An Integrated Strategy for Nutraceuticals from Haematoccus pluvialis: From Cultivation to Extraction
Abstract
1. Introduction
2. Materials and Methods
2.1. Microalgae and Growth Medium
2.2. Vertical Bubble Column Photobioreactor Conditions
2.3. Cell Growth Measurements
2.4. Growth Conditions and Inoculum Reuse
2.5. Accelerated Solvent Extraction
2.6. Analytical Methods
3. Results
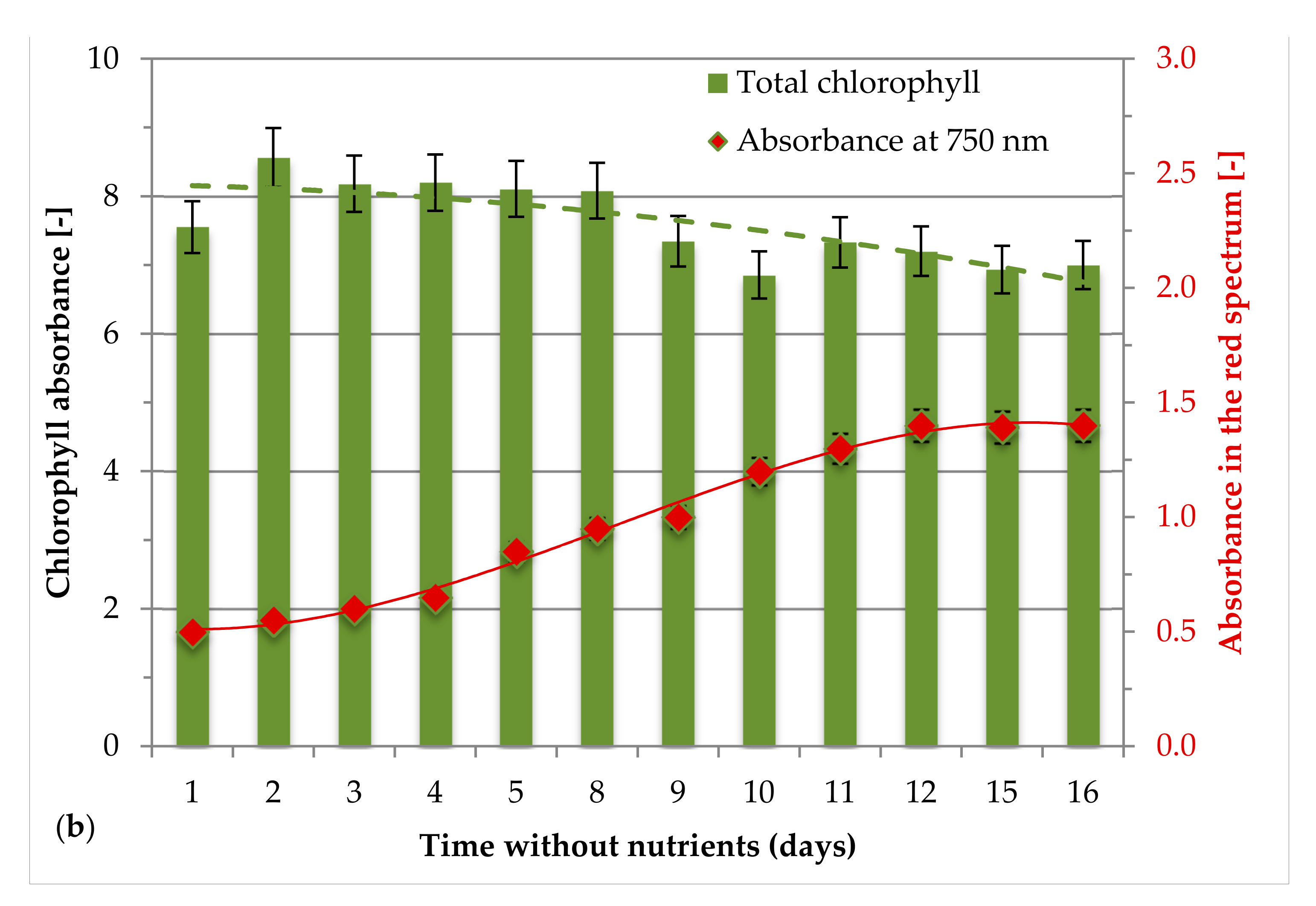
3.1. Effect of Nutrients during the Growth of H. pluvialis Green Phase
3.2. Effect of Nutrients and Light Intensity during the Switch from Green to Red Phase
3.3. Extraction of Bioactive Compounds from H. pluvialis Red Phase
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Adarme-Vega, T.C.; Lim, D.K.Y.; Timmins, M. Microalgal biofactories: A promising approach towards sustainable omega-3 fatty acid production. Microb. Cell Fact. 2012, 11, 96. [Google Scholar] [CrossRef]
- Fassett, R.G.; Coombes, J.S. Astaxanthin: A potential therapeutic agent in cardiovascular disease. Mar. Drugs 2011, 9, 447–465. [Google Scholar] [CrossRef]
- Shah, M.M.R.; Liang, Y.; Cheng, J.J.; Daroch, M. Astaxanthin-producing green microalga Haematococcus pluvialis: From single cell to high value commercial products. Front. Plant. Sci. 2016, 7, 531. [Google Scholar] [CrossRef]
- Butler, T.O.; McDougall, G.J.; Campbell, R.; Stanley, M.S.; Day, J.G. Media screening for obtaining Haematococcus pluvialis red motile macrozooids rich in astaxanthin and fatty acids. Biology 2018, 7, 2. [Google Scholar] [CrossRef]
- Butler, T.; Golan, Y. Astaxanthin Production from Microalgae. In Microalgae Biotechnology for Food, Health and High Value Products; Alam, M., Xu, J.L., Wang, Z., Eds.; Springer: Singapore, 2020. [Google Scholar] [CrossRef]
- Molino, A.; Iovine, A.; Casella, P.; Mehariya, S.; Chianese, S.; Cerbone, A.; Rimauro, J.; Musmarra, D. Microalgae characterization for consolidated and new application in human food, animal feed and nutraceuticals. Int. J. Environ. Res. Public Health 2018, 15, 2436. [Google Scholar] [CrossRef]
- Imamoglu, E.; Dalay, M.C.; Sukan, F.V. Influences of different stress media and high light intensities on accumulation of astaxanthin in the green alga Haematococcus pluvialis. New Biotechnol. 2009, 26, 199–204. [Google Scholar] [CrossRef]
- Kang, C.D.; Lee, J.S.; Park, T.H.; Sim, S.J. Comparison of heterotrophic and photoautotrophic induction on astaxanthin production by Haematococcus pluvialis. Appl. Microbiol. Biotechnol. 2005, 68, 237–241. [Google Scholar] [CrossRef]
- Feng, L.; Nie, K.; Jiang, H.; Fan, W. Effects of lutein supplementation in age-related macular degeneration. PLoS ONE 2019, 14, e0227048. [Google Scholar] [CrossRef] [PubMed]
- Ekpe, L.; Inaku, K.; Ekpe, V. Antioxidant effects of astaxanthin in various diseases—A review. J. Mol. Pathophysiol. 2018, 7, 1–6. [Google Scholar] [CrossRef]
- Guerin, M.; Huntley, M.E.; Olaizola, M. Haematococcus astaxanthin: Applications for human health and nutrition. Trends Biotechnol. 2003, 21, 210–216. [Google Scholar] [CrossRef]
- Davinelli, S.; Nielsen, M.E.; Scapagnini, G. Astaxanthin in skin health, repair, and disease: A comprehensive review. Nutrients 2018, 10, 522. [Google Scholar] [CrossRef]
- Novoveská, L.; Ross, M.E.; Stanley, M.S.; Pradelles, R.; Wasiolek, V.; Sassi, J.F. Microalgal carotenoids: A review of production, current markets, regulations, and future direction. Mar. Drugs 2019, 17, 640. [Google Scholar] [CrossRef]
- Ranard, K.M.; Jeon, S.; Mohn, E.S.; Griffiths, J.C.; Johnson, E.J.; Erdman, J.W. Dietary guidance for lutein: Consideration for intake recommendations is scientifically supported. Eur. J. Nutr. 2017, 56, 37–42. [Google Scholar] [CrossRef] [PubMed]
- EFSA Panel on Food Additives and Nutrient Sources added to Food (ANS). Scientific opinion on the reevaluation of lutein (e 161b) as a food additive on request of the european commission. EFSA J. 2010, 8, 1678. [Google Scholar] [CrossRef]
- EFSA NDA Panel (EFSA Panel on Nutrition, Novel Foods and Food Allergens); Turck, D.; Castenmiller, J.; de Henauw, S.; Hirsch-Ernst, K.I.; Kearney, J.; Maciuk, A.; Mangelsdorf, I.; McArdle, H.J.; Naska, A.; et al. Scientific opinion on the safety of astaxanthin for its use as a novel food in food supplements. EFSA J. 2020, 18, 5993. [Google Scholar] [CrossRef]
- White, B. Dietary fatty acids. Am. Fam. Phys. 2009, 80, 345–350. [Google Scholar]
- Ruxton, C.H.S.; Reed, S.C.; Simpson, M.J.A.; Millington, K.J. The health benefits of omega-3 polyunsaturated fatty acids: A review of the evidence. J. Hum. Nutr. Diet. 2004, 17, 449–459. [Google Scholar] [CrossRef]
- da Silva Ferreira, V.; Sant’Anna, C. Impact of culture conditions on the chlorophyll content of microalgae for biotechnological applications. World J. Microbiol. Biotechnol. 2016, 33, 20. [Google Scholar] [CrossRef]
- Hadj-Romdhane, F.; Zheng, X.; Jaouen, P.; Pruvost, J.; Grizeau, D.; Croué, J.P.; Bourseau, P. The culture of Chlorella vulgaris in a recycled supernatant: Effects on biomass production and medium quality. Bioresour. Technol. 2013, 132, 285–292. [Google Scholar] [CrossRef]
- Velichkova, K.; Sirakov, I. Growth parameters, protein and photosynthetic pigment content of Chlorella vulgaris cultivated under photoautotrophic and mixotrophic conditions. Bulg. J. Agric. Sci. 2018, 24, 150–155. [Google Scholar]
- Choi, Y.Y.; Joun, J.M.; Lee, J.; Hong, M.E.; Pham, H.-M.; Chang, W.S.; Sim, S.J. Development of large-scale and economic pH control system for outdoor cultivation of microalgae Haematococcus pluvialis using industrial flue gas. Bioresour. Technol. 2017, 244, 1235–1244. [Google Scholar] [CrossRef] [PubMed]
- Panis, G.; Carreon, J.R. Commercial astaxanthin production derived by green alga Haematococcus pluvialis: A microalgae process model and a techno-economic assessment all through production line. Algal. Res. 2016, 18, 175–190. [Google Scholar] [CrossRef]
- Park, J.C.; Choi, S.P.; Hong, M.-E.; Sim, S.J. Enhanced astaxanthin production from microalga, Haematococcus pluvialis by two-stage perfusion culture with stepwise light irradiation. Bioprocess. Biosyst. Eng. 2014, 37, 2039–2047. [Google Scholar] [CrossRef] [PubMed]
- Boussiba, S.; Vonshak, A. Astaxanthin accumulation in the green alga Haematococcus pluvialis 1. Plant. Cell Physiol. 1991, 32, 1077–1082. [Google Scholar] [CrossRef]
- Choi, Y.-E.; Yun, Y.-S.; Park, J.M.; Yang, J.-W. Determination of the time transferring cells for astaxanthin production considering two-stage process of Haematococcus pluvialis cultivation. Bioresour. Technol. 2011, 102, 11249–11253. [Google Scholar] [CrossRef]
- Fábregas, J.; Otero, A.; Maseda, A.; Domínguez, A. Two-stage cultures for the production of Astaxanthin from Haematococcus pluvialis. J. Biotechnol. 2001, 89, 65–71. [Google Scholar] [CrossRef]
- Aflalo, C.; Meshulam, Y.; Zarka, A.; Boussiba, S. On the relative efficiency of two- vs. one-stage production of astaxanthin by the green alga Haematococcus pluvialis. Biotechnol. Bioeng. 2007, 98, 300–305. [Google Scholar] [CrossRef]
- Río, E.D.; Acién, F.G.; García-Malea, M.C.; Rivas, J.; Molina-Grima, E.; Guerrero, M.G. Efficient one-step production of astaxanthin by the microalga Haematococcus pluvialis in continuous culture. Biotechnol. Bioeng. 2005, 91, 808–815. [Google Scholar] [CrossRef]
- Saha, S.K.; McHugh, E.; Hayes, J.; Moane, S.; Walsh, D.; Murray, P. Effect of various stress-regulatory factors on biomass and lipid production in microalga Haematococcus pluvialis. Bioresour. Technol. 2013, 128, 118–124. [Google Scholar] [CrossRef]
- Zhang, W.W.; Zhou, X.F.; Zhang, Y.L.; Cheng, P.F.; Ma, R.; Cheng, W.L.; Chu, H.Q. Enhancing astaxanthin accumulation in Haematococcus pluvialis by coupled light intensity and nitrogen starvation in column photobioreactors. J. Microbiol. Biotechnol. 2018, 28, 2019–2028. [Google Scholar] [CrossRef]
- Nahidian, B.; Ghanati, F.; Shahbazi, M.; Soltani, N. Effect of nutrients on the growth and physiological features of newly isolated Haematococcus pluvialis TMU1. Bioresour. Technol. 2018, 255, 229–237. [Google Scholar] [CrossRef] [PubMed]
- Scibilia, L.; Girolomoni, L.; Berteotti, S.; Alboresi, A.; Ballottari, M. Photosynthetic response to nitrogen starvation and high light in Haematococcus pluvialis. Algal. Res. 2015, 12, 170–181. [Google Scholar] [CrossRef]
- Cheng, J.; Li, K.; Yang, Z.; Zhou, J.; Cen, K. Enhancing the growth rate and astaxanthin yield of Haematococcus pluvialis by nuclear irradiation and high concentration of carbon dioxide stress. Bioresour. Technol. 2016, 204, 49–54. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.; Li, K.; Yang, Z.; Lu, H.; Zhou, J.; Cen, K. Gradient domestication of Haematococcus pluvialis mutant with 15% CO2 to promote biomass growth and astaxanthin yield. Bioresour. Technol. 2016, 216, 340–344. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.; Li, K.; Zhu, Y.; Yang, W.; Zhou, J.; Cen, K. Transcriptome sequencing and metabolic pathways of astaxanthin accumulated in Haematococcus pluvialis mutant under 15% CO2. Bioresour. Technol. 2017, 228, 99–105. [Google Scholar] [CrossRef]
- Choi, Y.Y.; Hong, M.E.; Jin, E.S.; Woo, H.M.; Sim, S.J. Improvement in modular scalability of polymeric thin-film photobioreactor for autotrophic culturing of Haematococcus pluvialis using industrial flue gas. Bioresour. Technol. 2018, 249, 519–526. [Google Scholar] [CrossRef]
- Wang, H.-C.; Cho, M.-G.; Riznichenko, G.; Rubin, A.B.; Lee, J.-H. Investigation of the maximum quantum yield of PS II in Haematococcus pluvialis cell cultures during growth: Effects of chemical or high-intensity light treatment. J. Photochem. Photobiol. B Biol. 2011, 104, 394–398. [Google Scholar] [CrossRef]
- Katsuda, T.; Shimahara, K.; Shiraishi, H.; Yamagami, K.; Ranjbar, R.; Katoh, S. Effect of flashing light from blue light emitting diodes on cell growth and astaxanthin production of Haematococcus pluvialis. J. Biosci. Bioeng. 2006, 102, 442–446. [Google Scholar] [CrossRef]
- Lababpour, A.; Hada, K.; Shimahara, K.; Katsuda, T.; Katoh, S. Effects of nutrient supply methods and illumination with blue light emitting diodes (LEDs) on astaxanthin production by Haematococcus pluvialis. J. Biosci. Bioeng. 2004, 98, 452–456. [Google Scholar] [CrossRef]
- Sun, H.; Liu, B.; Lu, X.; Cheng, K.W.; Chen, F. Staged cultivation enhances biomass accumulation in the green growth phase of Haematococcus pluvialis. Bioresour. Technol. 2017, 233, 326–331. [Google Scholar] [CrossRef]
- Witono, J.R.; Gunadi, A.; Santoso, H.; Miryanti, A.; Kumalaputri, A.J. The optimal condition on the growth of green Haematococcus pluvialis as one of the future natural resources. In Proceedings of the 26th Regional Symposium on Chemical Engineering (RSCE 2019), Kuala Lumpur, Malaysia, 30–31 October 2019. [Google Scholar]
- Zhang, C.; Chen, X.; Too, H.P. Microbial astaxanthin biosynthesis: Recent achievements, challenges, and commercialization outlook. Appl. Microbiol. Biot. 2020, 1–13. [Google Scholar] [CrossRef]
- Khoo, K.S.; Lee, S.Y.; Ooi, C.W.; Fu, X.; Miao, X.; Ling, T.C.; Show, P.L. Recent advances in biorefinery of astaxanthin from Haematococcus pluvialis. Bioresour. Technol. 2019, 288, 121606. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.-A.; Oh, Y.-K.; Lee, J.; Sim, S.J.; Hong, M.E.; Park, J.-Y.; Kim, M.-S.; Kim, S.W.; Lee, J.-S. High-efficiency cell disruption and astaxanthin recovery from Haematococcus pluvialis cyst cells using room-temperature imidazolium-based ionic liquid/water mixtures. Bioresour. Technol. 2019, 274, 120–126. [Google Scholar] [CrossRef]
- Ba, F.; Ursu, A.V.; Laroche, C.; Djelveh, G. Haematococcus pluvialis soluble proteins: Extraction, characterization, concentration/fractionation and emulsifying properties. Bioresour. Technol. 2016, 200, 147–152. [Google Scholar] [CrossRef] [PubMed]
- Molino, A.; Mehariya, S.; Di Sanzo, G.; Larocca, V.; Martino, M.; Leone, G.P.; Marino, T.; Chianese, S.; Balducchi, R.; Musmarra, D. Recent developments in supercritical fluid extraction of bioactive compounds from microalgae: Role of key parameters, technological achievements and challenges. J. CO2 Util. 2020, 36, 196–209. [Google Scholar] [CrossRef]
- Molino, A.; Mehariya, S.; Karatza, D.; Chianese, S.; Iovine, A.; Casella, P.; Marino, T.; Musmarra, D. Bench-scale cultivation of microalgae scenedesmus almeriensis for CO2 capture and Lutein production. Energies 2019, 12, 2806. [Google Scholar] [CrossRef]
- Molino, A.; Mehariya, S.; Iovine, A.; Casella, P.; Marino, T.; Karatza, D.; Chianese, S.; Musmarra, D. Enhancing biomass and Lutein production from Scenedesmus almeriensis: Effect of carbon dioxide concentration and culture medium reuse. Front. Plant. Sci. 2020, 11. [Google Scholar] [CrossRef]
- Molino, A.; Rimauro, J.; Casella, P.; Cerbone, A.; Larocca, V.; Chianese, S.; Karatza, D.; Mehariya, S.; Ferraro, A.; Hristoforou, E.; et al. Extraction of astaxanthin from microalga Haematococcus pluvialis in red phase by using generally recognized as safe solvents and accelerated extraction. J. Biotechnol. 2018, 283, 51–61. [Google Scholar] [CrossRef]
- ISO UNI. 12966-2 Animal and Vegetables Fat and Oils. Gas Chromatography of Fatty Acid Methyl Esters. Part 2: Preparation of Methyl Esters of Fatty Acids; International Organization for Standardization: Geneva, Switzerland, 2011. [Google Scholar]
- ISO UNI. 12966-4. Animal and Vegetables Fat and Oils. Gas Chromatography of Fatty Acid Methyl Esters. Part 4: Determination by Capillary Chromatography; International Organization for Standardization: Geneva, Switzerland, 2015. [Google Scholar]
- Molino, A.; Mehariya, S.; Iovine, A.; Larocca, V.; Di Sanzo, G.; Martino, M.; Casella, P.; Chianese, S.; Musmarra, D. Extraction of astaxanthin and lutein from microalga Haematococcus pluvialis in the red phase using CO2 supercritical fluid extraction Technology with ethanol as Co-Solvent. Mar. Drugs 2018, 16, 432. [Google Scholar] [CrossRef]
- Mehariya, S.; Iovine, A.; Di Sanzo, G.; Larocca, V.; Martino, M.; Leone, G.; Casella, P.; Karatza, D.; Marino, T.; Musmarra, D.; et al. Supercritical fluid extraction of lutein from Scenedesmus almeriensis. Molecules 2019, 24, 1324. [Google Scholar] [CrossRef]
- Casella, P.; Musmarra, D.; Dimatteo, S.; Chianese, S.; Karatza, D.; Mehariya, S.; Molino, A. Purification of astaxanthin from microalgae by using commercial activated carbon. Chem. Eng. Trans. 2020, 79, 295–300. [Google Scholar] [CrossRef]
- Sanzo, G.D.; Mehariya, S.; Martino, M.; Larocca, V.; Casella, P.; Chianese, S.; Musmarra, D.; Balducchi, R.; Molino, A. Supercritical carbon dioxide extraction of Astaxanthin, Lutein, and Fatty Acids from Haematococcus pluvialis microalgae. Mar. Drugs 2018, 16, 334. [Google Scholar] [CrossRef] [PubMed]
- Steinbrenner, J.; Linden, H. Regulation of two carotenoid biosynthesis genes coding for phytoene synthase and carotenoid hydroxylase during stress-induced astaxanthin formation in the green alga Haematococcus pluvialis. Plant Physiol. 2001, 125, 810–817. [Google Scholar] [CrossRef] [PubMed]
- Lababpour, A.; Shimahara, K.; Hada, K.; Kyoui, Y.; Katsuda, T.; Katoh, S. Fed-batch culture under illumination with blue light emitting diodes (LEDs) for astaxanthin production by Haematococcus pluvialis. J. Biosci. Bioeng. 2005, 100, 339–342. [Google Scholar] [CrossRef]
- Lam, M.K.; Lee, K.T. Potential of using organic fertilizer to cultivate Chlorella vulgaris for biodiesel production. Appl. Energy 2012, 94, 303–308. [Google Scholar] [CrossRef]
- Deniz, I. Scaling-up of Haematococcus pluvialis production in stirred tank photobioreactor. Bioresour. Technol. 2020, 123434. [Google Scholar] [CrossRef]
- Christian, D.; Zhang, J.; Sawdon, A.J.; Peng, C.-A. Enhanced astaxanthin accumulation in Haematococcus pluvialis using high carbon dioxide concentration and light illumination. Bioresour. Technol. 2018, 256, 548–551. [Google Scholar] [CrossRef]
- Wan, M.; Hou, D.; Li, Y.; Fan, J.; Huang, J.; Liang, S.; Wang, W.; Pan, R.; Wang, J.; Li, S. The effective photoinduction of Haematococcus pluvialis for accumulating astaxanthin with attached cultivation. Bioresour. Technol. 2014, 163, 26–32. [Google Scholar] [CrossRef]
- Zhang, W.; Wang, J.; Wang, J.; Liu, T. Attached cultivation of Haematococcus pluvialis for astaxanthin production. Bioresour. Technol. 2014, 158, 329–335. [Google Scholar] [CrossRef]
- Kim, J.Y.; Lee, C.; Jeon, M.S.; Park, J.; Choi, Y.-E. Enhancement of microalga Haematococcus pluvialis growth and astaxanthin production by electrical treatment. Bioresour. Technol. 2018, 268, 815–819. [Google Scholar] [CrossRef]








| Nutrients | 1st GP-C (mM) | 1st GP-Q (mg) | 2nd GP-C (mM) | 2nd GP-Q (mg) |
|---|---|---|---|---|
| Mg2+ | 0.22 ± 0.01 | 36.12 ± 1.63 | 0.21 ± 0.01 | 55.12 ± 2.65 |
| SO42− | 0.12 ± 0.01 | 92.68 ± 4.63 | 0.11 ± 0.01 | 106.71 ± 5.34 |
| Na+ | 10.36 ± 0.26 a | 5049.8 ± 126.25 | 7.98 ± 0.36 a | 4842.6 ± 217.92 |
| NO3− | 1.71 ± 0.07 b | 2256.8 ± 90.27 | 1.31 ± 0.06 b | 2136.2 ± 96.13 |
| NO2− | 0.10 ± 0.00 | 56.00 ± 2.52 | 0.08 ± 0.00 | 49.64 ± 2.23 |
| Ca2+ | 1.25 ± 0.06 c | 597.52 ± 26.89 | 1.08 ± 0.05 c | 513.85 ± 3.12 |
| Cl− | 2.10 ± 0.08 d | 1449.6 ± 57.98 | 1.66 ± 0.08 d | 1359.5 ± 67.98 |
| K+ | 0.36 ± 0.02 | 223.16 ± 10.04 | 0.30 ± 0.02 | 187.9 ± 9.40 |
| PO43− | 0.17 ± 0.01 | 386.4 ± 17.39 | 0.12 ± 0.01 | 299.06 ± 14.95 |
| Nutrients | 1st RP-C (mM) | 1st RP-Q (mg) | 2nd RP-C (mM) | 2nd RP-Q (mg) |
|---|---|---|---|---|
| Mg2+ | 0.17 ± 0.01 | 3.6 ± 0.16 | 0.13 ± 0.01 | 0.23 ± 0.01 |
| SO42− | 0.09 ± 0.00 | 6.82 ± 0.34 | 0.07 ± 0.00 | 0.20 ± 0.01 |
| Na+ | 2.52 ± 0.06 a | 54.17 ± 1.35 a | 0.46 ± 0.02 a | 6.36 ± 0.29 a |
| NO3− | 0.41 ± 0.02 b | 28.99 ± 1.16 b | 0.08 ± 0.00 b | 0.20 ± 0.01 b |
| NO2− | 0.05 ± 0.00 | 0.68 ± 0.03 | 0.05 ± 0.00 | 0.35 ± 0.02 |
| Ca2+ | 0.71 ± 0.03 | 27.55 ± 1.24 | 0.63 ± 0.03 | 6.14 ± 0.28 |
| Cl− | 0.64 ± 0.03 c | 12.72 ± 0.51 c | 0.29 ± 0.01 c | 5.80 ± 0.29 c |
| K+ | 0.16 ± 0.01 | 5.71 ± 0.26 | 0.13 ± 0.01 | 2.33 ± 0.12 |
| PO43− | 0.02 ± 0.00 | 2.24 ± 0.10 | 0.01 ± 0.00 | 0.25 ± 0.01 |
| % FAs | Switch a to 2500 Lux | Switch b to 500 Lux |
|---|---|---|
| Butyric acid | 0.38 ± 0.02 | 0.86 ± 0.04 |
| Myristic acid | 0.21 ± 0.01 | 0.02 ± 0.00 |
| Palmitic acid | 35.48 ± 1.61 | 45.43 ± 2.22 |
| Pentadecanoic acid | 0.27 ± 0.01 | 0.62 ± 0.04 |
| Arachidic acid | 8.96 ± 0.32 | 6.28 ± 0.37 |
| Heneicosanoic | 0.59 ± 0.03 | 0.37 ± 0.01 |
| cis-10-Pentadecenoic acid | 0.27 ± 0.01 | 0.37 ± 0.01 |
| Palmitoleic acid | 0.38 ± 0.02 | 1.35 ± 0.07 |
| cis-10-Heptadecenoic acid | 0.38 ± 0.02 | 0.49 ± 0.02 |
| Elaidic acid | 2.04 ± 0.11 | 3.45 ± 0.12 |
| Myristoleic acid | 0.21 ± 0.01 | 0.86 ± 0.04 |
| cis-11-Eicosenoic acid | 9.02 ± 0.43 | 8.62 ± 0.37 |
| Linolenic acid | 0.05 ± 0.00 | 0.00 ± 0.00 |
| Linoelaidic acid | 40.79 ± 1.83 | 31.27 ± 1.60 |
| γ-Linolenic acid | 0.97 ± 0.05 | 0.00 ± 0.00 |
| Cultivation Conditions | Production of Bioactive Compounds (mg/g) | Reference | ||||||
|---|---|---|---|---|---|---|---|---|
| LI (Lux) | CT (Days) | WV (mL) | AFR (mL/min) | Astaxanthin | Lutein | β-Carotene | Fatty Acids | |
| 7290 | 15 | 400 | 120 | ~4 | na * | na | Na | [35] |
| 5832 | 15 | 400 | 120 | ~6.4 | na | na | Na | [36] |
| 5400 | 4 | 30 | ∼7 | 8.87 ± 2.7 | na | na | Na | [61] |
| 16,200 | 4 | 30 | ∼7 | 9.27 ± 1.0 | na | na | Na | [61] |
| 2500 | 14 | 1200 | 50 | 3.12 ± 0.1 | 1.03 ± 0.1 | 1.07 ± 0.1 | 19.62 ± 0.6 | This study |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mehariya, S.; Sharma, N.; Iovine, A.; Casella, P.; Marino, T.; Larocca, V.; Molino, A.; Musmarra, D. An Integrated Strategy for Nutraceuticals from Haematoccus pluvialis: From Cultivation to Extraction. Antioxidants 2020, 9, 825. https://doi.org/10.3390/antiox9090825
Mehariya S, Sharma N, Iovine A, Casella P, Marino T, Larocca V, Molino A, Musmarra D. An Integrated Strategy for Nutraceuticals from Haematoccus pluvialis: From Cultivation to Extraction. Antioxidants. 2020; 9(9):825. https://doi.org/10.3390/antiox9090825
Chicago/Turabian StyleMehariya, Sanjeet, Neeta Sharma, Angela Iovine, Patrizia Casella, Tiziana Marino, Vincenzo Larocca, Antonio Molino, and Dino Musmarra. 2020. "An Integrated Strategy for Nutraceuticals from Haematoccus pluvialis: From Cultivation to Extraction" Antioxidants 9, no. 9: 825. https://doi.org/10.3390/antiox9090825
APA StyleMehariya, S., Sharma, N., Iovine, A., Casella, P., Marino, T., Larocca, V., Molino, A., & Musmarra, D. (2020). An Integrated Strategy for Nutraceuticals from Haematoccus pluvialis: From Cultivation to Extraction. Antioxidants, 9(9), 825. https://doi.org/10.3390/antiox9090825










