MnTE-2-PyP Suppresses Prostate Cancer Cell Growth via H2O2 Production
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture and Reagents
2.2. Animal Husbandry
2.3. Orthotopic Implantation of PC3 Tumor Cells
2.4. Radiotherapy Protocol for Mice
2.5. Tumor Harvesting and Tumor Size Measurement
2.6. Cell Growth and Viability Assays
2.7. Cellular H2O2 Level Measurements
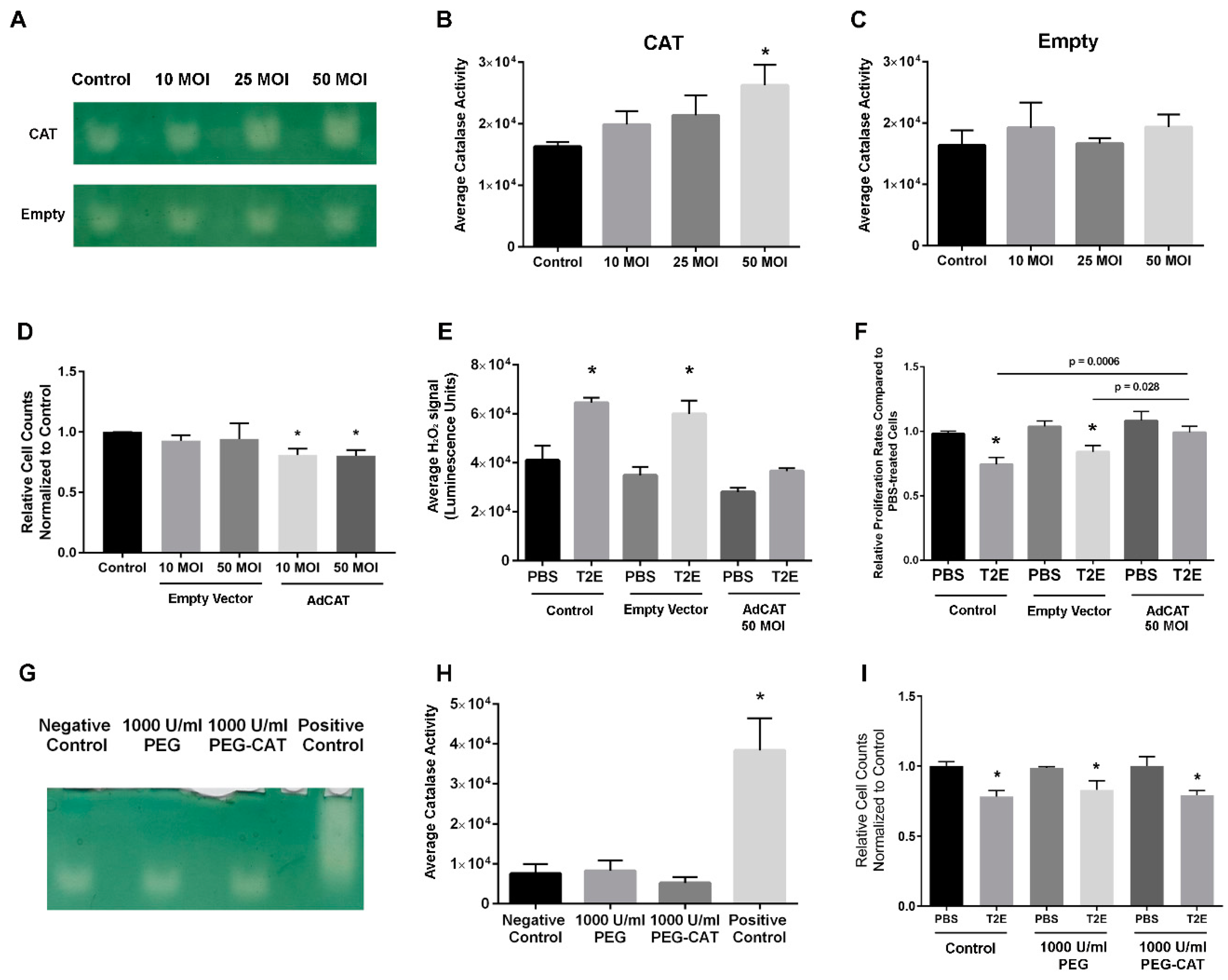
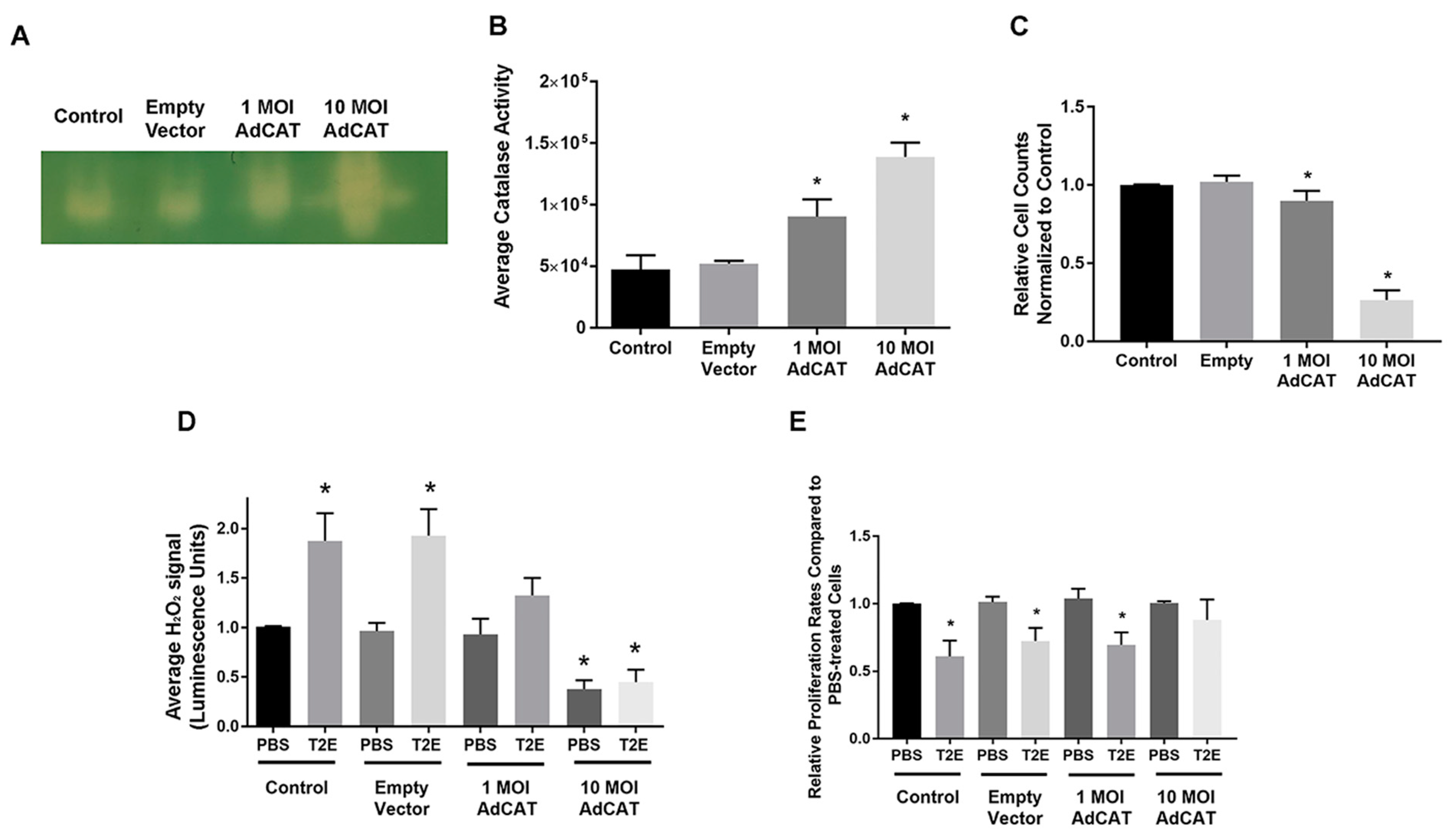
2.8. Altering Intracellular or Extracellular Catalase Activity
2.9. Catalase Activity Measurement
2.10. Thiol Oxidation Detection
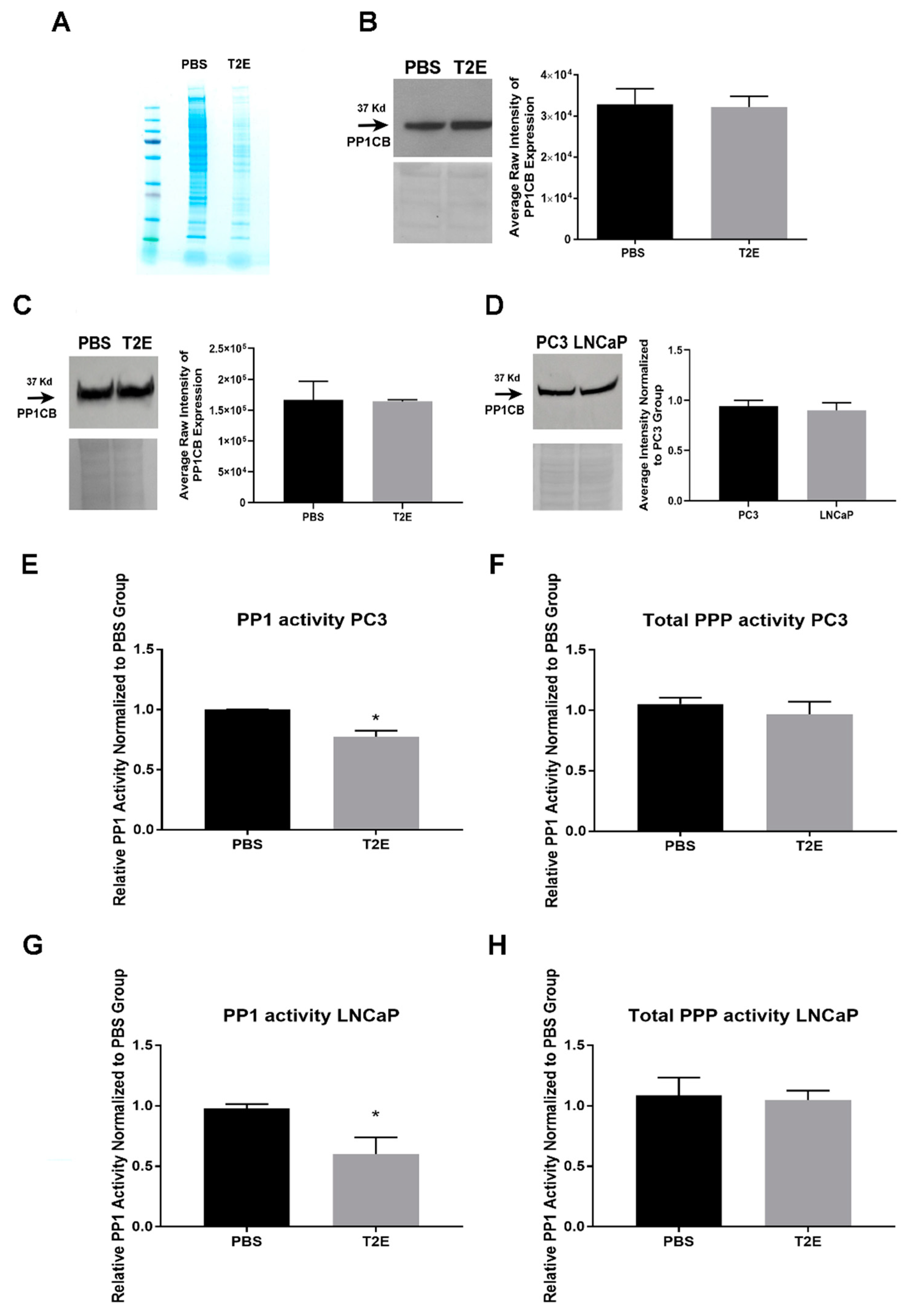
2.11. Ser/Thr Phosphoprotein Phosphatase (PPP) Activity Measurement
2.12. Western Blot Analysis
2.13. Cell Cycle Analysis
2.14. Nuclear Abnormality and DNA Damage Imaging
2.15. Statistical Analysis
3. Results
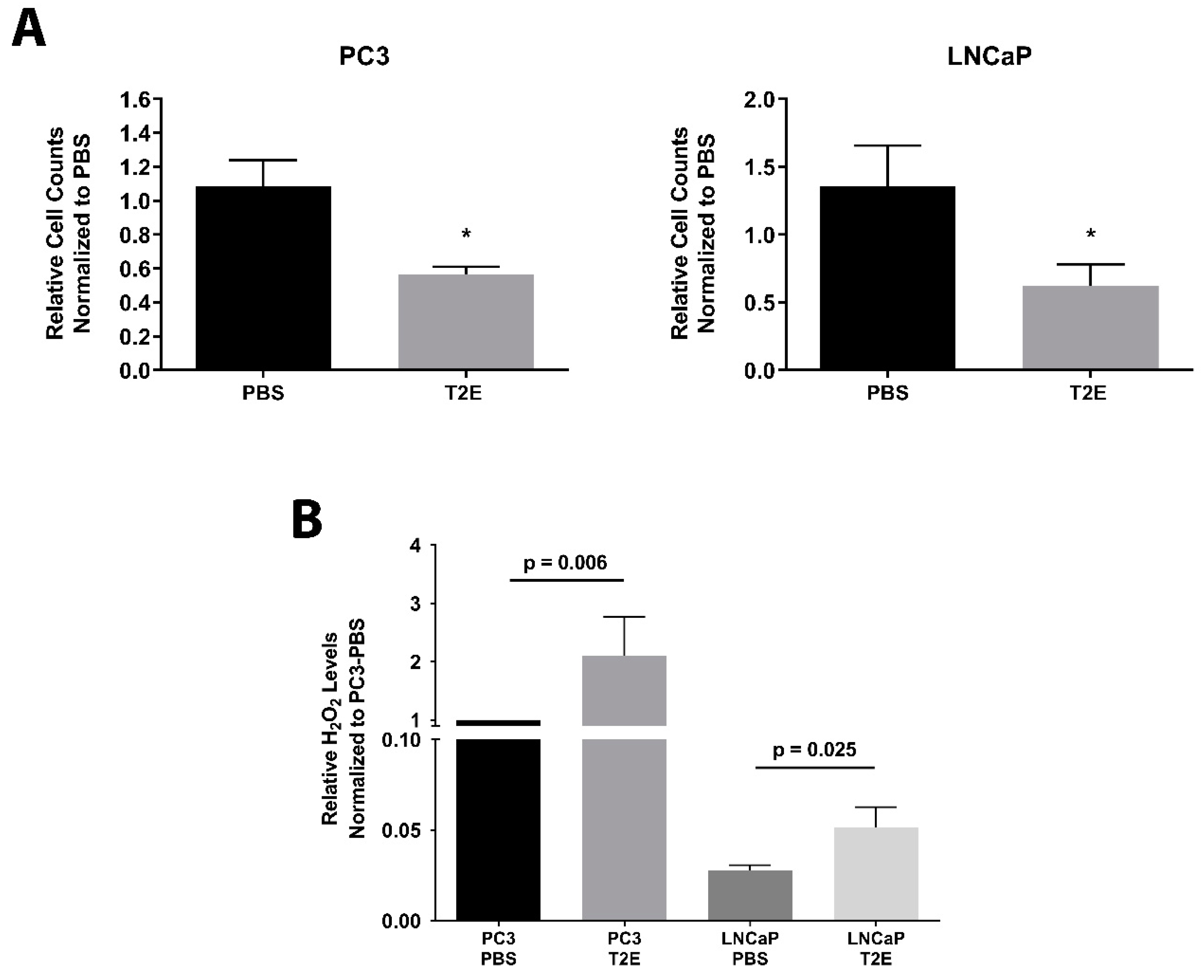
3.1. MnTE-2-PyP Inhibits Prostate Cancer Cell Growth and Enhances H2O2 Levels
3.2. Intracellular H2O2 Balance Is Critical for Prostate Cancer Cell Growth
3.3. MnTE-2-PyP Modulates Thiol Modifications in PC3 Cells
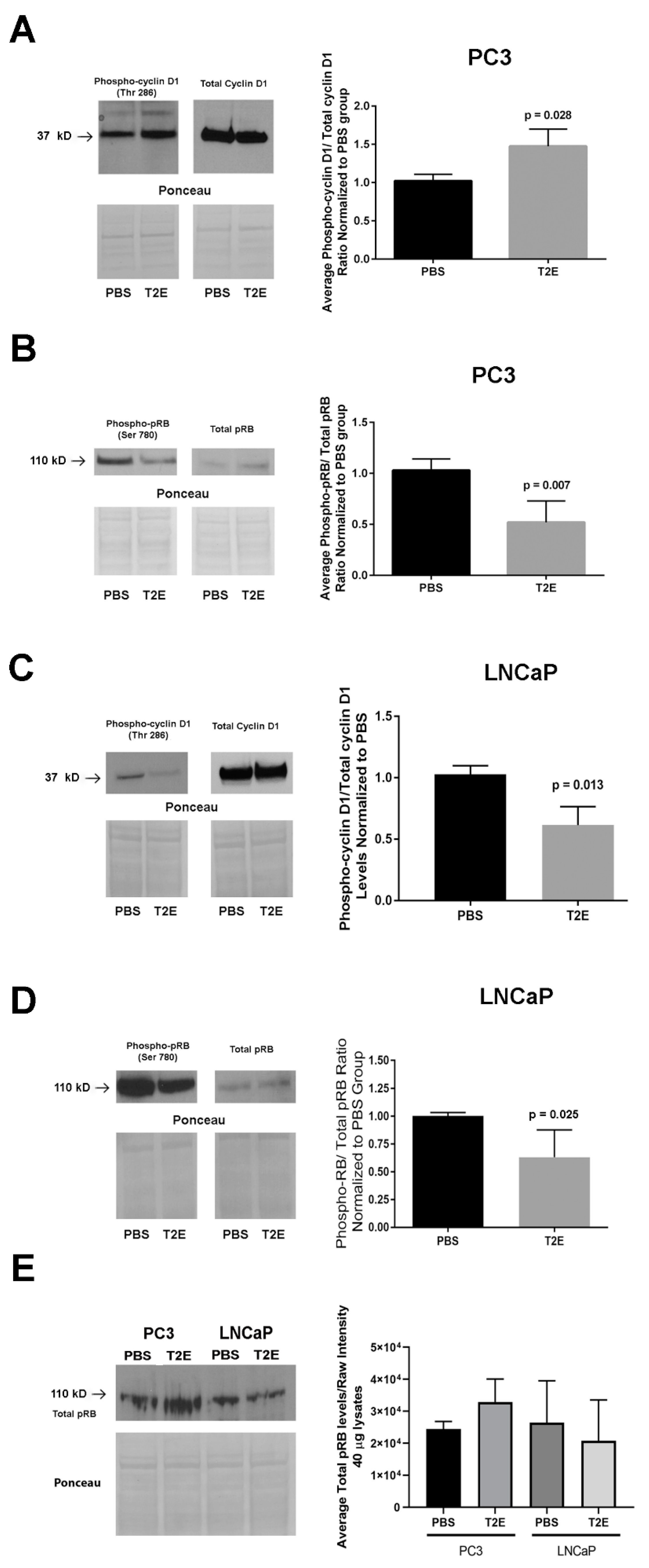
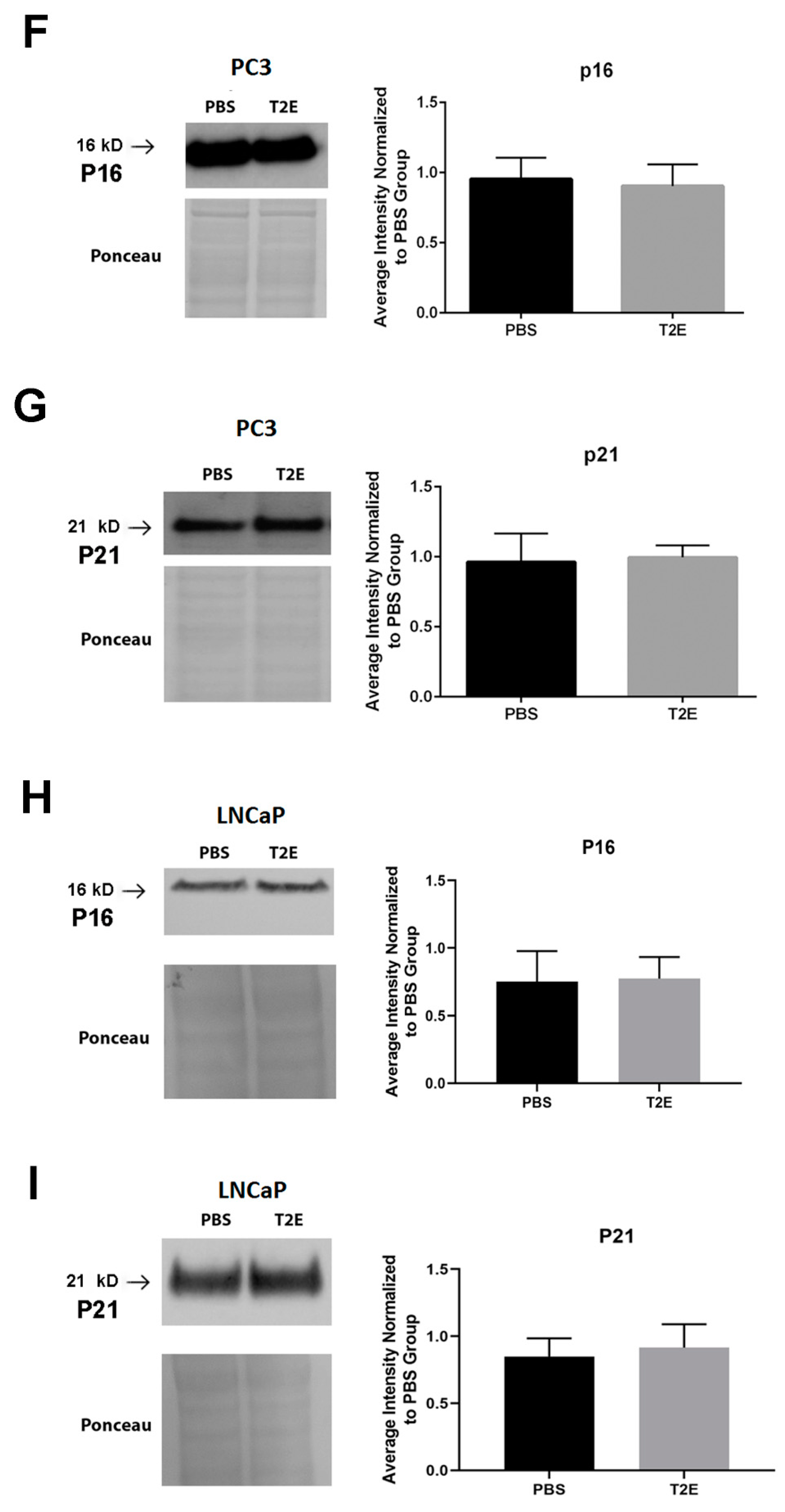
3.4. MnTE-2-PyP Affects PPP-Regulated Pathways
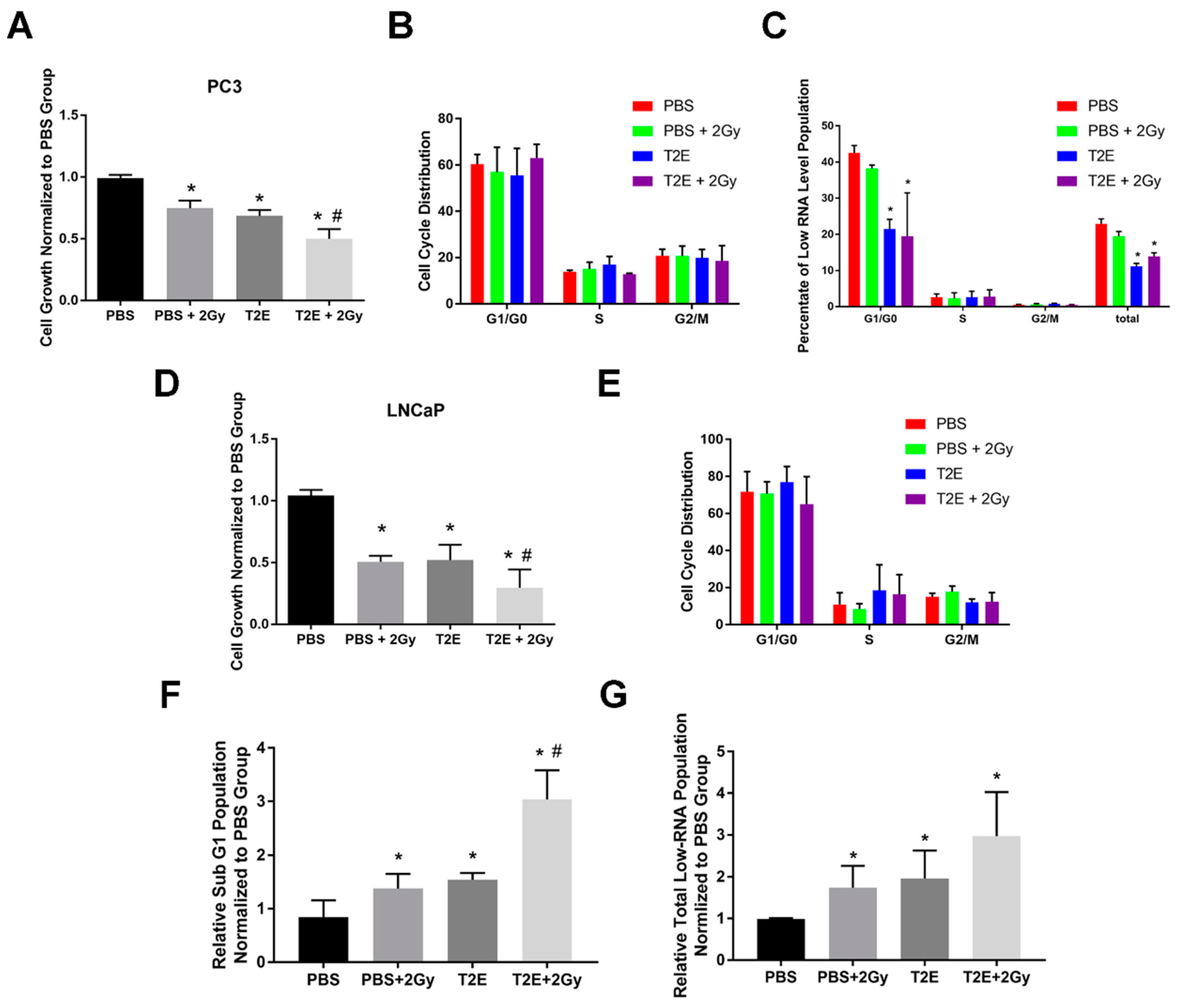
3.5. MnTE-2-PyP Enhances Radiation-Induced Cell Growth Arrest in Prostate Cancer Cells
3.6. MnTE-2-PyP Does Not Affect Overall Cell Cycle Distribution in Prostate Cancer Cells
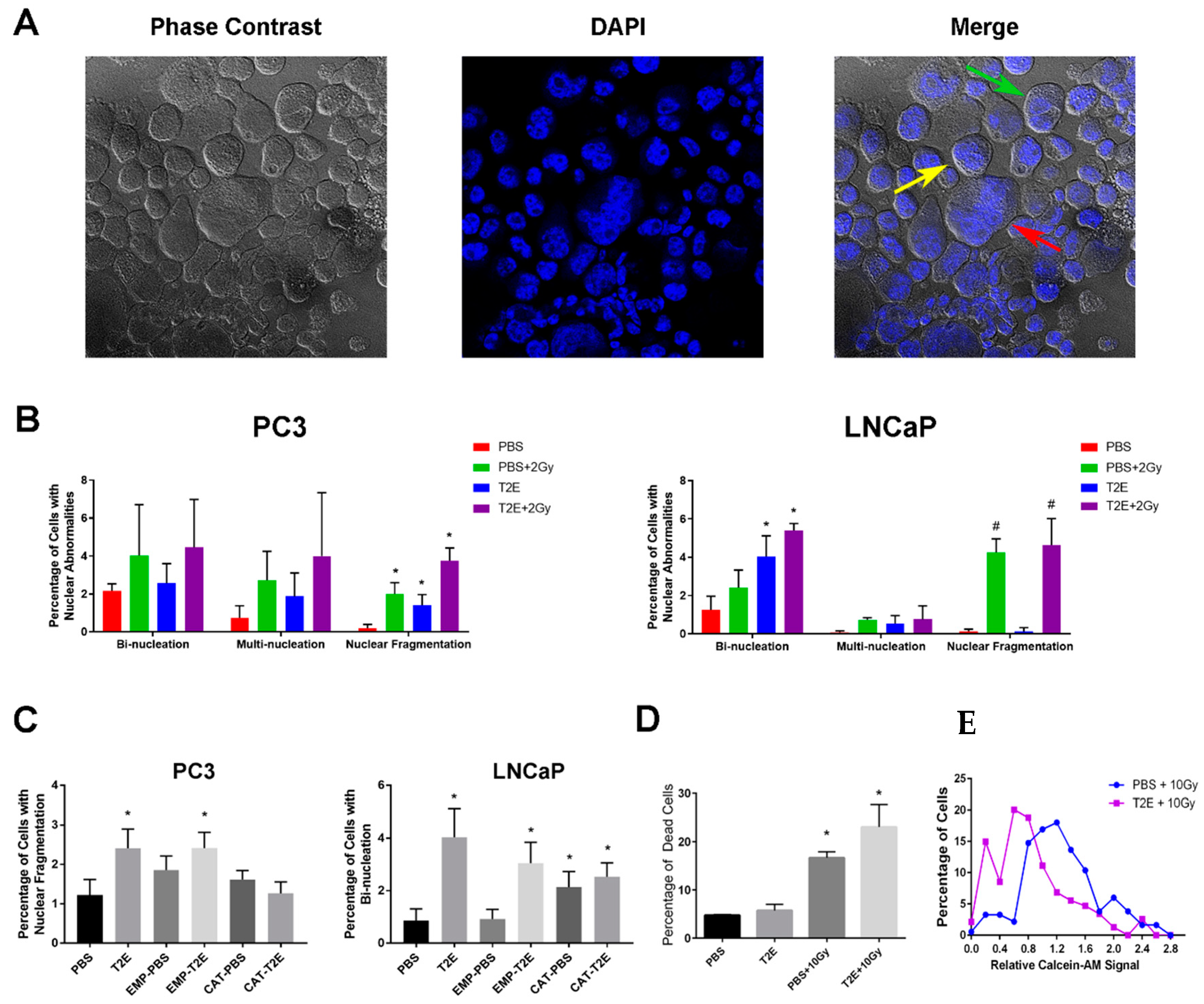
3.7. MnTE-2-PyP Treatment Induces Nuclear Abnormalities in Prostate Cancer Cells
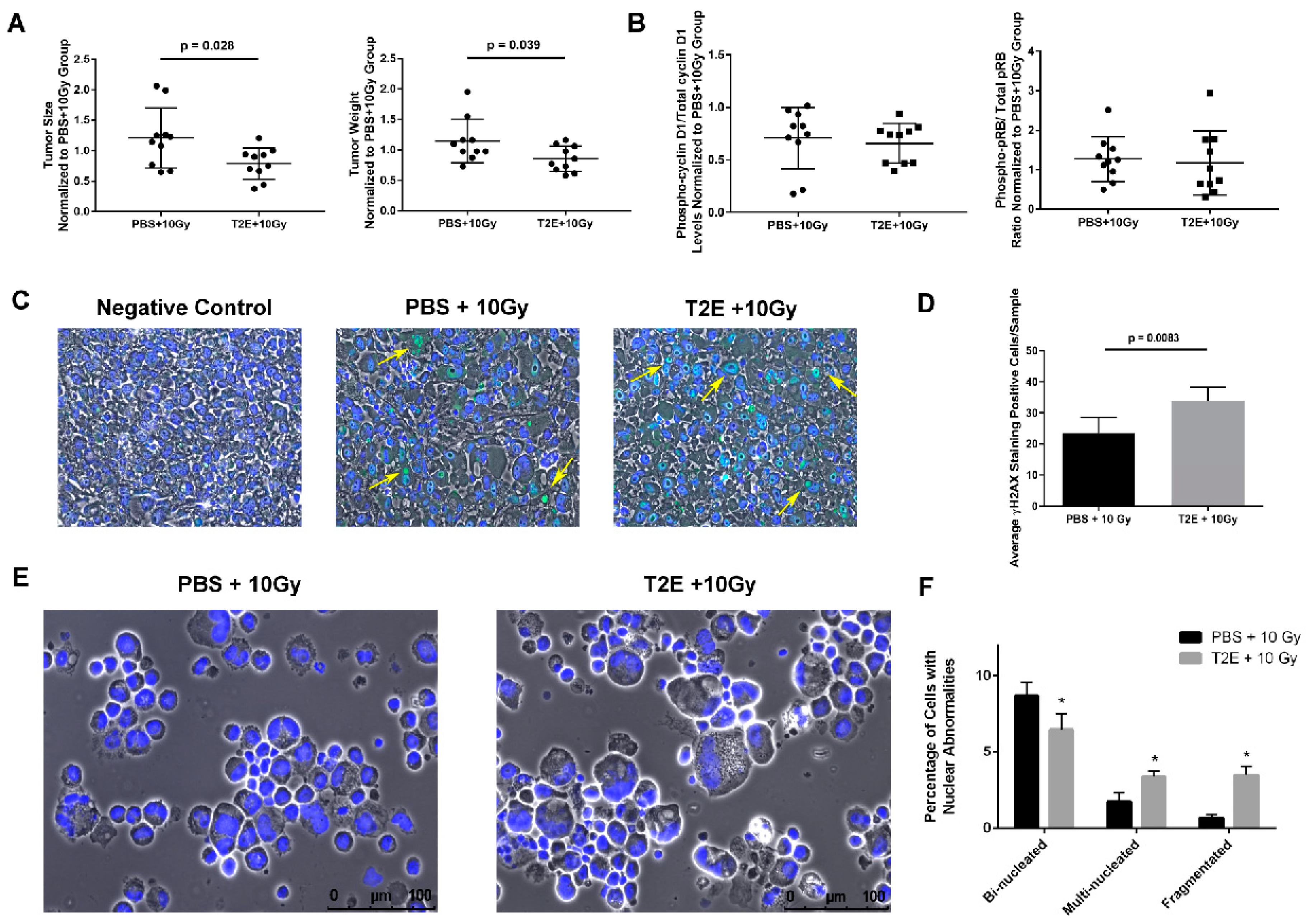
3.8. MnTE-2-PyP treatment induces nuclear abnormalities in PC3 tumors in vivo
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Denmeade, S.R.; Isaacs, J.T. A history of prostate cancer treatment. Nat. Rev. Cancer 2002, 2, 389–396. [Google Scholar] [CrossRef]
- Miller, K.D.; Siegel, R.L.; Lin, C.C.; Mariotto, A.B.; Kramer, J.L.; Rowland, J.H.; Stein, K.D.; Alteri, R.; Jemal, A. Cancer treatment and survivorship statistics, 2016. Ca Cancer J. Clin. 2016, 66, 271–289. [Google Scholar] [CrossRef] [PubMed]
- D'Autreaux, B.; Toledano, M.B. ROS as signalling molecules: mechanisms that generate specificity in ROS homeostasis. Nat. Rev. Mol. Cell Biol. 2007, 8, 813–824. [Google Scholar] [CrossRef] [PubMed]
- Schieber, M.; Chandel, N.S. ROS function in redox signaling and oxidative stress. Curr. Biol. 2014, 24, R453–R462. [Google Scholar] [CrossRef] [PubMed]
- Koukourakis, M.I. Radiation damage and radioprotectants: new concepts in the era of molecular medicine. Br. J. Radiol. 2012, 85, 313–330. [Google Scholar] [CrossRef]
- Alberti, C. Prostate cancer: radioresistance molecular target-related markers and foreseeable modalities of radiosensitization. Eur. Rev. Med. Pharm. Sci. 2014, 18, 2275–2282. [Google Scholar]
- Batinic-Haberle, I.; Tovmasyan, A.; Spasojevic, I. Mn Porphyrin-Based Redox-Active Drugs: Differential Effects as Cancer Therapeutics and Protectors of Normal Tissue Against Oxidative Injury. Antioxid. Redox Signal. 2018, 29, 1691–1724. [Google Scholar] [CrossRef]
- Batinic-Haberle, I.; Cuzzocrea, S.; Reboucas, J.S.; Ferrer-Sueta, G.; Mazzon, E.; Di Paola, R.; Radi, R.; Spasojevic, I.; Benov, L.; Salvemini, D. Pure MnTBAP selectively scavenges peroxynitrite over superoxide: comparison of pure and commercial MnTBAP samples to MnTE-2-PyP in two models of oxidative stress injury, an SOD-specific Escherichia coli model and carrageenan-induced pleurisy. Free Radic. Biol. Med. 2009, 46, 192–201. [Google Scholar] [CrossRef]
- Batinic-Haberle, I.; Reboucas, J.S.; Spasojevic, I. Superoxide dismutase mimics: chemistry, pharmacology, and therapeutic potential. Antioxid. Redox Signal. 2010, 13, 877–918. [Google Scholar] [CrossRef]
- Archambeau, J.O.; Tovmasyan, A.; Pearlstein, R.D.; Crapo, J.D.; Batinic-Haberle, I. Superoxide dismutase mimic, MnTE-2-PyP(5+) ameliorates acute and chronic proctitis following focal proton irradiation of the rat rectum. Redox Biol. 2013, 1, 599–607. [Google Scholar] [CrossRef]
- Oberley-Deegan, R.E.; Steffan, J.J.; Rove, K.O.; Pate, K.M.; Weaver, M.W.; Spasojevic, I.; Frederick, B.; Raben, D.; Meacham, R.B.; Crapo, J.D.; et al. The antioxidant, MnTE-2-PyP, prevents side-effects incurred by prostate cancer irradiation. PLoS ONE 2012, 7, e44178. [Google Scholar] [CrossRef] [PubMed]
- Barbosa, A.M.; Sarmento-Neto, J.F.; Menezes Filho, J.E.R.; Jesus, I.C.G.; Souza, D.S.; Vasconcelos, V.M.N.; Gomes, F.D.L.; Lara, A.; Araujo, J.S.S.; Mattos, S.S.; et al. Redox-Active Drug, MnTE-2-PyP(5+), Prevents and Treats Cardiac Arrhythmias Preserving Heart Contractile Function. Oxidative Med. Cell. Longev. 2020, 2020, 4850697. [Google Scholar] [CrossRef] [PubMed]
- Gad, S.C.; Sullivan, D.W., Jr.; Mujer, C.V.; Spainhour, C.B.; Crapo, J.D. Nonclinical Safety and Toxicokinetics of MnTE-2-PyP (BMX-010), a Topical Agent in Phase 2 Trials for Psoriasis and Atopic Dermatitis. Int. J. Toxicol. 2019, 38, 291–302. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, A.; Zhu, Y.; Tong, Q.; Kosmacek, E.A.; Lichter, E.Z.; Oberley-Deegan, R.E. The Addition of Manganese Porphyrins during Radiation Inhibits Prostate Cancer Growth and Simultaneously Protects Normal Prostate Tissue from Radiation Damage. Antioxidants 2018, 7, 21. [Google Scholar] [CrossRef]
- Tong, Q.; Weaver, M.R.; Kosmacek, E.A.; O'Connor, B.P.; Harmacek, L.; Venkataraman, S.; Oberley-Deegan, R.E. MnTE-2-PyP reduces prostate cancer growth and metastasis by suppressing p300 activity and p300/HIF-1/CREB binding to the promoter region of the PAI-1 gene. Free Radic. Biol. Med. 2016, 94, 185–194. [Google Scholar] [CrossRef]
- Miao, L.; St Clair, D.K. Regulation of superoxide dismutase genes: implications in disease. Free Radic. Biol. Med. 2009, 47, 344–356. [Google Scholar] [CrossRef]
- Sarsour, E.H.; Kalen, A.L.; Goswami, P.C. Manganese superoxide dismutase regulates a redox cycle within the cell cycle. Antioxid. Redox Signal. 2014, 20, 1618–1627. [Google Scholar] [CrossRef]
- Oberley, L.W.; Buettner, G.R. Role of superoxide dismutase in cancer: a review. Cancer Res. 1979, 39, 1141–1149. [Google Scholar]
- Oberley, L.W.; Oberley, T.D.; Buettner, G.R. Cell differentiation, aging and cancer: the possible roles of superoxide and superoxide dismutases. Med. Hypotheses 1980, 6, 249–268. [Google Scholar] [CrossRef]
- Venkataraman, S.; Jiang, X.; Weydert, C.; Zhang, Y.; Zhang, H.J.; Goswami, P.C.; Ritchie, J.M.; Oberley, L.W.; Buettner, G.R. Manganese superoxide dismutase overexpression inhibits the growth of androgen-independent prostate cancer cells. Oncogene 2005, 24, 77–89. [Google Scholar] [CrossRef]
- Zmijewski, J.W.; Banerjee, S.; Bae, H.; Friggeri, A.; Lazarowski, E.R.; Abraham, E. Exposure to hydrogen peroxide induces oxidation and activation of AMP-activated protein kinase. J. Biol. Chem. 2010, 285, 33154–33164. [Google Scholar] [CrossRef] [PubMed]
- Veal, E.A.; Day, A.M.; Morgan, B.A. Hydrogen peroxide sensing and signaling. Mol. Cell. 2007, 26, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Sikka, S.C. Role of oxidative stress response elements and antioxidants in prostate cancer pathobiology and chemoprevention--a mechanistic approach. Curr. Med. Chem. 2003, 10, 2679–2692. [Google Scholar] [CrossRef] [PubMed]
- Oh, B.; Figtree, G.; Costa, D.; Eade, T.; Hruby, G.; Lim, S.; Elfiky, A.; Martine, N.; Rosenthal, D.; Clarke, S.; et al. Oxidative stress in prostate cancer patients: A systematic review of case control studies. Prostate Int. 2016, 4, 71–87. [Google Scholar] [CrossRef]
- Arsova-Sarafinovska, Z.; Eken, A.; Matevska, N.; Erdem, O.; Sayal, A.; Savaser, A.; Banev, S.; Petrovski, D.; Dzikova, S.; Georgiev, V.; et al. Increased oxidative/nitrosative stress and decreased antioxidant enzyme activities in prostate cancer. Clin. Biochem. 2009, 42, 1228–1235. [Google Scholar] [CrossRef]
- Baker, A.M.; Oberley, L.W.; Cohen, M.B. Expression of antioxidant enzymes in human prostatic adenocarcinoma. Prostate 1997, 32, 229–233. [Google Scholar] [CrossRef]
- Wang, C.D.; Sun, Y.; Chen, N.; Huang, L.; Huang, J.W.; Zhu, M.; Wang, T.; Ji, Y.L. The Role of Catalase C262T Gene Polymorphism in the Susceptibility and Survival of Cancers. Sci. Rep. 2016, 6, 26973. [Google Scholar] [CrossRef]
- Liwei, L.; Chunyu, L.; Ruifa, H. Association between manganese superoxide dismutase gene polymorphism and risk of prostate cancer: a meta-analysis. Urology 2009, 74, 884–888. [Google Scholar] [CrossRef]
- Kang, D.; Lee, K.M.; Park, S.K.; Berndt, S.I.; Peters, U.; Reding, D.; Chatterjee, N.; Welch, R.; Chanock, S.; Huang, W.Y.; et al. Functional variant of manganese superoxide dismutase (SOD2 V16A) polymorphism is associated with prostate cancer risk in the prostate, lung, colorectal, and ovarian cancer study. Cancer Epidemiol. Biomark. Prev. 2007, 16, 1581–1586. [Google Scholar] [CrossRef]
- Kumar, B.; Koul, S.; Khandrika, L.; Meacham, R.B.; Koul, H.K. Oxidative stress is inherent in prostate cancer cells and is required for aggressive phenotype. Cancer Res. 2008, 68, 1777–1785. [Google Scholar] [CrossRef]
- Tong, Q.; Zhu, Y.; Galaske, J.W.; Kosmacek, E.A.; Chatterjee, A.; Dickinson, B.C.; Oberley-Deegan, R.E. MnTE-2-PyP modulates thiol oxidation in a hydrogen peroxide-mediated manner in a human prostate cancer cell. Free Radic. Biol. Med. 2016, 101, 32–43. [Google Scholar] [CrossRef]
- Swingle, M.; Ni, L.; Honkanen, R.E. Small-molecule inhibitors of ser/thr protein phosphatases: specificity, use and common forms of abuse. Methods Mol. Biol. 2007, 365, 23–38. [Google Scholar] [PubMed]
- Arnold, R.S.; Shi, J.; Murad, E.; Whalen, A.M.; Sun, C.Q.; Polavarapu, R.; Parthasarathy, S.; Petros, J.A.; Lambeth, J.D. Hydrogen peroxide mediates the cell growth and transformation caused by the mitogenic oxidase Nox1. Proc. Natl. Acad. Sci. USA 2001, 98, 5550–5555. [Google Scholar] [CrossRef] [PubMed]
- Preston, T.J.; Muller, W.J.; Singh, G. Scavenging of extracellular H2O2 by catalase inhibits the proliferation of HER-2/Neu-transformed rat-1 fibroblasts through the induction of a stress response. J. Biol. Chem. 2001, 276, 9558–9564. [Google Scholar] [CrossRef] [PubMed]
- Smit-de Vries, M.P.; van der Toorn, M.; Bischoff, R.; Kauffman, H.F. Resistance of quiescent and proliferating airway epithelial cells to H2O2 challenge. Eur. Respir. J. 2007, 29, 633–642. [Google Scholar] [CrossRef]
- Santos, C.X.; Hafstad, A.D.; Beretta, M.; Zhang, M.; Molenaar, C.; Kopec, J.; Fotinou, D.; Murray, T.V.; Cobb, A.M.; Martin, D.; et al. Targeted redox inhibition of protein phosphatase 1 by Nox4 regulates eIF2alpha-mediated stress signaling. Embo J. 2016, 35, 319–334. [Google Scholar] [CrossRef]
- Zhang, H.; Ma, Y.; Liu, K.; Yu, J.G. Theoretical studies on the reaction mechanism of PP1 and the effects of different oxidation states of the Mn-Mn center on the mechanism. J. Biol. Inorg. Chem. 2013, 18, 451–459. [Google Scholar] [CrossRef]
- Virshup, D.M.; Shenolikar, S. From promiscuity to precision: protein phosphatases get a makeover. Mol. Cell 2009, 33, 537–545. [Google Scholar] [CrossRef]
- Kolupaeva, V.; Janssens, V. PP1 and PP2A phosphatases--cooperating partners in modulating retinoblastoma protein activation. FEBS J. 2013, 280, 627–643. [Google Scholar] [CrossRef]
- Cohen, P.T. Protein phosphatase 1--targeted in many directions. J. Cell Sci. 2002, 115 Pt 2, 241–256. [Google Scholar]
- Kim, H.S.; Fernandes, G.; Lee, C.W. Protein Phosphatases Involved in Regulating Mitosis: Facts and Hypotheses. Mol. Cells 2016, 39, 654–662. [Google Scholar] [CrossRef] [PubMed]
- Hsu, L.C.; Huang, X.; Seasholtz, S.; Potter, D.M.; Gollin, S.M. Gene amplification and overexpression of protein phosphatase 1alpha in oral squamous cell carcinoma cell lines. Oncogene 2006, 25, 5517–5526. [Google Scholar] [CrossRef] [PubMed]
- Edelson, J.R.; Brautigan, D.L. The Discodermia calyx toxin calyculin a enhances cyclin D1 phosphorylation and degradation, and arrests cell cycle progression in human breast cancer cells. Toxins (Basel) 2011, 3, 105–119. [Google Scholar] [CrossRef] [PubMed]
- Cicchillitti, L.; Fasanaro, P.; Biglioli, P.; Capogrossi, M.C.; Martelli, F. Oxidative stress induces protein phosphatase 2A-dependent dephosphorylation of the pocket proteins pRb, p107, and p130. J. Biol. Chem. 2003, 278, 19509–19517. [Google Scholar] [CrossRef] [PubMed]
- Junttila, M.R.; Puustinen, P.; Niemela, M.; Ahola, R.; Arnold, H.; Bottzauw, T.; Ala-aho, R.; Nielsen, C.; Ivaska, J.; Taya, Y.; et al. CIP2A inhibits PP2A in human malignancies. Cell 2007, 130, 51–62. [Google Scholar] [CrossRef] [PubMed]
- Remmerie, M.; Janssens, V. PP2A: A Promising Biomarker and Therapeutic Target in Endometrial Cancer. Front. Oncol. 2019, 9, 462. [Google Scholar] [CrossRef]
- Alt, J.R.; Cleveland, J.L.; Hannink, M.; Diehl, J.A. Phosphorylation-dependent regulation of cyclin D1 nuclear export and cyclin D1-dependent cellular transformation. Genes Dev. 2000, 14, 3102–3114. [Google Scholar] [CrossRef]
- Guo, Y.; Yang, K.; Harwalkar, J.; Nye, J.M.; Mason, D.R.; Garrett, M.D.; Hitomi, M.; Stacey, D.W. Phosphorylation of cyclin D1 at Thr 286 during S phase leads to its proteasomal degradation and allows efficient DNA synthesis. Oncogene 2005, 24, 2599–2612. [Google Scholar] [CrossRef]
- Kusume, T.; Tsuda, H.; Kawabata, M.; Inoue, T.; Umesaki, N.; Suzuki, T.; Yamamoto, K. The p16-cyclin D1/CDK4-pRb pathway and clinical outcome in epithelial ovarian cancer. Clin. Cancer Res. 1999, 5, 4152–4157. [Google Scholar]
- Dai, M.; Al-Odaini, A.A.; Fils-Aime, N.; Villatoro, M.A.; Guo, J.; Arakelian, A.; Rabbani, S.A.; Ali, S.; Lebrun, J.J. Cyclin D1 cooperates with p21 to regulate TGFbeta-mediated breast cancer cell migration and tumor local invasion. Breast Cancer Res. 2013, 15, R49. [Google Scholar] [CrossRef]
- Stein, G.H.; Drullinger, L.F.; Soulard, A.; Dulic, V. Differential roles for cyclin-dependent kinase inhibitors p21 and p16 in the mechanisms of senescence and differentiation in human fibroblasts. Mol. Cell Biol. 1999, 19, 2109–2117. [Google Scholar] [CrossRef] [PubMed]
- Shrishrimal, S.; Kosmacek, E.A.; Chatterjee, A.; Tyson, M.J.; Oberley-Deegan, R.E. The SOD Mimic, MnTE-2-PyP, Protects from Chronic Fibrosis and Inflammation in Irradiated Normal Pelvic Tissues. Antioxidants 2017, 6, 87. [Google Scholar] [CrossRef] [PubMed]
- Thyagarajan, A.; Sahu, R.P. Potential Contributions of Antioxidants to Cancer Therapy: Immunomodulation and Radiosensitization. Integr. Cancer 2018, 17, 210–216. [Google Scholar] [CrossRef] [PubMed]
- Sznarkowska, A.; Kostecka, A.; Meller, K.; Bielawski, K.P. Inhibition of cancer antioxidant defense by natural compounds. Oncotarget 2017, 8, 15996–16016. [Google Scholar] [CrossRef]
- Kong, Q.; Lillehei, K.O. Antioxidant inhibitors for cancer therapy. Med. Hypotheses 1998, 51, 405–409. [Google Scholar] [CrossRef]
- Li, P.; Wu, M.; Wang, J.; Sui, Y.; Liu, S.; Shi, D. NAC selectively inhibit cancer telomerase activity: A higher redox homeostasis threshold exists in cancer cells. Redox Biol. 2016, 8, 91–97. [Google Scholar] [CrossRef]
- Wu, C.W.; Liu, H.C.; Yu, Y.L.; Hung, Y.T.; Wei, C.W.; Yiang, G.T. Combined treatment with vitamin C and methotrexate inhibits triple-negative breast cancer cell growth by increasing H2O2 accumulation and activating caspase-3 and p38 pathways. Oncol. Rep. 2017, 37, 2177–2184. [Google Scholar] [CrossRef]
- Wegman-Points, L.J.; Teoh-Fitzgerald, M.L.; Mao, G.; Zhu, Y.; Fath, M.A.; Spitz, D.R.; Domann, F.E. Retroviral-infection increases tumorigenic potential of MDA-MB-231 breast carcinoma cells by expanding an aldehyde dehydrogenase (ALDH1) positive stem-cell like population. Redox Biol. 2014, 2, 847–854. [Google Scholar] [CrossRef][Green Version]
- Hahm, E.R.; Sakao, K.; Singh, S.V. Honokiol activates reactive oxygen species-mediated cytoprotective autophagy in human prostate cancer cells. Prostate 2014, 74, 1209–1221. [Google Scholar] [CrossRef]
- Hyoudou, K.; Nishikawa, M.; Umeyama, Y.; Kobayashi, Y.; Yamashita, F.; Hashida, M. Inhibition of metastatic tumor growth in mouse lung by repeated administration of polyethylene glycol-conjugated catalase: quantitative analysis with firefly luciferase-expressing melanoma cells. Clin. Cancer Res. 2004, 10, 7685–7691. [Google Scholar] [CrossRef]
- Chen, X.; Ko, S.K.; Kim, M.J.; Shin, I.; Yoon, J. A thiol-specific fluorescent probe and its application for bioimaging. Chem. Commun. (Camb) 2010, 46, 2751–2753. [Google Scholar] [CrossRef] [PubMed]
- Langford, T.F.; Huang, B.K.; Lim, J.B.; Moon, S.J.; Sikes, H.D. Monitoring the action of redox-directed cancer therapeutics using a human peroxiredoxin-2-based probe. Nat. Commun. 2018, 9, 3145. [Google Scholar] [CrossRef] [PubMed]
- Seo, Y.H.; Carroll, K.S. Profiling protein thiol oxidation in tumor cells using sulfenic acid-specific antibodies. Proc. Natl. Acad. Sci. USA 2009, 106, 16163–16168. [Google Scholar] [CrossRef] [PubMed]
- Shakir, S.; Vinh, J.; Chiappetta, G. Quantitative analysis of the cysteine redoxome by iodoacetyl tandem mass tags. Anal. Bioanal. Chem. 2017, 409, 3821–3830. [Google Scholar] [CrossRef]
- Zambrano, C.A.; Egana, J.T.; Nunez, M.T.; Maccioni, R.B.; Gonzalez-Billault, C. Oxidative stress promotes tau dephosphorylation in neuronal cells: the roles of cdk5 and PP1. Free Radic. Biol. Med. 2004, 36, 1393–1402. [Google Scholar] [CrossRef] [PubMed]
- Kurimchak, A.; Grana, X. PP2A Counterbalances Phosphorylation of pRB and Mitotic Proteins by Multiple CDKs: Potential Implications for PP2A Disruption in Cancer. Genes Cancer 2012, 3, 739–748. [Google Scholar] [CrossRef]
- Roche, B.; Arcangioli, B.; Martienssen, R. Transcriptional reprogramming in cellular quiescence. RNA Biol. 2017, 14, 843–853. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, Y.; Kosmacek, E.A.; Chatterjee, A.; Oberley-Deegan, R.E. MnTE-2-PyP Suppresses Prostate Cancer Cell Growth via H2O2 Production. Antioxidants 2020, 9, 490. https://doi.org/10.3390/antiox9060490
Zhu Y, Kosmacek EA, Chatterjee A, Oberley-Deegan RE. MnTE-2-PyP Suppresses Prostate Cancer Cell Growth via H2O2 Production. Antioxidants. 2020; 9(6):490. https://doi.org/10.3390/antiox9060490
Chicago/Turabian StyleZhu, Yuxiang, Elizabeth A. Kosmacek, Arpita Chatterjee, and Rebecca E. Oberley-Deegan. 2020. "MnTE-2-PyP Suppresses Prostate Cancer Cell Growth via H2O2 Production" Antioxidants 9, no. 6: 490. https://doi.org/10.3390/antiox9060490
APA StyleZhu, Y., Kosmacek, E. A., Chatterjee, A., & Oberley-Deegan, R. E. (2020). MnTE-2-PyP Suppresses Prostate Cancer Cell Growth via H2O2 Production. Antioxidants, 9(6), 490. https://doi.org/10.3390/antiox9060490



