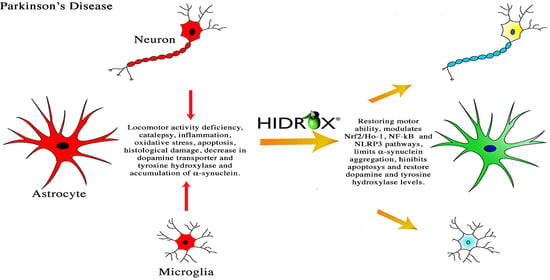
Anti-inflammatory and Anti-oxidant Activity of Hidrox® in Rotenone-Induced Parkinson’s Disease in Mice
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Rotenone-Induced PD and Treatment
2.3. Experimental Group
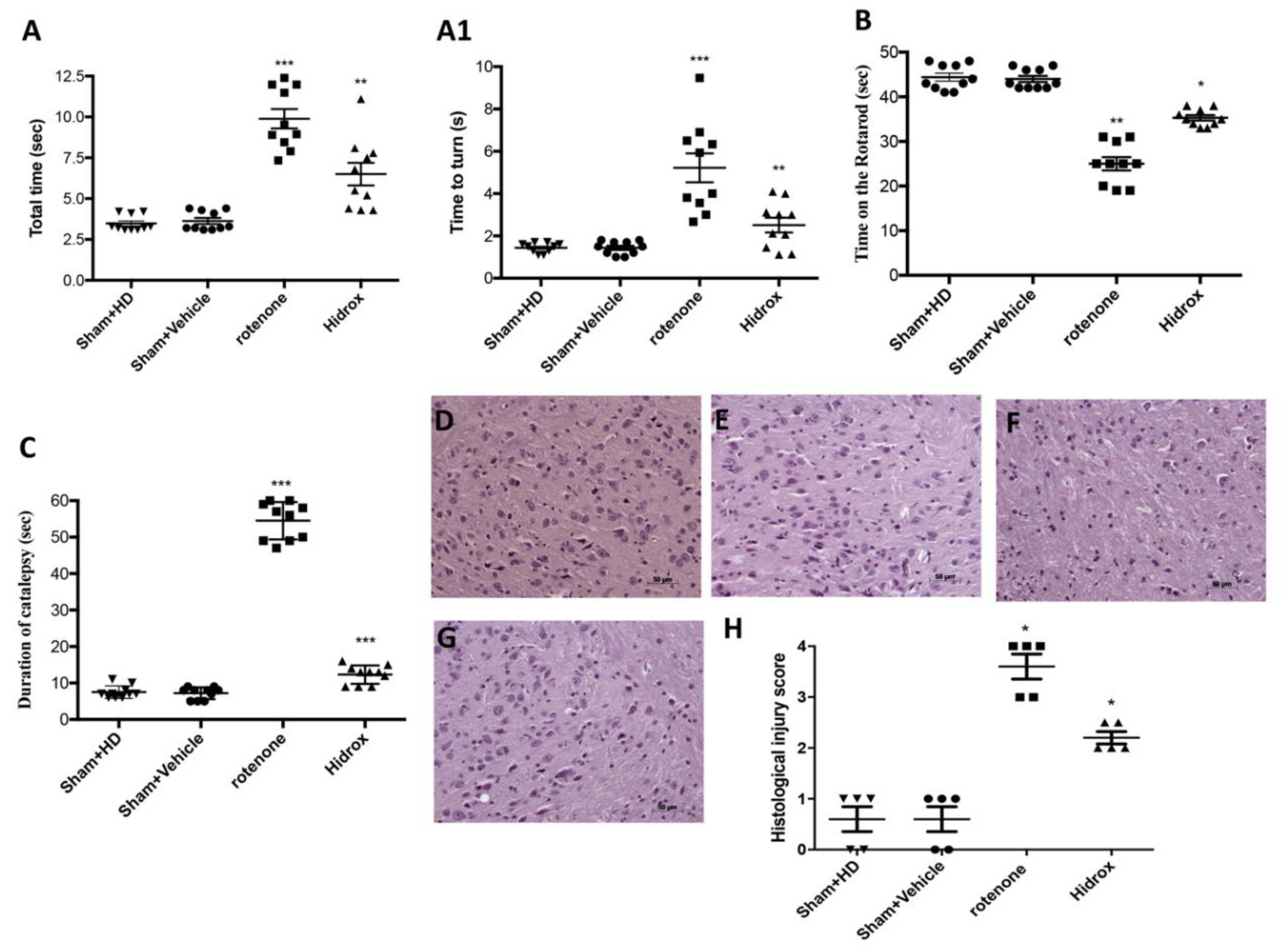
2.4. Behavioral Testing
2.5. Pole Test
2.6. Rotarod Test
2.7. Catalepsy Test
2.8. Histology
2.9. Stereological Analysis
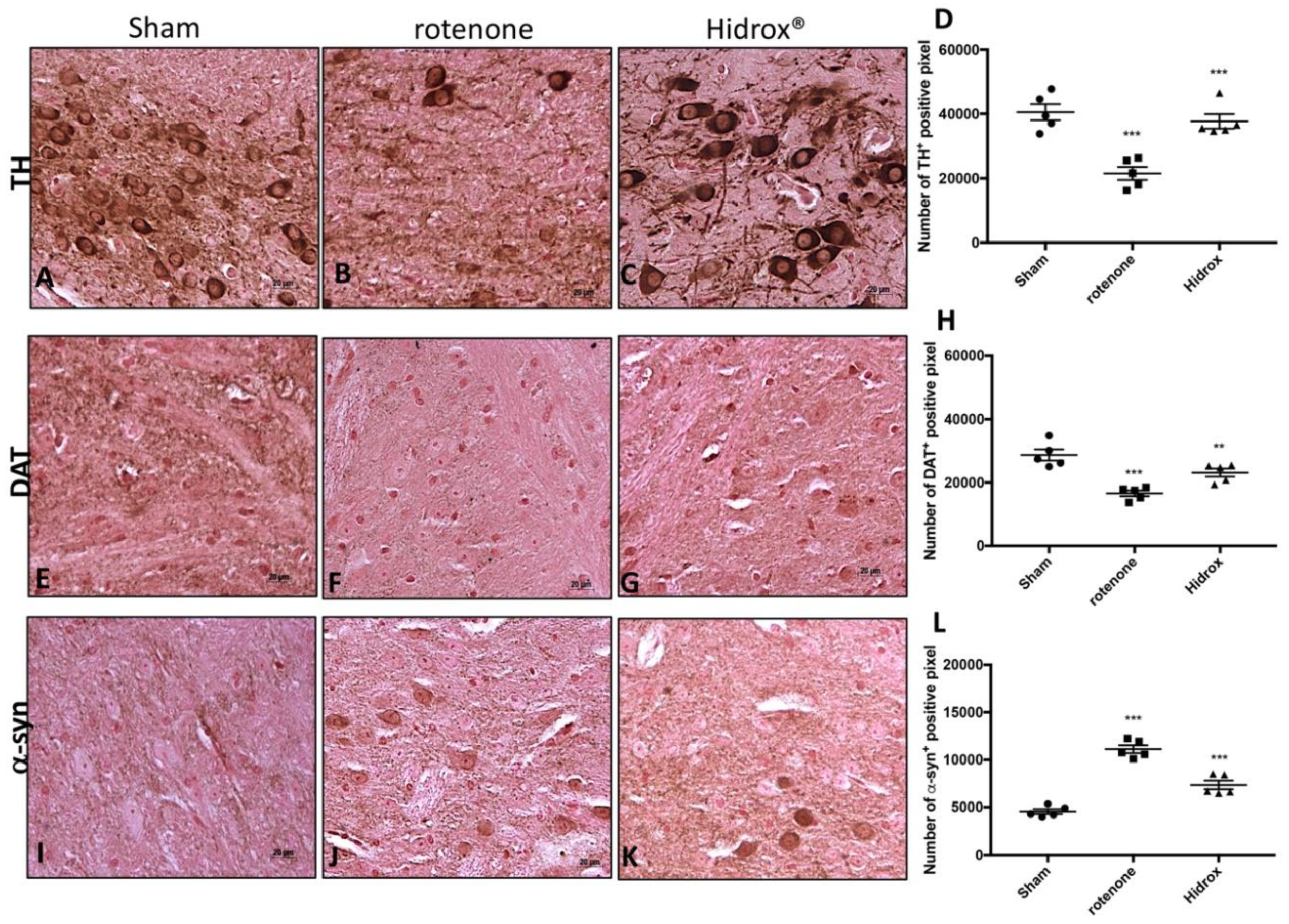
2.10. Immunohistochemical Localization of Tyrosine Hydroxylase (TH), Dopamine Transporter (DAT) and α-Synuclein (α-syn)
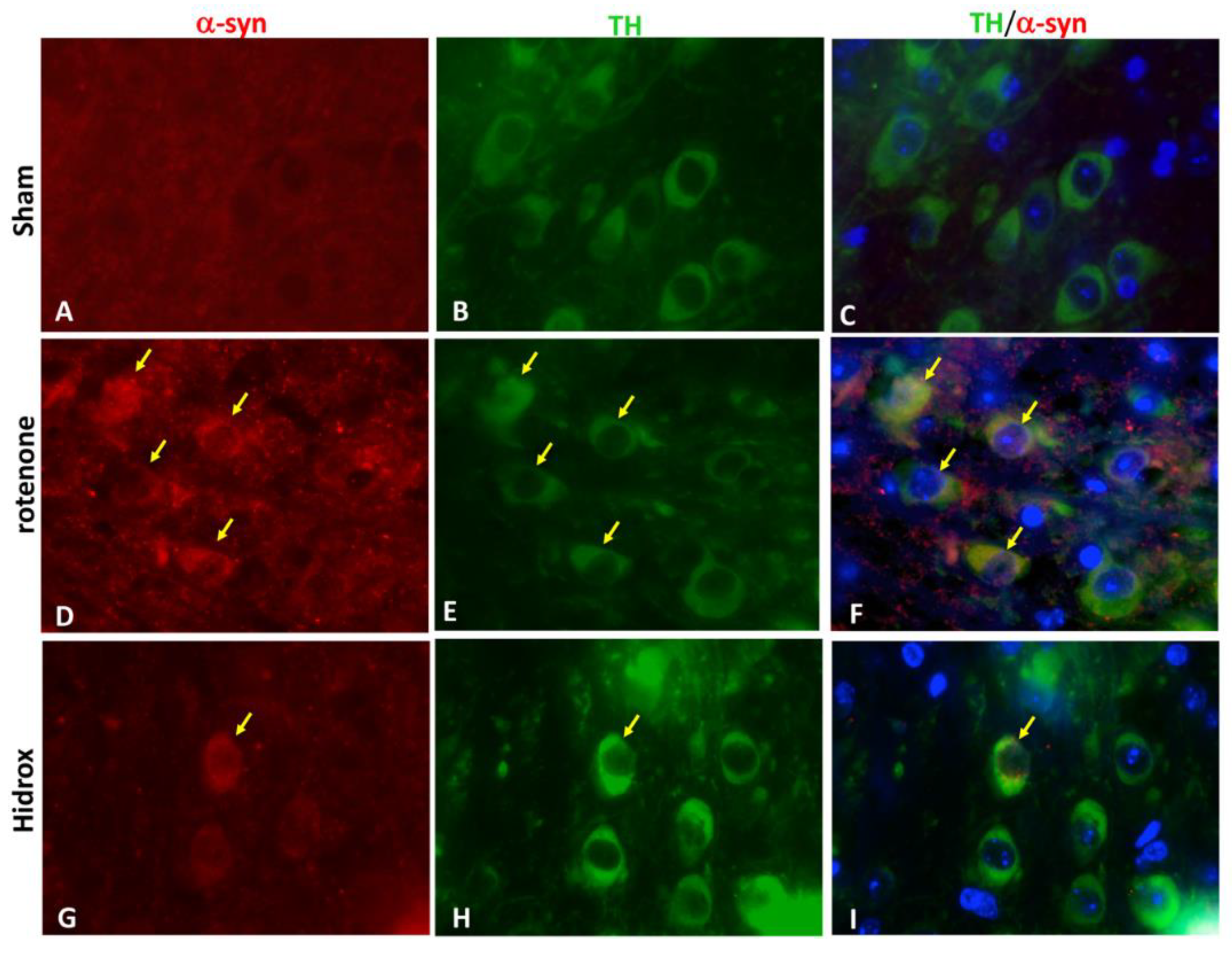
2.11. Immunofluorescence Co-localization of TH/α-syn
2.12. Western Blot Analysis for IkB-α, NF-kB, Bax, Bcl-2, iNOS, NRLP3, ASC, Caspase-1, IL-18, IL-1β, Hsp70, Sirt-1 and HO1
2.13. Protein Carbonyl Assay
2.14. Statistical Evaluation
3. Results
3.1. Effect of HD Treatment on Behavioral Impairments and on the Neuronal Degeneration of the Dopaminergic Tract Induced by Rotenone Administration
3.2. HD Treatment Reduced the Loss of TH, DAT and α-Synuclein Expression in the SN Induced by Rotenone Administration
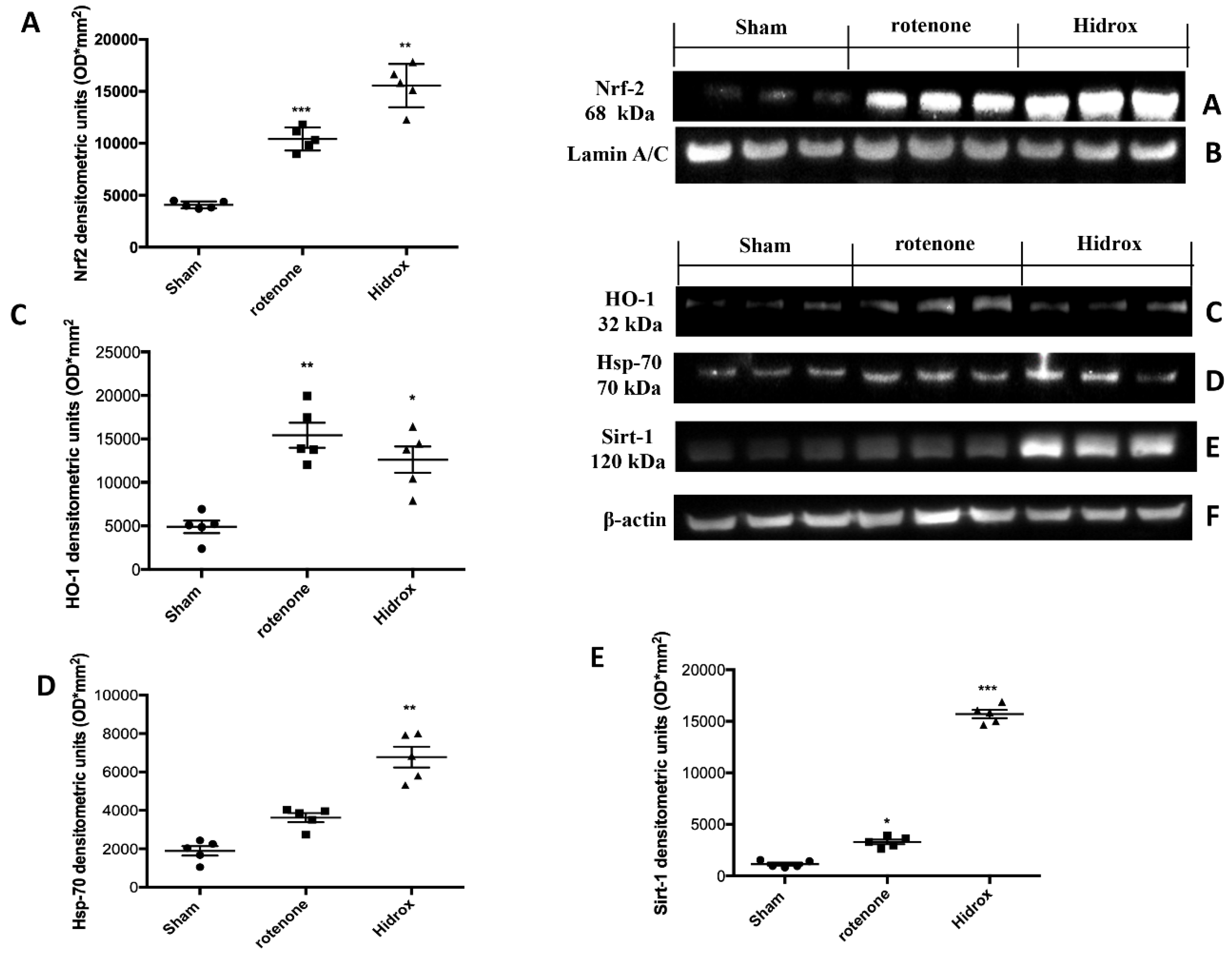
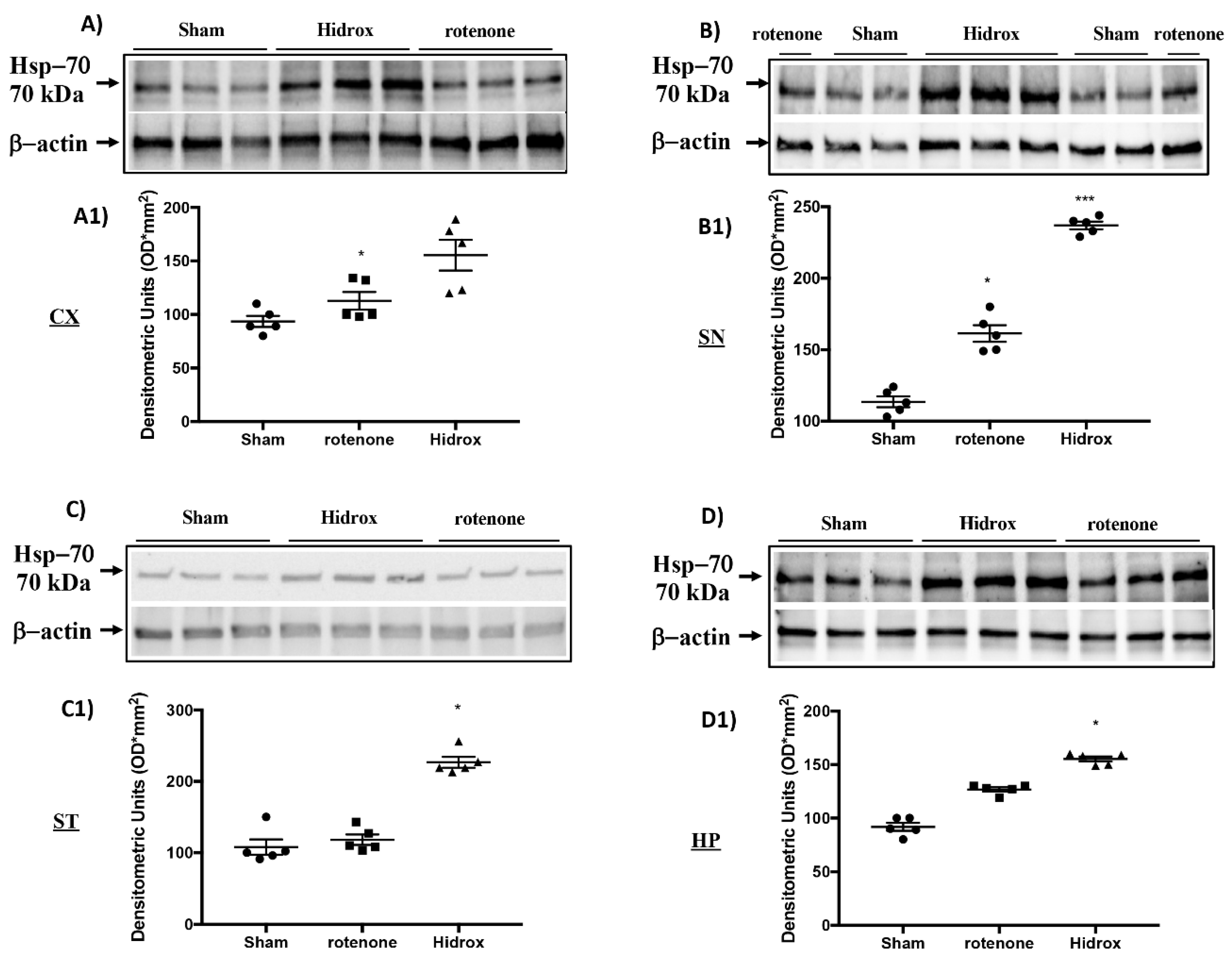
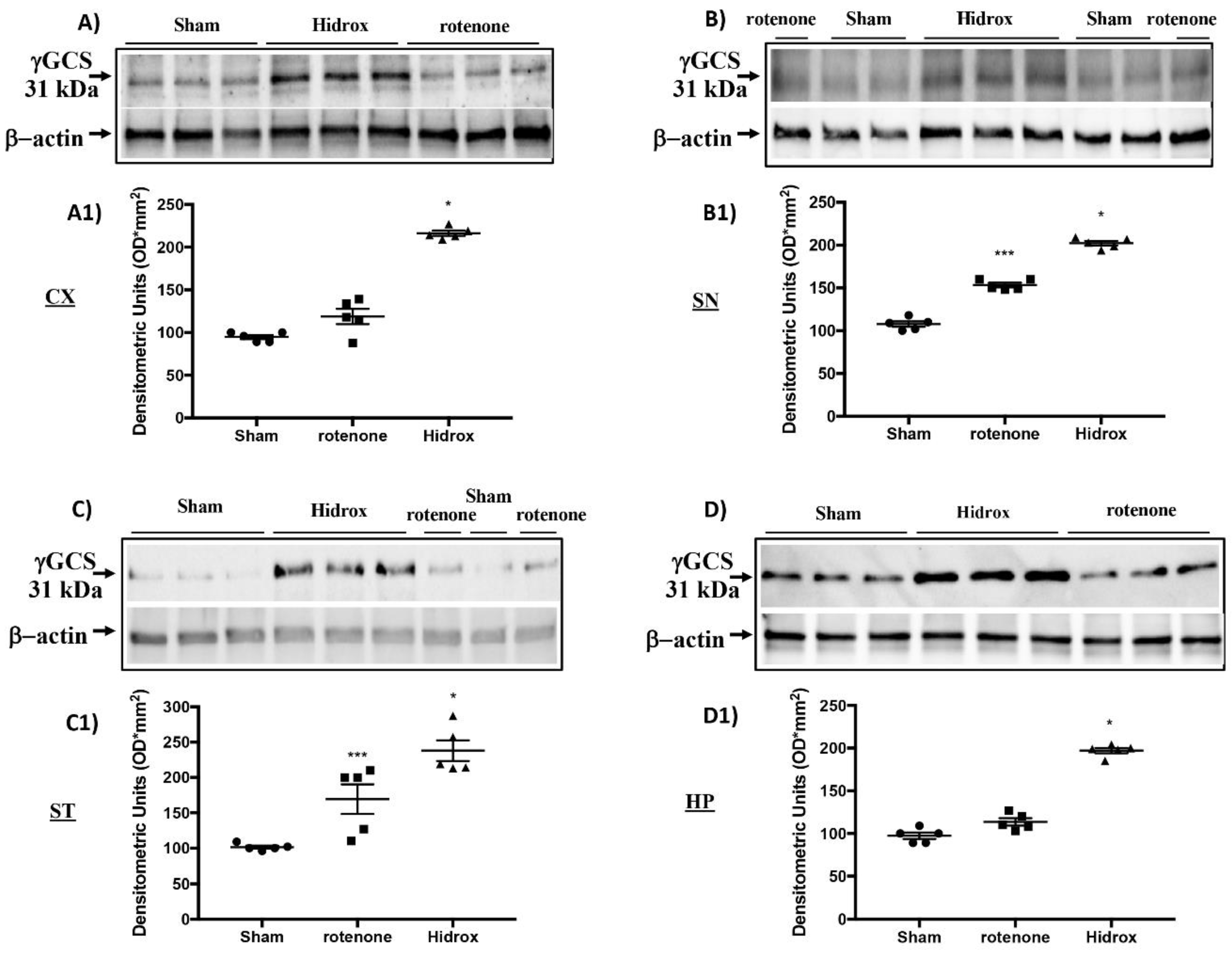
3.3. Effect of HD on Cellular Stress Response after Rotenone Administration
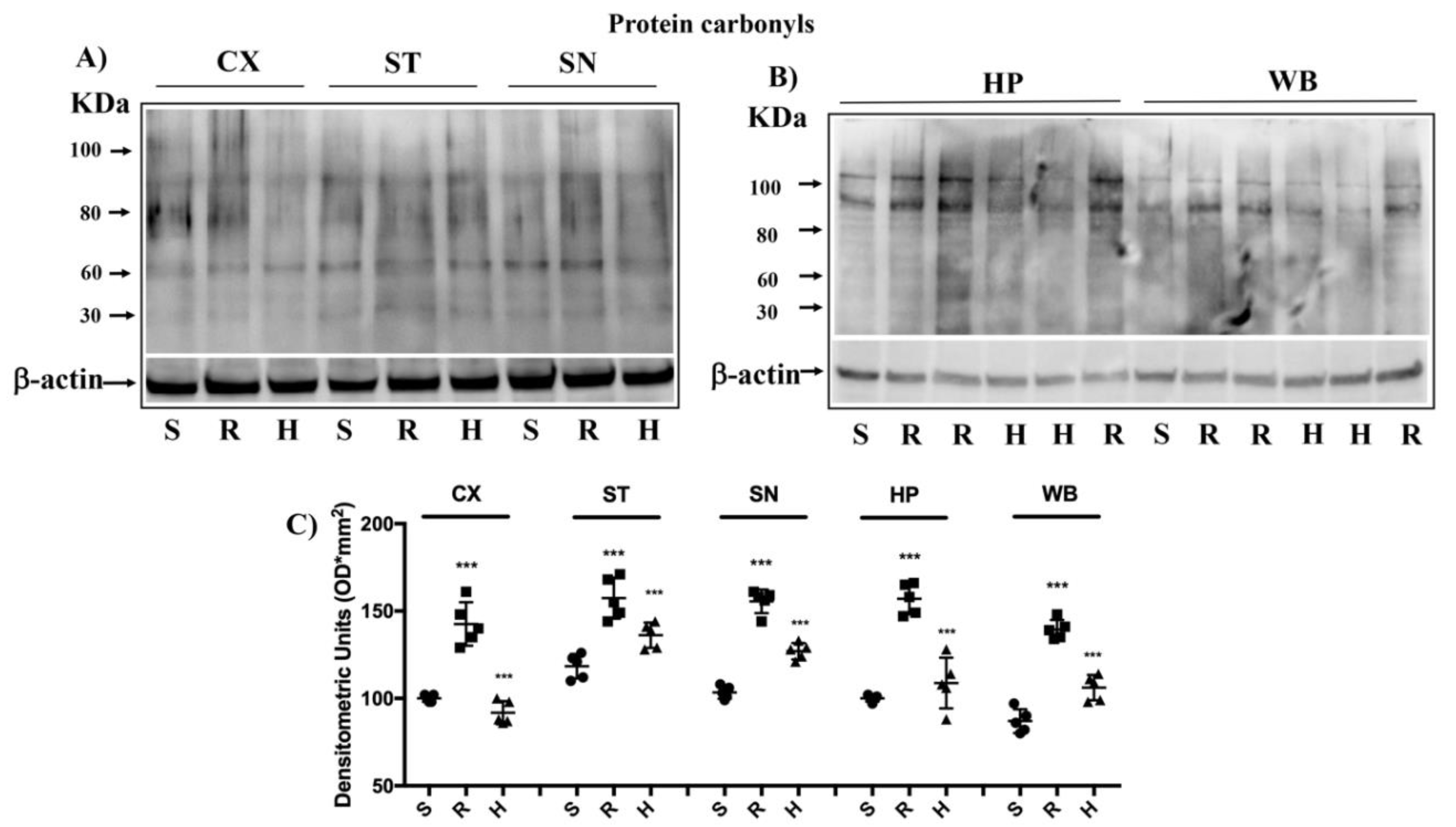
3.4. Modulation of Protein Carbonyls in Mice Brain after Rotenone Treatment and HD Supplementation
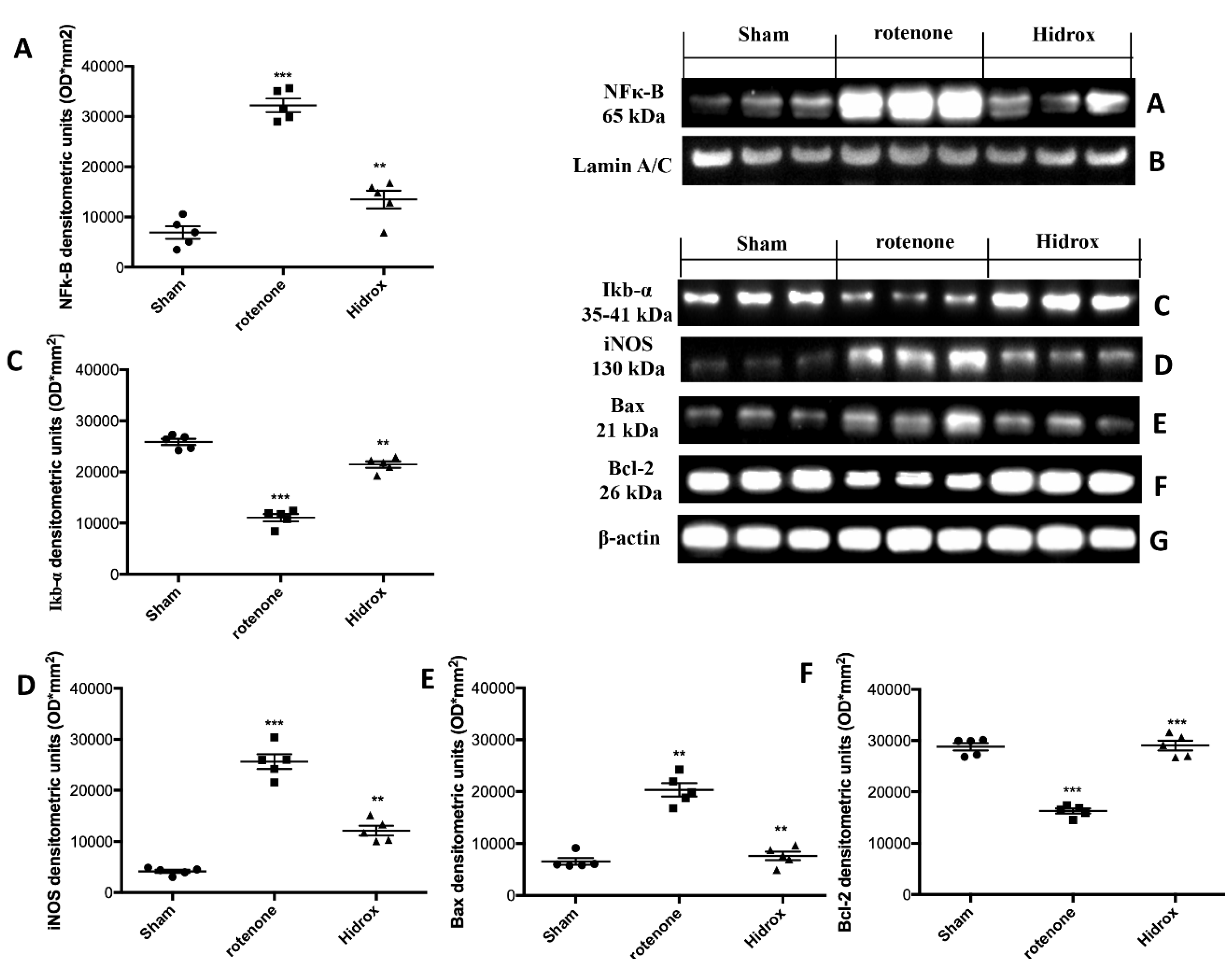
3.5. Effect of HD Treatment on NF-κB, IκB-α, iNOS Expression and on Apoptosis Induced by Rotenone Administration
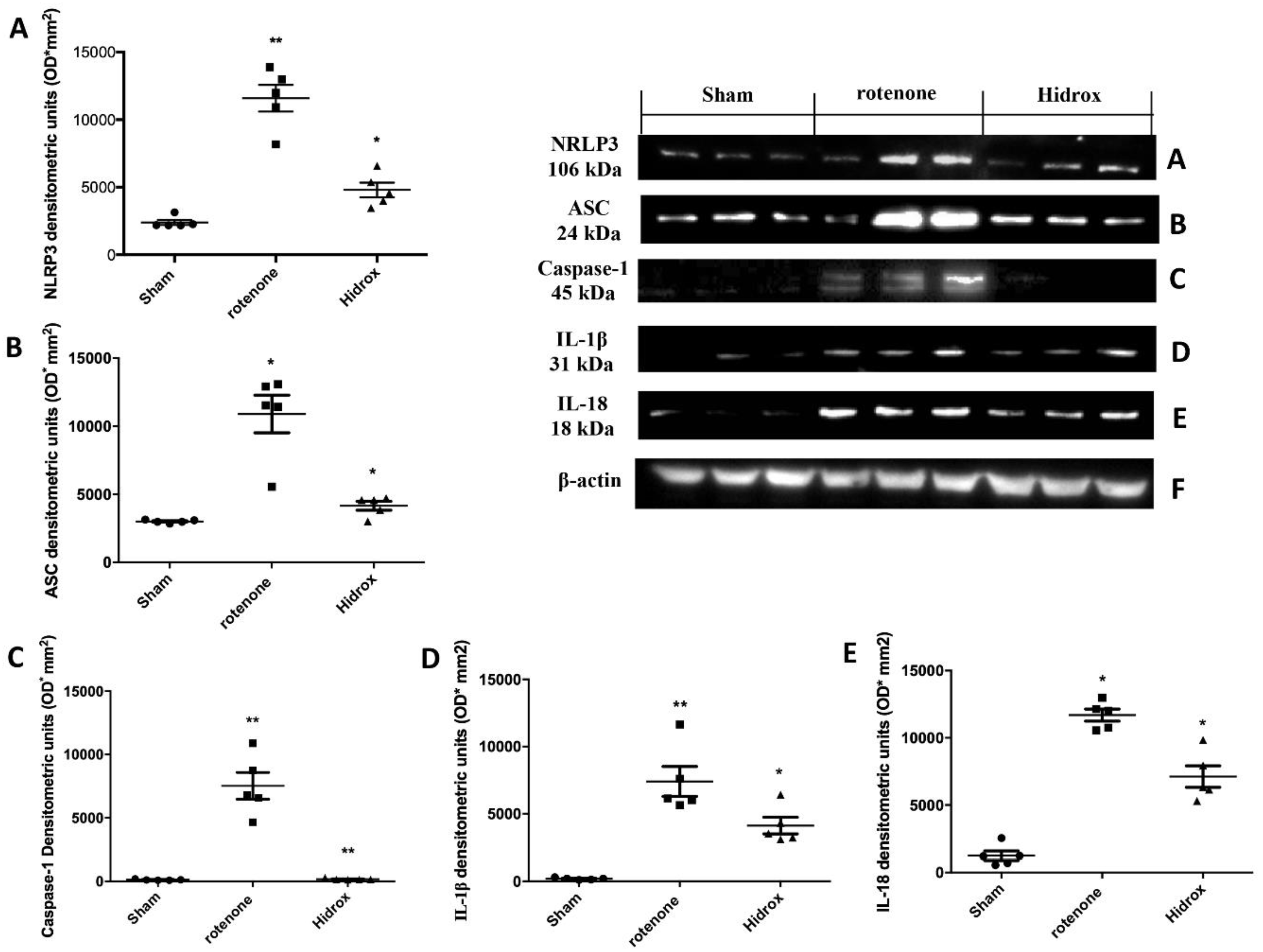
3.6. Effect of HD Treatment on Inflammasome Pathway and Caspase-1, IL-1β, IL18 Expression Induced by Rotenone Administration
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Shimohama, S.; Sawada, H.; Kitamura, Y.; Taniguchi, T. Disease model: Parkinson’s disease. Trends Mol. Med. 2003, 9, 360–365. [Google Scholar] [CrossRef]
- Spillantini, M.G.; Schmidt, M.L.; Lee, V.M.-Y.; Trojanowski, J.Q.; Jakes, R.; Goedert, M. α-Synuclein in Lewy bodies. Nature 1997, 388, 839–840. [Google Scholar] [CrossRef]
- Duda, J.E.; Lee, V.M.-Y.; Trojanowski, J.Q. Neuropathology of synuclein aggregates. J. Neurosci. Res. 2000, 61, 121–127. [Google Scholar] [CrossRef]
- Ha, D.; Stone, D.K.; Mosley, R.L.; Gendelman, H.E. Immunization strategies for Parkinson’s disease. Park. Relat. Disord. 2012, 18 (Suppl. 1), S218–S221. [Google Scholar] [CrossRef]
- Hirsch, E.C.; Hunot, S. Neuroinflammation in Parkinson’s disease: A target for neuroprotection? Lancet Neurol. 2009, 8, 382–397. [Google Scholar] [CrossRef]
- Nalbandian, A.; Khan, A.A.; Srivastava, R.; Llewellyn, K.J.; Tan, B.; Shukr, N.; Fazli, Y.; Kimonis, V.E.; Benmohamed, L. Activation of the NLRP3 Inflammasome Is Associated with Valosin-Containing Protein Myopathy. Inflammation 2016, 40, 21–41. [Google Scholar] [CrossRef]
- Siracusa, R.; Paterniti, I.; Impellizzeri, D.; Cordaro, M.; Crupi, R.; Navarra, M.; Cuzzocrea, S.; Esposito, E. The association of palmitoylethanolamide with luteolin decreases neuroinflammation and stimulates autophagy in Parkinson’s disease model. CNS Neurol. Disord. Drug Targets 2015, 14, 1350–1366. [Google Scholar] [CrossRef]
- Nagatsu, T.; Mogi, M.; Ichinose, H.; Togari, A. Cytokines in Parkinson’s disease. J. Neural Transm. Suppl. 2000, 143–151. [Google Scholar] [CrossRef]
- Jenner, P. Oxidative mechanisms in nigral cell death in Parkinson’s disease. Mov. Disord. 1998, 13 (Suppl. 1), 24–34. [Google Scholar]
- Greenamyre, J.T.; MacKenzie, G.; Peng, T.I.; Stephans, S.E. Mitochondrial dysfunction in Parkinson’s disease. Biochem. Soc. Symp. 1999, 66, 85–97. [Google Scholar]
- Tarozzi, A.; Angeloni, C.; Malaguti, M.; Morroni, F.; Hrelia, S.; Hrelia, P. Sulforaphane as a potential protective phytochemical against neurodegenerative diseases. Oxidative Med. Cell. Longev. 2013, 2013, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Vauzour, D.; Ravaioli, G.; Vafeiadou, K.; Rodriguez-Mateos, A.; Angeloni, C.; Spencer, J.P. Peroxynitrite induced formation of the neurotoxins 5-S-cysteinyl-dopamine and DHBT-1: Implications for Parkinson’s disease and protection by polyphenols. Arch. Biochem. Biophys. 2008, 476, 145–151. [Google Scholar] [CrossRef] [PubMed]
- Aruoma, O.I.; Bahorun, T.; Jen, L.-S. Neuroprotection by bioactive components in medicinal and food plant extracts. Mutat. Res. 2003, 544, 203–215. [Google Scholar] [CrossRef] [PubMed]
- Vauzour, D.; Vafeiadou, K.; Rodriguez-Mateos, A.; Rendeiro, C.; Spencer, J.P. The neuroprotective potential of flavonoids: A multiplicity of effects. Genes Nutr. 2008, 3, 115–126. [Google Scholar] [CrossRef]
- Kelsey, N.A.; Wilkins, H.M.; Linseman, D.A. Nutraceutical antioxidants as novel neuroprotective agents. Molecules 2010, 15, 7792–7814. [Google Scholar] [CrossRef]
- Singh, A.; Tripathi, P.; Yadawa, A.K.; Singh, S. Promising polyphenols in Parkinson’s disease therapeutics. Neurochem. Res. 2020, 45, 1731–1745. [Google Scholar] [CrossRef]
- Robles-Almazan, M.; Pulido-Morán, M.; Moreno-Fernandez, J.; Ramirez-Tortosa, C.; Rodríguez-García, C.; Quiles, J.L.; Ramirez-Tortosa, M. Hydroxytyrosol: Bioavailability, toxicity, and clinical applications. Food Res. Int. 2018, 105, 654–667. [Google Scholar] [CrossRef]
- Hoffman, R.; Gerber, M. Food processing and the Mediterranean diet. Nutrients 2015, 7, 7925–7964. [Google Scholar] [CrossRef]
- Angeloni, C.; Malaguti, M.; Barbalace, M.C.; Hrelia, S. Bioactivity of olive oil phenols in Neuroprotection. Int. J. Mol. Sci. 2017, 18, 2230. [Google Scholar] [CrossRef]
- Peyrol, J.; Riva, C.; Amiot, M.-J. Hydroxytyrosol in the prevention of the metabolic syndrome and related disorders. Nutrients 2017, 9, 306. [Google Scholar] [CrossRef]
- Féart, C.; Samieri, C.; Barberger-Gateau, P. Mediterranean diet and cognitive function in older adults. Curr. Opin. Clin. Nutr. Metab. Care 2010, 13, 14–18. [Google Scholar] [CrossRef] [PubMed]
- Scarmeas, N.; Stern, Y.; Mayeux, R.; Manly, J.J.; Schupf, N.; Luchsinger, J.A. Mediterranean diet and mild cognitive impairment. Arch. Neurol. 2009, 66, 216–225. [Google Scholar] [CrossRef] [PubMed]
- Tasset, I.; Pontes, A.J.; Hinojosa, A.J.; De La Torre, R.; Tunez, I. Olive oil reduces oxidative damage in a 3-nitropropionic acid-induced Huntington’s disease-like rat model. Nutr. Neurosci. 2011, 14, 106–111. [Google Scholar] [CrossRef] [PubMed]
- Grossi, C.; Rigacci, S.; Ambrosini, S.; Dami, T.E.; Luccarini, I.; Traini, C.; Failli, P.; Berti, A.; Casamenti, F.; Stefani, M. The polyphenol Oleuropein Aglycone Protects TgCRND8 Mice against Aß plaque pathology. PLoS ONE 2013, 8, e71702. [Google Scholar] [CrossRef]
- Casamenti, F.; Grossi, C.; Rigacci, S.; Pantano, D.; Luccarini, I.; Stefani, M. Oleuropein Aglycone: A possible drug against degenerative conditions. In vivo evidence of its effectiveness against Alzheimer’s disease. J. Alzheimer’s Dis. 2015, 45, 679–688. [Google Scholar] [CrossRef]
- Arunsundar, M.; Shanmugarajan, T.S.; Ravichandran, V. 3,4-Dihydroxyphenylethanol attenuates Spatio-cognitive deficits in an Alzheimer’s disease mouse model: Modulation of the molecular signals in neuronal survival-apoptotic programs. Neurotox. Res. 2014, 27, 143–155. [Google Scholar] [CrossRef]
- Leri, M.; Scuto, M.; Ontario, M.L.; Calabrese, V.; Calabrese, V.; Bucciantini, M.; Stefani, M. Calabrese healthy effects of plant polyphenols: Molecular mechanisms. Int. J. Mol. Sci. 2020, 21, 1250. [Google Scholar] [CrossRef]
- Montoya, T.; Aparicio-Soto, M.; Castejón, M.L.; Rosillo, M.Á.; Sanchez-Hidalgo, M.; Begines, P.; Fernández-Bolaños, J.G.; Alarcón, C.; Sánchez, M. Peracetylated hydroxytyrosol, a new hydroxytyrosol derivate, attenuates LPS-induced inflammatory response in murine peritoneal macrophages via regulation of non-canonical inflammasome, Nrf2/HO1 and JAK/STAT signaling pathways. J. Nutr. Biochem. 2018, 57, 110–120. [Google Scholar] [CrossRef]
- Peng, S.; Zhang, B.; Yao, J.; Duan, D.; Fang, J. Dual protection of hydroxytyrosol, an olive oil polyphenol, against oxidative damage in PC12 cells. Food Funct. 2015, 6, 2091–2100. [Google Scholar] [CrossRef]
- Kim, S.; Viswanath, A.N.I.; Park, J.-H.; Lee, H.E.; Park, A.; Choi, J.W.; Kim, H.J.; Londhe, A.M.; Jang, B.K.; Lee, J.; et al. Nrf2 activator via interference of Nrf2-Keap1 interaction has antioxidant and anti-inflammatory properties in Parkinson’s disease animal model. Neuropharmacology 2020, 167, 107989. [Google Scholar] [CrossRef]
- Calabrese, V.; Cornelius, C.; Dinkova-Kostova, A.T.; Calabrese, E.J.; Mattson, M.P. Cellular stress responses, the hormesis paradigm, and vitagenes: Novel targets for therapeutic intervention in neurodegenerative disorders. Antioxid. Redox Signal. 2010, 13, 1763–1811. [Google Scholar] [CrossRef] [PubMed]
- Calabrese, E.J.; Calabrese, V.; Tsatsakis, A.; Giordano, J. Hormesis and Ginkgo biloba (GB): Numerous biological effects of GB are mediated via hormesis. Ageing Res. Rev. 2020, 101019. [Google Scholar] [CrossRef] [PubMed]
- Gorell, J.M.; Johnson, C.C.; Rybicki, B.A.; Peterson, E.L.; Richardson, R.J. The risk of Parkinson’s disease with exposure to pesticides, farming, well water, and rural living. Neurology 1998, 50, 1346–1350. [Google Scholar] [CrossRef] [PubMed]
- Betarbet, R.; Sherer, T.B.; MacKenzie, G.; Garcia-Osuna, M.; Panov, A.V.; Greenamyre, J.T. Chronic systemic pesticide exposure reproduces features of Parkinson’s disease. Nat. Neurosci. 2000, 3, 1301–1306. [Google Scholar] [CrossRef]
- Chinta, S.J.; A Lieu, C.; DeMaria, M.; Laberge, R.-M.; Campisi, J.; Andersen, J.K. Environmental stress, ageing and glial cell senescence: A novel mechanistic link to Parkinson’s disease? J. Intern. Med. 2013, 273, 429–436. [Google Scholar] [CrossRef]
- Sherer, T.B.; Betarbet, R.; Kim, J.H.; Greenamyre, J.T. Selective microglial activation in the rat rotenone model of Parkinson’s disease. Neurosci. Lett. 2003, 341, 87–90. [Google Scholar] [CrossRef]
- Yadava, N.; Nicholls, D.G. Spare respiratory capacity rather than oxidative stress regulates glutamate Excitotoxicity after partial respiratory inhibition of mitochondrial complex I with rotenone. J. Neurosci. 2007, 27, 7310–7317. [Google Scholar] [CrossRef]
- Soni, M.; Burdock, G.; Christian, M.; Bitler, C.; Crea, R. Safety assessment of aqueous olive pulp extract as an antioxidant or antimicrobial agent in foods. Food Chem. Toxicol. 2006, 44, 903–915. [Google Scholar] [CrossRef]
- Miraglia, N.; Bianchi, D.; Trentin, A.; Volpi, N.; Soni, M.G. Safety assessment of non-animal chondroitin sulfate sodium: Subchronic study in rats, genotoxicity tests and human bioavailability. Food Chem. Toxicol. 2016, 93, 89–101. [Google Scholar] [CrossRef]
- Liu, Y.; Sun, J.-D.; Song, L.-K.; Li, J.; Chu, S.-F.; Yuan, Y.-H.; Chen, N.-H. Environment-contact administration of rotenone: A new rodent model of Parkinson’s disease. Behav. Brain Res. 2015, 294, 149–161. [Google Scholar] [CrossRef]
- Zheng, A.; Li, H.; Cao, K.; Xu, J.; Zou, X.; Li, Y.; Chen, C.; Liu, J.; Feng, Z. Maternal hydroxytyrosol administration improves neurogenesis and cognitive function in prenatally stressed offspring. J. Nutr. Biochem. 2015, 26, 190–199. [Google Scholar] [CrossRef] [PubMed]
- Sedelis, M.; Schwarting, R.K.; Huston, J.P. Behavioral phenotyping of the MPTP mouse model of Parkinson’s disease. Behav. Brain Res. 2001, 125, 109–125. [Google Scholar] [CrossRef]
- Cordaro, M.; Siracusa, R.; Crupi, R.; Impellizzeri, D.; Peritore, A.F.; D’Amico, R.; Gugliandolo, E.; Di Paola, R.; Cuzzocrea, S. 2-Pentadecyl-2-Oxazoline Reduces Neuroinflammatory Environment in the MPTP Model of Parkinson Disease. Mol. Neurobiol. 2018, 55, 9251–9266. [Google Scholar] [CrossRef] [PubMed]
- Fleming, S.M.; Mulligan, C.K.; Richter, F.; Mortazavi, F.; Lemesre, V.; Frias, C.; Zhu, C.; Stewart, A.; Gozes, I.; Morimoto, B.; et al. A pilot trial of the microtubule-interacting peptide (NAP) in mice overexpressing alpha-synuclein shows improvement in motor function and reduction of alpha-synuclein inclusions. Mol. Cell. Neurosci. 2011, 46, 597–606. [Google Scholar] [CrossRef]
- Siracusa, R.; Paterniti, I.; Cordaro, M.; Crupi, R.; Bruschetta, G.; Campolo, M.; Cuzzocrea, S.; Esposito, E. Neuroprotective effects of Temsirolimus in animal models of Parkinson’s disease. Mol. Neurobiol. 2017, 55, 2403–2419. [Google Scholar] [CrossRef]
- Araki, T.; Kumagai, T.; Tanaka, K.; Matsubara, M.; Kato, H.; Itoyama, Y.; Imai, Y. Neuroprotective effect of riluzole in MPTP-treated mice. Brain Res. 2001, 918, 176–181. [Google Scholar] [CrossRef]
- Paterniti, I.; Campolo, M.; Siracusa, R.; Cordaro, M.; Di Paola, R.; Calabrese, V.; Navarra, M.; Cuzzocrea, S.; Esposito, E. Liver X receptors activation, through TO901317 binding, reduces neuroinflammation in Parkinson’s disease. PLoS ONE 2017, 12, e0174470. [Google Scholar] [CrossRef]
- Kawai, T.; Akira, S. Signaling to NF-κB by Toll-like receptors. Trends Mol. Med. 2007, 13, 460–469. [Google Scholar] [CrossRef]
- Lee, K.-W.; Zhao, X.; Im, J.-Y.; Grosso, H.; Jang, W.H.; Chan, T.W.; Sonsalla, P.K.; German, D.C.; Ichijo, H.; Junn, E.; et al. Apoptosis signal-regulating kinase 1 mediates MPTP toxicity and regulates glial activation. PLoS ONE 2012, 7, e29935. [Google Scholar] [CrossRef]
- Paterniti, I.; Briguglio, E.; Mazzon, E.; Galuppo, M.; Oteri, G.; Cordasco, G.; Cuzzocrea, S. Effects of Hypericum Perforatum, in a rodent model of periodontitis. BMC Complement. Altern. Med. 2010, 10, 73. [Google Scholar] [CrossRef]
- Cordaro, M.; Impellizzeri, D.; Gugliandolo, E.; Siracusa, R.; Crupi, R.; Esposito, E.; Cuzzocrea, S. Adelmidrol, a Palmitoylethanolamide analogue, as a new pharmacological treatment for the management of inflammatory bowel disease. Mol. Pharmacol. 2016, 90, 549–561. [Google Scholar] [CrossRef] [PubMed]
- Sawant, S.; Gokulan, R.; Dongre, H.; Vaidya, M.; Chaukar, D.; Prabhash, K.; Ingle, A.; Joshi, S.; Dange, P.; Joshi, S.; et al. Prognostic role of Oct4, CD44 and c-Myc in radio–chemo-resistant oral cancer patients and their tumourigenic potential in immunodeficient mice. Clin. Oral Investig. 2015, 20, 43–56. [Google Scholar] [CrossRef] [PubMed]
- Varghese, F.; Bukhari, A.B.; Malhotra, R.; De, A. IHC profiler: An open source plugin for the quantitative evaluation and automated scoring of immunohistochemistry images of human tissue samples. PLoS ONE 2014, 9, e96801. [Google Scholar] [CrossRef] [PubMed]
- Siracusa, R.; Paterniti, I.; Bruschetta, G.; Cordaro, M.; Impellizzeri, D.; Crupi, R.; Cuzzocrea, S.; Esposito, E. The association of Palmitoylethanolamide with Luteolin decreases autophagy in spinal cord injury. Mol. Neurobiol. 2015, 53, 3783–3792. [Google Scholar] [CrossRef] [PubMed]
- Petrosino, S.; Cordaro, M.; Verde, R.; Moriello, A.S.; Marcolongo, G.; Schievano, C.; Siracusa, R.; Piscitelli, F.; Peritore, A.F.; Crupi, R.; et al. Oral Ultramicronized Palmitoylethanolamide: Plasma and tissue levels and spinal anti-hyperalgesic effect. Front. Pharmacol. 2018, 9. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, N.; Hirose, Y.; Ohara, S.; Ono, T.; Watanabe, Y. A simple quantitative bradykinesia test in MPTP-treated mice. Res. Commun. Chem. Pathol. Pharmacol. 1985, 50, 435–441. [Google Scholar]
- Weber, D.; Davies, M.J.; Grune, T. Determination of protein carbonyls in plasma, cell extracts, tissue homogenates, isolated proteins: Focus on sample preparation and derivatization conditions. Redox Biol. 2015, 5, 367–380. [Google Scholar] [CrossRef]
- Calabrese, V.; Mancuso, C.; Calvani, M.; Rizzarelli, E.; Butterfield, D.A.; Stella, A.M.G. Nitric oxide in the central nervous system: Neuroprotection versus neurotoxicity. Nat. Rev. Neurosci. 2007, 8, 766–775. [Google Scholar] [CrossRef]
- Park, H.-A.; Ellis, A. Dietary antioxidants and Parkinson’s disease. Antioxidants 2020, 9, 570. [Google Scholar] [CrossRef]
- Féart, C.; Samieri, C.; Allès, B.; Barberger-Gateau, P. Potential benefits of adherence to the Mediterranean diet on cognitive health. Proc. Nutr. Soc. 2012, 72, 140–152. [Google Scholar] [CrossRef]
- Scarmeas, N.; Luchsinger, J.A.; Stern, Y.; Gu, Y.; He, J.; DeCarli, C.; Brown, T.; Brickman, A.M. Mediterranean diet and magnetic resonance imaging-assessed cerebrovascular disease. Ann. Neurol. 2011, 69, 257–268. [Google Scholar] [CrossRef] [PubMed]
- Stupans, I.; Kirlich, A.; Tuck, K.L.; Hayball, P.J. Comparison of radical scavenging effect, inhibition of microsomal oxygen free radical generation, and serum lipoprotein oxidation of several natural antioxidants. J. Agric. Food Chem. 2002, 50, 2464–2469. [Google Scholar] [CrossRef] [PubMed]
- Richard, N.; Arnold, S.; Hoeller, U.; Kilpert, C.; Wertz, K.; Schwager, J. Hydroxytyrosol is the major anti-inflammatory compound in aqueous olive extracts and impairs cytokine and chemokine production in macrophages. Planta Medica 2011, 77, 1890–1897. [Google Scholar] [CrossRef]
- Lavelli, V. Comparison of the antioxidant activities of extra virgin olive oils. J. Agric. Food Chem. 2002, 50, 7704–7708. [Google Scholar] [CrossRef] [PubMed]
- Owen, R.; Giacosa, A.; E Hull, W.; Haubner, R.; Spiegelhalder, B.; Bartsch, H. The antioxidant/anticancer potential of phenolic compounds isolated from olive oil. Eur. J. Cancer 2000, 36, 1235–1247. [Google Scholar] [CrossRef]
- De La Puerta, R.; Domínguez, M.E.M.; Ruíz-Gutíerrez, V.; Flavill, J.A.; Hoult, J.S. Effects of virgin olive oil phenolics on scavenging of reactive nitrogen species and upon nitrergic neurotransmission. Life Sci. 2001, 69, 1213–1222. [Google Scholar] [CrossRef]
- Visioli, F.; Wolfram, R.; Richard, D.; Abdullah, M.I.C.B.; Crea, R. Olive Phenolics increase glutathione levels in healthy volunteers. J. Agric. Food Chem. 2009, 57, 1793–1796. [Google Scholar] [CrossRef]
- Lins, P.G.; Pugine, S.M.P.; Scatolini, A.M.; De Melo, M.P. In vitro antioxidant activity of olive leaf extract (Olea europaea L.) and its protective effect on oxidative damage in human erythrocytes. Heliyon 2018, 4, e00805. [Google Scholar] [CrossRef]
- Crea, R.; Bitler, C.M.; Bolin, L.M.; Pontoniere, P. Anti-inflammatory activity of hydroxytyrosol. Eur. J. Nutraceuticals Funct. Foods 2012, 23, 26–29. [Google Scholar]
- Brunetti, G.; Di Rosa, G.; Scuto, M.; Leri, M.; Stefani, M.; Schmitz-Linneweber, C.; Calabrese, V.; Saul, N. Healthspan maintenance and prevention of Parkinson’s-like phenotypes with Hydroxytyrosol and Oleuropein Aglycone in C. elegans. Int. J. Mol. Sci. 2020, 21, 2588. [Google Scholar] [CrossRef]
- Miller, I.N.; Cronin-Golomb, A. Gender differences in Parkinson’s disease: Clinical characteristics and cognition. Mov. Disord. 2010, 25, 2695–2703. [Google Scholar] [CrossRef] [PubMed]
- Bagli, E.; Goussia, A.; Moschos, M.M.; Agnantis, N.; Kitsos, G. Natural compounds and Neuroprotection: Mechanisms of action and novel delivery systems. In Vivo 2016, 30, 535–547. [Google Scholar] [PubMed]
- Jing, X.; Wei, X.; Ren, M.; Wang, L.; Zhang, X.; Lou, H. Neuroprotective effects of Tanshinone I against 6-OHDA-induced oxidative stress in cellular and mouse model of Parkinson’s disease through Upregulating Nrf2. Neurochem. Res. 2015, 41, 779–786. [Google Scholar] [CrossRef] [PubMed]
- Zhao, F.; Wu, T.; Lau, A.; Jiang, T.; Huang, Z.; Wang, X.-J.; Chen, W.; Wong, P.K.; Zhang, D.D. Nrf2 promotes neuronal cell differentiation. Free. Radic. Biol. Med. 2009, 47, 867–879. [Google Scholar] [CrossRef] [PubMed]
- Calkins, M.J.; Johnson, D.A.; Townsend, J.A.; Vargas, M.R.; Dowell, J.A.; Williamson, T.P.; Kraft, A.D.; Lee, J.-M.; Li, J.; Johnson, J.A. The Nrf2/ARE pathway as a potential therapeutic target in neurodegenerative disease. Antioxid. Redox Signal. 2009, 11, 497–508. [Google Scholar] [CrossRef]
- Belcher, J.D.; Chen, C.; Nguyen, J.; Zhang, P.; Abdulla, F.; Nguyen, P.; Killeen, T.; Xu, P.; O’Sullivan, G.; Nath, K.A.; et al. Control of oxidative stress and inflammation in sickle cell disease with the Nrf2 activator dimethyl Fumarate. Antioxid. Redox Signal. 2017, 26, 748–762. [Google Scholar] [CrossRef]
- Lastres-Becker, I.; García-Yagüe, A.J.; Scannevin, R.H.; Casarejos, M.J.; Kügler, S.; Rábano, A.; Cuadrado, A. Repurposing the NRF2 activator dimethyl Fumarate as therapy against Synucleinopathy in Parkinson’s disease. Antioxid. Redox Signal. 2016, 25, 61–77. [Google Scholar] [CrossRef]
- Mutter, F.E.; Park, B.K.; Copple, I.M. Value of monitoring Nrf2 activity for the detection of chemical and oxidative stress. Biochem. Soc. Trans. 2015, 43, 657–662. [Google Scholar] [CrossRef]
- Zagoura, D.; Canovas-Jorda, D.; Pistollato, F.; Bremer-Hoffmann, S.; Bal-Price, A. Evaluation of the rotenone-induced activation of the Nrf2 pathway in a neuronal model derived from human induced pluripotent stem cells. Neurochem. Int. 2017, 106, 62–73. [Google Scholar] [CrossRef]
- Scuto, M.; Di Mauro, P.; Ontario, M.L.; Amato, C.; Modafferi, S.; Ciavardelli, D.; Trovato-Salinaro, A.; Maiolino, L.; Calabrese, V. Nutritional mushroom treatment in Meniere’s disease with Coriolus versicolor: A rationale for therapeutic intervention in Neuroinflammation and Antineurodegeneration. Int. J. Mol. Sci. 2019, 21, 284. [Google Scholar] [CrossRef]
- Miquel, S.; Champ, C.; Day, J.; Aarts, E.; Bahr, B.A.; Bakker, M.; Bánáti, D.; Calabrese, V.; Cederholm, T.; Cryan, J.; et al. Poor cognitive ageing: Vulnerabilities, mechanisms and the impact of nutritional interventions. Ageing Res. Rev. 2018, 42, 40–55. [Google Scholar] [CrossRef] [PubMed]
- Bayram, B.; Özçelik, B.; Grimm, S.; Roeder, T.; Schrader, C.; Ernst, I.M.; Wagner, A.E.; Grune, T.; Frank, J.; Rimbach, G. A diet rich in olive oil Phenolics reduces oxidative stress in the heart of SAMP8 Mice by induction of Nrf2-dependent gene expression. Rejuvenation Res. 2012, 15, 71–81. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Morató, J.; Xicota, L.; Fitó, M.; Farré, M.; Dierssen, M.; De La Torre, R. Potential role of olive oil phenolic compounds in the prevention of neurodegenerative diseases. Molecules 2015, 20, 4655–4680. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Huélamo, M.; Rodríguez-Morató, J.; Boronat, A.; De La Torre, R. Modulation of Nrf2 by olive oil and wine polyphenols and Neuroprotection. Antioxidants 2017, 6, 73. [Google Scholar] [CrossRef] [PubMed]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Siracusa, R.; Scuto, M.; Fusco, R.; Trovato, A.; Ontario, M.L.; Crea, R.; Di Paola, R.; Cuzzocrea, S.; Calabrese, V. Anti-inflammatory and Anti-oxidant Activity of Hidrox® in Rotenone-Induced Parkinson’s Disease in Mice. Antioxidants 2020, 9, 824. https://doi.org/10.3390/antiox9090824
Siracusa R, Scuto M, Fusco R, Trovato A, Ontario ML, Crea R, Di Paola R, Cuzzocrea S, Calabrese V. Anti-inflammatory and Anti-oxidant Activity of Hidrox® in Rotenone-Induced Parkinson’s Disease in Mice. Antioxidants. 2020; 9(9):824. https://doi.org/10.3390/antiox9090824
Chicago/Turabian StyleSiracusa, Rosalba, Maria Scuto, Roberta Fusco, Angela Trovato, Maria Laura Ontario, Roberto Crea, Rosanna Di Paola, Salvatore Cuzzocrea, and Vittorio Calabrese. 2020. "Anti-inflammatory and Anti-oxidant Activity of Hidrox® in Rotenone-Induced Parkinson’s Disease in Mice" Antioxidants 9, no. 9: 824. https://doi.org/10.3390/antiox9090824
APA StyleSiracusa, R., Scuto, M., Fusco, R., Trovato, A., Ontario, M. L., Crea, R., Di Paola, R., Cuzzocrea, S., & Calabrese, V. (2020). Anti-inflammatory and Anti-oxidant Activity of Hidrox® in Rotenone-Induced Parkinson’s Disease in Mice. Antioxidants, 9(9), 824. https://doi.org/10.3390/antiox9090824