Increasing the Magnesium Concentration in Various Dialysate Solutions Differentially Modulates Oxidative Stress in a Human Monocyte Cell Line
Abstract
1. Introduction
2. Materials and Methods
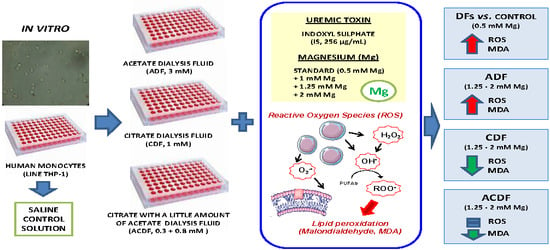
2.1. Hemodialysis Fluids
2.2. Culture of Human Monocytes (THP-1)
2.3. Quantification of Reactive Oxygen Species Levels
2.4. Lipid Peroxidation Assay
2.5. Cell Viability Assay
2.6. Statistical Analysis
3. Results
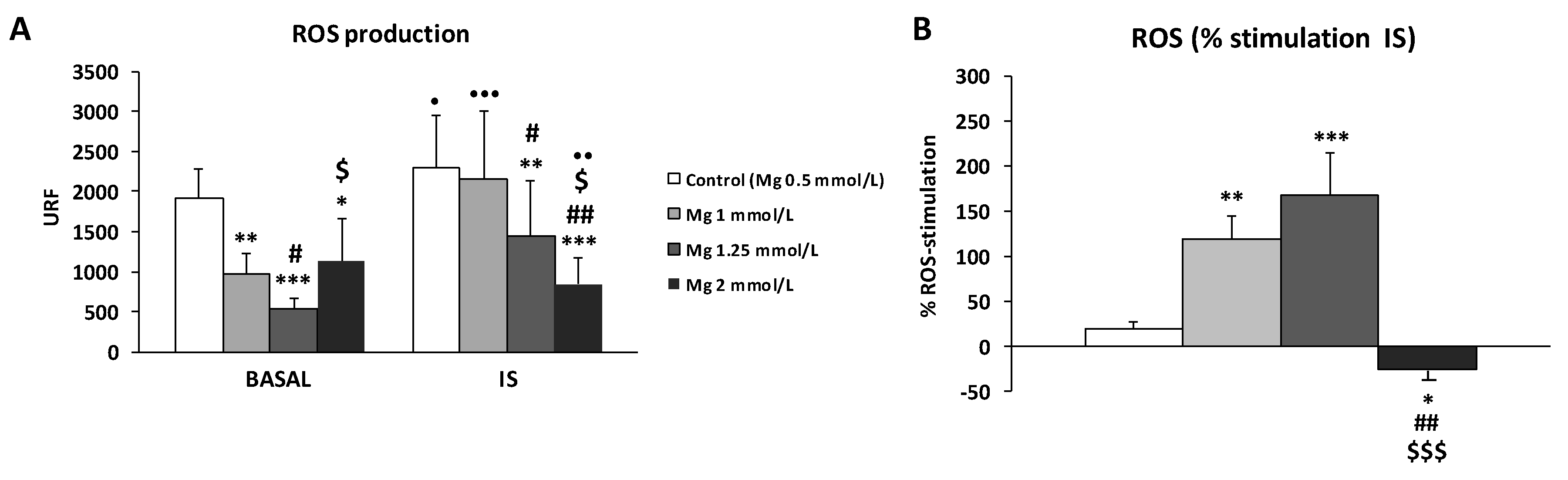
3.1. ROS Production in THP-1 Cells Induced by IS Treatment in Different Dialysis Fluids
3.2. High Concentration of Mg Attenuates ROS Production in IS-Stimulated THP-1 Cells
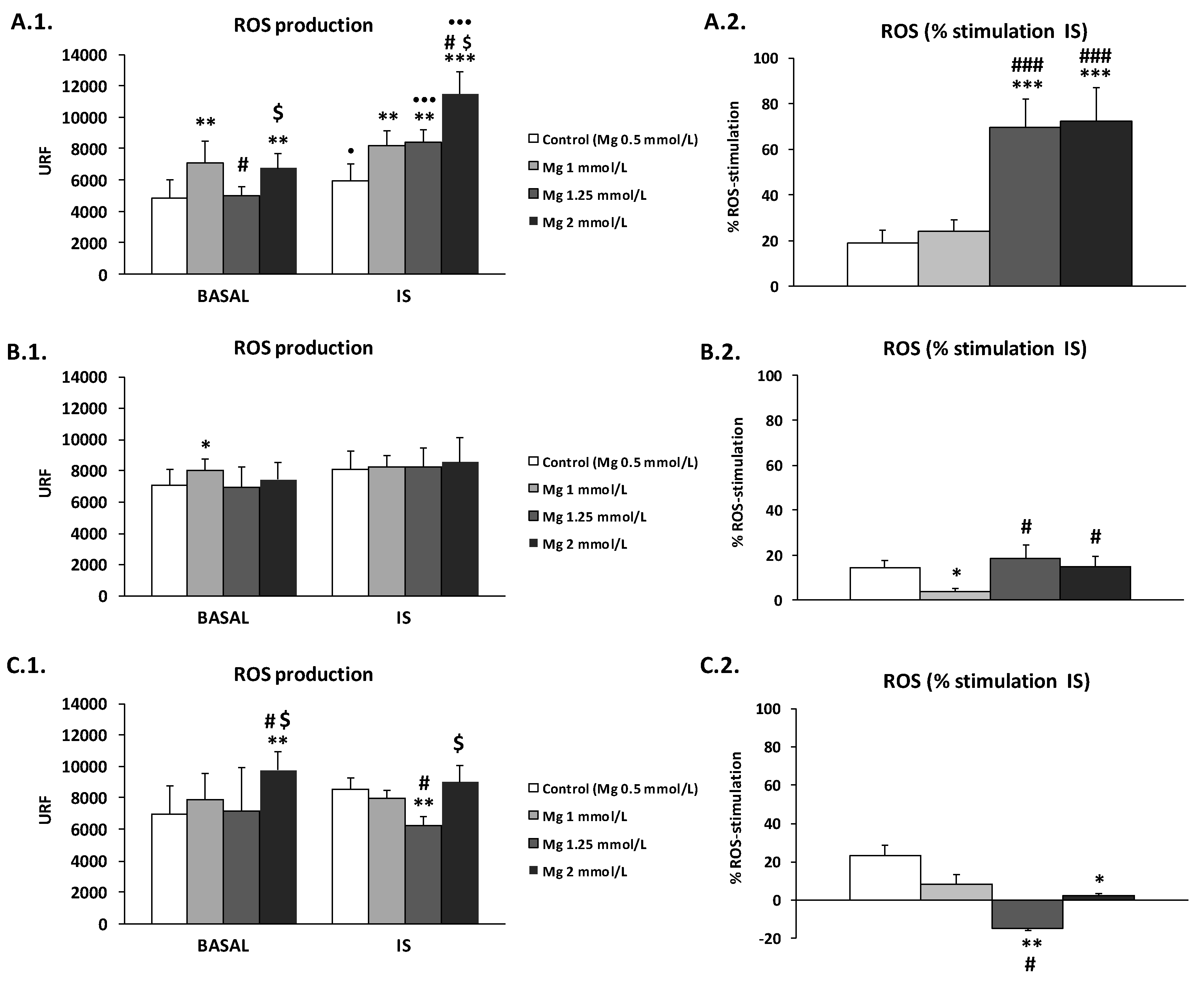
3.3. Effect of High Mg Concentration on Different Dialysate-Induced ROS Production in IS-Stimulated THP-1 Cells
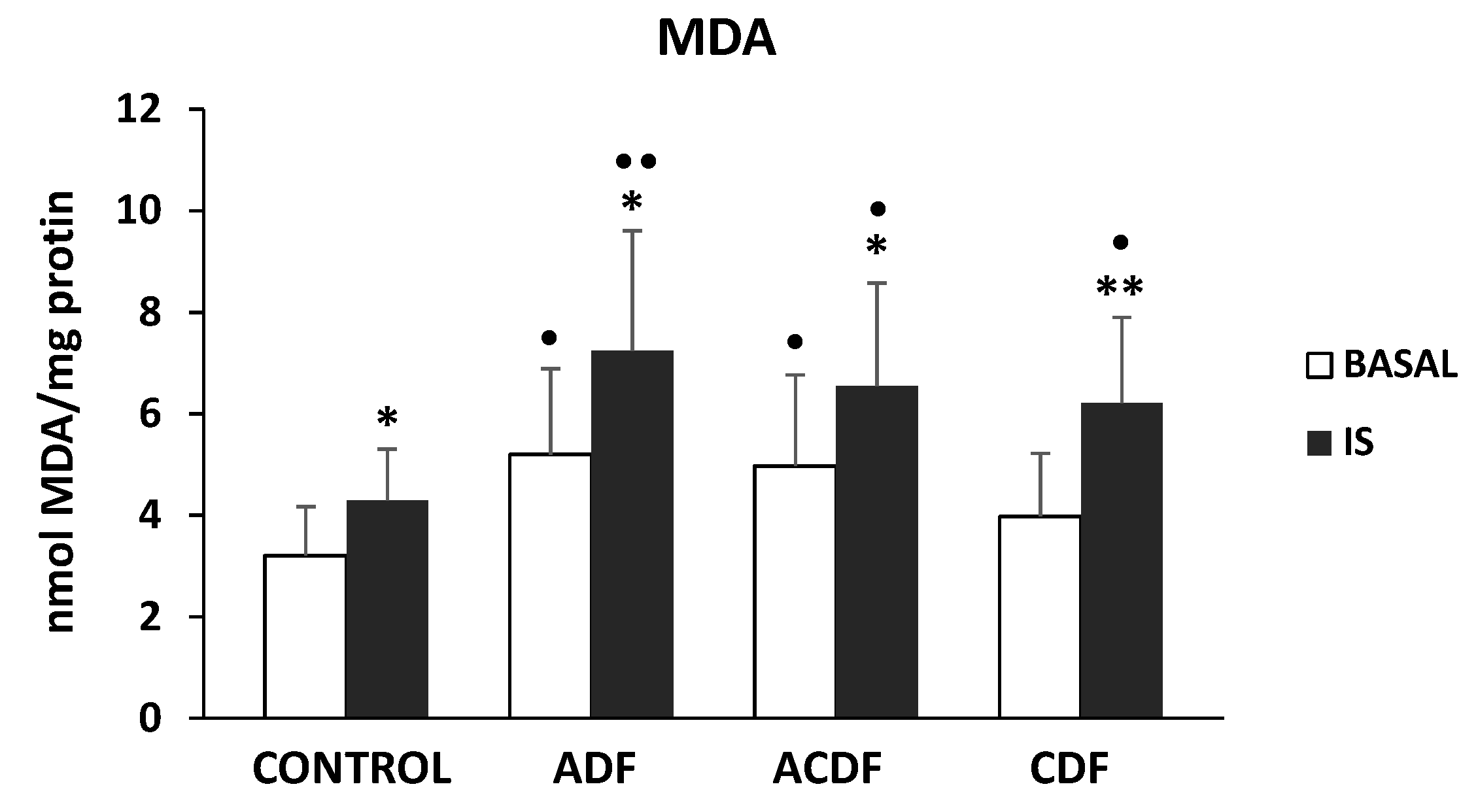
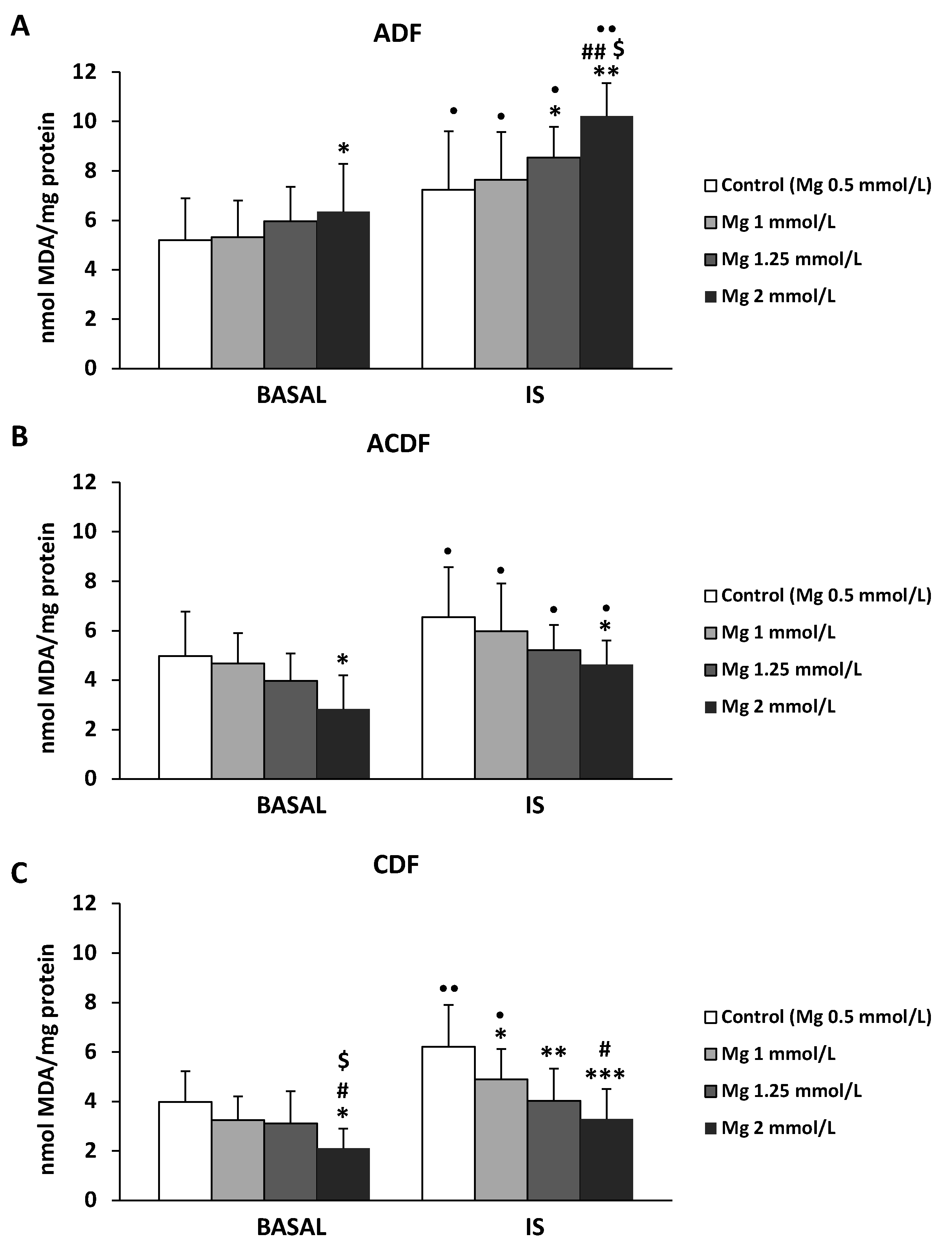
3.4. Effect of High Mg Concentration on Different Dialysate-Induced Lipid Oxidative Damage in IS-Stimulated THP-1 Cells
3.5. Effect of High Mg Containing Dialysates on the Viability of IS-Treated THP-1 Monocytes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Di Angelantonio, E.; Chowdhury, R.; Sarwar, N.; Aspelund, T.; Danesh, J.; Gudnason, V. Chronic kidney disease and risk of major cardiovascular disease and non-vascular mortality: Prospective population based cohort study. BMJ 2010, 341, c4986. [Google Scholar] [CrossRef]
- Chien, K.L.; Lin, H.J.; Lee, B.C.; Hsu, H.C.; Lee, Y.T.; Chen, M.F. A prediction model for the risk of incident chronic kidney disease. Am. J. Med. 2010, 123, 836–846. [Google Scholar] [CrossRef]
- Cozzolino, M.; Mangano, M.; Stucchi, A.; Ciceri, P.; Conte, F.; Galassi, A. Cardiovascular disease in dialysis patients. Nephrol. Dial. Transplant. 2018, 33 (Suppl. 3), iii28–iii34. [Google Scholar] [CrossRef]
- Assem, M.; Lando, M.; GrissFi, M.; Kamel, S.; MassyI, Z.A.; Chillon, J.M.; Hénaut, L. The Impact of Uremic Toxins on Cerebrovascular and Cognitive Disorders. Toxins 2018, 10, 303. [Google Scholar] [CrossRef]
- Niwa, T.; Ise, M. Indoxyl sulfate, a circulating uremic toxin, stimulates the progression of glomerular sclerosis. J. Lab. Clin. Med. 1994, 124, 96–104. [Google Scholar]
- Barreto, F.C.; Barreto, D.V.; Liabeuf, S.; Meert, N.; Glorieux, G.; Temmar, M.; Choukroun, G.; Vanholder, R.; Massy, Z.A.; European Uremic Toxin Work Group (EUTox). Serum indoxyl sulfate is associated with vascular disease and mortality in chronic kidney disease patients. Clin. J. Am. Soc. Nephrol. 2009, 4, 1551–1558. [Google Scholar] [CrossRef] [PubMed]
- Moradi, H.; Sica, D.A.; Kalantar-Zadeh, K. Cardiovascular burden associated with uremic toxins in patients with chronic kidney disease. Am. J. Nephrol. 2013, 38, 136–148. [Google Scholar] [CrossRef] [PubMed]
- Vanholder, R.; Argiles, A.; Baurmeister, U.; Brunet, P.; Clark, W.; Cohen, G.; Dedeyn, P.P.; Deppisch, R.; Descamps-Latscha, B.; Henle, T.; et al. Uremic toxicity: Present state of the art. Int. J. Artif. Organs 2001, 24, 695–725. [Google Scholar] [CrossRef]
- Liakopoulos, V.; Roumeliotis, S.; Zarogiannis, S.; Eleftheriadis, T.; Mertens, P.R. Oxidative stress in hemodialysis: Causative mechanisms, clinical implications, and possible therapeutic interventions. Semin. Dial. 2019, 32, 58–71. [Google Scholar] [CrossRef] [PubMed]
- Varan, H.I.; Dursun, B.; Dursun, E.; Ozben, T.; Suleymanlar, G. Acute effects of hemodialysis on oxidative stress parameters in chronic uremic patients: Comparison of two dialysis membranes. Int. J. Nephrol. Renovasc. Dis. 2010, 3, 39–45. [Google Scholar]
- Liakopoulos, V.; Roumeliotis, S.; Gorny, X.; Dounousi, E.; Mertens, P.R. Oxidative Stress in Hemodialysis Patients: A Review of the Literature. Oxid. Med. Cell. Longev. 2017, 2017, 3081856. [Google Scholar] [CrossRef] [PubMed]
- Liakopoulos, V.; Roumeliotis, S.; Bozikas, A.; Eleftheriadis, T.; Dounousi, E. Antioxidant Supplementation in Renal Replacement Therapy Patients: Is There Evidence? Oxid. Med. Cell. Longev. 2019, 2019, 9109473. [Google Scholar] [CrossRef] [PubMed]
- Todeschini, M.; Macconi, D.; Fernandez, N.G.; Ghilardi, M.; Anabaya, A.; Binda, E.; Morigi, M.; Cattaneo, D.; Perticucci, E.; Remuzzi, G.; et al. Effect of acetate-free biofiltration and bicarbonate hemodialysis on neutrophil activation. Am. J. Kidney Dis. 2002, 40, 783–793. [Google Scholar] [CrossRef] [PubMed]
- Masuda, A.; Hagiwara, S.; Tanimoto, M.; Kodama, F.; Okumura, K.; Nohara, N.; Matsumoto, M.; Maiguma, M.; Omote, K.; Io, H.; et al. Effects of Acetate-Free Citrate Dialysate on Glycoxidation and Lipid Peroxidation Products in Hemodialysis Patients. Nephron Extra 2012, 2, 256–268. [Google Scholar] [CrossRef]
- Himmelfarb, J.; Lazarus, J.M.; Hakim, R. Reactive oxygen species production by monocytes and polymorphonuclear leukocytes during dialysis. Am. J. Kidney Dis. 1991, 17, 271–276. [Google Scholar] [CrossRef]
- Pérez-García, R.; Ramírez, R.; de Sequera, P.; Albalate, M.; Puerta, M.; Ortega, M.; Ruiz, M.C.; Alcazar-Arroyo, R. Citrate dialysate does not induce oxidative stress or inflammation in vitro as compared to acetate dialysate. Nefrologia 2017, 37, 630–637. [Google Scholar] [CrossRef]
- Misra, M. Basic mechanisms governing solute and fluid transport in hemodialysis. Hemodial. Int. 2008, 12 (Suppl 2), S25–S28. [Google Scholar] [CrossRef]
- Pizzarelli, F.; Cerrai, T.; Dattolo, P.; Ferro, G. On-line hemodiafiltration with and without acetate. Nephrol. Dial. Trasplant. 2006, 21, 1648–1651. [Google Scholar] [CrossRef]
- De Sequera, P.; Albalate, M.; Peréz-García, R.R.; Corchete, E.; Arribas, P.; Alcázar, R.; Ortega, M.; Puerta, M. Acute effect of citrate bath on postdialysis alkalaemia. Nefrología (Engl. Ed.) 2015, 35, 164–171. [Google Scholar] [CrossRef]
- Dellepiane, S.; Medica, D.; Guarena, C.; Musso, T.; Quercia, A.D.; Leonardi, G.; Marengo, M.; Migliori, M.; Panichi, V.; Biancone, L.; et al. Citrate anion improves chronic dialysis efficacy, reduces systemic inflammation and prevents Chemerin-mediated microvascular injury. Sci. Rep. 2019, 9, 10622. [Google Scholar] [CrossRef]
- Ikee, R. Cardiovascular disease, mortality, and magnesium in chronic kidney disease: Growing interest in magnesium-related interventions. Ren. Replace. Ther. 2018, 4, 1. [Google Scholar] [CrossRef]
- Nielsen, F.H. Magnesium deficiency and increased inflammation: Current perspectives. J. Inflamm. Res. 2018, 11, 25–34. [Google Scholar] [CrossRef] [PubMed]
- Leenders, N.H.J.; Vervloet, M.G. Magnesium: A Magic Bullet for Cardiovascular Disease in Chronic Kidney Disease? Nutrients 2019, 11, 455. [Google Scholar] [CrossRef] [PubMed]
- Muñoz-Castañeda, J.R.; Pendón-Ruiz de Mier, M.V.; Rodríguez, M.; Rodríguez-Ortiz, M.E. Magnesium Replacement to Protect Cardiovascular and Kidney Damage? Lack of Prospective Clinical Trials. Int. J. Mol. Sci. 2018, 19, 664. [Google Scholar] [CrossRef]
- Pun, P.H.; Middleton, J.P. Dialysate Potassium, Dialysate Magnesium, and Hemodialysis Risk. J. Am. Soc. Nephrol. 2017, 28, 3441–3451. [Google Scholar] [CrossRef]
- Cunningham, J.; Rodríguez, M.; Messa, P. Magnesium in chronic kidney disease Stages 3 and 4 and in dialysis patients. Clin. Kidney 2012, 5 (Suppl. 1), i39–i51. [Google Scholar] [CrossRef]
- Alhosaini, M.; Leehey, D.J. Magnesium and Dialysis: The Neglected Cation. Am. J. Kidney Dis. 2015, 66, 523–531. [Google Scholar] [CrossRef]
- Floege, J. Magnesium Concentration in Dialysate Is Higher Better? Clin. J. Am. Soc. Nephrol. 2018, 13, 1309–1310. [Google Scholar] [CrossRef]
- Navarro-González, J.F.; Mora-Fernández, C.; García-Pérez, J. Clinical implications of disordered magnesium homeostasis in chronic renal failure and dialysis. Semin. Dial. 2009, 22, 37–44. [Google Scholar] [CrossRef]
- De Sequera, P.; Pérez-García, R.; Molina-Nuñez, M.; Muñoz-González, R.I.; Álvarez-Fernández, G.; Mérida-Herrero, E.; Camba-Caride, M.J.; Blázquez-Collado, L.A.; Alcaide-Lara, M.P.; Echarri-Carrillo, R. Prospective randomised multicentre study to demonstrate the benefits of haemodialysis without acetate (with citrate): ABC-treat Study. Acute effect of citrate. Nefrología 2019, 39, 424–433. [Google Scholar]
- Tumlin, J.A.; Roy-Chaudhury, P.; Koplan, B.A.; Koplan, B.A.; Costea, A.I.; Kher, V.; Williamson, D.; Pokhariyal, S.; Charytan, D.M.; MiD Investigators and Committees. Relationship between dialytic parameters and reviewer confirmed arrhythmias in hemodialysis patients in the monitoring in dialysis study. BMC Nephrol. 2019, 20, 80. [Google Scholar] [CrossRef]
- Schmaderer, C.; Braunisch, M.C.; Suttmann, Y.; Lorenz, G.; Pham, D.; Haller, B.; Angermann, S.; Matschkal, J.; Renders, L.; Baumann, M.; et al. Reduced Mortality in Maintenance Haemodialysis Patients on High versus Low Dialysate Magnesium: A Pilot Study. Nutrients 2017, 9, 926. [Google Scholar] [CrossRef] [PubMed]
- Carmona, A.; Guerrero, F.; Buendia, P.; Obrero, T.; Aljama, P.; Carracedo, J. Microvesicles Derived from Indoxyl Sulfate Treated Endothelial Cells Induce Endothelial Progenitor Cells Dysfunction. Front. Physiol. 2017, 8, 666. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Joseph, J.A. Quantifying cellular oxidative stress by dichlorofluorescein assay using microplate reader. Free Radic. Biol. Med. 1999, 27, 612–616. [Google Scholar] [CrossRef]
- Gabutti, L.; Lucchini, B.; Marone, C.; Alberio, L.; Burnier, M. Citrate- vs. acetate-based dialysate in bicarbonate haemodialysis: Consequences on haemodynamics, coagulation, acid-base status, and electrolytes. BMC Nephrol. 2009, 10, 7. [Google Scholar] [CrossRef] [PubMed]
- Zheltova, A.A.; Kharitonova, M.V.; Iezhitsa, I.N.; Spasov, A.A. Magnesium deficiency and oxidative stress: An update. Biomedicine (Taipei) 2016, 6, 20. [Google Scholar] [CrossRef] [PubMed]
- Dou, L.; Bertrand, E.; Cerini, C.; Faure, V.; Sampol, J.; Vanholder, R.; Berland, Y.; Brunet, P. The uremic solutes p-cresol and indoxyl sulfate inhibit endothelial proliferation and wound repair. Kidney Int. 2004, 65, 442–451. [Google Scholar] [CrossRef]
- Lekawanvijit, S.; Adrahtas, A.; Kelly, D.J.; Kompa, A.R.; Wang, B.H.; Krum, H. Does indoxyl sulfate, a uraemic toxin, have direct effects on cardiac fibroblasts and myocytes? Eur. Heart J. 2010, 31, 1771–1779. [Google Scholar] [CrossRef]
- Niwa, T. Role of indoxyl sulfate in the progression of chronic kidney disease and cardiovascular disease: Experimental and clinical effects of oral sorbent AST-120. Ther. Apher. Dial. 2011, 15, 120–124. [Google Scholar] [CrossRef]
- Adesso, S.; Popolo, A.; Bianco, G.; Sorrentino, R.; Pinto, A.; Autore, G.; Marzocco, S. The uremic toxin indoxyl sulphate enhances macrophage response to LPS. PLoS ONE 2013, 8, e76778. [Google Scholar] [CrossRef]
- Yang, K.; Xu, X.; Nie, L.; Xiao, T.; Guan, X.; He, T.; Yu, Y.; Liu, L.; Huang, Y.; Zhang, J.; et al. Indoxyl sulfate induces oxidative stress and hypertrophy in cardiomyocytes by inhibiting the AMPK/UCP2 signaling pathway. Toxicol. Lett. 2015, 234, 110–119. [Google Scholar] [CrossRef] [PubMed]
- Gritters, M.; Grooteman, M.P.; Schoorl, M.; Schoorl, M.; Bartels, P.C.; Scheffer, P.G.; Teerlink, T.; Schalkwijk, C.G.; Spreeuwenberg, M.; Nubé, M.J. Citrate anticoagulation abolishes degranulation of polymorphonuclear cells and platelets and reduces oxidative stress during haemodialysis. Nephrol. Dial. Transplant. 2006, 21, 153–159. [Google Scholar] [CrossRef] [PubMed]
- Carozzi, S.; Nasini, M.G.; Caviglia, P.M.; Schelotto, C.; Santoni, O.; Atti, M. Acetate free biofiltration. Effects on peripheral blood monocyte activation and cytokine release. ASAIO J. 1992, 38, 52–54. [Google Scholar] [CrossRef] [PubMed]
- Higuchi, T.; Yamamoto, C.; Kuno, T.; Okada, K.; Soma, M.; Fukuda, N.; Nagura, Y.; Takahashi, S.; Matsumoto, K. A comparison of bicarbonate hemodialysis, hemodiafiltration, and acetate-free biofiltration on cytokine production. Ther. Apher. Dial. 2004, 8, 460–467. [Google Scholar] [CrossRef]
- Wu, X.; Dai, H.; Liu, L.; Xu, C.; Yin, Y.; Yi, J.; Bielec, M.D.; Han, Y.; Lia, S. Citrate reduced oxidative damage in stem cells by regulating cellular redox signaling pathways and represent a potential treatment for oxidative stress-induced diseases. Redox Biol. 2019, 21, 101057. [Google Scholar] [CrossRef]
- Van de Wal-Visscher, E.R.; Kooman, J.P.; Van der Sande, F.M. Magnesium in Chronic Kidney Disease: Should We Care? Blood Purif. 2018, 45, 173–178. [Google Scholar] [CrossRef]
- Küchle, C.; Suttmann, Y.; Reichelt, A.L.; Apfelböck, J.; Zoller, V.; Heemann, U. Correcting low magnesia levels in hemodialysis by higher dialysate magnesium. Cogent Med. 2017, 4, 1302544. [Google Scholar] [CrossRef]
- Van Laecke, S.; Van Biesen, W.; Vanholder, R. Hypomagnesaemia, the kidney and the vessels. Nephrol. Dial. Transplant. 2012, 27, 4003–4010. [Google Scholar] [CrossRef]
- Wolf, F.I.; Trapani, V.; Simonacci, M.; Ferré, S.; Maier, J.A. Magnesium deficiency and endothelial dysfunction: Is oxidative stress involved? Magnes. Res. 2008, 21, 58–64. [Google Scholar]
- Dickens, B.F.; Weglicki, W.B.; Li, Y.S.; Mak, I.T. Magnesium deficiency in vitro enhances free radical-induced intracellular oxidation and cytotoxicity in endothelial cells. FEBS Lett. 1992, 311, 187–191. [Google Scholar] [CrossRef]
- Maier, J.A.; Bernardini, D.; Rayssiguier, Y.; Mazur, A. High concentrations of magnesium modulate vascular endothelial cell behaviour in vitro. Biochim. Biophys. Acta 2004, 1689, 6–12. [Google Scholar] [CrossRef] [PubMed]
- Ling, X.C.; Kuo, K.L. Oxidative stress in chronic kidney disease. Ren. Replace Ther. 2018, 4, 53. [Google Scholar] [CrossRef]
- Daenen, K.; Andries, A.; Mekahli, D.; Van Schepdael, A.; Jouret, F.; Bammens, B. Oxidative stress in chronic kidney disease. Pediatr. Nephrol. 2019, 34, 975–991. [Google Scholar] [CrossRef] [PubMed]

| CS or DF Reference | Hank’s Medium | Acetate SoftPac® Baxter | Citrate SelectBag Citrate® Baxter | Acetate + Citrate Citrasate® Nipro |
|---|---|---|---|---|
| Na, mM | 140 | 140 | 140 | 140 |
| K, mM | 2 | 2 | 2 | 2 |
| Ca, mM | 1.50 | 1.50 | 1.65 | 1.50 |
| Mg, mM | 0.50 | 0.50 | 0.50 | 0.50 |
| Cl, mM | 106 | 109.5 | 106.5 | 105.7 |
| Citrate, mM | 0 | 0 | 1 | 0.8 |
| Acetate, mM | 0 | 3 | 0 | 0.3 |
| Bicarbonate, mM | 34 | 34 | 34 | 34 |
| Glucose, mg/dL | 100 | 100 | 100 | 100 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vida, C.; Carracedo, J.; de Sequera, P.; Bodega, G.; Pérez, R.; Alique, M.; Ramírez, R. Increasing the Magnesium Concentration in Various Dialysate Solutions Differentially Modulates Oxidative Stress in a Human Monocyte Cell Line. Antioxidants 2020, 9, 319. https://doi.org/10.3390/antiox9040319
Vida C, Carracedo J, de Sequera P, Bodega G, Pérez R, Alique M, Ramírez R. Increasing the Magnesium Concentration in Various Dialysate Solutions Differentially Modulates Oxidative Stress in a Human Monocyte Cell Line. Antioxidants. 2020; 9(4):319. https://doi.org/10.3390/antiox9040319
Chicago/Turabian StyleVida, Carmen, Julia Carracedo, Patricia de Sequera, Guillermo Bodega, Rafael Pérez, Matilde Alique, and Rafael Ramírez. 2020. "Increasing the Magnesium Concentration in Various Dialysate Solutions Differentially Modulates Oxidative Stress in a Human Monocyte Cell Line" Antioxidants 9, no. 4: 319. https://doi.org/10.3390/antiox9040319
APA StyleVida, C., Carracedo, J., de Sequera, P., Bodega, G., Pérez, R., Alique, M., & Ramírez, R. (2020). Increasing the Magnesium Concentration in Various Dialysate Solutions Differentially Modulates Oxidative Stress in a Human Monocyte Cell Line. Antioxidants, 9(4), 319. https://doi.org/10.3390/antiox9040319