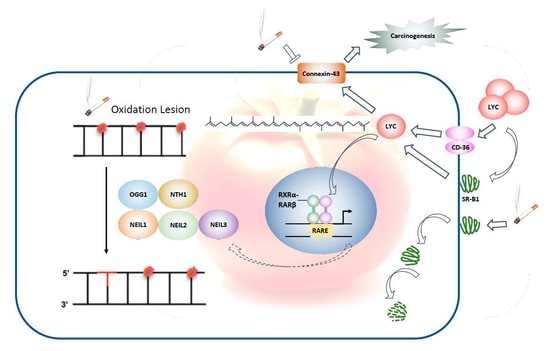
Lycopene Protects against Smoking-Induced Lung Cancer by Inducing Base Excision Repair
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
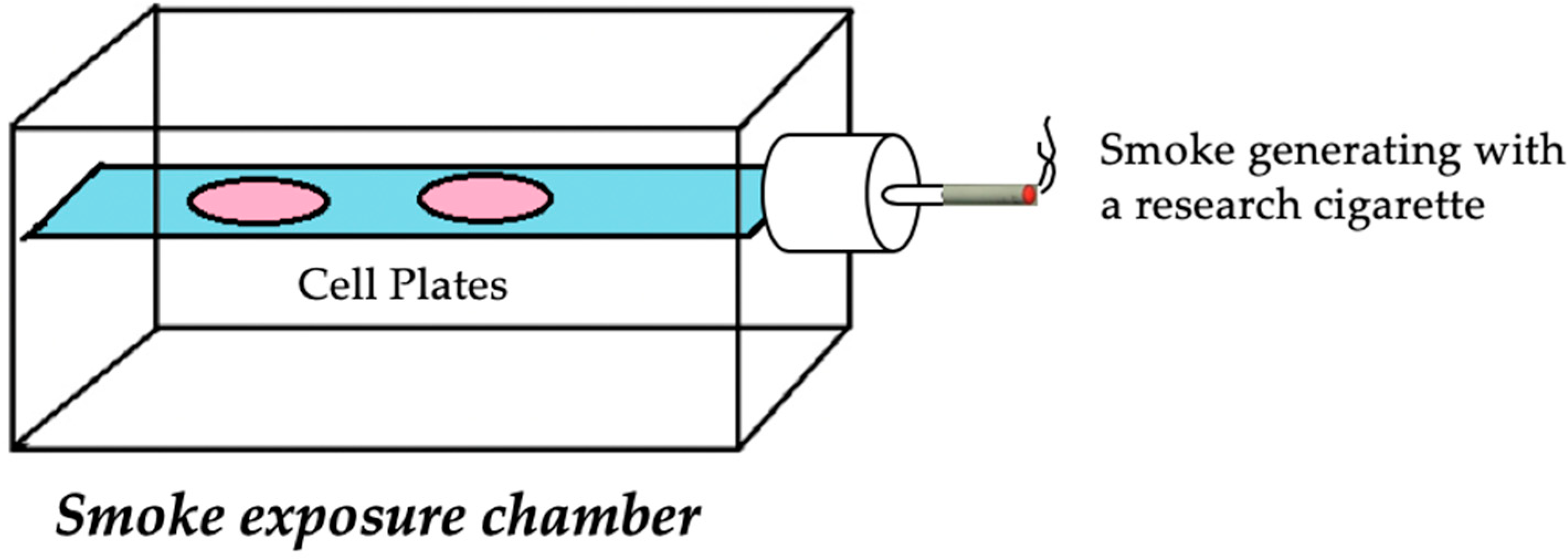
2.2. Cell Culture
2.3. RNA Isolation
2.4. cDNA Synthesis and Quantitative PCR
2.5. Western Blotting
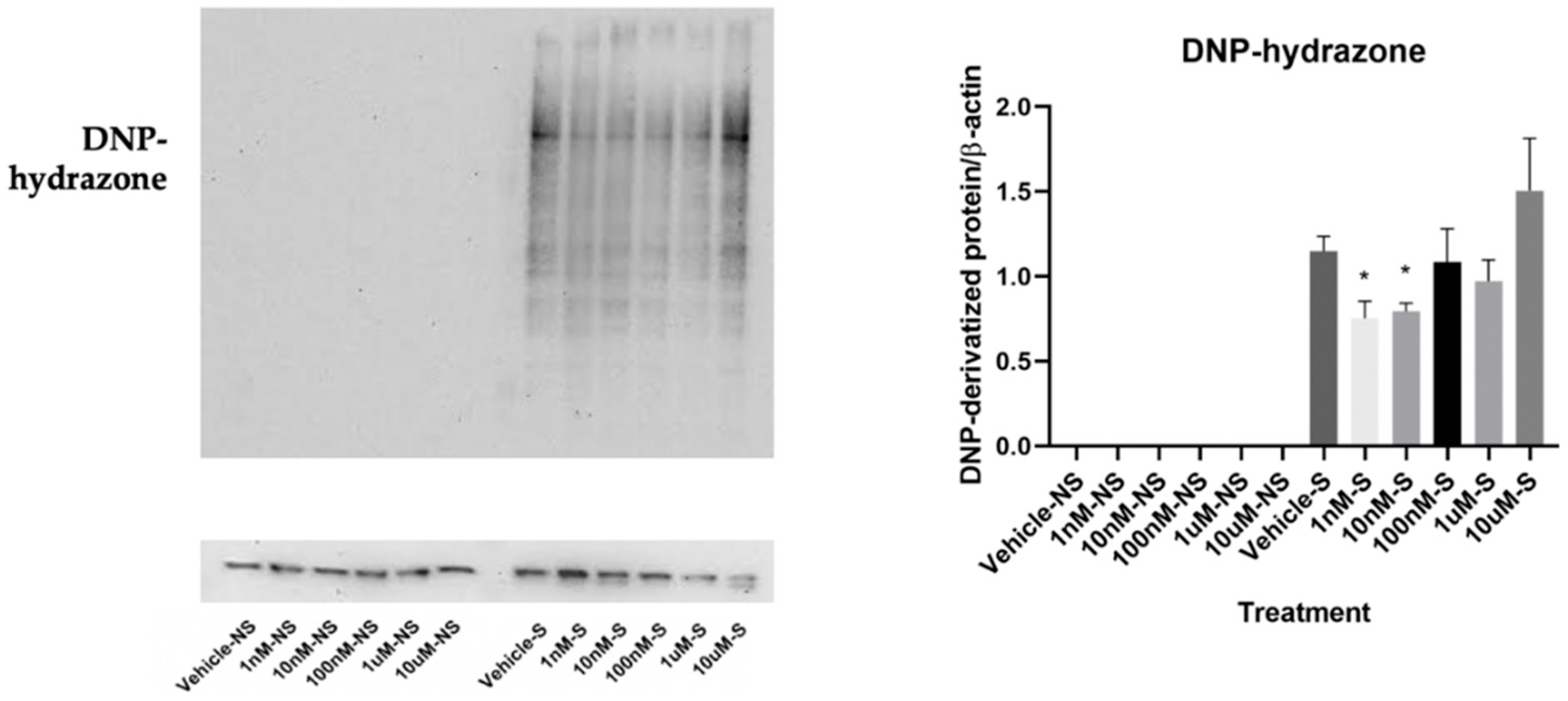
2.6. OxyBlot
2.7. Statistical Analysis
3. Results
3.1. Lycopene Inhibited Smoking-Induced Oxidative Stress
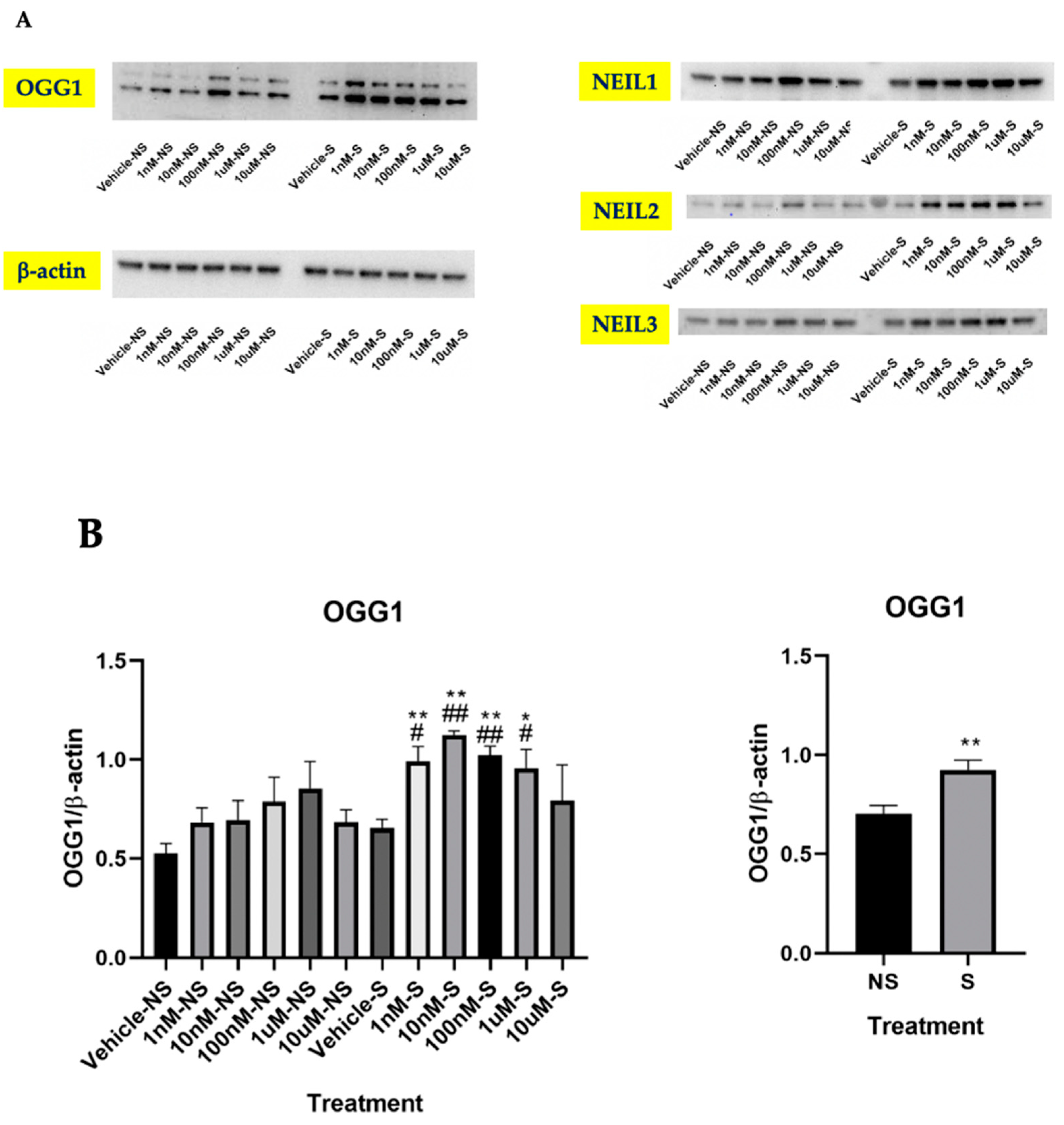
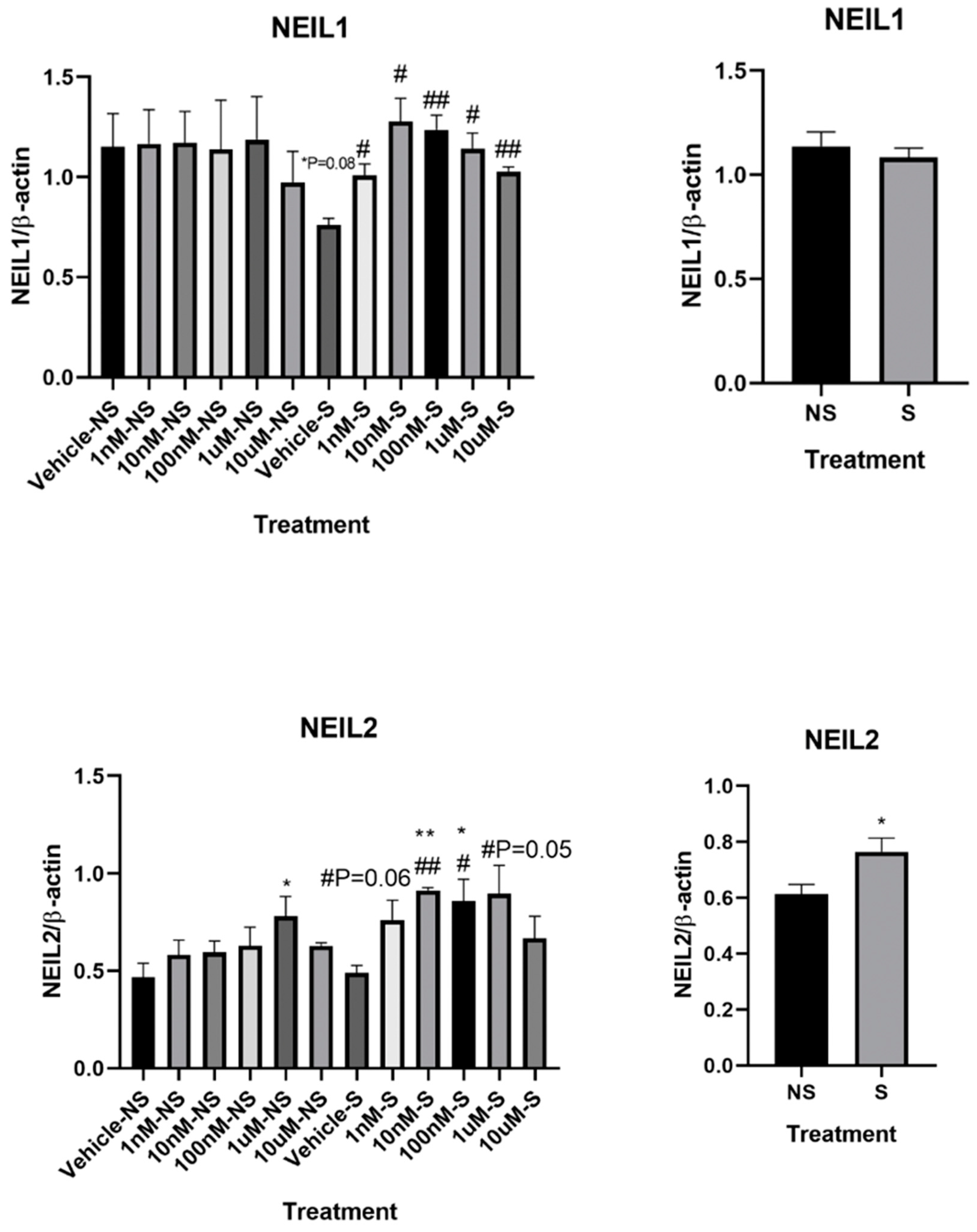
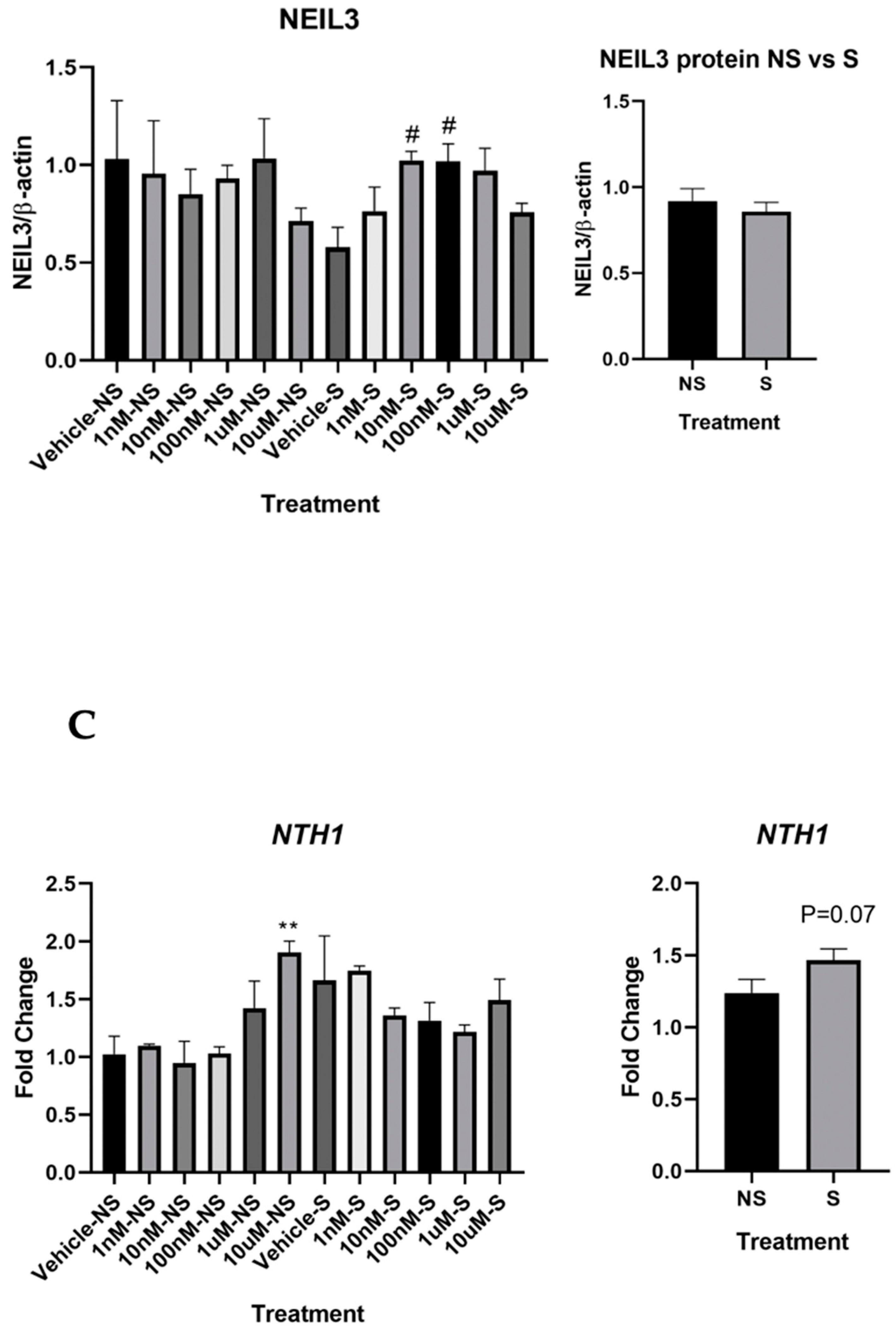
3.2. Lycopene Increased Base Excision Repair in the Cells Exposed to Smoke
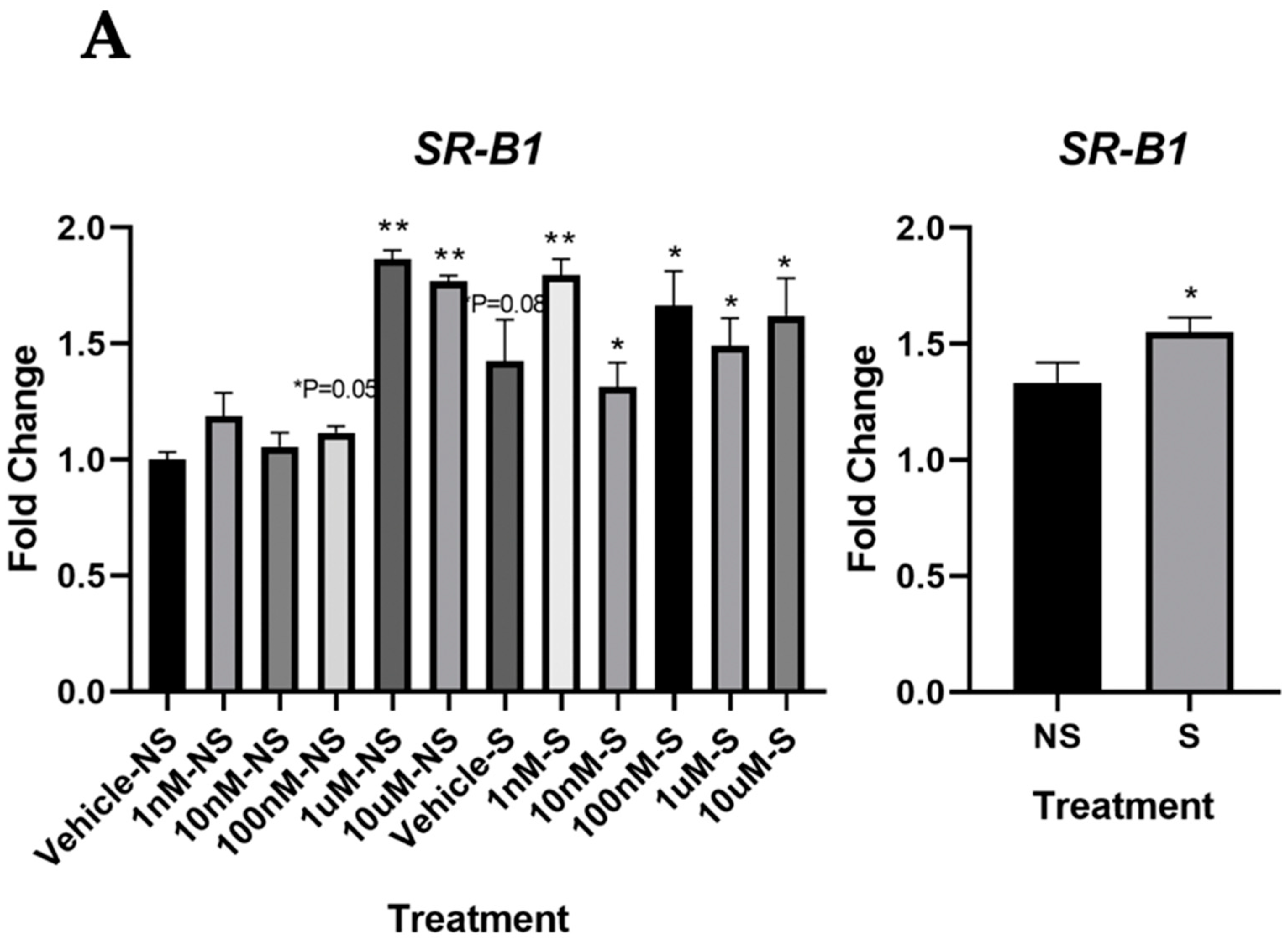
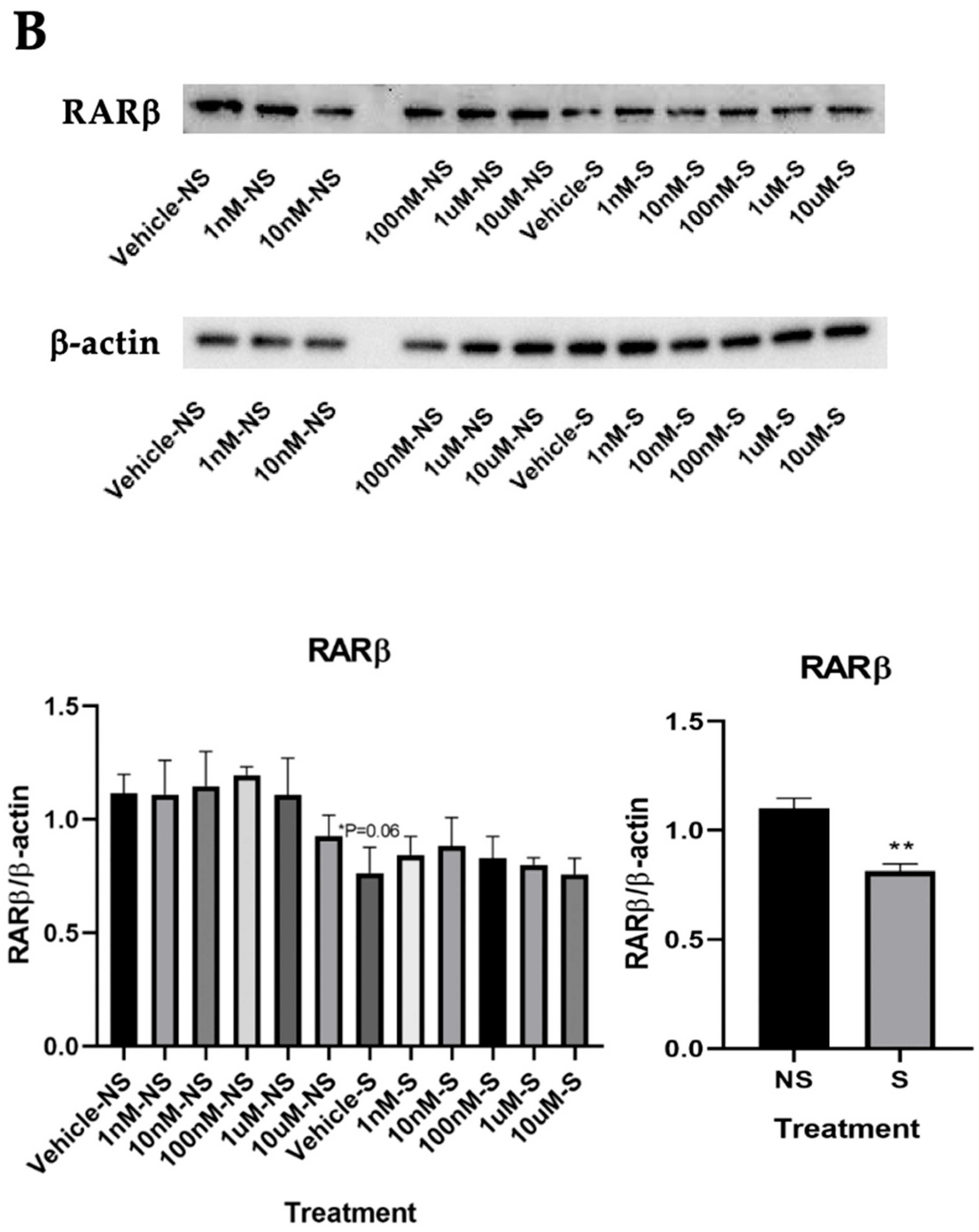
3.3. Lycopene Uptake Was Increased in the Cells Exposed to Smoke
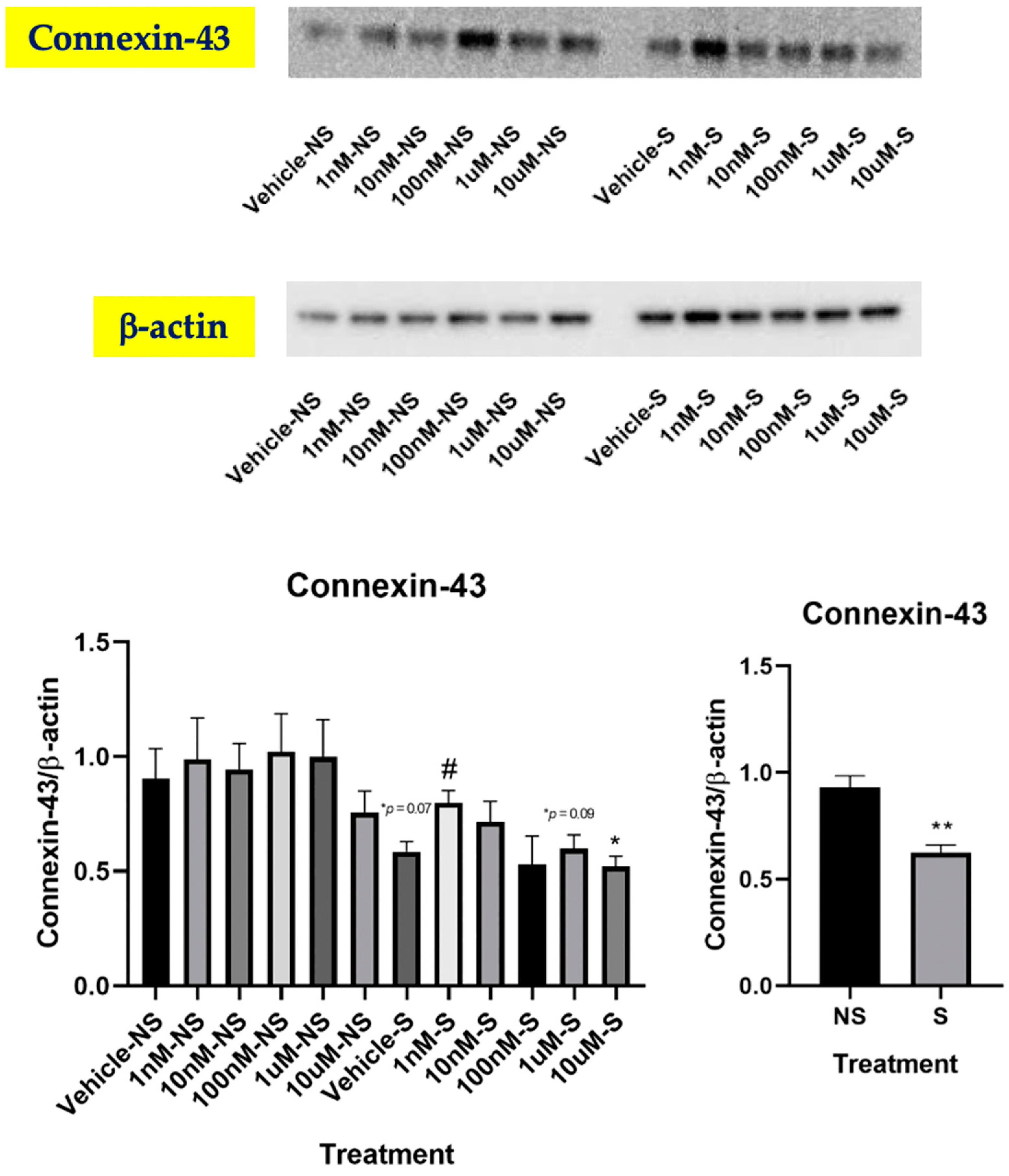
3.4. Lycopene Increased Gap Junctional Communication
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Organization WH: Tobacco. 2019. Available online: https://www.who.int/news-room/fact-sheets/detail/cancer (accessed on 19 July 2020).
- O’Keeffe, L.M.; Taylor, G.; Huxley, R.R.; Mitchell, P.; Woodward, M.; Peters, S.A.E. Smoking as a risk factor for lung cancer in women and men: A systematic review and meta-analysis. BMJ Open 2018, 8, e021611. [Google Scholar] [CrossRef]
- Klaunig, J.E. Oxidative Stress and Cancer. Curr. Pharm. Des. 2018, 24, 4771–4778. [Google Scholar] [CrossRef] [PubMed]
- Eroglu, A.; Harrison, E.H. Carotenoid metabolism in mammals, including man: Formation, occurrence, and function of apocarotenoids. J. Lipid Res. 2013, 54, 1719–1730. [Google Scholar] [CrossRef] [PubMed]
- De Mejia, E.G.; Zhang, Q.; Penta, K.; Eroglu, A.; Lila, M.A. The Colors of Health: Chemistry, Bioactivity, and Market Demand for Colorful Foods and Natural Food Sources of Colorants. Annu. Rev. Food Sci. Technol. 2020, 11, 145–182. [Google Scholar] [CrossRef] [PubMed]
- Romanchik, J.E.; Morel, D.W.; Harrison, E.H. Distributions of carotenoids and alpha-tocopherol among lipoproteins do not change when human plasma is incubated in vitro. J. Nutr. 1995, 125, 2610–2617. [Google Scholar]
- Shyam, R.; Vachali, P.; Gorusupudi, A.; Nelson, K.; Bernstein, P.S. All three human scavenger receptor class B proteins can bind and transport all three macular xanthophyll carotenoids. Arch. Biochem. Biophys. 2017, 634, 21–28. [Google Scholar] [CrossRef]
- Wang, X.D. Lycopene metabolism and its biological significance. Am. J. Clin. Nutr. 2012, 96, 1214s–1222s. [Google Scholar] [CrossRef]
- Krinsky, N.I.; Yeum, K.J. Carotenoid-radical interactions. Biochem. Biophys. Res. Commun. 2003, 305, 754–760. [Google Scholar] [CrossRef]
- Kaulmann, A.; Bohn, T. Carotenoids, inflammation, and oxidative stress--implications of cellular signaling pathways and relation to chronic disease prevention. Nutr. Res. 2014, 34, 907–929. [Google Scholar] [CrossRef]
- Harrison, E.H.; dela Sena, C.; Eroglu, A.; Fleshman, M.K. The formation, occurrence, and function of beta-apocarotenoids: Beta-carotene metabolites that may modulate nuclear receptor signaling. Am. J. Clin. Nutr. 2012, 96, 1189s–1192s. [Google Scholar] [CrossRef]
- Chatterjee, M.; Roy, K.; Janarthan, M.; Das, S.; Chatterjee, M. Biological activity of carotenoids: Its implications in cancer risk and prevention. Curr. Pharm. Biotechnol. 2012, 13, 180–190. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; Zhang, W.; Wang, X.; Zhao, K.; Negi, D.S.; Zhuo, L.; Qi, M.; Wang, X.; Zhang, X. Lycopene and Risk of Prostate Cancer: A Systematic Review and Meta-Analysis. Medicine (Baltimore) 2015, 94, e1260. [Google Scholar] [CrossRef] [PubMed]
- Li, C.C.; Liu, C.; Fu, M.; Hu, K.Q.; Aizawa, K.; Takahashi, S.; Hiroyuki, S.; Cheng, J.; von Lintig, J.; Wang, X.D. Tomato Powder Inhibits Hepatic Steatosis and Inflammation Potentially Through Restoring SIRT1 Activity and Adiponectin Function Independent of Carotenoid Cleavage Enzymes in Mice. Mol. Nutr. Food Res. 2018, 62, e1700738. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.; Miao, B.; Hu, K.Q.; Fu, X.; Wang, X.D. Apo-10′-lycopenoic acid inhibits cancer cell migration and angiogenesis and induces peroxisome proliferator-activated receptor gamma. J. Nutr. Biochem. 2018, 56, 26–34. [Google Scholar] [CrossRef] [PubMed]
- Ip, B.C.; Liu, C.; Ausman, L.M.; von Lintig, J.; Wang, X.D. Lycopene attenuated hepatic tumorigenesis via differential mechanisms depending on carotenoid cleavage enzyme in mice. Cancer Prev. Res. 2014, 7, 1219–1227. [Google Scholar] [CrossRef]
- Kim, J.H.; Lee, J.; Choi, I.J.; Kim, Y.I.; Kwon, O.; Kim, H.; Kim, J. Dietary Carotenoids Intake and the Risk of Gastric Cancer: A Case-Control Study in Korea. Nutrients 2018, 10, 1031. [Google Scholar] [CrossRef]
- Lim, J.Y.; Wang, X.D. Mechanistic understanding of beta-cryptoxanthin and lycopene in cancer prevention in animal models. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2020. [Google Scholar] [CrossRef]
- Nara, E.; Hayashi, H.; Kotake, M.; Miyashita, K.; Nagao, A. Acyclic carotenoids and their oxidation mixtures inhibit the growth of HL-60 human promyelocytic leukemia cells. Nutr. Cancer 2001, 39, 273–283. [Google Scholar] [CrossRef]
- Kotake-Nara, E.; Kushiro, M.; Zhang, H.; Sugawara, T.; Miyashita, K.; Nagao, A. Carotenoids affect proliferation of human prostate cancer cells. J. Nutr. 2001, 131, 3303–3306. [Google Scholar] [CrossRef]
- Lian, F.; Smith, D.E.; Ernst, H.; Russell, R.M.; Wang, X.D. Apo-10′-lycopenoic acid inhibits lung cancer cell growth in vitro, and suppresses lung tumorigenesis in the A/J mouse model in vivo. Carcinogenesis 2007, 28, 1567–1574. [Google Scholar] [CrossRef]
- Kotake-Nara, E.; Kim, S.J.; Kobori, M.; Miyashita, K.; Nagao, A. Acyclo-retinoic acid induces apoptosis in human prostate cancer cells. Anticancer Res. 2002, 22, 689–695. [Google Scholar] [PubMed]
- Stahl, W.; von Laar, J.; Martin, H.D.; Emmerich, T.; Sies, H. Stimulation of gap junctional communication: Comparison of acyclo-retinoic acid and lycopene. Arch. Biochem. Biophys. 2000, 373, 271–274. [Google Scholar] [CrossRef] [PubMed]
- Veeramachaneni, S.; Wang, X.D. Carotenoids and lung cancer prevention. Front. Biosci. 2009, 1, 258–274. [Google Scholar] [CrossRef]
- Torres-Gonzalez, M.; Gawlowski, T.; Kocalis, H.; Scott, B.T.; Dillmann, W.H. Mitochondrial 8-oxoguanine glycosylase decreases mitochondrial fragmentation and improves mitochondrial function in H9C2 cells under oxidative stress conditions. Am. J. Physiol. Cell Physiol. 2014, 306, C221–C229. [Google Scholar] [CrossRef]
- Arnett, S.D.; Osbourn, D.M.; Moore, K.D.; Vandaveer, S.S.; Lunte, C.E. Determination of 8-oxoguanine and 8-hydroxy-2′-deoxyguanosine in the rat cerebral cortex using microdialysis sampling and capillary electrophoresis with electrochemical detection. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2005, 827, 16–25. [Google Scholar] [CrossRef][Green Version]
- Katerji, M.; Filippova, M.; Duerksen-Hughes, P. Approaches and Methods to Measure Oxidative Stress in Clinical Samples: Research Applications in the Cancer Field. Oxid Med. Cell Longev. 2019, 2019, 1279250. [Google Scholar] [CrossRef]
- Wang, R.; Hao, W.; Pan, L.; Boldogh, I.; Ba, X. The roles of base excision repair enzyme OGG1 in gene expression. Cell Mol. Life Sci. 2018, 75, 3741–3750. [Google Scholar] [CrossRef]
- Wallace, S.S. Base excision repair: A critical player in many games. DNA Repair 2014, 19, 14–26. [Google Scholar] [CrossRef]
- Hazra, T.K.; Das, A.; Das, S.; Choudhury, S.; Kow, Y.W.; Roy, R. Oxidative DNA damage repair in mammalian cells: A new perspective. DNA Repair 2007, 6, 470–480. [Google Scholar] [CrossRef]
- Boesch, P.; Weber-Lotfi, F.; Ibrahim, N.; Tarasenko, V.; Cosset, A.; Paulus, F.; Lightowlers, R.N.; Dietrich, A. DNA repair in organelles: Pathways, organization, regulation, relevance in disease and aging. Biochim. Biophys. Acta 2011, 1813, 186–200. [Google Scholar] [CrossRef]
- Bravard, A.; Vacher, M.; Gouget, B.; Coutant, A.; de Boisferon, F.H.; Marsin, S.; Chevillard, S.; Radicella, J.P. Redox regulation of human OGG1 activity in response to cellular oxidative stress. Mol. Cell Biol. 2006, 26, 7430–7436. [Google Scholar] [CrossRef] [PubMed]
- Paz-Elizur, T.; Sevilya, Z.; Leitner-Dagan, Y.; Elinger, D.; Roisman, L.C.; Livneh, Z. DNA repair of oxidative DNA damage in human carcinogenesis: Potential application for cancer risk assessment and prevention. Cancer Lett. 2008, 266, 60–72. [Google Scholar] [CrossRef] [PubMed]
- Hamosh, M.; Shechter, Y.; Hamosh, P. Effect of tobacco smoke on the metabolism of rat lung. Arch. Environ. Health 1979, 34, 17–23. [Google Scholar] [CrossRef] [PubMed]
- Muresan, X.M.; Cervellati, F.; Sticozzi, C.; Belmonte, G.; Chui, C.H.; Lampronti, I.; Borgatti, M.; Gambari, R.; Valacchi, G. The loss of cellular junctions in epithelial lung cells induced by cigarette smoke is attenuated by corilagin. Oxid Med. Cell Longev. 2015, 2015, 631758. [Google Scholar] [CrossRef]
- Valacchi, G.; Davis, P.A.; Khan, E.M.; Lanir, R.; Maioli, E.; Pecorelli, A.; Cross, C.E.; Goldkorn, T. Cigarette smoke exposure causes changes in Scavenger Receptor B1 level and distribution in lung cells. Int. J. Biochem. Cell Biol. 2011, 43, 1065–1070. [Google Scholar] [CrossRef]
- Sies, H.; Stahl, W. Carotenoids and intercellular communication via gap junctions. Int. J. Vitam Nutr. Res. 1997, 67, 364–367. [Google Scholar]
- Ford, N.A.; Elsen, A.C.; Zuniga, K.; Lindshield, B.L.; Erdman, J.W., Jr. Lycopene and apo-12’-lycopenal reduce cell proliferation and alter cell cycle progression in human prostate cancer cells. Nutr. Cancer 2011, 63, 256–263. [Google Scholar] [CrossRef]
- Gitenay, D.; Lyan, B.; Talvas, J.; Mazur, A.; Georgé, S.; Caris-Veyrat, C.; Rock, E. Serum from rats fed red or yellow tomatoes induces Connexin43 expression independently from lycopene in a prostate cancer cell line. Biochem. Biophys. Res. Commun. 2007, 364, 578–582. [Google Scholar] [CrossRef]
- Aizawa, K.; Liu, C.; Tang, S.; Veeramachaneni, S.; Hu, K.Q.; Smith, D.E.; Wang, X.D. Tobacco carcinogen induces both lung cancer and non-alcoholic steatohepatitis and hepatocellular carcinomas in ferrets which can be attenuated by lycopene supplementation. Int. J. Cancer 2016, 139, 1171–1181. [Google Scholar] [CrossRef]
- Campos, K.K.D.; Araujo, G.R.; Martins, T.L.; Bandeira, A.C.B.; Costa, G.P.; Talvani, A.; Garcia, C.C.M.; Oliveira, L.A.M.; Costa, D.C.; Bezerra, F.S. The antioxidant and anti-inflammatory properties of lycopene in mice lungs exposed to cigarette smoke. J. Nutr. Biochem. 2017, 48, 9–20. [Google Scholar] [CrossRef]
- Campos, K.K.D.; de Oliveira Ramos, C.; Martins, T.L.; Costa, G.P.; Talvani, A.; Garcia, C.C.M.; Oliveira, L.A.M.; Cangussu, S.D.; Costa, D.C.; Bezerra, F.S. Lycopene mitigates pulmonary emphysema induced by cigarette smoke in a murine model. J. Nutr. Biochem. 2019, 65, 93–100. [Google Scholar] [CrossRef] [PubMed]
- Elvira-Torales, L.I.; Garcia-Alonso, J.; Periago-Caston, M.J. Nutritional Importance of Carotenoids and Their Effect on Liver Health: A Review. Antioxidants 2019, 8, 229. [Google Scholar] [CrossRef] [PubMed]
- Gann, H.G.; Dai, Y.; Giangreco, A.A.; Deaton, R.; van Breemen, R.; Lu, Y.; Rueter, E.E.; Nonn, L. Abstract LB-217: Lycopene and gene expression in benign prostate: A phase II randomized trial. In Proceedings of the AACR 103rd Annual Meeting, Chicago, IL, USA, 31 March–4 April 2012. [Google Scholar] [CrossRef]
- Rao, A.V.; Agarwal, S. Bioavailability and in vivo antioxidant properties of lycopene from tomato products and their possible role in the prevention of cancer. Nutr. Cancer 1998, 31, 199–203. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, S.; Rao, A.V. Tomato lycopene and its role in human health and chronic diseases. CMAJ 2000, 163, 739–744. [Google Scholar] [PubMed]
- Aasen, T.; Mesnil, M.; Naus, C.C.; Lampe, P.D.; Laird, D.W. Gap junctions and cancer: Communicating for 50 years. Nat. Rev. Cancer 2016, 16, 775–788. [Google Scholar] [CrossRef]
- Aasen, T.; Leithe, E.; Graham, S.V.; Kameritsch, P.; Mayan, M.D.; Mesnil, M.; Pogoda, K.; Tabernero, A. Connexins in cancer: Bridging the gap to the clinic. Oncogene 2019, 38, 4429–4451. [Google Scholar] [CrossRef]
- Novo, R.; Freire, C.M.; Felisbino, S.; Minicucci, M.F.; Azevedo, P.S.; Zornoff, L.A.; Paiva, S.A. Smoking is associated with remodeling of gap junction in the rat heart: Smoker’s paradox explanation? ARQ Bras. Cardiol. 2013, 100, 274–280. [Google Scholar] [CrossRef]
- Zhu, Y.; Guo, L.; Wang, S.; Yu, Q.; Lu, J. Association of Smoking and XPG, CYP1A1, OGG1, ERCC5, ERCC1, MMP2, and MMP9 Gene Polymorphisms with the early detection and occurrence of Laryngeal Squamous Carcinoma. J. Cancer 2018, 9, 968–977. [Google Scholar] [CrossRef]
- Xue, Y.; Harris, E.; Wang, W.; Baybutt, R.C. Vitamin A depletion induced by cigarette smoke is associated with an increase in lung cancer-related markers in rats. J. Biomed. Sci. 2015, 22, 84. [Google Scholar] [CrossRef][Green Version]
- Chan, E.C.; Lam, S.Y.; Fu, K.H.; Kwong, Y.L. Polymorphisms of the GSTM1, GSTP1, MPO, XRCC1, and NQO1 genes in Chinese patients with non-small cell lung cancers: Relationship with aberrant promoter methylation of the CDKN2A and RARB genes. Cancer Genet. Cytogenet. 2005, 162, 10–20. [Google Scholar] [CrossRef]
- Izhar, L.; Adamson, B.; Ciccia, A.; Lewis, J.; Pontano-Vaites, L.; Leng, Y.; Liang, A.C.; Westbrook, T.F.; Harper, J.W.; Elledge, S.J. A Systematic Analysis of Factors Localized to Damaged Chromatin Reveals PARP-Dependent Recruitment of Transcription Factors. Cell Rep. 2015, 11, 1486–1500. [Google Scholar] [CrossRef] [PubMed]
- Figueroa-González, G.; Pérez-Plasencia, C. Strategies for the evaluation of DNA damage and repair mechanisms in cancer. Oncol. Lett. 2017, 13, 3982–3988. [Google Scholar] [CrossRef] [PubMed]
- Limpose, K.L.; Trego, K.S.; Li, Z.; Leung, S.W.; Sarker, A.H.; Shah, J.A.; Ramalingam, S.S.; Werner, E.M.; Dynan, W.S.; Cooper, P.K.; et al. Overexpression of the base excision repair NTHL1 glycosylase causes genomic instability and early cellular hallmarks of cancer. Nucleic. Acids Res. 2018, 46, 4515–4532. [Google Scholar] [CrossRef] [PubMed]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cheng, J.; Miller, B.; Balbuena, E.; Eroglu, A. Lycopene Protects against Smoking-Induced Lung Cancer by Inducing Base Excision Repair. Antioxidants 2020, 9, 643. https://doi.org/10.3390/antiox9070643
Cheng J, Miller B, Balbuena E, Eroglu A. Lycopene Protects against Smoking-Induced Lung Cancer by Inducing Base Excision Repair. Antioxidants. 2020; 9(7):643. https://doi.org/10.3390/antiox9070643
Chicago/Turabian StyleCheng, Junrui, Baxter Miller, Emilio Balbuena, and Abdulkerim Eroglu. 2020. "Lycopene Protects against Smoking-Induced Lung Cancer by Inducing Base Excision Repair" Antioxidants 9, no. 7: 643. https://doi.org/10.3390/antiox9070643
APA StyleCheng, J., Miller, B., Balbuena, E., & Eroglu, A. (2020). Lycopene Protects against Smoking-Induced Lung Cancer by Inducing Base Excision Repair. Antioxidants, 9(7), 643. https://doi.org/10.3390/antiox9070643