Smart Method for Carotenoids Characterization in Haematococcus pluvialis Red Phase and Evaluation of Astaxanthin Thermal Stability
Abstract
1. Introduction
2. Materials and Methods
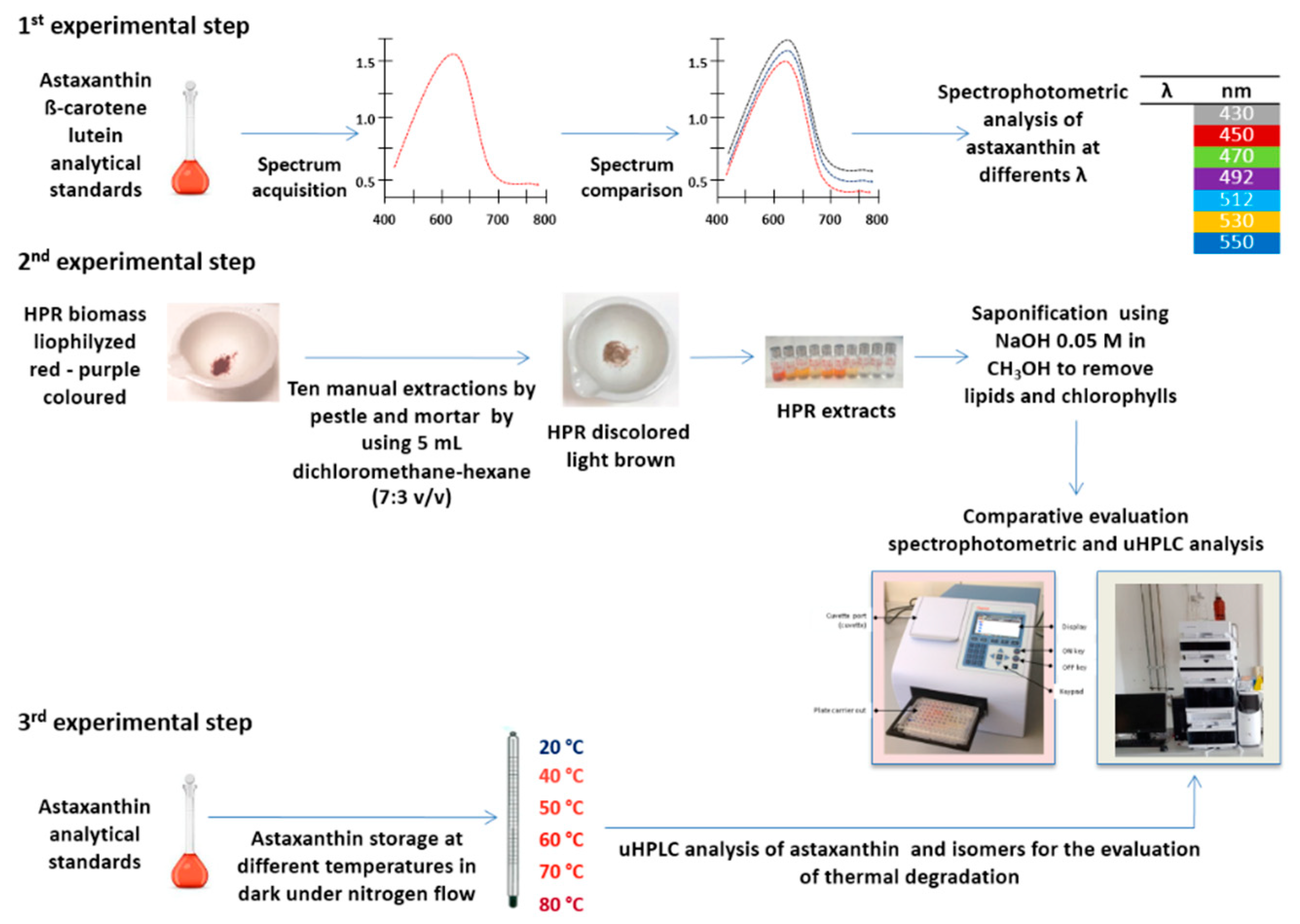
2.1. Experimental Design
2.2. uHPLC Analysis
2.3. Thermal Degradation of Astaxanthin
2.4. Statistical Analysis
3. Results
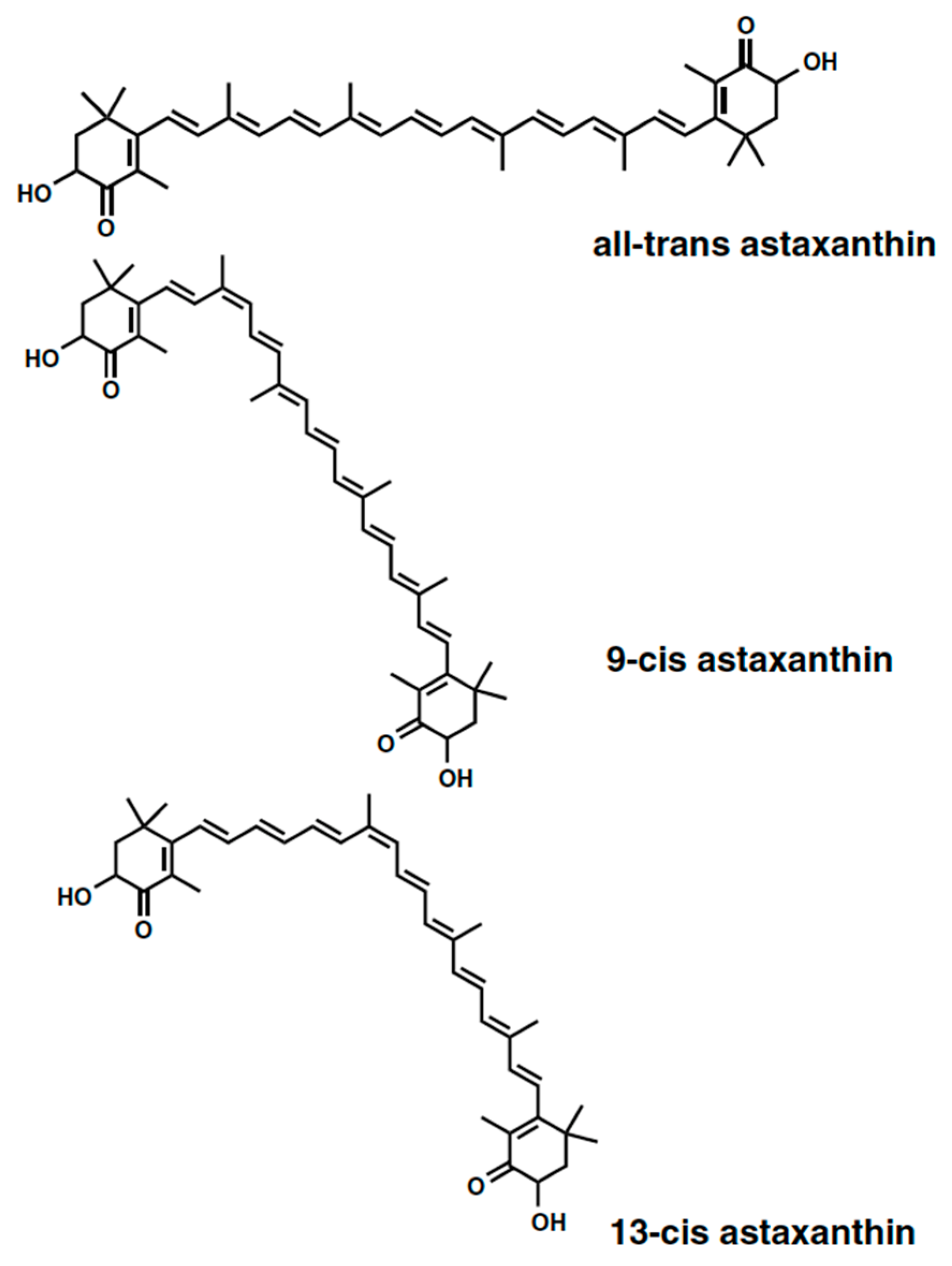
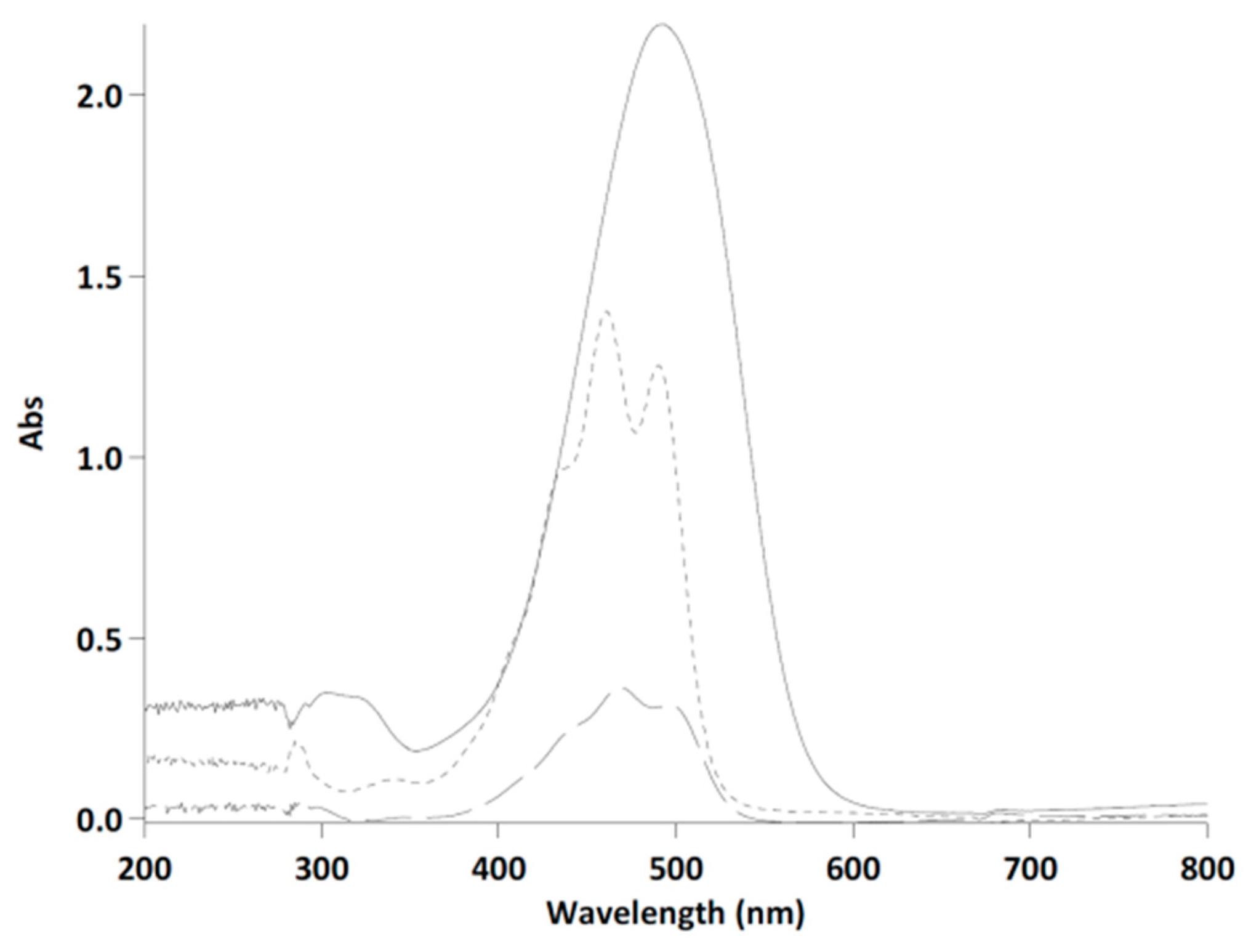
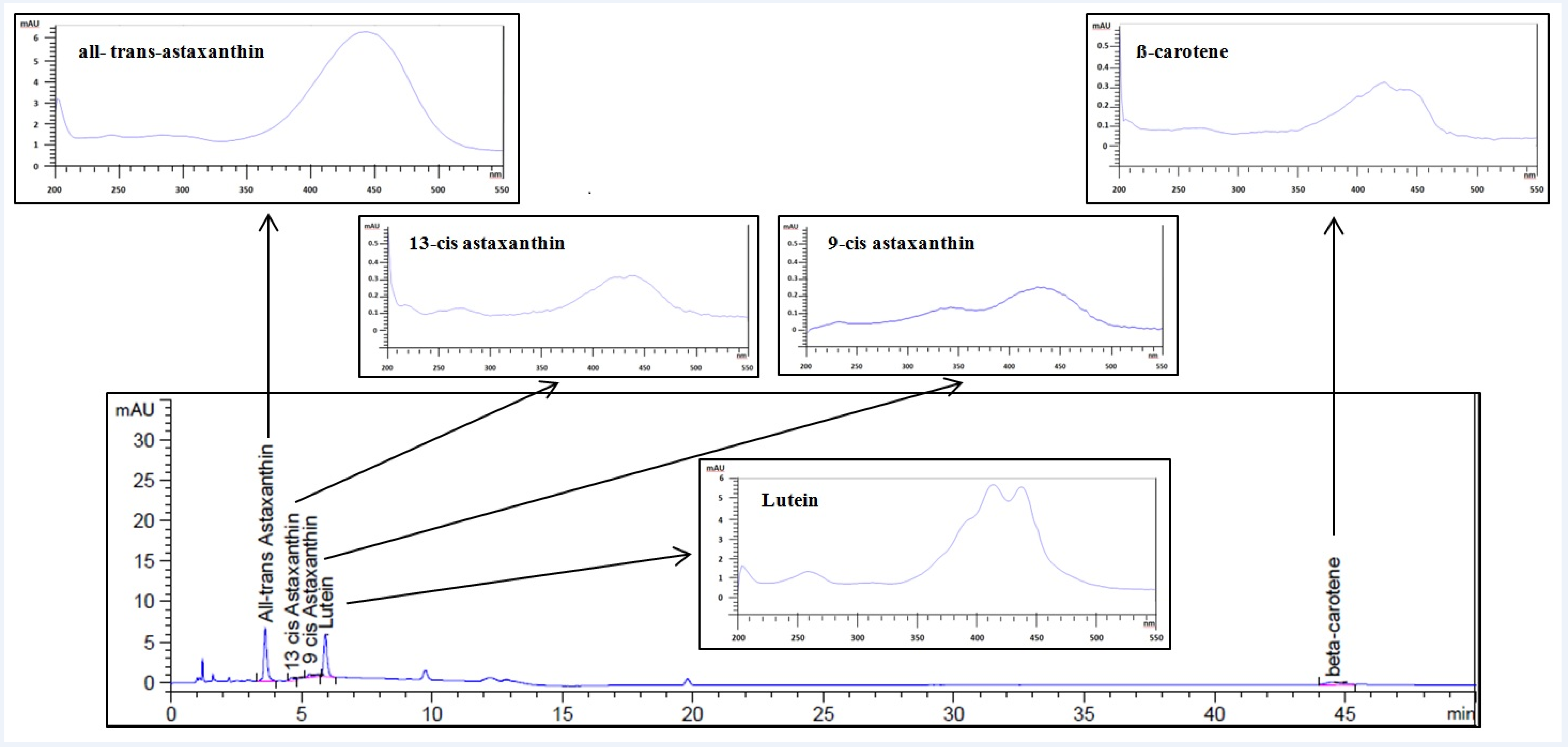
3.1. Astaxanthin and Carotenoids Characterization
3.2. Thermal Stability of Astaxanthin
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Britton, G.; Liaaen-Jensen, S.; Pfander, H. Section II: Main List Natural carotenoids. In Handbook, Carotenoids, 1st ed.; Britton, G., Pfander, H., Eds.; Springer: Basel, Switzerland, 2004; Volume 1, pp. 29–296. [Google Scholar] [CrossRef]
- Ambati, R.R.; Phang, S.M.; Ravi, S.; Aswathanarayana, R.G. Astaxanthin: Sources, extraction, stability, biological activities and its commercial applications—A review. Mar. Drugs 2014, 12, 128–152. [Google Scholar] [CrossRef] [PubMed]
- Spolaore, P.; Joannis-Cassan, C.; Duran, E.; Isambert, A. Commercial applications of microalgae. J. Biosci. Bioeng. 2006, 101, 87–96. [Google Scholar] [CrossRef] [PubMed]
- Noviendri, D.; Hasrini, R.F.; Octavianti, F. Carotenoids: Sources, medicinal properties and their application in food and nutraceutical industry. J. Med. Plants Res. 2011, 5, 7119–7131. [Google Scholar] [CrossRef]
- Rizwan, M.; Mujtaba, G.; Memon, S.A.; Lee, K.; Rashid, N. Exploring the potential of microalgae for new biotechnology applications and beyond: A review. Renew. Sustain. Energy Rev. 2018, 92, 394–404. [Google Scholar] [CrossRef]
- Carotenoids Market Analysis by Source (Natural, Synthetic), By Product (Beta-Carotene, Lutein, Lycopene, Astaxanthin, Zeaxanthin, Canthaxanthin), by Application (Food, Supplements, Feed, Pharmaceuticals, Cosmetics), and Segment Forecasts, 2014–2025. 2016. Available online: https://www.grandviewresearch.com/industry-analysis/carotenoids-market (accessed on 16 August 2018).
- Gammone, M.A.; Riccioni, G.; D’Orazio, N. Marine carotenoids against oxidative stress: Effects on human health. Mar. Drugs 2015, 13, 6226–6246. [Google Scholar] [CrossRef]
- Dose, J.; Matsugo, S.; Yokokawa, H.; Koshida, Y.; Okazaki, S.; Seidel, U.; Esatbeyoglu, T. Free radical scavenging and cellular antioxidant properties of astaxanthin. Int. J. Mol. Sci. 2016, 17, 103. [Google Scholar] [CrossRef]
- Rahman, M.M.; Khosravi, S.; Chang, K.H.; Lee, S.M. Effects of dietary inclusion of astaxanthin on growth, muscle pigmentation and antioxidant capacity of juvenile rainbow trout (Oncorhynchus mykiss). Prev. Nutr. Food Sci. 2016, 21, 281. [Google Scholar] [CrossRef]
- Liu, X.; Osawa, T. Cis astaxanthin and especially 9-cis astaxanthin exhibits a higher antioxidant activity in vitro compared to the all-trans isomer. Biochem. Biophys. Res. Commun. 2007, 357, 187–193. [Google Scholar] [CrossRef]
- Shah, M.; Mahfuzur, R.; Liang, Y.; Cheng, J.J.; Daroch, M. Astaxanthin-producing green microalga Haematococcus pluvialis: From single cell to high value commercial products. Front. Plant Sci. 2016, 7, 531. [Google Scholar] [CrossRef]
- Ekpe, L.; Inaku, K.; Ekpe, V. Antioxidant effects of astaxanthin in various diseases—A review. J. Mol. Pathophysiol. 2018, 7, 1–6. [Google Scholar] [CrossRef]
- Chintong, S.; Phatvej, W.; Rerk-Am, U.; Waiprib, Y.; Klaypradit, W. In vitro antioxidant, antityrosinase, and cytotoxic activities of astaxanthin from shrimp waste. Antioxidants 2019, 8, 128. [Google Scholar] [CrossRef] [PubMed]
- Davinelli, S.; Nielsen, M.E.; Scapagnini, G. Astaxanthin in skin health, repair, and disease: A comprehensive review. Nutrients 2018, 10, 522. [Google Scholar] [CrossRef] [PubMed]
- Zuluaga, M.; Gueguen, V.; Letourneur, D.; Pavon-Djavid, G. Astaxanthin-antioxidant impact on excessive Reactive Oxygen Species generation induced by ischemia and reperfusion injury. Chem.-Biol. Interact. 2018, 279, 145–158. [Google Scholar] [CrossRef] [PubMed]
- McCall, B.; McPartland, C.K.; Moore, R.; Frank-Kamenetskii, A.; Booth, B.W. Effects of astaxanthin on the proliferation and migration of breast cancer cells in vitro. Antioxidants 2018, 7, 135. [Google Scholar] [CrossRef]
- Commission Implementing Regulation (EU) 2017/2470 of 20 December 2017 Establishing the Union List of Novel Foods in Accordance with Regulation (EU) 2015/2283 of the European Parliament and of the Council on Novel Foods. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX%3A32017R2470 (accessed on 8 March 2020).
- Commission Implementing Regulation (EU) 2015/1415 of 20 August 2015 Concerning the Authorisation of Astaxanthin as a Feed Additive for Fish, Crustaceans and Ornamental Fish. Available online: https://eur-lex.europa.eu/eli/reg_impl/2015/1415/oj (accessed on 8 March 2020).
- Tibbetts, S.M.; Whitney, C.G.; MacPherson, M.J.; Bhatti, S.; Banskota, A.H.; Stefanova, R.; McGinn, P.J. Biochemical characterization of microalgal biomass from freshwater species isolated in Alberta, Canada for animal feed applications. Algal Res. 2015, 11, 435–447. [Google Scholar] [CrossRef]
- Matos, Â.P.; Feller, R.; Moecke, E.H.S.; de Oliveira, J.V.; Junior, A.F.; Derner, R.B.; Sant’Anna, E.S. Chemical Characterization of Six Microalgae with Potential Utility for Food Application. J. AOCS J. Am. Oil Chem. Soc. 2016, 93, 963–972. [Google Scholar] [CrossRef]
- Molino, A.; Iovine, A.; Casella, P.; Mehariya, S.; Chianese, S.; Cerbone, A.; Musmarra, D. Microalgae Characterization for Consolidated and New Application in Human Food, Animal Feed and Nutraceuticals. Int. J. Environ. Res. Public Health 2018, 15, 2436. [Google Scholar] [CrossRef]
- FAD-2009-0054. CR Evaluation Report on Analytical Method Submitted in Connection with the Application for the Authorisation of a Feed Additive According to the Regulation (EC) No 1831/2003. Available online: https://ec.europa.eu/jrc/sites/jrcsh/files/FinRep-FAD-2009-0054.pdf (accessed on 8 March 2020).
- Davies, B.H. Carotenoids. In Chemistry and Biochemistry of Plant Pigments; Goodwin, T.W., Ed.; Academic Press: London, UK, 1976; Volume 2, pp. 38–166. [Google Scholar]
- Lichtenthaler, H.K. Chlorophylls and carotenoids: Pigments of photosynthetic biomembranes. In Methods in Enzymology; Academic Press: London, Uk, 1987; Volume 148, pp. 350–382. [Google Scholar]
- Chen, Y.; Li, D.; Lu, W.; Xing, J.; Hui, B.; Han, Y. Screening and characterization of astaxanthin-hyperproducing mutants of Haematococcus pluvialis. Biotechnol. Lett. 2003, 25, 527–529. [Google Scholar] [CrossRef]
- Giannelli, L.; Yamada, H.; Katsuda, T.; Yamaji, H. Effects of temperature on the astaxanthin productivity and light harvesting characteristics of the green alga Haematococcus pluvialis. J. Biosci. Bioeng. 2015, 119, 345–350. [Google Scholar] [CrossRef]
- Sarada, R.; Tripathi, U.; Ravishankar, G.A. Influence of stress on astaxanthin production in Haematococcuspluvialis grown under different culture conditions. Process Biochem. 2002, 37, 623–627. [Google Scholar] [CrossRef]
- Hong, M.E.; Hwang, S.K.; Chang, W.S.; Kim, B.W.; Lee, J.; Sim, S.J. Enhanced autotrophic astaxanthin production from Haematococcus pluvialis under high temperature via heat stress-driven Haber–Weiss reaction. Appl. Microbiol. Biotechnol. 2015, 99, 5203–5215. [Google Scholar] [CrossRef] [PubMed]
- López, M.G.M.; Sanchez, E.D.R.; López, J.C.; Fernández, F.A.; Sevilla, J.F.; Rivas, J.; Grima, E.M. Comparative analysis of the outdoor culture of Haematococcus pluvialis in tubular and bubble column photobioreactors. J. Biotechnol. 2006, 123, 329–342. [Google Scholar] [CrossRef] [PubMed]
- Vidhyavathi, R.; Venkatachalam, L.; Kamath, B.S.; Sarada, R.; Ravishankar, G.A. Differential expression of carotenogenic genes and associated changes in pigment profile during regeneration of Haematococcus pluvialis cysts. Appl. Microbiol. Biotechnol. 2007, 75, 879–887. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Miao, F.; Geng, Y.; Lu, D.; Zhang, C.; Zeng, M. Accurate quantification of astaxanthin from Haematococcus crude extract spectrophotometrically. Chin. J. Oceanol. Limn. 2012, 30, 627–637. [Google Scholar] [CrossRef]
- Wang, S.; Meng, Y.; Liu, J.; Cao, X.; Xue, S. Accurate quantification of astaxanthin from Haematococcus pluvialis using DMSO extraction and lipase-catalyzed hydrolysis pretreatment. Algal Res. 2018, 35, 427–431. [Google Scholar] [CrossRef]
- Kittikaiwan, P.; Powthongsook, S.; Pavasant, P.; Shotipruk, A. Encapsulation of Haematococcus pluvialis using chitosan for astaxanthin stability enhancement. Carbohydr. Polym. 2007, 70, 378–385. [Google Scholar] [CrossRef]
- Martínez-Delgado, A.A.; Khandual, S.; Villanueva-Rodríguez, S.J. Chemical stability of astaxanthin integrated into a food matrix: Effects of food processing and methods for preservation. Food Chem. 2017, 225, 23–30. [Google Scholar] [CrossRef]
- Denery, J.R.; Dragull, K.; Tang, C.S.; Li, Q.X. Pressurized fluid extraction of carotenoids from Haematococcus pluvialis and Dunaliella salina and kavalactones from Piper methysticum. Anal. Chim. Acta 2004, 501, 175–181. [Google Scholar] [CrossRef]
- Molino, A.; Rimauro, J.; Casella, P.; Cerbone, A.; Larocca, V.; Chianese, S.; Musmarra, D. Extraction of astaxanthin from microalga Haematococcus pluvialis in red phase by using generally recognized as safe solvents and accelerated extraction. J. Biotechnol. 2018, 283, 51–61. [Google Scholar] [CrossRef]
- Di Sanzo, G.; Mehariya, S.; Martino, M.; Larocca, V.; Casella, P.; Chianese, S.; Musmarra, D.; Balducchi, R.; Molino, A. Supercritical Carbon Dioxide Extraction of Astaxanthin, Lutein, and Fatty Acids from Haematococcus pluvialis Microalgae. Mar. Drugs 2018, 16, 334. [Google Scholar] [CrossRef]
- Molino, A.; Mehariya, S.; Iovine, A.; Larocca, V.; Di Sanzo, G.; Martino, M.; Musmarra, D. Extraction of Astaxanthin and Lutein from Microalga Haematococcus pluvialis in the Red Phase Using CO2 Supercritical Fluid Extraction Technology with Ethanol as Co-Solvent. Mar. Drugs 2018, 16, 432. [Google Scholar] [CrossRef] [PubMed]
- Yuan, J.P.; Chen, F. Isomerization of trans-astaxanthin to cis-isomers in organic solvents. J. Agric. Food Chem. 1999, 47, 3656–3660. [Google Scholar] [CrossRef] [PubMed]
- Yuan, J.P.; Chen, F. Kinetics for the reversible isomerization reaction of trans-astaxanthin. Food Chem. 2001, 73, 131–137. [Google Scholar] [CrossRef]
- Villalobos-Castillejos, F.; Cerezal-Mezquita, P.; Hernández-De Jesús, M.L.; Barragán-Huerta, B.E. Production and stability of water-dispersible astaxanthin oleoresin from Phaffia rhodozyma. Int. J. Food Sci. Technol. 2013, 48, 1243–1251. [Google Scholar] [CrossRef]
- Raposo, M.F.J.; Morais, A.M.; Morais, R.M. Effects of spray-drying and storage on astaxanthin content of Haematococcus pluvialis biomass. World J. Microbiol. Biotechnol. 2012, 28, 1253–1257. [Google Scholar] [CrossRef]
- Ahmed, F.; Li, Y.; Fanning, K.; Netzel, M.; Schenk, P.M. Effect of drying, storage temperature and air exposure on astaxanthin stability from Haematococcus pluvialis. Food Res. Int. 2015, 74, 231–236. [Google Scholar] [CrossRef]
- Armenta, R.E.; Guerrero-Legarreta, I. Stability studies on astaxanthin extracted from fermented shrimp byproducts. J. Agric. Food Chem. 2009, 57, 6095–6100. [Google Scholar] [CrossRef]
- Gómez-Estaca, J.; Calvo, M.M.; Álvarez-Acero, I.; Montero, P.; Gómez-Guillén, M.C. Characterization and storage stability of astaxanthin esters, fatty acid profile and α-tocopherol of lipid extract from shrimp (L. vannamei) waste with potential applications as food ingredient. Food Chem. 2017, 216, 37–44. [Google Scholar] [CrossRef]
- Ruen-ngam, D.; Shotipruk, A.; Pavasant, P. Comparison of extraction methods for recovery of astaxanthin from Haematococcus pluvialis. Sep. Sci. Technol. 2011, 46, 64–70. [Google Scholar] [CrossRef]
- Buchwald, M.; Jencks, W.P. Optical properties of astaxanthin solutions and aggregates. Biochemistry 1986, 7, 834–843. [Google Scholar] [CrossRef]
- Craft, N.E.; Soares, J.H. Relative solubility, stability, and absorptivity of lutein and. beta.-carotene in organic solvents. J. Agric. Food Chem. 1992, 40, 431–434. [Google Scholar] [CrossRef]
- Hong, M.E.; Choi, Y.Y.; Sim, S.J. Effect of red cyst cell inoculation and iron (II) supplementation on autotrophic astaxanthin production by Haematococcus pluvialis under outdoor summer conditions. J. Biotechnol. 2016, 218, 25–33. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Hassan, Y.I.; Liu, R.; Zhang, H.; Chen, Y.; Zhang, L.; Tsao, R. Anti-inflammatory effects of different astaxanthin isomers and the roles of lipid transporters in the cellular transport of astaxanthin isomers in Caco-2 cell monolayers. J. Agric. Food Chem. 2019, 67, 6222–6231. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Zhou, Q.; Yang, L.; Xue, Y.; Xu, J.; Xue, C. Effect of thermal processing on astaxanthin and astaxanthin esters in pacific white shrimp Litopenaeus vannamei. J. Oleo Sci. 2015, 64, 243–253. [Google Scholar] [CrossRef]
- Holtin, K.; Kuehnle, M.; Rehbein, J.; Schuler, P.; Nicholson, G.; Albert, K. Determination of astaxanthin and astaxanthin esters in the microalgae Haematococcus pluvialis by LC-(APCI) MS and characterization of predominant carotenoid isomers by NMR spectroscopy. Anal. Bioanal. Chem. 2009, 395, 1613. [Google Scholar] [CrossRef]

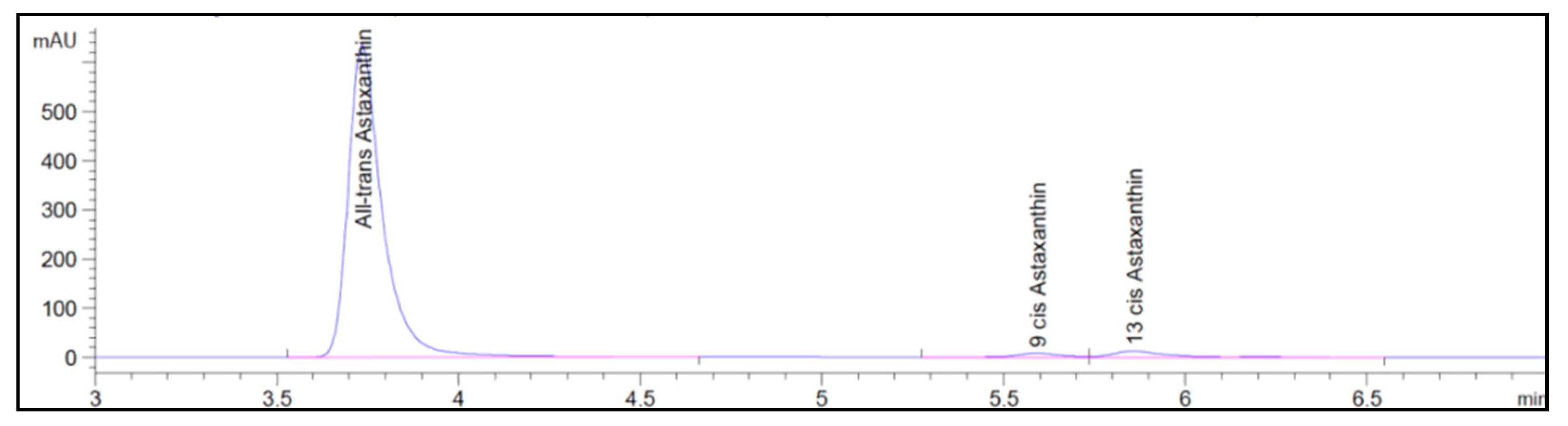
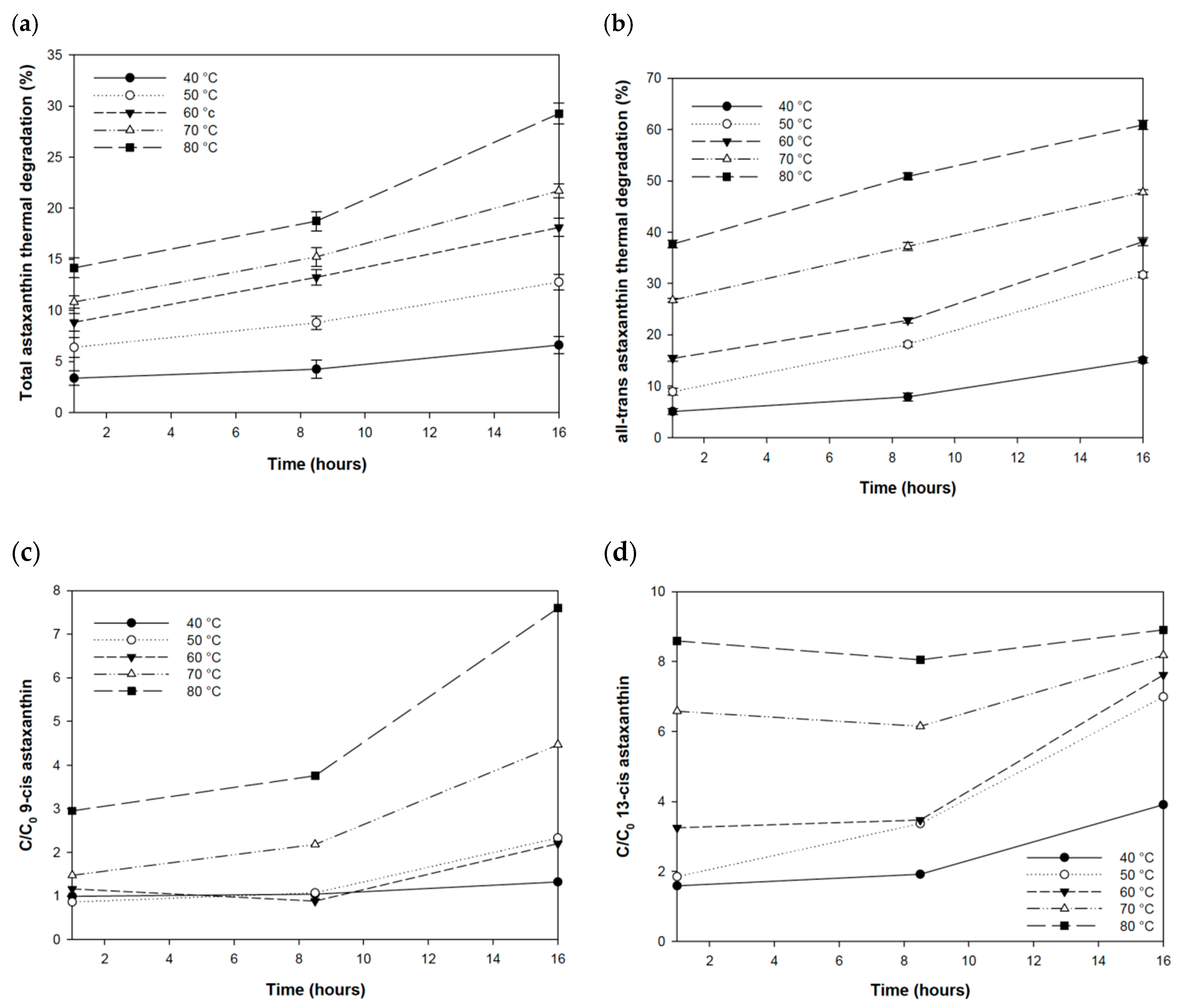
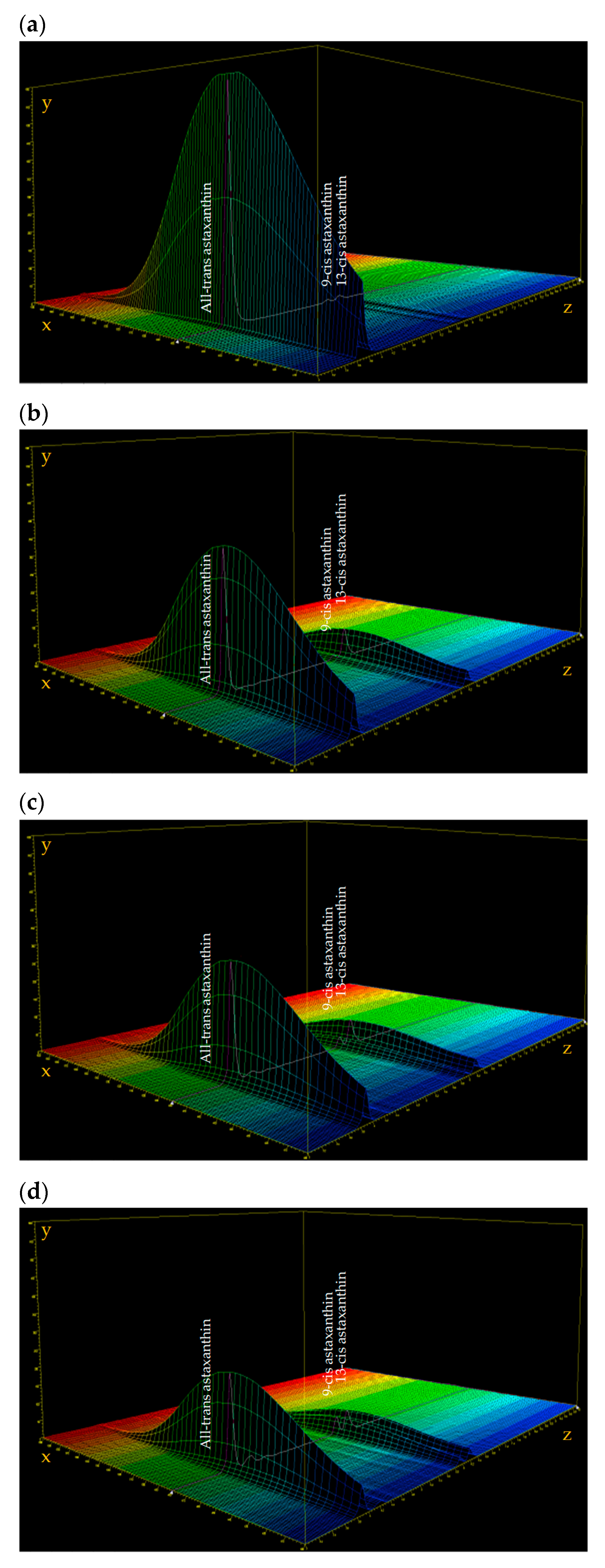
| Test n° | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Temperature (°C) | 40 | 40 | 40 | 50 | 50 | 50 | 60 | 60 | 60 | 70 | 70 | 70 | 80 | 80 | 80 |
| Time (hours) | 1 | 8.5 | 16 | 1 | 8.5 | 16 | 1 | 8.5 | 16 | 1 | 8.5 | 16 | 1 | 8.5 | 16 |
| Wavelength λ (nm) | Equations in this Work | Literature [31] |
|---|---|---|
| 492 | y = 0.2176x + 0.0262 (1) | y = 0.2220x + 0.0104 |
| 512 | y = 0.2x + 0.0254 (2) | y = 0.2096x + 0.0110 |
| 530 | y = 0.1498x + 0.0191 (3) | y = 0.1556x + 0.0107 |
| 550 | y = 0.0715x + 0.0086 (4) | y = 0.0759x + 0.005 |
| Analysis | Astaxanthin Concentration (mg/L) | Astaxanthin Content (mg/g) |
|---|---|---|
| Spectrophotometer | 8.34 ± 0.15 | 20.02 ± 0.20 |
| uHPLC-DAD | 8.42 ± 0.04 | 20.00 ± 0.05 |
| Carotenoids | Concentration (mg/l) | Content (mg/g) |
|---|---|---|
| All-trans-astaxanthin | 3.46 ± 0.03 | 8.30 ± 0.04 |
| 9-Cis astaxanthin | 2.59 ± 0.05 | 6.24 ± 0.05 |
| 13-Cis astaxanthin | 2.27 ± 0.05 | 5.46 ± 0.06 |
| Total astaxanthin | 8.42 ± 0.04 | 20.00 ± 0.05 |
| Lutein | 3.20 ± 0.04 | 7.70 ± 0.04 |
| β-Carotene | 0.41 ± 0.02 | 0.98 ± 0.04 |
| Total | 12.03 ± 0.04 | 28.70 ± 0.05 |
| Astaxanthin Isomers | Concentration (C0) |
|---|---|
| (mg/L) | |
| All-trans | 59.42 ± 0.34 |
| 9-Cis | 0.85 ± 0.10 |
| 13-Cis | 1.58 ± 0.15 |
| Total | 61.85 ± 0.79 |
| Time (days) | Astaxanthin Thermal Degradation |
|---|---|
| % | |
| 0.6 | 0.16 ± 0.08 |
| 2 | 2.29 ± 0.13 |
| 5 | 4.74 ± 0.23 |
| 7 | 7.07 ± 0.44 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Casella, P.; Iovine, A.; Mehariya, S.; Marino, T.; Musmarra, D.; Molino, A. Smart Method for Carotenoids Characterization in Haematococcus pluvialis Red Phase and Evaluation of Astaxanthin Thermal Stability. Antioxidants 2020, 9, 422. https://doi.org/10.3390/antiox9050422
Casella P, Iovine A, Mehariya S, Marino T, Musmarra D, Molino A. Smart Method for Carotenoids Characterization in Haematococcus pluvialis Red Phase and Evaluation of Astaxanthin Thermal Stability. Antioxidants. 2020; 9(5):422. https://doi.org/10.3390/antiox9050422
Chicago/Turabian StyleCasella, Patrizia, Angela Iovine, Sanjeet Mehariya, Tiziana Marino, Dino Musmarra, and Antonio Molino. 2020. "Smart Method for Carotenoids Characterization in Haematococcus pluvialis Red Phase and Evaluation of Astaxanthin Thermal Stability" Antioxidants 9, no. 5: 422. https://doi.org/10.3390/antiox9050422
APA StyleCasella, P., Iovine, A., Mehariya, S., Marino, T., Musmarra, D., & Molino, A. (2020). Smart Method for Carotenoids Characterization in Haematococcus pluvialis Red Phase and Evaluation of Astaxanthin Thermal Stability. Antioxidants, 9(5), 422. https://doi.org/10.3390/antiox9050422










