The Profile of Selected Antioxidants in Two Courgette Varieties from Organic and Conventional Production
Abstract
1. Introduction
2. Materials and Methods
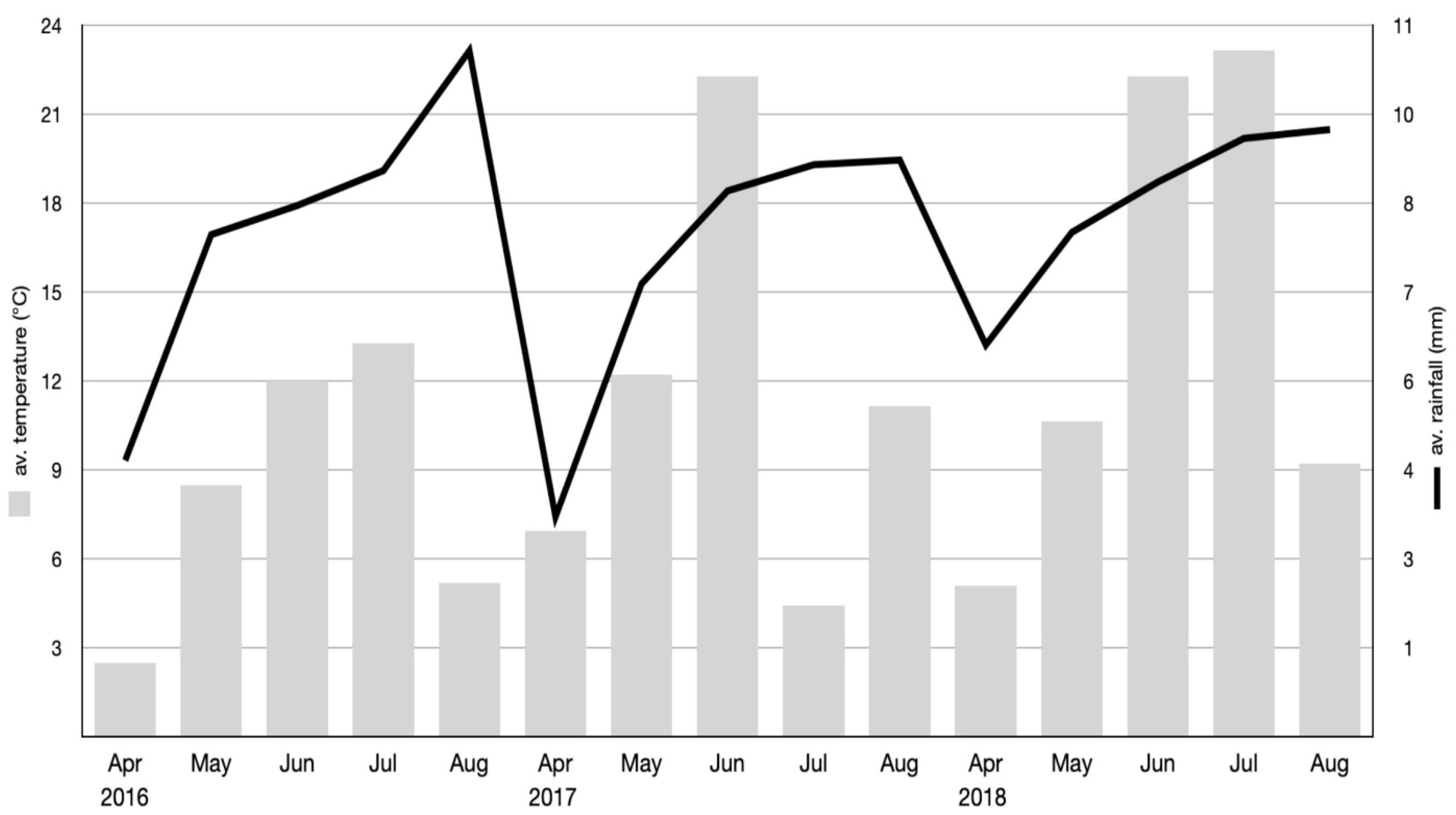
2.1. Study Design and Plant Material
2.2. Preparation of Samples
2.3. Chemicals
2.4. Dry Matter
2.5. Phenolic Compounds Extraction and Identification
2.6. Carotenoids and Chlorophylls Extraction and Identification
2.7. Vitamin C Extraction and Identificiation
2.8. Statistical Analysis
3. Results
3.1. Dry Matter Content
3.2. Vitamin C Content
3.3. Phenolic Acids Content
3.4. Flavonoids Content
3.5. Carotenoids Content
3.6. Chlorophylls Content
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Ben-nun, L. Characteristics of Zucchini; Ben-nun, L., Ed.; B. N. Publication House: Beer-Sheva, Israel, 2019. [Google Scholar]
- Salehi, B.; Sharifi-rad, J.; Capanoglu, E.; Adrar, N. Cucurbita Plants: From Farm to Industry. Appl. Sci. 2019, 1010, 3387. [Google Scholar] [CrossRef]
- Paris, H.S. History of the cultivar-groups of Cucurbita pepo. In Horticultural Reviews; Janick, J., Ed.; John Wiley & Sons: Hoboken, NJ, USA, 2001; Volume 25, pp. 71–170. [Google Scholar]
- Paris, H.S. Germplasm enhancement of Cucurbita pepo (pumpkin, squash, gourd: Cucurbitaceae): Progress and challenges. Euphytica 2016, 208, 415–438. [Google Scholar] [CrossRef]
- Verdone, M.; Rao, R.; Coppola, M.; Corrado, G. Identification of zucchini varieties in commercial food products by DNA typing. Food Control 2018, 84, 197–204. [Google Scholar] [CrossRef]
- Occhino, E.; Hernando, I.; Llorca, E.; Neri, L.; Pittia, P. Effect of Vacuum Impregnation Treatments to Improve Quality and Texture of Zucchini (Cucurbita Pepo, L). Procedia Food Sci. 2011, 1, 829–835. [Google Scholar] [CrossRef]
- Lust, T.A.; Paris, H.S. Italian horticultural and culinary records of summer squash (Cucurbita pepo, Cucurbitaceae) and emergence of the zucchini in 19th-century Milan. Ann. Bot. 2016, 118, 53–69. [Google Scholar] [CrossRef]
- Ciaccia, C.; Montemurro, F.; Campanelli, G.; Diacono, M.; Fiore, A.; Canali, S. Legume cover crop management and organic amendments application: Effects on organic zucchini performance and weed competition. Sci. Hortic. 2015, 185, 48–58. [Google Scholar] [CrossRef]
- Oloyede, F.M.; Agbaje, G.O.; Obuotor, E.M.; Obisesan, I.O. Nutritional and antioxidant profiles of pumpkin (Cucurbita pepo Linn.) immature and mature fruits as influenced by NPK fertilizer. Food Chem. 2012, 135, 460–463. [Google Scholar] [CrossRef]
- Peters, R.; Kelsey, J.W.; White, J.C. Differences in p,p′-DDE bioaccumulation from compost and soil by the plants Cucurbita pepo and Cucurbita maxima and the earthworms Eisenia fetida and Lumbricus terrestris. Environ. Pollut. 2007, 148, 539–545. [Google Scholar] [CrossRef]
- Mahabir, V.; Verma, V. Application of Atomic Absorption Spectroscopy in Food Sciences: (A Study on Cucurbita Maxima). APCBEE Procedia 2012, 2, 135–140. [Google Scholar] [CrossRef][Green Version]
- Yok, M.C.K.; Gisong, S.A.D.; Modon, B.A.; Rusim, R. Creating New Market in Integrated Agriculture Development Area in Samarahan, Sarawak, Malaysia—Case Study in the Supply Chain of Cucurbita sp. (Pumpkin). Procedia Soc. Behav. Sci. 2016, 224, 516–522. [Google Scholar] [CrossRef][Green Version]
- Sinha, S.; Kumar, B.; Luqman, S.; Singh, D.K. Neuroprotective potential of Cucurbita maxima Duchesne ex Poir, Caeselpenia bunduc (L.) Roxb and Bombax ceiba Linn extracts. South Afr. J. Bot. 2019, 120, 319–325. [Google Scholar] [CrossRef]
- Amin, M.Z.; Islam, T.; Uddin, M.R.; Uddin, M.J.; Rahman, M.M.; Satter, M.A. Comparative study on nutrient contents in the different parts of indigenous and hybrid varieties of pumpkin (Cucurbita maxima Linn.). Heliyon 2019, 5, 02462. [Google Scholar] [CrossRef] [PubMed]
- Benbrook, C.M. Elevating antioxidant levels in food through organic farming and food processing. In An Organic Center State of Science Review; The Organic Center: Washington, DC, USA, 2005. [Google Scholar]
- Eissa, H.A.; Bareh, G.F.; Ibrahim, A.A.; Moawad, R.K.; Ali, H.S. The effect of different drying methods on the nutrients and non-nutrients composition of zucchini (green squash) rings. J. Appl. Sci. Res. 2013, 9, 5380–5389. [Google Scholar]
- Rouphael, Y.; Colla, G. Growth, yield, fruit quality and nutrient uptake of hydroponically cultivated zucchini squash as affected by irrigation systems and growing seasons. Sci. Hortic. 2005, 105, 177–195. [Google Scholar] [CrossRef]
- U.S. Department of Agriculture FoodData Central. Available online: https://fdc.nal.usda.gov/ (accessed on 14 April 2020).
- Pomares-Viciana, T.; Martinez-Valdivieso, D.; Font, R.; Gomez, P.; Del Rio-Celestino, M. Characterisation and prediction of carbohydrate content in zucchini fruit using near infrared spectroscopy. J. Sci. Food Agric. 2018, 98, 1703–1711. [Google Scholar] [CrossRef]
- Rolnik, A.; Olas, B. Vegetables from Cucurbitaceae family and their products; positive effect on human health. Nutrition 2020, in press. [Google Scholar] [CrossRef]
- Hamissou, M.; Smith, A.C.; Carter, R.E.; Triplett, J.K. Antioxidative properties of bitter gourd (Momordica charantia) and zucchini (Cucurbita pepo). Emir. J. Food Agric. 2013, 25, 641–647. [Google Scholar] [CrossRef]
- European Parliament; European Council. Regulation (EU) 2018/848 of the European Parliament and of the Council of 30 May 2018 on Organic Production and Labelling of Organic Products and Repealing Council Regulation (EC) No 834/2007. 2018. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=uriserv%3AOJ.L_.2018.150.01.0001.01.ENG (accessed on 9 May 2020).
- Aschemann-Witzel, J.; Zielke, S. Can’t Buy Me Green? A Review of Consumer Perceptions of and Behavior Toward the Price of Organic Food. J. Consum. Aff. 2017, 51, 211–251. [Google Scholar] [CrossRef]
- Mørk, T.; Bech-Larsen, T.; Grunert, K.G.; Tsalis, G. Determinants of citizen acceptance of environmental policy regulating consumption in public settings: Organic food in public institutions. J. Clean. Prod. 2017, 148, 407–414. [Google Scholar] [CrossRef]
- Azzurra, A.; Massimiliano, A.; Angela, M. Measuring sustainable food consumption: A case study on organic food. Sustain. Prod. Consum. 2019, 17, 95–107. [Google Scholar] [CrossRef]
- Rouphael, Y.; Kyriacou, M.C.; Petropoulos, S.A.; De Pascale, S.; Colla, G. Improving vegetable quality in controlled environments. Sci. Hortic. 2018, 234, 275–289. [Google Scholar] [CrossRef]
- Sanchez-Santed, F.; Colomina, M.T.; Herrero Hernandez, E. Organophosphate pesticide exposure and neurodegeneration. Cortex 2016, 74, 417–426. [Google Scholar] [CrossRef] [PubMed]
- Lukowicz, C.; Ellero-Simatos, S.; Regnier, M.; Polizzi, A.; Lasserre, F.; Montagner, A.; Lippi, Y.; Jamin, E.L.; Martin, J.-F.; Naylies, C.; et al. Metabolic Effects of a Chronic Dietary Exposure to a Low-Dose Pesticide Cocktail in Mice: Sexual Dimorphism and Role of the Constitutive Androstane Receptor. Environ. Health Perspect. 2018, 126, 67007. [Google Scholar] [CrossRef] [PubMed]
- Barański, M.; Średnicka-Tober, D.; Volakakis, N.; Seal, C.; Sanderson, R.; Stewart, G.B.; Benbrook, C.; Biavati, B.; Markellou, E.; Giotis, C.; et al. Higher antioxidant and lower cadmium concentrations and lower incidence of pesticide residues in organically grown crops: A systematic literature review and meta-analyses. Br. J. Nutr. 2014, 112, 794–811. [Google Scholar] [CrossRef] [PubMed]
- Barański, M.; Rempelos, L.; Iversen, P.O.; Leifert, C. Effects of organic food consumption on human health; the jury is still out! Food Nutr. Res. 2017, 61, 1–5. [Google Scholar] [CrossRef]
- Barends, C.; Weenen, H.; Warren, J.; Hetherington, M.M.; de Graaf, C.; de Vries, J.H.M. A systematic review of practices to promote vegetable acceptance in the first three years of life. Appetite 2019, 137, 174–197. [Google Scholar] [CrossRef]
- Maggio, A.; De Pascale, S.; Paradiso, R.; Barbieri, G. Quality and nutritional value of vegetables from organic and conventional farming. Sci. Hortic. 2013, 164, 532–539. [Google Scholar] [CrossRef]
- Kazimierczak, R.; Hallmann, E.; Kazimierczyk, M.; Rembiałkowska, E. Antioxidants Content in Chosen Spice Plants from Organic and Conventional Cultivation. J. Res. Appl. Agric. Eng. 2010, 55, 164–170. [Google Scholar]
- Bilsborrow, P.; Cooper, J.; Tétard-Jones, C.; Średnicka-Tober, D.; Barański, M.; Eyre, M.; Schmidt, C.; Shotton, P.; Volakakis, N.; Cakmak, I.; et al. The effect of organic and conventional management on the yield and quality of wheat grown in a long-term field trial. Eur. J. Agron. 2013, 51, 71–80. [Google Scholar] [CrossRef]
- Pedro, A.C.; Sánchez-Mata, M.C.; Pérez-Rodríguez, M.L.; Cámara, M.; López-Colón, J.L.; Bach, F.; Bellettini, M.; Haminiuk, C.W.I. Qualitative and nutritional comparison of goji berry fruits produced in organic and conventional systems. Sci. Hortic. 2019, 257, 108660. [Google Scholar] [CrossRef]
- Ponder, A.; Hallmann, E. The effects of organic and conventional farm management and harvest time on the polyphenol content in different raspberry cultivars. Food Chem. 2019, 301, 125295. [Google Scholar] [CrossRef] [PubMed]
- Carrillo, C.; Wilches-Pérez, D.; Hallmann, E.; Kazimierczak, R.; Rembiałkowska, E. Organic versus conventional beetroot. Bioactive compounds and antioxidant properties. Lwt 2019, 116, 108552. [Google Scholar] [CrossRef]
- PlantiCo. Available online: https://plantico.pl/ (accessed on 30 April 2020).
- PNOS Ożarów Mazowiecki. Available online: http://www.pnos.pl/ (accessed on 30 April 2020).
- Siebeneicher, G.E. Podręcznik Rolnictwa Ekologicznego; Wydawnictwo Naukowe PWN: Warszawa, Poland, 1999. [Google Scholar]
- Maćkowiak, C.; Żebrowski, J. Skład chemiczny obornika w Polsce. Nawozy i Nawożenie 2000, 4, 119–130. [Google Scholar]
- The Polish Committee for Standardization Polish Norm PN-EN 12145. Fruits and Vegetable Juices—Determination of Dry Matter—Gravimetric Method; The Polish Committee for Standardization: Warsaw, Poland, 2001.
- Hallmann, E.; Kazimierczak, R.; Marszałek, K.; Drela, N.; Kiernozek, E.; Toomik, P.; Matt, D.; Luik, A.; Rembiałkowska, E. The nutritive value of organic and conventional white cabbage (Brassica oleracea L. var. Capitata) and anti-apoptotic activity in gastric adenocarcinoma cells of sauerkraut juice produced therof. J. Agric. Food Chem. 2017, 65, 8171–8183. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.-Q.; Ye, X.-Q.; Fang, Z.-X.; Chen, J.-C.; Xu, G.-H.; Liu, D.-H. Phenolic compounds and antioxidant activity of extracts from ultrasonic treatment of Satsuma mandarin (Citrus unshiu Marc.) peels. J. Agric. Food Chem. 2008, 56, 5682–5690. [Google Scholar] [CrossRef]
- Nishiyama, I.; Fukuda, T.; Oota, T. Genotypic differences in chlorophyll, lutein, and beta-carotene contents in the fruits of actinidia species. J. Agric. Food Chem. 2005, 53, 6403–6407. [Google Scholar] [CrossRef]
- Singh, D.; Puri, M.; Wilkens, S.; Mathur, A.S.; Tuli, D.K.; Barrow, C.J. Characterization of a new zeaxanthin producing strain of Chlorella saccharophila isolated from New Zealand marine waters. Bioresour. Technol. 2013, 143, 308–314. [Google Scholar] [CrossRef]
- Nojavan, S.; Khalilian, F.; Kiaie, F.M.; Rahimi, A.; Arabanian, A.; Chalavi, S. Extraction and quantitative determination of ascorbic acid during different maturity stages of Rosa canina L. fruit. J. Food Compos. Anal. 2008, 21, 300–305. [Google Scholar] [CrossRef]
- Van de Velde, F.; Pirovani, M.; Cámara, M.; Güemes, D.; Bernardi, C. Optimization and validation of a UV–HPLC method for vitamin C determination in strawberries (Fragaria ananassa Duch.), using experimental designs. Food Anal. Methods 2012, 5, 1097–1104. [Google Scholar] [CrossRef]
- Kazimierczak, R.; Srednicka-Tober, D.; Hallmann, E.; Kopczynska, K.; Zarzynska, K. The impact of organic vs. conventional agricultural practices on selected quality features of eight potato cultivars. Agronomy 2019, 9, 799. [Google Scholar] [CrossRef]
- R Core Team. An Introduction to R; R Foundation for Statistical Computing: Vienna, Austria, 2019. [Google Scholar]
- Mattila, P.; Hellström, J. Phenolic acids in potatoes, vegetables, and some of their products. J. Food Compos. Anal. 2007, 20, 152–160. [Google Scholar] [CrossRef]
- Iswaldi, I.; Gómez-Caravaca, A.M.; Lozano-Sánchez, J.; Arráez-Román, D.; Segura-Carretero, A.; Fernández-Gutiérrez, A. Profiling of phenolic and other polar compounds in zucchini (Cucurbita pepo L.) by reverse-phase high-performance liquid chromatography coupled to quadrupole time-of-flight mass spectrometry. Food Res. Int. 2013, 50, 77–84. [Google Scholar] [CrossRef]
- Neocleous, D.; Savvas, D. Modelling Ca2+ accumulation in soilless zucchini crops: Physiological and agronomical responses. Agric. Water Manag. 2018, 203, 197–206. [Google Scholar] [CrossRef]
- Ilić, Z.S.; Fallik, E. Light quality manipulation improves vegetable quality at harvest and postharvest: A review. Environ. Exp. Bot. 2017, 139, 79–90. [Google Scholar] [CrossRef]
- Ombódi, A.O.; Pék, Z.; Szuvandzsiev, P.; Lugasi, A.; Ledóné Darázsi, H.; Helyes, L. Effect of coloured shade nets on some nutritional characteristics of a kapia type pepper grown in plastic tunnel. Columella J. Agric. Environ. Sci. 2016, 3, 25–33. [Google Scholar] [CrossRef]
- Baslam, M.; Garmendia, I.; Goicoechea, N. Enhanced accumulation of vitamins, nutraceuticals and minerals in lettuces associated with arbuscular mycorrhizal fungi (Amf): A question of interest for both vegetables and humans. Agriculture 2013, 3, 188–209. [Google Scholar] [CrossRef]
- Gomiero, T. Food quality assessment in organic vs. conventional agricultural produce: Findings and issues. Appl. Soil Ecol. 2018, 123, 714–728. [Google Scholar] [CrossRef]
- Buchanan, A.L.; Hooks, C.R.R. Influence of winter cover crop mulch on arthropods in a reduced tillage Cucurbit system. Environ. Entomol. 2018, 47, 292–299. [Google Scholar] [CrossRef]
- Kopta, T.; Híc, P.; Šlosár, M.; Pokluda, R. Quality changes in organic and conventional Hokkaido pumpkin (Cucurbita maxima Duch.) during storage. Biol. Agric. Hortic. 2018, 34, 1–9. [Google Scholar] [CrossRef]
- Armesto, J.; Rocchetti, G.; Senizza, B.; Pateiro, M.; Barba, F.J.; Domínguez, R.; Lucini, L.; Lorenzo, J.M. Nutritional characterization of Butternut squash (Cucurbita moschata D.): Effect of variety (Ariel vs. Pluto) and farming type (conventional vs. organic). Food Res. Int. 2020, 132, 109052. [Google Scholar] [CrossRef]
| Crop Production Practice | Organic | Conventional | ||
|---|---|---|---|---|
| Crop rotation | sugar beet + sheep manure-> winter spelt undersown with red clover-> red clover-> winter spelt | winter wheat-> winter rape-> spring barley | ||
| Fertilizer (dose) | Sheep manure (20t/ha) | Polifoska (625 kg/ha) | Ammonium sulphate (219 kg/ha) | Superphosphate (263 kg/ha) |
| The content of main compounds in fertilizers (%) | N (total): 0.76 P2O5: 0.4 K2O: 1.25 | N (total): 8 P2O5 2: 24 P2O5 1: 21 K2O 1: 24 SO3: 9 | N-NH: 17.2 N-NO: 17.2 | P2O5: 40 CaO: 10 Si: 5 |
| Sum of N, P and K applied to soil (kg/ha) [41] | N: 45.6 | N-NH: 37.7, N-NO: 37.7 | ||
| P: 13.9 | P: 124.6 | |||
| K: 166.7 | K: 125 | |||
| Factor | Dry Matter 1 | DHA 2 | l-ASC 3 | Vitamin C 4 | Polyphenols (Sum) 5 | Phenolic Acids (Sum) 5 | Flavonoids (Sum) 5 |
|---|---|---|---|---|---|---|---|
| Cultivation Year (CY) | |||||||
| 2016 | 5.29 ± 0.12 6 b 7 | 0.88 ± 0.07 b | 3.73 ± 0.19 a | 4.61 ± 0.22 c | 19.18 ± 0.85 c | 18.44 ± 0.83 c | 0.73 ± 0.03 c |
| 2017 | 5.77 ± 0.13 a | 1.31 ± 0.07 b | 4.40 ± 0.32 a | 5.70 ± 0.35 b | 45.74 ± 2.70 a | 40.69 ± 2.25 a | 5.05 ± 0.48 a |
| 2018 | 4.14 ± 0.10 c | 7.02 ± 0.32 a | 1.36 ± 0.10 b | 8.37 ± 0.32 a | 33.34 ± 1.81 b | 29.76 ± 1.54 b | 3.58 ± 0.32 b |
| Variety (VR) | |||||||
| Astra Polka | 5.06 ± 0.11 | 2.78 ± 0.28 | 3.14 ± 0.20 | 5.91 ± 0.29 | 32.76 ± 1.78 | 29.76 ± 1.52 | 3.00 ± 0.29 |
| Nimba | 5.13 ± 0.11 | 3.14 ± 0.28 | 3.31 ± 0.23 | 6.45 ± 0.27 | 32.81 ± 1.94 | 29.58 ± 1.62 | 3.23 ± 0.34 |
| Agronomic System (AS) | |||||||
| conventional | 4.83 ± 0.10 | 3.12 ± 0.26 | 3.22 ± 0.18 | 6.34 ± 0.25 | 23.51 ± 1.02 | 21.44 ± 0.88 | 2.07 ± 0.16 |
| organic | 5.37 ± 0.13 | 2.77 ± 0.31 | 3.22 ± 0.25 | 5.99 ± 0.31 | 42.63 ± 2.17 | 38.40 ± 1.80 | 4.22 ± 0.40 |
| ANOVA p-values | |||||||
| CY | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 |
| VR | NS 8 | 0.002 | NS | NS | NS | NS | NS |
| AS | 0.003 | 0.020 | NS | NS | <0.001 | <0.001 | <0.001 |
| CY × VR | NS | NS | NS | NS | NS | NS | NS |
| CY × AS | NS | NS | NS | NS | NS | NS | 0.012 |
| VR × AS | NS | NS | NS | NS | NS | NS | NS |
| CY × VR × AS | NS | NS | NS | NS | NS | NS | NS |
| Factor | Gallic Acid | Chlorogenic Acid | Caffeic Acid | p-Coumaric Acid | Ferulic Acid | Quercetin-3-O-Rutinoside | Kaempferol-3-O-Glucoside |
|---|---|---|---|---|---|---|---|
| Cultivation Year (CY) | |||||||
| 2016 | 6.02 ± 0.56 1 c 2 | 0.53 ± 0.03 c | 2.26 ± 0.31 a | 9.15 ± 0.36 a | 0.47 ± 0.02 c | 0.22 ± 0.02 c | 0.51 ± 0.01 c |
| 2017 | 18.82 ± 0.96 a | 6.98 ± 0.42 a | 2.91 ± 0.24 a | 8.22 ± 0.49 a | 3.76 ± 0.37 a | 2.88 ± 0.29 a | 2.16 ± 0.20 a |
| 2018 | 12.16 ± 0.62 b | 5.57 ± 0.30 b | 3.09 ± 0.37 a | 6.48 ± 0.37 b | 2.65 ± 0.24 b | 1.98 ± 0.18 b | 1.63 ± 0.15 b |
| Variety (VR) | |||||||
| Astra Polka | 12.55 ± 0.71 | 4.15 ± 0.35 | 2.62 ± 0.24 | 8.19 ± 0.37 | 2.24 ± 0.24 | 1.64 ± 0.18 | 1.36 ± 0.12 |
| Nimba | 12.17 ± 0.82 | 4.50 ± 0.35 | 2.88 ± 0.27 | 7.80 ± 0.33 | 2.34 ± 0.24 | 1.74 ± 0.20 | 1.50 ± 0.14 |
| Agronomic System (AS) | |||||||
| conventional | 8.50 ± 0.48 | 3.20 ± 0.23 | 1.63 ± 0.13 | 6.69 ± 0.28 | 1.42 ± 0.12 | 1.15 ± 0.11 | 0.92 ± 0.06 |
| organic | 16.47 ± 0.85 | 5.54 ± 0.42 | 3.93 ± 0.31 | 9.39 ± 0.38 | 3.21 ± 0.31 | 2.27 ± 0.24 | 1.97 ± 0.17 |
| ANOVA p-values | |||||||
| CY | <0.001 | <0.001 | 0.011 | <0.001 | <0.001 | <0.001 | <0.001 |
| VR | NS 3 | NS | NS | NS | NS | NS | NS |
| AS | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | 0.001 | <0.001 |
| CY × VR | NS | NS | NS | NS | NS | NS | NS |
| CY × AS | NS | <0.001 | 0.019 | <0.001 | 0.001 | NS | 0.002 |
| VR × AS | NS | NS | NS | NS | NS | NS | NS |
| CY × VR × AS | NS | NS | NS | 0.007 | NS | NS | NS |
| CY 1 | AS 2 | Chlorogenic Acid 3 | Caffeic Acid 3 | Ferulic Acid 3 | Flavonoids (Sum) 3 | Kaempferol-3-O-Glucoside 3 | Lutein 4 | β-Carotene 4 |
|---|---|---|---|---|---|---|---|---|
| 2016 | conventional | 0.43 ± 0.04 5 c 6 | 0.63 ± 0.04 c | 0.45 ± 0.03 d | 0.66 ± 0.02 d | 0.48 ± 0.02 c | 0.100 ± 0.003 b,c | 0.50 ± 0.02 b |
| 2016 | organic | 0.64 ± 0.05 c | 3.89 ± 0.53 a | 0.49 ± 0.02 d | 0.81 ± 0.04 d | 0.55 ± 0.02 c | 0.115 ± 0.004 a | 0.62 ± 0.03 b |
| 2017 | conventional | 5.27 ± 0.39 b | 2.16 ± 0.21 b,c | 2.21 ± 0.24 c | 3.23 ± 0.33 c | 1.32 ± 0.12 b | 0.106 ± 0.003 a,b | 0.60 ± 0.03 b |
| 2017 | organic | 8.81 ± 0.67 a | 3.72 ± 0.42 a,b | 5.42 ± 0.64 a | 6.98 ± 0.83 a | 3.06 ± 0.34 a | 0.100 ± 0.002 b,c | 1.03 ± 0.04 a |
| 2018 | conventional | 3.84 ± 0.24 b | 2.11 ± 0.29 b,c | 1.58 ± 0.16 c,d | 2.27 ± 0.22 c,d | 0.94 ± 0.08 b,c | 0.083 ± 0.003 d | 0.85 ± 0.08 a |
| 2018 | organic | 7.69 ± 0.36 a | 4.21 ± 0.68 a | 3.86 ± 0.40 b | 5.07 ± 0.54 b | 2.40 ± 0.24 a | 0.091 ± 0.003 c,d | 1.06 ± 0.11 a |
| ANOVA p-values | ||||||||
| CY | <0.001 | 0.010 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | |
| AS | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | 0.013 | <0.001 | |
| CY × AS | <0.001 | 0.016 | 0.001 | 0.011 | 0.002 | 0.018 | 0.037 | |
| CY 1 | AS 2 | p-Coumaric Acid 3 | Chlorophylls (Sum) 4 | Chlorophyll b 4 | Chlorophyll a 4 |
|---|---|---|---|---|---|
| Variety Astra Polka | |||||
| 2016 | conventional | 10.48 ± 0.54 5 a 6 | 2.34 ± 0.11 b, c | 0.55 ± 0.03 a,b | 1.79 ± 0.10 a,b |
| 2016 | organic | 8.77 ± 0.91 a | 2.78 ± 0.12 a, b | 0.62 ± 0.03 a | 2.16 ± 0.10 a |
| 2017 | conventional | 5.55 ± 0.54 b | 2.46 ± 0.14 a,b,c | 0.60 ± 0.02 a,b | 1.86 ± 0.13 a, b |
| 2017 | organic | 11.09 ± 1.18 a | 2.87 ± 0.11 a | 0.62 ± 0.03 a,b | 2.22 ± 0.09 a |
| 2018 | conventional | 3.92 ± 0.38 b | 1.62 ± 0.10 d | 0.44 ± 0.02 c | 1.17 ± 0.08 c |
| 2018 | organic | 9.51 ± 0.64 a | 2.19 ± 0.15 c | 0.52 ± 0.03 b,c | 1.67 ± 0.13 b |
| Variety Nimba | |||||
| 2016 | conventional | 8.30 ± 0.55 a | 1.97 ± 0.09 c | 0.48 ± 0.02 b | 1.48 ± 0.07 b |
| 2016 | organic | 8.96 ± 0.79 a | 2.99 ± 0.13 a | 0.68 ± 0.03 a | 2.31 ± 0.12 a |
| 2017 | conventional | 7.01 ± 0.57 a,b | 2.14 ± 0.11 b,c | 0.59 ± 0.03 a,b | 1.55 ± 0.09 b |
| 2017 | organic | 9.45 ± 1.12 a | 2.56 ± 0.18 a,b | 0.64 ± 0.03 a | 1.92 ± 0.16 a,b |
| 2018 | conventional | 4.65 ± 0.31 b | 1.98 ± 0.16 c | 0.49 ± 0.03 b | 1.49 ± 0.13 b |
| 2018 | organic | 8.36 ± 0.79 a | 1.94 ± 0.13 c | 0.50 ± 0.03 b | 1.44 ± 0.11 b |
| ANOVA p-values | |||||
| CY | 0.002 | <0.001 | <0.001 | <0.001 | |
| AS | 0.016 | 0.004 | 0.008 | 0.006 | |
| CY × AS | NS 7 | <0.001 | 0.004 | 0.003 | |
| Factor | Carotenoids (Sum) | Lutein | Zeaxanthin | β-Carotene | Chlorophylls (Sum) | Chlorophyll b | Chlorophyll a |
|---|---|---|---|---|---|---|---|
| Cultivation Year (CY) | |||||||
| 2016 | 0.66 ± 0.02 1 b 2 | 0.107 ± 0.003 a | - | 0.56 ± 0.02 b | 2.53 ± 0.07 a | 0.59 ± 0.01 a | 1.94 ± 0.06 a |
| 2017 | 0.94 ± 0.04 a | 0.103 ± 0.002 a | 0.036 ± 0.001 | 0.80 ± 0.04 a | 2.50 ± 0.07 a | 0.61 ± 0.01 a | 1.89 ± 0.06 a |
| 2018 | 1.05 ± 0.07 a | 0.087 ± 0.002 b | 0.027 ± 0.001 | 0.95 ± 0.06 a | 1.93 ± 0.07 b | 0.49 ± 0.01 b | 1.44 ± 0.06 b |
| Variety (VR) | |||||||
| Astra Polka | 0.88 ± 0.04 | 0.099 ± 0.002 | 0.031 ± 0.001 | 0.77 ± 0.03 | 2.34 ± 0.06 | 0.55 ± 0.01 | 1.78 ± 0.05 |
| Nimba | 0.86 ± 0.04 | 0.099 ± 0.002 | 0.031 ± 0.001 | 0.75 ± 0.04 | 2.28 ± 0.07 | 0.56 ± 0.01 | 1.71 ± 0.06 |
| Agronomic System (AS) | |||||||
| conventional | 0.76 ± 0.03 | 0.095 ± 0.002 | 0.030 ± 0.001 | 0.65 ± 0.03 | 2.06 ± 0.05 | 0.52 ± 0.01 | 1.54 ± 0.04 |
| organic | 0.99 ± 0.04 | 0.103 ± 0.002 | 0.032 ± 0.001 | 0.87 ± 0.04 | 2.57 ± 0.07 | 0.60 ± 0.01 | 1.97 ± 0.06 |
| ANOVA p-values | |||||||
| CY | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 |
| VR | NS 3 | NS | NS | NS | NS | NS | NS |
| AS | <0.001 | 0.015 | NS | <0.001 | <0.001 | <0.001 | <0.001 |
| CY × VR | NS | NS | NS | NS | NS | NS | NS |
| CY × AS | NS | 0.020 | NS | 0.039 | 0.036 | 0.026 | NS |
| VR × AS | NS | NS | NS | NS | NS | NS | NS |
| CY × VR × AS | NS | NS | NS | NS | 0.003 | 0.028 | 0.006 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kopczyńska, K.; Kazimierczak, R.; Średnicka-Tober, D.; Barański, M.; Wyszyński, Z.; Kucińska, K.; Perzanowska, A.; Szacki, P.; Rembiałkowska, E.; Hallmann, E. The Profile of Selected Antioxidants in Two Courgette Varieties from Organic and Conventional Production. Antioxidants 2020, 9, 404. https://doi.org/10.3390/antiox9050404
Kopczyńska K, Kazimierczak R, Średnicka-Tober D, Barański M, Wyszyński Z, Kucińska K, Perzanowska A, Szacki P, Rembiałkowska E, Hallmann E. The Profile of Selected Antioxidants in Two Courgette Varieties from Organic and Conventional Production. Antioxidants. 2020; 9(5):404. https://doi.org/10.3390/antiox9050404
Chicago/Turabian StyleKopczyńska, Klaudia, Renata Kazimierczak, Dominika Średnicka-Tober, Marcin Barański, Zdzisław Wyszyński, Katarzyna Kucińska, Aneta Perzanowska, Paweł Szacki, Ewa Rembiałkowska, and Ewelina Hallmann. 2020. "The Profile of Selected Antioxidants in Two Courgette Varieties from Organic and Conventional Production" Antioxidants 9, no. 5: 404. https://doi.org/10.3390/antiox9050404
APA StyleKopczyńska, K., Kazimierczak, R., Średnicka-Tober, D., Barański, M., Wyszyński, Z., Kucińska, K., Perzanowska, A., Szacki, P., Rembiałkowska, E., & Hallmann, E. (2020). The Profile of Selected Antioxidants in Two Courgette Varieties from Organic and Conventional Production. Antioxidants, 9(5), 404. https://doi.org/10.3390/antiox9050404