Natural Antioxidants from Seeds and Their Application in Meat Products
Abstract
:1. Introduction
2. Phenolic Compounds Found in Seeds
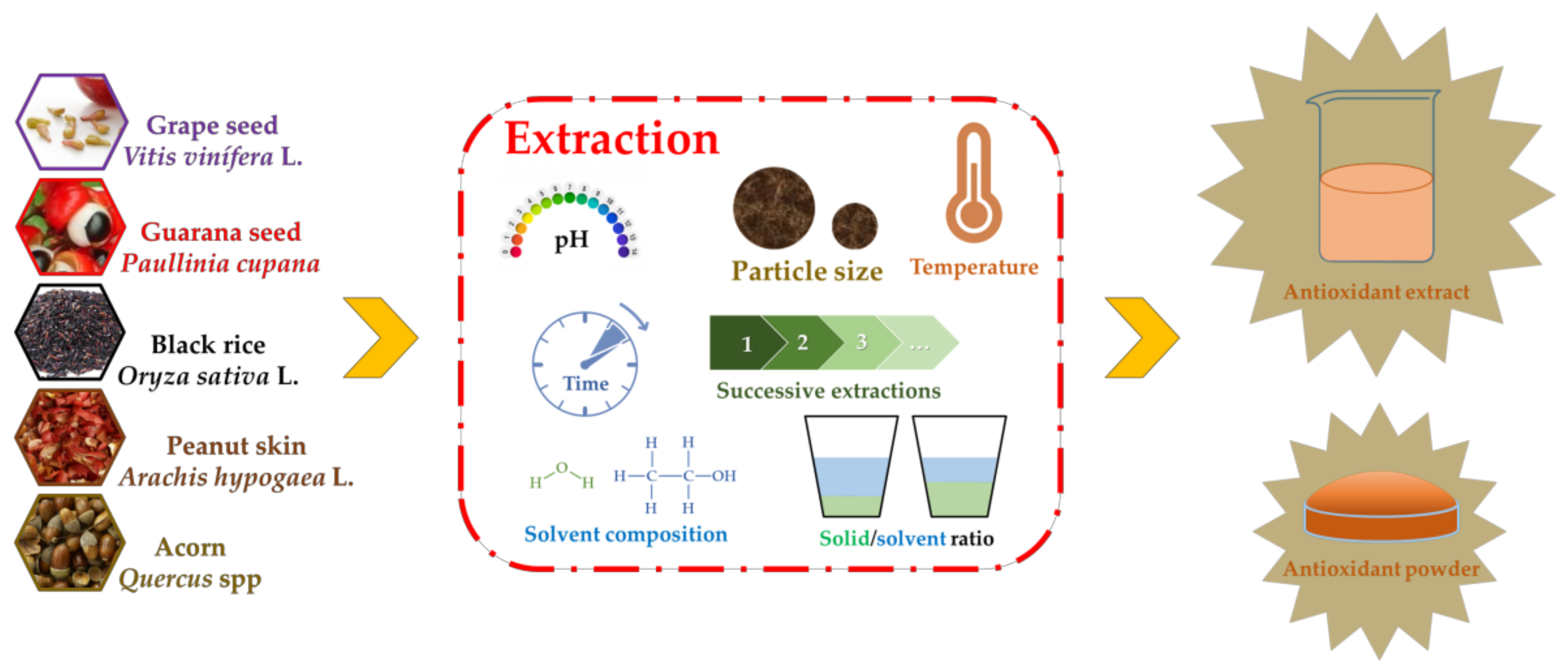
3. Influence of Extraction Conditions in the Polyphenol Content and Antioxidant Activity of Seed Extracts
4. Color of Meat Products
5. Lipid and Protein Oxidation
6. Sensory Attributes
7. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Cunha, L.C.M.; Monteiro, M.L.G.; Lorenzo, J.M.; Munekata, P.E.S.; Muchenje, V.; de Carvalho, F.A.L.; Conte-Junior, C.A. Natural antioxidants in processing and storage stability of sheep and goat meat products. Food Res. Int. 2018, 111, 379–390. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, J.S.; Santos, M.J.M.C.; Silva, L.K.R.; Pereira, L.C.L.; Santos, I.A.; da Silva-Lannes, S.C.; da Silva, M.V. Natural antioxidants used in meat products: A brief review. Meat Sci. 2019, 148, 181–188. [Google Scholar] [CrossRef]
- Lorenzo, J.M.; Gómez, M. Shelf life of fresh foal meat under MAP, overwrap and vacuum packaging conditions. Meat Sci. 2012, 92, 610–618. [Google Scholar] [CrossRef] [PubMed]
- Domínguez, R.; Pateiro, M.; Gagaoua, M.; Barba, F.J.; Zhang, W.; Lorenzo, J.M. A comprehensive review on lipid oxidation in meat and meat products. Antioxidants 2019, 8, 429. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fernandes, R.P.P.; Trindade, M.A.; Tonin, F.G.; Pugine, S.M.P.; Lima, C.G.; Lorenzo, J.M.; de Melo, M.P. Evaluation of oxidative stability of lamb burger with Origanum vulgare extract. Food Chem. 2017, 233, 101–109. [Google Scholar] [CrossRef] [PubMed]
- Zamuz, S.; López-Pedrouso, M.; Barba, F.J.; Lorenzo, J.M.; Domínguez, H.; Franco, D. Application of hull, bur and leaf chestnut extracts on the shelf-life of beef patties stored under MAP: Evaluation of their impact on physicochemical properties, lipid oxidation, antioxidant, and antimicrobial potential. Food Res. Int. 2018, 112, 263–273. [Google Scholar] [CrossRef]
- Fernandes, R.P.P.; Trindade, M.A.; Lorenzo, J.M.; de Melo, M.P. Assessment of the stability of sheep sausages with the addition of different concentrations of Origanum vulgare extract during storage. Meat Sci. 2018, 137, 244–257. [Google Scholar] [CrossRef]
- Lorenzo, J.M.; Vargas, F.C.; Strozzi, I.; Pateiro, M.; Furtado, M.M.; Sant’Ana, A.S.; Rocchetti, G.; Barba, F.J.; Dominguez, R.; Lucini, L.; et al. Influence of pitanga leaf extracts on lipid and protein oxidation of pork burger during shelf-life. Food Res. Int. 2018, 114, 47–54. [Google Scholar] [CrossRef]
- Alirezalu, K.; Pateiro, M.; Yaghoubi, M.; Alirezalu, A.; Peighambardoust, S.H.; Lorenzo, J.M. Phytochemical constituents, advanced extraction technologies and techno-functional properties of selected Mediterranean plants for use in meat products. A comprehensive review. Trends Food Sci. Technol. 2020, 100, 292–306. [Google Scholar] [CrossRef]
- Domínguez, R.; Gullón, P.; Pateiro, M.; Munekata, P.E.S.; Zhang, W.; Lorenzo, J.M. Tomato as potential source of natural additives for meat industry. A review. Antioxidants 2020, 9, 73. [Google Scholar] [CrossRef] [Green Version]
- Halliwell, B.; Gutteridge, J.M.C. The definition and measurement of antioxidants in biological systems. Free Radic. Biol. Med. 1995, 18, 125–126. [Google Scholar] [CrossRef]
- Lorenzo, J.M.; Munekata, P.E.S.; Baldin, J.C.; Franco, D.; Domínguez, R.; Trindade, M.A. The use of natural antioxidants to replace chemical antioxidants in foods. In Strategies for Obtaining Healthier Foods; Lorenzo, J.M., Carballo, F.J., Eds.; Nova Science Publishers: New York, NY, USA, 2017; pp. 205–228. ISBN 9781536121599. [Google Scholar]
- Lorenzo, J.M.; Pateiro, M.; Domínguez, R.; Barba, F.J.; Putnik, P.; Kovačević, D.B.; Shpigelman, A.; Granato, D.; Franco, D. Berries extracts as natural antioxidants in meat products: A review. Food Res. Int. 2018, 106, 1095–1104. [Google Scholar] [CrossRef] [PubMed]
- Nikmaram, N.; Budaraju, S.; Barba, F.J.; Lorenzo, J.M.; Cox, R.B.; Mallikarjunan, K.; Roohinejad, S. Application of plant extracts to improve the shelf-life, nutritional and health-related properties of ready-to-eat meat products. Meat Sci. 2018, 145, 245–255. [Google Scholar] [CrossRef] [PubMed]
- Munekata, P.E.S.; Rocchetti, G.; Pateiro, M.; Lucini, L.; Domínguez, R.; Lorenzo, J.M. Addition of plant extracts to meat and meat products to extend shelf-life and health-promoting attributes: An overview. Curr. Opin. Food Sci. 2020, 31, 81–87. [Google Scholar] [CrossRef]
- Oswell, N.J.; Thippareddi, H.; Pegg, R.B. Practical use of natural antioxidants in meat products in the U.S.: A review. Meat Sci. 2018, 145, 469–479. [Google Scholar] [CrossRef]
- Fernandes, R.P.P.; Trindade, M.A.; Lorenzo, J.M.; Munekata, P.E.S.; de Melo, M.P. Effects of oregano extract on oxidative, microbiological and sensory stability of sheep burgers packed in modified atmosphere. Food Control 2016, 63, 65–75. [Google Scholar] [CrossRef]
- Pateiro, M.; Domínguez, R.; Bermúdez, R.; Munekata, P.E.S.; Zhang, W.; Gagaoua, M.; Lorenzo, J.M. Antioxidant active packaging systems to extend the shelf life of sliced cooked ham. Curr. Res. Food Sci. 2019, 1, 24–30. [Google Scholar] [CrossRef]
- Das, A.K.; Das, A.; Nanda, P.K.; Madane, P.; Biswas, S.; Zhang, W.; Lorenzo, J.M. A comprehensive review on antioxidant dietary fibre enriched meat-based functional foods. Trends Food Sci. Technol. 2020, 99, 323–336. [Google Scholar] [CrossRef]
- Lorenzo, J.M.; Sineiro, J.; Amado, I.R.; Franco, D. Influence of natural extracts on the shelf life of modified atmosphere-packaged pork patties. Meat Sci. 2014, 96, 526–534. [Google Scholar] [CrossRef]
- Gullón, B.; Gagaoua, M.; Barba, F.J.; Gullón, P.; Zhang, W.; Lorenzo, J.M. Seaweeds as promising resource of bioactive compounds: Overview of novel extraction strategies and design of tailored meat products. Trends Food Sci. Technol. 2020, 100, 1–18. [Google Scholar] [CrossRef]
- Paja̧k, P.; Socha, R.; Gałkowska, D.; Roznowski, J.; Fortuna, T. Phenolic profile and antioxidant activity in selected seeds and sprouts. Food Chem. 2014, 143, 300–306. [Google Scholar] [CrossRef] [PubMed]
- Lorenzo, J.M.; Mousavi-Khaneghah, A.; Gavahian, M.; Marszałek, K.; Es, I.; Munekata, P.E.S.; Ferreira, I.C.F.R.; Barba, F.J. Understanding the potential benefits of thyme and its derived products for food industry and consumer health: From extraction of value-added compounds to the evaluation of bioaccessibility, bioavailability, anti-inflammatory, and antimicrobial activities. Crit. Rev. Food Sci. Nutr. 2019, 59, 2879–2895. [Google Scholar] [CrossRef]
- Quideau, S.; Deffieux, D.; Douat-Casassus, C.; Pouységu, L. Plant polyphenols: Chemical properties, biological activities, and synthesis. Angew. Chemie Int. Ed. 2011, 50, 586–621. [Google Scholar] [CrossRef] [PubMed]
- Singh, B.; Singh, J.P.; Kaur, A.; Singh, N. Phenolic composition and antioxidant potential of grain legume seeds: A review. Food Res. Int. 2017, 101, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Arjeh, E.; Akhavan, H.R.; Barzegar, M.; Carbonell-Barrachina, Á.A. Bio-active compounds and functional properties of pistachio hull: A review. Trends Food Sci. Technol. 2020, 97, 55–64. [Google Scholar] [CrossRef]
- Lorenzo, J.M.; Munekata, P.E.S.; Sant’Ana, A.S.; Carvalho, R.B.; Barba, F.J.; Toldrá, F.; Mora, L.; Trindade, M.A. Main characteristics of peanut skin and its role for the preservation of meat products. Trends Food Sci. Technol. 2018, 77, 1–10. [Google Scholar] [CrossRef]
- Mojzer, E.B.; Hrnčič, M.K.; Škerget, M.; Knez, Ž.; Bren, U. Polyphenols: Extraction methods, antioxidative action, bioavailability and anticarcinogenic effects. Molecules 2016, 21, 901. [Google Scholar] [CrossRef]
- European Food Safety Authority Use of rosemary extracts as a food additive-Scientific Opinion of the Panel on Food Additives, Flavourings, Processing Aids and Materials in Contact with Food. EFSA J. 2008, 6, 1–29.
- U.S. Department of Health and Human Services GRAS Notices No. In 446 Red Grape Pomace Extract. Available online: https://www.accessdata.fda.gov/scripts/fdcc/index.cfm?set=GRASNotices (accessed on 17 August 2020).
- U.S. Department of Health and Human Services GRAS Notices No. 93 Grape Seed Extract and Grape Skin Extract. Available online: https://www.accessdata.fda.gov/scripts/fdcc/index.cfm?set=GRASNotices (accessed on 17 August 2020).
- U.S. Department of Health and Human Services GRAS Notices No. 459 Olive Pulp Extract. Available online: https://www.accessdata.fda.gov/scripts/fdcc/index.cfm?set=GRASNotices (accessed on 17 August 2020).
- Zhang, H.; Shao, Y.; Bao, J.; Beta, T. Phenolic compounds and antioxidant properties of breeding lines between the white and black rice. Food Chem. 2015, 172, 630–639. [Google Scholar] [CrossRef]
- Pang, Y.; Ahmed, S.; Xu, Y.; Beta, T.; Zhu, Z.; Shao, Y.; Bao, J. Bound phenolic compounds and antioxidant properties of whole grain and bran of white, red and black rice. Food Chem. 2018, 240, 212–221. [Google Scholar] [CrossRef]
- Kiprovski, B.; Mikulic-Petkovsek, M.; Slatnar, A.; Veberic, R.; Stampar, F.; Malencic, D.; Latkovic, D. Comparison of phenolic profiles and antioxidant properties of European Fagopyrum esculentum cultivars. Food Chem. 2015, 185, 41–47. [Google Scholar] [CrossRef]
- Zhang, W.; Zhu, Y.; Liu, Q.; Bao, J.; Liu, Q. Identification and quantification of polyphenols in hull, bran and endosperm of common buckwheat (Fagopyrum esculentum) seeds. J. Funct. Foods 2017, 38, 363–369. [Google Scholar] [CrossRef]
- Alcântara, M.A.; Polari, I.; Meireles, B.R.L.; de Lima, A.E.A.; da Silva-Junior, J.C.; Viera, E.A.; dos Santos, N.A.; Cordeiro, A.M.T. Effect of the solvent composition on the profile of phenolic compounds extracted from chia seeds. Food Chem. 2019, 275, 489–496. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.J.; de Camargo, A.C.; Shahidi, F. Phenolic and polyphenolic profiles of chia seeds and their in vitro biological activities. J. Funct. Foods 2017, 35, 622–634. [Google Scholar] [CrossRef]
- Hernández-Hernández, C.; Viera-Alcaide, I.; Morales-Sillero, A.M.; Fernández-Bolaños, J.; Rodríguez-Gutiérrez, G. Bioactive compounds in Mexican genotypes of cocoa cotyledon and husk. Food Chem. 2018, 240, 831–839. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hernández-Hernández, C.; Morales-Sillero, A.; Fernández-Bolaños, J.; Bermúdez-Oria, A.; Morales, A.A.; Rodríguez-Gutiérrez, G. Cocoa bean husk: Industrial source of antioxidant phenolic extract. J. Sci. Food Agric. 2019, 99, 325–333. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.F.; Zhang, H. Phytochemical constituents, health benefits, and industrial applications of grape seeds: A mini-review. Antioxidants 2017, 6, 71. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aquilani, C.; Sirtori, F.; Flores, M.; Bozzi, R.; Lebret, B.; Pugliese, C. Effect of natural antioxidants from grape seed and chestnut in combination with hydroxytyrosol, as sodium nitrite substitutes in Cinta Senese dry-fermented sausages. Meat Sci. 2018, 145, 389–398. [Google Scholar] [CrossRef] [PubMed]
- Ribas-Agustí, A.; Gratacós-Cubarsí, M.; Sárraga, C.; Guàrdia, M.D.; García-Regueiro, J.A.; Castellari, M. Stability of phenolic compounds in dry fermented sausages added with cocoa and grape seed extracts. LWT Food Sci. Technol. 2014, 57, 329–336. [Google Scholar] [CrossRef]
- da Silva, G.S.; Canuto, K.M.; Ribeiro, P.R.V.; de Brito, E.S.; Nascimento, M.M.; Zocolo, G.J.; Coutinho, J.P.; de Jesus, R.M. Chemical profiling of guarana seeds (Paullinia cupana) from different geographical origins using UPLC-QTOF-MS combined with chemometrics. Food Res. Int. 2017, 102, 700–709. [Google Scholar] [CrossRef]
- Pinaffi, A.C.; Sampaio, G.R.; Soares, M.J.; Shahidi, F.; de Camargo, A.C.; Torres, E.A.F.S.F.S. Insoluble-bound polyphenols released from guarana powder: Inhibition of alpha-glucosidase and proanthocyanidin profile. Molecules 2020, 25, 679. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pateiro, M.; Vargas, F.C.; Chincha, A.A.I.A.; Sant’Ana, A.S.; Strozzi, I.; Rocchetti, G.; Barba, F.J.; Domínguez, R.; Lucini, L.; do Amaral Sobral, P.J.; et al. Guarana seed extracts as a useful strategy to extend the shelf life of pork patties: UHPLC-ESI/QTOF phenolic profile and impact on microbial inactivation, lipid and protein oxidation and antioxidant capacity. Food Res. Int. 2018, 114, 55–63. [Google Scholar] [CrossRef]
- Yeo, J.D.; Shahidi, F. Identification and quantification of soluble and insoluble-bound phenolics in lentil hulls using HPLC-ESI-MS/MS and their antioxidant potential. Food Chem. 2020, 315, 126202. [Google Scholar] [CrossRef] [PubMed]
- Munekata, P.E.S.; Domínguez, R.; Franco, D.; Bermúdez, R.; Trindade, M.A.; Lorenzo, J.M. Effect of natural antioxidants in Spanish salchichón elaborated with encapsulated n-3 long chain fatty acids in konjac glucomannan matrix. Meat Sci. 2017, 124, 54–60. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Kosińska-Cagnazzo, A.; Kerr, W.L.; Amarowicz, R.; Swanson, R.B.; Pegg, R.B. Separation and characterization of phenolic compounds from dry-blanched peanut skins by liquid chromatography-electrospray ionization mass spectrometry. J. Chromatogr. A 2014, 1356, 64–81. [Google Scholar] [CrossRef]
- Grace, M.H.; Esposito, D.; Timmers, M.A.; Xiong, J.; Yousef, G.; Komarnytsky, S.; Lila, M.A. Chemical composition, antioxidant and anti-inflammatory properties of pistachio hull extracts. Food Chem. 2016, 210, 85–95. [Google Scholar] [CrossRef]
- Garavand, F.; Madadlou, A.; Moini, S. Determination of phenolic profile and antioxidant activity of pistachio hull using high-performance liquid chromatography–diode array detector–electro-spray ionization–mass spectrometry as affected by ultrasound and microwave. Int. J. Food Prop. 2017, 20, 19–29. [Google Scholar] [CrossRef] [Green Version]
- Mayengbam, S.; Aachary, A.; Thiyam-Holländer, U. Endogenous phenolics in hulls and cotyledons of mustard and canola: A comparative study on its sinapates and antioxidant capacity. Antioxidants 2014, 3, 544–558. [Google Scholar] [CrossRef] [Green Version]
- Cantos, E.; Espín, J.C.; López-Bote, C.; De la Hoz, L.D.; Ordóñez, J.A.; Tomás-Barberán, F.A. Phenolic compounds and fatty acids from acorns (Quercus spp.), the main dietary constituent of free-ranged Iberian pigs. J. Agric. Food Chem. 2003, 51, 6248–6255. [Google Scholar] [CrossRef]
- Zhang, C. In vitro antioxidant properties of Euryale ferox seed shell extracts and their preservation effects on pork sausages. J. Food Process. Preserv. 2015, 39, 1172–1182. [Google Scholar] [CrossRef]
- Siva, N.; Thavarajah, D.; Johnson, C.R.; Duckett, S.; Jesch, E.D.; Thavarajah, P. Can lentil (Lens culinaris Medikus) reduce the risk of obesity? J. Funct. Foods 2017, 38, 706–715. [Google Scholar] [CrossRef]
- Arya, S.S.; Salve, A.R.; Chauhan, S. Peanuts as functional food: A review. J. Food Sci. Technol. 2016, 53, 31–41. [Google Scholar] [CrossRef] [Green Version]
- Dreher, M.L. Pistachio nuts: Composition and potential health benefits. Nutr. Rev. 2012, 70, 234–240. [Google Scholar] [CrossRef]
- Boscaro, V.; Boffa, L.; Binello, A.; Amisano, G.; Fornasero, S.; Cravotto, G.; Gallicchio, M. Antiproliferative, proapoptotic, antioxidant and antimicrobial effects of Sinapis nigra L. and Sinapis alba L. extracts. Molecules 2018, 23, 3004. [Google Scholar] [CrossRef] [Green Version]
- Özünlü, O.; Ergezer, H.; Gökçe, R. Improving physicochemical, antioxidative and sensory quality of raw chicken meat by using acorn extracts. Lwt 2018, 98, 477–484. [Google Scholar] [CrossRef]
- Lee, S.E.; Ju, E.M.; Kim, J.H. Antioxidant activity of extracts from Euryale ferox seed. Exp. Mol. Med. 2002, 34, 100–106. [Google Scholar] [CrossRef] [Green Version]
- Gallo, M.; Conte, E.; Naviglio, D. Analysis and comparison of the antioxidant component of Portulaca oleracea leaves obtained by different solid-liquid extraction techniques. Antioxidants 2017, 6, 64. [Google Scholar] [CrossRef] [Green Version]
- Munekata, P.E.S.; Alcántara, C.; Žugčić, T.; Abdelkebir, R.; Collado, M.C.; García-Pérez, J.V.; Jambrak, A.R.; Gavahian, M.; Barba, F.J.; Lorenzo, J.M. Impact of ultrasound-assisted extraction and solvent composition on bioactive compounds and in vitro biological activities of thyme and rosemary. Food Res. Int. 2020, 134, 109242. [Google Scholar] [CrossRef]
- Bouaoudia-Madi, N.; Boulekbache-Makhlouf, L.; Madani, K.; Silva, A.M.S.; Dairi, S.; Oukhmanou-Bensidhoum, S.; Cardoso, S.M. Optimization of ultrasound-assisted extraction of polyphenols from Myrtus communis L. pericarp. Antioxidants 2019, 8, 205. [Google Scholar] [CrossRef] [Green Version]
- Domínguez, R.; Zhang, L.; Rocchetti, G.; Lucini, L.; Pateiro, M.; Munekata, P.E.S.; Lorenzo, J.M. Elderberry (Sambucus nigra L.) as potential source of antioxidants. Characterization, optimization of extraction parameters and bioactive properties. Food Chem. 2020, 330, 127266. [Google Scholar] [CrossRef]
- Pedro, A.C.; Granato, D.; Rosso, N.D. Extraction of anthocyanins and polyphenols from black rice (Oryza sativa L.) by modeling and assessing their reversibility and stability. Food Chem. 2016, 191, 12–20. [Google Scholar] [CrossRef] [Green Version]
- Das, A.B.; Goud, V.V.; Das, C. Extraction of phenolic compounds and anthocyanin from black and purple rice bran (Oryza sativa L.) using ultrasound: A comparative analysis and phytochemical profiling. Ind. Crops Prod. 2017, 95, 332–341. [Google Scholar] [CrossRef]
- Inglett, G.E.; Rose, D.J.; Chen, D.; Stevenson, D.G.; Biswas, A. Phenolic content and antioxidant activity of extracts from whole buckwheat (Fagopyrum esculentum Möench) with or without microwave irradiation. Food Chem. 2010, 119, 1216–1219. [Google Scholar] [CrossRef]
- Saphier, O.; Silberstein, T.; Kamer, H.; Ben-Abu, Y.; Tavor, D. Chia seeds are richer in polyphenols compared to flax seeds. Integr. Food, Nutr. Metab. 2017, 4, 1–4. [Google Scholar] [CrossRef] [Green Version]
- Okiyama, D.C.G.; Soares, I.D.; Cuevas, M.S.; Crevelin, E.J.; Moraes, L.A.B.; Melo, M.P.; Oliveira, A.L.; Rodrigues, C.E.C. Pressurized liquid extraction of flavanols and alkaloids from cocoa bean shell using ethanol as solvent. Food Res. Int. 2018, 114, 20–29. [Google Scholar] [CrossRef]
- da Porto, C.; Porretto, E.; Decorti, D. Comparison of ultrasound-assisted extraction with conventional extraction methods of oil and polyphenols from grape (Vitis vinifera L.) seeds. Ultrason. Sonochem. 2013, 20, 1076–1080. [Google Scholar] [CrossRef]
- Li, Y.; Skouroumounis, G.K.; Elsey, G.M.; Taylor, D.K. Microwave-assistance provides very rapid and efficient extraction of grape seed polyphenols. Food Chem. 2011, 129, 570–576. [Google Scholar] [CrossRef]
- Santana, Á.L.; Macedo, G.A. Effects of hydroalcoholic and enzyme-assisted extraction processes on the recovery of catechins and methylxanthines from crude and waste seeds of guarana (Paullinia cupana). Food Chem. 2019, 281, 222–230. [Google Scholar] [CrossRef]
- Marques, L.L.M.; Panizzon, G.P.; Aguiar, B.A.A.; Simionato, A.S.; Cardozo-Filho, L.; Andrade, G.; de Oliveira, A.G.; Guedes, T.A.; Mello, J.C.P. de Guaraná (Paullinia cupana) seeds: Selective supercritical extraction of phenolic compounds. Food Chem. 2016, 212, 703–711. [Google Scholar] [CrossRef]
- Franco, D.; Rodríguez-Amado, I.; Agregán, R.; Munekata, P.E.S.; Vázquez, J.A.; Barba, F.J.; Lorenzo, J.M. Optimization of antioxidants extraction from peanut skin to prevent oxidative processes during soybean oil storage. LWT Food Sci. Technol. 2018, 88, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Bodoira, R.; Rossi, Y.; Montenegro, M.; Maestri, D.; Velez, A. Extraction of antioxidant polyphenolic compounds from peanut skin using water-ethanol at high pressure and temperature conditions. J. Supercrit. Fluids 2017, 128, 57–65. [Google Scholar] [CrossRef]
- Erşan, S.; Güçlü-Üstündağ, Ö.; Carle, R.; Schweiggert, R.M. Subcritical water extraction of phenolic and antioxidant constituents from pistachio (Pistacia vera L.) hulls. Food Chem. 2018, 253, 46–54. [Google Scholar] [CrossRef] [PubMed]
- Kyriakidou, K.; Mourtzinos, I.; Biliaderis, C.G.; Makris, D.P. Optimization of a green extraction/inclusion complex formation process to recover antioxidant polyphenols from oak acorn husks (Quercus robur) using aqueous 2-hydroxypropyl-β-cyclodextrin/glycerol mixtures. Environments 2016, 3, 3. [Google Scholar] [CrossRef] [Green Version]
- Castro-Puyana, M.; Marina, M.L.; Plaza, M. Water as green extraction solvent: Principles and reasons for its use. Curr. Opin. Green Sustain. Chem. 2017, 5, 31–36. [Google Scholar] [CrossRef]
- M’hiri, N.; Ioannou, I.; Ghoul, M.; Boudhrioua, N.M. Extraction methods of citrus peel phenolic compounds. Food Rev. Int. 2014, 30, 265–290. [Google Scholar] [CrossRef]
- Faustman, C.; Cassens, R.G. The biochemical basis for discoloration in fresh meat: A review. J. Muscle Foods 1990, 1, 217–243. [Google Scholar] [CrossRef]
- Suman, S.P.; Joseph, P. Myoglobin chemistry and meat color. Annu. Rev. Food Sci. Technol. 2013, 4, 79–99. [Google Scholar] [CrossRef] [Green Version]
- Hammes, W.P. Metabolism of nitrate in fermented meats: The characteristic feature of a specific group of fermented foods. Food Microbiol. 2012, 29, 151–156. [Google Scholar] [CrossRef]
- Gassara, F.; Kouassi, A.P.; Brar, S.K.; Belkacemi, K. Green alternatives to nitrates and nitrites in meat-based products–A review. Crit. Rev. Food Sci. Nutr. 2016, 56, 2133–2148. [Google Scholar] [CrossRef] [Green Version]
- Aminzare, M.; Tajik, H.; Aliakbarlu, J.; Hashemi, M.; Raeisi, M. Effect of cinnamon essential oil and grape seed extract as functional-natural additives in the production of cooked sausage-impact on microbiological, physicochemical, lipid oxidation and sensory aspects, and fate of inoculated Clostridium perfringens. J. Food Saf. 2018, 38, e12459. [Google Scholar] [CrossRef]
- Pateiro, M.; Bermúdez, R.; Lorenzo, J.; Franco, D. Effect of Addition of Natural Antioxidants on the Shelf-Life of “Chorizo”, a Spanish Dry-Cured Sausage. Antioxidants 2015, 4, 42–67. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- de Carvalho, F.A.L.; Lorenzo, J.M.; Pateiro, M.; Bermúdez, R.; Purriños, L.; Trindade, M.A. Effect of guarana (Paullinia cupana) seed and pitanga (Eugenia uniflora L.) leaf extracts on lamb burgers with fat replacement by chia oil emulsion during shelf life storage at 2 °C. Food Res. Int. 2019, 125, 108554. [Google Scholar] [CrossRef] [PubMed]
- Munekata, P.E.S.; Fernandes, R.; de Malo, M.P.; Trindade, M.A.; Lorenzo, J.M. Influence of peanut skin extract on shelf-life of sheep patties. Asian Pac. J. Trop. Biomed. 2016, 6, 586–596. [Google Scholar] [CrossRef] [Green Version]
- Munekata, P.E.S.; Calomeni, A.V.; Rodrigues, C.E.C.; Fávaro-Trindade, C.S.; Alencar, S.M.; Trindade, M.A. Peanut skin extract reduces lipid oxidation in cooked chicken patties. Poult. Sci. 2015, 94, 442–446. [Google Scholar] [CrossRef]
- Munekata, P.E.S.; Domínguez, R.; Campagnol, P.C.B.P.C.B.; Franco, D.; Trindade, M.A.M.A.; Lorenzo, J.M.J.M. Effect of natural antioxidants on physicochemical properties and lipid stability of pork liver pâté manufactured with healthy oils during refrigerated storage. J. Food Sci. Technol. 2017, 54, 4324–4334. [Google Scholar] [CrossRef]
- Prommachart, R.; Belem, T.S.; Uriyapongson, S.; Rayas-Duarte, P.; Uriyapongson, J.; Ramanathan, R. The effect of black rice water extract on surface color, lipid oxidation, microbial growth, and antioxidant activity of beef patties during chilled storage. Meat Sci. 2020, 164, 108091. [Google Scholar] [CrossRef]
- Çağlar, M.Y.; Gök, V.; Tomar, O.; Akarca, G. Determination of the effect of different ground mustard seeds on quality characteristics of meatballs. Korean J. Food Sci. Anim. Resour. 2018, 38, 530–543. [Google Scholar]
- Scapin, G.; Schimdt, M.M.; Prestes, R.C.; Ferreira, S.; Silva, A.F.C.; da Rosa, C.S. Effect of extract of chia seed (Salvia hispanica) as an antioxidant in fresh pork sausage. Int. Food Res. J. 2015, 22, 1195–1202. [Google Scholar]
- Choi, J.; Kim, N.; Choi, H.Y.; Han, Y.S. Effect of cacao bean husk powder on the quality properties of pork sausages. Food Sci. Anim. Resour. 2019, 39, 742–755. [Google Scholar] [CrossRef]
- Al-Juhaimi, F.; Adiamo, O.Q.; Alsawmahi, O.N.; Gahfoor, K.; Mohamed, Z.I.S.; Mohamed-Ahmed, I.A.; Babiker, E.E. Effect of pistachio seed hull extracts on quality attributes of chicken burger. CYTA J. Food 2017, 15, 9–14. [Google Scholar] [CrossRef]
- Jiménez-Colmenero, F.; Serrano, A.; Ayo, J.; Solas, M.T.; Cofrades, S.; Carballo, J. Physicochemical and sensory characteristics of restructured beef steak with added walnuts. Meat Sci. 2003, 65, 1391–1397. [Google Scholar] [CrossRef]
- Papuc, C.; Goran, G.V.; Predescu, C.N.; Nicorescu, V.; Stefan, G. Plant polyphenols as antioxidant and antibacterial agents for shelf-life extension of meat and meat products: Classification, structures, sources, and action mechanisms. Compr. Rev. Food Sci. Food Saf. 2017, 16, 1243–1268. [Google Scholar] [CrossRef] [Green Version]
- Lorenzo, J.M.; Batlle, R.; Gómez, M. Extension of the shelf-life of foal meat with two antioxidant active packaging systems. LWT Food Sci. Technol. 2014, 59, 181–188. [Google Scholar] [CrossRef]
- Echegaray, N.; Domínguez, R.; Franco, D.; Lorenzo, J.M.; Carballo, J. Effect of the use of chestnuts (Castanea sativa Miller) in the finishing diet of Celta pig breed on the shelf-life of meat refrigerated and frozen. Food Res. Int. 2018, 114, 114–122. [Google Scholar] [CrossRef] [PubMed]
- Amaral, A.B.; Silva, M.V.; Lannes, S.C.S. Lipid oxidation in meat: Mechanisms and protective factors–a review. Food Sci. Technol. 2018, 38, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Domínguez, R.; Purriños, L.; Pérez-Santaescolástica, C.; Pateiro, M.; Barba, F.J.; Tomasevic, I.; Campagnol, P.C.B.; Lorenzo, J.M. Characterization of Volatile Compounds of Dry-Cured Meat Products Using HS-SPME-GC/MS Technique. Food Anal. Methods 2019, 12, 1263–1284. [Google Scholar] [CrossRef]
- Barriuso, B.; Astiasarán, I.; Ansorena, D. A review of analytical methods measuring lipid oxidation status in foods: A challenging task. Eur. Food Res. Technol. 2013, 236, 1–15. [Google Scholar] [CrossRef]
- Domínguez, R.; Gómez, M.; Fonseca, S.; Lorenzo, J.M. Influence of thermal treatment on formation of volatile compounds, cooking loss and lipid oxidation in foal meat. LWT Food Sci. Technol. 2014, 58, 439–445. [Google Scholar] [CrossRef]
- Lund, M.N.; Heinonen, M.; Baron, C.P.; Estévez, M. Protein oxidation in muscle foods: A review. Mol. Nutr. Food Res. 2011, 55, 83–95. [Google Scholar] [CrossRef]
- Soladoye, O.P.; Juárez, M.L.; Aalhus, J.L.; Shand, P.; Estévez, M. Protein oxidation in processed meat: Mechanisms and potential implications on human health. Compr. Rev. Food Sci. Food Saf. 2015, 14, 106–122. [Google Scholar] [CrossRef]
- Loypimai, P.; Moongngarm, A.; Naksawat, S. Application of natural colorant from black rice bran for fermented Thai pork sausage-Sai Krok Isan. Int. Food Res. J. 2017, 24, 1529–1537. [Google Scholar]
- Tajik, H.; Aminzare, M.; Mounesi-Raad, T.; Hashemi, M.; Hassanzad-Azar, H.; Raeisi, M.; Naghili, H. Effect of Zataria multiflora Boiss essential oil and grape seed extract on the shelf life of raw buffalo patty and fate of inoculated Listeria monocytogenes. J. Food Process. Preserv. 2015, 39, 3005–3013. [Google Scholar] [CrossRef]
- El-Samahy, K.; Embaby, H.; Mokhtar, S.; Mostafa, A.; Gaballah, A. Effect of lentil (Lens culinaris) coat powder addition on lipid oxidation and quality characteristics of beef burgers stored at 4 oC. Suez Canal Univ. J. Food Sci. 2016, 3, 35–44. [Google Scholar]
- Hęś, M.; Szwengiel, A.; Dziedzic, K.; le Thanh-Blicharz, J.; Kmiecik, D.; Górecka, D. The effect of buckwheat hull extract on lipid oxidation in frozen-stored meat products. J. Food Sci. 2017, 82, 882–889. [Google Scholar] [CrossRef] [PubMed]
- Falowo, A.B.; Fayemi, P.O.; Muchenje, V. Natural antioxidants against lipid-protein oxidative deterioration in meat and meat products: A review. Food Res. Int. 2014, 64, 171–181. [Google Scholar] [CrossRef]
| Source | Main Polyphenols (Concentration) | Ref. |
|---|---|---|
| CEREALS AND PSEUDO-CEREALS | ||
| Black rice (Oryza sativa L.) | Ferulic acid (26.1–215.2 μg/g), p-coumaric acid (6.6–28.5 μg/g), isoferulic acid (6.3–45.3 μg/g), cyanidin-3-glucoside (0.56–0.79 mg/g), and peonidin-3-glucoside (0.11–0.16 mg/g | [33] |
| Ferulic acid (23.0–43.9 mg/100 g), vanillic acid (4.4–10.5 mg/100 g), and p-coumaric acid (2.0–6.6 mg/100 g) | [34] | |
| Buckwheat (Fagopyrum esculentum Moench) | Quercetin-3-rutinoside (3.3–151.4 mg/100 g), isoorientin (0.1–2.7 mg/100 g), caffeic acid-pentoside (0.1–6.7 mg/100 g), procyanidin trimer (0.1–5.5 mg/100 g), and epiafzelechin-epicatechin (0.2–5.3 mg/100 g) | [35] |
| 1-O-Caffeoyl-6-O-rhamnopyranosyl-glycopyranoside (0.3–9.1 mg/100 g), epicatechin-gallate (25.4–91.3 mg/100 g), vitexin/isovitexin (101.6–188.8 mg/100 g), hyperin (53.5–274.1 mg/100 g), rutin (62.4–173.6 mg/100 g), and orientin/isorientin (8.4–26.3 mg/100 g) | [36] | |
| Chia (Salvia hispanica L.) | Myricetin (7.0–1198.6 mg/g), quercetin (4.8–29.6 mg/g), protocatechuic acid (3.0–29.6 mg/g), and salicylic acid (6.2–241.2 mg/g) | [37] |
| Rosmarinic acid (738.2 μg/g), caffeic acid (178.6 μg/g), quercetin (309.5 μg/g), and daidzein (110.5 μg/g) | [38] | |
| FRUITS | ||
| Cacao (Theobroma cacao L.) | Epicatechin (4.4–35.0 mg/g) and catechin (0.6–4.7mg/g) | [40] |
| Epicatechin (7.0–15.8 mg/g) and catechin (1.0–6.2 mg/g) | [39] | |
| Grape seed (Vitis vinífera L.) | Epicatechin gallate trimer (150.0–383.0 mg/g), epicatechin gallate (180.6 mg/g), and epicatechin (13.6 mg/g) | [42] |
| Guarana seed (Paullinia cupana) | Tyrosol (14.7 g/kg) | [46] |
| Epicatechin (3.7–9.2 mg/g) and catechin (5.2–11.6 mg/g) | [44] | |
| PULSES AND NUTS | ||
| Lentil (Lens culinaris) | Catechin glucoside (2.2–6.6 mg/g), quercetin-O-pentoside (2.1–3.3 mg/g), prodelphinidin dimer (1.4–5.8 mg/g), and procyanidin dimer (1.6–4.3 mg/g) | [47] |
| Peanut skin (Arachis hypogaea L.) | Catechin (20.7 mg/100 g) and protocatechuic acid (3.8 mg/100 g) | [48] |
| Proanthocyanidins (0.8–8.3 mg/100 g), di-p-coumaroyltartaric acid (13.8 mg/100 g), and p-coumaroylsinapoyltartaric acid (6.3 mg/100 g) | [49] | |
| Pistachio (Pistacia vera L.) | Isoquercetin (27.3–578.2 mg/g), myrcitin-3-glucoside (62.7–75.3 mg/g), and quercetin-3-glucuronide (6.0–106.1 mg/g) | [50] |
| Naringenin (28.1–49.9 μg/g), catechin (29.1–53.6 μg/g), and gallic acid (2.3–38.6 μg/g) | [51] | |
| CRUCIFEROUS VEGETABLES | ||
| Mustard (Sinapis alba) | Sinapine (10.2 mg/g), sinapoyl glucose (0.66 mg/g), and sinapic acid (0.19 mg/g) | [52] |
| OTHER SOURCES | ||
| Acorn (Quercus spp.) | Gallic acid (3.6–417.5 μg/g), trigalloyl glucose (2.5–150.2 μg/g), trigalloyl-hexahydrodiphenoyl-glucose (1.4–240.8 μg/g), and valoneic acid dilactone (1.7–40.4 μg/g) | [53] |
| Euryale ferox | Gallic acid (107.5–392.2 mg/g), rutin (44.8–85.3 mg/g), and catechin (21.2–162.3 mg/g) | [54] |
| Source | Extraction Conditions | Phenolic Content and Antioxidant Activity | Ref. |
|---|---|---|---|
| CEREALS AND PSEUDOCEREALS | |||
| Black rice (Oryza sativa L.) | Conventional extraction: temperature (10, 30, and 50 °C); time (20, 50 and 80 min); and solid/solvent ratio (1 g/15, 30, and 45 mL) | TPC 1: 520.17 mg GAE 2/100 g; AA 3: 46.5% of DPPH 4 inhibition; optimum conditions: 34.7 °C, 80 min, 1 g/30 mL | [65] |
| Ultrasound: frequency (35 kHz); temperature (30–60°C); pH (2–4); solvent (20–60% ethanol); and time (10–60 min) | TPC: 2124.98 mg GAE/100 g; optimum conditions: 36.0 °C, pH 2.5, 23.8% ethanol, and 22.9 min | [66] | |
| Buckwheat (Fagopyrum esculentum Moench) | Microwave irradiation: time (15 min); solid/solvent ratio (1 g/50 mL); rotation (320 rpm); temperature (23–150 °C); and solvent (0, 50, and 100% ethanol) | TPC: 18.5 mg GAE/g; optimum conditions: 150 °C, 50% ethanol | [67] |
| Chia (Salvia hispanica L.) | Conventional extraction: solid/solvent ratio (1 g/10 mL); solvent (0, 20, 50, 80, and 100% ethanol); extraction cycles (1–4); and time (1–72 h) | TPC: 42% increase; optimum conditions: 50% ethanol, and 4 cycles of 1 h each | [68] |
| FRUITS | |||
| Cacao bean shell (Theobroma cacao L.) | Pressurized liquid extraction: solid/solvent ratio (1 g/3 mL); pressure (10.35 MPa); time (5, 30; 50 min); and temperature (60–90 °C) | Increasing time and temperature increased the extraction of flavonols (90 °C, 50 min) and antioxidant activity (DPPH and FRAP 5 assays); optimum conditions: 90 °C and 50 min for polyphenols; and 75 °C and 50 min for antioxidant activity | [69] |
| Grape seed (Vitis vinífera L.) | Ultrasound: frequency (20 kHz); power (150 W); time (15 min); temperature (<30 °C); and solvent (methanol); Soxhlet: temperature (RT 6); time (12 h); solvent (methanol) | TPC: 104.19 mg GAE/g; AA: 109.3 aTocE 7/g for DPPH radical assay; defatted seeds with ultrasound followed by Soxhlet extraction of polyphenols | [70] |
| Microwave irradiation: time (15 min); solid/solvent ratio (1 g/10–50 mL); solvent (10–90% ethanol); time (2-32 min); and temperature (40–60 °C) | TPC: 96.3 mg GAE/g; optimum conditions: 47.2% ethanol, 1 g/45.3 mL, ratio, 4.6 min | [71] | |
| Guarana seed (Paullinia cupana) | Conventional extraction (cold): solid/solvent ratio (1 g/3 g); temperature (25 °C); time (24 h); particle size (25, 125, and 1680 μm); and solvent (0, 50, 65, 80, and 100% ethanol) | Highest TPC was obtained with 1680 μm particles and 50% ethanol solution | [72] |
| Conventional extraction (hot): solid/solvent ratio (1 g/3 g); agitation (48 rpm); temperature (40, 50, and 60 °C); and time (1–6 h) | Highest TPC was obtained after 6 h, no effect of temperature (40–60 °C) | [72] | |
| Enzyme-assisted extraction: solid/solvent ratio (1 g/3 g); time (4 h); agitation (48 rpm); pH (4.8); time (4 h); enzyme (pectinase:celulase, 1:0, 1:1, 0:1); and temperature (40 and 50 °C) | Highest TPC was obtained using pectinase alone 50 °C | [72] | |
| Super-critical CO2: flow (6 mL/min); pressure (100, 200, and 300 bar); temperature (40, 50, and 60 °C); co-solvent (0, 10, 20, 40% of methanol, ethanol, and their 1:1 combination); and time (20, 40, and 60 min) | TPC: 105.76 mg PE 8/g; optimum conditions: 300 bar, 40 °C, 40% of 1:1 ethanol:methanol, and 40 min | [73] | |
| NUTS | |||
| Peanut skin (Arachis hypogaea L.) | Conventional extraction: time (5–150 min); temperature (25–90 °C); and solvent (20–100% ethanol) | TPC: 0.39 g GAE/g; AA: 79.9% of inhibition in the DPPH radical assay and IC50 of 0.26 μg/mL in the ABTS 9 radical assay; optimum conditions: 71.6 °C and 74% ethanol | [74] |
| Sub-critical water: temperature (140, 180 and 220 °C); flow (3, 5, and 7 g/min); and solvent (0, 50 and 95% ethanol) | TPC: 136.9 mg/g; AA: IC50 of 10.5 μg/mL in DPPH radical assay and IC50 of 17.05 μg/mL optimum conditions: 220 °C, 7 g/min, and 60.5% ethanol | [75] | |
| Pistachio (Pistacia vera L.) | Sub-critical water: solid/solvent ratio (1 g/25 mL); pressure (6.9 MPa); flow (4 mL/min); and temperature (110–190 °C) | TPC: 39.5 and 39.4 g/kg at 150 and 170 °C, respectively; AA: ABTS radical (1.18 mmol TE 10/g), DPPH radical (0.84 mmol TE/g) and FRAP (1.20 mmol TE/g) assays at 190 °C | [76] |
| Ultrasound: solid/solvent ratio (1 g/60 mL); and solvent (methanol:water:formic acid; 80:19:1) | TPC of 81.8 g/kg; AA: 0.47, 0.51, and 0.49 mmol TE/g for ABTS, DPPH, and FRAP assays, respectively | [76] | |
| OTHER SOURCES | |||
| Acorn (Quercus spp.) | Eco-friendly extraction: solid/solvent ratio (1 g/50 mL); time (10 min); solvent (water:glycerol:CD; 40–100%:0–60%:1–13%); and temperature (40, 60, and 80 °C) | TPC: 122.2 mg/g; AA: DPPH radical (1209.8 μmol TE/g) and reducing power (555.8 µmol AAE 11/g) assays optimum conditions: water: glycerol: CD 12, 27:60:13 and 80 °C | [77] |
| Source and Concentration | Meat Product | Sampling Point or Storage Conditions | Effect on Color | Ref. |
|---|---|---|---|---|
| Grape seed (0.08 and 0.16%) | Cooked chicken sausage | 40 days at 4 °C | Improved the preservation of redness | [84] |
| Grape seed (10 g/kg) | Dry-fermented pork sausages | After ripening | Similar to control with nitrite | [42] |
| Grape seed (50, 200 and 1000 mg/kg) | Dry-cured sausage | During ripening and during 7 months at 4 °C | Effect was dependent of extract concentration; higher redness values were obtained from 200 mg/kg treatment | [85] |
| Guarana seed (250, 500, and 1000 mg/kg) | Raw pork patty | 18 days at 2 °C | Improved the preservation of redness and reduced the formation of metmyoglobin (1000 mg/kg) | [46] |
| Guarana seed (250 mg/kg) | Raw lamb burgers | 18 days at 2 °C | Slowed the reduction of redness and formation of metmyoglobin | [86] |
| Peanut skin (1000 mg/kg) | Raw sheep patties | 20 days at 2 °C | Improved the stability of redness | [87] |
| Peanut skin (70 mg GAE1/kg) | Cooked chicken patties | 15 days at 1 °C | Reduced the loss of redness | [88] |
| Peanut skin (2000 mg/kg) | Dry-cured sausage (Spanish salchichón) | After ripening | Not significant effect | [48] |
| Peanut skin (1000 mg/kg) | Pork liver pâté | 60 days at 4 °C | Slight differences | [89] |
| Acorn (1000 mg phenolics/L) | Raw chicken meat | 14 days at 2 °C | Preservation of redness | [59] |
| Black rice (0.4%, 0.8%, and 1.2%) | Raw beef patties | 6 days at 2 °C | Reduced the loss of redness | [90] |
| Euryale ferox seed kernels (500 mg/kg) | Cooked pork sausage | 10 weeks at 8 °C | Not significant effect on redness | [54] |
| Yellow, brown, and black mustard seeds (2.0%) | Raw beef meatballs | 15 days at 4 °C | Reduced redness | [91] |
| Chia seed (1.0%, 1.5%, and 2.0%) | Fresh pork sausage | 28 days at 4 °C | Lower redness than control | [92] |
| Source | Meat Product | Sampling Point or Storage Conditions | Effect on Lipid and Protein Oxidation | Ref. |
|---|---|---|---|---|
| Black rice (0.4%, 0.8%, and 1.2%) | Raw beef patties | Six days at 2 °C | All extract inhibited the lipid oxidation | [90] |
| Black rice (0.2–1.0 g/100 g) | Sai Krok Isan | Four days at 4 °C | Reduced the formation of peroxides and TBARS1 | [105] |
| Grape seed (0.1% and 0.2%) | Raw buffalo patties | Nine days at 8 °C | All extracts inhibited lipid oxidation | [106] |
| Grape seed (0.08% and 0.16%) | Cooked chicken sausage | 40 days at 4 °C | Lower values than control | [84] |
| Grape seed (10 g/kg) | Dry-fermented pork sausages | After ripening | Similar antioxidant effect to sodium nitrite; higher formation of volatile compounds from lipid oxidation | [42] |
| Grape seed (50, 200, and 1000 mg/kg) | Dry-cured sausage | During ripening and during 7 months at 4 °C | Inhibited lipid oxidation during both ripening and storage periods | [85] |
| Guarana seed (250, 500, and 1000 mg/kg) | Raw pork patties | 18 days at 2 °C | All extract reduced the formation of carbonyls and TBARS | [46] |
| Guarana seed (250 mg/kg) | Raw lamb burgers | 18 days at 2 °C | Slowed lipid and protein oxidation up to 12 days; inhibited the formation of volatile aldehydes | [86] |
| Peanut skin (1000 mg/kg) | Raw sheep patties | 20 days at 2 °C | Slowed both lipid and protein oxidation | [87] |
| Peanut skin (70 mg GAE2/kg) | Cooked chicken patties | 15 days at 1 °C | Inhibited lipid oxidation | [88] |
| Peanut skin (2000 mg/kg) | Dry-cured sausage (Spanish salchichón) | After ripening | No effect on lipid oxidation; reduction of protein oxidation | [48] |
| Peanut skin (1000 mg/kg) | Pork liver pâté | 160 days at 4 °C | Not significant effect | [89] |
| Pistachio seed hull (2.0%, 5.0%, and 7.0%) | Cooked chicken burger | 10 days 4 °C | Lowest TBARS values sing 5 and 7% | [94] |
| Lentil coat powder (1.0, 2.0 and 3.0%) | Raw beef burgers | 12 days at 4 °C | Slowed the evolution of lipid oxidation | [107] |
| Buckwheat hulls (0.5%) | Cooked pork meatballs | 180 days at −18 °C | Reduced the formation of peroxides and TBARS | [108] |
| Cacao bean husk (0.25%, 0.5%, 1.0%, and 2.0%) | Cooked pork sausage | Seven days at 4 °C | Inhibition in a concentration-dependent manner | [93] |
| Euryale ferox seed kernels (500 mg/kg) | Cooked pork sausage | 10 weeks at 8 °C | Inhibited lipid oxidation throughout storage | [54] |
| Acorn (1000 mg phenolics/L) | Raw chicken meat | 14 days at 2 °C | All extracts reduced lipid and protein oxidation | [59] |
| Yellow, brown, and black mustard seeds (2.0%) | Raw beef meatballs | 15 days at 4 °C | All powders slowed lipid oxidation | [91] |
| Chia seeds (1.0%, 1.5%, and 2.0%) | Fresh pork sausage | During 28 days at 4 °C | Slowed the generation of lipid oxidation products | [92] |
| Source | Meat Product | Sampling Point or Storage Conditions | Effect on Sensory Attributes | Ref. |
|---|---|---|---|---|
| Grape seed (0.1% and 0.2%) | Raw buffalo patties | After nine days at 8 °C | 0.1% extract preserved the odor and overall acceptance | [106] |
| Grape seed (0.08% and 0.16%) | Cooked chicken sausage | After cooking | Similar scores for odor, color and overall acceptance | [84] |
| Grape seed (10 g/kg) | Dry-fermented pork sausages | After ripening | Lower acceptance for color uniformity, redness than control; rancidity was not perceived | [42] |
| Cacao bean husk (0.25%, 0.5%, 0.75%, 1.0%, and 2.0%) | Cooked pork sausage | After cooking | Preserved color, flavor and overall acceptance; optimum concentration: 0.75% | [93] |
| Peanut skin (1000 mg/kg) | Raw sheep patties | 20 days at 2 °C | Extended the acceptance of red color, reduced superficial discoloration and formation of off-odor | [87] |
| Lentil coat powder (1.0%, 2.0%, and 3.0%) | Cooked beef burgers | 12 days at 4 °C | Improved the preservation of color, odor and overall acceptability; negative influence of concentration | [107] |
| Acorn (1000 mg phenolics/L) | Raw chicken meat | 14 days at 2 °C | Not significant effect on color acceptance | [59] |
| Chia seed (1.0%, 1.5%, and 2.0%) | Fresh pork sausage | After processing | Similar scores to control with antioxidant for appearance, color, odor and intention to purchase; slightly effect in taste (2%) | [92] |
| Yellow, brown, and black mustard seeds (2.0%) | Raw beef meatballs | 15 days at 4 °C | Decreased during storage; yellow mustard burgers received the highest scores | [91] |
| Pistachio seed hull (2.0%, 5.0%, and 7.0%) | Cooked chicken burger | After cooking | Reduced acceptance of color (5 and 7%) | [94] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Munekata, P.E.S.; Gullón, B.; Pateiro, M.; Tomasevic, I.; Domínguez, R.; Lorenzo, J.M. Natural Antioxidants from Seeds and Their Application in Meat Products. Antioxidants 2020, 9, 815. https://doi.org/10.3390/antiox9090815
Munekata PES, Gullón B, Pateiro M, Tomasevic I, Domínguez R, Lorenzo JM. Natural Antioxidants from Seeds and Their Application in Meat Products. Antioxidants. 2020; 9(9):815. https://doi.org/10.3390/antiox9090815
Chicago/Turabian StyleMunekata, Paulo E. S., Beatriz Gullón, Mirian Pateiro, Igor Tomasevic, Ruben Domínguez, and José M. Lorenzo. 2020. "Natural Antioxidants from Seeds and Their Application in Meat Products" Antioxidants 9, no. 9: 815. https://doi.org/10.3390/antiox9090815









