Effects of Darkness and Light Spectra on Nutrients and Pigments in Radish, Soybean, Mung Bean and Pumpkin Sprouts
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials, Seed Germination and Light Treatments
2.2. Determination of Dry Matter Content
2.3. Anthocyanin Content
2.4. Determination of the Chlorophyll and Carotenoid Contents
2.5. Vitamin C Content
2.6. Soluble Protein Assay
2.7. Soluble Carbohydrates and Starch Measurements
2.8. Determination of the Polyphenol Contents
2.9. Starch Qualitative Analysis
2.10. Statistical Analyses
3. Results and Discussion
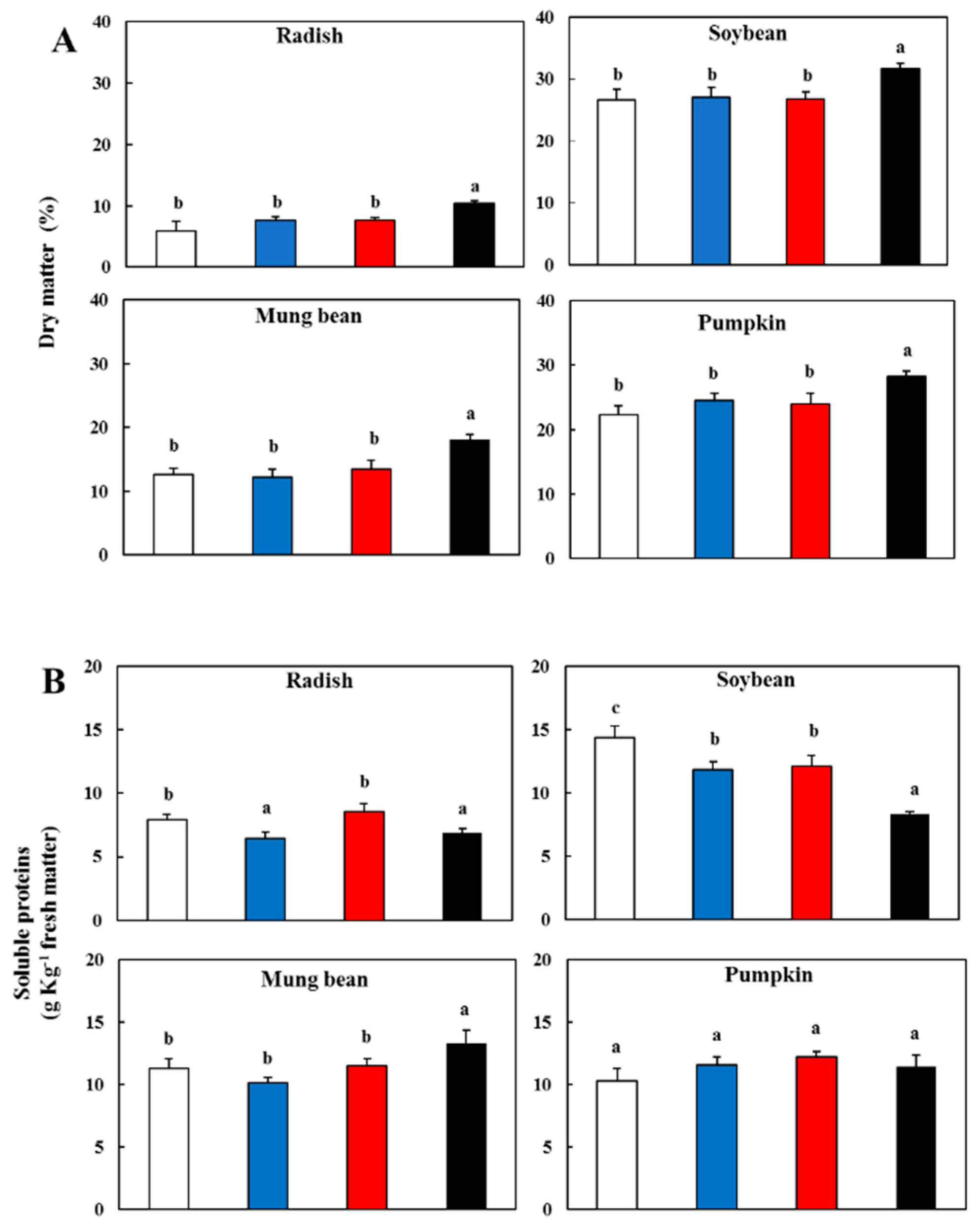
3.1. Effect of Light Spectral Properties on Dry Matter and Soluble Proteins
3.2. Effect of Light on Sugars and Starch
3.3. Changes in the Vitamin C and Total Phenolic Contents
3.4. Changes in the Anthocyanins, Chlorophylls and Carotenoids After Light and Dark Treatment
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Mbithi–Mwikya, S.; Van Camp, J.; Rodriguez, R.; Huyghebaert, A. Effects of sprouting on nutrient and antinutrient composition of kidney beans (Phaseolus vulgaris var. Rose coco). Eur. Food Res. Technol. 2001, 212, 188–191. [Google Scholar] [CrossRef]
- Donkor, O.N.; Henriksson, A.; Vasiljevic, T.; Shah, N.P. Probiotic strains as starter cultures improve angiotensin–converting enzyme inhibitory activity in soy yoghurt. J. Food Sci. 2005, 70, M375–M381. [Google Scholar] [CrossRef]
- Bamdad, F.; Dokhani, S.; Keramat, J.; Zareie, R. The impact of germination and in vitro digestion on the formation of angiotensin converting enzyme (ACE) inhibitory peptides from lentil proteins compared to whey proteins. J. Eng. Technol. 2009, 49, 36–46. [Google Scholar]
- Shah, S.A.; Zeb, A.; Masood, T.; Noreen, N.; Abbas, S.J.; Samiullah, M.; Alim, M.A.; Muhammad, A. Effects of sprouting time on biochemical and nutritional qualities of mungbean varieties. Acad J. Agric. Res. 2011, 6, 5091–5098. [Google Scholar]
- Nakamura, K.; Naramoto, K.; Koyama, M. Blood-pressure-lowering effect of fermented buckwheat sprouts in spontaneously hypertensive rats. J. Funct. Foods 2013, 5, 406–415. [Google Scholar] [CrossRef]
- Kumar, A.; Kaur, H.; Devi, P.; Mohan, V. Role of coenzyme Q10 (CoQ10) in cardiac disease, hypertension and Meniere—like syndrome. Pharmacol. Ther. 2009, 124, 259–268. [Google Scholar] [CrossRef]
- Mamilla, R.K.; Mishra, V.K. Effect of germination on antioxidant and ACE inhibitory activities of legumes. LWT 2017, 75, 51–58. [Google Scholar] [CrossRef]
- Kubo, H.; Fujii, K.; Kawabe, T.; Matsumoto, H.K.; Hosoe, K. Food content of ubiquinol–10 and ubiquinone–10 in the Japanese diet. J. Food Compost. Anal. 2008, 21, 199–210. [Google Scholar] [CrossRef]
- Lopez-Amoros, M.L.; Hernandez, T.; Estrella, I. Effect of germination on legume phenolic compounds and their antioxidant activity. J. Food Compost. Anal. 2006, 19, 277–283. [Google Scholar] [CrossRef]
- Wu, M.C.; Hou, C.Y.; Jiang, C.M.; Wang, Y.T.; Wang, C.Y.; Chen, H.H.; Chang, H.M. A novel approach of LED light radiation improves the antioxidant activity of pea seedlings. Food Chem. 2007, 101, 1753–1758. [Google Scholar] [CrossRef]
- Xu, H.L.; Xu, Q.C.; Li, F.L.; Feng, Y.Z.; Qin, F.F.; Fang, W. Applications of xerophytophysiology in plant production-LED blue light as a stimulus improved the tomato crop. Sci. Hortic. 2012, 148, 190–196. [Google Scholar] [CrossRef]
- Lin, K.H.; Huang, M.Y.; Huang, W.D.; Hsu, M.H.; Yang, Z.W.; Yang, C.M. The effects of red, blue, and white light–emitting diodes on the growth, development, and edible quality of hydroponically grown lettuce (Lactuca sativa L. var. capitata). Sci. Hortic. 2013, 150, 86–91. [Google Scholar] [CrossRef]
- Mastropasqua, L.; Borraccino, G.; Bianco, L.; Paciolla, C. Light qualities and dose influence ascorbate pool size in detached oat leaves. Plant Sci. 2012, 183, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Ma, G.; Zhang, L.; Setiawan, C.K.; Yamawaki, K.; Asai, T.; Nishikawa, F.; Maezawa, S.; Sato, H.; Kanemitsu, N.; Kato, M. Effect of red and blue LED light irradiation on ascorbate content and expression of genes related to ascorbate metabolism in postharvest broccoli. Postharvest Biol. Technol. 2014, 94, 97–103. [Google Scholar] [CrossRef]
- Mastropasqua, L.; Tanzarella, P.; Paciolla, C. Effects of postharvest light spectra on quality and health–related parameters in green Asparagus officinalis L. Postharvest Biol. Technol. 2016, 112, 143–151. [Google Scholar] [CrossRef]
- Chen, Z.; Zhang, H.; Jablonowski, D.; Zhou, X.; Ren, X.; Hong, X.; Schaffrath, R.; Zhu, J.K.; Gong, Z. Mutations in ABO1/ELO2, a subunit of holo–Elongator, increase abscisic acid sensitivity and drought tolerance in Arabidopsis thaliana. Mol. Cell. Biol. 2006, 26, 6902–6912. [Google Scholar] [CrossRef] [PubMed]
- Kadomura–Ishikawa, Y.; Miyawaki, K.; Noji, S.; Takahashi, A. Phototropin 2 is involved in blue light–induced anthocyanin accumulation in Fragaria × ananassa fruits. J. Plant Res. 2013, 126, 847–857. [Google Scholar] [CrossRef]
- Rodriguez–Villalon, A.; Gas, E.; Rodriguez–Concepcion, M. Colors in the dark: A model for the regulation of carotenoid biosynthesis in etioplasts. Plant Signal Behav. 2009, 4, 965–967. [Google Scholar] [CrossRef]
- Serafini–Fracassini, D.; Del Duca, S.; Monti, F.; Poli, F.; Sacchetti, G.; Bregoli, A.M.; Biondi, S.; Della Mea, M. Transglutaminase activity during senescence and programmed cell death in the corolla of tobacco (Nicotiana tabacum) flowers. Cell Death Differ. 2002, 9, 309–321. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K. Chlorophylls and carotenoids: Pigments of photosynthetic biomembranes. Methods Enzymol. 1987, 148, 350–382. [Google Scholar] [CrossRef]
- Cozzi, G.; Paciolla, C.; Haidukowski, M.; De Leonardis, S.; Mulé, G.; Logrieco, A. Increase of fumonisin B2 and ochratoxin a production by black aspergillus species and oxidative stress in grape berries damaged by powdery mildew. J. Food Prot. 2013, 76, 2031–2036. [Google Scholar] [CrossRef] [PubMed]
- Bradford, M.M. A Rapid and Sensitive Method for the Quantitation of Microgram Quantities of Protein Utilizing the Principle of Protein–Dye Binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Volden, J.; Borge, G.I.A.; Bengtsson, G.B.; Hansen, M.; Thygesen, I.E.; Wicklund, T. Effect of thermal treatment on glucosinolates and antioxidant related parameters in red cabbage (Brassica oleracea L. ssp. capitata f. rubra). Food Chem. 2008, 109, 595–605. [Google Scholar] [CrossRef]
- Chen, Y.; Chang, S.K. Macronutrients, Phytochemicals, and Antioxidant Activity of Soybean Sprout Germinated with or without Light Exposure. J. Food Sci. 2015, 80, 1391–1398. [Google Scholar] [CrossRef] [PubMed]
- Zhong, H.B.; Qi, C.Y.; Wen, K.L. Effects of light quality on the accumulation of phytochemicals in vegetables produced in controlled environments: A review. Sci. Food Agric. 2014, 95, 869–877. [Google Scholar]
- Chen, X.L.; Guo, W.Z.; Xue, X.Z.; Wang, L.C.; Qiao, X.J. Growth and quality responses of ‘Green Oak Leaf’ lettuce as affected by monochromic or mixed radiation provided by fluorescent lamp (FL) and light-emitting diode (LED). Sci. Hortic. 2014, 172, 168–175. [Google Scholar] [CrossRef]
- Matsuda, R.; Yamano, T.; Murakami, K.; Fujiwara, K. Effects of spectral distribution and photosynthetic photon flux density for overnight LED light irradiation on tomato seedling growth and leaf injury. Sci. Hortic. 2016, 198, 363–369. [Google Scholar] [CrossRef]
- Folta, K.M.; Edgar, P.S. Unexpected roles for cryptochrome 2 and phototropin revealed by high-resolution analysis of blue lightmediated hypocotyl growth inhibition. Plant J. 2001, 26, 471–478. [Google Scholar] [CrossRef]
- Wu, Q.; Su, N.N.; Shen, W.B.; Cui, J. Analyzing photosynthetic activity and growth of Solanum lycopersicum seedlings exposed to different light qualities. Acta Physiol. Plant. 2014, 36, 1411–1420. [Google Scholar] [CrossRef]
- Li, Y.; Xin, G.; Wei, M.; Shi, Q.; Yang, F.; Wang, X. Carbohydrate accumulation and sucrose metabolism responses in tomato seedling leaves when subjected to different light qualities. Sci. Hortic. 2017, 225, 490–497. [Google Scholar] [CrossRef]
- Sæbø, A.; Krekling, T.; Appelgren, M. Light quality affects photosynthesis and leaf anatomy of birch plantlets in vitro. Plant Cell Tissue Organ Cult. 1995, 41, 177–185. [Google Scholar] [CrossRef]
- Xu, M.J.; Dong, J.F.; Zhu, M.Y. Effects of germination conditions on ascorbic acid level and yield of soybean sprouts. J. Sci. Food Agric. 2005, 85, 943–947. [Google Scholar] [CrossRef]
- De Gara, L.; de Pinto, M.C.; Arrigoni, O. Ascorbate synthesis and ascorbate peroxidase activity during the early stage of wheat germination. Physiol. Plant. 1997, 100, 894–900. [Google Scholar] [CrossRef]
- Lee, S.W.; Seo, J.M.; Lee, M.K.; Chun, J.H.; Antonisamy, P.; Arasu, M.V.; Suzuki, T.; Al–Dhabi, N.A.; Kim, S.J. Influence of different LED lamps on the production of phenolic compounds in common and Tartary buckwheat sprouts. Ind. Crop. Prod. 2014, 54, 320–326. [Google Scholar] [CrossRef]
- Shoji, K.; Goto, E.; Hashida, S.; Goto, F.; Yoshihara, T. Effect of light quality on the polyphenol content and antioxidant activity of Sweet basil (Ocimum basilicum L.). Acta Hortic. 2011, 907, 95–100. [Google Scholar] [CrossRef]
- Qian, H.; Liu, T.; Deng, M.; Miao, H.; Cai, C.; Shen, W.; Wang, Q. Effects of light quality on main health–promoting compounds and antioxidant capacity of Chinese kale sprouts. Food Chem. 2016, 196, 1232–1238. [Google Scholar] [CrossRef]
- Mancinelli, A.L. Light dependent anthocyanin synthesis: A model system for the study of plant photomorphogenesis. Bot. Rev. 1985, 51, 107–157. [Google Scholar] [CrossRef]
- Shi, L.; Cao, S.; Chen, W.; Yang, Z. Blue light induced anthocyanin accumulation and expression of associated genes in Chinese bayberry fruit. Sci. Hortic. 2014, 179, 98–102. [Google Scholar] [CrossRef]
- Saure, M.C. External control of anthocyanin formation in apple. Sci. Hortic. 1990, 42, 181–218. [Google Scholar] [CrossRef]
- Xu, F.; Cao, S.; Shi, L.; Chen, W.; Su, X.; Yang, Z. Blue light irradiation affects anthocyanin content and enzyme activities involved in postharvest strawberry fruit. J. Agric. Food Chem. 2014, 62, 4778–4783. [Google Scholar] [CrossRef]
- Konczak, I.; Zhang, W. Anthocyanins—More Than Nature’s Colours. J. Biomed. Biotechnol. 2004, 5, 239–240. [Google Scholar] [CrossRef] [PubMed]
- Goodwin, T.W. Functions of Carotenoids. In The Biochemistry of the Carotenoids; Goodwin, T.W., Ed.; Springer: Dordrecht, The Netherlands, 1980; pp. 77–95. [Google Scholar]
- Simkin, A.J.; Zhu, C.; Kuntz, M.; Sandmann, G. Light–dark regulation of carotenoid biosynthesis in pepper (Capsicum annuum) leaves. J. Plant Physiol. 2003, 160, 439–443. [Google Scholar] [CrossRef] [PubMed]

 ); blue light, (
); blue light, ( ); red light (
); red light ( ), and dark (
), and dark ( ).
).
 ); blue light, (
); blue light, ( ); red light (
); red light ( ), and dark (
), and dark ( ).
).
 ), d-glucose (
), d-glucose ( ), sucrose (
), sucrose ( )—In sprouts 5 d after germination following various treatments. White light, WL; blue light, BL; red light, RL, and dark, D. (B) Starch content in the sprouts 5 d after germination following various treatments. White light (
)—In sprouts 5 d after germination following various treatments. White light, WL; blue light, BL; red light, RL, and dark, D. (B) Starch content in the sprouts 5 d after germination following various treatments. White light ( ); blue light (
); blue light ( ); red light (
); red light ( ), and dark (
), and dark ( ). Values represent the means of at least three replications from four independent experiments. Identical letters over the columns indicate non-significant differences between the treatments within each segment (Tukey’s HSD test, p < 0.05).
). Values represent the means of at least three replications from four independent experiments. Identical letters over the columns indicate non-significant differences between the treatments within each segment (Tukey’s HSD test, p < 0.05).
 ), d-glucose (
), d-glucose ( ), sucrose (
), sucrose ( )—In sprouts 5 d after germination following various treatments. White light, WL; blue light, BL; red light, RL, and dark, D. (B) Starch content in the sprouts 5 d after germination following various treatments. White light (
)—In sprouts 5 d after germination following various treatments. White light, WL; blue light, BL; red light, RL, and dark, D. (B) Starch content in the sprouts 5 d after germination following various treatments. White light ( ); blue light (
); blue light ( ); red light (
); red light ( ), and dark (
), and dark ( ). Values represent the means of at least three replications from four independent experiments. Identical letters over the columns indicate non-significant differences between the treatments within each segment (Tukey’s HSD test, p < 0.05).
). Values represent the means of at least three replications from four independent experiments. Identical letters over the columns indicate non-significant differences between the treatments within each segment (Tukey’s HSD test, p < 0.05).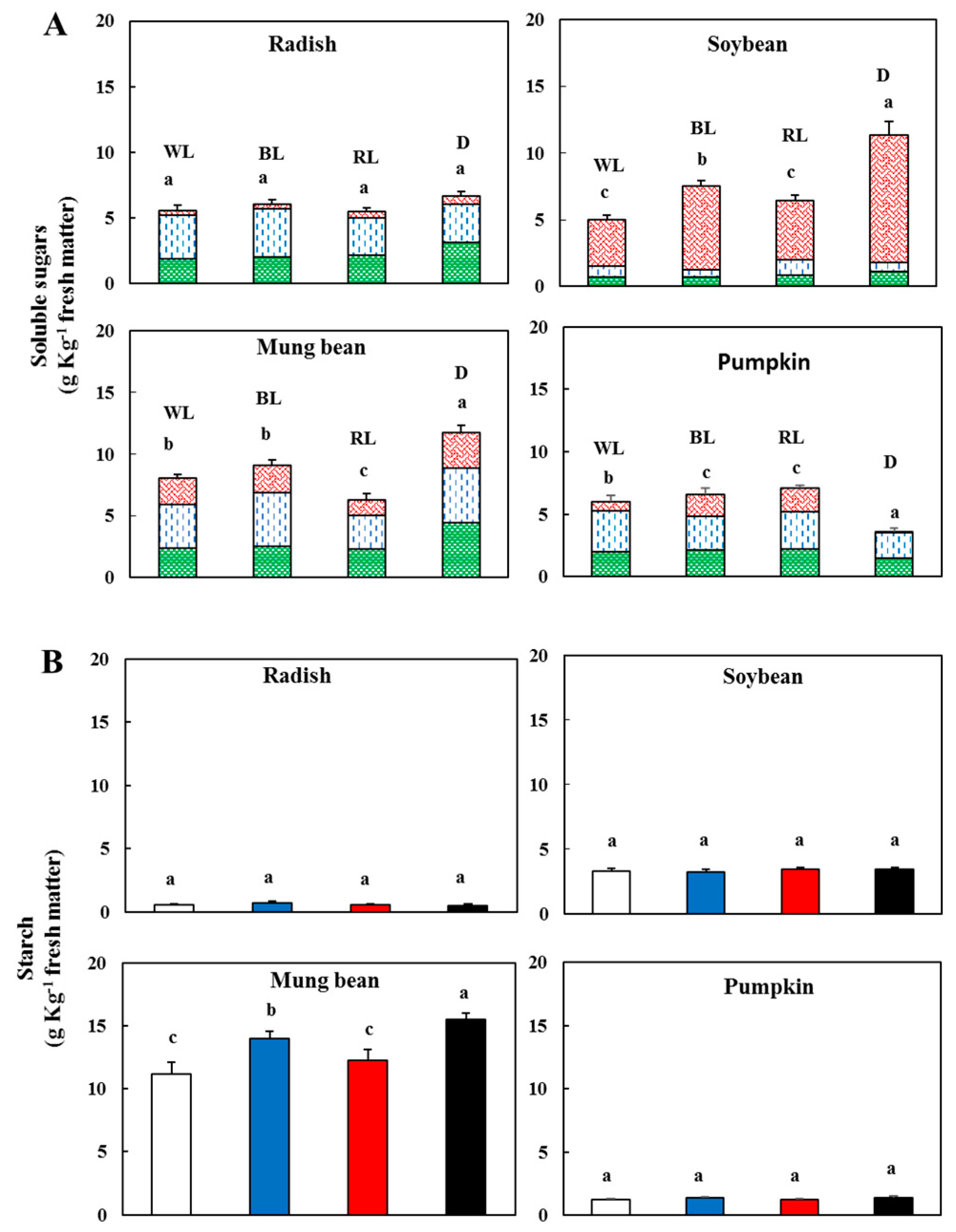
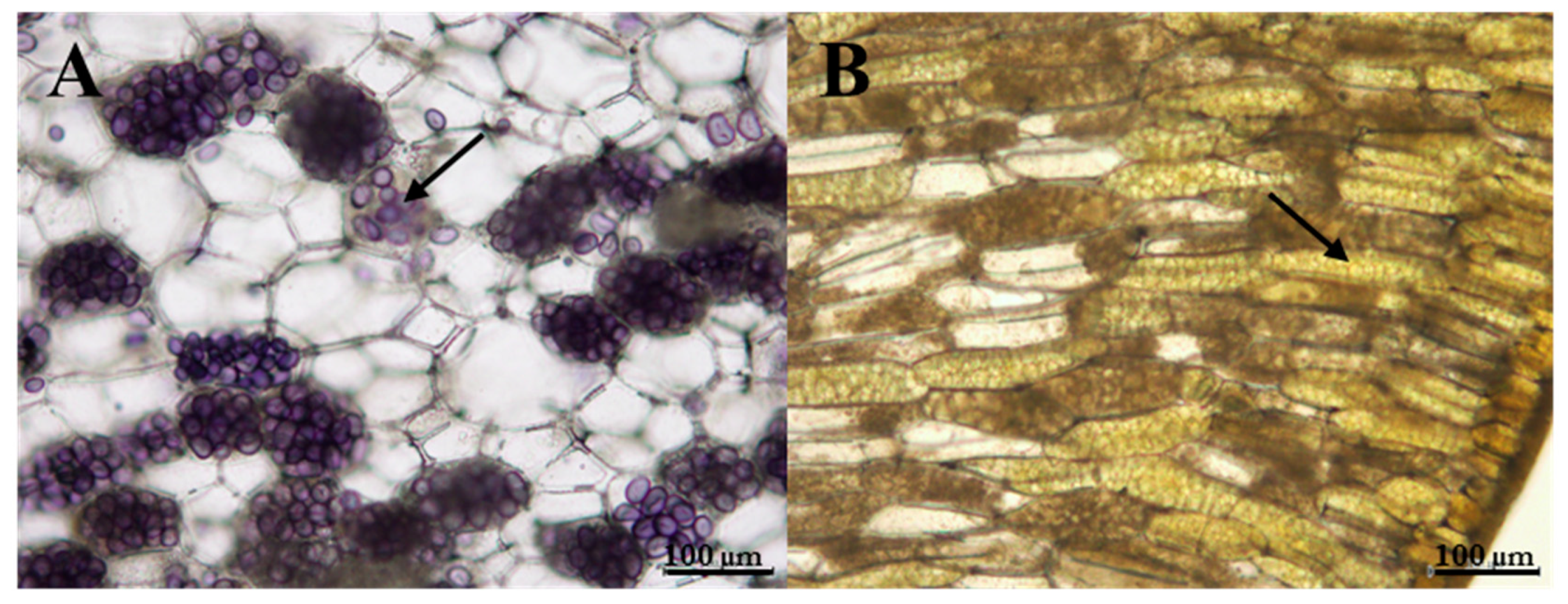
 ) and l-dehydroascorbic acid (DHA,
) and l-dehydroascorbic acid (DHA,  )—In the sprouts 5 d after germination following various treatments. White light, WL; blue light, BL; red light, RL, and dark, D. (B) Total polyphenolic content in the sprouts 5 d after the germination of various treatments. White light (
)—In the sprouts 5 d after germination following various treatments. White light, WL; blue light, BL; red light, RL, and dark, D. (B) Total polyphenolic content in the sprouts 5 d after the germination of various treatments. White light ( ); blue light (
); blue light ( ); red light (
); red light ( ), and dark (
), and dark ( ). Values represent the mean of at least three replicates from four independent experiments. Identical letters over the columns indicate non-significant differences between treatments within each segment (Tukey’s HSD test, p < 0.05).
). Values represent the mean of at least three replicates from four independent experiments. Identical letters over the columns indicate non-significant differences between treatments within each segment (Tukey’s HSD test, p < 0.05).
 ) and l-dehydroascorbic acid (DHA,
) and l-dehydroascorbic acid (DHA,  )—In the sprouts 5 d after germination following various treatments. White light, WL; blue light, BL; red light, RL, and dark, D. (B) Total polyphenolic content in the sprouts 5 d after the germination of various treatments. White light (
)—In the sprouts 5 d after germination following various treatments. White light, WL; blue light, BL; red light, RL, and dark, D. (B) Total polyphenolic content in the sprouts 5 d after the germination of various treatments. White light ( ); blue light (
); blue light ( ); red light (
); red light ( ), and dark (
), and dark ( ). Values represent the mean of at least three replicates from four independent experiments. Identical letters over the columns indicate non-significant differences between treatments within each segment (Tukey’s HSD test, p < 0.05).
). Values represent the mean of at least three replicates from four independent experiments. Identical letters over the columns indicate non-significant differences between treatments within each segment (Tukey’s HSD test, p < 0.05).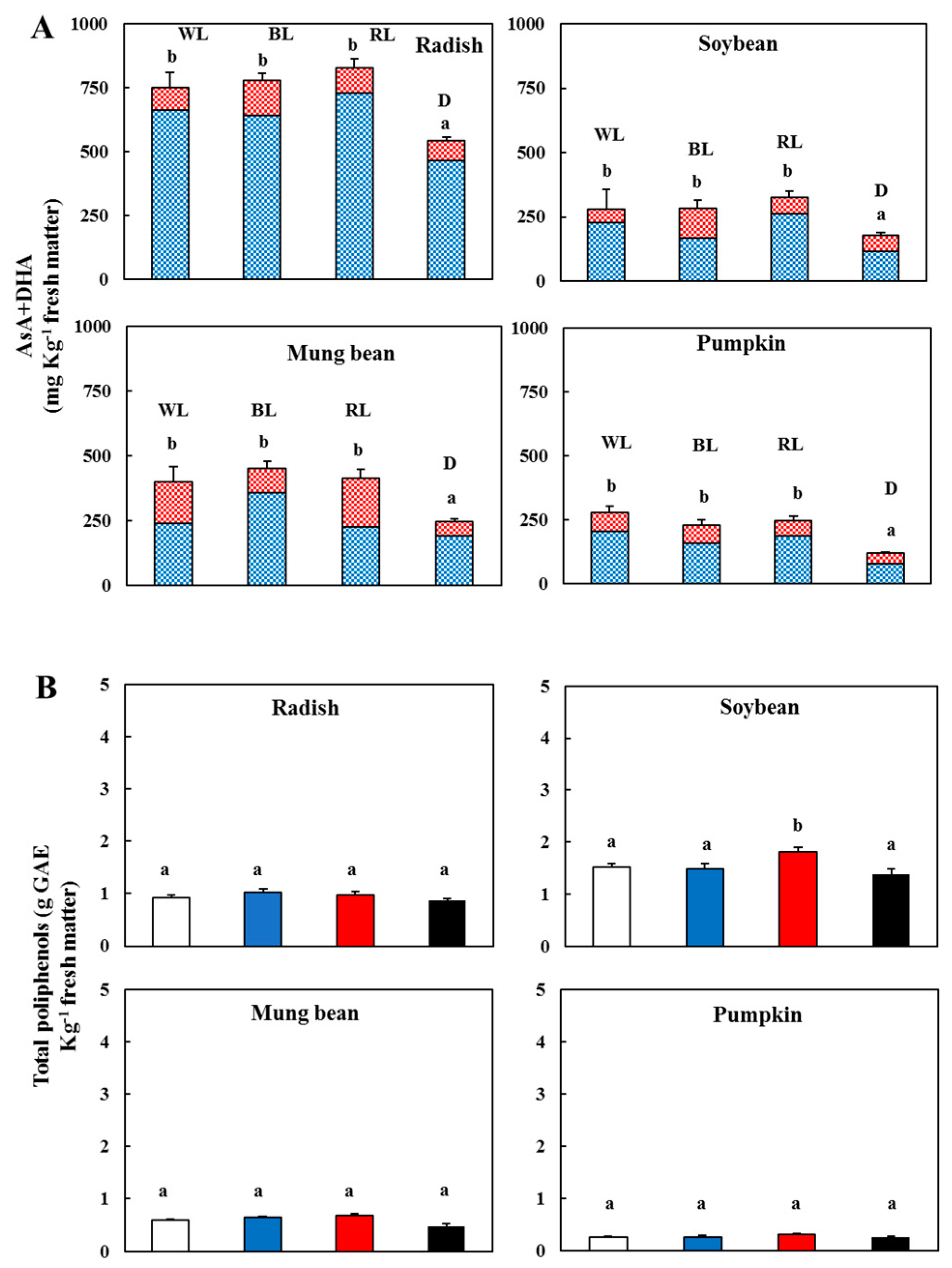
 ); blue light (
); blue light ( ); red light (
); red light ( ), and dark (
), and dark ( ). (C) Contents of chlorophyll a (
). (C) Contents of chlorophyll a ( ) and b (
) and b ( ) in the sprouts 5 d after germination following various treatments. White light, WL; blue light, BL; red light, RL, and dark, D. Values represent the means of at least three replicates from four independent experiments. Identical letters over the columns indicate non-significant differences between the treatments within each segment (Tukey’s HSD test, p < 0.05).
) in the sprouts 5 d after germination following various treatments. White light, WL; blue light, BL; red light, RL, and dark, D. Values represent the means of at least three replicates from four independent experiments. Identical letters over the columns indicate non-significant differences between the treatments within each segment (Tukey’s HSD test, p < 0.05).
 ); blue light (
); blue light ( ); red light (
); red light ( ), and dark (
), and dark ( ). (C) Contents of chlorophyll a (
). (C) Contents of chlorophyll a ( ) and b (
) and b ( ) in the sprouts 5 d after germination following various treatments. White light, WL; blue light, BL; red light, RL, and dark, D. Values represent the means of at least three replicates from four independent experiments. Identical letters over the columns indicate non-significant differences between the treatments within each segment (Tukey’s HSD test, p < 0.05).
) in the sprouts 5 d after germination following various treatments. White light, WL; blue light, BL; red light, RL, and dark, D. Values represent the means of at least three replicates from four independent experiments. Identical letters over the columns indicate non-significant differences between the treatments within each segment (Tukey’s HSD test, p < 0.05).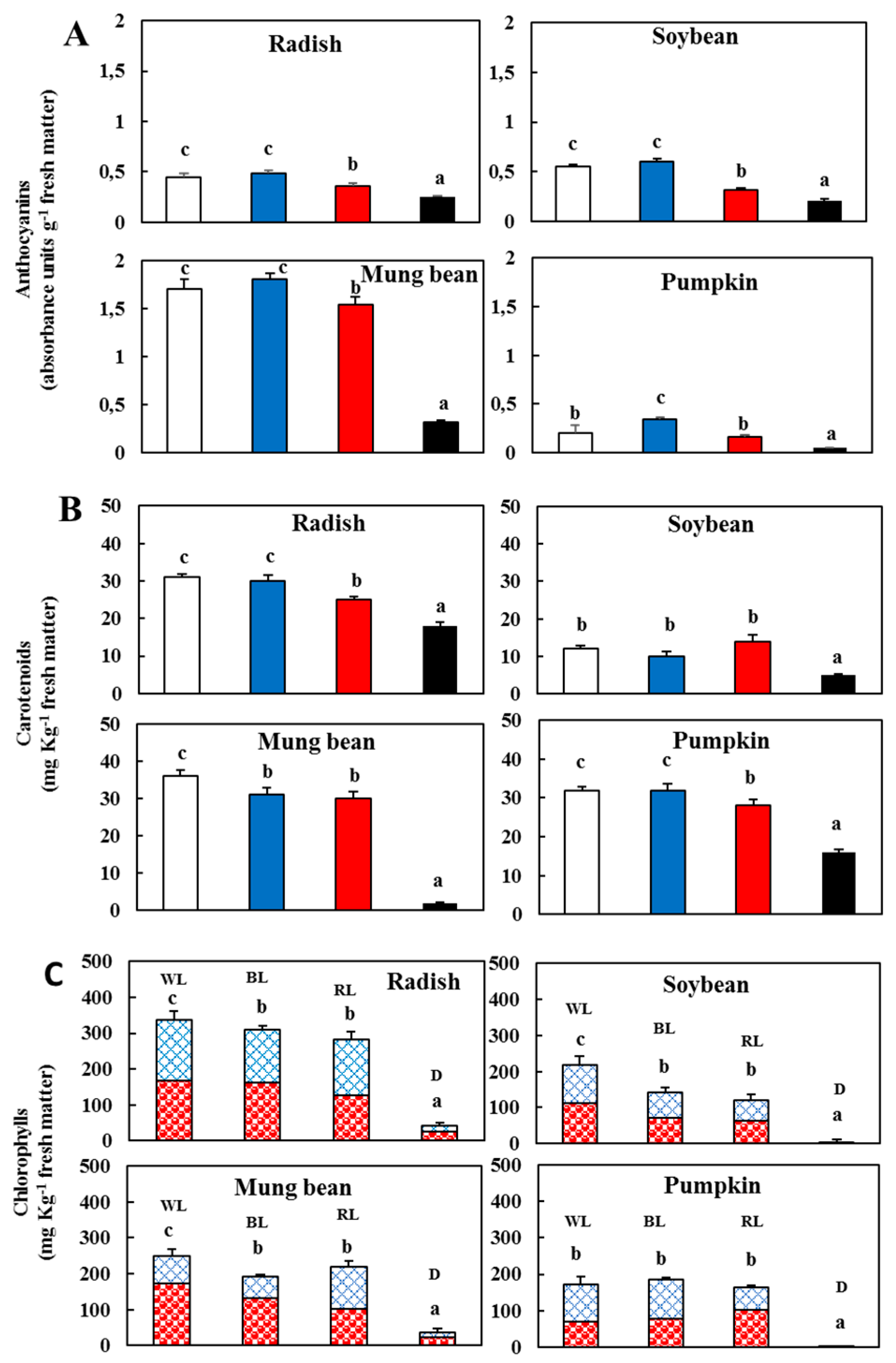
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mastropasqua, L.; Dipierro, N.; Paciolla, C. Effects of Darkness and Light Spectra on Nutrients and Pigments in Radish, Soybean, Mung Bean and Pumpkin Sprouts. Antioxidants 2020, 9, 558. https://doi.org/10.3390/antiox9060558
Mastropasqua L, Dipierro N, Paciolla C. Effects of Darkness and Light Spectra on Nutrients and Pigments in Radish, Soybean, Mung Bean and Pumpkin Sprouts. Antioxidants. 2020; 9(6):558. https://doi.org/10.3390/antiox9060558
Chicago/Turabian StyleMastropasqua, Linda, Nunzio Dipierro, and Costantino Paciolla. 2020. "Effects of Darkness and Light Spectra on Nutrients and Pigments in Radish, Soybean, Mung Bean and Pumpkin Sprouts" Antioxidants 9, no. 6: 558. https://doi.org/10.3390/antiox9060558
APA StyleMastropasqua, L., Dipierro, N., & Paciolla, C. (2020). Effects of Darkness and Light Spectra on Nutrients and Pigments in Radish, Soybean, Mung Bean and Pumpkin Sprouts. Antioxidants, 9(6), 558. https://doi.org/10.3390/antiox9060558






