Anti-Inflammatory and Reactive Oxygen Species Suppression through Aspirin Pretreatment to Treat Hyperoxia-Induced Acute Lung Injury in NF-κB–Luciferase Inducible Transgenic Mice
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Murine Model of Hyperoxia-Induced Acute Lung Injury
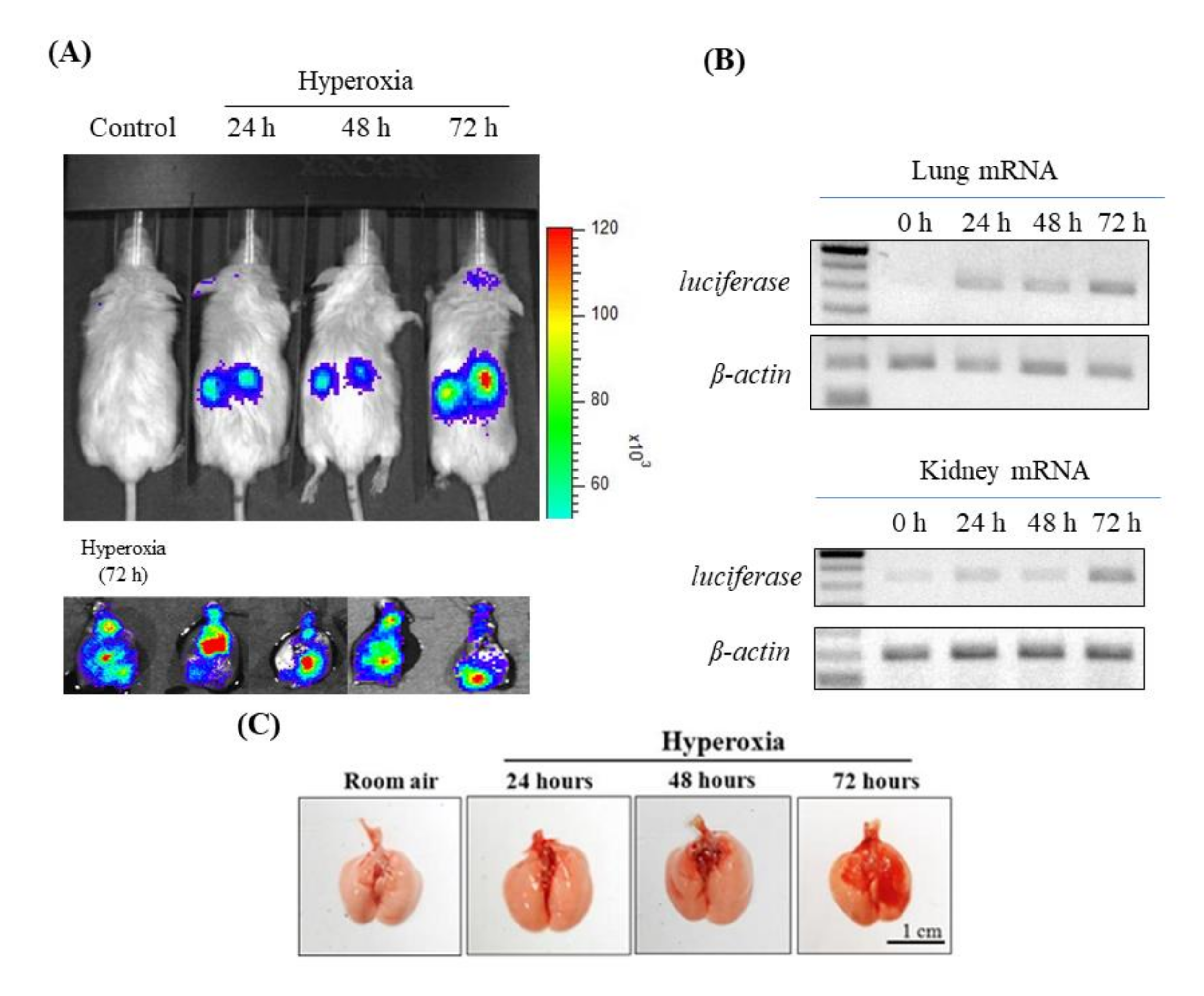
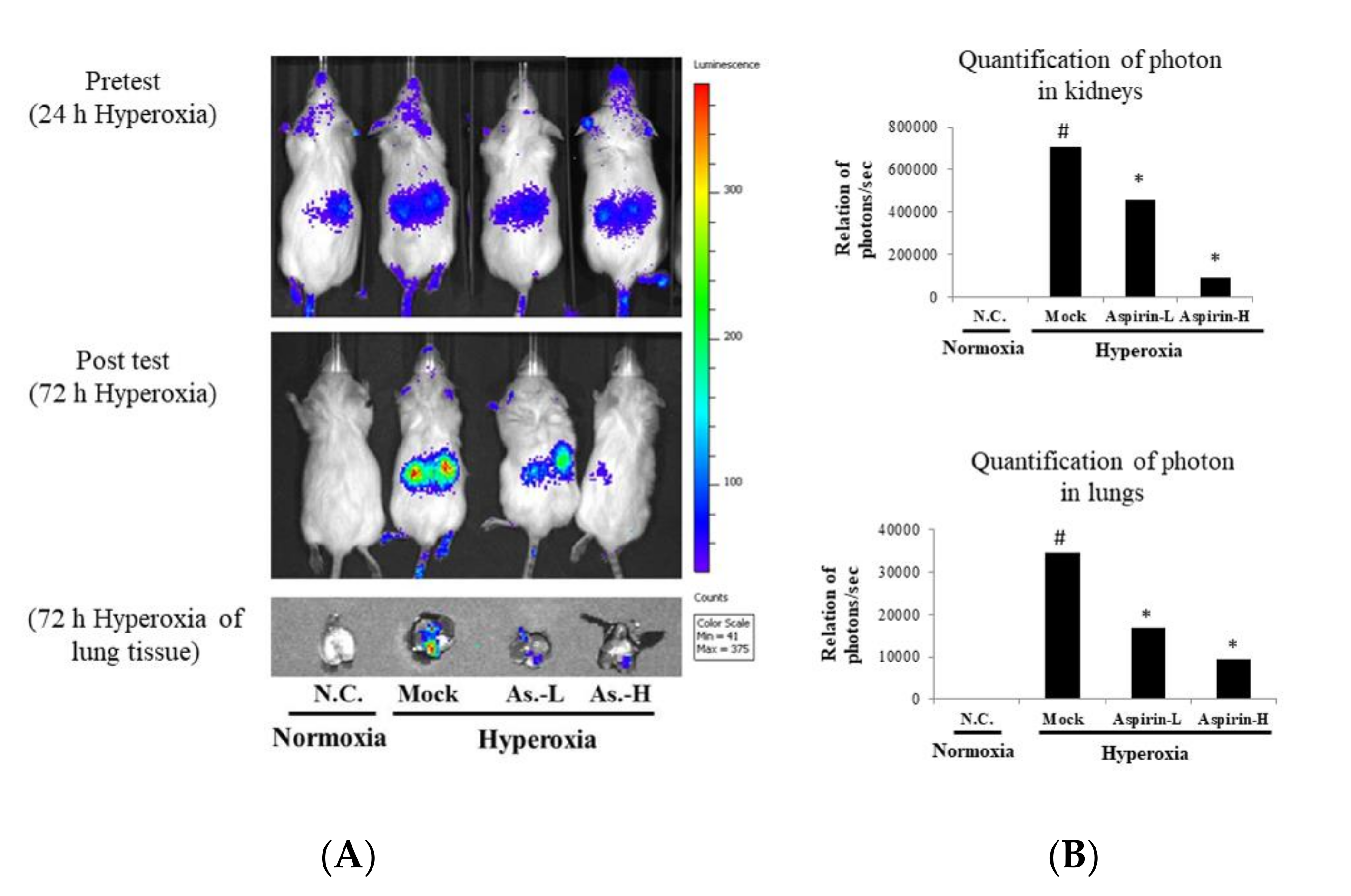
2.3. Imaging of Luciferase Activity
2.4. Reverse Transcription Polymerase Chain Reaction
2.5. Western Blot Analysis
2.6. Pathological Histology
2.7. Measurement of ROS Generation
2.8. Analysis of Airway Inflammation in Bronchoalveolar Lavage Fluid
2.9. Statistical Analysis
3. Results
3.1. Time Course of Lung Damage Induced by Hyperoxia in Mouse Lung in NF-κB-Luciferase+/+ Transgenic Mice
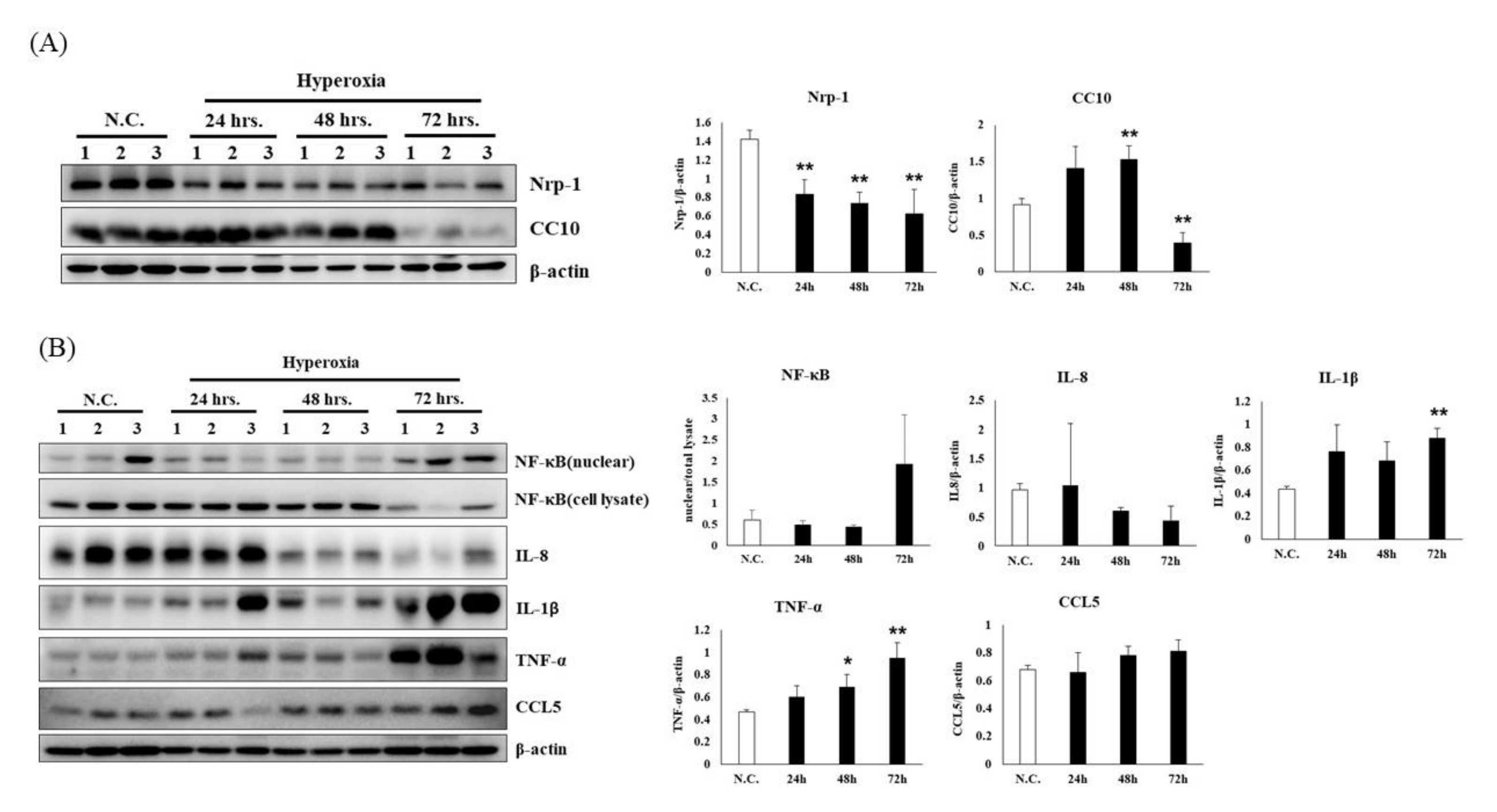
3.2. Time Course of Hyperoxia in Relation to Alveolar Injury and Inflammation in NF-κB-Luciferase+/+ Transgenic Mice
3.3. Inhibition of Hyperoxia-Induced NF-κB Activation by Using Aspirin Pretreatment in NF-κB–Luciferase+/+ Transgenic Mice
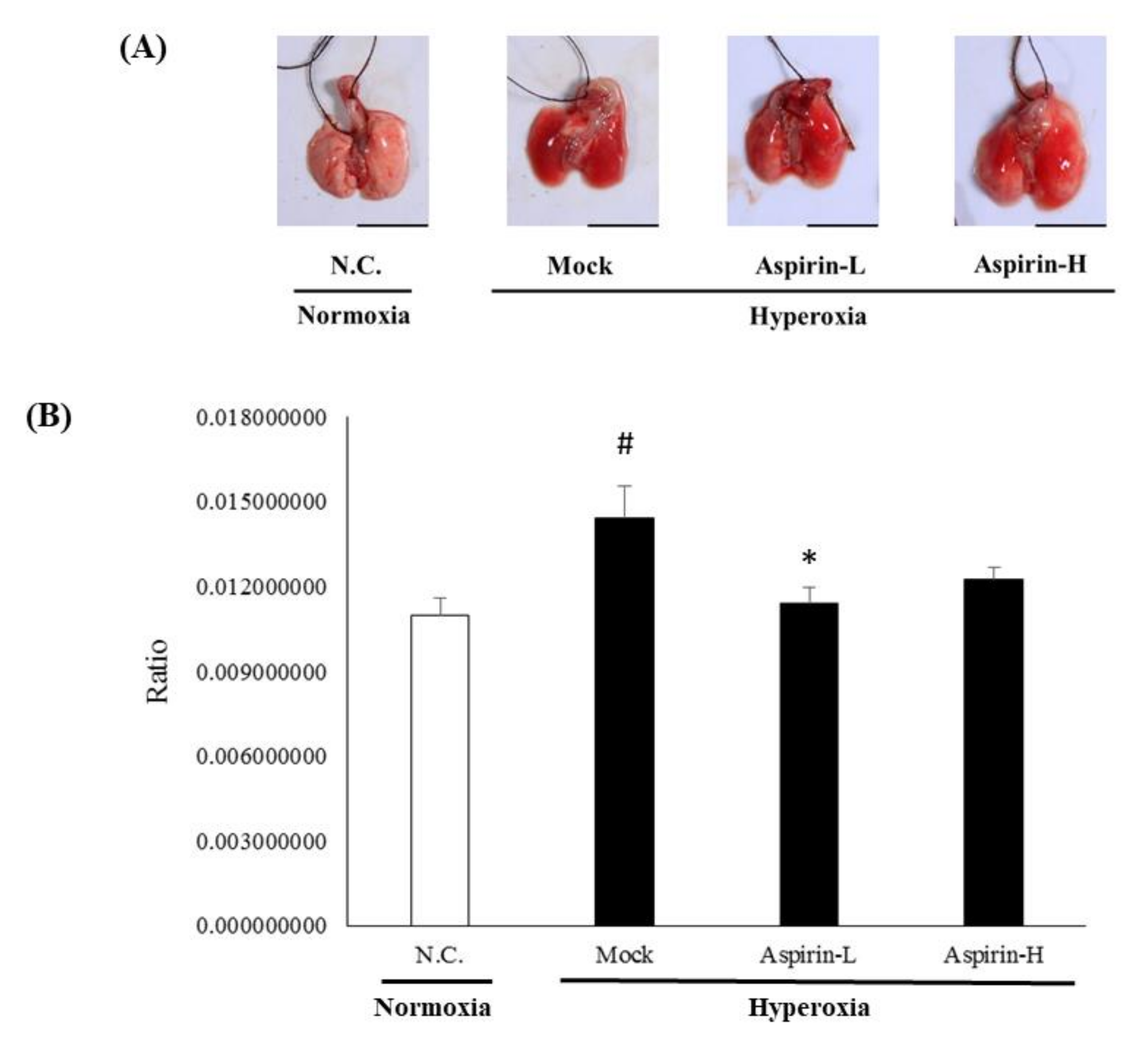
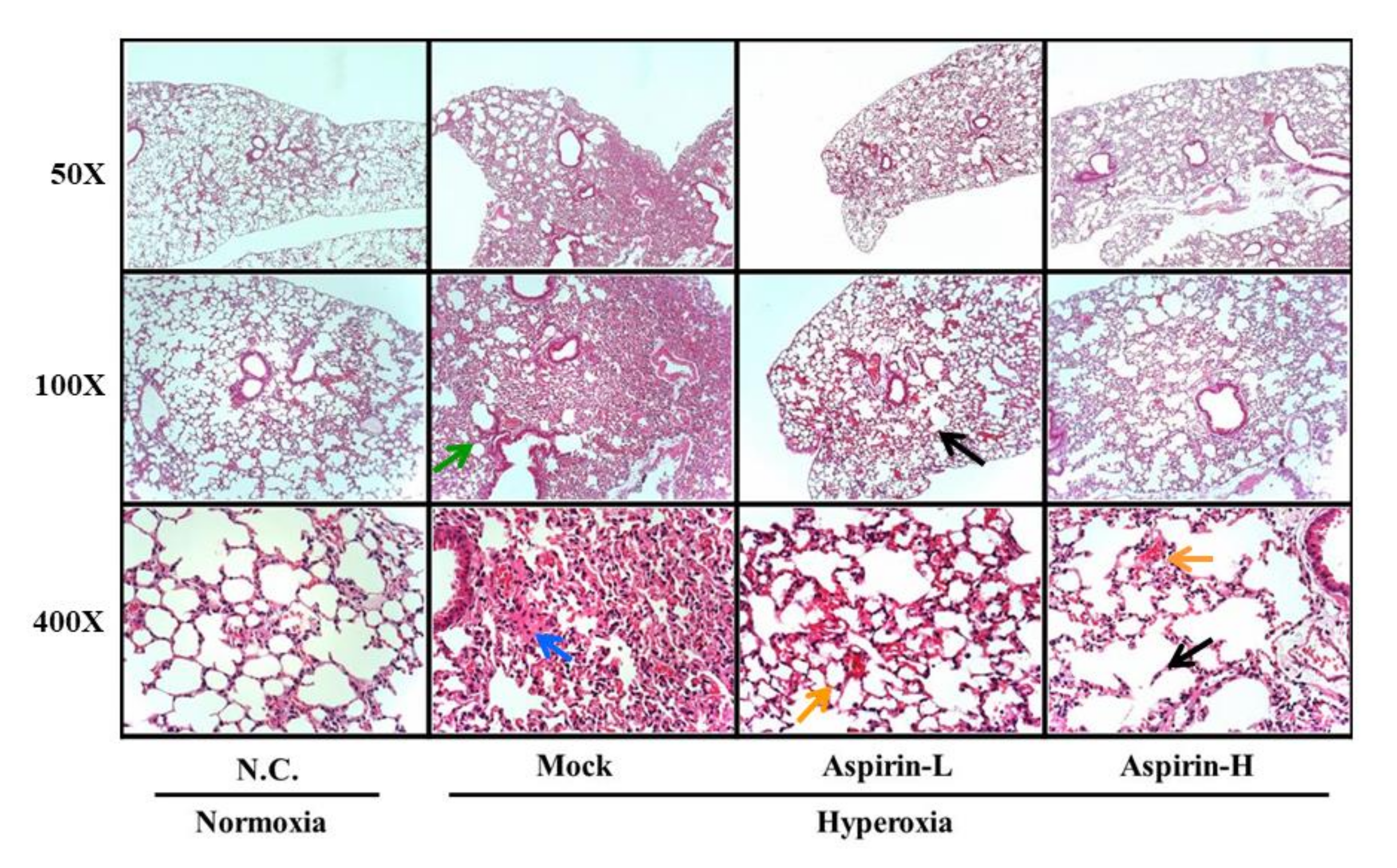
3.4. Effect of Pretreatment on Histological Changes in the Lung in NF-κB–Luciferase+/+ Transgenic Mice
3.5. Effects of Aspirin on the Generation of ROS and the Number of Macrophages in NF-κB–Luciferase+/+ Transgenic Mice
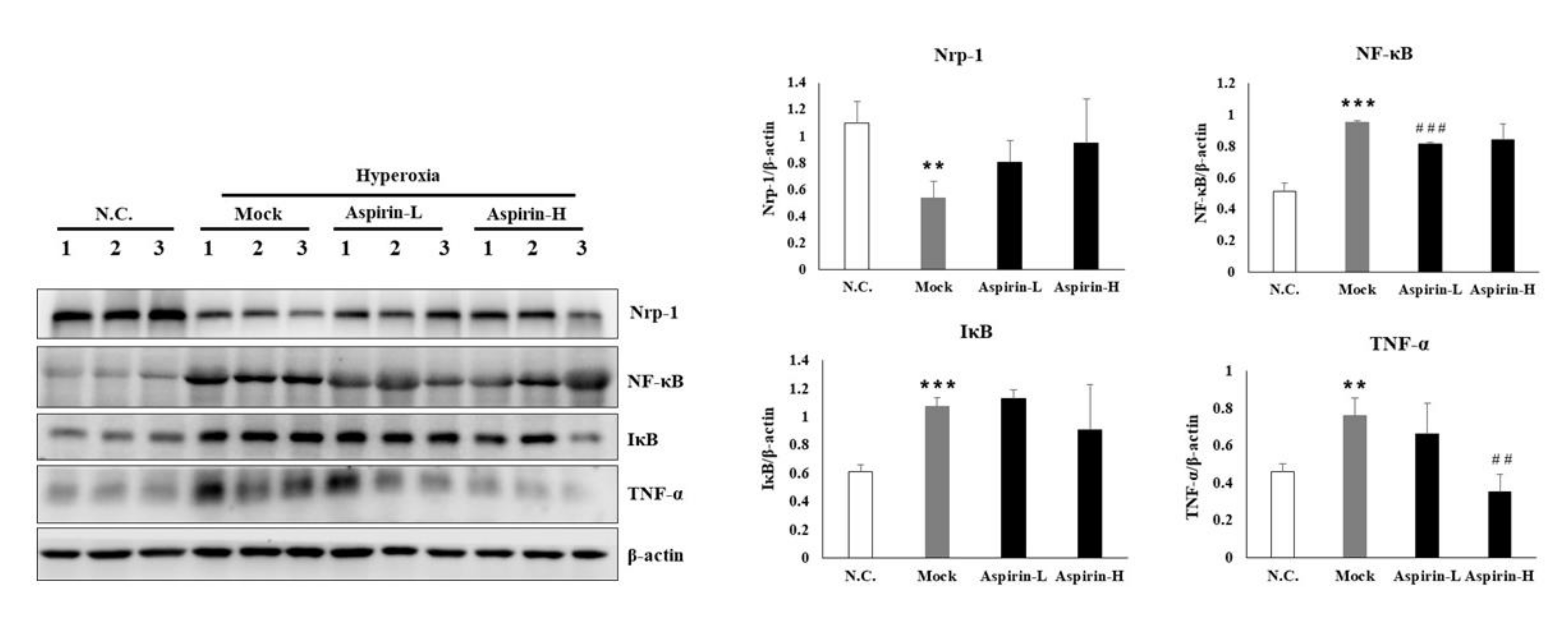
3.6. Effects of Aspirin on the Inflammatory Signaling in NF-κB-Luciferase+/+ Transgenic Mice
4. Discussion
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| ALI | Acute lung injury |
| ARDS | Acute respiratory distress syndrome |
| BALF | Bronchoalveolar lavage fluid |
| CC10 | Clara cell 10 kilodalton protein |
| FVB | Friend leukemia virus B |
| H&E | Hematoxylin and eosin |
| IVIS | In vivo imaging system |
| LPS | Lipopolysaccharide |
| MMP | Matrix metalloproteinase |
| NRP-1 | Neuropilin 1 |
| PBS | Phosphate buffered saline |
| ROS | Reactive oxygen species |
References
- Ware, L.B.; Matthay, M.A. The acute respiratory distress syndrome. N. Engl. J. Med. 2000, 342, 1334–1349. [Google Scholar] [CrossRef]
- Matthay, M.A.; Zimmerman, G.A.; Esmon, C.; Bhattacharya, J.; Coller, B.; Doerschuk, C.M.; Floros, J.; Gimbrone, M.A.; Hoffman, E.; Hubmayr, R.D.; et al. Future Research Directions in Acute Lung Injury. Am. J. Respir. Crit. Care Med. 2003, 167, 1027–1035. [Google Scholar] [CrossRef]
- Matthay, M.A.; Ware, L.B.; Zimmerman, G.A. The acute respiratory distress syndrome. J. Clin. Investig. 2012, 122, 2731–2740. [Google Scholar] [CrossRef]
- Kallet, R.H.; Matthay, M.A. Hyperoxic acute lung injury. Respir. Care 2013, 58, 123–141. [Google Scholar] [CrossRef]
- Crapo, J.D. Morphologic changes in pulmonary oxygen toxicity. Annu. Rev. Physiol. 1986, 48, 721–731. [Google Scholar] [CrossRef]
- Bhandari, V.; Choo-Wing, R.; Lee, C.G.; Zhu, Z.; Nedrelow, J.H.; Chupp, G.L.; Zhang, X.; Matthay, M.A.; Ware, L.B.; Homer, R.; et al. Hyperoxia causes angiopoietin 2–mediated acute lung injury and necrotic cell death. Nat. Med. 2006, 12, 1286–1293. [Google Scholar] [CrossRef]
- Mach, W.J.; Thimmesch, A.R.; Pierce, J.T.; Pierce, J.D. Consequences of Hyperoxia and the Toxicity of Oxygen in the Lung. Nurs. Res. Pr. 2011, 2011, 1–7. [Google Scholar] [CrossRef]
- Altemeier, W.A.; Sinclair, S.E. Hyperoxia in the intensive care unit: Why more is not always better. Curr. Opin. Crit. Care 2007, 13, 73–78. [Google Scholar] [CrossRef]
- Bhandari, V. Molecular mechanisms of hyperoxia-induced acute lung injury. Front. Biosci. 2008, 13, 6653. [Google Scholar] [CrossRef]
- Matute-Bello, G.; Frevert, C.W.; Martin, T.R. Animal models of acute lung injury. Am. J. Physiol. Cell. Mol. Physiol. 2008, 295, L379–L399. [Google Scholar] [CrossRef]
- Jepsen, S.; Herlevsen, P.; Knudsen, P.; Bud, M.I.; Klausen, N.O. Antioxidant treatment with N-acetylcysteine during adult respiratory distress syndrome: A prospective, randomized, placebo-controlled study. Crit. Care Med. 1992, 20, 918–923. [Google Scholar] [CrossRef]
- Meade, M.O.; Jacka, M.J.; Cook, D.J.; Dodek, P.; Griffith, L.E.; Guyatt, G.H. Survey of interventions for the prevention and treatment of acute respiratory distress syndrome. Crit. Care Med. 2004, 32, 946–954. [Google Scholar] [CrossRef]
- Calfee, C.S.; Matthay, M.A. Nonventilatory treatments for acute lung injury and ARDS. Chest 2007, 131, 913–920. [Google Scholar] [CrossRef]
- Kiefmann, R.; Heckel, K.; Schenkat, S.; Dörger, M.; Wesierska-Gadek, J.; Goetz, A.E. Platelet-endothelial cell interaction in pulmonary micro-circulation: The role of PARS. Thromb. Haemost. 2004, 91, 761–770. [Google Scholar] [CrossRef]
- Kiefmann, R.; Heckel, K.; Schenkat, S.; Dörger, M.; Goetz, A. Role of P-Selectin in Platelet Sequestration in Pulmonary Capillaries during Endotoxemia. J. Vasc. Res. 2006, 43, 473–481. [Google Scholar] [CrossRef]
- Zarbock, A.; Singbartl, K.; Ley, K. Complete reversal of acid-induced acute lung injury by blocking of platelet-neutrophil aggregation. J. Clin. Investig. 2006, 116, 3211–3219. [Google Scholar] [CrossRef]
- Zarbock, A.; Polanowska-Grabowska, R.K.; Ley, K. Platelet-neutrophil-interactions: Linking hemostasis and inflammation. Blood Rev. 2007, 21, 99–111. [Google Scholar] [CrossRef]
- Kario, K.; Eguchi, K.; Hoshide, S.; Hoshide, Y.; Umeda, Y.; Mitsuhashi, T.; Shimada, K. U-curve relationship between orthostatic blood pressure change and silent cerebrovascular disease in elderly hypertensives: Orthostatic hypertension as a new cardiovascular risk factor. J. Am. Coll. Cardiol. 2002, 40, 133–141. [Google Scholar] [CrossRef]
- Chen, Z.T.; Li, S.L.; Cai, E.Q.; Wu, W.L.; Jin, J.S.; Zhu, B. LPS induces pulmonary intravascular macrophages producing inflammatory mediators via activating NF-kappaB. J. Cell Biochem. 2003, 89, 1206–1214. [Google Scholar] [CrossRef]
- Looney, M.; Nguyen, J.X.; Hu, Y.; Van Ziffle, J.A.; Lowell, C.A.; Matthay, M.A. Platelet depletion and aspirin treatment protect mice in a two-event model of transfusion-related acute lung injury. J. Clin. Investig. 2009, 119, 3450–3461. [Google Scholar] [CrossRef]
- Caudrillier, A.; Kessenbrock, K.; Gilliss, B.M.; Nguyen, J.X.; Marques, M.; Monestier, M.; Toy, P.; Werb, Z.; Looney, M. Platelets induce neutrophil extracellular traps in transfusion-related acute lung injury. J. Clin. Investig. 2012, 122, 2661–2671. [Google Scholar] [CrossRef]
- Eickmeier, O.; Seki, H.; Haworth, O.; Hilberath, J.N.; Gao, F.; Uddin, M.; Croze, R.H.; Carlo, T.; Pfeffer, M.A.; Levy, B. Aspirin-triggered resolvin D1 reduces mucosal inflammation and promotes resolution in a murine model of acute lung injury. Mucosal Immunol. 2012, 6, 256–266. [Google Scholar] [CrossRef]
- Tuinman, P.R.; Müller, M.C.; Jongsma, G.; Hegeman, M.A.; Juffermans, N.P. High-dose acetylsalicylic acid is superior to low-dose as well as to clopidogrel in preventing lpopolysaccharide-induced lung injury in mice. Shock 2013, 40, 334–338. [Google Scholar] [CrossRef]
- Britt, R.D.; Velten, M.; Tipple, T.; Nelin, L.D.; Rogers, L.K. Cyclooxygenase-2 in newborn hyperoxic lung injury. Free. Radic. Boil. Med. 2013, 61, 502–511. [Google Scholar] [CrossRef]
- Cox, R., Jr.; Phillips, O.; Fukumoto, J.; Fukumoto, I.; Parthasarathy, P.T.; Arias, S.; Cho, Y.; Lockey, R.F.; Kolliputi, N. Aspirin-triggered resolvin D1 treatment enhances resolution of hyperoxic acute lung injury. Am. J. Respir. Cell Mol. Biol. 2015, 53, 422–435. [Google Scholar] [CrossRef]
- Chen, W.; Janz, D.R.; Bastarache, J.A.; May, A.K.; O’Neal, H.R.; Bernard, G.R.; Ware, L.B. Prehospital Aspirin Use Is Associated with Reduced Risk of Acute Respiratory Distress Syndrome in Critically Ill Patients. Crit. Care Med. 2015, 43, 801–807. [Google Scholar] [CrossRef]
- Yen, C.; Lin, C.; Chong, K.-Y.; Tsai, T.; Shen, C.-J.; Lin, M.; Su, C.; Chen, H.; Chen, C.; Lim, C.-Y. Lactoferrin as a Natural Regimen for Selective Decontamination of the Digestive Tract: Recombinant Porcine Lactoferrin Expressed in the Milk of Transgenic Mice Protects Neonates from Pathogenic Challenge in the Gastrointestinal Tract. J. Infect. Dis. 2009, 199, 590–598. [Google Scholar] [CrossRef]
- Yen, C.-C.; Lai, Y.-W.; Chen, H.-L.; Lai, C.-W.; Lin, C.-Y.; Chen, W.; Kuan, Y.-P.; Hsu, W.-H.; Chen, C.-M. Aerosolized Human Extracellular Superoxide Dismutase Prevents Hyperoxia-Induced Lung Injury. PLOS ONE 2011, 6, e26870. [Google Scholar] [CrossRef]
- Wu, S.-C.; Chen, H.-L.; Yen, C.-C.; Kuo, M.-F.; Yang, T.-S.; Wang, S.-R.; Weng, C.-N.; Chen, C.-M.; Cheng, W.T.K. Recombinant Porcine Lactoferrin Expressed in the Milk of Transgenic Mice Enhances Offspring Growth Performance. J. Agric. Food Chem. 2007, 55, 4670–4677. [Google Scholar] [CrossRef]
- Wen, S.-T.; Chen, W.; Chen, H.-L.; Lai, C.-W.; Yen, C.-C.; Lee, K.-H.; Wu, S.-C.; Chen, C.-M. Amniotic Fluid Stem Cells from EGFP Transgenic Mice Attenuate Hyperoxia-Induced Acute Lung Injury. PLoS ONE 2013, 8, e75383. [Google Scholar] [CrossRef]
- Al-Mehdi, A.; Shuman, H.; Fisher, A.B. Fluorescence microtopography of oxidative stress in lung ischemia-reperfusion. Lab. Investig. 1994, 70, 579–587. [Google Scholar]
- Nagato, A.; Bezerra, F.S.; Lanzetti, M.; Lopes, A.D.A.; Silva, M.A.D.S.; Porto, L.C.; Valenca, S. Time course of inflammation, oxidative stress and tissue damage induced by hyperoxia in mouse lungs. Int. J. Exp. Pathol. 2012, 93, 269–278. [Google Scholar] [CrossRef]
- Ho, T.; Chen, Y.; Hsiang, C.-Y. Noninvasive nuclear factor-κB bioluminescence imaging for the assessment of host–biomaterial interaction in transgenic mice. Biomaterials 2007, 28, 4370–4377. [Google Scholar] [CrossRef]
- Hsiang, C.-Y.; Chen, Y.-S.; Ho, T.-Y. Nuclear factor-κB bioluminescence imaging-guided transcriptomic analysis for the assessment of host–biomaterial interaction in vivo. Biomaterials 2009, 30, 3042–3049. [Google Scholar] [CrossRef]
- Reddy, N.M.; Kleeberger, S.R.; Kensler, T.W.; Yamamoto, M.; Hassoun, P.M.; Reddy, S.P. Disruption of Nrf2 impairs the resolution of hyperoxia-induced acute lung injury and inflammation in mice. J. Immunol. 2009, 182, 7264–7271. [Google Scholar] [CrossRef]
- Valko, M.; Leibfritz, D.; Moncol, J.; Cronin, M.T.; Mazúr, M.; Telser, J. Free radicals and antioxidants in normal physiological functions and human disease. Int. J. Biochem. Cell Boil. 2007, 39, 44–84. [Google Scholar] [CrossRef]
- Hamid, U.; Krasnodembskaya, A.; Fitzgerald, M.; Shyamsundar, M.; Kissenpfennig, A.; Scott, C.; Lefrançais, E.; Looney, M.R.; Verghis, R.; Scott, J.; et al. Aspirin reduces lipopolysaccharide-induced pulmonary inflammation in human models of ARDS. Thorax 2017, 72, 971–980. [Google Scholar] [CrossRef]
- Boyle, A.J.; Di Gangi, S.; Hamid, U.I.; Mottram, L.-J.; McNamee, L.; White, G.; Cross, L.J.M.; McNamee, J.J.; O’Kane, C.; McAuley, D.F. Aspirin therapy in patients with acute respiratory distress syndrome (ARDS) is associated with reduced intensive care unit mortality: A prospective analysis. Crit. Care 2015, 19, 109. [Google Scholar] [CrossRef]
- Kor, D.J.; Carter, R.E.; Park, P.K.; Festic, E.; Banner-Goodspeed, V.M.; Hinds, R.; Talmor, D.; Gajic, O.; Ware, L.B.; Gong, M.N.; et al. Effect of Aspirin on Development of ARDS in At-Risk Patients Presenting to the Emergency Department: The LIPS-A Randomized Clinical Trial. JAMA 2016, 315, 2406–2414. [Google Scholar] [CrossRef]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, C.-M.; Tung, Y.-T.; Wei, C.-H.; Lee, P.-Y.; Chen, W. Anti-Inflammatory and Reactive Oxygen Species Suppression through Aspirin Pretreatment to Treat Hyperoxia-Induced Acute Lung Injury in NF-κB–Luciferase Inducible Transgenic Mice. Antioxidants 2020, 9, 429. https://doi.org/10.3390/antiox9050429
Chen C-M, Tung Y-T, Wei C-H, Lee P-Y, Chen W. Anti-Inflammatory and Reactive Oxygen Species Suppression through Aspirin Pretreatment to Treat Hyperoxia-Induced Acute Lung Injury in NF-κB–Luciferase Inducible Transgenic Mice. Antioxidants. 2020; 9(5):429. https://doi.org/10.3390/antiox9050429
Chicago/Turabian StyleChen, Chuan-Mu, Yu-Tang Tung, Chi-Hsuan Wei, Po-Ying Lee, and Wei Chen. 2020. "Anti-Inflammatory and Reactive Oxygen Species Suppression through Aspirin Pretreatment to Treat Hyperoxia-Induced Acute Lung Injury in NF-κB–Luciferase Inducible Transgenic Mice" Antioxidants 9, no. 5: 429. https://doi.org/10.3390/antiox9050429
APA StyleChen, C.-M., Tung, Y.-T., Wei, C.-H., Lee, P.-Y., & Chen, W. (2020). Anti-Inflammatory and Reactive Oxygen Species Suppression through Aspirin Pretreatment to Treat Hyperoxia-Induced Acute Lung Injury in NF-κB–Luciferase Inducible Transgenic Mice. Antioxidants, 9(5), 429. https://doi.org/10.3390/antiox9050429






