Abstract
Schisandra rubriflora is a dioecious, underestimated medicinal plant species known from traditional Chinese medicine. The present study was aimed at characterising the polyphenolic profile composition and the related antioxidant capacity of S. rubriflora fruit, stem and leaf and in vitro microshoot culture extracts. Separate analyses of material from female and male specimens were carried out. This study was specifically aimed at detailed characterisation of the contribution of phenolic compounds to overall antioxidant activity using ultra-high-performance liquid chromatography with a photodiode array detector coupled to electrospray ionization ion trap mass spectrometry (UHPLC-DAD-ESI-MS3) and a high-performance liquid chromatography-diode array detector (HPLC-DAD). Using UHPLC-DAD-ESI-MS3, twenty-seven phenolic compounds from among phenolic acids and flavonoids were identified. Concentrations of three phenolic acids (neochlorogenic, chlorogenic and cryptochlorogenic acids) and eight flavonoids (hyperoside, rutoside, isoquercitrin, guaijaverin, trifolin, quercetin, kaempferol, and isorhamnetin) were determined using HPLC-DAD using reference standards. The highest total phenolic content was confirmed for the stem and leaf extracts collected in spring. The contents of phenolic compounds of in vitro biomasses were comparable to that in the fruit extracts. The methanolic extracts from the studied plant materials were evaluated for their antioxidant properties using various in vitro assays, namely free radicals scavenging estimation using 2,2-diphenyl-1-picryl-hydrazyl-hydrate (DPPH), ferric-reducing antioxidant power (FRAP) and cupric-reducing antioxidant capacity (CUPRAC) as well as QUick, Easy, New, CHEap, and Reproducible CUPRAC (QUENCHER-CUPRAC) assays. A close relationship between the content of polyphenolic compounds in S. rubriflora and their antioxidant potential has been documented.
1. Introduction
Currently, the attention of scientific research has been focused on plants as a source of phytochemicals with antioxidant potential. Different groups of plant secondary metabolites have been identified as responsible for this activity. These natural compounds comprise different structures and involve several protective mechanisms. Plant secondary metabolites most likely to exhibit health-promoting effects include polyphenols such as flavonoids, phenolic acids, catechins, tannins, and proanthocyanidins [1,2,3,4,5,6]. Several studies reported that these compounds show a preventive effect, associated with an excess of free radicals, e.g., against cancer, atherosclerosis, Alzheimer’s disease, Parkinson’s disease, and ischemic and cardiovascular diseases, etc. [7,8]. Furthermore, the latest studies of plant biotechnology proved that different in vitro systems of various plant species could be a rich, alternative source of polyphenolic compounds of strong antioxidant power, even higher than that of the intact plants [9,10,11].
Schisandra chinensis (Turcz.) Baill is a well-known plant species, used in the traditional Chinese medicine, whose importance is increasing nowadays in European and American countries as well [12,13,14,15,16]. Its fruits are a widely-consumed nutraceutical providing beneficial nutritional and bioactive properties [17,18]. S. chinensis fruits are known for their hepatoprotective, anticancer, antiaging, and stimulant effects as well as use as a sedative and tonic drug [12,17]. In the Schisandra genus, the most recognised active components are lignans (especially from dibenzocyclooctadiene group) including, among others, schisandrin, gomisins A, C and G, deoxyschisandrin, schisanhenol, and schisantherins A and B [12,19,20]. This plant has been frequently studied for its antioxidant potential [21,22,23,24]. The results of the scientific studies have shown that lignans are not the main components responsible for the antioxidant activity. The polyphenolic fraction of the studied extracts have been indicated as responsible for this activity [24,25,26,27,28,29]. This is a very interesting aspect, as there is little research on S. chinensis polyphenolic composition [23,24,30].
Schisandra rubriflora (Franch.) Rehd. et Wils is another species of the genus Schisandra known from East Asian phytotherapy. The species seems to be closely related to S. chinensis but less known, as it is the endemic species (typical for the Sichuan province of China) [31,32]. The planting of S. rubriflora outside the East Asian region is limited [14,32]. S. rubriflora is a dioecious vine, whose fruits are known for their use in traditional Chinese medicine as sedatives and toning agents. This species is also traditionally used in the treatment of hepatitis, chronic gastroenteritis and neurasthenia [32,33]. To date, the potential pharmacological applications of S. rubriflora fruit been described only by the Chinese research groups. AntiHIV-1 studies (inhibition of HIV-1 replication in H9 lymphocytes) were performed for fruit extracts [34,35]. Shoot extracts have been shown to be useful in the treatment of liver and bile duct disorders, through studies assessing their impact on the level of glutamin-pyruvate transaminase (GPT) in blood [32,36]. According to these studies, compounds from dibenzocyclooctadiene lignans as well as triterpenoids are responsible for these properties [34,37,38]. Recently, we have studied the lignan profiling of fruits, leaves and stems of female (F) and male (M) S. rubriflora plants [39]. In the same study, strong antiinflammatory properties in this species were also noted, based on inhibitory activity against 15-lipooxygenase (15-LOX), phospholipase A2 (sPLA2), cyclooxygenase 1 and 2 (COX-1; COX-2) enzyme assays have been indicated [39].
The aim of this study was to investigate the polyphenol profile and the antioxidant activity of S. rubriflora to fill this gap in the literature. The present study has presented the phytochemical qualitative and quantitative characteristics of the polyphenol content of S. rubriflora dividing the material into female (F) and male (M) specimens for the first time. For the estimations an ultra-high-performance liquid chromatography with a photodiode array detector coupled to electrospray ionization ion trap mass spectrometry (UHPLC-DAD-ESI-MS3) and a high-performance liquid chromatography-diode array detector (HPLC-DAD) were used. Fruits, stems and leaves of the soil-grown plants as well F and M lines of S. rubriflora in vitro microshoot cultures were studied. Moreover, total phenolic content was measured with Folin-Ciocalteu reagent. The antioxidant potential in the tested plant materials has been investigated for the first time using different methods, namely scavenging of free radicals using 2,2-Diphenyl-1-Picryl-Hydrazyl-Hydrate (DPPH), Ferric-Reducing Antioxidant Power (FRAP), Cupric-Reducing Antioxidant Capacity (CUPRAC) as well as QUick, Easy, New, CHEap, and Reproducible CUPRAC (QUENCHER-CUPRAC).
2. Materials and Methods
2.1. Reagents
Acetic acid, ethanol 96%, methanol, and sucrose were from Chempur (Piekary Śląskie, Poland). HPLC-grade methanol and acetonitrile were purchased in Merck (Darmstadt, Germany).
Plant culture media components, plant growth regulators BA (6-benzyladenine) and NAA (1-naphthaleneacetic acid) and agar were purchased in Duchefa Biochemie (Haarlem, Netherlands). Cultures were grown in the plant tissue-dedicated glass containers (V8630, Sigma-Aldrich, Saint Louis, MI, USA).
Commercially available standards: chlorogenic acid, cryptochlorogenic acid, and neochlorogenic acid, hyperoside (quercetin 3-galactoside), isoquercitrin (quercetin 3-glucoside), isorhamnetin, kaempferol, guaijaverin (quercetin 3-arabinoside), quercetin, rutoside (quercetin 3-rutinoside), and trifolin (kaempferol-3-galactoside) of HPLC grade (≥95.0%) purity were acquired in Sigma-Aldrich Saint Louis, MI, USA. Ammonium acetate, CuCl2⋅2H2O, DPPH, FeCl3⋅6H2O, Folin-Ciocalteu reagent, hydrochloric acid (HCl), Na2CO3, 2,9-dimethyl-1,10-phenanthroline (neocuprine), 2,4,6-tris(2-pyridyl)-s-triazine (TPTZ), and (±)-6-hydroxy-2,5,7,8-tetramethylchromane-2-carboxylic acid (trolox) were also provided by Sigma-Aldrich. Deionised water (>15 MΩ) was produced in house (PureLab OptionR, Elga, High Wycombe, UK).
2.2. Plant Material
Plant material for establishing in vitro cultures, as well as stems, leaves and fruits of the intact plants, were obtained from Clematis—the Source of Good Climbers Ltd. (Źródło Dobrych Pnączy Spółka z o.o., Pruszków, Poland) [40]. The plant species were identified by PhD Szczepan Marczyński (the head of the Clematis arboretum). Plant material was harvested in 2018 during three vegetative periods from 10-year-old female (F) and male (M) individuals of Schisandra rubriflora (Franch.) Rehd. et Wils specimens. Leaves and stems (stems were collected with leaves as they grow) were harvested in spring (May), summer (July) and autumn (September) from F and M specimens (approximately 50 individuals each). Fruits were collected in September 2018 from F specimens.
Leaf buds which were used for the initiation of in vitro cultures were collected in April 2018. Fruits were lyophilised and leaves and stems were air dried (at 25–30 °C). The dry plant material was pulverised in a mixing mill (MM 400, Retch, Germany).
2.3. Establishment of In Vitro Cultures
S. rubriflora leaf buds of F and M specimens were degreased with 70% ethanol (30 s) and then subjected to further sterilisation steps. HgCl2 (mercury chloride II) at a concentration of 0.1% was used for sterilisation for 7 min. Sterile buds were rinsed with sterile redistilled water and transferred to the agar medium according to Murashige and Skoog (1962) (MS) [41] and supplemented with the following plant growth regulators (PGRs): 1 mg/L 6-benzyladenine (BA) and 0.5 mg/L 1-naphthaleneacetic acid (NAA). Microshoots appeared after four weeks, and then (after eight weeks) stable in vitro cultures were obtained. Cultures were subcultured every 30 days.
2.4. Experimental Microshoot Cultures
Experimental agar microshoot cultures were maintained on MS medium [41] with 0.72% (w/v) agar and 3% (w/v) sucrose. In vitro cultures were cultured at 25 ± 2 °C under continuous artificial illumination of LED white light, with the photosynthetic photon flux density (PPFD) of 40 μmol m−2 s−1, and subcultured at 30 day intervals.
For the experiment, 0.5 g of inoculum (initial fresh weight of microshoots) per vessel was used. For experimental microshoot cultures under optimisation of PGR composition performed before [unpublished], the best medium for their cultivation contained 1 mg/L BA and 1 mg/L indole-3-butyric acid (IBA). The duration of the growth cycle time was 30 days; three series (n = 10) were performed.
2.5. UHPLC-DAD-ESI-MS3
Extracts from female (F) and male (M) individuals of S. rubriflora were prepared through sonication (15 min, room temperature) of 100 mg of the dry plant material with 2 mL of methanol:water (1:1, v/v). The samples were centrifuged and the supernatant was filtrated through a 0.45 μm polyvinylidene fluoride (PVDF) syringe filter before UHPLC analysis.
UHPLC-DAD-ESI-MS3 was performed using the Ultimate 3000 series system (Dionex, Idstein, Germany) coupled with an amaZon SL ion trap mass spectrometer (Bruker Daltonik GmbH, Bremen, Germany). The analysis of compounds was carried out using a Kinetex XB-C18 analytical column (150 mm × 2.1 mm × 1.9 µm), Phenomenex (Torrance, CA, USA). The column temperature was 25 °C. Elution was conducted using a mobile phase A (0.1% methanol in water) and a mobile phase B (0.1% methanol in acetonitrile) with a three-step gradient: 0 min 4% B, 60 min 26% B and 90 min 95% B. The flow rate was 0.300 mL/min during all the analyses. A volume of 3 µL of the prepared extract was injected to the column. UV-Vis spectra were recorded in the range of 200–450 nm. The chromatogram was read at 254 nm. The eluate was introduced into the mass spectrometer without splitting. The amaZon SL ion trap mass spectrometer was equipped with an ESI source. The parameters for the source were set as follows: nebuliser pressure 40 psi; dry gas flow 9 L/min; dry temperature 145 °C; and capillary voltage 4500 V. The analysis was carried in a scan range of 70–2200 m/z. Compounds were analysed in the negative and positive ion modes. MS2 and MS3 fragmentations were performed using the Smart Frag mode. Compounds were tentatively identified by determination of their molecular mass, UV-Vis spectra and fragmentation profiles in respect to the literature data and by comparison to available standards.
2.6. HPLC-DAD
After conducting UHPLC-DAD-ESI-MS3 for quantification of the detected compounds, HPLC-DAD was performed.
In order to prepare the methanolic extracts, 0.3 g samples of the dry powder in vitro biomass and in vivo-derived plant material were weighed (three samples in three replications). The material was subjected to extraction with HPLC-grade methanol (5 mL). The extraction was carried out in an ultrasonic bath (Sonic-2, POLSONIC, Warsaw, Poland; ultrasonic power 2 × 100 W, 40 kHz, 1.6 L volume) twice for 30 min at 25 ± 2 °C. The extracts were centrifuged (MPW-223E, MPW, Warsaw, Poland) at 4000 rpm for 5 min. The extracts were then filtered through the syringe filters (0.22 µm Millex®GP, Millipore, Merck, Darmstadt, Germany).
Quantitative analysis of phenolic compounds in the methanolic extracts was performed according to a validated method [42,43], using Merck-Hitachi liquid chromatograph (LaChrom Elite, Darmstadt, Germany) with a DAD L-2455 detector. The separation was conducted on a Purospher RP-18 (250 × 4 mm; 5 μm, Merck, Germany) column. The mobile phase consisted of A—methanol, 0.5% acetic acid 1:4; B—methanol (v/v). The flow rate was 1 mL/min at 25 °C. The gradient was as follows: 100% A for 0–20 min; 100–80% A for 20–35 min; 80–70% A and 20–30% B for 35–45 min; 70–60% A and 30–40% B for 45–55 min; 60–50% A and 40–50% B for 55–60 min; 50–25% A and 50–75% B for 60–65 min; 25–0% A and 75–100% B for 65–70 min; 0–0% A and 100–100% B for 70–75 min; 0–100% A and 100–0% B for 75–80 min; 100–100% A and 0–0% B for 80–90 min. The injection volume was 10 μL and the compounds were detected at 254 nm. Identification and quantification were performed by comparison to retention times of parameters and confirmed later by fragmentation spectra (UHPLC-DAD-ESI-MS3). Quantification was performed based on calibration curves. The results were expressed in mg/100 g dry weight (DW).
2.7. The Total Phenolic Assay
Total phenolic content was measured for microshoot cultures, fruits and plant material (stems and leaves) collected in spring, with the highest phenolic content determined by HPLC-DAD.
Approximately 3 mg of the weighted samples was extracted in 1 mL of analytical-grade methanol (5 min, 15 Hz, MM400 RetschHaan, Germany), and then centrifuged (3 min, 15 °C, 33,000× g, 32R, Hettich, Balingen, Germany). Total phenolic content was measured according to the method by Singelton et al. [44] with modifications reported by Bach et al. [45]. A volume of 100 μL of extract was mixed with 0.45 mL of Folin-Ciocalteu reagent in deionised water (5/2 v/v), and then 0.45 mL of saturated Na2CO3 was added. Samples were incubated in the dark (2 h, 25 °C), and then centrifuged and transferred to well plates. The absorbance was measured at 760 nm. The analyses were carried out in a 96-well plate using Synergy II (Biotek, Winooski, VT, USA) reader. The antioxidant response was expressed as mg trolox equivalents (TE)/100 g of dry weight (DW).
2.8. The Antioxidant Potential Estimation
The extract collected for the total phenolic assay was used to carry out the antioxidant assays. All measurements were performed in a 96-well plate using Synergy II (Biotek, Winooski, VT, USA) reader. The antioxidant response was expressed as TE in mg/100 g DW. All measurements were performed in five replicates.
2.8.1. The FRAP Assay
The ferric-reducing ability of the extracts was assessed using the FRAP assay [46]. A solution of TPTZ (10 mmol/L in 40 mmol/L HCl) was mixed with 20 mmol/L FeCl3⋅6H2O and 300 mmol/L acetate buffer at pH = 3.6 (1/1/10 v/v/v). A volume of 50 μL of the studied extract was added to 150 μL of this mixture. The samples were incubated for 5 min at 25 °C. The absorbance was read at 593 nm.
2.8.2. The DPPH Assay
Free radical-scavenging activity was estimated using DPPH [47]. Plant extract (50 μL) was mixed with 150 μL of the DPPH methanolic solution. After incubation on a horizontal shaker in the dark (1 h, 25 °C), the absorbance was read at 517 nm.
2.8.3. The CUPRAC Assay of the Total Antioxidant Capacity
The cupric-reducing antioxidant capacity (CUPRAC) assay [48] adapted by Biesaga-Kościelniak et al. [49] was used. A volume of 50 μL of 1 mol/L ammonia acetate buffer (pH = 7) and 50 μL of 7.5 mmol neocuprine in methanol were mixed with 50 μL of sample extract followed by 50 μL of 10 mmol Cu2+. After incubation on a horizontal shaker (15 min, 25 °C), the absorbance was read at 450 nm.
2.8.4. The QUENCHER-CUPRAC Assay of the Total Antioxidant Capacity
The QUENCHER-CUPRAC assay [50] was used for estimation of the aggregate antioxidant response of plant material. The 1 mL aliquots of 1 mol/L ammonia acetate buffer (pH = 7.0), 7.5 mmol/L neocuprine, and 10 mmol/L Cu2+ were added sequentially to 1 mg of accurately weighed pulverised samples. After a 2 h incubation (shaking on a rotator, 25 °C), the samples were centrifuged and transferred to 96-well plates. The absorbance was measured at λ = 425 nm.
2.9. The Statistical Analysis
The quantitative HPLC results were expressed in mg/100 g dry weight (DW). The total phenolic and antioxidant assays of trolox equivalents (TE) in mg/100 g DW. The results were expressed as the mean ± standard deviation (SD) of four or five samples (n = 4, n = 5, p < 0.05) for experiments that were repeated three times.
3. Results
3.1. The Characteristics of In Vitro Cultures
The morphological differences in the appearance of microshoot cultures of F and M culture lines grown on MS medium variant with 1 mg/L BA and 1 mg/L IBA were not measurable (Figure 1). It was possible to observe a significant number of dark green, strongly spread microshoots. The dry biomass increments were expressed by the growth index (Gi) (Gi = (Dw1 − Dw0)/Dw0, where Dw1—dry weight of microshoots at the end of the experiment; Dw0—dry weight of the inoculum) [51]. After a 30 day growth period, Gi was 2.91 ± 0.28 and 3.22 ± 0.31 for the F and M lines, respectively.

Figure 1.
The morphological appearance of Schisandra rubriflora microshoots from agar in vitro cultures (MS medium with 1 mg/L 6-benzyladenine (BA) and 0.5 mg/L 1-naphthaleneacetic acid (NAA), after a 30 day growth period): (A) female (F) line; (B) male (M) line.
3.2. UHPLC-DAD-ESI-MS3 Analysis of Phenolics
The qualitative profiles of phenolic compounds from in vitro biomass extracts as well as from the intact plants (stems and leaves) were the same. In the fruit extracts, neochlorogenic acid was not detected.
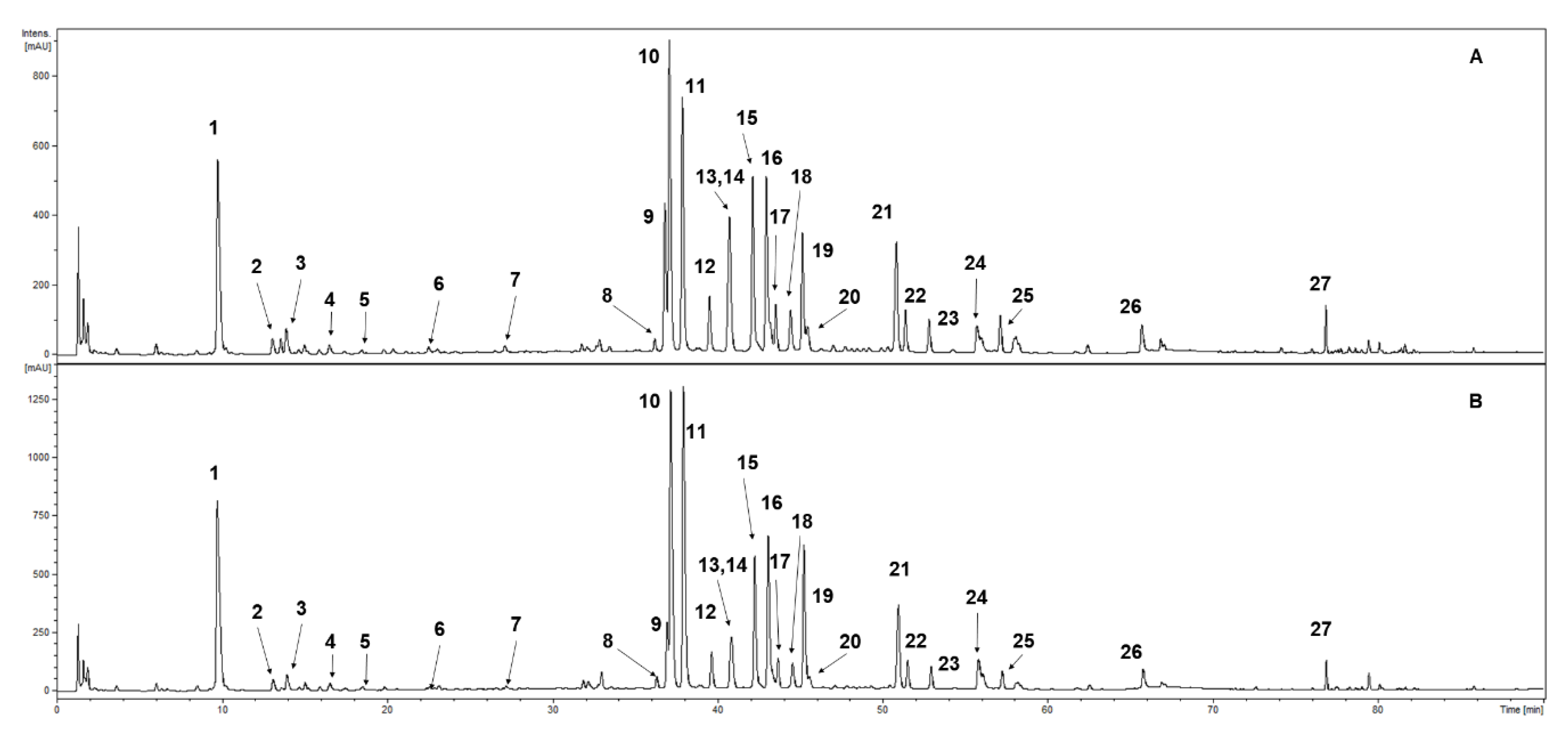
UHPLC-DAD-ESI-MS3 of the methanolic extracts confirmed the presence of 27 phenolic compounds (Table 1, Figure 1). Compounds were classified into photochemical groups based on the profile of UV-Vis spectra recorded during the analysis. Constituents with an absorption maxima in UV at ca. 300 and ca. 325 nm were classed as caffeic acid derivatives (1, 4 and 5). The characteristic maxima at ca. 300 and ca. 310 nm were tentatively identified as p-coumaroyl acid derivatives (2, 3, 6, and 7). Compounds with a strong absorption maxima at ca. 340–360 nm were classed as flavonoid derivatives. Further characterisation was performed using analysis of MS and MS/MS spectra. Compounds with an aglycone signal in MS2 or MS3 spectra at m/z = 301 in the negative ion mode were identified as quercetin derivatives (8–12, 14, and 19). Compounds exhibiting a strong signal in MS/MS spectra at m/z = 285 in the negative ion mode were classed as kaempferol glycosides (13, 15, 16, 18, 20, 21, and 25) with various substitution patterns. Constituents an aglycone signal in the fragmentation spectra at m/z = 315 in the negative ion mode were identified as isorhamentin derivatives (17 and 22). Three free aglycones were detected and identified as quercetin (24), kaempferol (26) and isorhamnetin (27). As a result of the comparison to the chemical standards, some of the detected natural products were fully identified as neochlorogenic acid (1), chlorogenic acid (4), cryptochlorogenic acid (5), hyperoside (quercetin 3-galactoside) (9), rutoside (quercetin 3-rutinoside) (10), isoquercitrin (quercetin 3-glucoside) (11), guaijaverin (quercetin 3-arabinoside) (12), trifolin (kaempferol 3-O-galactoside) (13), avicularin (quercetin 3-O-α-L-arabinopyranoside) (14), and astragalin (kaempferol 3-glucoside) (16) (the numbers correspond with Figure 2 and Table 1).

Table 1.
The phenolic profile of S. rubriflora extracts determined by UHPLC-DAD-ESI-MS3.
Figure 2.
UHPLC-DAD-ESI-MS3 chromatogram of S. rubriflora leaf extract separation of the phenolic compounds (at 254 nm): (A) female (F) specimen; (B) male (M) specimen. For compound numbers, refer to Table 1.
3.3. HPLC-DAD Phenolic Profile Quantitative Characterisation
3.3.1. Fruits
From among phenolic acids present in the studied fruit extracts of S. rubriflora, quantification was performed for chlorogenic acid and cryptochlorogenic acid. The presence of neochlorogenic acid was not detected. Total phenolic content was 78 mg/100 g DW (Table 2). Cryptochlorogenic acid was the dominant compound (69 mg/100 g DW). The amount of chlorogenic acid was lower (9 mg/100 g DW) (Table 2).

Table 2.
The content (mg/100 g DW ± SD) of main detected phenolic compounds of S. rubriflora fruit extracts (n = 5, p < 0.05).
Five flavonoid glycosides—hyperoside, rutoside, isoquercitrin, guaijaverin, and trifolin—and three aglycones—quercetin, kaempferol and isorhamnetin—were quantitatively estimated in the fruit extracts (Table 2). The total content of flavonoids amounted to 353 mg/100 g DW. The dominant compounds were kaempferol (139 mg/100 g DW), guaijaverin (52 mg/100 g DW), rutoside (42 mg/100 g DW), and isorhamnetin (39 mg/100 g DW). The content of other compounds was as follows: quercetin (32 mg/100 g DW), isoquercitrin (27 mg/100 g DW) and trifolin (19 mg/100 g DW); the lowest content was found for hyperoside (42 mg/100 g DW) (Table 2).
3.3.2. Stems
The individual and total amounts of the studied phenolic acids and flavonoids were dependent on the stem harvesting vegetation period and on the sex of S. rubriflora individuals.
The total phenolic acid content in the stem extracts ranged from 260 mg/100 g DW (M, summer) to 590 mg/100 g DW (F, spring) (Table 3). The quantitative estimations in the stem extracts were performed for neochlorogenic acid, chlorogenic acid and cryptochlorogenic acid. Neochlorogenic acid was the quantitatively dominant compound both in female (F) and male (M) stems collected during the vegetative season (Table 3). The amounts of this compound ranged from 185 mg/100 g DW (M, summer) to 457 mg/100 g DW (F, spring). The amounts of cryptochlorogenic and chlorogenic acids were also high, ranging from 20 to 80 mg/100 g DW and from 18 to 53 mg/100 g DW, respectively. The minimal and maximal contents of cryptochlorogenic and chlorogenic acids was confirmed in the same samples collected from F specimens in spring and in autumn, respectively.

Table 3.
The content (mg/100 g DW ± SD) of main detected phenolic compounds of S. rubriflora stem extracts (F—female; M—male) (n = 5, p < 0.05).
Five flavonoid glycosides—hyperoside, rutoside, isoquercitrin, guaijaverin, and trifolin—and three aglycones—quercetin, kaempferol and isorhamnetin—were quantitatively estimated in the stem extracts (Table 3). The total flavonoid content in the stem extracts varied from 967 (M, autumn) to 2693 mg/100 g DW (F, spring). Rutoside, isoquercitrin and trifolin were the main compounds both in female (F) and male (M) stems collected during the vegetative season (Table 3). The amounts of these compounds ranged from 213 (M, summer) to 932 mg/100 g DW (F, spring), 160 (M, autumn) to 624 mg/100 g DW (F, spring) and from 189 (M, autumn) to 605 mg/100 g DW (F, spring), respectively. The highest contents of quercetin (135 mg/100 g DW), kaempferol (113 mg/100 g DW) and isorhamnetin (53 mg/100 g DW) were detected in the extracts of stems collected in spring from F specimens. The maximal content of hyperoside (130 mg/100 g DW) was detected in stems harvested from M specimens in autumn, while the maximal content of guaijaverin (190 mg/100 g DW) was confirmed for stems harvested in spring, also from M specimens (Table 3).
3.3.3. Leaves
The individual and total amounts of the researched phenolic acids and flavonoids were dependent on the leaf harvesting vegetation period and on the sex of the studied S. rubriflora individuals.
The total phenolic acid content in the leaf extracts ranged from 262 (F, summer) to 758 mg/100 g DW (F, autumn) (Table 4). In the stem extracts, the quantitative estimations were performed for neochlorogenic acid, chlorogenic acid and cryptochlorogenic acid. The estimations revealed that neochlorogenic acid was the dominant compound both in female (F) and male (M) stems collected during the vegetative season (Table 4). The amounts of this compound ranged from 217 (F, summer) to 607 mg/100 g DW (F, autumn). The amounts of cryptochlorogenic and chlorogenic acids were lower and they ranged from 19 (F, spring) to 94 mg/100 g DW (M, autumn) and from 17 DW (F, summer) to 93 mg/100 g DW (F, autumn), respectively.

Table 4.
The content (mg/100 g DW ± SD) of main detected phenolic compounds of S. rubriflora leaf extracts (F—female; M—male) (n = 5, p < 0.05).
Five flavonoid glycosides—hyperoside, rutoside, isoquercitrin, guaijaverin, and trifolin—and three aglycones—quercetin, kaempferol and isorhamnetin—were quantitatively estimated in the leaf extracts (Table 4). The total flavonoid content in the stem extracts varied from 1248 (M, autumn) to 2838 mg/100 g DW (M, spring). Rutoside, isoquercitrin and trifolin were the dominant compounds both in female (F) and male (M) stems collected during the vegetative season (Table 4). The amounts of these compounds ranged from 311 (M, autumn) to 841 mg/100 g DW (M, spring), from 180 (M, autumn) to 503 mg/100 g DW (F, spring) and from 251 (M, autumn) to 714 mg/100 g DW (M, spring), respectively. The highest contents of guaijaverin (269 mg/100 g DW) and quercetin (213 mg/100 g DW) were detected in the extracts of leaves collected in spring from M specimens. The highest contents of hyperoside (164 mg/100 g DW) and isorhamnetin (59 mg/100 g DW) were detected in the extracts of leaves collected in autumn, also from M specimens. The highest content of kaempferol (111 mg/100 g DW) was detected in the extracts of leaves collected from F specimens in autumn (Table 4).
3.3.4. In Vitro Cultures
In the studied S. rubriflora F and M line microshoot culture extracts, quantification was performed for neochlorogenic acid, chlorogenic acid and cryptochlorogenic acid from among phenolic acids. Total phenolic content was 188 (F) and 211 mg/100 g DW (M) (Table 5). The highest amounts were confirmed for neochlorogenic acid (F—81 mg/100 g DW; M—103 mg/100 g DW) and cryptochlorogenic acid (F—87 mg/100 g DW; M—91 mg/100 g DW). The amount of chlorogenic acid was lower (F—20 mg/100 g DW; M—16 mg/100 g DW).

Table 5.
The content (mg/100 g DW ± SD) of main detected phenolic compounds of S. rubriflora microshoot in vitro culture extracts (F—female; M—male) (n = 10, p < 0.05).
Five flavonoid glycosides—hyperoside, rutoside, isoquercitrin, guaijaverin, and trifolin—and three aglycones—quercetin, kaempferol and isorhamnetin—were quantitatively estimated in the fruit extracts (Table 5). The total contents of flavonoids were 328 (F) and 420 mg/100 g DW (M). The dominant compounds were aglycones: quercetin (F—56 mg/100 g DW; M—87 mg/100 g DW), kaempferol (F—72 mg/100 g DW; M—91 mg/100 g DW) and isorhamnetin (F—72 mg/100 g DW; M—80 mg/100 g DW). From among glycosides, guaijaverin (F—38 mg/100 g DW; M—44 mg/100 g DW), rutoside (F—29 mg/100 g DW; M—42 mg/100 g DW) and trifolin (F—27 mg/100 g DW; M—37 mg/100 g DW) were detected in large quantities. The amounts of isoquercitrin (F—21 mg/100 g DW; M—26 mg/100 g DW) and hyperoside (F—14 mg/100 g DW; M—12 mg/100 g DW) were slightly lower (Table 5).
3.4. The Total Phenolic Assay
The total phenolic assay, performed in accordance with the Folin-Ciocalteu method, indicated the highest results for the leaf extracts (F—36,633 mg/100 g DW; M—31,207 mg/100 g DW) and the lowest for the fruit extracts (5181 mg/100 g DW) (Table 6). In the stem extracts, total phenolic content was 22,763 mg/100 g DW for F and 18,129 mg/100 g DW for M specimens. In vitro cultured microshoots contained 4729 mg/100 g DW for F and 6374 mg/100 g DW for M lines (Table 6).

Table 6.
Total phenolic content of plant material determined by Folin-Ciocaletu assay. Antioxidant response was expressed as TE in mg/100 g DW ± SD; F—female; M—male (n = 4, p < 0.05).
3.5. The Antioxidant Potential Assays
3.5.1. The FRAP Assay
The antioxidant potential, measured with the FRAP assay, was highest for the leaf extracts (F—19,720 mg/100 g DW; M—28,179 mg/100 g DW) (Table 7). Further, a high antioxidant potential was indicated for the fruit extracts—10,075 mg/100 g DW. The antioxidant content in the stem extracts estimated with the FRAP assay amounted to 7474 mg/100 g DW for F and 5893 mg/100 g DW for M specimens. The antioxidant potential for in vitro cultured microshoots was 2869 mg/100 g DW for F and 5178 mg/100 g DW for M lines (Table 7).

Table 7.
Antioxidant potential of plant material determined by Ferric-Reducing Antioxidant Power (FRAP), 2,2-Diphenyl-1-Picryl-Hydrazyl-Hydrate (DPPH), Cupric-Reducing Antioxidant Capacity (CUPRAC) and QUick, Easy, New, CHEap, and Reproducible CUPRAC (QUENCHER-CUPRAC) assays. Antioxidant response was expressed as TE in mg/100 g DW ± SD; F—female; M—male (n = 4, p < 0.05).
3.5.2. The DPPH Assay
The antioxidant potential measured with DPPH indicated the highest results for the leaf and stem extracts: 8405 and 8363 mg/100 g DW and 7967 and 8340 mg/100 g DW for F and M specimens, respectively (Table 7). A lower antioxidant potential was indicated for the fruit extracts—2984 mg/100 g DW. The antioxidant potential measured with DPPH for in vitro cultured microshoots was 2964 mg/100 g DW for F and 3750 mg/100 g DW for M lines (Table 7).
3.5.3. The CUPRAC Assay
The antioxidant potential measured with the CUPRAC assay showed the highest results for the leaf and stem extracts: 19,720 and 28,179 mg/100 g DW and 22,270 and 17,920 mg/100 g DW for F and M specimens, respectively (Table 7). A lower antioxidant potential was indicated for the fruit extracts—10,075 mg/100 g DW. The antioxidant potential measured with the CUPRAC assay for in vitro cultured microshoots was 2869 mg/100 g DW for F and 5178 mg/100 g DW for M lines (Table 7).
3.5.4. The QUENCHER-CUPRAC Assay
The antioxidant potential measured with the QUENCHER-CUPRAC assay revealed the highest results for the leaf and stem extracts: 29,539 and 29,303 mg/100 g DW and 27,147 and 28,317 mg/100 g DW for F and M specimens, respectively (Table 7). A lower antioxidant potential was indicated for the fruit extracts (6083 mg/100 g DW). The QUENCHER-CUPRAC antioxidant potential measured for in vitro cultured microshoots was 5994 mg/100 g DW for F and 5822 mg/100 g DW for M lines (Table 7).
4. Discussion
In our study, the phenolic profile of S. rubriflora female (F) and male (M) specimens has been evaluated for the first time. This study divided specimens into F and M individuals as well as dividing different parts of plants and different vegetative periods of their harvesting. The research showed a relationship between the content of polyphenolic compounds in the extracts and their antioxidant potential, which was measured using four methods: DPPH, FRAP, CUPRAC and QUENCHER-CUPRAC. In addition, the F and M lines of S. rubriflora in vitro microshoot cultures were initiated and evaluated for phenolic content as well as antioxidant potential.
UHPLC-DAD-ESI-MS3 analyses provided the qualitative profile of S. rubriflora samples. The qualitative profiles of phenolic compounds from in vitro biomass extracts as well as from stems and leaves were the same. Neochlorogenic acid was the only absent compound in the fruit extracts. The analyses confirmed the presence of 27 phenolic compounds (Table 1, Figure 2). S. rubriflora has not been studied for phenolic compounds before. There is only a single study dealing with extracts from stems collected in Lincang County, China, in autumn. Li et al. [52], using various column chromatography methods (silica gel, Sephadex LH-20 and RP-18 high-resolution electrospray ionisation mass spectrometry (HRESIMS)) detected only one phenolic acid—glucosyringic acid—and three flavonoids—naringin, didimin (acinoside, isosakuranetin-7-O-rutinoside) and maesopsin-6-O-glucopyranoside—in the samples. These rare compounds were not detected in our research.
Under the present study, we confirmed that fruits are a poorer source of phenols than stems and leaves of S. rubriflora (Table 2, Table 3 and Table 4). Total phenolic content based on HPLC-DAD results was 431 mg/100 g DW for the fruit extracts (Table 2), and was 3–8-fold lower than the minimal and maximal contents detected for the investigated leaf and stem extracts. Total phenolic content ranged from 1233 (M, autumn) to 3283 mg/100 g DW (F, spring) (Table 3) for the stem extracts, and from 1689 (M, autumn) to 3452 mg/100 g DW (M, spring) for the leaf extracts (Table 4). The results of the chromatographic quantification fully correlated with the results of the total phenolic content assay carried out spectrophotometrically according to the Folin-Ciocaletu assay (Table 6). The highest total phenolic content was indicated for the extracts of leaves collected in spring (F—36,633 mg/100 g DW; M—31,207 mg/100 g DW), and was 7- and 6-fold lower for the fruit extracts (5181 mg/100 g DW) (Table 6). In the stem extracts, total phenolic content was marginally lower in comparison to leaves—22,764 mg/100 g DW for F and 18,129 mg/100 g DW for M specimens (Table 6).
Neochlorogenic acid, indicated as the most abundant compound in the stem and leaf extracts, was not detected in the fruit extracts. The high amount was confirmed for chlorogenic acid (69 mg/100 g DW) and kaempferol (138 mg/100 g DW) (Table 2). Fruits of S. chinensis, were investigated by Mocan et al. [23]. This team studied the material supplied by a local producer from Cluj-Napoca (Romania). It was confirmed with HPLC-UV-MS that fruits are a poor source of phenolic compounds. From among phenolic acids, only chlorogenic acid was detected in the amount of 0.33 mg/100 g DW and traces of genistic acid and p-coumaric acid were also found. The amount of chlorogenic acid was 27-fold lower than that detected in our fruit samples. Mocan et al. confirmed the presence of four compounds from among flavonoids, namely rutoside (1.3 mg/100 g DW), isoquercitrin (0.66 mg/100 g DW), hyperoside (0.2 mg/100 g DW), and quercetin (0.2 mg/100 g DW). These amounts were many times lower than that detected by us for S. rubriflora—they were 32-, 41-, 24-, and 180-fold lower, respectively. Further, these studies did not detect guaijaverin and kaempferol, which were the main compounds confirmed in the fruit extracts of S. rubriflora (Table 2). The differences in fruit extract phenolic composition between S. rubriflora and S. chinensis were also confirmed by our team. Using HPLC-DAD, we were able to confirm different compounds from among phenolic acids, including gallic acid, p-hydroxybenzoic acid, protocatechuic acid, salicylic acid, syringic acid, and vanillic acid, in S. chinensis fruits of Polish origin. Only chlorogenic acid was confirmed in fruits of both species, but in S. chinensis its amount was 2-fold lower (4.55 mg/100 g DW) than in S. rubriflora. Our former studies did not confirm the presence of flavonoid compounds in S. chinensis fruits [30,53,54,55,56].
S. rubriflora stem extracts proved to be abundant in phenolic compounds (Table 3). Their individual and total amounts were dependent on the harvesting time, vegetation period and the sex of the studied S. rubriflora individuals. Total phenolic content was very high, and in each vegetation period, higher content was found for stems collected from female specimens. Spring: F—3283 mg/100 g DW; M—2266 mg/100 g DW. Summer: F—2213 mg/100 g DW; M—1510 mg/100 g DW. Autumn: F—1756 mg/100 g DW; M—1233 mg/100 g DW (Table 3). The highest total phenolic content was found in the extracts of stems harvested in spring from F specimens, both for individual compounds and for the total pools. Neochlorogenic acid was clearly the main compound (max. 457 mg/100 g DW, F, spring) among the detected phenolic acids. Rutoside (max. 932 mg/100 g DW), isoquercitrin (624 mg/100 g DW) and trifolin (605 mg/100 g DW) were the quantitative dominant compounds among flavonoids. Their maximal content was confirmed in stems harvested in spring from F specimens. The stem extracts of S. chinensis of Romanian origin were studied by Mocan et al. [24]. In their research, identification of compounds was performed using LC-DAD-ESI-ToF-MS, while quantification was carried out using HPLC-DAD. From among phenolic acids, similarly to S. rubriflora, compounds from depside group were indicated in the extracts. The 3-O-p-coumarylquinic acid (63 mg/100 g DW) and neochlorogenic acid (37 mg/100 g DW) were the dominant compounds. Chlorogenic acid (24 mg/100 g DW) and 4-O-p-coumarylquinic acid (17 mg/100 g DW) were also estimated. From among flavonoids in S. chinensis stem extracts, Mocan et al. estimated mainly 7-O-rhamnosides—quercetin-3-O-glucoside-7-O-rhamnoside (78 mg/100 g DW) and kaempferol-3-O-glucoside-7-O-rhamnoside (77 mg/100 g DW)—as well as 3-O-glucosides—quercetin-3-O-glucoside (59 mg/100 g DW) and kempferol-3-O-glucoside (37 mg/100 g DW) [24].
S. rubriflora leaf extracts were the most abundant in phenolic compounds, which was confirmed by both chromatographic and spectrophotometric analyses (Table 4 and Table 6). HPLC-DAD estimations indicated that the individual as well as the total amounts of phenolic acids and flavonoids were dependent on the harvesting vegetation period and on the sex of the studied S. rubriflora individuals. Total phenolic content was very high, and the highest content was found for leaves collected in spring: F—2814 mg/100 g DW; M—3452 mg/100 g DW. In the samples collected in summer, these values were also very high: F—2145 mg/100 g DW; M—2165 mg/100 g DW, respectively. Leaves collected in autumn were a good source of phenolic compounds: F—2718 mg/100 g DW; M—1689 mg/100 g DW (Table 4). Neochlorogenic acid was the main compound (607 mg/100 g DW, F, autumn; 529 mg/100 g DW, F, spring; and 530 mg/100 g DW, M, spring) among the detected phenolic acids. Among flavonoids, the maximal content of rutoside (max. 841 mg/100 g DW), trifolin (714 mg/100 g DW), isoquercitrin (499 mg/100 g DW), and guaijaverin (269 mg/100 g DW) was found in leaves harvested in spring from M specimens. The maximal content—164 and 59 mg/100 g DW, respectively—of hyperoside and isorhamnetin was detected in extracts from M leaves collected in autumn. The leaf extracts of S. chinensis of Romanian origin were studied for phenolic acids and flavonoids by Mocan et al. as well [24]. The contents of these phenolic compounds in the leaf extracts were higher than in stems or fruits. This team also found more compounds quantitatively. Among phenolic acids, the dominant compounds were chlorogenic acid (499 mg/100 g DW), neochlorogenic acid (295 mg/100 g DW), trans-5-O-p-coumaroylquinic acid (105 mg/100 g DW), and protocatechuic acid (53 mg/100 g DW). The quantitatively dominant compounds among flavonoids in S. chinensis leaf extracts were kaempferol 3-O-glucoside-7-O-rhamnoside (568 mg/100 g DW), kaempferol 3-O-glucoside (512 mg/100 g DW) and quercetin 3-O-galactoside (249 mg/100 g DW). As part of our earlier research on a S. chinensis specimen of Polish origin, the phenolic acid and flavonoid contents in the leaf extracts were also determined using HPLC-DAD [30,53,54,55]. They were quantitatively similar to those found by Mocan et al. Chlorogenic acid (48 mg/100 g DW) and protocatechuic acid (25 mg/100 g DW) were the dominant compounds among phenolic acids, while quercitrin (100 mg/100 g DW), quercetin (70 mg/100 g DW) and myricetin (42 mg/100 g DW) were detected among flavonoids [30,53,54,55,56]. The high contents of chlorogenic acid (max. 635 mg/100 g DW) and rutoside (103 mg/100 g DW) were confirmed also in the leaf extracts of Lycium barbarum and Lycium chinense cultivated in Italy [57]. In this study, the qualitative and quantitative differences between Lycium species were noted—cryptochlorogenic acid was found only in L. barbarum, while quercetin-3-O-rutinoside-7-O-glucoside and quercetin-3-O-sophoroside-7-O-rhamnoside were found only in L. chinense leaves.
The initiation of S. rubriflora microshoot cultures and testing of the accumulation of the phenolic content in their biomass performed in this study aimed at assessing their usefulness in relation to the material derived from the field conditions. The results of this research turned out to be ultimately innovative. According to the literature review, there are no papers on S. rubriflora in vitro cultures. The results obtained prove to be very interesting from a cognitive and practical point of view. The two lines (F and M) of in vitro microshoot cultures were successfully initiated from leaf buds. In vitro microshoot cultures grew well on MS medium supplemented with 1 mg/L BA and 1 mg/L IBA as the plant growth regulators. Biomass growth was satisfactory; the estimated growth index was approximately three for a 30 day growth period. That was similar to S. chinensis in vitro cultures cultured in our laboratory before [30,55,56]. Total phenolic content detected by HPLC-DAD (Table 5) was 515 mg/100 g DW for F and 631 mg/100 g DW for M lines. These results corresponded to the outcomes achieved by the spectrophotometric assay with Folin-Ciocaletu reagent, which indicated that total phenolic content was 4729 mg/100 g DW for F and 6374 mg/100 g DW for M lines (Table 6). In accordance with this method, total phenolic content of in vitro microshoots was slightly lower for F lines and marginally higher for M lines than for fruits of mother plants (5181 mg/100 g DW) (Table 6). In comparison to the stem and leaf extracts, total phenolic content was 4.8- and 2.8-fold and 7.7- and 4.9-fold lower for F and M microshoot lines, respectively. Based on the chromatographic estimations carried out with UHPLC-DAD-ESI-MS3, the qualitative profile of phenolic compounds was the same for in vitro cultures as well as for the intact plant material (Table 1). It was evident from the quantitative analysis carried out using HPLC-DAD that neochlorogenic acid and cryptochlorogenic acid were the dominant compounds among phenolic acids: 81 and 103 mg/100 g DW, and 87 and 91 mg/100 g DW for F and M lines, respectively (Table 5). In in vitro culture extracts, aglycones were the quantitative dominant compounds among flavonoids: kaempferol (F—72 mg/100 g DW; M—91 mg/100 g DW), isorhamnetin (F—72 mg/100 g DW; M—80 mg/100 g DW) and quercetin (F—56 mg/100 g DW and M—87 mg/100 g DW) (Table 5). In order to carry out the comparative assessment of the results from S. rubriflora in vitro cultures, our previous long-term studies on S. chinensis in vitro cultures were taken into consideration [30,53,54,55,56]. In these studies on S. chinensis agar microshoot cultures, chlorogenic acid (max. 13 mg/100 g DW) and protocatechuic acid (max. 36 mg/100 g DW) were the main compounds from phenolic acid group, similarly to S. rubriflora [30,53,54,55,56]. The main compound from among flavonoids was quercitrin (max. 27 mg/100 g DW), which was not estimated in the S. rubriflora microshoots.
To sum up the phenolic content estimations, it is important to state that in plant material (fruits, stems and leaves) of S. rubriflora and S. chinensis, it is S. rubriflora that is a richer source of compounds with antioxidant potential. Fruits of both species are a poor source of both phenolic acids and flavonoids. However, the leaf and stem extracts seem to be promising in this respect. The noticeably high contents of these compounds were noted using chromatographic and spectrophotometric tests in the leaf extracts collected in spring. Furthermore, stems collected at this time of the year also indicated good results.
The contents of these compounds in extracts from in vitro cultures were lower than in the leaf or stem extracts. However, they were comparable to the results obtained for fruits which are Schisandra pharmacopeial raw material. The outcome of our research resulted in carrying out the comparative analyses using four assays, namely FRAP, DPPH, CUPRAC, and QUENCHER-CUPRAC, for extracts from leaves, stems and fruits, as well as also evaluating our biotechnological research using in vitro cultures.
The antioxidant potential estimations revealed that S. rubriflora has very high antioxidant potential (Table 7). The highest antioxidant power was indicated by means of all the applied methods for the leaf extracts. For the leaf extracts, the highest antioxidant capability was found for M specimens when using the FARP and CUPRAC assays; the results for F and M specimens were almost the same using the DPPH and the QENCHER-CUPRAC assays. High potential was also demonstrated for the stem extracts. The antioxidant capacity of in vitro cultured microshoots measured with the DPPH and the QENCHER-CUPRAC assays was comparable to the power of the fruit extracts (Table 7).
Results from the antioxidant activity assays fully corresponded with the results from the quantification of phenols described above.
Many authors claim that phenolic secondary metabolites, especially from among phenolic acids and flavonoids, are primarily responsible for the antioxidant capacity of different plant raw materials [3,4,58,59,60]. Lignans (especially dibenzocyclooctadiene lignans) are the most characteristic and the most frequently marked compounds for the Schisandra genus [12,19,32]. There are only a few publications on the analysis of phenolic compounds and lignans in Schisandra species, which mainly concern S. chinensis species. Mocan et al. [24] characterised the contribution of the single constituents, namely lignans and further phenolic compounds, to the overall antioxidant activity. The results of the complex study contributed to similar conclusions of our research. The team measured the antioxidant activity of leaves, stems and fruits using the trolox equivalent antioxidant capacity assay of different extracts against the stable synthetic ABTS•+ radical cation. In general, not lignans, but chlorogenic acid isomers and quercetin glycosides contributed over 80% of the total antioxidant activity for S. chinensis. [24].
Choi et al. [29] assessed the antioxidant activity of lignans using the 2′,7′-dichlorodihydrofluorescein diacetate (DCFH-DA) cellular-based analysis. The structure–activity relationships of various dibenzocyclooctadiene lignans exhibited that the exocyclic methylene functionality, which occurs only in individual structures of lignans, was essential for the antioxidant activity, with the benzoyloxy group probably enhancing such effects. Only one structure—schisandrene—was indicated as a responsible lignan. Zhang et al. [26] estimated the antioxidant and antiproliferative activities of five compounds from among lignans and terpenoids (d-epigalbacin, machilin G, chicanine, anwulignan, and epi-anwuweizic acid) and the extract from S. chinensis fruits. The antioxidant capacity was evaluated by the DPPH radical-scavenging assay. The result of DPPH measures indicated that S. chinensis crude fruit extract exhibited 7-fold higher activity (IC50 = 188) than the individual tested compounds (max. chicanine IC50 = 26). This is a premise that lignans and terpenoids are less responsible for Schisandra antioxidant activity. The close influence of polyphenolic compounds on the antioxidant activity was also indicated by other authors for Thymus vulgaris, Salvia officinalis and Origanum majorana [61], Moringa oleifera [62], Rhus coriaria [63], or Malus sp. [64], Juglans sp. [65], and Quercus sp. [66]. The results of our research based on the determination of phenolic compounds fully correlated with the antioxidant activity studies carried out by our team as well.
The antioxidant activity for in vitro cultures is also often compared to in vivo material [9]. Hakkim et al. [67] conducted a comparative study of the chemical composition and antioxidant property of Ocimum sanctum leaves, stems, and inflorescences and their in vitro callus cultures. The callus cultures were maintained on MS medium with 1 mg/L of 2,4-dichlorophenoxyacetic acid (2,4-D) with different concentrations of kinetin (0.1–0.5 mg/L). The distribution of phenolic compounds in these extracts was analysed using HPLC-PDA. In the intact plant extracts, more compounds were detected than in the callus extracts. These included isothymusin, ursolic acid, carnosic acid, eugenol, sinapic acid, and rosmarinic acid. However, rosmarinic acid was found to be the predominant phenolic acid in the callus extracts, with an amount (ca. 220 mg/100 g DW) that was 11-fold higher than in the studied leaves, stems, and inflorescences (ca. 20 mg/100 g DW). In this study, the antioxidant activity of the extracts was evaluated, among others, by means of the DPPH assay. The IC50 (expressed in mg of extract/mL) of the callus cultures was ca. 0.4 and was higher—ca. 0.6—for the intact plant parts.
Costa et al. performed a comparative study of in vitro and in vivo material of Thymus lotocephalus [68] and Lavandula viridis [69]. The study compared phenolic metabolites using HPLC-DAD and also antioxidant activities using the oxygen radical absorbance capacity (ORAC) assay. The main phenols in T. lotocephalus were phenolic acids—caffeic acid and rosmarinic acid—and flavones—luteolin and apigenin. In vitro cultures accumulated the large amounts of rosmarinic acid. The research indicated that in vitro cultures of T. lotocephalus showed slightly lower activity than a wild plant expressed with the ORAC assay—1.30 and 1.19 (mol trolox/g of extract) for the herb and callus cultures extracts, respectively. HPLC-DAD analyses for L. viridis showed that the dominant phenols were; from phenolic acids: 3-O-caffeoylquinic, 4-O-caffeoylquinic, 5-O-caffeoylquinic, and rosmarinic acids, and from flavonoids luteolin and pinocembrin. The water/ethanol extract from in vitro cultures contained the highest amount of the identified phenolics (51,653 mg/kg). The antioxidant tests showed that extracts from L. viridis (both wild plants and in vitro cultures) showed the ability to chelate Fe2+, scavenge free radicals and protect against lipid peroxidation.
Kuhlmann and Röhl [70] compared the phenolic content using HPLC-DAD and the antioxidant capacity with DPPH of different in vitro culture types, namely shoot culture, callus culture and cell suspension of Rosmarinus officinalis. The dominant compounds found were diterpenes (carnosic acid and carnosol) and depside (rosmarinic acid). The level of carnosic acid in the suspension culture was 3-fold lower than for the callus culture. The amounts of rosmarinic acid produced in the shoot and callus cultures were similar, whereas a higher amount of rosmarinic acid was measured in the suspension culture than in the shoot and callus cultures. The results of the DPPH radical-scavenging activity of the extracts showed that this activity was dependent in particular on the amount of rosmarinic acid.
Taveira et al. [71] also made a comparison of the phenolic composition only of in vitro material from shoot, callus and root cultures of Brassica oleracea var. costata and its antioxidant capacity using DPPH. The determination of phenolic compounds was carried out by HPLC-DAD. No phenolic compounds were identified in callus and root cultures. The presence of 36 compounds, which included flavonoids (kaempferol and quercetin derivatives), hydroxycinnamic acids, and hydroxycinnamic acyl glycosides, was confirmed in shoots. MS liquid medium with 0.1 mg/L NAA (1-naphthaleneacetic acid) and 2 mg/L BA was the best condition to produce the shoot culture biomass with the highest phenolic content and the antioxidant potential. The authors indicated that the phenolic content was responsible for the antioxidant power of B. oleracea var. costata in vitro shoots.
Królicka et al. [72] evaluated the antioxidant activity as well as the secondary metabolites of Drosera aliciae shoot cultures grown in vitro. The methanol extract from D. aliciae proved to be an effective antioxidant in both the DPPH and the FRAP assays. The antiradical potential was dependent on the estimated flavonoid contents.
To conclude, the biotechnological research results confirmed our observations regarding in vitro cultures of S. rubriflora. The accumulation of phenolic compounds in in vitro cultured biomass was lower in comparison to the field-grown plants parts, which is connected with its antioxidant potential. However, S. rubriflora in vitro cultured biomass could be an alternative, valuable source of natural antioxidants and an efficient tool for in vitro biosynthesis of phenolic acids (neochlorogenic and cryptochlorogenic acids) as well as for flavonoids (kaempferol, isorhamnetin), which would allow for avoiding the need to exploit populations of wild, endemic plants.
5. Conclusions
The present study is the first comparative, complex, qualitative and quantitative analysis of the polyphenol composition as well as of the antioxidant potential of S. rubriflora fruits, stems and leaves and in vitro microshoot cultures. The qualitative estimations of phenolic acids and flavonoids were performed using UHPLC-DAD-ESI-MS3. The phenolic profile revealed the presence of 27 compounds. The contents of the main compounds have been determined for the first time using HPLC-DAD. This study quantitatively characterised from phenolic acids: chlorogenic acid, cryptochlorogenic acid and neochlorogenic acid, from flavonoid glycosides: hyperoside, rutoside, isoquercitrin, guaijaverin, and trifolin, and from flavonoid aglycones: quercetin, kaempferol and isorhamnetin. The qualitative (fruits—no presence of neochlorogenic acid) and quantitative differences in the phenolic compound composition were recorded and they were dependent on the sex of specimens, the vegetation period, and parts of the intact plant material—fruits, leaves and stems. The qualitative and quantitative differences were also indicated for the first time in in vitro cultures of S. rubriflora initiated within this study.
Additionally, the antioxidant activity, based on four in vitro assays (DPPH, FRAP, CUPRAC and QUENCHER-CUPRAC), of S. rubriflora samples with the highest content of phenolic compounds (stems and leaves collected in spring), fruits and in vitro microshoots has been determined for the first time. The results of the antioxidant assays agreed with the results of total phenolic content measured spectroscopically with the Folin–Ciocalteu assay as well as with the chromatographic analyses. A close relationship between total phenolic content in the studied materials of S. rubriflora and their antioxidant potential has been documented for the first time. Moreover, the results revealed the high competitiveness of S. rubriflora in relation to the known, pharmacopeial plant species—S. chinensis.
As a result of our research, the extracts of S. rubriflora (fruit, stems and leaves) should be considered as a rich, valuable source of phenolic compounds, with promising very strong antioxidant potential. In vitro cultures exhibited very interesting differences and showed new research directions involving plant biotechnology solutions for obtaining this endemic plant material.
Author Contributions
Conceptualization, A.S.; methodology, A.S., M.D., and S.G.; formal analysis, A.S.; investigation, A.S., M.D., A.W., M.K.-S., K.J., P.K. and S.G.; data curation, A.S., M.D., and S.G.; writing—original draft preparation, A.S.; writing—review and editing, A.S., M.D., H.E., and S.G.; visualization, A.S., M.D., P.K. and S.G.; supervision, A.S., M.D., S.G., and H.E.; project administration, A.S.; funding acquisition, A.S. All authors have read and agreed to the published version of the manuscript.
Funding
This study was funded by National Science Centre, Poland (grant number 2016/23/D/NZ7/01316).
Conflicts of Interest
All authors declare that they have no conflict of interest.
References
- Apak, R.; Güçlü, K.; Demirata, B.; Özyürek, M.; Çelik, S.; Bektaşoğlu, B.; Berker, K.; Özyurt, D. Comparative Evaluation of Various Total Antioxidant Capacity Assays Applied to Phenolic Compounds with the CUPRAC Assay. Molecules 2007, 12, 1496. [Google Scholar] [CrossRef] [PubMed]
- Krolicka, A.; Szpitter, A.; Gilgenast, E.; Romanik, G.; Kaminski, M.; Lojkowska, E.; Kakkar, S.; Bais, S.; Muthukumaran, J.; Srinivasan, S.; et al. Syringic acid, a novel natural phenolic acid, normalizes hyperglycemia with special reference to glycoprotein components in experimental diabetic rats. J. Acute Dis. 2013, 2014, 952943. [Google Scholar] [CrossRef]
- Stevenson, D.E.; Hurst, R.D. Polyphenolic phytochemicals—Just antioxidants or much more? Cell. Mol. Life Sci. 2007, 64, 2900–2916. [Google Scholar] [CrossRef] [PubMed]
- Agati, G.; Azzarello, E.; Pollastri, S.; Tattini, M. Flavonoids as antioxidants in plants: Location and functional significance. Plant Sci. 2012, 196, 67–76. [Google Scholar] [CrossRef] [PubMed]
- Lombardi, G.; Vannini, S.; Blasi, F.; Marcotullio, M.C.; Dominici, L.; Villarini, M.; Cossignani, L.; Moretti, M. In Vitro Safety/Protection Assessment of Resveratrol and Pterostilbene in a Human Hepatoma Cell Line (HepG2). Nat. Prod. Commun. 2015, 10, 1403–1408. [Google Scholar]
- Thilakarathna, S.; Rupasinghe, H. Flavonoid Bioavailability and Attempts for Bioavailability Enhancement. Nutrients 2013, 5, 3367–3387. [Google Scholar] [CrossRef]
- Perron, N.R.; Brumaghim, J.L. A review of the antioxidant mechanisms of polyphenol compounds related to iron binding. Cell Biochem. Biophys. 2009, 53, 75–100. [Google Scholar] [CrossRef]
- Choi, D.Y.; Lee, Y.J.; Hong, J.T.; Lee, H.J. Antioxidant properties of natural polyphenols and their therapeutic potentials for Alzheimer’s disease. Brain Res. Bull. 2012, 87, 144–153. [Google Scholar] [CrossRef]
- Matkowski, A. Plant in vitro culture for the production of antioxidants—A review. Biotechnol. Adv. 2008, 26, 548–560. [Google Scholar] [CrossRef]
- Dias, M.I.; Sousa, M.J.; Alves, R.C.; Ferreira, I.C.F.R. Exploring plant tissue culture to improve the production of phenolic compounds: A review. Ind. Crops Prod. 2016, 82, 9–22. [Google Scholar] [CrossRef]
- Smetanska, I. Sustainable Production of Polyphenols and Antioxidants by Plant In Vitro Cultures; Springer: Cham, Switzerland, 2018; pp. 225–269. [Google Scholar]
- Szopa, A.; Ekiert, R.; Ekiert, H. Current knowledge of Schisandra chinensis (Turcz.) Baill. (Chinese magnolia vine) as a medicinal plant species: A review on the bioactive components, pharmacological properties, analytical and biotechnological studies. Phytochem. Rev. 2017, 16, 195–218. [Google Scholar] [CrossRef]
- Chinese Pharmacopoeia Commission Pharmacopoeia of the People’s Republic of China; China Chemical Industry Press: Beijing, China, 2005.
- European Directorate for the Quality of Medicines. Schisandra fruit. In European Pharmacopoeia 9.0; European Directorate for the Quality of Medicines: Strasburg, France, 2017; p. 1514. [Google Scholar]
- Upton, R.; Graff, A.; Jolliffe, G.; Länger, R.; Williamson, E. American Herbal Pharmacopoeia: Botanical Pharmacognosy—Microscopic Characterization of Botanical Medicines; CRC Press: Boca Raton, FL, USA, 2011; ISBN 1420073281. [Google Scholar]
- Barnes, J.; Anderson, L.A. Herbal Medicines, 3rd ed.; Pharmaceutical Press: London, UK, 2007; ISBN 9780853696230. [Google Scholar]
- Hancke, J.L.; Burgos, R.A.; Ahumada, F. Schisandra chinensis (Turcz.) Baill. Fitoterapia 1999, 70, 451–471. [Google Scholar] [CrossRef]
- Ekiert, R.J.; Szopa, A.; Ekiert, H.; Krzek, J.; Dzik, E. Analysis of lignans in Schisandra chinensis fruits, leaves, biomasses from in vitro cultures and food supplements. J. Funct. Foods 2013, 5, 1576–1581. [Google Scholar] [CrossRef]
- Opletal, L.; Sovová, H.; Bártlová, M. Dibenzo[a,c]cyclooctadiene lignans of the genus Schisandra: Importance, isolation and determination. J. Chromatogr. B 2004, 812, 357–371. [Google Scholar] [CrossRef]
- Chang, J.; Reiner, J.; Xie, J. Progress on the chemistry of dibenzocyclooctadiene lignans. Chem. Rev. 2005, 105, 4581–4609. [Google Scholar] [CrossRef] [PubMed]
- Cheng, N.; Ren, N.; Gao, H.; Lei, X.; Zheng, J.; Cao, W. Antioxidant and hepatoprotective effects of Schisandra chinensis pollen extract on CCl4-induced acute liver damage in mice. Food Chem. Toxicol. 2013, 55, 234–240. [Google Scholar] [CrossRef]
- Sökmen, M.; Serkedjieva, J.; Daferera, D.; Gulluce, M.; Polissiou, M.; Tepe, B.; Akpulat, H.A.; Sahin, F.; Sokmen, A. In vitro antioxidant, antimicrobial, and antiviral activities of the essential oil and various extracts from herbal parts and callus cultures of Origanum acutidens. J. Agric. Food Chem. 2004, 52, 3309–3312. [Google Scholar] [CrossRef] [PubMed]
- Mocan, A.; Crișan, G.; Vlase, L.; Crișan, O.; Vodnar, D.C.; Raita, O.; Gheldiu, A.M.; Toiu, A.; Oprean, R.; Tilea, I. Comparative studies on polyphenolic composition, antioxidant and antimicrobial activities of Schisandra chinensis leaves and fruits. Molecules 2014, 19, 15162–15179. [Google Scholar] [CrossRef]
- Mocan, A.; Schafberg, M.; Crisan, G.; Rohn, S. Determination of lignans and phenolic components of Schisandra chinensis (Turcz.) Baill. using HPLC-ESI-ToF-MS and HPLC-online TEAC: Contribution of individual components to overall antioxidant activity and comparison with traditional antioxidant assays. J. Funct. Foods 2016, 24, 579–594. [Google Scholar] [CrossRef]
- Wu, X.; Yu, X.; Jing, H. Optimization of phenolic antioxidant extraction from wuweizi (Schisandra chinensis) pulp using random-centroid optimazation methodology. Int. J. Mol. Sci. 2011, 12, 6255–6266. [Google Scholar] [CrossRef]
- Zhang, L.; Chen, H.; Tian, J.; Chen, S. Antioxidant and anti-proliferative activities of five compounds from Schisandra chinensis fruit. Ind. Crops Prod. 2013, 50, 690–693. [Google Scholar] [CrossRef]
- Döring, A.S.; Petersen, M. Production of caffeic, chlorogenic and rosmarinic acids in plants and suspension cultures of Glechoma hederacea. Phytochem. Lett. 2014, 10, cxi–cxvii. [Google Scholar] [CrossRef]
- Jang, H.I.; Do, G.M.; Lee, H.M.; Ok, H.M.; Shin, J.H.; Kwon, O. Schisandra chinensis Baillon regulates the gene expression of phase II antioxidant/detoxifying enzymes in hepatic damage induced rats. Nutr. Res. Pract. 2014, 8, 272–277. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.-W.; Takamatsu, S.; Khan, S.I.; Srinivas, P.V.; Ferreira, D.; Zhao, J.; Khan, I.A. Schisandrene, a dibenzocyclooctadiene lignan from Schisandra chinensis: Structure−antioxidant activity relationships of dibenzocyclooctadiene lignans. J. Nat. Prod. 2006, 69, 356–359. [Google Scholar] [CrossRef] [PubMed]
- Szopa, A.; Ekiert, H. In vitro cultures of Schisandra chinensis (Turcz.) Baill. (Chinese magnolia vine)—A potential biotechnological rich source of therapeutically important phenolic acids. Appl. Biochem. Biotechnol. 2012, 166, 1941–1948. [Google Scholar] [CrossRef] [PubMed]
- Saunders, R.M.K. Monograph of Schisandra (Schisandraceae). In Systematic Botany Monographs; American Society of Plant Taxonomists: Ann Arbor, MI, USA, 2000; Volume 58, pp. 1–146. ISBN 978-0912861586. [Google Scholar]
- Szopa, A.; Barnaś, M.; Ekiert, H. Phytochemical studies and biological activity of three Chinese Schisandra species (Schisandra sphenanthera, Schisandra henryi and Schisandra rubriflora): Current findings and future applications. Phytochem. Rev. 2019, 18, 109–128. [Google Scholar] [CrossRef]
- Tang, J.L.; Liu, B.Y.; Ma, K.W. Traditional Chinese medicine. Lancet. 2008, 372, 1938–1940. [Google Scholar] [CrossRef]
- Mu, H.X.; Li, X.S.; Fan, P.; Yang, G.Y.; Pu, J.X.; Sun, H.D.; Hu, Q.F.; Xiao, W.L. Dibenzocyclooctadiene lignans from the fruits of Schisandra rubriflora and their anti-HIV-1 activities. J. Asian Nat. Prod. Res. 2011, 13, 393–399. [Google Scholar] [CrossRef]
- Xiao, W.L.; Wang, R.R.; Zhao, W.; Tian, R.R.; Shang, S.Z.; Yang, L.M.; Yang, J.H.; Pu, J.X.; Zheng, Y.T.; Sun, H.D. Anti-HIV-1 activity of lignans from the fruits of Schisandra rubriflora. Arch. Pharm. Res. 2010, 33, 697–701. [Google Scholar] [CrossRef]
- Lu, H.; Liu, G.T. Anti-oxidant activity of dibenzocyclooctene lignans isolated from Schisandraceae. Planta Med. 1992, 58, 311–313. [Google Scholar] [CrossRef]
- Chen, M.; Kilgore, N.; Lee, K.H.; Chen, D.F. Rubrisandrins A and B, lignans and related anti-HIV compounds from Schisandra rubriflora. J. Nat. Prod. 2006, 69, 1697–1701. [Google Scholar] [CrossRef] [PubMed]
- Xiao, W.L.; Li, X.; Wang, R.R.; Yang, L.M.; Li, M.; Huang, S.X.; Pu, J.X.; Zheng, Y.T.; Li, R.T.; Sun, H.D. Triterpenoids from Schisandra rubriflora. J. Nat. Prod. 2007, 70, 1056–1059. [Google Scholar] [CrossRef] [PubMed]
- Szopa, A.; Dziurka, M.; Warzecha, A.; Kubica, P.; Klimek-Szczykutowicz, M.; Ekiert, H. Targeted Lignan Profiling and Anti-Inflammatory Properties of Schisandra rubriflora and Schisandra chinensis Extracts. Molecules 2018, 23, 3103. [Google Scholar] [CrossRef]
- Available online: http://www.clematis.com.pl/pl/ (accessed on 15 April 2020).
- Murashige, T.; Skoog, F. A Revised Medium for Rapid Growth and Bio Assays with Tobacco Tissue Cultures. Physiol. Plant 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Ellnain-Wojtaszek, M.; Zgorka, G. High-performance liquid chromatography and thin-layer chromatography of phenolic acids from Ginkgo biloba L. leaves collected within vegetative period. J. Liq. Chromatogr. Relat. Technol. 1999, 22, 1457–1471. [Google Scholar] [CrossRef]
- Sułkowska-Ziaja, K.; Maślanka, A.; Szewczyk, A.; Muszyńska, B. Physiologically active compounds in four species of Phellinus. Nat. Prod. Commun. 2017, 12, 363–366. [Google Scholar] [CrossRef]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-Ciocalteu reagent. Methods Enzymol. 1999, 299, 152–178. [Google Scholar] [CrossRef]
- Bach, A.; Kapczyńska, A.; Dziurka, K.; Dziurka, M. Phenolic compounds and carbohydrates in relation to bulb formation in Lachenalia ‘Ronina’ and ‘Rupert’ in vitro cultures under different lighting environments. Sci. Hortic. 2015, 188, 23–29. [Google Scholar] [CrossRef]
- Benzie, I.F.F.; Strain, J.J. The Ferric Reducing Ability of Plasma (FRAP) as a Measure of “Antioxidant Power”: The FRAP Assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef]
- Blios, M.S. Antioxidant Determinations by the Use of a Stable Free Radical. Nature 1958, 181, 1199–1200. [Google Scholar] [CrossRef]
- Özyürek, M.; Güçlü, K.; Bektaşoğlu, B.; Apak, R. Spectrophotometric determination of ascorbic acid by the modified CUPRAC method with extractive separation of flavonoids–La(III) complexes. Anal. Chim. Acta 2007, 588, 88–95. [Google Scholar] [CrossRef] [PubMed]
- Biesaga-Kościelniak, J.; Dziurka, M.; Ostrowska, A.; Mirek, M.; Kościelniak, J.; Janeczko, A. Brassinosteroid improves content of antioxidants in seeds of selected Leguminous plants. AJCS 2014, 8, 378–388. [Google Scholar]
- Tufan, A.N.; Çelik, S.E.; Özyürek, M.; Güçlü, K.; Apak, R. Direct measurement of total antioxidant capacity of cereals: QUENCHER-CUPRAC method. Talanta 2013, 108, 136–142. [Google Scholar] [CrossRef] [PubMed]
- Kokotkiewicz, A.; Bucinski, A.; Luczkiewicz, M. Xanthone, benzophenone and bioflavonoid accumulation in Cyclopia genistoides (L.) Vent. (honeybush) shoot cultures grown on membrane rafts and in a temporary immersion system. Plant Cell Tissue Organ Cult. 2015, 120, 373–378. [Google Scholar] [CrossRef]
- Li, G.P.; Zhao, J.F.; Tu, Y.Q.; Yang, X.D.; Zhang, H.B.; Li, L. Chemical constituents of Schisandra rubriflora Rehd. et Wils. J. Integr. Plant Biol. 2005, 47, 362–367. [Google Scholar] [CrossRef]
- Szopa, A.; Klimek-Szczykutowicz, M.; Kokotkiewicz, A.; Dziurka, M.; Luczkiewicz, M.; Ekiert, H. Phenolic acid and flavonoid production in agar, agitated and bioreactor-grown microshoot cultures of Schisandra chinensis cv. Sadova No. 1—A valuable medicinal plant. J. Biotechnol. 2019, 305, 61–70. [Google Scholar] [CrossRef]
- Szopa, A.; Kokotkiewicz, A.; Bednarz, M.; Jafernik, K.; Luczkiewicz, M.; Ekiert, H. Bioreactor type affects the accumulation of phenolic acids and flavonoids in microshoot cultures of Schisandra chinensis (Turcz.) Baill. Plant Cell. Tiss. Organ Cult. 2019, 139, 199–206. [Google Scholar] [CrossRef]
- Szopa, A.; Kokotkiewicz, A.; Bednarz, M.; Luczkiewicz, M.; Ekiert, H. Studies on the accumulation of phenolic acids and flavonoids in different in vitro culture systems of Schisandra chinensis (Turcz.) Baill. using a DAD-HPLC method. Phytochem. Lett. 2017, 20, 462–469. [Google Scholar] [CrossRef]
- Szopa, A.; Kokotkiewicz, A.; Klimek-Szczykutowicz, M.; Luczkiewicz, M.; Ekiert, H. Different Types of In vitro Cultures of Schisandra chinensis and Its Cultivar (S. chinensis cv. Sadova): A Rich Potential Source of Specific Lignans and Phenolic Compounds. In Plant Cell and Tissue Differentiation and Secondary Metabolites; Reference Series in Phytochemistry; Ramawat, K., Ekiert, H., Goyal, S., Eds.; Springer: Cham, Switzerland, 2020; pp. 1–28. [Google Scholar]
- Pollini, L.; Rocchi, R.; Cossignani, L.; Mañes, J.; Compagnone, D.; Blasi, F. Phenol Profiling and Nutraceutical Potential of Lycium spp. Leaf Extracts Obtained with Ultrasound and Microwave Assisted Techniques. Antioxidants 2019, 8, 260. [Google Scholar] [CrossRef]
- Williamson, G.; Faulkner, K.; Plumb, G.W. Glucosinolates and phenolics as antioxidants from plant foods. Eur. J. Cancer Prev. 1998, 7, 17–21. [Google Scholar]
- Piazzon, A.; Vrhovsek, U.; Masuero, D.; Mattivi, F.; Mandoj, F.; Nardini, M. Antioxidant activity of phenolic acids and their metabolites: Synthesis and antioxidant properties of the sulfate derivatives of ferulic and caffeic acids and of the acyl glucuronide of ferulic acid. J. Agric. Food Chem. 2012, 60, 12312–12323. [Google Scholar] [CrossRef] [PubMed]
- Grace, S.C. Phenolics as Antioxidants. In Antioxidants and Reactive Oxygen Species in Plants; Blackwell Publishing Ltd.: Oxford, UK, 2007; pp. 141–168. [Google Scholar]
- Roby, M.H.H.; Sarhan, M.A.; Selim, K.A.H.; Khalel, K.I. Evaluation of antioxidant activity, total phenols and phenolic compounds in thyme (Thymus vulgaris L.), sage (Salvia officinalis L.), and marjoram (Origanum majorana L.) extracts. Ind. Crops Prod. 2013, 43, 827–831. [Google Scholar] [CrossRef]
- Saleem, A.; Saleem, M.; Akhtar, M.F. Antioxidant, anti-inflammatory and antiarthritic potential of Moringa oleifera Lam: An ethnomedicinal plant of Moringaceae family. S. Afr. J. Bot. 2020, 128, 246–256. [Google Scholar] [CrossRef]
- Kosar, M.; Bozan, B.; Temelli, F.; Baser, K.H.C. Antioxidant activity and phenolic composition of sumac (Rhus coriaria L.) extracts. Food Chem. 2007, 103, 952–959. [Google Scholar] [CrossRef]
- Elansary, H.O.; Szopa, A.; Kubica, P.; El-Ansary, D.O.; Ekiert, H.; Al-Mana, F.A. Malus baccata var. gracilis and Malus toringoides bark polyphenol studies and antioxidant, antimicrobial and anticancer activities. Processes 2020, 8, 283. [Google Scholar] [CrossRef]
- Fernández-Agulló, A.; Castro-Iglesias, A.; Freire, M.S.; González-Álvarez, J. Optimization of the Extraction of Bioactive Compounds from Walnut (Juglans major 209 x Juglans regia) Leaves: Antioxidant Capacity and Phenolic Profile. Antioxidants 2019, 9, 18. [Google Scholar] [CrossRef]
- Elansary, H.O.; Szopa, A.; Kubica, P.; Ekiert, H.; Mattar, M.A.; Al-Yafrasi, M.A.; El-Ansary, D.O.; Zin El-Abedin, T.K.; Yessoufou, K. Polyphenol profile and pharmaceutical potential of Quercus spp. bark extracts. Plants 2019, 8, 486. [Google Scholar] [CrossRef]
- Lukmanul Hakkim, F.; Gowri Shankar, C.; Girija, S. Chemical composition and antioxidant property of holy basil (Ocimum sanctum L.) leaves, stems, and inflorescence and their in vitro callus cultures. J. Agric. Food Chem. 2007, 55, 9109–9117. [Google Scholar] [CrossRef]
- Costa, P.; Gonçalves, S.; Valentão, P.; Andrade, P.B.; Coelho, N.; Romano, A. Thymus lotocephalus wild plants and in vitro cultures produce different profiles of phenolic compounds with antioxidant activity. Food Chem. 2012, 135, 1253–1260. [Google Scholar] [CrossRef]
- Costa, P.; Gonçalves, S.; Valentão, P.; Andrade, P.B.; Romano, A. Accumulation of phenolic compounds in in vitro cultures and wild plants of Lavandula viridis L’Hér and their antioxidant and anti-cholinesterase potential. Food Chem. Toxicol. 2013, 57, 69–74. [Google Scholar] [CrossRef]
- Kuhlmann, A.; Röhl, C. Phenolic antioxidant compounds produced by in vitro cultures of rosemary (Rosmarinus officinalis) and their anti-inflammatory effect on lipopolysaccharide-activated microglia. Pharm. Biol. 2006, 44, 401–410. [Google Scholar] [CrossRef]
- Taveira, M.; Pereira, D.M.; Sousa, C.; Ferreres, F.; Andrade, P.B.; Martins, A.; Pereira, J.A.; Valentão, P. In vitro cultures of Brassica oleracea L. var costata DC: Potential plant bioreactor for antioxidant phenolic compounds. J. Agric. Food Chem. 2009, 57, 1247–1252. [Google Scholar] [CrossRef] [PubMed]
- Krolicka, A.; Szpitter, A.; Maciag, M.; Biskup, E.; Gilgenast, E.; Wegrzyn, G.; Lojkowska, E. Antibacterial and antioxidant activity of the secondary metabolites from in vitro cultures of the Alice sundew (Drosera aliciae). Biotechnol. Appl. Biochem. 2009, 53, 175–184. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).