, H.; Takeda, M.; Kumada, K. Dextran sulfate inhibits E-selectin-mediated neutrophil adhesion to endotoxin-activated vascular endothelial cells. Life Sci. 1999, 64, 9–17. [Google Scholar] [CrossRef]
- Hagiwara, A.; Sawai, K.; Sakakura, C.; Shirasu, M.; Ohgaki, M.; Imanishi, T.; Yamasaki, J.; Togawa, T.; Takahashi, T. Prevention of peritoneal metastasis of cancer with dextran sulfate—An experimental study in mice. Anticancer Drugs 1997, 8, 894–897. [Google Scholar] [CrossRef]
- Fujishima, M.; Omae, T.; Tanaka, K.; Iino, K.; Matsuo, O.; Mihara, H. Controlled trial of combined urokinase and dextran sulfate therapy in patients with acute cerebral infarction. Angiology 1986, 37, 487–498. [Google Scholar] [CrossRef] [PubMed]
- Marmarou, A.; Foda, M.A.; van den Brink, W.; Campbell, J.; Kita, H.; Demetriadou, K. A new model of diffuse brain injury in rats. Part I: Pathophysiology and biomechanics. J. Neurosurg. 1994, 80, 291–300. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maldonado, E.N.; Lemasters, J.J. ATP/ADP ratio, the missed connection between mitochondria and the Warburg effect. Mitochondrion 2014, 19, 78–84. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Froese, L.; Batson, C.; Gomez, A.; Dian, J.; Zeiler, F.A. the limited impact of current therapeutic interventions on cerebrovascular reactivity in traumatic brain injury: A narrative overview. Neurocrit. Care 2020. [Google Scholar] [CrossRef]
- Ng, S.Y.; Lee, A.Y.W. Traumatic brain injuries: Pathophysiology and potential therapeutic targets. Front. Cell Neurosci. 2019, 13, 528. [Google Scholar] [CrossRef] [PubMed]
- Casey, P.A.; McKenna, M.C.; Fiskum, G.; Saraswati, M.; Robertson, C.L. Early and sustained alterations in cerebral metabolism after traumatic brain injury in immature rats. J. Neurotrauma 2008, 25, 603–614. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Signoretti, S.; Marmarou, A.; Aygok, G.A.; Fatouros, P.P.; Portella, G.; Bullock, R.M. Assessment of mitochondrial impairment in traumatic brain injury using high-resolution proton magnetic resonance spectroscopy. J. Neurosurg. 2008, 108, 42–52. [Google Scholar] [CrossRef] [PubMed]
- Sacoman, J.L.; Dagda, R.Y.; Burnham-Marusich, A.R.; Dagda, R.K.; Berninsone, P.M. Mitochondrial O-GlcNAc transferase (mOGT) regulates mitochondrial structure, function, and survival in HeLa cells. J. Biol. Chem. 2017, 292, 4499–4518. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Akella, N.M.; Ciraku, L.; Reginato, M.J. Fueling the fire: Emerging role of the hexosamine biosynthetic pathway in cancer. BMC Biol. 2019, 17, 52. [Google Scholar] [CrossRef] [Green Version]
- Wang, Q.; Zhao, M.; Parungao, G.G.; Viola, R.E. Purification and characterization of aspartate N-acetyltransferase: A critical enzyme in brain metabolism. Protein Expr. Purif. 2016, 119, 11–18. [Google Scholar] [CrossRef]
- Tahay, G.; Wiame, E.; Tyteca, D.; Courtoy, P.J.; van Schaftingen, E. Determinants of the enzymatic activity and the subcellular localization of aspartate N-acetyltransferase. Biochem. J. 2012, 441, 105–112. [Google Scholar] [CrossRef] [Green Version]
- Lazzarino, G.; Amorini, A.M.; Signoretti, S.; Musumeci, G.; Lazzarino, G.; Caruso, G.; Pastore, F.S.; di Pietro, V.; Tavazzi, B.; Belli, A. Pyruvate dehydrogenase and tricarboxylic acid cycle enzymes are sensitive targets of traumatic brain injury induced metabolic derangement. Int. J. Mol. Sci. 2019, 20, 5774. [Google Scholar] [CrossRef] [Green Version]
- Kulikova, V.A.; Gromyko, D.V.; Nikiforov, A.A. The regulatory role of NAD in human and animal cells. Biochem. Mosc. 2018, 83, 800–812. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Sauve, A.A. NAD+ metabolism: Bioenergetics, signaling and manipulation for therapy. Biochim. Biophys. Acta 2016, 1864, 1787–1800. [Google Scholar] [CrossRef] [Green Version]
- Xiao, W.; Loscalzo, J. Metabolic responses to reductive stress. Antioxid. Redox Signal 2020, 32, 1330–1347. [Google Scholar] [CrossRef] [Green Version]
- Mejía, S.Á.; Gutman, L.A.B.; Camarillo, C.O.; Navarro, R.M.; Becerra, M.C.S.; Santana, L.D.; Cruz, M.; Pérez, E.H.; Flores, M.D. Nicotinamide prevents sweet beverage-induced hepatic steatosis in rats by regulating the G6PD, NADPH/NADP+ and GSH/GSSG ratios and reducing oxidative and inflammatory stress. Eur. J. Pharmacol. 2018, 818, 499–507. [Google Scholar] [CrossRef]
- Bakthavachalam, P.; Shanmugam, P.S.T. Mitochondrial dysfunction–Silent killer in cerebral ischemia. J. Neurol. Sci. 2017, 375, 417–423. [Google Scholar] [CrossRef] [PubMed]
- Yonutas, H.M.; Vekaria, H.J.; Sullivan, P.G. Mitochondrial specific therapeutic targets following brain injury. Brain Res. 2016, 1640, 77–93. [Google Scholar] [CrossRef] [PubMed]
- Ma, M.W.; Wang, J.; Dhandapani, K.M.; Wang, R.; Brann, D.W. NADPH oxidases in traumatic brain injury-Promising therapeutic targets? Redox Biol. 2018, 16, 285–293. [Google Scholar] [CrossRef] [PubMed]
- Devanney, N.A.; Stewart, A.N.; Gensel, J.C. Microglia and macrophage metabolism in CNS injury and disease: The role of immunometabolism in neurodegeneration and neurotrauma. Exp. Neurol. 2020, 329, 113310. [Google Scholar] [CrossRef]
- Nisenbaum, E.J.; Novikov, D.S.; Lui, Y.W. The presence and role of iron in mild traumatic brain injury: An imaging perspective. J. Neurotrauma 2014, 31, 301–307. [Google Scholar] [CrossRef]
- Di Pietro, V.; Yakoub, K.M.; Caruso, G.; Lazzarino, G.; Signoretti, S.; Barbey, A.K.; Tavazzi, B.; Lazzarino, G.; Belli, A.; Amorini, A.M. Antioxidant therapies in traumatic brain injury. Antioxidants 2020, 9, 260. [Google Scholar] [CrossRef] [Green Version]
- Robinson, S.; Berglass, J.B.; Denson, J.L.; Berkner, J.; Anstine, C.V.; Winer, J.L.; Maxwell, J.R.; Qiu, J.; Yang, Y.; Sillerud, L.O.; et al. Microstructural and microglial changes after repetitive mild traumatic brain injury in mice. J. Neurosci. Res. 2017, 95, 1025–1035. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Josephine, A.; Veena, C.K.; Amudha, G.; Preetha, S.P.; Varalakshmi, P. Evaluating the effect of sulphated polysaccharides on cyclosporine a induced oxidative renal injury. Mol. Cell Biochem. 2006, 287, 101–108. [Google Scholar] [CrossRef]
- Chen, L.; Huang, G. Antioxidant activities of sulfated pumpkin polysaccharides. Int. J. Biol. Macromol. 2019, 126, 743–746. [Google Scholar] [CrossRef] [PubMed]
- Presa, F.B.; Marques, M.L.M.; Viana, R.L.S.; Nobre, L.T.D.B.; Costa, L.S.; Rocha, H.A.O. The protective role of sulfated polysaccharides from green seaweed udotea flabellum in cells exposed to oxidative damage. Mar. Drugs 2018, 16, 135. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, X.T.; Sun, X.Y.; Yu, K.; Gui, B.S.; Gui, Q.; Ouyang, J.M. Effect of content of sulfate groups in seaweed polysaccharides on antioxidant activity and repair effect of subcellular organelles in injured HK-2 cells. Oxid. Med. Cell Longev. 2017, 2017, 2542950. [Google Scholar] [CrossRef]
- Patel, R.K.; Prasad, N.; Kuwar, R.; Haldar, D.; Abdul-Muneer, P.M. Transforming growth factor-beta 1 signaling regulates neuroinflammation and apoptosis in mild traumatic brain injury. Brain Behav. Immun. 2017, 64, 244–258. [Google Scholar] [CrossRef]
- Barritault, D.; Gilbert-Sirieix, M.; Rice, K.L.; Siñeriz, F.; Papy-Garcia, D.; Baudouin, C.; Desgranges, P.; Zakine, G.; Saffar, J.L.; van Neck, J. RGTA® or ReGeneraTing Agents mimic heparan sulfate in regenerative medicine: From concept to curing patients. Glycoconj. J. 2017, 34, 325–338. [Google Scholar] [CrossRef] [Green Version]
- Schmidt, P.; Magnusson, C.; Lundgren, T.; Korsgren, O.; Nilsson, B. Low molecular weight dextran sulfate is well tolerated in humans and increases endogenous expression of islet protective hepatocyte growth factor. Transplantation 2008, 86, 1523–1530. [Google Scholar] [CrossRef]
- Sun, W.; Funakoshi, H.; Nakamura, T. Overexpression of HGF retards disease progression and prolongs life span in a transgenic mouse model of ALS. J. Neurosci. 2002, 22, 6537–6548. [Google Scholar] [CrossRef]
- Perdomo, G.; Martinez-Brocca, M.A.; Bhatt, B.A.; Brown, N.F.; O’Doherty, R.M.; Garcia-Ocaña, A. Hepatocyte growth factor is a novel stimulator of glucose uptake and metabolism in skeletal muscle cells. J. Biol. Chem. 2008, 283, 13700–13706. [Google Scholar] [CrossRef] [Green Version]
- Vasantharaja, R.; Stanley Abraham, L.; Gopinath, V.; Hariharan, D.; Smita, K.M. Attenuation of oxidative stress induced mitochondrial dysfunction and cytotoxicity in fibroblast cells by sulfated polysaccharide from Padina gymnospora. Int. J. Biol. Macromol. 2019, 124, 50–59. [Google Scholar] [CrossRef] [PubMed]
- Josephine, A.; Amudha, G.; Veena, C.K.; Preetha, S.P.; Rajeswari, A.; Varalakshmi, P. Beneficial effects of sulfated polysaccharides from Sargassum wightii against mitochondrial alterations induced by Cyclosporine A in rat kidney. Mol. Nutr. Food Res. 2007, 51, 1413–1422. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Zhu, M.; He, Z.Z. Low-molecular-weight fucoidan attenuates mitochondrial dysfunction and improves neurological outcome after traumatic brain injury in aged mice: Involvement of Sirt3. Cell Mol. Neurobiol. 2016, 36, 1257–1268. [Google Scholar] [CrossRef]
- Cunningham, T.L.; Cartagena, C.M.; Lu, X.C.; Konopko, M.; Dave, J.R.; Tortella, F.C.; Shear, D.A. Correlations between blood-brain barrier disruption and neuroinflammation in an experimental model of penetrating ballistic-like brain injury. J. Neurotrauma 2014, 31, 505–514. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barzó, P.; Marmarou, A.; Fatouros, P.; Corwin, F.; Dunbar, J. Magnetic resonance imaging-monitored acute blood-brain barrier changes in experimental traumatic brain injury. J. Neurosurg. 1996, 85, 1113–1121. [Google Scholar] [CrossRef]
- Nimmo, A.J.; Cernak, I.; Heath, D.L.; Hu, X.; Bennett, C.J.; Vink, R. Neurogenic inflammation is associated with development of edema and functional deficits following traumatic brain injury in rats. Neuropeptides 2004, 38, 40–47. [Google Scholar] [CrossRef]
- Farbood, Y.; Sarkaki, A.; Dianat, M.; Khodadadi, A.; Haddad, M.K.; Mashhadizadeh, S. Ellagic acid prevents cognitive and hippocampal long-term potentiation deficits and brain inflammation in rat with traumatic brain injury. Life Sci. 2015, 124, 120–127. [Google Scholar] [CrossRef]
- Si, D.; Li, J.; Liu, J.; Wang, X.; Wei, Z.; Tian, Q.; Wang, H.; Liu, G. Progesterone protects blood-brain barrier function and improves neurological outcome following traumatic brain injury in rats. Exp. Ther. Med. 2014, 8, 1010–1014. [Google Scholar] [CrossRef] [Green Version]
- Choi, J.J.; Wang, S.; Tung, Y.S.; Konofagou, E.E. Molecules of various pharmacologically-relevant sizes can cross the ultrasound-induced blood-brain barrier opening in vivo. Ultrasound Med. Biol. 2010, 36, 58–67. [Google Scholar] [CrossRef] [Green Version]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lazzarino, G.; Amorini, A.M.; Barnes, N.M.; Bruce, L.; Mordente, A.; Lazzarino, G.; Pietro, V.D.; Tavazzi, B.; Belli, A.; Logan, A. Low Molecular Weight Dextran Sulfate (ILB®) Administration Restores Brain Energy Metabolism Following Severe Traumatic Brain Injury in the Rat. Antioxidants 2020, 9, 850. https://doi.org/10.3390/antiox9090850
Lazzarino G, Amorini AM, Barnes NM, Bruce L, Mordente A, Lazzarino G, Pietro VD, Tavazzi B, Belli A, Logan A. Low Molecular Weight Dextran Sulfate (ILB®) Administration Restores Brain Energy Metabolism Following Severe Traumatic Brain Injury in the Rat. Antioxidants. 2020; 9(9):850. https://doi.org/10.3390/antiox9090850
Chicago/Turabian StyleLazzarino, Giacomo, Angela Maria Amorini, Nicholas M. Barnes, Lars Bruce, Alvaro Mordente, Giuseppe Lazzarino, Valentina Di Pietro, Barbara Tavazzi, Antonio Belli, and Ann Logan. 2020. "Low Molecular Weight Dextran Sulfate (ILB®) Administration Restores Brain Energy Metabolism Following Severe Traumatic Brain Injury in the Rat" Antioxidants 9, no. 9: 850. https://doi.org/10.3390/antiox9090850
APA StyleLazzarino, G., Amorini, A. M., Barnes, N. M., Bruce, L., Mordente, A., Lazzarino, G., Pietro, V. D., Tavazzi, B., Belli, A., & Logan, A. (2020). Low Molecular Weight Dextran Sulfate (ILB®) Administration Restores Brain Energy Metabolism Following Severe Traumatic Brain Injury in the Rat. Antioxidants, 9(9), 850. https://doi.org/10.3390/antiox9090850









