In vitro Antioxidant, Anti-inflammatory, Anti-metabolic Syndrome, Antimicrobial, and Anticancer Effect of Phenolic Acids Isolated from Fresh Lovage Leaves [Levisticum officinale Koch] Elicited with Jasmonic Acid and Yeast Extract
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Plant Material
2.3. Preparation of Extracts
2.3.1. Buffer Extracts
2.3.2. In Vitro Digestion
2.4. Analysis of Phenolic Compounds
HPLC Analysis
2.5. Antioxidant Activities
2.5.1. Free Radical Scavenging Assay
2.5.2. Ferric Reducing Antioxidant Power
2.5.3. Chelating Power
2.6. Determination of Potential Anti-Inflammatory Properties
2.6.1. Lipoxygenase Inhibitory Activity
2.6.2. Cyclooxygenase-2 Inhibitory Activity
2.7. Metabolic Syndrome Enzyme Inhibitory Activity Assay
2.7.1. Angiotensin Converting Enzyme (ACE) Inhibitory Assay
2.7.2. Lipase Inhibitory Activity Assay
2.7.3. α-Amylase Inhibitory Assay
2.7.4. α-Glucosidase Inhibitory Assay (αGIA)
2.8. Antimicrobial Properties
2.8.1. Determination of the Minimum Inhibitory Concentration (MIC) and Minimum Lethal Concentrations (MLC)
2.8.2. Estimation of the Biotoxicity of the Studied Samples Using Resazurin Reduction Assays
2.9. Anticancer Properties
2.9.1. Cell Culture
2.9.2. MTT and NR Tests
2.9.3. Cell Viability and Type of Cell Death
2.9.4. Cell Cycle
2.10. Statistical Analysis
3. Results
3.1. Phenolic Acid Analysis
3.2. Antioxidant Activities
3.3. Potential Anti-Inflammatory Properties
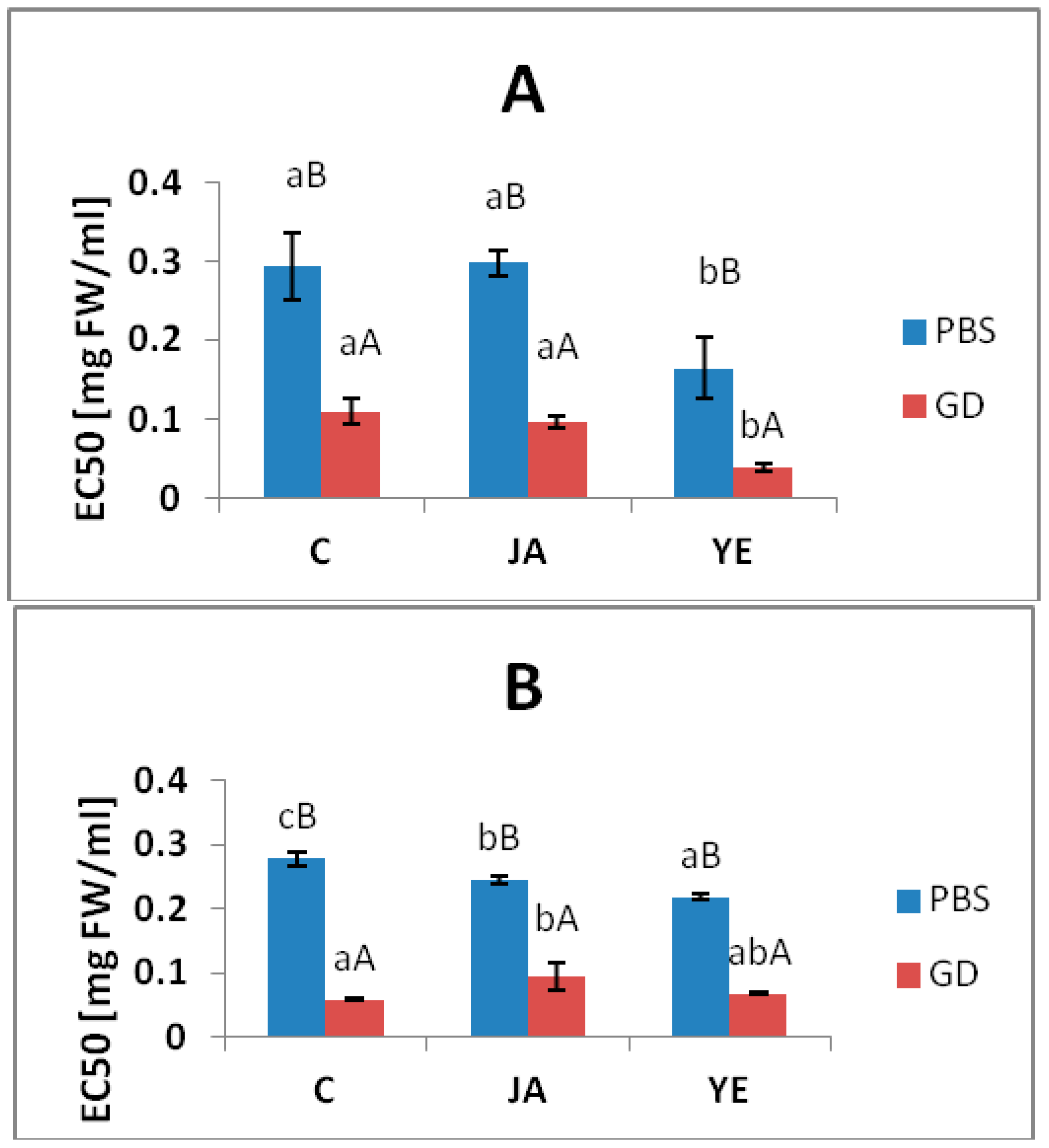
3.4. Inhibitory Activity against Metabolic Syndrome Enzymes
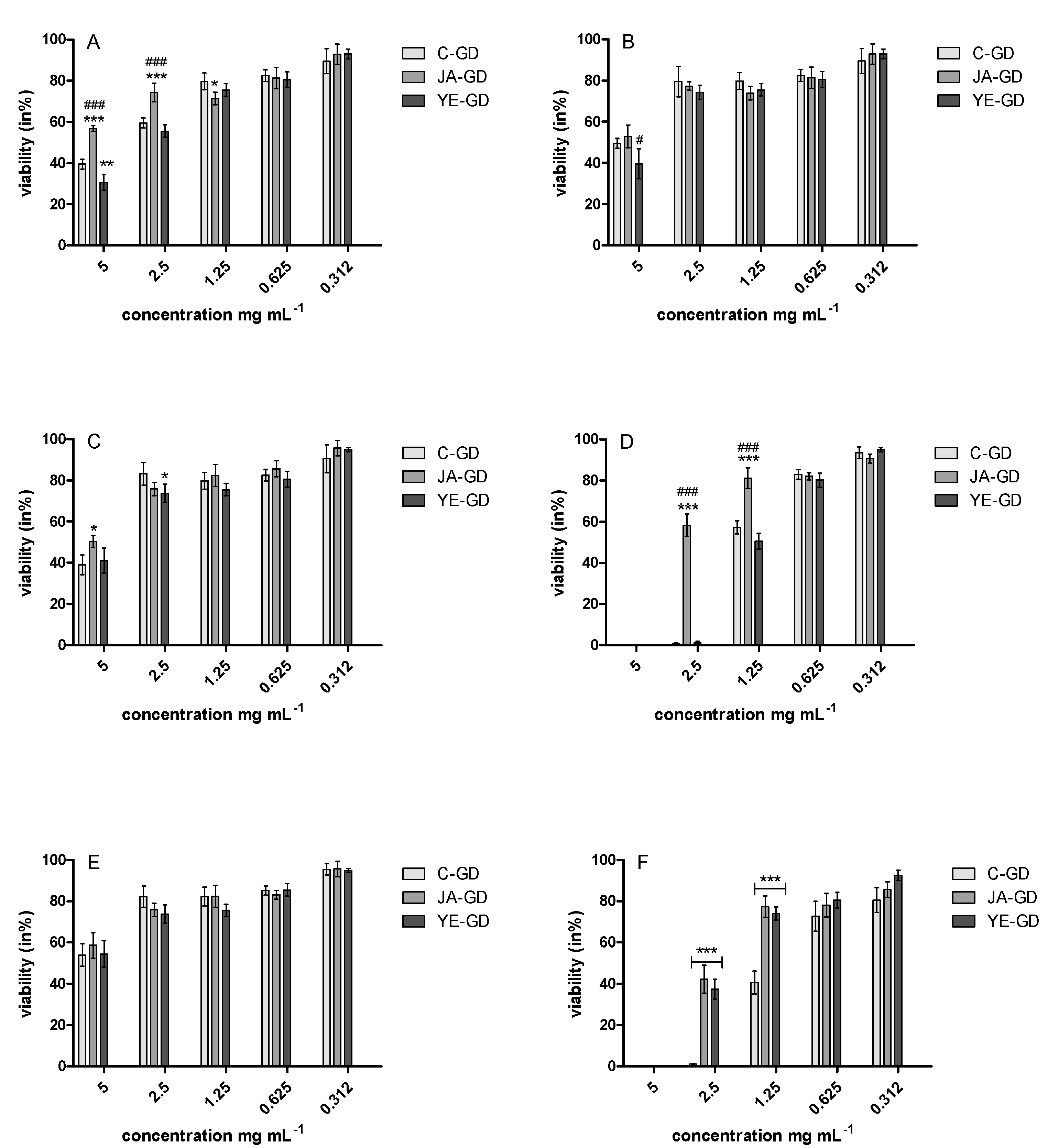
3.5. Antimicrobial Properties
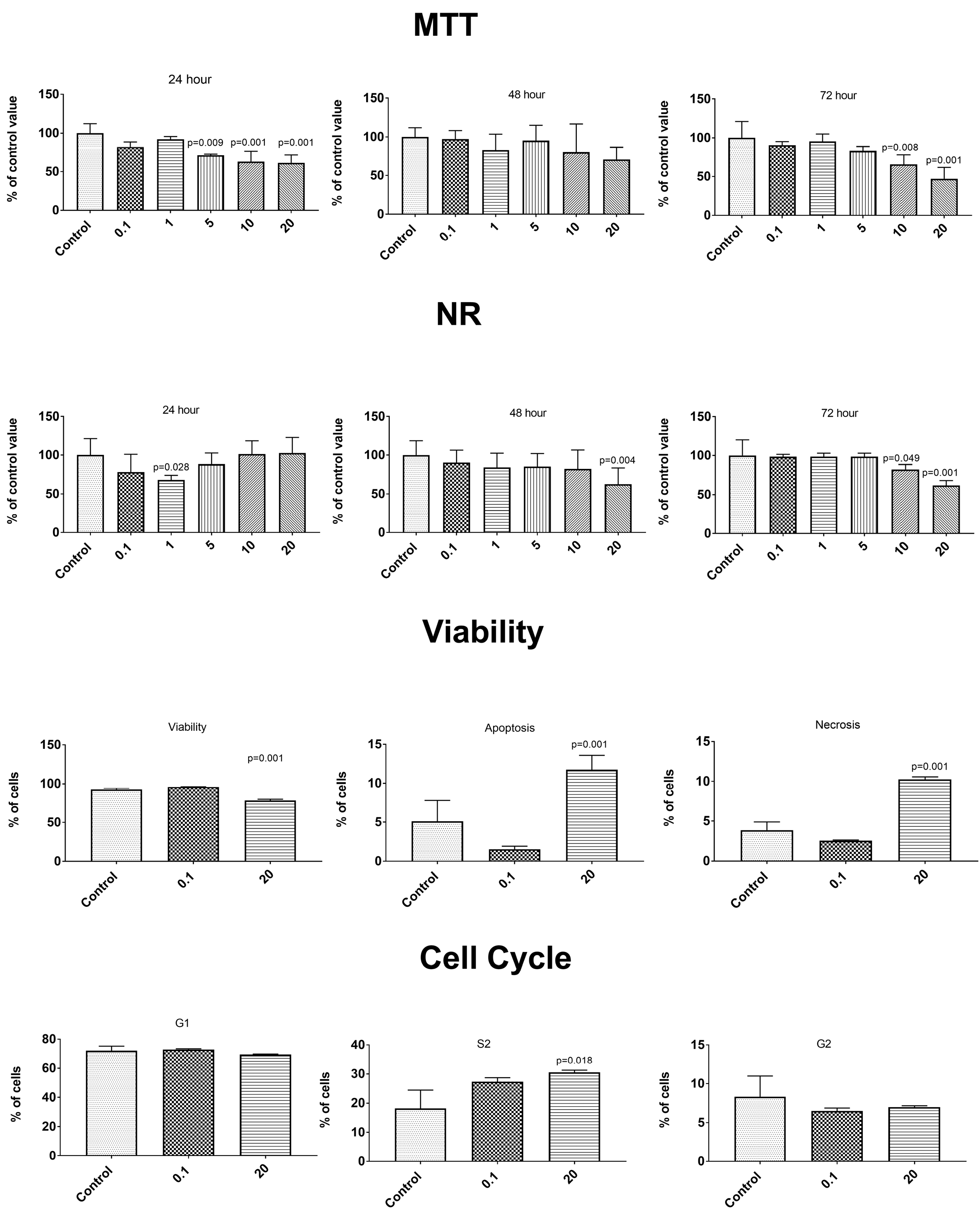
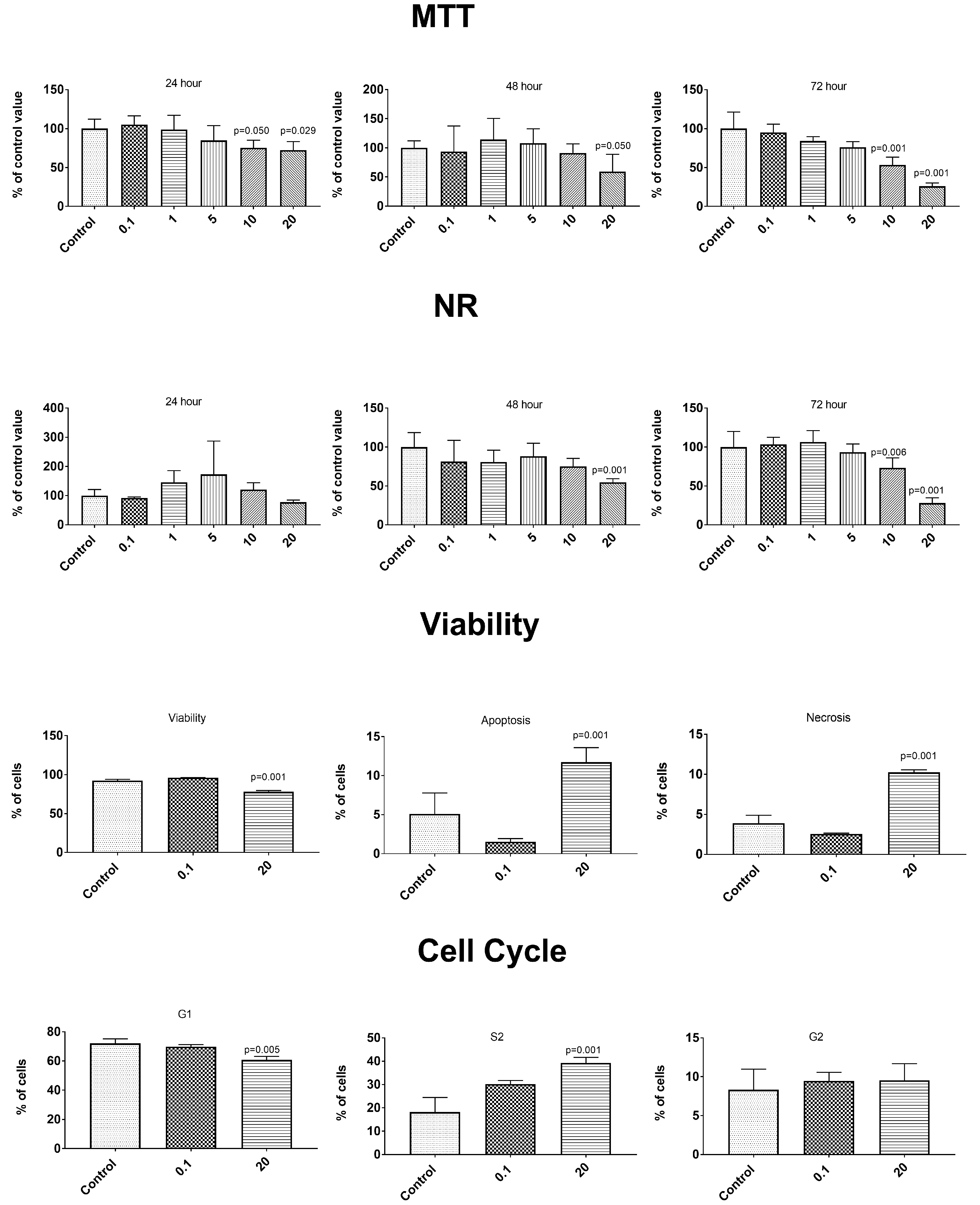
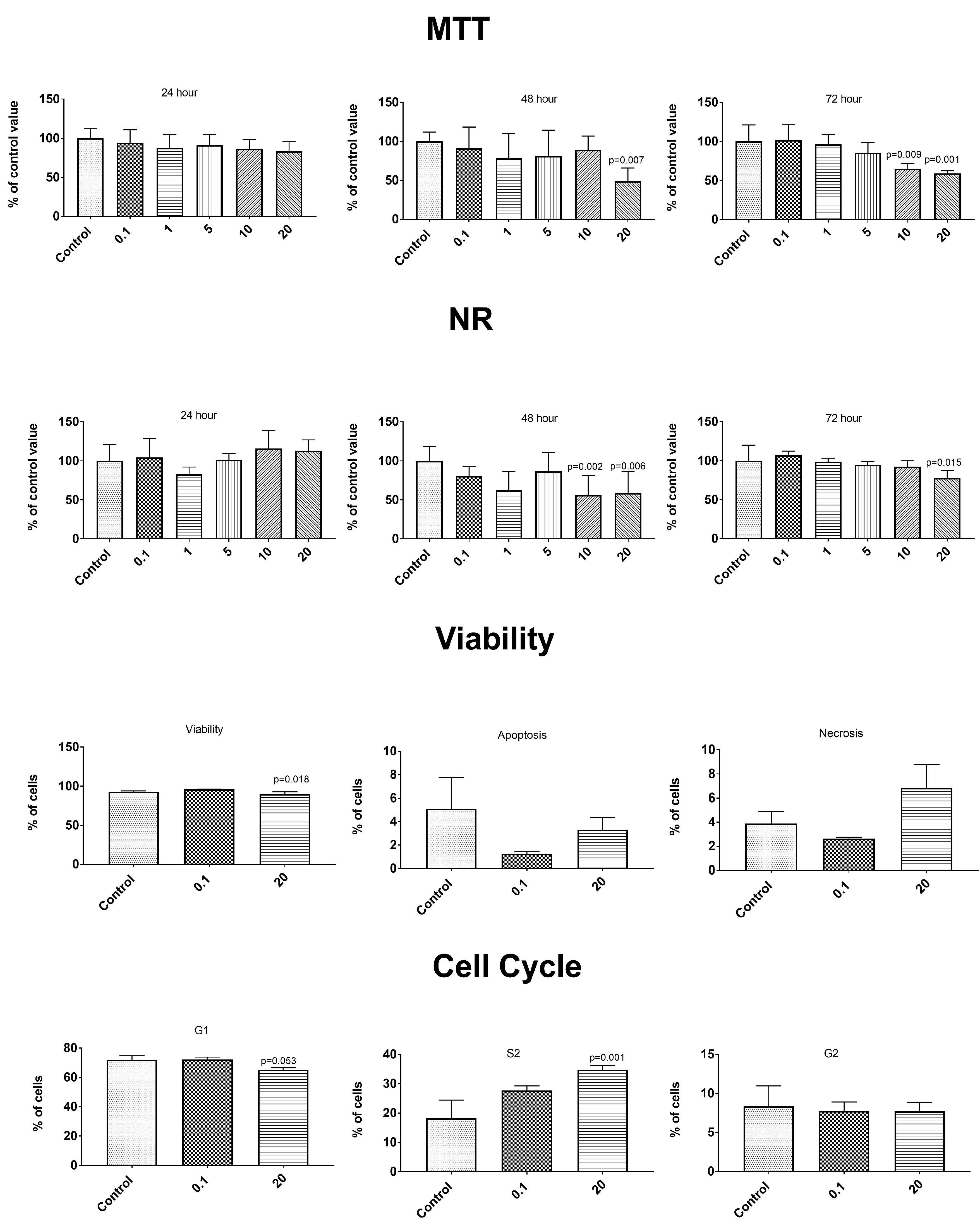
3.6. Anticancer Properties
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Nour, V.; Trandafir, I.; Cosmulescu, S. Bioactive compounds, antioxidant activity and nutritional quality of different culinary aromatic herbs. Not. Bot. Horti Agrobot. Cluj Napoca 2017, 45, 179–184. [Google Scholar] [CrossRef]
- Tajkarimi, M.M.; Ibrahim, S.A.; Cliver, D.O. Antimicrobial herb and spice compounds in food. Food Control 2010, 21, 1199–1218. [Google Scholar] [CrossRef]
- Spréa, R.M.; Fernandes, Â.; Calhelha, R.C.; Pereira, C.; Pires, T.C.S.P.; Alves, M.J.; Canan, C.; Barros, L.; Amaral, J.S.; Ferreira, I.C.F.R. Chemical and bioactive characterization of the aromatic plant: Levisticum officinale W.D.J. Koch: A comprehensive study. Food Funct. 2020, 11, 1292–1303. [Google Scholar] [CrossRef]
- Sertel, S.; Eichhorn, T.; Plinkert, P.K.; Efferth, T. Chemical composition and antiproliferative activity of essential oil from the leaves of a medicinal herb, Levisticum officinale, against UMSCC1 head and neck squamous carcinoma cells. Anticancer Res. 2011, 31, 185–191. [Google Scholar]
- Pandey, K.B.; Rizvi, S.I. Plant polyphenols as dietary antioxidants in human health and disease. Oxid. Med. Cell. Longev. 2009, 2, 270–278. [Google Scholar] [CrossRef]
- Lin, D.; Xiao, M.; Zhao, J.; Li, Z.; Xing, B.; Li, X.; Kong, M.; Li, L.; Zhang, Q.; Liu, Y.; et al. An overview of plant phenolic compounds and their importance in human nutrition and management of type 2 diabetes. Molecules 2016, 21, 1374. [Google Scholar] [CrossRef]
- Rathee, P.; Chaudhary, H.; Rathee, S.; Rathee, D.; Kumar, V.; Kohli, K. Mechanism of action of flavonoids as anti-inflammatory agents: A review. Inflamm. Allergy Drug Targets 2009, 8, 229–235. [Google Scholar] [CrossRef]
- García-Lafuente, A.; Guillamón, E.; Villares, A.; Rostagno, M.A.; Martínez, J.A. Flavonoids as anti-inflammatory agents: Implications in cancer and cardiovascular disease. Inflamm. Res. 2009, 58, 537–552. [Google Scholar] [CrossRef] [PubMed]
- Kakkar, S.; Bais, S. A review on protocatechuic acid and its pharmacological potential. ISRN Pharmacol. 2014, 2014, 952943. [Google Scholar] [CrossRef] [PubMed]
- Kumar, N.; Goel, N. Phenolic acids: Natural versatile molecules with promising therapeutic applications. Biotechnol. Rep. 2019, 24, e00370. [Google Scholar] [CrossRef] [PubMed]
- Ambriz-Perez, D.L.; Leyva-Lopez, N.; Gutierrez-Grijalva, E.P.; Heredia, J.B. Phenolic compounds: Natural alternative in inflammation treatment. A review. Cogent Food Agric. 2016, 2, 1131412. [Google Scholar]
- Azay-Milhau, J.; Ferrare, K.; Leroy, J.; Aubaterre, J.; Tournier, M.; Lajoix, A.-D.; Tousch, D. Antihyperglycemic effect of a natural chicoric acid extract of chicory (Cichorium intybus L.): A comparative in vitro study with the effects of caffeic and ferulic acids. J. Ethnopharmacol. 2013, 150, 755–760. [Google Scholar] [CrossRef] [PubMed]
- Sakulnarmrat, K.; Konczak, I. Composition of native Australian herbs polyphenolic-rich fractions and in vitro inhibitory activities against key enzymes relevant to metabolic syndrome. Food Chem. 2012, 134, 1011–1019. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Davis, L.C.; Verpoorte, R. Elicitor signal transduction leading to production of plant secondary metabolites. Biotechnol. Adv. 2005, 23, 283–333. [Google Scholar] [CrossRef] [PubMed]
- Baenas, N.; García-viguera, C.; Moreno, D.A. Elicitation: A Tool for enriching the bioactive composition of foods. Molecules 2014, 19, 13541–13563. [Google Scholar] [CrossRef]
- Złotek, U.; Wójcik, W. Effect of arachidonic acid elicitation on lettuce resistance towards Botrytis cinerea. Sci. Hortic. (Amst.) 2014, 179, 16–20. [Google Scholar] [CrossRef]
- Świeca, M. Potentially bioaccessible phenolics, antioxidant activity and nutritional quality of young buckwheat sprouts affected by elicitation and elicitation supported by phenylpropanoid pathway precursor feeding. Food Chem. 2016, 192, 625–632. [Google Scholar] [CrossRef]
- Złotek, U.; Szychowski, K.A.; Świeca, M. Potential in vitro antioxidant, anti-inflammatory, antidiabetic, and anticancer effect of arachidonic acid-elicited basil leaves. J. Funct. Foods 2017, 36, 290–299. [Google Scholar] [CrossRef]
- Złotek, U.; Szymanowska, U.; Karaś, M.; Świeca, M. Antioxidative and anti-inflammatory potential of phenolics from purple basil (Ocimum basilicum L.) leaves induced by jasmonic, arachidonic and β-aminobutyric acid elicitation. Int. J. Food Sci. Technol. 2016, 51, 163–170. [Google Scholar]
- Złotek, U.; Świeca, M. Elicitation effect of Saccharomyces cerevisiae yeast extract on main health-promoting compounds and antioxidant and anti-inflammatory potential of butter lettuce (Lactuca sativa L.). J. Sci. Food Agric. 2016, 96, 2565–2572. [Google Scholar]
- Złotek, U.; Szymanowska, U.; Pecio, Ł.; Kozachok, S.; Jakubczyk, A. Antioxidative and potentially anti-inflammatory activity of phenolics from lovage leaves Levisticum officinale koch elicited with jasmonic acid and yeast extract. Molecules 2019, 24, 1441. [Google Scholar] [CrossRef] [PubMed]
- Gawlik-Dziki, U.; Dziki, D.; Baraniak, B.; Lin, R. The effect of simulated digestion in vitro on bioactivity of wheat bread with Tartary buckwheat flavones addition. LWT Food Sci. Technol. 2009, 42, 137–143. [Google Scholar] [CrossRef]
- Świeca, M.; Baraniak, B. Nutritional and antioxidant potential of lentil sprouts affected by elicitation with temperature stress. J. Agric. Food Chem. 2014, 62, 3306–3313. [Google Scholar] [CrossRef] [PubMed]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Oyaizu, M. Studies on products of browning reaction antioxidative activities of products of browning reaction prepared from glucosamine.itle. Jpn. J. Nutr. Diet. 1986, 44, 307–315. [Google Scholar] [CrossRef]
- Guo, J.T.; Lee, H.L.; Chiang, S.H.; Lin, H.I.; Chang, C.Y. Antioxidant properties of the extracts from different parts of broccoli in Taiwan. J. Food Drug Anal. 2001, 9, 96–101. [Google Scholar]
- Szymanowska, U.; Jakubczyk, A.; Baraniak, B.; Kur, A. Characterisation of lipoxygenase from pea seeds (Pisum sativum var.Telephone L.). Food Chem. 2009, 116, 906–910. [Google Scholar] [CrossRef]
- Karaś, M.; Jakubczyk, A.; Szymanowska, U.; Jęderka, K.; Lewicki, S.; Złotek, U. Different temperature treatments of millet grains affect the biological activity of protein hydrolyzates and peptide fractions. Nutrients 2019, 11, 550. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, J.; Xu, J.H.; Fan, L.Q.; Li, S.X.; Zhao, L.L.; Mao, X.B. Significantly improved expression and biochemical properties of recombinant serratia marcescens lipase as robust biocatalyst for kinetic resolution of chiral ester. Appl. Biochem. Biotechnol. 2010, 162, 2387–2399. [Google Scholar] [CrossRef]
- Złotek, U.; Jakubczyk, A.; Rybczyńska-Tkaczyk, K.; Ćwiek, P.; Baraniak, B.; Lewicki, S. Characteristics of new peptides GQLGEHGGAGMG, GEHGGAGMGGGQFQPV, EQGFLPGPEESGR, RLARAGLAQ, YGNPVGGVGH, and GNPVGGVGHGTTGT as inhibitors of enzymes involved in metabolic syndrome and antimicrobial potential. Molecules 2020, 25, 2492. [Google Scholar] [CrossRef]
- Świeca, M.; Baraniak, B.; Gawlik-Dziki, U. In vitro digestibility and starch content, predicted glycemic index and potential in vitro antidiabetic effect of lentil sprouts obtained by different germination techniques. Food Chem. 2013, 138, 1414–1420. [Google Scholar] [CrossRef] [PubMed]
- Jakubczyk, A.; Świeca, M.; Gawlik-Dziki, U.; Dziki, D. Nutritional potential and inhibitory activity of bread fortified with green coffee beans against enzymes involved in metabolic syndrome pathogenesis. LWT 2018, 95, 78–84. [Google Scholar] [CrossRef]
- Osaka, I.; Hefty, P.S. Simple resazurin-based microplate assay for measuring chlamydia infections. Antimicrob. Agents Chemother. 2013, 57, 2838–2840. [Google Scholar] [CrossRef] [PubMed]
- Rokicki, D.; Zdanowski, R.; Lewicki, S.; Leśniak, M.; Suska, M.; Wojdat, E.; Skopińska-Rózewska, E.; Skopiński, P. Inhibition of proliferation, migration and invasiveness of endothelial murine cells culture induced by resveratrol. Cent. Eur. J. Immunol. 2014, 39, 449–454. [Google Scholar] [CrossRef]
- Leśniak, M.; Zdanowski, R.; Suska, M.; Brewczyńska, A.; Stankiewicz, W.; Kloc, M.; Kubiak, J.Z.; Lewicki, S. Effects of hexachlorophene, a chemical accumulating in adipose tissue, on mouse and human mesenchymal stem cells. Tissue Eng. Regen. Med. 2018, 15, 211–222. [Google Scholar] [CrossRef]
- Huang, W.Y.; Cai, Y.Z.; Zhang, Y. Natural phenolic compounds from medicinal herbs and dietary plants: Potential use for cancer prevention. Nutr. Cancer 2010, 62, 1–20. [Google Scholar] [CrossRef]
- Kim, H.-J.; Fonseca, J.M.; Choi, J.-H.; Kubota, C. Effect of methyl jasmonate on phenolic compounds and carotenoids of romaine lettuce (Lactuca sativa L.). J. Agric. Food Chem. 2007, 55, 10366–10372. [Google Scholar] [CrossRef]
- Złotek, U.; Michalak-Majewska, M.; Szymanowska, U. Effect of jasmonic acid elicitation on the yield, chemical composition, and antioxidant and anti-inflammatory properties of essential oil of lettuce leaf basil (Ocimum basilicum L.). Food Chem. 2016, 213, 1–7. [Google Scholar]
- Świeca, M.; Jakubczyk, A. Effect of abiotic elicitation on main health-promoting compounds, antioxidant activity and commercial quality of butter lettuce (Lactuca sativa L.). Food Chem. 2014, 148, 253–260. [Google Scholar]
- Karaś, M.; Jakubczyk, A.; Szymanowska, U.; Złotek, U.; Zielińska, E. Digestion and bioavailability of bioactive phytochemicals. Int. J. Food Sci. Technol. 2017, 52, 291–305. [Google Scholar] [CrossRef]
- Jakobek, L. Interactions of polyphenols with carbohydrates, lipids and proteins. Food Chem. 2015, 175, 556–567. [Google Scholar] [CrossRef]
- Ng, Z.X.; See, A.N. Effect of in vitro digestion on the total polyphenol and flavonoid, antioxidant activity and carbohydrate hydrolyzing enzymes inhibitory potential of selected functional plant-based foods. J. Food Process. Preserv. 2019, 43, e13903. [Google Scholar] [CrossRef]
- Jayawardena, N.; Watawana, M.I.; Waisundara, V.Y. Evaluation of the total antioxidant capacity, polyphenol contents and starch hydrolase inhibitory activity of ten edible plants in an in vitro model of digestion. Plant Foods Hum. Nutr. 2015, 70, 71–76. [Google Scholar] [CrossRef] [PubMed]
- Wootton-Beard, P.C.; Moran, A.; Ryan, L. Stability of the total antioxidant capacity and total polyphenol content of 23 commercially available vegetable juices before and after in vitro digestion measured by FRAP, DPPH, ABTS and Folin-Ciocalteu methods. Food Res. Int. 2011, 44, 217–224. [Google Scholar] [CrossRef]
- Wong, Y.H.; Tan, C.P.; Long, K.; Nyam, K.L. In vitro simulated digestion on the biostability of Hibiscus cannabinus L. seed extract. Czech J. Food Sci. 2014, 32, 177–181. [Google Scholar] [CrossRef]
- Velika, B.; Kron, I. Antioxidant properties of benzoic acid derivatives against superoxide radical. Free Radic. Antioxid. 2012, 2, 62–67. [Google Scholar] [CrossRef]
- Manuja, R.; Sachdeva, S.; Jain, A.; Chaudhary, J. A comprehensive review on biological activities of P-hydroxy benzoic acid and its derivatives. Int. J. Pharm. Sci. Rev. Res. 2013, 22, 109–115. [Google Scholar]
- Su, X.; Zhang, J.; Wang, H.; Xu, J.; He, J.; Liu, L.; Zhang, T.; Chen, R.; Kang, J. Phenolic acid profiling, antioxidant, and anti-inflammatory activities, and miRNA regulation in the polyphenols of 16 blueberry samples from China. Molecules 2017, 22, 312. [Google Scholar] [CrossRef]
- Taofiq, O.; Calhelha, R.C.; Heleno, S.; Barros, L.; Martins, A.; Santos-Buelga, C.; Queiroz, M.J.R.P.; Ferreira, I.C.F.R. The contribution of phenolic acids to the anti-inflammatory activity of mushrooms: Screening in phenolic extracts, individual parent molecules and synthesized glucuronated and methylated derivatives. Food Res. Int. 2015, 76, 821–827. [Google Scholar] [CrossRef]
- Roschek, B.; Fink, R.C.; Li, D.; McMichael, M.; Tower, C.M.; Smith, R.D.; Alberte, R.S. Pro-inflammatory enzymes, cyclooxygenase 1, cyclooxygenase 2, and 5-lipooxygenase, inhibited by stabilized rice bran extracts. J. Med. Food 2009, 12, 615–623. [Google Scholar] [CrossRef]
- Mahdi, J.G.; Al-Musayeib, N.; Mahdi, E.; Pepper, C. Pharmacological importance of simple phenolic compounds on inflammation, cell proliferation and apoptosis with a special reference to β-D-salicin and hydroxybenzoic acid. Eur. J. Inflamm. 2013, 11, 327–336. [Google Scholar] [CrossRef]
- Rosa, L.S.; Silva NJ, A.; Soares NC, P.; Monteiro, M.C.; Teodoro, A.J. Anticancer properties of phenolic acids in colon cancer—A review. J. Nutr. Food Sci. 2016, 6, 1–7. [Google Scholar]
- Golkar, P.; Taghizadeh, M.; Jalali, S.A.H. Determination of phenolic compounds, antioxidant and anticancer activity of Chrozophora tinctoria accessions collected from different regions of Iran. J. Food Biochem. 2019, 43, e13036. [Google Scholar] [CrossRef] [PubMed]
- Bacanlı, M.; Başaran, A.A.; Başaran, N. Cytotoxicity evaluation of some phenolic compounds in V79 Cells. Turk. J. Pharm. Sci. 2015, 12, 53–61. [Google Scholar] [CrossRef]
- Jaganathan, S.K.; Supriyanto, E. Antiproliferative and molecular mechanism of eugenol-induced apoptosis in cancer cells. Molecules 2012, 17, 6290–6304. [Google Scholar] [CrossRef]
- Soobrattee, M.A.; Neergheen, V.S.; Luximon-Ramma, A.; Aruoma, O.I.; Bahorun, T. Phenolics as potential antioxidant therapeutic agents: Mechanism and actions. Mutat. Res. Fundam. Mol. Mech. Mutagen. 2005, 579, 200–213. [Google Scholar] [CrossRef]
- Eguchi, S.; Kawai, T.; Scalia, R.; Rizzo, V. Understanding Angiotensin II type 1 receptor signaling in vascular pathophysiology. Hypertension 2018, 71, 804–810. [Google Scholar] [CrossRef]
- Akif, M.; Schwager, S.L.; Anthony, C.S.; Czarny, B.; Beau, F.; Dive, V.; Sturrock, E.D.; Acharya, K.R. Novel mechanism of inhibition of human angiotensin-l-converting enzyme (ACE) by a highly specific phosphinic tripeptide. Biochem. J. 2011, 436, 53–59. [Google Scholar] [CrossRef]
- Błaszczak, W.; Jeż, M.; Szwengiel, A. Polyphenols and inhibitory effects of crude and purified extracts from tomato varieties on the formation of advanced glycation end products and the activity of angiotensin-converting and acetylcholinesterase enzymes. Food Chem. 2020, 314, 126–181. [Google Scholar] [CrossRef]
- Wang, N.; Manabe, Y.; Sugawara, T.; Paul, N.A.; Zhao, J. Identification and biological activities of carotenoids from the freshwater alga Oedogonium intermedium. Food Chem. 2018, 242, 247–255. [Google Scholar] [CrossRef]
- Al Shukor, N.; Van Camp, J.; Gonzales, G.B.; Staljanssens, D.; Struijs, K.; Zotti, M.J.; Raes, K.; Smagghe, G. Angiotensin-converting enzyme inhibitory effects by plant phenolic compounds: A study of structure activity relationships. J. Agric. Food Chem. 2013, 61, 11832–11839. [Google Scholar] [CrossRef]
- Juurlink, B.H.J.; Azouz, H.J.; Aldalati, A.M.Z.; Altinawi, B.M.H.; Ganguly, P. Hydroxybenzoic acid isomers and the cardiovascular system. Nutr. J. 2014, 13, 63. [Google Scholar] [CrossRef]
- Kumar, S.; Prahalathan, P.; Raja, B. Syringic acid ameliorates L-NAME-induced hypertension by reducing oxidative stress. Naunyn Schmiedebergs Arch. Pharmacol. 2012, 385, 1175–1184. [Google Scholar] [CrossRef]
- Wu, X.; Feng, Y.; Lu, Y.; Li, Y.; Fan, L.; Liu, L.; Wu, K.; Wang, X.; Zhang, B.; He, Z. Effect of phenolic hydroxyl groups on inhibitory activities of phenylpropanoid glycosides against lipase. J. Funct. Foods 2017, 38, 510–518. [Google Scholar] [CrossRef]
- Birari, R.B.; Bhutani, K.K. Pancreatic lipase inhibitors from natural sources: Unexplored potential. Drug Discov. Today 2007, 12, 879–889. [Google Scholar] [CrossRef]
- Ghosh, S.; More, P.; Derle, A.; Patil, A.B.; Markad, P.; Asok, A.; Kumbhar, N.; Shaikh, M.L.; Ramanamurthy, B.; Shinde, V.S.; et al. Diosgenin from Dioscorea bulbifera: Novel hit for treatment of type II diabetes mellitus with inhibitory activity against α-amylase and α-glucosidase. PLoS ONE 2014, 9, e106039. [Google Scholar] [CrossRef]
- You, Q.; Chen, F.; Wang, X.; Jiang, Y.; Lin, S. Anti-diabetic activities of phenolic compounds in muscadine against alpha-glucosidase and pancreatic lipase. LWT Food Sci. Technol. 2012, 46, 164–168. [Google Scholar] [CrossRef]
- Saltan, F.Z.; Okutucu, B.; Canbay, H.S.; Ozel, D. In vitro α-glucosidase and α-amylase enzyme inhibitory effects in Elaeagnus angustifolia leaves extracts. Eurasian J. Anal. Chem. 2017, 12, 117–126. [Google Scholar] [CrossRef]
- Cen, Y.; Xiao, A.; Chen, X.; Liu, L. Isolation of α-amylase inhibitors from kadsura longipedunculata using a high-speed counter-current chromatography target guided by centrifugal ultrafiltration with LC-MS. Molecules 2016, 21, 1190. [Google Scholar] [CrossRef]
- Bintsis, T. Foodborne pathogens. AIMS Microbiol. 2017, 3, 529–563. [Google Scholar] [CrossRef]
- Tong, Y.; Tang, J. Candida albicans infection and intestinal immunity. Microbiol. Res. 2017, 198, 27–35. [Google Scholar] [CrossRef] [PubMed]
- Jahani, S.; Bazi, S.; Shahi, Z.; Sheykhzade Asadi, M.; Mosavi, F.; Sohil Baigi, G. Antifungal effect of the extract of the plants against Candida albicans. Int. J. Infect. 2016, 4. [Google Scholar] [CrossRef]
- Satana, D.; Genc, G.E.; Erturan, Z. The antifungal susceptibilities of oral Candida spp isolates from HIV-infected patients. Afr. J. Microbiol. Res. 2010, 4, 466–470. [Google Scholar]
- Mostafa, A.A.; Al-Askar, A.A.; Almaary, K.S.; Dawoud, T.M.; Sholkamy, E.N.; Bakri, M.M. Antimicrobial activity of some plant extracts against bacterial strains causing food poisoning diseases. Saudi J. Biol. Sci. 2018, 25, 361–366. [Google Scholar] [CrossRef]
- Daglia, M. Polyphenols as antimicrobial agents. Curr. Opin. Biotechnol. 2012, 23, 174–181. [Google Scholar] [CrossRef]
- Alves, M.J.; Ferreira, I.C.F.R.; Froufe, H.J.C.; Abreu, R.M.V.; Martins, A.; Pintado, M. Antimicrobial activity of phenolic compounds identified in wild mushrooms, SAR analysis and docking studies. J. Appl. Microbiol. 2013, 115, 346–357. [Google Scholar] [CrossRef]
- Teodoro, G.R.; Ellepola, K.; Seneviratne, C.J.; Koga-Ito, C.Y. Potential use of phenolic acids as anti-Candida agents: A review. Front. Microbiol. 2015, 6, 1420. [Google Scholar] [CrossRef]
- Mekinic, I.G.; Skroza, D.; Ljubenkov, I.; Krstulovic, L.; Mozina, S.S.; Katalinic, V. Phenolic acids profile, antioxidant and antibacterial activity of chamomile, common yarrow and immortelle (asteraceae). Nat. Prod. Commun. 2014, 9, 1745–1748. [Google Scholar] [CrossRef]
| Compounds (µg/g FW) | Sample | |||||
|---|---|---|---|---|---|---|
| C-PBS | C-GD | JA-PBS | JA-GD | YE-PBS | YE-GD | |
| Protocatechuic acid | 6.63 ± 0.92a | 4.85 ± 0.00a | 10.52 ± 0.08b | 5.23 ± 0.37a | nd | 4.33 ± 0.02a |
| Hydroxybenzoic acid | 3.56 ± 0.04a | 21.88 ± 0.00b | 3.74 ± 0.01a | 35.21 ± 4.04c | 3.06 ± 0.00a | 35.75 ± 1.65c |
| Siringic acid | 4.23 ± 0.28a | 4.15 ± 1.19a | 10.53 ± 0.04b | 4.47±0.88a | nd | 3.33 ± 0.03a |
| Vanillic acid | 5.54 ± 0.37a | nd | 10.10 ± 0.01b | nd | 5.58 ± 1.83a | nd |
| Sinapic acid | 18.78 ± 0.62b | 9.28 ± 1.82a | 40.48 ± 0.03d | 8.09 ± 1.51a | 33.80 ± 0.13c | 9.74 ± 0.25a |
| Salicylic acid | 9.59 ± 1.72a | nd | 52.57 ± 0.07b | 11.32 ± 6.01a | nd | nd |
| Caffeic acid | 5.80 ± 0.56a | nd | 18.16 ± 0.02b | nd | nd | nd |
| Antioxidant Activity | Sample | |||||
|---|---|---|---|---|---|---|
| C-PBS | C-GD | JA-PBS | JA-GD | YE-PBS | YE-GD | |
| ABTS (µM Trolox/g FW) | 3.20 ± 0.66a | 10.40 ± 1.34d | 4.60 ± 0.78ab | 7.27 ± 1.77c | 3.05 ± 0.43a | 6.49 ± 1.28bc |
| RP (mg Trolox/g FW) | 10.02 ± 2.01ab | 8.86 ± 1.07ab | 8.16 ± 1.23a | 12.87 ± 2.44b | 7.76 ± 1.13a | 11.21 ± 2.90ab |
| CHP (mg EDTA/g FW) | 711.71 ± 13.91b | 572.44 ± 73.86a | 725.62 ± 4.64b | 575.61 ± 8.95a | 687.37 ± 48.98b | 572.44 ± 15.92a |
| EC50 (mg FW/mL) | Sample | |||||
|---|---|---|---|---|---|---|
| C-PBS | C-GD | JA-PBS | JA-GD | YE-PBS | YE-GD | |
| ACE | 199.93 ± 5.65d | 8.19 ± 0.78a | 102.49 ± 4.78c | 33.88 ± 1.10 b | 97.68 ± 8.83c | 24.72 ± 1.72 b |
| lipase | 2.14 ± 0.15e | 0.27 ± 0.02a | 1.15 ± 0.05c | 0.78 ± 0.06b | 1.85 ± 0.13c | 0.79 ± 0.08 b |
| α-amylase | na | 20.82 ± 1.07b | na | 33.88 ± 1.49c | na | 17.63 ± 0.81a |
| α-glucosidase | 13.11 ± 1.85b | 2.64 ± 0.16a | 24.18 ± 1.54d | 3.10 ± 0.73a | 19.67 ± 1.02c | 2.52 ± 0.29a |
| Antimicrobial Activity | Sample | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| C-PBS | C-GD | JA-PBS | JA-GD | YE-PBS | YE-GD | Penicilin G *** | Streptomycin *** | Cyclohexamide *** | |
| E. coli ATCC 25922 | Nd */nd ** | 2.5 */>5.0 ** | Nd */nd ** | 5.0 */>5.0 ** | Nd */nd ** | 2.5 */>5.0 ** | 7.81/7.81 | 15.62/15.62 | ns |
| S. aureus ATCC 29737 | Nd */nd ** | 5.0 */>5.0 ** | Nd */nd ** | 5.0 */>5.0 ** | Nd */nd *** | 5.0 */>5.0 ** | >250/>250 | 7.81/7.81 | ns |
| S. enterica ATCC 4931 | Nd */nd ** | 5.0 */>5.0 ** | Nd */nd ** | 5.0 */>5.0 ** | Nd */nd ** | 5.0 */>5.0 ** | 7.81/7.81 | 3.90/3.90 | ns |
| B. cereus ATCC 14579 | Nd */nd ** | 1.25 */>2.5 ** | Nd */nd ** | 2.5 */5.0 ** | Nd */nd ** | 1.25 */2.5 ** | >250/>250 | 15.62/15.62 | ns |
| L. monocytogenes ATCC BAA-2660 | Nd */nd ** | 5.0 */>5.0 ** | Nd */nd ** | 5.0 */>5.0 ** | Nd */nd ** | 5.0 */>5.0 ** | 1.95/1.95 | 3.90/3.90 | ns |
| C. albicans ATCC 90028 | Nd */nd ** | 1.25 */2.5 ** | Nd */nd ** | 2.5 */5.0 *** | Nd */nd ** | 2.5 */5.0 ** | ns | ns | 15.62/15.62 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jakubczyk, A.; Złotek, U.; Szymanowska, U.; Rybczyńska-Tkaczyk, K.; Jęderka, K.; Lewicki, S. In vitro Antioxidant, Anti-inflammatory, Anti-metabolic Syndrome, Antimicrobial, and Anticancer Effect of Phenolic Acids Isolated from Fresh Lovage Leaves [Levisticum officinale Koch] Elicited with Jasmonic Acid and Yeast Extract. Antioxidants 2020, 9, 554. https://doi.org/10.3390/antiox9060554
Jakubczyk A, Złotek U, Szymanowska U, Rybczyńska-Tkaczyk K, Jęderka K, Lewicki S. In vitro Antioxidant, Anti-inflammatory, Anti-metabolic Syndrome, Antimicrobial, and Anticancer Effect of Phenolic Acids Isolated from Fresh Lovage Leaves [Levisticum officinale Koch] Elicited with Jasmonic Acid and Yeast Extract. Antioxidants. 2020; 9(6):554. https://doi.org/10.3390/antiox9060554
Chicago/Turabian StyleJakubczyk, Anna, Urszula Złotek, Urszula Szymanowska, Kamila Rybczyńska-Tkaczyk, Krystyna Jęderka, and Sławomir Lewicki. 2020. "In vitro Antioxidant, Anti-inflammatory, Anti-metabolic Syndrome, Antimicrobial, and Anticancer Effect of Phenolic Acids Isolated from Fresh Lovage Leaves [Levisticum officinale Koch] Elicited with Jasmonic Acid and Yeast Extract" Antioxidants 9, no. 6: 554. https://doi.org/10.3390/antiox9060554
APA StyleJakubczyk, A., Złotek, U., Szymanowska, U., Rybczyńska-Tkaczyk, K., Jęderka, K., & Lewicki, S. (2020). In vitro Antioxidant, Anti-inflammatory, Anti-metabolic Syndrome, Antimicrobial, and Anticancer Effect of Phenolic Acids Isolated from Fresh Lovage Leaves [Levisticum officinale Koch] Elicited with Jasmonic Acid and Yeast Extract. Antioxidants, 9(6), 554. https://doi.org/10.3390/antiox9060554







