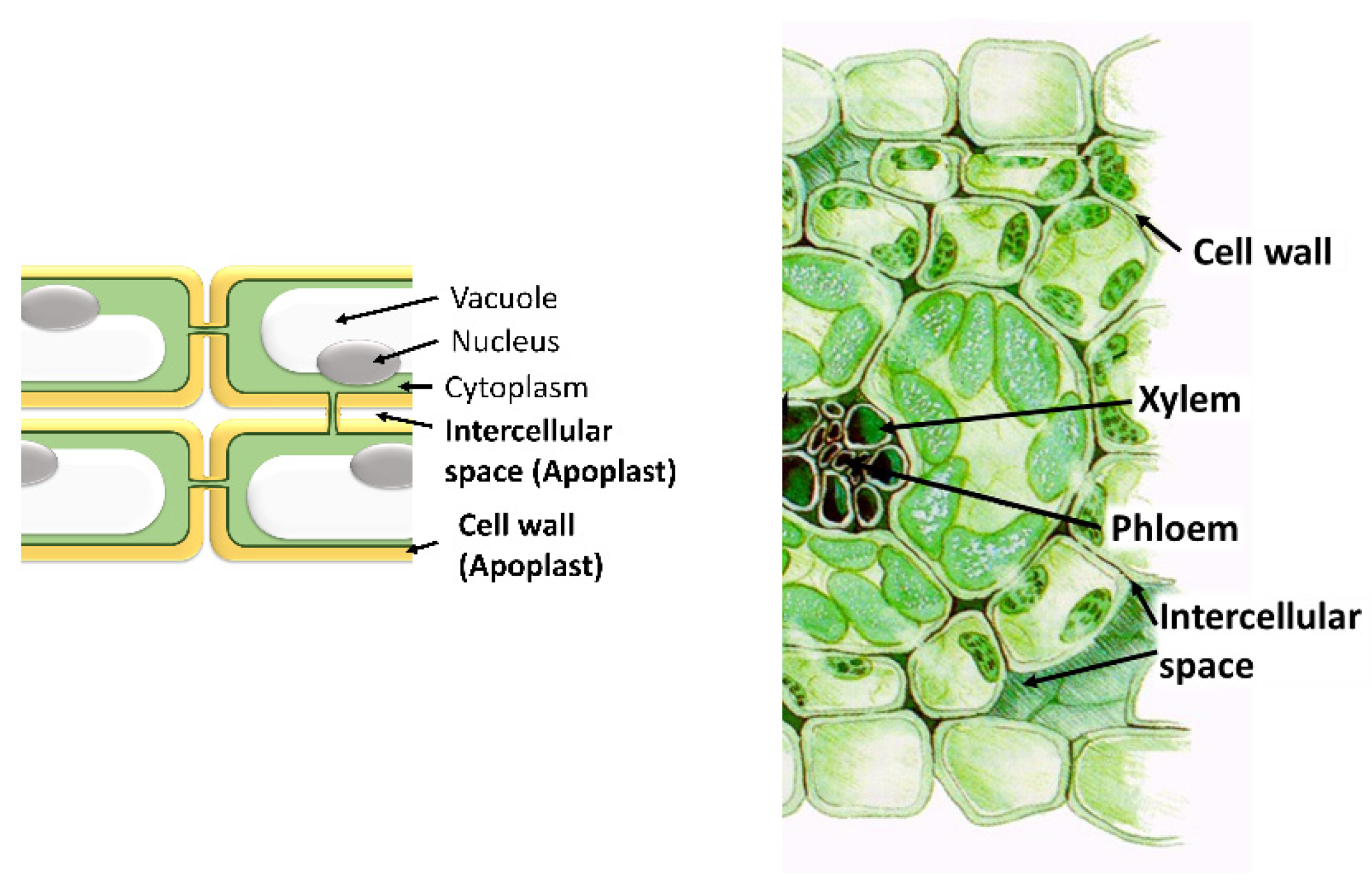
The Apoplast: A Key Player in Plant Survival
Abstract
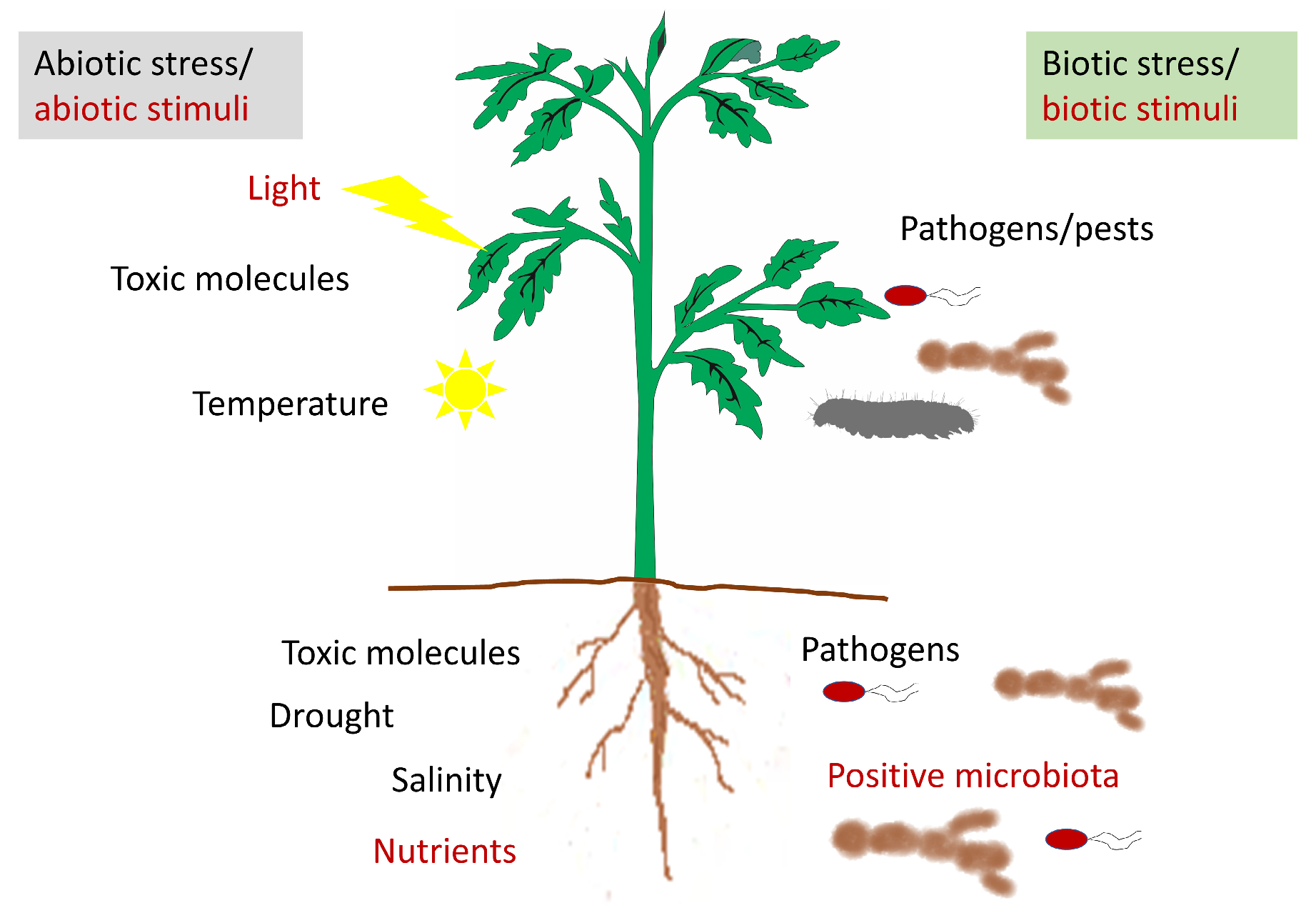
1. Introduction
2. Apoplastic ROS Production and Their Role to Overcome Stress
3. Antioxidants in the Apoplast
4. Apoplastic Proteins and Peptides in Modulating Plant–Pathogen Interactions. Microbe-Associated Molecular Patterns (MAMPs) of Proteic Nature
4.1. Apoplastic Proteins Related to Plant Defense
4.2. Apoplastic Peptides Related to Plant Defense
4.3. Microbe-Associated Molecular Patterns of Proteic Nature
5. Hormones Found in the Apoplast and Their Functions
6. Concluding Remarks
Funding
Conflicts of Interest
References
- Münch, E. Die Stoffbewegungen in der Pflanze; G. Fischer: Schaffhausen, Switzerland, 1930. [Google Scholar]
- Boyer, J.S. Cell wall biosynthesis and the molecular mechanism of plant enlargement. Funct. Plant Biol. 2009, 36, 383. [Google Scholar] [CrossRef]
- Pauly, M.; Keegstra, K. Biosynthesis of the Plant Cell Wall Matrix Polysaccharide Xyloglucan. Annu. Rev. Plant Biol. 2016, 67, 235–259. [Google Scholar] [CrossRef] [PubMed]
- Voiniciuc, C.; Pauly, M.; Usadel, B. Monitoring polysaccharide dynamics in the plant cell wall. Plant Physiol. 2018, 176, 2590–2600. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Luo, L.; Zheng, L. Lignins: Biosynthesis and Biological Functions in Plants. Int. J. Mol. Sci. 2018, 19, 335. [Google Scholar] [CrossRef] [PubMed]
- Koch, W.; Kwart, M.; Laubner, M.; Heineke, D.; Stransky, H.; Frommer, W.B.; Tegeder, M. Reduced amino acid content in transgenic potato tubers due to antisense inhibition of the leaf H+/amino acid symporter StAAP1. Plant J. 2003, 33, 211–220. [Google Scholar] [CrossRef] [PubMed]
- McCully, M.E. Niches for bacterial endophytes in crop plants: A plant biologist’s view. Aust. J. Plant Physiol. 2001, 28, 983–990. [Google Scholar] [CrossRef]
- Sattelmacher, B. The apoplast and its significance for plant mineral nutrition. New Phytol. 2001, 149, 167–192. [Google Scholar] [CrossRef]
- Rico, A.; Preston, G.M. Pseudomonas syringae pv. tomato DC3000 Uses Constitutive and Apoplast-Induced Nutrient Assimilation Pathways to Catabolize Nutrients That Are Abundant in the Tomato Apoplast. Mol. Plant-Microbe Interact. 2008, 21, 269–282. [Google Scholar] [CrossRef]
- Floerl, S.; Majcherczyk, A.; Possienke, M.; Feussner, K.; Tappe, H.; Gatz, C.; Feussner, I.; Kües, U.; Polle, A. Verticillium longisporum Infection Affects the Leaf Apoplastic Proteome, Metabolome, and Cell Wall Properties in Arabidopsis thaliana. PLoS ONE 2012, 7, e31435. [Google Scholar] [CrossRef]
- Scalschi, L.; Llorens, E.; González-Hernández, A.I.; Valcárcel, M.; Gamir, J.; García-Agustín, P.; Vicedo, B.; Camañes, G. 1-Methyltryptophan Modifies Apoplast Content in Tomato Plants Improving Resistance Against Pseudomonas syringae. Front. Microbiol. 2018, 9, 2056. [Google Scholar] [CrossRef]
- Hoson, T. Apoplast as the site of response to environmental signals. J. Plant Res. 1998, 111, 167–177. [Google Scholar] [CrossRef]
- Horst, W.J.; Wang, Y.; Eticha, D. The role of the root apoplast in aluminium-induced inhibition of root elongation and in aluminium resistance of plants: A review. Ann. Bot. 2010, 106, 185–197. [Google Scholar] [CrossRef] [PubMed]
- Horst, W.J. The role of the apoplast in aluminium toxicity and resistance of higher plants: A review. Zeitschrift für Pflanzenernährung und Bodenkd. 1995, 158, 419–428. [Google Scholar] [CrossRef]
- Geilfus, C.M. The pH of the Apoplast: Dynamic Factor with Functional Impact Under Stress. Mol. Plant 2017, 10, 1371–1386. [Google Scholar] [CrossRef] [PubMed]
- Rosenblueth, M.; Martínez-Romero, E. Bacterial endophytes and their interactions with hosts. Mol. Plant-Microbe Interact. 2006, 19, 827–837. [Google Scholar] [CrossRef] [PubMed]
- Bulgarelli, D.; Rott, M.; Schlaeppi, K.; Ver Loren van Themaat, E.; Ahmadinejad, N.; Assenza, F.; Rauf, P.; Huettel, B.; Reinhardt, R.; Schmelzer, E.; et al. Revealing structure and assembly cues for Arabidopsis root-inhabiting bacterial microbiota. Nature 2012, 488, 91–95. [Google Scholar] [CrossRef] [PubMed]
- Romero, F.M.; Rossi, F.R.; Gárriz, A.; Carrasco, P.; Ruíz, O.A. A Bacterial Endophyte from Apoplast Fluids Protects Canola Plants from Different Phytopathogens via Antibiosis and Induction of Host Resistance. Phytopathology 2019, 109, 375–383. [Google Scholar] [CrossRef]
- Turner, T.R.; James, E.K.; Poole, P.S. The plant microbiome. Genome Biol. 2013, 14, 1–10. [Google Scholar] [CrossRef]
- O’Leary, B.M.; Neale, H.C.; Geilfus, C.M.; Jackson, R.W.; Arnold, D.L.; Preston, G.M. Early changes in apoplast composition associated with defence and disease in interactions between Phaseolus vulgaris and the halo blight pathogen Pseudomonas syringae Pv. phaseolicola. Plant Cell Environ. 2016, 39. [Google Scholar]
- Doehlemann, G.; Hemetsberger, C. Apoplastic immunity and its suppression by filamentous plant pathogens. New Phytol. 2013, 198, 1001–1016. [Google Scholar] [CrossRef]
- Yan, J.; Yu, H.; Li, B.; Fan, A.; Melkonian, J.; Wang, X.; Zhou, T.; Hua, J. Cell autonomous and non-autonomous functions of plant intracellular immune receptors in stomatal defense and apoplastic defense. PLoS Pathog. 2019, 15, e1008094. [Google Scholar] [CrossRef] [PubMed]
- Das, K.; Roychoudhury, A. Reactive oxygen species (ROS) and response of antioxidants as ROS-scavengers during environmental stress in plants. Front. Environ. Sci. 2014, 2, 53. [Google Scholar] [CrossRef]
- Pogány, M.; Harrach, B.D.; Hafez, Y.M.; Barna, B.; Király, Z.; Páldi, E. Role of reactive oxygen species in abiotic and biotic stresses in plants. Acta Phytopathol. Entomol. Hungarica 2006, 41, 23–35. [Google Scholar] [CrossRef]
- Foyer, C.H.; Noctor, G. Redox regulation in photosynthetic organisms: Signaling, acclimation, and practical implications. Antioxidants Redox Signal. 2009, 11, 861–905. [Google Scholar] [CrossRef]
- Mittler, R. ROS Are Good. Trends Plant Sci. 2017, 22, 11–19. [Google Scholar] [CrossRef]
- Noctor, G.; Reichheld, J.P.; Foyer, C.H. ROS-related redox regulation and signaling in plants. Semin. Cell Dev. Biol. 2018, 80, 3–12. [Google Scholar] [CrossRef]
- Qi, J.; Wang, J.; Gong, Z.; Zhou, J.M. Apoplastic ROS signaling in plant immunity. Curr. Opin. Plant Biol. 2017, 38, 92–100. [Google Scholar] [CrossRef]
- Huang, H.; Ullah, F.; Zhou, D.X.; Yi, M.; Zhao, Y. Mechanisms of ROS regulation of plant development and stress responses. Front. Plant Sci. 2019, 10. [Google Scholar] [CrossRef]
- Choudhary, A.; Kumar, A.; Kaur, N. ROS and oxidative burst: Roots in plant development. Plant Divers. 2020, 42, 33–43. [Google Scholar] [CrossRef]
- Jubany-Marí, T.; Munné-Bosch, S.; López-Carbonell, M.; Alegre, L. Hydrogen peroxide is involved in the acclimation of the Mediterranean shrub, Cistus albidus L., to summer drought. J. Exp. Bot. 2009, 60, 107–120. [Google Scholar] [CrossRef]
- Roychoudhury, A.; Basu, S. Ascorbate-glutathione and plant tolerance to various abiotic stresses. In Oxidative Stress Plants Causes, Consequences Toler; I.K. International Publishing House Pvt. Ltd.: New Delhi, India, 2012; pp. 177–258. [Google Scholar]
- Podgórska, A.; Burian, M.; Szal, B. Extra-cellular but extra-ordinarily important for cells: Apoplastic reactive oxygen species metabolism. Front. Plant Sci. 2017, 8. [Google Scholar] [CrossRef] [PubMed]
- Minibayeva, F.; Kolesnikov, O.; Chasov, A.; Beckett, R.P.; LÜthje, S.; Vylegzhanina, N.; Buck, F.; BÖttger, M. Wound-induced apoplastic peroxidase activities: Their roles in the production and detoxification of reactive oxygen species. Plant. Cell Environ. 2009, 32, 497–508. [Google Scholar] [CrossRef] [PubMed]
- Almagro, L.; Gómez Ros, L.V.; Belchi-Navarro, S.; Bru, R.; Ros Barceló, A.; Pedreño, M.A. Class III peroxidases in plant defence reactions. J. Exp. Bot. 2009, 60, 377–390. [Google Scholar] [CrossRef] [PubMed]
- Shigeto, J.; Tsutsumi, Y. Diverse functions and reactions of class III peroxidases. New Phytol. 2016, 49, 1395–1402. [Google Scholar] [CrossRef] [PubMed]
- Survila, M.; Davidsson, P.R.; Pennanen, V.; Kariola, T.; Broberg, M.; Sipari, N.; Heino, P.; Palva, E.T. Peroxidase-generated apoplastic ROS impair cuticle integrity and contribute to DAMP-elicited defenses. Front. Plant Sci. 2016, 7. [Google Scholar] [CrossRef]
- Pandey, V.P.; Awasthi, M.; Singh, S.; Tiwari, S.; Dwivedi, U.N. A Comprehensive Review on Function and Application of Plant Peroxidases. Biochem. Anal. Biochem. 2017, 6, 308. [Google Scholar] [CrossRef]
- Van Loon, L.C.; Rep, M.; Pieterse, C.M.J. Significance of inducible defense-related proteins in infected plants. Annu. Rev. Phytopathol. 2006, 44, 135–162. [Google Scholar] [CrossRef]
- Passardi, F.; Cosio, C.; Penel, C.; Dunand, C. Peroxidases have more functions than a Swiss army knife. Plant Cell Rep. 2005, 24, 255–265. [Google Scholar] [CrossRef]
- Passardi, F.; Penel, C.; Dunand, C. Performing the paradoxical: How plant peroxidases modify the cell wall. Trends Plant Sci. 2004, 9, 534–540. [Google Scholar] [CrossRef]
- Kadota, Y.; Shirasu, K.; Zipfel, C. Regulation of the NADPH Oxidase RBOHD During Plant Immunity. Plant Cell Physiol. 2015, 56. [Google Scholar] [CrossRef]
- Torres, M.A.; Jones, J.D.G.; Dangl, J.L. Reactive Oxygen Species Signaling in Response to Pathogens. Plant Physiol. 2006, 141, 373–378. [Google Scholar] [CrossRef] [PubMed]
- Choudhury, F.K.; Rivero, R.M.; Blumwald, E.; Mittler, R. Reactive oxygen species, abiotic stress and stress combination. Plant J. 2017, 90, 856–867. [Google Scholar] [CrossRef] [PubMed]
- Mittler, R.; Vanderauwera, S.; Suzuki, N.; Miller, G.; Tognetti, V.B.; Vandepoele, K.; Gollery, M.; Shulaev, V.; Van Breusegem, F. ROS signaling: The new wave? Trends Plant Sci. 2011, 16, 300–309. [Google Scholar] [CrossRef] [PubMed]
- Sagi, M.; Fluhr, R. Production of reactive oxygen species by plant NADPH oxidases. Plant Physiol. 2006, 141, 336–340. [Google Scholar] [CrossRef] [PubMed]
- Marino, D.; Dunand, C.; Puppo, A.; Pauly, N. A burst of plant NADPH oxidases. Trends Plant Sci. 2012, 17, 9–15. [Google Scholar] [CrossRef]
- Hu, C.-H.; Wang, P.-Q.; Zhang, P.-P.; Nie, X.-M.; Li, B.-B.; Tai, L.; Liu, W.-T.; Li, W.-Q.; Chen, K.-M. NADPH Oxidases: The Vital Performers and Center Hubs during Plant Growth and Signaling. Cells 2020, 9, 437. [Google Scholar] [CrossRef]
- Zhou, J.; Xia, X.-J.; Zhou, Y.-H.; Shi, K.; Chen, Z.; Yu, J.-Q. RBOH1-dependent H2O2 production and subsequent activation of MPK1/2 play an important role in acclimation-induced cross-tolerance in tomato. J. Exp. Bot. 2013, 65, 595–607. [Google Scholar] [CrossRef]
- Fernández-Crespo, E.; Scalschi, L.; Llorens, E.; García-Agustín, P.; Camañes, G. NH4+ protects tomato plants against Pseudomonas syringae by activation of systemic acquired acclimation. J. Exp. Bot. 2015, 66, 6777–6790. [Google Scholar] [CrossRef]
- González-Hernández, A.I.; Fernández-Crespo, E.; Scalschi, L.; Hajirezaei, M.-R.; von Wirén, N.; García-Agustín, P.; Camañes, G. Ammonium mediated changes in carbon and nitrogen metabolisms induce resistance against Pseudomonas syringae in tomato plants. J. Plant Physiol. 2019, 239, 28–37. [Google Scholar] [CrossRef]
- Zhang, X.Y.; Nie, Z.H.; Wang, W.J.; Leung, D.W.M.; Xu, D.G.; Chen, B.L.; Chen, Z.; Zeng, L.X.; Liu, E.E. Relationship between Disease Resistance and Rice Oxalate Oxidases in Transgenic Rice. PLoS ONE 2013, 8. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zhang, B.; Oakes, A.D.; Newhouse, A.E.; Baier, K.M.; Maynard, C.A.; Powell, W.A. A threshold level of oxalate oxidase transgene expression reduces Cryphonectria parasitica-induced necrosis in a transgenic American chestnut (Castanea dentata) leaf bioassay. Transgenic Res. 2013, 22, 973–982. [Google Scholar] [CrossRef] [PubMed]
- Angelini, R.; Cona, A.; Federico, R.; Fincato, P.; Tavladoraki, P.; Tisi, A. Plant amine oxidases “on the move”: An update. Plant Physiol. Biochem. 2010, 48, 560–564. [Google Scholar] [CrossRef] [PubMed]
- Tavladoraki, P.; Cona, A.; Angelini, R. Copper-containing amine oxidases and FAD-dependent polyamine oxidases are key players in plant tissue differentiation and organ development. Front. Plant Sci. 2016, 7. [Google Scholar] [CrossRef] [PubMed]
- Saha, J.; Brauer, E.K.; Sengupta, A.; Popescu, S.C.; Gupta, K.; Gupta, B. Polyamines as redox homeostasis regulators during salt stress in plants. Front. Environ. Sci. 2015, 3, 21. [Google Scholar] [CrossRef]
- Angelini, R.; Bragaloni, M.; Federico, R.; Infantino, A.; Porta-Pugua, A. Involvement of Polyamines, Diamine Oxidase and Peroxidase in Resistance of Chickpea to Ascochyta rabiei. J. Plant Physiol. 1993, 142, 704–709. [Google Scholar] [CrossRef]
- Wang, W.; Paschalidis, K.; Feng, J.C.; Song, J.; Liu, J.H. Polyamine catabolism in plants: A universal process with diverse functions. Front. Plant Sci. 2019, 10. [Google Scholar] [CrossRef]
- Porta, H.; Rocha-Sosa, M. Plant lipoxygenases. Physiological and molecular features. Plant Physiol. 2002, 130, 15–21. [Google Scholar] [CrossRef]
- Sharma, P.; Jha, A.B.; Dubey, R.S.; Pessarakli, M.; Sharma, P.; Jha, A.B.; Dubey, R.S.; Pessarakli, M. Reactive Oxygen Species, Oxidative Damage, and Antioxidative Defense Mechanism in Plants under Stressful Conditions. J. Bot. 2012, 2012, 1–26. [Google Scholar] [CrossRef]
- Roach, T.; Colville, L.; Beckett, R.P.; Minibayeva, F.V.; Havaux, M.; Kranner, I. A proposed interplay between peroxidase, amine oxidase and lipoxygenase in the wounding-induced oxidative burst in Pisum sativum seedlings. Phytochemistry 2015, 112, 130–138. [Google Scholar] [CrossRef]
- Leone, A.; Melillo, M.T.; Bleve-Zacheo, T. Lipoxygenase in pea roots subjected to biotic stress. Plant Sci. 2001, 161, 703–717. [Google Scholar] [CrossRef]
- Denness, L.; McKenna, J.F.; Segonzac, C.; Wormit, A.; Madhou, P.; Bennett, M.; Mansfield, J.; Zipfel, C.; Hamann, T. Cell wall damage-induced lignin biosynthesis is regulated by a reactive oxygen species- and jasmonic acid-dependent process in arabidopsis. Plant Physiol. 2011, 156, 1364–1374. [Google Scholar] [CrossRef] [PubMed]
- Janku, M.; Luhová, L.; Petrivalský, M. On the origin and fate of reactive oxygen species in plant cell compartments. Antioxidants 2019, 8, 105. [Google Scholar] [CrossRef] [PubMed]
- Caverzan, A.; Casassola, A.; Patussi Brammer, S. Reactive Oxygen Species and Antioxidant Enzymes Involved in Plant Tolerance to Stress. In Abiotic and Biotic Stress in Plants—Recent Advances and Future Perspectives; InTech: Nappanee, IN, USA, 2016. [Google Scholar]
- Kangasjärvi, S.; Kangasjärvi, J. Towards Understanding Extracellular ROS Sensory and Signaling Systems in Plants. Adv. Bot. 2014, 2014, 538946. [Google Scholar] [CrossRef]
- Berwal, M.K.; Ram, C. Superoxide Dismutase: A Stable Biochemical Marker for Abiotic Stress Tolerance in Higher Plants. In Abiotic and Biotic Stress in Plants; IntechOpen: London, UK, 2019. [Google Scholar]
- Sgherri, C.; Quartacci, M.F.; Navari-Izzo, F. Early production of activated oxygen species in root apoplast of wheat following copper excess. J. Plant Physiol. 2007, 164, 1152–1160. [Google Scholar] [CrossRef]
- García, G.; Clemente-Moreno, M.J.; Díaz-Vivancos, P.; García, M.; Hernández, J.A. The apoplastic and symplastic antioxidant system in onion: Response to long-term salt stress. Antioxidants 2020, 9, 67. [Google Scholar] [CrossRef]
- Vanacker, H.; Harbinson, J.; Ruisch, J.; Carver, T.L.W.; Foyer, C.H. Antioxidant defences of the apoplast. Protoplasma 1998, 205, 129–140. [Google Scholar] [CrossRef]
- Vanacker, H.; Carver, T.L.W.; Foyer, C.H. Pathogen-induced changes in the antioxidant status of the apoplast in barley leaves. Plant Physiol. 1998, 117, 1103–1114. [Google Scholar] [CrossRef]
- Singh, B.N.; Singh, A.; Singh, S.P.; Singh, H.B. Trichoderma harzianum- mediated reprogramming of oxidative stress response in root apoplast of sunflower enhances defence against Rhizoctonia solani. Eur. J. Plant Pathol. 2011, 131, 121–134. [Google Scholar] [CrossRef]
- Sharma, I.; Ahmad, P. Catalase: A Versatile Antioxidant in Plants. In Oxidative Damage to Plants: Antioxidant Networks and Signaling; Elsevier Inc.: Amsterdam, The Netherlands, 2014; pp. 131–148. ISBN 9780127999630. [Google Scholar]
- Caverzan, A.; Passaia, G.; Rosa, S.B.; Ribeiro, C.W.; Lazzarotto, F.; Margis-Pinheiro, M. Plant responses to stresses: Role of ascorbate peroxidase in the antioxidant protection. Genet. Mol. Biol. 2012, 35, 1011–1019. [Google Scholar] [CrossRef]
- Sharova, E.I.; Medvedev, S.S.; Demidchik, V.V. Ascorbate in the Apoplast: Metabolism and Functions. Russ. J. Plant Physiol. 2020, 67, 207–220. [Google Scholar] [CrossRef]
- Bindschedler, L.V.; Palmblad, M.; Cramer, R. Hydroponic isotope labelling of entire plants (HILEP) for quantitative plant proteomics; an oxidative stress case study. Phytochemistry 2008, 69, 1962–1972. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Gallie, D.R. Dehydroascorbate reductase affects leaf growth, development, and function. Plant Physiol. 2006, 142, 775–787. [Google Scholar] [CrossRef] [PubMed]
- Ding, H.; Wang, B.; Han, Y.; Li, S. The pivotal function of dehydroascorbate reductase in glutathione homeostasis in plants. J. Exp. Bot. 2020. [Google Scholar] [CrossRef] [PubMed]
- Yousuf, P.Y.; Hakeem, K.U.R.; Chandna, R.; Ahmad, P. Role of Glutathione Reductase in Plant Abiotic Stress. In Abiotic Stress Responses in Plants; Springer: New York, NY, USA, 2012; pp. 149–158. [Google Scholar]
- Zechmann, B. Subcellular distribution of ascorbate in plants. Plant Signal. Behav. 2011, 6, 360–363. [Google Scholar] [CrossRef] [PubMed]
- Hasanuzzaman, M.; Borhannuddin Bhuyan, M.H.M.; Anee, T.I.; Parvin, K.; Nahar, K.; Al Mahmud, J.; Fujita, M. Regulation of ascorbate-glutathione pathway in mitigating oxidative damage in plants under abiotic stress. Antioxidants 2019, 8, 384. [Google Scholar] [CrossRef]
- Saruhan Güler, N.; Sağlam, A.; Demiralay, M.; Kadioğlu, A.; Saruhan Güler, N.; Sağlam, A.; Demiralay, M.; Kadioğlu, A. Apoplastic and symplastic solute concentrations contribute to osmotic adjustment in bean genotypes during drought stress. Turk J Biol 2012, 36, 151–160. [Google Scholar]
- Dar, M.I.; Naikoo, M.I.; Rehman, F.; Naushin, F.; Khan, F.A. Proline accumulation in plants: Roles in stress tolerance and plant development. In Osmolytes and Plants Acclimation to Changing Environment: Emerging Omics Technologies; Springer: Delhi, India, 2015; pp. 155–166. ISBN 9788132226161. [Google Scholar]
- Babenko, L.M.; Smirnov, O.E.; Romanenko, K.O.; Trunova, O.K.; Kosakivska, I.V. Phenolic compounds in plants: Biogenesis and functions. Ukr. Biochem. J. 2019, 91, 5–18. [Google Scholar] [CrossRef]
- Chen, D.; Shao, Q.; Yin, L.; Younis, A.; Zheng, B. Polyamine function in plants: Metabolism, regulation on development, and roles in abiotic stress responses. Front. Plant Sci. 2019, 9, 1945. [Google Scholar] [CrossRef]
- Takahashi, T.; Kakehi, J.-I. Polyamines: Ubiquitous polycations with unique roles in growth and stress responses. Ann. Bot. 2009, 105, 1–6. [Google Scholar] [CrossRef]
- Salguero, J.; Böttger, M. Secreted catalase activity from roots of developing maize (Zea mays L.) seedlings. Protoplasma 1995, 184, 72–78. [Google Scholar] [CrossRef]
- Parra-Lobato, M.C.; Fernandez-Garcia, N.; Olmos, E.; Alvarez-Tinaut, M.C.; Gómez-Jiménez, M.C. Methyl jasmonate-induced antioxidant defence in root apoplast from sunflower seedlings. Environ. Exp. Bot. 2009, 66, 9–17. [Google Scholar] [CrossRef]
- Zhou, Y.; Liu, S.; Yang, Z.; Yang, Y.; Jiang, L.; Hu, L. CsCAT3, a catalase gene from Cucumis sativus, confers resistance to a variety of stresses to Escherichia coli. Biotechnol. Biotechnol. Equip. 2017, 31, 886–896. [Google Scholar] [CrossRef]
- Fernández-Crespo, E.; Gómez-Pastor, R.; Scalschi, L.; Llorens, E.; Camañes, G.; García-Agustín, P. NH4 + induces antioxidant cellular machinery and provides resistance to salt stress in citrus plants. Trees 2014, 28, 1693–1704. [Google Scholar] [CrossRef]
- De Pinto, M.C.; De Gara, L. Changes in the ascorbate metabolism of apoplastic and symplastic spaces are associated with cell differentiation. J. Exp. Bot. 2004, 55, 2559–2569. [Google Scholar] [CrossRef] [PubMed]
- Asada, K. Ascorbate peroxidase—A hydrogen peroxide-scavenging enzyme in plants. Physiol. Plant. 1992, 85, 235–241. [Google Scholar] [CrossRef]
- Pandey, S.; Fartyal, D.; Agarwal, A.; Shukla, T.; James, D.; Kaul, T.; Negi, Y.K.; Arora, S.; Reddy, M.K. Abiotic stress tolerance in plants: Myriad roles of ascorbate peroxidase. Front. Plant Sci. 2017, 8. [Google Scholar] [CrossRef]
- O’Brien, J.A.; Daudi, A.; Finch, P.; Butt, V.S.; Whitelegge, J.P.; Souda, P.; Ausubel, F.M.; Bolwell, G.P. A Peroxidase-Dependent Apoplastic Oxidative Burst in Cultured Arabidopsis Cells Functions in MAMP-Elicited Defense. Plant Physiol. 2012, 158, 2013–2027. [Google Scholar] [CrossRef]
- Saruhan, N.; Terzi, R.; Saǧlam, A.; Kadioǧlu, A. Scavenging of reactive oxygen species in apoplastic and symplastic areas of rolled leaves in Ctenanthe setosa under drought stress. Acta Biol. Hung. 2010, 61, 282–298. [Google Scholar] [CrossRef]
- Diaz-Vivancos, P.; Rubio, M.; Mesonero, V.; Periago, P.M.; Barceló, A.R.; Martínez-Gómez, P.; Hernández, J.A. The apoplastic antioxidant system in Prunus: Response to long-term plum pox virus infection. J. Exp. Bot. 2006, 57, 3813–3824. [Google Scholar] [CrossRef]
- Pandey, P.; Singh, J.; Achary, V.M.M.; Mallireddy Reddy, K. Redox homeostasis via gene families of ascorbate-glutathione pathway. Front. Environ. Sci. 2015, 3, 25. [Google Scholar] [CrossRef]
- Negi, B.; Salvi, P.; Bhatt, D.; Majee, M.; Arora, S. Molecular cloning, in-silico characterization and functional validation of monodehydroascorbate reductase gene in Eleusine coracana. PLoS ONE 2017, 12. [Google Scholar] [CrossRef] [PubMed]
- Eltayeb, A.E.; Kawano, N.; Badawi, G.H.; Kaminaka, H.; Sanekata, T.; Shibahara, T.; Inanaga, S.; Tanaka, K. Overexpression of monodehydroascorbate reductase in transgenic tobacco confers enhanced tolerance to ozone, salt and polyethylene glycol stresses. Planta 2007, 225, 1255–1264. [Google Scholar] [CrossRef] [PubMed]
- Akram, N.A.; Shafiq, F.; Ashraf, M. Ascorbic acid-a potential oxidant scavenger and its role in plant development and abiotic stress tolerance. Front. Plant Sci. 2017, 8, 613. [Google Scholar] [CrossRef] [PubMed]
- Hasanuzzaman, M.; Nahar, K.; Anee, T.I.; Fujita, M. Glutathione in plants: Biosynthesis and physiological role in environmental stress tolerance. Physiol. Mol. Biol. Plants 2017, 23, 249–268. [Google Scholar] [CrossRef] [PubMed]
- Cheng, M.C.; Ko, K.; Chang, W.L.; Kuo, W.C.; Chen, G.H.; Lin, T.P. Increased glutathione contributes to stress tolerance and global translational changes in Arabidopsis. Plant J. 2015, 83, 926–939. [Google Scholar] [CrossRef] [PubMed]
- Hasanuzzaman, M.; Nahar, K.; Alam, M.M.; Roychowdhury, R.; Fujita, M. Physiological, biochemical, and molecular mechanisms of heat stress tolerance in plants. Int. J. Mol. Sci. 2013, 14, 9643–9684. [Google Scholar] [CrossRef]
- Sairam, R.K.; Srivastava, G.C.; Saxena, D.C. Increased antioxidant activity under elevated temperatures: A mechanism of heat stress tolerance in wheat genotypes. Biol. Plant. 2000, 43, 245–251. [Google Scholar] [CrossRef]
- Dash, S.; Mohanty, N. Response of seedlings to heat-stress in cultivars of wheat: Growth temperature-dependent differential modulation of photosystem 1 and 2 activity, and foliar antioxidant defense capacity. J. Plant Physiol. 2002, 159, 49–59. [Google Scholar] [CrossRef]
- Hayat, S.; Hayat, Q.; Alyemeni, M.N.; Wani, A.S.; Pichtel, J.; Ahmad, A. Role of proline under changing environments: A review. Plant Signal. Behav. 2012, 7. [Google Scholar] [CrossRef]
- Cuin, T.A.; Shabala, S. Compatible solutes reduce ROS-induced potassium efflux in Arabidopsis roots. Plant. Cell Environ. 2007, 30, 875–885. [Google Scholar] [CrossRef]
- Gadallah, M.A.A. Effects of proline and glycinebetaine on Vicia faba responses to salt stress. Biol. Plant. 1999, 42, 249–257. [Google Scholar] [CrossRef]
- Mafakheri, A.; Siosemardeh, A.; Bahramnejad, B.; Struik, P.C.; Sohrabi, Y. Effect of drought stress on yield, proline and chlorophyll contents in three chickpea cultivars. Aust. J. Crop Sci. 2010, 4. [Google Scholar]
- Roychoudhury, A.; Basu, S.; Sarkar, S.N.; Sengupta, D.N. Comparative physiological and molecular responses of a common aromatic indica rice cultivar to high salinity with non-aromatic indica rice cultivars. Plant Cell Rep. 2008, 27, 1395–1410. [Google Scholar] [CrossRef] [PubMed]
- Saglam, A.; Terzi, R.; Nar, H.; Saruhan, N.; Faik, A.A.; Kadioglu, A. Inorganic and organic solutes in apoplastic and symplastic spaces contribute to osmotic adjustment during leaf rolling in Ctenanthe setosa. Acta Biol. Cracoviensia Ser. Bot. 2010, 52, 37–44. [Google Scholar] [CrossRef]
- Amarowicz, R.; Weidner, S. Biological activity of grapevine phenolic compounds. In Grapevine Molecular Physiology and Biotechnology, 2nd ed.; Springer: Dordrecht, The Netherlands, 2009; pp. 389–405. ISBN 9789048123049. [Google Scholar]
- Caverzan, A.; Piasecki, C.; Chavarria, G.; Stewart, C.N.; Vargas, L. Defenses against ROS in crops and weeds: The effects of interference and herbicides. Int. J. Mol. Sci. 2019, 20, 86. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Shahzad, B.; Rehman, A.; Bhardwaj, R.; Landi, M.; Zheng, B. Response of phenylpropanoid pathway and the role of polyphenols in plants under abiotic stress. Molecules 2019, 24, 2452. [Google Scholar] [CrossRef] [PubMed]
- Guerra-Guimarães, L.; Tenente, R.; Pinheiro, C.; Chaves, I.; do Céu Silva, M.; Cardoso, F.M.H.; Planchon, S.; Barros, D.R.; Renaut, J.; Ricardo, C.P. Proteomic analysis of apoplastic fluid of Coffea arabica leaves highlights novel biomarkers for resistance against Hemileia vastatrix. Front. Plant Sci. 2015, 6, 478. [Google Scholar]
- Tuzun, S.; Somanchi, A.; der Hoorn, R. The Possible Role of PR proteins in Multigenic and Induced Systemic Resistance. In Multigenic and Induced Systemic Resistance in Plants; Springer: Berlin, Germany, 2008; Volume 59 SRC-, pp. 112–142. [Google Scholar]
- Dempsey, D.A.; Silva, H.; Klessig, D.F. Engineering disease and pest resistance in plants. Trends Microbiol. 1998, 6, 54–61. [Google Scholar] [CrossRef]
- Sels, J.; Mathys, J.; De Coninck, B.M.A.; Cammue, B.P.A.; De Bolle, M.F.C. Plant pathogenesis-related (PR) proteins: A focus on PR peptides. Plant Physiol. Biochem. 2008, 46, 941–950. [Google Scholar] [CrossRef]
- Breen, S.; Williams, S.J.; Outram, M.; Kobe, B.; Solomon, P.S. Emerging Insights into the Functions of Pathogenesis-Related Protein 1. Trends Plant Sci. 2017, 22, 871–879. [Google Scholar] [CrossRef]
- Hong, T.; Meng, M. Biochemical characterization and antifungal activity of an endo-3-beta-glucanase of Paenibacillus sp. Isol. from Gard. soil. Appl. Microbiol. Biotechnol. 2003, 61, 472–478. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Gong, Z. Purification and characterization of an ethylene-induced antifungal protein from leaves of guilder rose (Hydrangea macrophylla). Protein Expr. Purif. 2002, 24, 76–82. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.-X.; Ahammed, G.; Wu, C.; Fan, S.; Zhou, Y.-H. Crosstalk among Jasmonate, Salicylate and Ethylene Signaling Pathways in Plant Disease and Immune Responses. Curr. Protein Pept. Sci. 2015, 16, 450–461. [Google Scholar] [CrossRef] [PubMed]
- Naz, R.; Bano, A.; Wilson, N.L.; Guest, D.; Roberts, T.H. Pathogenesis-related protein expression in the apoplast of wheat leaves protected against leaf rust following application of plant extracts. Phytopathology 2014, 104, 933–944. [Google Scholar] [CrossRef] [PubMed]
- Tornero, P.; Conejero, V.; Vera, P. Identification of a new pathogen-induced member of the subtilisin-like processing protease family from plants. J. Biol. Chem. 1997, 272, 14412–14419. [Google Scholar] [CrossRef]
- Simmons, C. The Physiology and Molecular Biology of Plant 1,3-β-D-Glucanases and 1,3;1,4-β-D-Glucanases. Crit. Rev. Plant Sci. 1994, 13, 325–387. [Google Scholar]
- Van Loon, L.C. Induced resistance in plants and the role of pathogenesis-related proteins. Eur. J. Plant Pathol. 1997, 103, 753–765. [Google Scholar] [CrossRef]
- Sugawara, T.; Trifonova, E.A.; Kochetov, A.V.; Kanayama, Y. Expression of an extracellular ribonuclease gene increases resistance to Cucumber mosaic virus in tobacco. BMC Plant Biol. 2016, 16. [Google Scholar] [CrossRef]
- Filipenko, E.; Kochetov, A.; Kanayama, Y.; Malinovsky, V.; Shumny, V. PR-proteins with ribonuclease activity and plant resistance against pathogenic fungi. Russ. J. Genet. Appl. Res. 2013, 10 SRC-, 474–480. [Google Scholar] [CrossRef]
- Bravo, J.M.; Campo, S.; Murillo, I.; Coca, M.; San Segundo, B. Fungus- and wound-induced accumulation of mRNA containing a class II chitinase of the pathogenesis-related protein 4 (PR-4) family of maize. Plant Mol. Biol. 2003, 52, 745–759. [Google Scholar] [CrossRef]
- Penninckx, I.A.; Eggermont, K.; Terras, F.R.; Thomma, B.P.; De Samblanx, G.W.; Buchala, A.; Métraux, J.P.; Manners, J.M.; Broekaert, W.F. Pathogen-induced systemic activation of a plant defensin gene in Arabidopsis follows a salicylic acid-independent pathway. Plant Cell 1996, 8, 2309–2323. [Google Scholar] [PubMed]
- Stec, B. Plant thionins--the structural perspective. Cell. Mol. Life Sci. 2006, 63, 1370–1385. [Google Scholar] [CrossRef] [PubMed]
- Kido, E.A.; Pandolfi, V.; Houllou-Kido, L.M.; Andrade, P.P.; Marcelino, F.C.; Nepomuceno, A.L.; Abdelnoor, R.V.; Burnquist, W.L.; Benko-Iseppon, A.M. Plant Antimicrobial Peptides: An Overview of SuperSAGE Transcriptional Profile and a Functional Review. Curr. Protein Pept. Sci. 2010, 11, 220–230. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Bidney, D.L.; Yalpani, N.; Duvick, J.P.; Crasta, O.; Folkerts, O.; Lu, G. Overexpression of a gene encoding hydrogen peroxide-generating oxalate oxidase evokes defense responses in sunflower. Plant Physiol. 2003, 133, 170–181. [Google Scholar] [CrossRef] [PubMed]
- Christensen, A.B.; Cho, B.H.O.; Næsby, M.; Gregersen, P.L.; Brandt, J.; Madriz-Ordeñana, K.; Collinge, D.B.; Thordal-Christensen, H. The molecular characterization of two barley proteins establishes the novel PR-17 family of pathogenesis-related proteins. Mol. Plant Pathol. 2002, 3, 135–144. [Google Scholar] [CrossRef] [PubMed]
- Jones, J.D.; Dangl, J.L. The plant immune system. Nature 2006, 444, 323–329. [Google Scholar] [CrossRef]
- Van Loon, L.C.; Van Strien, E.A. The families of pathogenesis-related proteins, their activities, and comparative analysis of PR-1 type proteins. Physiol. Mol. Plant Pathol. 1999, 55, 85–97. [Google Scholar] [CrossRef]
- Liu, Q.; Xue, Q. Computational identification of novel PR-1-type genes in Oryza sativa. J. Genet. 2006, 85, 193–198. [Google Scholar] [CrossRef]
- Payne, G.; Ward, E.; Gaffney, T.; Goy, P.A.; Moyer, M.; Harper, A.; Meins, F.; Ryals, J. Evidence for a third structural class of β-1,3-glucanase in tobacco. Plant Mol. Biol. 1990, 15, 797–808. [Google Scholar] [CrossRef]
- Leubner-Metzger, G.; Meins, F. 3. Functions and Regulation of Plant ß-1,3-glucanases (PR-2); CRC Press LLC: Boca Raton, FL, USA, 1999. [Google Scholar]
- Dyakov, Y.T.; Dzhavakhiya, V.G.; Korpela, T. Comprehensive and Molecular Phytopathology; Elsevier: Amsterdam, The Netherlands, 2007; ISBN 9780080469331. [Google Scholar]
- Castresana, C.; de Carvalho, F.; Gheysen, G.; Habets, M.; Inzé, D.; Van Montagu, M. Tissue-specific and pathogen-induced regulation of a Nicotiana plumbaginifolia beta-1,3-glucanase gene. Plant Cell 1990, 2, 1131–1143. [Google Scholar] [CrossRef][Green Version]
- Alonso, E.; de Carvalho Niebel, F.; Obregon, P.; Gheysen, G.; Inze, D.; Van Montagu, M.; Castresana, C. Differential in vitro DNA binding activity to a promoter element of the gn1 beta-1,3-glucanase gene in hypersensitively reacting tobacco plants. Plant J. 1995, 7, 309–320. [Google Scholar] [CrossRef] [PubMed]
- Balasubramanian, V.; Vashisht, D.; Cletus, J.; Sakthivel, N. Plant β-1,3-glucanases: Their biological functions and transgenic expression against phytopathogenic fungi. Biotechnol. Lett. 2012, 34, 1983–1990. [Google Scholar] [CrossRef] [PubMed]
- Vázquez-Garcidueñas, S.; Leal-Morales, C.A.; Herrera-Estrella, A. Analysis of the beta-1,3-Glucanolytic System of the Biocontrol Agent Trichoderma harzianum. Appl. Environ. Microbiol. 1998, 64, 1442–1446. [Google Scholar] [CrossRef] [PubMed]
- Ait-Lahsen, H.; Soler, A.; Rey, M.; De La Cruz, J.; Monte, E.; Llobell, A. An Antifungal Exo-α-1,3-Glucanase (AGN13.1) from the Biocontrol Fungus Trichoderma harzianum. Appl. Environ. Microbiol. 2001, 67, 5833–5839. [Google Scholar] [CrossRef] [PubMed]
- Kasprzewska, A. Plant chitinases--regulation and function. Cell. Mol. Biol. Lett. 2003, 8, 809–824. [Google Scholar] [PubMed]
- Saikia, R.; Singh, B.P.; Kumar, R.; Arora, D.K. Detection of pathogenesis-related proteins-chitinase and β-1,3-glucanase in induced chickpea. Curr. Sci. 2005, 84, 659–663. [Google Scholar]
- Garg, N.; Gupta, H. Isolation and purification of fungal pathogen (Macrophomina phaseolina) induced chitinase from moth beans (Phaseolus aconitifolius). J. Pharm. Bio Allied Sci. 2010, 2, 38. [Google Scholar]
- Fukamizo, T.; Chie, S.; Masahiro, T. Plant chitinases: Structure-function relationships and their physiology. Foods Food Ingredients J. 2003, 208, 631–632. [Google Scholar]
- Gooday, G.W. Aggressive and defensive roles for chitinases. EXS 1999, 87, 157–169. [Google Scholar]
- Afroz, A.; Ali, G.M.; Mir, A.; Komatsu, S. Application of proteomics to investigate stress-induced proteins for improvement in crop protection. Plant Cell Rep. 2011, 30, 745–763. [Google Scholar] [CrossRef]
- Cantu, D.; Vicente, A.R.; Labavitch, J.M.; Bennett, A.B.; Powell, A.L.T. Strangers in the matrix: Plant cell walls and pathogen susceptibility. Trends Plant Sci. 2008, 13, 610–617. [Google Scholar] [CrossRef] [PubMed]
- Petriccione, M.; Salzano, A.M.; Di Cecco, I.; Scaloni, A.; Scortichini, M. Proteomic analysis of the Actinidia deliciosa leaf apoplast during biotrophic colonization by Pseudomonas syringae pv. actinidiae. J. Proteom. 2014, 101, 43–62. [Google Scholar] [CrossRef] [PubMed]
- Pechanova, O.; Hsu, C.-Y.; Adams, J.P.; Pechan, T.; Vandervelde, L.; Drnevich, J.; Jawdy, S.; Adeli, A.; Suttle, J.C.; Lawrence, A.M.; et al. Apoplast proteome reveals that extracellular matrix contributes to multistress response in poplar. BMC Genom. 2010, 11, 674. [Google Scholar] [CrossRef] [PubMed]
- Delaunois, B.; Colby, T.; Belloy, N.; Conreux, A.; Harzen, A.; Baillieul, F.; Clément, C.; Schmidt, J.; Jeandet, P.; Cordelier, S. Large-scale proteomic analysis of the grapevine leaf apoplastic fluid reveals mainly stress-related proteins and cell wall modifying enzymes. BMC Plant Biol. 2013, 13, 24. [Google Scholar] [CrossRef] [PubMed]
- Floerl, S.; Druebert, C.; Majcherczyk, A.; Karlovsky, P.; Kües, U.; Polle, A. Defence reactions in the apoplastic proteome of oilseed rape (Brassica napus var. napus) attenuate Verticillium longisporum growth but not disease symptoms. BMC Plant Biol. 2008, 8, 129. [Google Scholar] [CrossRef] [PubMed]
- Segarra, C.I.; Casalongué, C.A.; Pinedo, M.L.; Ronchi, V.P.; Conde, R.D. A germin-like protein of wheat leaf apoplast inhibits serine proteases. J. Exp. Bot. 2003, 54, 1335–1341. [Google Scholar] [CrossRef]
- Joosten, M.H.A.J.; De Wit, P.J.G.M. Identification of Several Pathogenesis-Related Proteins in Tomato Leaves Inoculated with Cladosporium fulvum (syn. Fulvia fulva ) as 1,3-β-Glucanases and Chitinases. Plant Physiol. 1989, 89, 945–951. [Google Scholar] [CrossRef]
- Han, L.B.; Li, Y.B.; Wang, F.X.; Wang, W.Y.; Liu, J.; Wu, J.H.; Zhong, N.Q.; Wu, S.J.; Jiao, G.L.; Wang, H.Y.; et al. The Cotton Apoplastic Protein CRR1 stabilizes chitinase 28 to facilitate defense against the fungal pathogen verticillium dahliae. Plant Cell 2019, 31, 520–536. [Google Scholar] [CrossRef]
- Hon, W.-C.; Griffith, M.; Mlynarz, A.; Kwok, Y.C.; Yang, D.S. Antifreeze proteins in winter rye are similar to pathogenesis-related proteins. Plant Physiol. 1995, 109, 879–889. [Google Scholar] [CrossRef]
- Rajam, M.V.; Chandola, N.; Goud, P.S.; Singh, D.; Kashyap, V.; Choudhary, M.L.; Sihachakr, D. Thaumatin gene confers resistance to fungal pathogens as well as tolerance to abiotic stresses in transgenic tobacco plants. Biol. Plant. 2007, 51, 135–141. [Google Scholar] [CrossRef]
- Islam, M.A.; Sturrock, R.N.; Holmes, T.A.; Ekramoddoullah, A.K.M. Ultrastructural studies of Phellinus sulphurascens infection of Douglas-fir roots and immunolocalization of host pathogenesis-related proteins. Mycol. Res. 2009, 113, 700–712. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Tang, C.; Deng, L.; Cai, G.; Liu, X.; Liu, B.; Han, Q.; Buchenauer, H.; Wei, G.; Han, D.; et al. Characterization of a pathogenesis-related thaumatin-like protein gene TaPR5 from wheat induced by stripe rust fungus. Physiol. Plant. 2010, 139, 27–38. [Google Scholar] [CrossRef] [PubMed]
- Shatters, R.G.; Boykin, L.M.; Lapointe, S.L.; Hunter, W.B.; Weathersbee, A.A. Phylogenetic and structural relationships of the PR5 gene family reveal an ancient multigene family conserved in plants and select animal taxa. J. Mol. Evol. 2006, 63, 12–29. [Google Scholar] [CrossRef] [PubMed]
- Dunwell, J.M.; Khuri, S.; Gane, P.J. Microbial Relatives of the Seed Storage Proteins of Higher Plants: Conservation of Structure and Diversification of Function during Evolution of the Cupin Superfamily. Microbiol. Mol. Biol. Rev. 2000, 64, 153–179. [Google Scholar] [CrossRef] [PubMed]
- Godfrey, D.; Able, A.J.; Dry, I.B. Induction of a grapevine germin-like protein (VvGLP3) gene is closely linked to the site of Erysiphe necator infection: A possible role in defense? Mol. Plant. Microbe. Interact. 2007, 20, 1112–1125. [Google Scholar] [CrossRef] [PubMed]
- Regente, M.; Pinedo, M.; San Clemente, H.; Balliau, T.; Jamet, E.; de la Canal, L. Plant extracellular vesicles are incorporated by a fungal pathogen and inhibit its growth. J. Exp. Bot. 2017, 68, 5485–5495. [Google Scholar] [CrossRef]
- Wojtaszek, P.; Stobiecki, M.; Bolwell, G.P. Changes in the composition of exocellular proteins of suspension-cultured Lupinus albus cells in response to fungal elicitors or CuCl2. J. Exp. Bot. 1997, 48, 2015–2021. [Google Scholar] [CrossRef]
- Dani, V.; Simon, W.J.; Duranti, M.; Croy, R.R.D.R.D. Changes in the tobacco leaf apoplast proteome in response to salt stress. Proteomics 2005, 5, 737–745. [Google Scholar] [CrossRef]
- Jaswanthi, N.; Krishna, M.S.R.; Sahitya, U.L.; Suneetha, P. Apoplast proteomic analysis reveals drought stress-responsive protein datasets in chilli (Capsicum annuum L.). Data Br. 2019, 25, 104041. [Google Scholar] [CrossRef]
- Dunwell, J.M.; Gibbings, J.G.; Mahmood, T.; Saqlan Naqvi, S.M. Germin and germin-like proteins: Evolution, structure, and function. CRC. Crit. Rev. Plant Sci. 2008, 27, 342–375. [Google Scholar] [CrossRef]
- Somssich, I.; Hahlbrock, K. Pathogen defence in plants — a paradigm of biological complexity. Trends Plant Sci. 1998, 3, 86–90. [Google Scholar] [CrossRef]
- Bowler, C.; Montagu, M.V.; Inze, D. Superoxide Dismutase and Stress Tolerance. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1992, 43, 83–116. [Google Scholar] [CrossRef]
- Khuri, S.; Bakker, F.T.; Dunwell, J.M. Phylogeny, function, and evolution of the cupins, a structurally conserved, functionally diverse superfamily of proteins. Mol. Biol. Evol. 2001, 18, 593–605. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Chen, X.; Zhu, F.; Li, H.; Li, L.; Yang, Q.; Chi, X.; Yu, S.; Liang, X. Characterization of Peanut Germin-Like Proteins, AhGLPs in Plant Development and Defense. PLoS ONE 2013, 8, e61722. [Google Scholar] [CrossRef] [PubMed]
- Amini, F.; Ehsanpour, A.A.; Hoang, Q.T.; Shin, J.S. Protein pattern changes in tomato under in vitro salt stress. Russ. J. Plant Physiol. 2007, 54, 464–471. [Google Scholar] [CrossRef]
- Van der Hoorn, R.A.L. Plant Proteases: From Phenotypes to Molecular Mechanisms. Annu. Rev. Plant Biol. 2008, 59, 191–223. [Google Scholar] [CrossRef]
- Tian, M.; Win, J.; Song, J.; Van Der Hoorn, R.; van der Knaap, E.; Kamoun, S. A Phytophthora infestans Cystatin-Like Protein Targets a Novel Tomato Papain-Like Apoplastic Protease. Plant Physiol. 2006, 143, 364–377. [Google Scholar] [CrossRef]
- Figueiredo, A.; Monteiro, F.; Sebastiana, M. Subtilisin-like proteases in plant–pathogen recognition and immune priming: A perspective. Front. Plant Sci. 2014, 5, 739. [Google Scholar] [CrossRef]
- Tian, M.; Benedetti, B.; Kamoun, S. A second Kazal-like protease inhibitor from Phytophthora infestans inhibits and interacts with the apoplastic pathogenesis-related protease P69B of tomato. Plant Physiol. 2005, 138, 1785–1793. [Google Scholar] [CrossRef]
- Ramírez, V.; López, A.; Mauch-Mani, B.; Gil, M.J.; Vera, P. An Extracellular Subtilase Switch for Immune Priming in Arabidopsis. PLoS Pathog. 2013, 9, e1003445. [Google Scholar] [CrossRef]
- Terras, F.R.G.; Schoofs, H.M.E.; Thevissen, K.; Osborn, R.W.; Vanderleyden, J.; Cammue, B.P.A.; Broekaert, W.F. Synergistic enhancement of the antifungal activity of wheat and barley thionins by radish and oilseed rape 2S albumins and by barley trypsin inhibitors. Plant Physiol. 1993, 103, 1311–1319. [Google Scholar] [CrossRef] [PubMed]
- Boller, T.; Felix, G. A Renaissance of Elicitors: Perception of Microbe-Associated Molecular Patterns and Danger Signals by Pattern-Recognition Receptors. Annu. Rev. Plant Biol. 2009, 60, 379–406. [Google Scholar] [CrossRef] [PubMed]
- Planas-Marquès, M.; Bernardo-Faura, M.; Paulus, J.; Kaschani, F.; Kaiser, M.; Valls, M.; Van Der Hoorn, R.A.L.; Coll, N.S. Protease Activities Triggered by Ralstonia solanacearum Infection in Susceptible and Tolerant Tomato Lines. Mol. Cell. Proteom. 2018, 17, 1112–1125. [Google Scholar] [CrossRef] [PubMed]
- Ziemann, S.; van der Linde, K.; Lahrmann, U.; Acar, B.; Kaschani, F.; Colby, T.; Kaiser, M.; Ding, Y.; Schmelz, E.; Huffaker, A.; et al. An apoplastic peptide activates salicylic acid signalling in maize. Nat. Plants 2018, 4, 172–180. [Google Scholar] [CrossRef] [PubMed]
- Schulze Hüynck, J.; Kaschani, F.; van der Linde, K.; Ziemann, S.; Müller, A.N.; Colby, T.; Kaiser, M.; Misas Villamil, J.C.; Doehlemann, G. Proteases underground: Analysis of the maize root apoplast identifies organ specific papain-like cysteine protease activity. Front. Plant Sci. 2019, 10, 473. [Google Scholar] [CrossRef] [PubMed]
- Krüger, J.; Thomas, C.M.; Golstein, C.; Dixon, M.S.; Smoker, M.; Tang, S.; Mulder, L.; Jones, J.D.G. A tomato cysteine protease required for Cf-2-dependent disease resistance and suppression of autonecrosis. Science 2002, 296, 744–747. [Google Scholar] [CrossRef] [PubMed]
- Lozano-Torres, J.L.; Wilbers, R.H.P.; Gawronski, P.; Boshoven, J.C.; Finkers-Tomczak, A.; Cordewener, J.H.G.; America, A.H.P.; Overmars, H.A.; Van ’t Klooster, J.W.; Baranowski, L.; et al. Dual disease resistance mediated by the immune receptor Cf-2 in tomato requires a common virulence target of a fungus and a nematode. Proc. Natl. Acad. Sci. USA 2012, 109, 10119–10124. [Google Scholar] [CrossRef] [PubMed]
- Lay, F.; Anderson, M. Defensins - Components of the Innate Immune System in Plants. Curr. Protein Pept. Sci. 2005, 6, 85–101. [Google Scholar] [CrossRef]
- Bohlmann, H. The role of thionins in plant protection. CRC. Crit. Rev. Plant Sci. 1994, 13, 1–16. [Google Scholar] [CrossRef]
- Broekaert, W.F.; Terras, F.R.G.; Cammue, B.P.A.; Osborn, R.W. Plant defensins: Novel antimicrobial peptides as components of the host defense system. Plant Physiol. 1995, 108, 1353–1358. [Google Scholar] [CrossRef]
- Kaur, J.; Fellers, J.; Adholeya, A.; Velivelli, S.L.S.; El-Mounadi, K.; Nersesian, N.; Clemente, T.; Shah, D. Expression of apoplast-targeted plant defensin MtDef4.2 confers resistance to leaf rust pathogen Puccinia triticina but does not affect mycorrhizal symbiosis in transgenic wheat. Transgenic Res. 2017, 26, 37–49. [Google Scholar] [CrossRef] [PubMed]
- Hsiao, P.Y.; Cheng, C.P.; Koh, K.W.; Chan, M.T. The Arabidopsis defensin gene, AtPDF1.1, mediates defence against Pectobacterium carotovorum subsp. carotovorum via an iron-withholding defence system. Sci. Rep. 2017, 7. [Google Scholar] [CrossRef] [PubMed]
- Koike, M.; Okamoto, T.; Tsuda, S.; Imai, R. A novel plant defensin-like gene of winter wheat is specifically induced during cold acclimation. Biochem. Biophys. Res. Commun. 2002, 298, 46–53. [Google Scholar] [CrossRef]
- Boutrot, F.; Chantret, N.; Gautier, M.F. Genome-wide analysis of the rice and arabidopsis non-specific lipid transfer protein (nsLtp) gene families and identification of wheat nsLtp genes by EST data mining. BMC Genom. 2008, 9. [Google Scholar] [CrossRef] [PubMed]
- Kader, J.-C. LIPID-TRANSFER PROTEINS IN PLANTS. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1996, 47, 627–654. [Google Scholar] [CrossRef] [PubMed]
- De O. Carvalho, A.; de S. Teodoro, C.E.; Da Cunha, M.; Okorokova-Facanha, A.L.; Okorokov, L.A.; Fernandes, K.V.S.; Gomes, V.M. Intracellular localization of a lipid transfer protein in Vigna unguiculata seeds. Physiol. Plant. 2004, 122, 328–336. [Google Scholar] [CrossRef]
- Kusumawati, L.; Imin, N.; Djordjevic, M.A. Characterization of the secretome of suspension cultures of medicago species reveals proteins important for defense and development. J. Proteome Res. 2008, 7, 4508–4520. [Google Scholar] [CrossRef]
- Pagnussat, L.; Burbach, C.; Baluska, F.; de la Canal, L. An extracellular lipid transfer protein is relocalized intracellularly during seed germination. J. Exp. Bot. 2012, 63, 6555–6563. [Google Scholar] [CrossRef] [PubMed]
- Finkina, E.I.; Melnikova, D.N.; Bogdanov, I.V.; Ovchinnikova, T.V. Lipid Transfer Proteins As Components of the Plant Innate Immune System: Structure, Functions, and Applications. Acta Naturae 2016, 8, 47–61. [Google Scholar] [CrossRef]
- Sakurai, N. Dynamic function and regulation of apoplast in the plant body. J. Plant Res. 1998, 111, 133–148. [Google Scholar] [CrossRef]
- Lee, S.B.; Go, Y.S.; Bae, H.J.; Park, J.H.; Cho, S.H.; Cho, H.J.; Lee, D.S.; Park, O.K.; Hwang, I.; Suh, M.C. Disruption of glycosylphosphatidylinositol-anchored lipid transfer protein gene altered cuticular lipid composition, increased plastoglobules, and enhanced susceptibility to infection by the fungal pathogen alternaria brassicicola. Plant Physiol. 2009, 150, 42–54. [Google Scholar] [CrossRef] [PubMed]
- Sarowar, S.; Kim, Y.J.; Kim, K.D.; Hwang, B.K.; Ok, S.H.; Shin, J.S. Overexpression of lipid transfer protein (LTP) genes enhances resistance to plant pathogens and LTP functions in long-distance systemic signaling in tobacco. Plant Cell Rep. 2009, 28, 419–427. [Google Scholar] [CrossRef] [PubMed]
- Maldonado, A.M.; Doerner, P.; Dixonk, R.A.; Lamb, C.J.; Cameron, R.K. A putative lipid transfer protein involved in systemic resistance signalling in Arabidopsis. Nature 2002, 419, 399–403. [Google Scholar] [CrossRef]
- Asai, T.; Tena, G.; Plotnikova, J.; Willmann, M.R.; Chiu, W.-L.; Gomez-Gomez, L.; Boller, T.; Ausubel, F.M.; Sheen, J. MAP kinase signalling cascade in Arabidopsis innate immunity. Nature 2002, 415, 977–983. [Google Scholar] [CrossRef] [PubMed]
- Nürnberger, T.; Brunner, F.; Kemmerling, B.; Piater, L. Innate immunity in plants and animals: Striking similarities and obvious differences. Immunol. Rev. 2004, 198. [Google Scholar]
- Felix, G.; Duran, J.D.; Volko, S.; Boller, T. Plants have a sensitive perception system for the most conserved domain of bacterial flagellin. Plant J. 1999, 18, 265–276. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Gómez, L.; Felix, G.; Boller, T. A single locus determines sensitivity to bacterial flagellin in Arabidopsis thaliana. Plant J. 1999, 18, 277–284. [Google Scholar] [CrossRef]
- Gómez-Gómez, L.; Boller, T. FLS2: An LRR Receptor–like Kinase Involved in the Perception of the Bacterial Elicitor Flagellin in Arabidopsis. Mol. Cell 2000, 2, 1003–1011. [Google Scholar] [CrossRef]
- Sun, Y.; Li, L.; Macho, A.P.; Han, Z.; Hu, Z.; Zipfel, C.; Zhou, J.M.; Chai, J. Structural basis for flg22-induced activation of the Arabidopsis FLS2-BAK1 immune complex. Science 2013, 342, 624–628. [Google Scholar] [CrossRef]
- Zipfel, C.; Robatzek, S.; Navarro, L.; Oakeley, E.J.; Jones, J.D.G.; Felix, G.; Boller, T. Bacterial disease resistance in Arabidopsis through flagellin perception. Nature 2004, 428, 764–767. [Google Scholar] [CrossRef]
- Kunze, G.; Zipfel, C.; Robatzek, S.; Niehaus, K.; Boller, T.; Felix, G. The N terminus of bacterial elongation factor Tu elicits innate immunity in Arabidopsis plants. Plant Cell 2004, 16, 3496–3507. [Google Scholar] [CrossRef] [PubMed]
- Mott, G.A.; Middleton, M.A.; Desveaux, D.; Guttman, D.S. Peptides and small molecules of the plant-pathogen apoplastic arena. Front. Plant Sci. 2014, 5, 677. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Albert, M.; Einig, E.; Fürst, U.; Krust, D.; Felix, G. The pattern-recognition receptor CORE of Solanaceae detects bacterial cold-shock protein. Nat. Plants 2016, 2. [Google Scholar] [CrossRef] [PubMed]
- Felix, G.; Grosskopf, D.G.; Regenass, M.; Basse, C.W.; Boller, T. Elicitor-induced ethylene biosynthesis in tomato cells: Characterization and use as a bioassay for elicitor action. Plant Physiol. 1991, 97, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Yano, A.; Suzuki, K.; Uchimiya, H.; Shinshi, H. Induction of hypersensitive cell death by a fungal protein in cultures of tobacco cells. Mol. Plant-Microbe Interact. 1998, 11, 115–123. [Google Scholar] [CrossRef]
- Enkerli, J.; Felix, G.; Boller, T. The enzymatic activity of fungal xylanase is not necessary for its elicitor activity. Plant Physiol. 1999, 121, 391–397. [Google Scholar] [CrossRef] [PubMed]
- Ron, M.; Avni, A. The receptor for the fungal elicitor ethylene-inducing xylanase is a member of a resistance-like gene family in tomato. Plant Cell 2004, 16, 1604–1615. [Google Scholar] [CrossRef]
- Ranf, S.; Gisch, N.; Schäffer, M.; Illig, T.; Westphal, L.; Knirel, Y.A.; Sánchez-Carballo, P.M.; Zähringer, U.; Hückelhoven, R.; Lee, J.; et al. A lectin S-domain receptor kinase mediates lipopolysaccharide sensing in Arabidopsis thaliana. Nat. Immunol. 2015, 16, 426–433. [Google Scholar] [CrossRef]
- Willmann, R.; Lajunen, H.M.; Erbs, G.; Newman, M.-A.; Kolb, D.; Tsuda, K.; Katagiri, F.; Fliegmann, J.; Bono, J.-J.; Cullimore, J.V.; et al. Arabidopsis lysin-motif proteins LYM1 LYM3 CERK1 mediate bacterial peptidoglycan sensing and immunity to bacterial infection. Proc. Natl. Acad. Sci. USA 2011, 108, 19824–19829. [Google Scholar] [CrossRef]
- Gust, A.A.; Willmann, R.; Desaki, Y.; Grabherr, H.M.; Nürnberger, T. Plant LysM proteins: Modules mediating symbiosis and immunity. Trends Plant Sci. 2012, 17, 495–502. [Google Scholar] [CrossRef]
- Kramer, E.M. How far can a molecule of weak acid travel in the apoplast or xylem? Plant Physiol. 2006, 141, 1233–1236. [Google Scholar] [CrossRef] [PubMed]
- Hager, A. Role of the plasma membrane H+-ATPase in auxin-induced elongation growth: Historical and new aspects. J. Plant Res. 2003, 116, 483–505. [Google Scholar] [CrossRef] [PubMed]
- Barbez, E.; Dünser, K.; Gaidora, A.; Lendl, T.; Busch, W. Auxin steers root cell expansion via apoplastic pH regulation in Arabidopsis thaliana. Proc. Natl. Acad. Sci. USA 2017, 114, E4884–E4893. [Google Scholar] [CrossRef] [PubMed]
- Hartung, W.; Sauter, A.; Hose, E. Abscisic acid in the xylem: Where does it come from, where does it go to? J. Exp. Bot. 2002, 53, 27–32. [Google Scholar] [CrossRef] [PubMed]
- Sauter, A.; Hartung, W. Radial transport of abscisic acid conjugates in maize roots: Its implication for long distance stress signals. J. Exp. Bot. 2000, 51, 929–935. [Google Scholar] [CrossRef]
- Moubayidin, L.; Di Mambro, R.; Sabatini, S. Cytokinin-auxin crosstalk. Trends Plant Sci. 2009, 14, 557–562. [Google Scholar] [CrossRef]
- Naseem, M.; Dandekar, T. The Role of Auxin-Cytokinin Antagonism in Plant-Pathogen Interactions. PLoS Pathog. 2012, 8, e1003026. [Google Scholar] [CrossRef]
- Pignocchi, C.; Kiddle, G.; Hernández, I.; Foster, S.J.; Asensi, A.; Taybi, T.; Barnes, J.; Foyer, C.H. Ascorbate oxidase-dependent changes in the redox state of the apoplast modulate gene transcript accumulation leading to modified hormone signaling and orchestration of defense processes in tobacco. Plant Physiol. 2006, 141, 423–435. [Google Scholar] [CrossRef]
- Chen, Z.; Silva, H.; Klessig, D.F. Active oxygen species in the induction of plant systemic acquired resistance by salicylic acid. Science 1993, 262, 1883–1886. [Google Scholar] [CrossRef]
- Cano, A.; Alcaraz, O.; Arnao, M.B. Free radical-scavenging activity of indolic compounds in aqueous and ethanolic media. Anal. Bioanal. Chem. 2003, 376, 33–37. [Google Scholar] [CrossRef]
- Schopfer, P.; Plachy, C.; Frahry, G. Release of reactive oxygen intermediates (superoxide radicals, hydrogen peroxide, and hydroxyl radicals) and peroxidase in germinating radish seeds controlled by light, gibberellin, and abscisic acid. Plant Physiol. 2001, 125, 1591–1602. [Google Scholar] [CrossRef] [PubMed]
| ENZYMATIC ANTIOXIDANTS | ||||
|---|---|---|---|---|
| Enzyme | Chemical Reaction | Involved in | Cellular Location | Ref. |
| Superoxide Dismutase (SOD) | O2•−+O2•−+2H+→2H2O2+O2 | Regulation of oxidative stress. Stress resistance or tolerance mechanisms | Apoplast, cytosol, mitochondria, chloroplast, peroxisomes | [23,69] |
| Catalase (CAT) | H2O2→H2O+(1/2) O2 | Regulation of oxidative stress. Stress resistance or tolerance mechanisms. Plant metabolism | Apoplast, cytosol, chloroplast, mitochondria, peroxisomes | [23,27,73] |
| Ascorbate Peroxidase (APX) | H2O2+Asc→2H2O+DHA | Regulation of oxidative stress. Stress resistance or tolerance mechanisms Plant growth and physiology | Apoplast, cytosol, mitochondria, peroxisomes, chloroplast | [74,75] |
| Monodehydroascorbate Reductase (MDHAR) | MDHA+NADPH→Asc+NADP+ | Regulation of oxidative stress. Stress resistance or tolerance mechanisms | Apoplast, cytosol, mitochondria, chloroplast | [23,76,77] |
| Dehydroascorbate Reductase (DHAR) | DHA+2GSH→Asc+GSSG | Regulation of oxidative stress Stress resistance or tolerance mechanisms Plant growth and development | Apoplast, cytoplasm, mitochondria, chloroplast, peroxisomes | [23,78,79] |
| Glutathione Reductase (GR) | GSSG+NADPH→2GSH+NADP+ | Regulation of oxidative stress. Stress resistance or tolerance mechanisms | Apoplast, cytoplasm, mitochondria, chloroplast | [80] |
| NON-ENZYMATIC ANTIOXIDANTS | |||
|---|---|---|---|
| Enzyme | Functions | Location | Ref. |
| Ascorbic Acid (AsA) | -Stress perception -Redox homeostasis -Regulation of oxidative stress -Improvement of plant stress tolerance | Apoplast, cytosol mitochondria, chloroplast, vacuoles, peroxisomes, nucleus | [23,81] |
| Glutathione (GSH) | -Protect membranes. -Prevent protein oxidative denaturation under stress conditions -Substrate for glutathione peroxidase and gluthatione S-transferase -Metal chelator | Apoplast, cytosol, chloroplast, mitochondria, vacuole, peroxisome, nucleus | [81] |
| Proline (Pro) | -Osmoprotectant activity -Antioxidant capacity -Metal chelator -Signalling under abiotic and biotic stresses -Plant growth and development | Apoplast, cytosol, mitochondria, chloroplast | [82,83] |
| Phenolic Compounds | -Antioxidant activity -Metal chelator -Protective and signalling functions against different stresses -Plant growth and development | Ubiquitous | [84] |
| Polyamines | -Antioxidant capacity -Plant growth and development. -Biotic and abiotic stress responses. -Osmotic adjustment ability | Ubiquitous | [85,86] |
| Families | Properties | References |
|---|---|---|
| PR-1 | Antifungal | [119] |
| PR-2 | β-1,3-glucanase | [120] |
| PR-3 | Chitinase type I, II, IV, V, VI, VII | [121] |
| PR-4 | Chitinase type I, II | [122] |
| PR-5 | Thaumatin- like | [39] |
| PR-6 | Proteinase- inhibitor | [123] |
| PR-7 | Endoproteinase | [124] |
| PR-8 | Chitinase type III | [125] |
| PR-9 | Peroxidase | [126] |
| PR-10 | Ribonuclease like | [127,128] |
| PR-11 | Chitinase, type I | [129] |
| PR-12 | Defensin | [130] |
| PR-13 | Thionin | [131] |
| PR-14 | Lipid- transfer protein | [132] |
| PR-15 | Oxalate oxidase | [133] |
| PR-16 | Oxalate oxidase-like | [123] |
| PR-17 | PRp27 Unknown | [134] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Farvardin, A.; González-Hernández, A.I.; Llorens, E.; García-Agustín, P.; Scalschi, L.; Vicedo, B. The Apoplast: A Key Player in Plant Survival. Antioxidants 2020, 9, 604. https://doi.org/10.3390/antiox9070604
Farvardin A, González-Hernández AI, Llorens E, García-Agustín P, Scalschi L, Vicedo B. The Apoplast: A Key Player in Plant Survival. Antioxidants. 2020; 9(7):604. https://doi.org/10.3390/antiox9070604
Chicago/Turabian StyleFarvardin, Atefeh, Ana Isabel González-Hernández, Eugenio Llorens, Pilar García-Agustín, Loredana Scalschi, and Begonya Vicedo. 2020. "The Apoplast: A Key Player in Plant Survival" Antioxidants 9, no. 7: 604. https://doi.org/10.3390/antiox9070604
APA StyleFarvardin, A., González-Hernández, A. I., Llorens, E., García-Agustín, P., Scalschi, L., & Vicedo, B. (2020). The Apoplast: A Key Player in Plant Survival. Antioxidants, 9(7), 604. https://doi.org/10.3390/antiox9070604





