Resveratrol in Autism Spectrum Disorders: Behavioral and Molecular Effects
Abstract
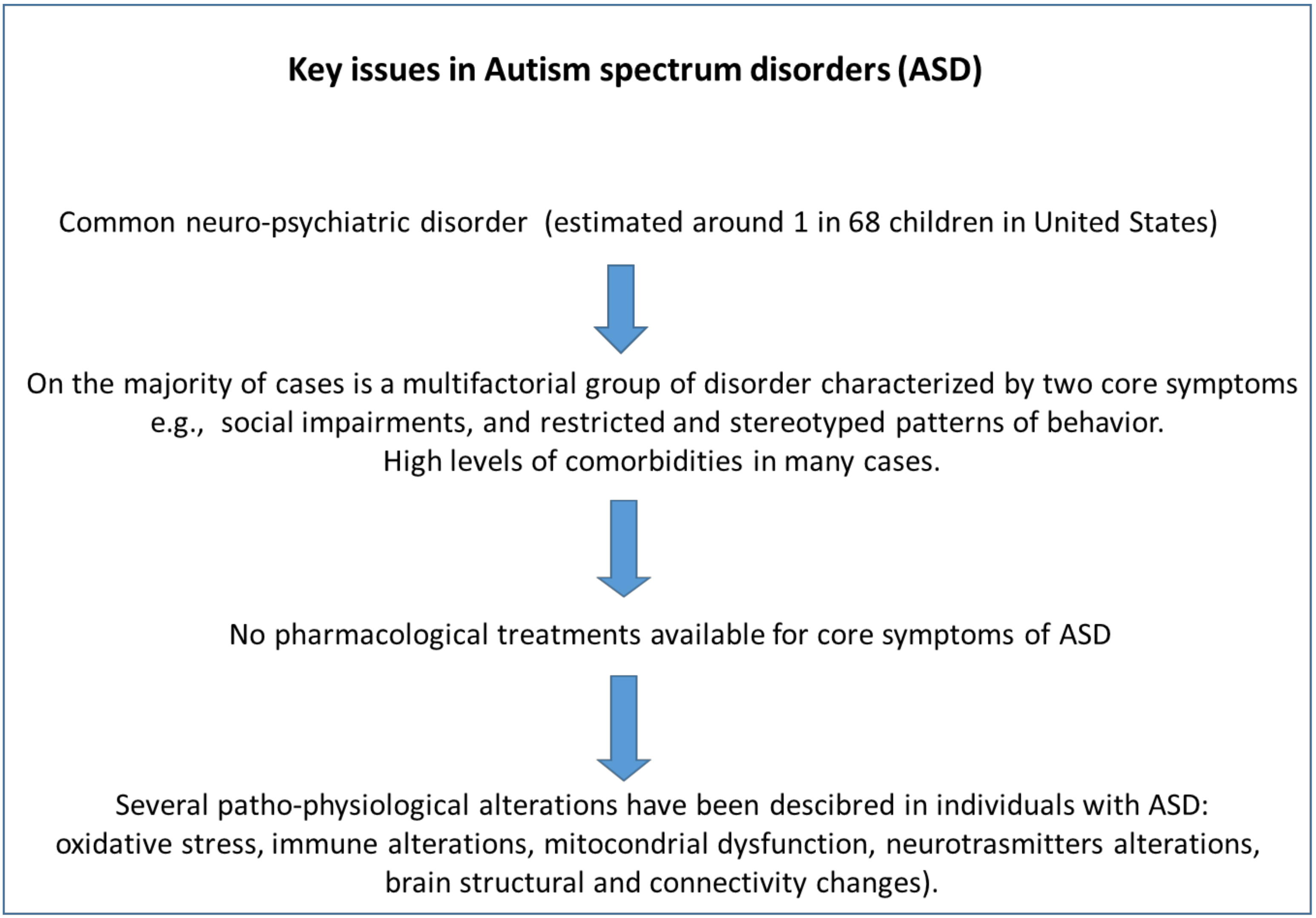
1. Introduction
- (1)
- To evaluate the behavioral effects of RSV in animal models of ASD.
- (2)
- To summarize the molecular mechanisms by which RSV administration improves behavioral deficits in animal models of ASD.
- (3)
- To review clinical studies with RSV in patients with ASD and its adverse effects.
2. Results
2.1. Behavioral Effects of RSV in Animal Models of ASD
2.2. Molecular Effects of RSV in Animal Models of ASD
2.3. Clinical Studies with RSV in Patients with ASD
3. Discussion
Author Contributions
Funding
Conflicts of Interest
References
- Posar, A.; Resca, F.; Visconti, P. Autism according to diagnostic and statistical manual of mental disorders 5th edition: The need for further improvements. J. Pediatr. Neurosci. 2015, 10, 146–148. [Google Scholar] [CrossRef]
- Baio, J.; Wiggins, L.; Christensen, D.L.; Maenner, M.J.; Daniels, J.; Warren, Z.; Kurzius-Spencer, M.; Zahorodny, W.; Robinson Rosenberg, C.; White, T.; et al. Correction and Republication: Prevalence and Characteristics of Autism Spectrum Disorder among Children Aged 8 Years—Autism and Developmental Disabilities Monitoring Network, 11 Sites, United States, 2012. MMWR. Morb. Mortal. Wkly. Rep. 2018, 67, 1279. [Google Scholar]
- Kanner, L. Autistic disturbances of affective contact. Nerv. Child. 1943, 2, 217–250. [Google Scholar]
- Miles, J.H. Autism spectrum disorders—A genetics review. Genet. Med. 2011, 13, 278–294. [Google Scholar] [CrossRef] [PubMed]
- Rossignol, D.A.; Frye, R.E. Mitochondrial dysfunction in autism spectrum disorders: A systematic review and meta-analysis. Mol. Psychiatry 2012, 7, 290–314. [Google Scholar] [CrossRef] [PubMed]
- James, S.J.; Melnyk, S.; Jernigan, S.; Cleves, M.A.; Halsted, C.H.; Wong, D.H.; Cutler, P.; Bock, K.; Boris, M.; Bradstreet, J.J.; et al. Metabolic endophenotype and related genotypes are associated with oxidative stress in children with autism. Am. J. Med. Genet. B Neuropsychiatr. Genet. 2006, 141B, 947–956. [Google Scholar] [CrossRef]
- Garbett, K.; Ebert, P.J.; Mitchell, A.; Lintas, C.; Manzi, B.; Mirnics, K.; Persico, A.M. Immune transcriptome alterations in the temporal cortex of subjects with autism. Neurobiol. Dis. 2008, 30, 303–311. [Google Scholar] [CrossRef]
- Ashwood, P.; Krakowiak, P.; Hertz-Picciotto, I.; Hansen, R.; Pessah, I.; Van de Water, J. Elevated plasma cytokines in autism spectrum disorders provide evidence of immune dysfunction and are associated with impaired behavioral outcome. Brain Behav. Immun. 2011, 25, 40–45. [Google Scholar] [CrossRef]
- Mostafa, G.A.; El-Hadidi, E.S.; Hewedi, D.H.; Abdou, M.M. Oxidative stress in Egyptian children with autism: Relation to autoimmunity. J. Neuroimmunol. 2010, 219, 114–118. [Google Scholar] [CrossRef]
- Rossignol, D.A.; Frye, R.E. Evidence linking oxidative stress, mitochondrial dysfunction, and inflammation in the brain of individuals with autism. Front. Physiol. 2014, 5, 150. [Google Scholar] [CrossRef]
- Barone, R.; Rizzo, R.; Tabbí, G.; Malaguarnera, M.; Frye, R.E.; Bastin, J. Nuclear Peroxisome Proliferator-Activated Receptors (PPARs) as therapeutic targets of resveratrol for autism spectrum disorder. Int. J. Mol. Sci. 2019, 20, 1878. [Google Scholar] [CrossRef]
- Weber, K.; Schulz, B.; Ruhnke, M. Resveratrol and its antifungal activity against Candida species. Mycoses 2011, 54, 30–33. [Google Scholar] [CrossRef] [PubMed]
- Malaguarnera, L. Influence of resveratrol on the immune response. Nutrients 2019, 11, 946. [Google Scholar] [CrossRef] [PubMed]
- Andrade, S.; Ramalho, M.J.; Pereira, M.D.C.; Loureiro, J.A. Resveratrol brain delivery for neurological disorders prevention and treatment. Front. Pharmacol. 2018, 9, 1261. [Google Scholar] [CrossRef] [PubMed]
- Quadros Gomes, B.A.; Bastos Silva, J.P.; Rodrigues Romeiro, C.F.; dos Santos, S.M.; Rodrigues, C.A.; Gonçalves, P.R.; Sakai, J.T.; Santos Mendes, P.F.; Pompeu Varela, E.L.; Monteiro, M.C. Neuroprotective mechanisms of resveratrol in Alzheimer’s disease: Role of SIRT1. Oxid. Med. Cell. Longev. 2018, 2018, 8152373. [Google Scholar] [CrossRef]
- Malaguarnera, G.; Pennisi, M.; Bertino, G.; Motta, M.; Borzì, A.M.; Vicari, E.; Bella, R.; Drago, F.; Malaguarnera, M. Resveratrol in Patients with Minimal Hepatic Encephalopathy. Nutrients 2018, 10, 329. [Google Scholar] [CrossRef]
- Turner, R.S.; Thomas, R.G.; Craft, S.; Van Dyck, C.H.; Mintzer, J.; Reynolds, B.A.; Brewer, J.B.; Rissman, R.A.; Raman, R.; Aisen, P.S. A randomized, double-blind, placebo-controlled trial of resveratrol for Alzheimer disease. Neurology 2015, 85, 1383–1391. [Google Scholar] [CrossRef]
- Nicolini, C.; Fahnestock, M. The valproic acid-induced rodent model of autism. Exp. Neurol. 2018, 299, 217–227. [Google Scholar] [CrossRef]
- Löscher, W. Basic pharmacology of valproate: A review after 35 years of clinical use for the treatment of epilepsy. CNS Drugs 2002, 16, 669–694. [Google Scholar] [CrossRef]
- Lambert, P.A.; Carraz, G.; Borselli, S.; Bouchardy, M. Dipropylacetamide in the treatment of manic-depressive psychosis. Encephale 1975, 1, 25–31. [Google Scholar]
- Emrich, H.M.; von Zerssen, D.; Kissling, W.; Möller, H.J.; Windorfer, A. Effect of sodium valproate on mania. The GABA-hypothesis of affective disorders. Arch. Psychiatr. Nervenkr. 1980, 229, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Bromley, R.L.; Mawer, G.; Clayton-Smith, J.; Baker, G.A. Autism spectrum disorders following in utero exposure to antiepileptic drugs. Neurology 2008, 71, 1923–1924. [Google Scholar] [CrossRef] [PubMed]
- Dufour-Rainfray, D.; Vourc’h, P.; Le Guisquet, A.M.; Garreau, L.; Ternant, D.; Bodard, S.; Jaumain, E.; Gulhan, Z.; Belzung, C.; Andres, C.R.; et al. Behavior and serotonergic disorders in rats exposed prenatally to valproate: A model for autism. Neurosci. Lett. 2010, 470, 55–59. [Google Scholar] [CrossRef] [PubMed]
- Bambini-Junior, V.; Zanatta, G.; Della Flora Nunes, G.; Mueller de Melo, G.; Michels, M.; Fontes-Dutra, M.; Nogueira Freire, V.; Riesgo, R.; Gottfried, C. Resveratrol prevents social deficts in animal model of autism induced by valproic acid. Neurosci. Lett. 2014, 583, 176–181. [Google Scholar] [CrossRef]
- Fontes-Dutra, M.; Santos-Terra, J.; Deckmann, I.; Schwingel, G.B.; Nunes, G.D.F.; Hirsch, M.M.; Bauer-Negrini, G.; Riesgo, R.S.; Bambini-Júnior, V.; Hedin-Pereira, C.; et al. Resveratrol prevents cellular and behavioral sensory alterations in the animal model of autism induced by valproic acid. Front. Synaptic Neurosci. 2018, 10, 1–12. [Google Scholar] [CrossRef]
- Hirsch, M.M.; Deckmann, I.; Fontes-Dutra, M.; Bauer-Negrini, G.; Della-Flora Nunes, G.; Nunes, W.; Rabelo, B.; Riesgo, R.; Margis, R.; Bambini-Junior, V.; et al. Behavioral alterations in autism model induced by valproic acid and translational analysis of circulating microRNA. Food Chem. Toxicol. 2018, 115, 336–343. [Google Scholar] [CrossRef]
- Fontes-Dutra, M.; Della-Flora Nunes, G.; Santos-Terra, J.; Souza-Nunes, W.; Bauer-Negrini, G.; Hirsch, M.M.; Green, L.; Riesgo, R.; Gottfried, C.; Bambini-Junior, V. Abnormal empathy-like pro-social behaviour in the valproic acid model of autism spectrum disorder. Behav. Brain Res. 2019, 364, 11–18. [Google Scholar] [CrossRef]
- Shultz, S.R.; MacFabe, D.F.; Ossenkopp, K.P.; Scratch, S.; Whelan, J.; Taylor, R.; Cain, D.P. Intracerebroventricular injection of propionic acid, an enteric bacterial metabolic end-product, impairs social behavior in the rat: Implications for an animal model of autism. Neuropharmacology 2008, 54, 901–911. [Google Scholar] [CrossRef]
- MacFabe, D.F.; Cain, D.P.; Rodriguez-Capote, K.; Franklin, A.E.; Hoffman, J.E.; Boon, F.; Taylor, A.R.; Kavaliers, M.; Ossenkopp, K.P. Neurobiological effects of intraventricular propionic acid in rats: Possible role of short chain fatty acids on the pathogenesis and characteristics of autism spectrum disorders. Behav. Brain Res. 2007, 176, 149–169. [Google Scholar] [CrossRef]
- Bhandari, R.; Kuhad, A. Resveratrol suppresses neuroinflammation in the experimental paradigm of autism spectrum disorders. Neurochem. Int. 2017, 103, 8–23. [Google Scholar] [CrossRef]
- Galef, B.G.; Kennett, D.J. Different mechanisms for social transmission of diet preference in rat pups of different ages. Dev. Psychobiol. 1987, 20, 209–215. [Google Scholar] [CrossRef] [PubMed]
- Bakheet, S.A.; Alzahrani, M.Z.; Nadeem, A.; Ansari, M.A.; Zoheir, K.M.A.; Attia, S.M.; AL-Ayadhi, L.Y.; Ahmad, S.F. Resveratrol treatment attenuates chemokine receptor expression in the BTBR T + tf/J mouse model of autism. Mol. Cell. Neurosci. 2016, 77, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Bakheet, S.A.; Alzahrani, M.Z.; Ansari, M.A.; Nadeem, A.; Zoheir, K.M.A.; Attia, S.M.; Al-Ayadhi, L.Y.; Ahmad, S.F. Resveratrol Ameliorates Dysregulation of Th1, Th2, Th17, and T Regulatory Cell-Related Transcription Factor Signaling in a BTBR T + tf/J Mouse Model of Autism. Mol. Neurobiol. 2017, 54, 5201–5212. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, S.F.; Ansari, M.A.; Nadeem, A.; Alzahrani, M.Z.; Bakheet, S.A.; Attia, S.M. Resveratrol Improves Neuroimmune Dysregulation Through the Inhibition of Neuronal Toll-Like Receptors and COX-2 Signaling in BTBR T+ Itpr3tf/J Mice. Neuromol. Med. 2018, 20, 133–146. [Google Scholar] [CrossRef]
- Ahmad, S.F.; Ansari, M.A.; Nadeem, A.; Bakheet, S.A.; Alzahrani, M.Z.; Alshammari, M.A.; Alanazi, W.A.; Alasmari, A.F.; Attia, S.M. Resveratrol attenuates pro-inflammatory cytokines and activation of JAK1-STAT3 in BTBR T(+) Itpr3(tf)/J autistic mice. Eur. J. Pharmacol. 2018, 829, 70–78. [Google Scholar] [CrossRef]
- Meyza, K.Z.; Blanchard, D.C. The BTBR mouse model of idiopathic autism—Current view on mechanisms. Neurosci. Biobehav. Rev. 2017, 76, 99–110. [Google Scholar] [CrossRef]
- Bolivar, V.J.; Walters, S.R.; Phoenix, J.L. Assessing autism-like behavior in mice: Variations in social interactions among inbred strains. Behav. Brain Res. 2007, 176, 21–26. [Google Scholar] [CrossRef]
- Moy, S.S.; Nadler, J.J.; Young, N.B.; Perez, A.; Holloway, L.P.; Barbaro, R.P.; Barbaro, J.R.; Wilson, L.M.; Threadgill, D.W.; Lauder, J.M.; et al. Mouse behavioral tasks relevant to autism: Phenotypes of 10 inbred strains. Behav. Brain Res. 2007, 176, 4–20. [Google Scholar] [CrossRef]
- Moy, S.S.; Nadler, J.J. Advances in behavioral genetics: Mouse models of autism. Mol. Psychiatry 2008, 13, 4–26. [Google Scholar] [CrossRef]
- Geschwind, D.H. Advances in Autism. Annu. Rev. Med. 2009. [Google Scholar] [CrossRef]
- Mamidala, M.P.; Polinedi, A.; Kumar, P.P.; Rajesh, N.; Vallamkonda, O.R.; Udani, V.; Singhal, N.; Rajesh, V. Maternal hormonal interventions as a risk factor for Autism Spectrum Disorder: An epidemiological assessment from India. J. Biosci. 2013, 38, 887–892. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Li, M.; Lu, J.; Ge, X.; Xie, W.; Wang, Z.; Li, X.; Li, C.; Wang, X.; Han, Y.; et al. Prenatal Progestin Exposure Is Associated With Autism Spectrum Disorders. Front. Psychiatry 2018, 9, 611. [Google Scholar] [CrossRef] [PubMed]
- Xie, W.; Ge, X.; Li, L.; Yao, A.; Wang, X.; Li, M.; Gong, X.; Chu, Z.; Lu, Z.; Huang, X.; et al. Resveratrol ameliorates prenatal progestin exposure-induced autism-like behavior through ERβ activation. Mol. Autism 2018, 9, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Palmieri, L.; Persico, A.M. Mitochondrial dysfunction in autism spectrum disorders: Cause or effect? Biochim. Biophys. Acta Bioenerg. 2010, 1797, 1130–1137. [Google Scholar] [CrossRef] [PubMed]
- Rose, S.; Melnyk, S.; Pavliv, O.; Bai, S.; Nick, T.G.; Frye, R.E.; James, S.J. Evidence of oxidative damage and inflammation associated with low glutathione redox status in the autism brain. Transl. Psychiatry 2012, 2, e134. [Google Scholar] [CrossRef] [PubMed]
- Chen-Bee, C.H.; Zhou, Y.; Jacobs, N.S.; Lim, B.; Frostig, R.D. Whisker array functional representation in rat barrel cortex: Transcendence of one-to-one topography and its underlying mechanism. Front. Neural Circuits 2012, 6, 93. [Google Scholar] [CrossRef] [PubMed]
- Paul, S.; Reyes, P.R.; Garza, B.S.; Sharma, A. MicroRNAs and Child Neuropsychiatric Disorders: A Brief Review. Neurochem. Res. 2020, 45, 232–240. [Google Scholar] [CrossRef]
- Hirsch, M.M.; Deckmann, I.; Fontes-Dutra, M.; Bauer-Negrini, G.; Nunes, G.D.F.; Nunes, W.; Rabelo, B.; Riesgo, R.; Margis, R.; Bambini-Junior, V.; et al. Data on social transmission of food preference in a model of autism induced by valproic acid and translational analysis of circulating microRNA. Data Br. 2018, 18, 1433–1440. [Google Scholar] [CrossRef]
- Hendouei, F.; Sanjari Moghaddam, H.; Mohammadi, M.R.; Taslimi, N.; Rezaei, F.; Akhondzadeh, S. Resveratrol as adjunctive therapy in treatment of irritability in children with autism: A double-blind and placebo-controlled randomized trial. J. Clin. Pharm. Ther. 2019. [Google Scholar] [CrossRef]
- Bhandari, R.; Paliwal, J.K.; Kuhad, A. Dietary Phytochemicals as Neurotherapeutics for Autism Spectrum Disorder: Plausible Mechanism and Evidence. Adv. Neurobiol. 2020, 24, 615–646. [Google Scholar]
- Saqib, U.; Kelley, T.T.; Panguluri, S.K.; Liu, D.; Savai, R.; Baig, M.S.; Schürer, S.C. Polypharmacology or Promiscuity? Structural Interactions of Resveratrol With Its Bandwagon of Targets. Front. Pharmacol. 2018, 9, 1201. [Google Scholar] [CrossRef] [PubMed]
- Bjørklund, G.; Meguid, N.A.; El-bana, M.A.; Tinkov, A.A.; Saad, K. Oxidative Stress in Autism Spectrum Disorder. Mol. Neurobiol. 2020. [Google Scholar] [CrossRef] [PubMed]
- Osredkar, J.; Gosar, D.; Maček, J.; Kumer, K.; Fabjan, T.; Finderle, P.; Šterpin, S.; Zupan, M.; Vrhovšek, M.J. Urinary markers of oxidative stress in children with autism spectrum disorder (ASD). Antioxidants 2019, 8, 187. [Google Scholar] [CrossRef] [PubMed]
- El-Ansary, A.; Bjørklund, G.; Chirumbolo, S.; Alnakhli, O.M. Predictive value of selected biomarkers related to metabolism and oxidative stress in children with autism spectrum disorder. Metab. Brain Dis. 2017, 32, 1209–1221. [Google Scholar] [CrossRef]
- El-Ansary, A.; Hassan, W.M.; Daghestani, M.; Al-Ayadhi, L.; Ben Bacha, A. Preliminary evaluation of a novel nine-biomarker profile for the prediction of autism spectrum disorder. PLoS ONE 2020, 15, e0227626. [Google Scholar] [CrossRef]
- Endres, D.; Tebartz van Elst, L.; Meyer, S.A.; Feige, B.; Nickel, K.; Bubl, A.; Riedel, A.; Ebert, D.; Lange, T.; Glauche, V.; et al. Glutathione metabolism in the prefrontal brain of adults with high-functioning autism spectrum disorder: An MRS study. Mol. Autism 2017, 8, 10. [Google Scholar] [CrossRef]
- Salehi, B.; Mishra, A.P.; Nigam, M.; Sener, B.; Kilic, M.; Sharifi-Rad, M.; Fokou, P.V.T.; Martins, N.; Sharifi-Rad, J. Resveratrol: A double-edged sword in health benefits. Biomedicines 2018, 6, 91. [Google Scholar] [CrossRef]
- Kode, A.; Rajendrasozhan, S.; Caito, S.; Yang, S.R.; Megson, I.L.; Rahman, I. Resveratrol induces glutathione synthesis by activation of Nrf2 and protects against cigarette smoke-mediated oxidative stress in human lung epithelial cells. Am. J. Physiol. Lung Cell. Mol. Physiol. 2008, 294. [Google Scholar] [CrossRef]
- Al-Yafee, Y.A.; Al-Ayadhi, L.Y.; Haq, S.H.; El-Ansary, A.K. Novel metabolic biomarkers related to sulfur-dependent detoxification pathways in autistic patients of Saudi Arabia. BMC Neurol. 2011, 11, 139. [Google Scholar] [CrossRef]
- Bennuri, S.C.; Rose, S.; Frye, R.E. Mitochondrial dysfunction is inducible in lymphoblastoid cell lines from children with autism and may involve the TORC1 pathway. Front. Psychiatry 2019, 10, 269. [Google Scholar] [CrossRef]
- Bu, X.; Wu, D.; Lu, X.; Yang, L.; Xu, X.; Wang, J.; Tang, J. Role of SIRT1/PGC-1α in mitochondrial oxidative stress in autistic spectrum disorder. Neuropsychiatr. Dis. Treat. 2017, 13, 1633–1645. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, M.F.; Elwell, C.; Johnson, M.H. Mitochondrial Dysfunction in Autism Spectrum Disorders. Autism. Open Access 2016, 6. [Google Scholar] [CrossRef] [PubMed]
- Corona, J.C.; Duchen, M.R. PPARγ as a therapeutic target to rescue mitochondrial function in neurological disease. Free Radic. Biol. Med. 2016, 100, 153–163. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, S.; Yadav, A.; Chaturvedi, R.K. Peroxisome proliferator-activated receptors (PPARs) as therapeutic target in neurodegenerative disorders. Biochem. Biophys. Res. Commun. 2017, 483, 1166–1177. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Liang, S.; Fujisawa, T.X.; Nishitani, S.; Tomoda, A.; Zou, M.; Li, Y.; Wu, L.; Shinohara, K. Association of estrogen receptor alpha polymorphisms with symptoms of autism among Chinese Han children. Neuroendocrinol. Lett. 2016, 37, 439–444. [Google Scholar] [PubMed]
- Chakrabarti, B.; Dudbridge, F.; Kent, L.; Wheelwright, S.; Hill-Cawthorne, G.; Allison, C.; Banerjee-Basu, S.; Baron-Cohen, S. Genes related to sex steroids, neural growth, and social-emotional behavior are associated with autistic traits, empathy, and asperger syndrome. Autism Res. 2009, 2, 157–177. [Google Scholar] [CrossRef]
- Crider, A.; Thakkar, R.; Ahmed, A.O.; Pillai, A. Dysregulation of estrogen receptor beta (ERβ), aromatase (CYP19A1), and ER co-activators in the middle frontal gyrus of autism spectrum disorder subjects. Mol. Autism 2014, 5, 46. [Google Scholar] [CrossRef]
- Nwachukwu, J.C.; Srinivasan, S.; Bruno, N.E.; Parent, A.A.; Hughes, T.S.; Pollock, J.A.; Gjyshi, O.; Cavett, V.; Nowak, J.; Garcia-Ordonez, R.D.; et al. Resveratrol modulates the inflammatory response via an estrogen receptor-signal integration network. Elife 2014, 2014, e02057. [Google Scholar] [CrossRef]
- Ashwood, P.; Corbett, B.A.; Kantor, A.; Schulman, H.; Van de Water, J.; Amaral, D.G. In search of cellular immunophenotypes in the blood of children with autism. PLoS ONE 2011, 6, e19299. [Google Scholar] [CrossRef]
- Basheer, S.; Venkataswamy, M.M.; Christopher, R.; Van Amelsvoort, T.; Srinath, S.; Girimaji, S.C.; Ravi, V. Immune aberrations in children with Autism Spectrum Disorder: A case-control study from a tertiary care neuropsychiatric hospital in India. Psychoneuroendocrinology 2018, 94, 162–167. [Google Scholar] [CrossRef]
- Choi, C.H.; Schoenfeld, B.P.; Bell, A.J.; Hinchey, J.; Rosenfelt, C.; Gertner, M.J.; Campbell, S.R.; Emerson, D.; Hinchey, P.; Kollaros, M.; et al. Multiple Drug Treatments That Increase cAMP Signaling Restore Long-Term Memory and Aberrant Signaling in Fragile X Syndrome Models. Front. Behav. Neurosci. 2016, 10, 136. [Google Scholar] [CrossRef] [PubMed]
- Hu, V.W.; Nguyen, A.T.; Kim, K.S.; Steinberg, M.E.; Sarachana, T.; Scully, M.A.; Soldin, S.J.; Luu, T.; Lee, N.H. Gene expression profiling of lymphoblasts from autistic and nonaffected sib pairs: Altered pathways in neuronal development and steroid biosynthesis. PLoS ONE 2009, 4, e5775. [Google Scholar] [CrossRef] [PubMed]
- Enstrom, A.M.; Onore, C.E.; Van de Water, J.A.; Ashwood, P. Differential monocyte responses to TLR ligands in children with autism spectrum disorders. Brain Behav. Immun. 2010, 24, 64–71. [Google Scholar] [CrossRef]
- Roberts, V.H.J.; Pound, L.D.; Thorn, S.R.; Gillingham, M.B.; Thornburg, K.L.; Friedman, J.E.; Frias, A.E.; Grove, K.L. Beneficial and cautionary outcomes of resveratrol supplementation in pregnant nonhuman primates. FASEB J. 2014, 28, 2466–2477. [Google Scholar] [CrossRef] [PubMed]
- Fung, L.K.; Mahajan, R.; Nozzolillo, A.; Bernal, P.; Krasner, A.; Jo, B.; Coury, D.; Whitaker, A.; Veenstra-Vanderweele, J.; Hardan, A.Y. Pharmacologic treatment of severe irritability and problem behaviors in Autism: A systematic review and meta-analysis. Pediatrics 2016, 137, S124–S135. [Google Scholar] [CrossRef]
- Howes, O.D.; Group, E.P.F.; Rogdaki, M.; Findon, J.L.; Wichers, R.H.; Charman, T.; King, B.H.; Loth, E.; Mcalonan, G.M.; Mccracken, J.T.; et al. Autism spectrum disorder: Consensus guidelines on assessment, treatment and research from the British Association for Psychopharmacology. J. Psychopharmacol. 2018, 32, 3–29. [Google Scholar] [CrossRef]
- Goldstein, S.; Schwebach, A.J. The comorbidity of pervasive developmental disorder and attention deficit hyperactivity disorder: Results of a retrospective chart review. J. Autism Dev. Disord. 2004, 34, 329–339. [Google Scholar] [CrossRef]
- Ghanizadeh, A. Co-morbidity and factor analysis on attention deficit hyperactivity disorder and autism spectrum disorder DSM-IV-derived items. J. Res. Med. Sci. 2012, 17, 368–372. [Google Scholar]
- Lee, D.O.; Ousley, O.Y. Attention-deficit hyperactivity disorder symptoms in a clinic sample of children and adolescents with pervasive developmental disorders. J. Child Adolesc. Psychopharmacol. 2006, 16, 737–746. [Google Scholar] [CrossRef]
- Ghanizadeh, A.; Molla, M.; Olango, G.J. The effect of stimulants on irritability in autism comorbid with ADHD: A systematic review. Neuropsychiatr. Dis. Treat. 2019, 15, 1547–1555. [Google Scholar] [CrossRef]
| Comorbidity | Drug Class |
|---|---|
| Hyperactivity/inattention | Psychostimulants |
| Non-stimulants | |
| Sleep alterations | Hormone (Melatonin) |
| Antihistamines | |
| Irritability | Atypical antipsychotics |
| Epilepsy | Antiepileptics |
| Aggression | Atypical antipsychotics |
| Miscellaneous | Antidepressants (selective serotonin reuptake inhibitors) |
| Mood stabilizers |
| Study | Animal Model | Dose RSV, Treatment Duration and Route of Administration | Behavioral Alterations | Main Outcome |
|---|---|---|---|---|
| Bambini-Junior et al., 2014 [24] | Prenatal exposure of valproic acid in Wistar rats | 3.6 mg/kg, subcutaneous. Administered daily for 13 days. | Social memory and preferences (Three chamber sociability and social novelty test). | RSV prevents autistic-like social behaviors. |
| Bakheet et al., 2016 [32] | BTBR model | 20–40 mg/kg, intraperitoneally For 7 days. | Self-Grooming (repetitive behavior). | RSV reduced repetitive behavior. |
| Bhandari and Kuhad 2017 [30] | Propanoic acid (PPA) infused into the anterior portion of the lateral ventricle in Sprague-Dawley rats | 5, 10, 15 mg/kg. Oral treatment. Administered daily for 27 days after PPA infusion. | Social interaction, stereotypy, locomotor activity, anxiety, spatial learning, memory, depression-like behaviors. | RSV normalizes the social interaction, stereotypy, locomotor activity, anxiety, spatial learning, memory and depression-like behaviors. |
| Fontes-Dutra et al., 2018 [25,27] | Prenatal exposure of valproic acid in Wistar rats | 3.6 mg/kg, subcutaneous. Administered daily for 12 days. | Effect on sensory behavior (Nest-seeking behavior and in whisker nuisance task). | RSV improves the percentage of correct choices (reach the nest shavings) per litter. |
| Hirsch et al., 2018 [26] | Prenatal exposure to valproic acid in Wistar rats | 3.6 mg/kg, subcutaneous. Administered daily for 12 days. | Reciprocal social interaction test (Social Transmission of Food Preference test). | RSV counteracts the deficit in the social interaction test. |
| Xie et al. 2018 [43] | Prenatal and Postnatal exposure to different progestins, and then just norethindrone (20 mg) in Sprague-Dawley rats | 20 mg/kg administered through oral gavage for 28 days (two protocol: prenatal and postnatal treatment). | Repetitive behavior, anxiety, and social interaction test. | RSV recovers the repetitive behavior (Marble burying test) and the deficit found in social interaction test. |
| Study | Animal Model of ASD | Dose RSV, Treatment Duration and Route of Administration | Molecular Effects of RSV in ASD Models |
|---|---|---|---|
| Bakheet et al., 2016 [32] | BTBR model | 20–40 mg/kg, intraperitoneally administered for 7 days. | Decreases the expression (mRNA) levels of CCR and CXCR in the spleen and brain tissues and downregulated the chemokine receptor levels in CD4+ T cells. |
| Bakheet et al., 2017 [33] | BTBR model | 20–40 mg/kg, intraperitoneally administered for 7 days. | Suppression of upregulation of T helper 17 (Th17), T helper 2, and T helper 1 cell-related transcription factors and induction of T-reg cell-related transcription factor such as FOX-p3, GATA. |
| Bhandari and Kuhad 2017 [30] | Propanoic acid (PPA) infused into the anterior portion of the lateral ventricle in Sprague-Dawley rats | 5, 10, 15 mg/kg. Oral treatment. Administered daily for 27 days after PPA infusion. | Increase the concentration of reduced glutathione, superoxide dismutase and catalase in the brain. Reduction of oxidative stress markers (lipid hydroperoxyde and nitrites). Normalizes brain levels MMP-9 and TNF-alpha. |
| Ahmad et al., 2018 [34] | BTBR model | 20–40 mg/kg, intraperitoneally administered for 7 days. | Decreases TLR2, TLR3, TLR4, NF-κB, iNOS, and COX-2 mRNA and protein expression levels in brain. |
| Ahmad et al., 2018 [35] | BTBR model | 20–40 mg/kg, intraperitoneally administered for 7 days. | Decreases IL-6, TNF-alpha, IFN-gamma and STAT-3 expression in spleen and in the brain. |
| Fontes-Dutra et al., 2018 [25,27] | Prenatal exposure of valproic acid in Wistar rats | 3.6 mg/kg, subcutaneous. Administered daily for 12 days. | Restoration of GABAergic neurons and cortical organization in the primary somato-sensory area and in the amygdala. |
| Hirsch et al., 2018 [26] | Prenatal exposure of valproic acid in Wistar rats | 3.6 mg/kg, subcutaneous. Administered daily for 12 days. | Prevention of the augmentation of miR134–5p levels induced by valproic acid. |
| Xie et al. 2018 [43] | Prenatal and postnatal exposure to different progestins in rats | 20 mg/kg of through oral gavage for 28 days (two protocol: prenatal and postnatal treatment) | Augmentation of estrogen receptor (ERβ) expression and its target genes by demethylation of DNA and histone on the ERβ promoter. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Malaguarnera, M.; Khan, H.; Cauli, O. Resveratrol in Autism Spectrum Disorders: Behavioral and Molecular Effects. Antioxidants 2020, 9, 188. https://doi.org/10.3390/antiox9030188
Malaguarnera M, Khan H, Cauli O. Resveratrol in Autism Spectrum Disorders: Behavioral and Molecular Effects. Antioxidants. 2020; 9(3):188. https://doi.org/10.3390/antiox9030188
Chicago/Turabian StyleMalaguarnera, Michele, Haroon Khan, and Omar Cauli. 2020. "Resveratrol in Autism Spectrum Disorders: Behavioral and Molecular Effects" Antioxidants 9, no. 3: 188. https://doi.org/10.3390/antiox9030188
APA StyleMalaguarnera, M., Khan, H., & Cauli, O. (2020). Resveratrol in Autism Spectrum Disorders: Behavioral and Molecular Effects. Antioxidants, 9(3), 188. https://doi.org/10.3390/antiox9030188







