Plant Phenolics and Phenolic-Enriched Extracts as Antimicrobial Agents against Food-Contaminating Microorganisms
Abstract
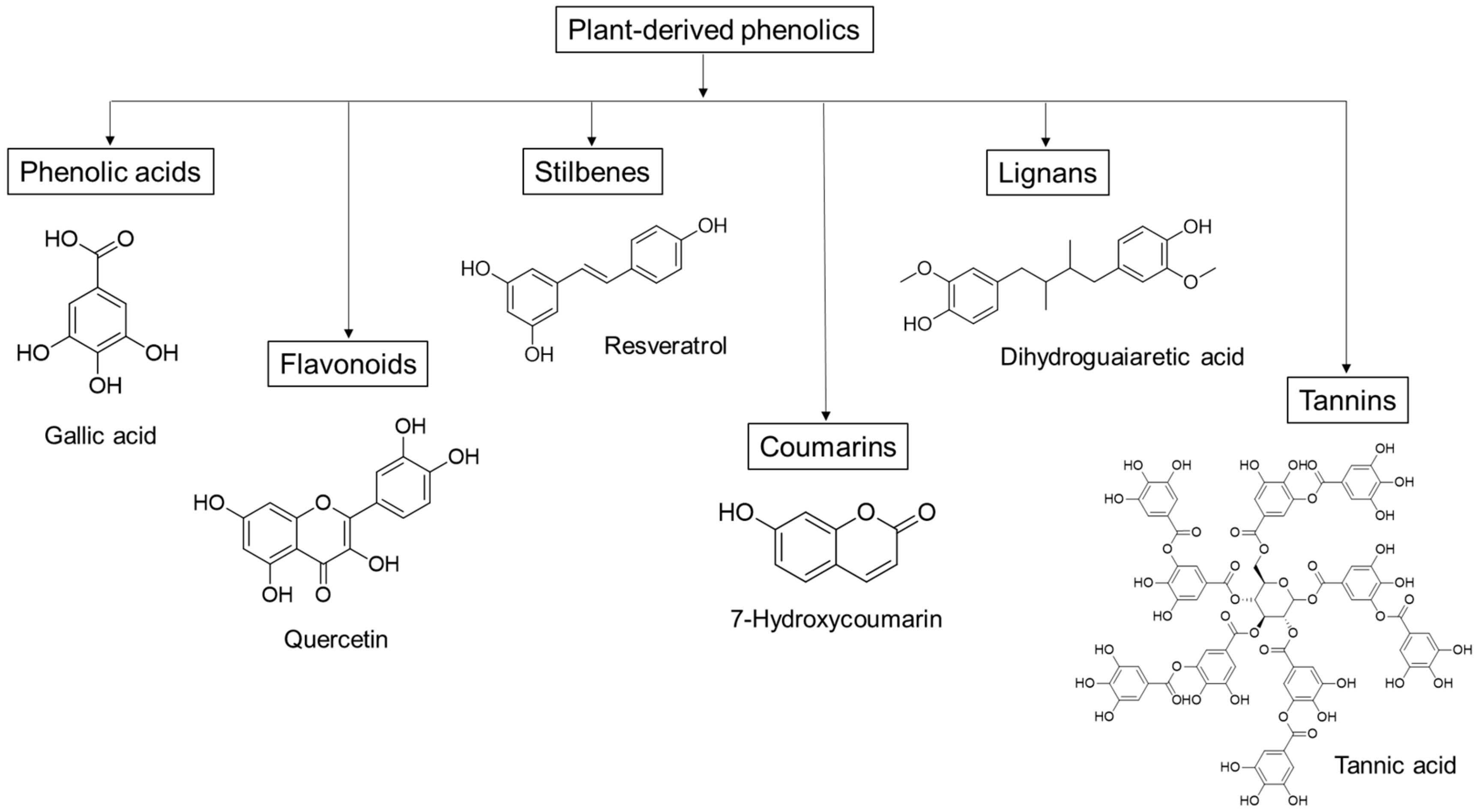
1. Introduction
2. Foodborne Pathogens and Food Spoilage Organisms
3. Antimicrobial Activity of Plant Phenolics
4. Quorum Sensing Systems and Biofilm Formation in Food Related Bacteria
5. Anti-Quorum Sensing and Antibiofilm Effects of Plant Phenolics
6. Anti-Enterotoxin Effect of Plant Phenolics
7. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Del Rio, D.; Rodriguez-Mateos, A.; Spencer, J.P.E.; Tognolini, M.; Borges, G.; Crozier, A. Dietary (poly) phenolics in human health: Structures, bioavailability, and evidence of protective effects against chronic diseases. Antioxid. Redox Signal. 2013, 18, 1818–1892. [Google Scholar] [CrossRef]
- Gyawali, R.; Ibrahim, S.A. Natural products as antimicrobial agents. Food Control 2014, 46, 412–429. [Google Scholar] [CrossRef]
- Zambrano, C.; Kerekes, E.B.; Kotogán, A.; Papp, T.; Vágvölgyi, C.; Krisch, J.; Takó, M. Antimicrobial activity of grape, apple and pitahaya residue extracts after carbohydrase treatment against food-related bacteria. LWT Food Sci. Technol. 2019, 100, 416–425. [Google Scholar] [CrossRef]
- Nazzaro, F.; Fratianni, F.; Coppola, R. Quorum sensing and phytochemicals. Int. J. Mol. Sci. 2013, 14, 12607–12619. [Google Scholar] [CrossRef] [PubMed]
- Kowalska, H.; Czajkowska, K.; Cichowska, J.; Lenart, A. What’s new in biopotential of fruit and vegetable by-products applied in the food processing industry. Trends Food Sci. Technol. 2017, 67, 150–159. [Google Scholar] [CrossRef]
- Aires, A. Phenolics in foods: Extraction, analysis and measurements. In Phenolic Compounds—Natural Sources, Importance and Applications; Soto-Hernandez, M., Palma-Tenango, M., Del Rosario Garcia-Mateos, M., Eds.; IntechOpen: London, UK, 2017; pp. 61–88. [Google Scholar]
- Xu, D.-P.; Li, Y.; Meng, X.; Zhou, T.; Zhou, Y.; Zheng, J.; Zhang, J.-J.; Li, H.-B. Natural antioxidants in foods and medicinal plants: Extraction, assessment and resources. Int. J. Mol. Sci. 2017, 18, 96. [Google Scholar] [CrossRef] [PubMed]
- Zambrano, C.; Kotogán, A.; Bencsik, O.; Papp, T.; Vágvölgyi, C.; Mondal, K.C.; Krisch, J.; Takó, M. Mobilization of phenolic antioxidants from grape, apple and pitahaya residues via solid state fungal fermentation and carbohydrase treatment. LWT Food Sci. Technol. 2018, 89, 457–465. [Google Scholar] [CrossRef]
- Ngwoke, K.G.; Odimegwu, D.C.; Esimone, C.O. Antimicrobial natural products. In Science Against Microbial Pathogens: Communicating Current Research and Technology Advances; Méndez-Vilas, A., Ed.; FORMATEX: Badajoz, Spain, 2011; pp. 1011–1026. [Google Scholar]
- Stojković, D.; Petrović, J.; Soković, M.; Glamočlija, J.; Kukić-Marković, J.; Petrović, S. In situ antioxidant and antimicrobial activities of naturally occurring caffeic acid, p-coumaric acid and rutin, using food systems. J. Sci. Food Agric. 2013, 93, 3205–3208. [Google Scholar] [CrossRef]
- Papuc, C.; Goran, G.V.; Predescu, C.N.; Nicorescu, V.; Stefan, G. Plant polyphenols as antioxidant and antibacterial agents for shelf-life extension of meat and meat products: Classification, structures, sources, and action mechanisms. Compr. Rev. Food Sci. Food Saf. 2017, 16, 1243–1268. [Google Scholar] [CrossRef]
- Shahidi, F.; Ambigaipalan, P. Phenolics and polyphenolics in foods, beverages and spices: Antioxidant activity and health effects—A review. J. Funct. Foods 2015, 18, 820–897. [Google Scholar] [CrossRef]
- Bintsis, T. Foodborne pathogens. AIMS Microbiol. 2017, 3, 529–563. [Google Scholar] [CrossRef] [PubMed]
- Lai, C.-K.; Chen, Y.-A.; Lin, C.-J.; Lin, H.-J.; Kao, M.-C.; Huang, M.-Z.; Lin, Y.-H.; Chiang-Ni, C.; Chen, C.-J.; Lo, U.-G.; et al. Molecular mechanisms and potential clinical applications of Campylobacter jejuni cytolethal distending toxin. Front. Cell. Infect. Microbiol. 2016, 6, 9. [Google Scholar] [CrossRef] [PubMed]
- Heredia, N.; García, S. Animals as sources of food-borne pathogens: A review. Anim. Nutr. 2018, 4, 250–255. [Google Scholar] [CrossRef] [PubMed]
- Hamon, M.A.; Ribet, D.; Stavru, F.; Cossart, P. Listeriolysin O: The Swiss army knife of Listeria. Trends Microbiol. 2012, 20, 360–368. [Google Scholar] [CrossRef]
- King, J.C.; Black, R.E.; Doyle, M.P.; Fritsche, K.L.; Halbrook, B.H.; Levander, O.A.; Meydani, S.N.; Walker, W.A.; Woteki, C.E. Foodborne illnesses and nutritional status: A statement from an American Society for Nutritional Sciences Working Group. J. Nutr. 2000, 130, 2613–2617. [Google Scholar] [CrossRef][Green Version]
- Shallcross, L.J.; Davies, D.S.C. Antibiotic overuse: A key driver of antimicrobial resistance. Br. J. Gen. Pract. 2014, 64, 604–605. [Google Scholar] [CrossRef]
- Hashempour-Baltork, F.; Hosseini, H.; Shojaee-Aliabadi, S.; Torbati, M.; Alizadeh, A.M.; Alizadeh, M. Drug resistance and the prevention strategies in food borne bacteria: An update review. Adv. Pharm. Bull. 2019, 9, 335–347. [Google Scholar] [CrossRef]
- Rawat, S. Food spoilage: Microorganisms and their prevention. Asian J. Plant Sci. Res. 2015, 5, 47–56. [Google Scholar]
- Sperber, W.H. Introduction to the microbiological spoilage of foods and beverages. In Compendium of the Microbiological Spoilage of Foods and Beverages; Sperber, W.H., Doyle, M.P., Eds.; Springer: New York, NY, USA, 2009; pp. 1–40. [Google Scholar]
- Garnier, L.; Valence, F.; Mounier, J. Diversity and control of spoilage fungi in dairy products: An update. Microorganisms 2017, 5, 42. [Google Scholar] [CrossRef]
- Mandal, P.K.; Biswas, A.K.; Choi, K.; Pal, U.K. Methods for rapid detection of foodborne pathogens: An overview. Am. J. Food Technol. 2011, 6, 87–102. [Google Scholar] [CrossRef]
- Jadhav, S.R.; Shah, R.M.; Karpe, A.V.; Morrison, P.D.; Kouremenos, K.; Beale, D.J.; Palombo, E.A. Detection of foodborne pathogens using proteomics and metabolomics-based approaches. Front. Microbiol. 2018, 9, 3132. [Google Scholar] [CrossRef] [PubMed]
- Fletcher, B.; Mullane, K.; Platts, P.; Todd, E.; Power, A.; Roberts, J.; Chapman, J.; Cozzolino, D.; Chandra, S. Advances in meat spoilage detection: A short focus on rapid methods and technologies. CYTA J. Food 2018, 16, 1037–1044. [Google Scholar] [CrossRef]
- Daglia, M. Polyphenols as antimicrobial agents. Curr. Opin. Biotechnol. 2012, 23, 174–181. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, T.J.; Khalid, S.A.; Romanha, A.J.; Alves, T.M.A.; Biavatti, M.W.; Brun, R.; Da Costa, F.B.; De Castro, S.L.; Ferreira, V.F.; De Lacerda, M.V.G.; et al. The potential of secondary metabolites from plants as drugs or leads against protozoan neglected diseases-part II. Curr. Med. Chem. 2012, 19, 2176–2228. [Google Scholar] [CrossRef]
- Li, A.-N.; Li, S.; Zhang, Y.-J.; Xu, X.-R.; Chen, Y.-M.; Li, H.-B. Resources and biological activities of natural polyphenols. Nutrients 2014, 6, 6020–6047. [Google Scholar] [CrossRef]
- Górniak, I.; Bartoszewski, R.; Króliczewski, J. Comprehensive review of antimicrobial activities of plant flavonoids. Phytochem. Rev. 2019, 18, 241–272. [Google Scholar] [CrossRef]
- Özkan, G.; Sagdiç, O.; Göktürk Baydar, N.; Kurumahmutoglu, Z. Antibacterial activities and total phenolic contents of grape pomace extracts. J. Sci. Food Agric. 2004, 84, 1807–1811. [Google Scholar] [CrossRef]
- Kabir, F.; Sultana, M.S.; Kurnianta, H. Antimicrobial activities of grape (Vitis vinifera L.) pomace polyphenols as a source of naturally occurring bioactive components. Afr. J. Biotechnol. 2015, 14, 2157–2161. [Google Scholar]
- Jayaprakasha, G.K.; Selvi, T.; Sakariah, K.K. Antibacterial and antioxidant activities of grape (Vitis vinifera) seed extracts. Food Res. Int. 2003, 36, 117–122. [Google Scholar] [CrossRef]
- Silván, J.M.; Mingo, E.; Hidalgo, M.; De Pascual-Teresa, S.; Carrascosa, A.V.; Martinez-Rodriguez, A.J. Antibacterial activity of a grape seed extract and its fractions against Campylobacter spp. Food Control 2013, 29, 25–31. [Google Scholar] [CrossRef]
- Levy, J.; Boyer, R.R.; Neilson, A.P.; O’Keefe, S.F.; Chu, H.S.S.; Williams, R.C.; Dorenkott, M.R.; Goodrich, K.M. Evaluation of peanut skin and grape seed extracts to inhibit growth of foodborne pathogens. Food Sci. Nutr. 2017, 5, 1130–1138. [Google Scholar] [CrossRef] [PubMed]
- Tenore, G.C.; Novellino, E.; Basile, A. Nutraceutical potential and antioxidant benefits of red pitaya (Hylocereus polyrhizus) extracts. J. Funct. Foods 2012, 4, 129–136. [Google Scholar] [CrossRef]
- Yong, Y.Y.; Dykes, G.; Lee, S.M.; Choo, W.S. Comparative study of betacyanin profile and antimicrobial activity of red pitahaya (Hylocereus polyrhizus) and red spinach (Amaranthus dubius). Plant Food. Hum. Nutr. 2017, 72, 41–47. [Google Scholar] [CrossRef] [PubMed]
- Tseng, A.; Zhao, Y. Effect of different drying methods and storage time on the retention of bioactive compounds and antibacterial activity of wine grape pomace (Pinot Noir and Merlot). J. Food Sci. 2012, 77, H192–H201. [Google Scholar] [CrossRef]
- Fratianni, F.; Coppola, R.; Nazzaro, F. Phenolic composition and antimicrobial and antiquorum sensing activity of an ethanolic extract of peels from the apple cultivar annurca. J. Med. Food 2011, 14, 957–963. [Google Scholar] [CrossRef]
- Xu, Y.; Burton, S.; Kim, C.; Sismour, E. Phenolic compounds, antioxidant, and antibacterial properties of pomace extracts from four Virginia-grown grape varieties. Food Sci. Nutr. 2016, 4, 125–133. [Google Scholar] [CrossRef]
- Usha, M.; Ragini, S.; Naqvi, S.M.A. Antibacterial activity of acetone and ethanol extracts of cinnamon (Cinnamomum zeylanicum) and Ajowan (Trachyspermum ammi) on four food spoilage bacteria. Int. Res. J. Biol. Sci. 2012, 1, 7–11. [Google Scholar]
- Salaheen, S.; Nguyen, C.; Hewes, D.; Biswas, D. Cheap extraction of antibacterial compounds of berry pomace and their mode of action against the pathogen Campylobacter jejuni. Food Control 2014, 46, 174–181. [Google Scholar] [CrossRef]
- Shen, X.; Sun, X.; Xie, Q.; Liu, H.; Zhao, Y.; Pan, Y.; Hwang, C.-A.; Wu, V.C.H. Antimicrobial effect of blueberry (Vaccinium corymbosum L.) extracts against the growth of Listeria monocytogenes and Salmonella Enteritidis. Food Control 2014, 35, 159–165. [Google Scholar] [CrossRef]
- Zhang, T.; Wei, X.; Miao, Z.; Hassan, H.; Song, Y.; Fan, M. Screening for antioxidant and antibacterial activities of phenolics from Golden Delicious apple pomace. Chem. Cent. J. 2016, 10, 47. [Google Scholar] [CrossRef]
- Ju, J.; Xie, Y.; Guo, Y.; Cheng, Y.; Qian, H.; Yao, W. Antibacterial activities of bayberry extract on foodborne pathogens and identification of its active components. Food Agric. Immunol. 2019, 30, 385–397. [Google Scholar] [CrossRef]
- Luchian, C.E.; Cotea, V.V.; Vlase, L.; Toiu, A.M.; Colibaba, L.C.; Răschip, I.E.; Nadăş, G.; Gheldiu, A.M.; Tuchiluş, C.; Rotaru, L. Antioxidant and antimicrobial effects of grape pomace extracts. BIO Web Conf. 2019, 15, 04006. [Google Scholar] [CrossRef]
- Radovanović, V.N.; Andjelković, M.; Arsić, B.; Radovanović, A.; Gojković-Bukarica, L. Cost-effective ultrasonic extraction of bioactive polyphenols from vine and wine waste in Serbia. S. Afr. J. Enol. Vitic. 2019, 40, 172–180. [Google Scholar] [CrossRef]
- Olejar, K.J.; Ricci, A.; Swift, S.; Zujovic, Z.; Gordon, K.C.; Fedrizzi, B.; Versari, A.; Kilmartin, P.A. Characterization of an antioxidant and antimicrobial extract from cool climate, white grape marc. Antioxidants 2019, 8, 232. [Google Scholar] [CrossRef] [PubMed]
- Raphaelli, C.O.; Dannenberg, G.; Dalmazo, G.O.; Pereira, E.S.; Radünz, M.; Vizzotto, M.; Fiorentini, A.M.; Gandra, E.A.; Nora, L. Antibacterial and antioxidant properties of phenolic-rich extracts from apple (Malus domestica cv. Gala). Int. Food Res. J. 2019, 26, 1133–1142. [Google Scholar]
- Mahboubi, A.; Asgarpanah, J.; Sadaghiyani, P.N.; Faizi, M. Total phenolic and flavonoid content and antibacterial activity of Punica granatum L. var. pleniflora flowers (Golnar) against bacterial strains causing foodborne diseases. BMC Complement. Altern. Med. 2015, 15, 366. [Google Scholar] [CrossRef]
- Al-Manhel, A.J.; Niamah, A.K. Effect of aqueous and alcoholic plant extracts on inhibition of some types of microbes and causing spoilage of food. J. Nutr. Food. Sci. 2015, S5, 104–109. [Google Scholar] [CrossRef]
- Arokiyaraj, S.; Bharanidharan, R.; Agastian, P.; Shin, H. Chemical composition, antioxidant activity and antibacterial mechanism of action from Marsilea minuta leaf hexane: Methanol extract. Chem. Cent. J. 2018, 12, 105. [Google Scholar] [CrossRef]
- Gonelimali, F.D.; Lin, J.; Miao, W.; Xuan, J.; Charles, F.; Chen, M.; Hatab, S.R. Antimicrobial properties and mechanism of action of some plant extracts against food pathogens and spoilage microorganisms. Front. Microbiol. 2018, 9, 1639. [Google Scholar] [CrossRef]
- Ibrahim, M.M.; El Ghani, S.A.; El-Moez, S.I.A. Phytochemical analysis and antimicrobial activities of different callus extracts of Pelargonium sidoides DC. against food borne pathogenic bacteria. J. Appl. Pharm. Sci. 2018, 8, 109–118. [Google Scholar]
- Generalić Mekinić, I.; Skroza, D.; Ljubenkov, I.; Katalinić, V.; Šimat, V. Antioxidant and antimicrobial potential of phenolic metabolites from traditionally used Mediterranean herbs and spices. Foods 2019, 8, 579. [Google Scholar] [CrossRef]
- Nas, F.S.; Ali, M.; Ahmad, A.M. In vitro antibacterial activity of different extracts of Zingiber officinale against bacterial isolates responsible for food spoilage. SOA Arch. Pharm. Pharmacol. 2018, 1, 001. [Google Scholar]
- Ouerghemmi, I.; Rebey, I.B.; Rahali, F.Z.; Bourgou, S.; Pistelli, L.; Ksouri, R.; Marzouk, B.; Tounsi, M.S. Antioxidant and antimicrobial phenolic compounds from extracts of cultivated and wild-grown Tunisian Ruta chalepensis. J. Food Drug Anal. 2017, 25, 350–359. [Google Scholar] [CrossRef]
- Ramli, S.; Radu, S.; Shaari, K.; Rukayadi, Y. Antibacterial activity of ethanolic extract of Syzygium polyanthum L. (Salam) leaves against foodborne pathogens and application as food sanitizer. BioMed Res. Int. 2017, 2017, 9024246. [Google Scholar] [CrossRef]
- Sagdic, O.; Ozturk, I.; Yilmaz, M.T.; Yetim, H. Effect of grape pomace extracts obtained from different grape varieties on microbial quality of beef patty. J. Food Sci. 2011, 76, M515–M521. [Google Scholar] [CrossRef]
- Sagdic, O.; Ozturk, I.; Kisi, O. Modeling antimicrobial effect of different grape pomace and extracts on S. aureus and E. coli in vegetable soup using artificial neural network and fuzzy logic system. Expert Syst. Appl. 2012, 39, 6792–6798. [Google Scholar] [CrossRef]
- Hayrapetyan, H.; Hazeleger, W.C.; Beumer, R.R. Inhibition of Listeria monocytogenes by pomegranate (Punica granatum) peel extract in meat paté at different temperatures. Food Control 2012, 23, 66–72. [Google Scholar] [CrossRef]
- Ahn, J.; Grün, I.U.; Mustapha, A. Effects of plant extracts on microbial growth, color change, and lipid oxidation in cooked beef. Food Microbiol. 2007, 24, 7–14. [Google Scholar] [CrossRef]
- Bouarab Chibane, L.; Degraeve, P.; Ferhout, H.; Bouajila, J.; Oulahal, N. Plant antimicrobial polyphenols as potential natural food preservatives. J. Sci. Food Agric. 2019, 99, 1457–1474. [Google Scholar] [CrossRef]
- García-Ruiz, A.; Moreno-Arribas, M.V.; Martín-Álvarez, P.J.; Bartolomé, B. Comparative study of the inhibitory effects of wine polyphenols on the growth of enological lactic acid bacteria. Int. J. Food Microbiol. 2011, 145, 426–431. [Google Scholar] [CrossRef]
- Pastorkova, E.; Zakova, T.; Landa, P.; Novakova, J.; Vadlejch, J.; Kokoska, L. Growth inhibitory effect of grape phenolics against wine spoilage yeasts and acetic acid bacteria. Int. J. Food Microbiol. 2013, 161, 209–213. [Google Scholar] [CrossRef] [PubMed]
- Favela-Hernández, J.M.J.; García, A.; Garza-González, E.; Rivas-Galindo, V.M.; Camacho-Corona, M.R. Antibacterial and antimycobacterial lignans and flavonoids from Larrea tridentata. Phytother. Res. 2012, 26, 1957–1960. [Google Scholar] [CrossRef] [PubMed]
- Rehman, S.-U.; Khan, R.; Bhat, K.A.; Raja, A.F.; Shawl, A.S.; Alam, M.S. Isolation, characterisation and antibacterial activity studies of coumarins from Rhododendron lepidotum Wall. ex G. Don, Ericaceae. Rev. Bras. Farmacogn. 2010, 20, 886–890. [Google Scholar] [CrossRef]
- Nitiema, L.W.; Savadogo, A.; Simpore, J.; Dianou, D.; Traore, A.S. In vitro antimicrobial activity of some phenolic compounds (coumarin and quercetin) against gastroenteritis bacterial strains. Int. J. Microbiol. Res. 2012, 3, 183–187. [Google Scholar]
- Díaz-Gómez, R.; Toledo-Araya, H.; López-Solís, R.; Obreque-Slier, E. Combined effect of gallic acid and catechin against Escherichia coli. LWT Food Sci. Technol. 2014, 59, 896–900. [Google Scholar] [CrossRef]
- Kerekes, E.B.; Vidács, A.; Jenei Török, J.; Gömöri, C.; Petkovits, T.; Chandrasekaran, M.; Kadaikunnan, S.; Alharbi, N.S.; Vágvölgyi, C.; Krisch, J. Anti-listerial effect of selected essential oils and thymol. Acta Biol. Hung. 2016, 67, 333–343. [Google Scholar] [CrossRef]
- Rúa, J.; Fernandez-Alvarez, L.; Gutierrez-Larrainzar, M.; Del Valle, P.; De Arriaga, D.; García-Armesto, M.R. Screening of phenolic antioxidants for their inhibitory activity against foodborne Staphylococcus aureus strains. Foodborne Pathog. Dis. 2010, 7, 695–705. [Google Scholar] [CrossRef]
- Quideau, S.; Deffieux, D.; Douat-Casassus, C.; Pouységu, L. Plant polyphenols: Chemical properties, biological activities, and synthesis. Angew. Chem. Int. Ed. Engl. 2011, 50, 586–621. [Google Scholar] [CrossRef]
- Sanhueza, L.; Melo, R.; Montero, R.; Maisey, K.; Mendoza, L.; Wilkens, M. Synergistic interactions between phenolic compounds identified in grape pomace extract with antibiotics of different classes against Staphylococcus aureus and Escherichia coli. PLoS ONE 2017, 12, e0172273. [Google Scholar] [CrossRef]
- Stefanović, O.D. Synergistic activity of antibiotics and bioactive plant extracts: A study against Gram-positive and Gram-negative bacteria. In Bacterial Pathogenesis and Antibacterial Control; Kırmusaoğlu, S., Ed.; IntechOpen: London, UK, 2018; pp. 23–48. [Google Scholar]
- Miklasińska-Majdanik, M.; Kępa, M.; Wojtyczka, R.D.; Idzik, D.; Wąsik, T.J. Phenolic compounds diminish antibiotic resistance of Staphylococcus aureus clinical strains. Int. J. Environ. Res. Public Health 2018, 15, 2321. [Google Scholar] [CrossRef]
- Figueiredo, A.R.; Campos, F.; De Freitas, V.; Hogg, T.; Couto, J.A. Effect of phenolic aldehydes and flavonoids on growth and inactivation of Oenococcus oeni and Lactobacillus hilgardii. Food Microbiol. 2008, 25, 105–112. [Google Scholar] [CrossRef]
- Oteiza, P.I.; Erlejman, A.G.; Verstraeten, S.V.; Keen, C.L.; Fraga, C.G. Flavonoid-membrane interactions: A protective role of flavonoids at the membrane surface? Clin. Dev. Immunol. 2005, 12, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Ultee, A.; Bennik, M.H.J.; Moezelaar, R. The phenolic hydroxyl group of carvacrol is essential for action against the food-borne pathogen Bacillus cereus. Appl. Environ. Microbiol. 2002, 68, 1561–1568. [Google Scholar] [CrossRef] [PubMed]
- Jeong, K.-W.; Lee, J.-Y.; Kang, D.-I.; Lee, J.-U.; Shin, S.Y.; Kim, Y. Screening of flavonoids as candidate antibiotics against Enterococcus faecalis. J. Nat. Prod. 2009, 72, 719–724. [Google Scholar] [CrossRef]
- Engels, C.; Knödler, M.; Zhao, Y.-Y.; Carle, R.; Gänzle, M.G.; Schieber, A. Antimicrobial activity of gallotannins isolated from mango (Mangifera indica L.) kernels. J. Agric. Food Chem. 2009, 57, 7712–7718. [Google Scholar] [CrossRef]
- Wu, T.; Zang, X.; He, M.; Pan, S.; Xu, X. Structure–activity relationship of flavonoids on their anti-Escherichia coli activity and inhibition of DNA gyrase. J. Agric. Food Chem. 2013, 61, 8185–8190. [Google Scholar] [CrossRef]
- Tsuchiya, H. Membrane interactions of phytochemicals as their molecular mechanism applicable to the discovery of drug leads from plants. Molecules 2015, 20, 18923–18966. [Google Scholar] [CrossRef]
- Sanver, D.; Murray, B.S.; Sadeghpour, A.; Rappolt, M.; Nelson, A.L. Experimental modeling of flavonoid–biomembrane interactions. Langmuir 2016, 32, 13234–13243. [Google Scholar] [CrossRef]
- Eumkeb, G.; Chukrathok, S. Synergistic activity and mechanism of action of ceftazidime and apigenin combination against ceftazidime-resistant Enterobacter cloacae. Phytomedicine 2013, 20, 262–269. [Google Scholar] [CrossRef]
- Soromou, L.W.; Zhang, Y.; Cui, Y.; Wei, M.; Chen, N.; Yang, X.; Huo, M.; Baldé, A.; Guan, S.; Deng, X.; et al. Subinhibitory concentrations of pinocembrin exert anti-Staphylococcus aureus activity by reducing α-toxin expression. J. Appl. Microbiol. 2013, 115, 41–49. [Google Scholar] [CrossRef]
- Ikigai, H.; Nakae, T.; Hara, Y.; Shimamura, T. Bactericidal catechins damage the lipid bilayer. BBA Biomembr. 1993, 1147, 132–136. [Google Scholar] [CrossRef]
- Taylor, P.W.; Hamilton-Miller, J.M.; Stapleton, P.D. Antimicrobial properties of green tea catechins. Food Sci. Technol. Bull. 2005, 2, 71–81. [Google Scholar] [CrossRef]
- Yam, T.S.; Hamilton-Miller, J.M.; Shah, S. The effect of a component of tea (Camellia sinensis) on methicillin resistance, PBP2′synthesis, and beta-lactamase production in Staphylococcus aureus. J. Antimicrob. Chemother. 1998, 42, 211–216. [Google Scholar] [CrossRef]
- Mandalari, G.; Bennett, R.N.; Bisignano, G.; Trombetta, D.; Saija, A.; Faulds, C.B.; Gasson, M.J.; Narbad, A. Antimicrobial activity of flavonoids extracted from bergamot (Citrus bergamia Risso) peel, a byproduct of the essential oil industry. J. Appl. Microbiol. 2007, 103, 2056–2064. [Google Scholar] [CrossRef]
- Puupponen-Pimiä, R.; Nohynek, L.; Ammann, S.; Oksman-Caldentey, K.-M.; Buchert, J. Enzyme-assisted processing increases antimicrobial and antioxidant activity of bilberry. J. Agric. Food Chem. 2008, 56, 681–688. [Google Scholar] [CrossRef]
- Ratz-Łyko, A.; Arct, J. Evaluation of antioxidant and antimicrobial properties of enzymatically hydrolysed Cucurbita pepo and Linum usitatissimum seedcakes. Food Sci. Biotechnol. 2015, 24, 1789–1796. [Google Scholar] [CrossRef]
- Skandamis, P.N.; Nychas, G.-J.E. Quorum sensing in the context of food microbiology. Appl. Environ. Microbiol. 2012, 78, 5473–5482. [Google Scholar] [CrossRef]
- Li, Y.-H.; Tian, X. Quorum sensing and bacterial social interactions in biofilms. Sensors 2012, 12, 2519–2538. [Google Scholar] [CrossRef]
- Schaefer, A.L.; Greenberg, E.P.; Oliver, C.M.; Oda, Y.; Huang, J.J.; Bittan-Banin, G.; Peres, C.M.; Schmidt, S.; Juhaszova, K.; Sufrin, J.R.; et al. A new class of homoserine lactone quorum-sensing signals. Nature 2008, 454, 595–599. [Google Scholar] [CrossRef]
- Ryan, R.P.; McCarthy, Y.; Watt, S.A.; Niehaus, K.; Dow, J.M. Intraspecies signaling involving the diffusible signal factor BDSF (cis-2-dodecenoic acid) influences virulence in Burkholderia cenocepacia. J. Bacteriol. 2009, 191, 5013–5019. [Google Scholar] [CrossRef]
- Liu, Y.-C.; Hussain, F.; Negm, O.; Paiva, A.C.; Halliday, N.; Dubern, J.-F.; Singh, S.; Muntaka, S.; Wheldon, L.; Luckett, J.; et al. Contribution of the alkylquinolone quorum-sensing system to the interaction of Pseudomonas aeruginosa with bronchial epithelial cells. Front. Microbiol. 2018, 9, 3018. [Google Scholar] [CrossRef] [PubMed]
- Krzyżek, P. Challenges and limitations of anti-quorum sensing therapies. Front. Microbiol. 2019, 10, 2473. [Google Scholar] [CrossRef] [PubMed]
- Lönn-Stensrud, J.; Landin, M.A.; Benneche, T.; Petersen, F.C.; Scheie, A.A. Furanones, potential agents for preventing Staphylococcus epidermidis biofilm infections? J. Antimicrob. Chemother. 2009, 63, 309–316. [Google Scholar] [CrossRef] [PubMed]
- Husain, A.; Khan, S.A.; Iram, F.; Iqbal, M.A.; Asif, M. Insights into the chemistry and therapeutic potential of furanones: A versatile pharmacophore. Eur. J. Med. Chem. 2019, 171, 66–92. [Google Scholar] [CrossRef]
- Galiè, S.; García-Gutiérrez, C.; Miguélez, E.M.; Villar, C.J.; Lombó, F. Biofilms in the food industry: Health aspects and control methods. Front. Microbiol. 2018, 9, 898. [Google Scholar] [CrossRef]
- Hoffman, L.R.; D’Argenio, D.A.; MacCoss, M.J.; Zhang, Z.; Jones, R.A.; Miller, S.I. Aminoglycoside antibiotics induce bacterial biofilm formation. Nature 2005, 436, 1171–1175. [Google Scholar] [CrossRef]
- Miquel, S.; Lagrafeuille, R.; Souweine, B.; Forestier, C. Anti-biofilm activity as a health issue. Front. Microbiol. 2016, 7, 592. [Google Scholar] [CrossRef]
- Brackman, G.; Coenye, T. Quorum sensing inhibitors as anti-biofilm agents. Curr. Pharm. Des. 2015, 21, 5–11. [Google Scholar] [CrossRef]
- Jiang, Q.; Chen, J.; Yang, C.; Yin, Y.; Yao, K. Quorum sensing: A prospective therapeutic target for bacterial diseases. BioMed Res. Int. 2019, 2019, 2015978. [Google Scholar] [CrossRef]
- Machado, I.; Silva, L.R.; Giaouris, E.D.; Melo, L.F.; Simões, M. Quorum sensing in food spoilage and natural-based strategies for its inhibition. Food Res. Int. 2020, 127, 108754. [Google Scholar] [CrossRef]
- Silva, L.N.; Zimmer, K.R.; Macedo, A.J.; Trentin, D.S. Plant natural products targeting bacterial virulence factors. Chem. Rev. 2016, 116, 9162–9236. [Google Scholar] [CrossRef] [PubMed]
- Slobodníková, L.; Fialová, S.; Rendeková, K.; Kováč, J.; Mučaji, P. Antibiofilm activity of plant polyphenols. Molecules 2016, 21, 1717. [Google Scholar] [CrossRef] [PubMed]
- Asfour, H.Z. Anti-quorum sensing natural compounds. J. Microsc. Ultrastruct. 2018, 6, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Borges, A.; Saavedra, M.J.; Simões, M. The activity of ferulic and gallic acids in biofilm prevention and control of pathogenic bacteria. Biofouling 2012, 28, 755–767. [Google Scholar] [CrossRef] [PubMed]
- Eydelnant, I.A.; Tufenkji, N. Cranberry derived proanthocyanidins reduce bacterial adhesion to selected biomaterials. Langmuir 2008, 24, 10273–10281. [Google Scholar] [CrossRef]
- Xu, X.; Zhou, X.D.; Wu, C.D. The tea catechin epigallocatechin gallate suppresses cariogenic virulence factors of Streptococcus mutans. Antimicrob. Agents Chemother. 2011, 55, 1229–1236. [Google Scholar] [CrossRef]
- Khokhani, D.; Zhang, C.; Li, Y.; Wang, Q.; Zeng, Q.; Yamazaki, A.; Hutchins, W.; Zhou, S.-S.; Chen, X.; Yang, C.-H. Discovery of plant phenolic compounds that act as type III secretion system inhibitors or inducers of the fire blight pathogen, Erwinia amylovora. Appl. Environ. Microbiol. 2013, 79, 5424–5436. [Google Scholar] [CrossRef]
- Vattem, D.A.; Mihalik, K.; Crixell, S.H.; McLean, R.J.C. Dietary phytochemicals as quorum sensing inhibitors. Fitoterapia 2007, 78, 302–310. [Google Scholar] [CrossRef]
- Priha, O.; Virkajärvi, V.; Juvonen, R.; Puupponen-Pimiä, R.; Nohynek, L.; Alakurtti, S.; Pirttimaa, M.; Storgårds, E. Quorum sensing signalling and biofilm formation of brewery-derived bacteria, and inhibition of signalling by natural compounds. Curr. Microbiol. 2014, 69, 617–627. [Google Scholar] [CrossRef]
- Vandeputte, O.M.; Kiendrebeogo, M.; Rajaonson, S.; Diallo, B.; Mol, A.; El Jaziri, M.; Baucher, M. Identification of catechin as one of the flavonoids from Combretum albiflorum bark extract that reduces the production of quorum-sensing-controlled virulence factors in Pseudomonas aeruginosa PAO1. Appl. Environ. Microbiol. 2010, 76, 243–253. [Google Scholar] [CrossRef]
- Vandeputte, O.M.; Kiendrebeogo, M.; Rasamiravaka, T.; Stévigny, C.; Duez, P.; Rajaonson, S.; Diallo, B.; Mol, A.; Baucher, M.; El Jaziri, M. The flavanone naringenin reduces the production of quorum sensing-controlled virulence factors in Pseudomonas aeruginosa PAO1. Microbiology 2011, 157, 2120–2132. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, J.; Sun, F.; Feng, W.; Sun, Y.; Qiu, X.; Xiong, L.; Liu, Y.; Chen, Y. Quercetin is an effective inhibitor of quorum sensing, biofilm formation and virulence factors in Pseudomonas aeruginosa. J. Appl. Microbiol. 2016, 120, 966–974. [Google Scholar] [CrossRef] [PubMed]
- Vasavi, H.S.; Arun, A.B.; Rekha, P.-D. Anti-quorum sensing activity of Psidium guajava L. flavonoids against Chromobacterium violaceum and Pseudomonas aeruginosa PAO1. Microbiol. Immunol. 2014, 58, 286–293. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-H.; Regmi, S.C.; Kim, J.-A.; Cho, M.H.; Yun, H.; Lee, C.-S.; Lee, J. Apple flavonoid phloretin inhibits Escherichia coli O157: H7 biofilm formation and ameliorates colon inflammation in rats. Infect. Immun. 2011, 79, 4819–4827. [Google Scholar] [CrossRef] [PubMed]
- Girennavar, B.; Cepeda, M.L.; Soni, K.A.; Vikram, A.; Jesudhasan, P.; Jayaprakasha, G.K.; Pillai, S.D.; Patil, B.S. Grapefruit juice and its furocoumarins inhibits autoinducer signaling and biofilm formation in bacteria. Int. J. Food Microbiol. 2008, 125, 204–208. [Google Scholar] [CrossRef]
- Vikram, A.; Jayaprakasha, G.K.; Jesudhasan, P.R.; Pillai, S.D.; Patil, B.S. Suppression of bacterial cell-cell signalling, biofilm formation and type III secretion system by citrus flavonoids. J. Appl. Microbiol. 2010, 109, 515–527. [Google Scholar] [CrossRef]
- Sheng, L.; Olsen, S.A.; Hu, J.; Yue, W.; Means, W.J.; Zhu, M.J. Inhibitory effects of grape seed extract on growth, quorum sensing, and virulence factors of CDC “top-six” non-O157 Shiga toxin producing E. coli. Int. J. Food Microbiol. 2016, 229, 24–32. [Google Scholar] [CrossRef]
- Luís, Â.; Silva, F.; Sousa, S.; Duarte, A.P.; Domingues, F. Antistaphylococcal and biofilm inhibitory activities of gallic, caffeic, and chlorogenic acids. Biofouling 2014, 30, 69–79. [Google Scholar] [CrossRef]
- Lee, J.-H.; Kim, Y.-G.; Ryu, S.Y.; Cho, M.H.; Lee, J. Ginkgolic acids and Ginkgo biloba extract inhibit Escherichia coli O157: H7 and Staphylococcus aureus biofilm formation. Int. J. Food Microbiol. 2014, 174, 47–55. [Google Scholar] [CrossRef]
- Bakkiyaraj, D.; Nandhini, J.R.; Malathy, B.; Pandian, S.K. The anti-biofilm potential of pomegranate (Punica granatum L.) extract against human bacterial and fungal pathogens. Biofouling 2013, 29, 929–937. [Google Scholar] [CrossRef]
- Slobodníková, L.; Fialová, S.; Hupková, H.; Grančai, D. Rosmarinic acid interaction with planktonic and biofilm Staphylococcus aureus. Nat. Prod. Commun. 2013, 8, 1747–1750. [Google Scholar] [CrossRef]
- Lin, M.-H.; Chang, F.-R.; Hua, M.-Y.; Wu, Y.-C.; Liu, S.-T. Inhibitory effects of 1,2,3,4,6-penta-O-galloyl-β-D-glucopyranose on biofilm formation by Staphylococcus aureus. Antimicrob. Agents Chemother. 2011, 55, 1021–1027. [Google Scholar] [CrossRef]
- Cho, H.S.; Lee, J.-H.; Cho, M.H.; Lee, J. Red wines and flavonoids diminish Staphylococcus aureus virulence with anti-biofilm and anti-hemolytic activities. Biofouling 2015, 31, 1–11. [Google Scholar] [CrossRef]
- Xu, C.; Yagiz, Y.; Hsu, W.-Y.; Simonne, A.; Lu, J.; Marshall, M.R. Antioxidant, antibacterial, and antibiofilm properties of polyphenols from muscadine grape (Vitis rotundifolia Michx.) pomace against selected foodborne pathogens. J. Agric. Food Chem. 2014, 62, 6640–6649. [Google Scholar] [CrossRef]
- Rahman, M.R.T.; Lou, Z.; Yu, F.; Wang, P.; Wang, H. Anti-quorum sensing and anti-biofilm activity of Amomum tsaoko (Amommum tsao-ko Crevost et Lemarie) on foodborne pathogens. Saudi J. Biol. Sci. 2017, 24, 324–330. [Google Scholar] [CrossRef] [PubMed]
- De Marco, S.; Piccioni, M.; Pagiotti, R.; Pietrella, D. Antibiofilm and antioxidant activity of propolis and bud poplar resins versus Pseudomonas aeruginosa. Evid. Based Complement. Altern. Med. 2017, 2017, 5163575. [Google Scholar] [CrossRef] [PubMed]
- Maia, D.S.V.; Haubert, L.; Kroning, I.S.; Dos Santos Soares, K.; Oliveira, T.L.; Da Silva, W.P. Biofilm formation by Staphylococcus aureus isolated from food poisoning outbreaks and effect of Butia odorata Barb. Rodr. extract on planktonic and biofilm cells. LWT Food Sci. Technol. 2020, 117, 108685. [Google Scholar] [CrossRef]
- Sharma, K.; Mahato, N.; Lee, Y.R. Systematic study on active compounds as antibacterial and antibiofilm agent in aging onions. J. Food Drug Anal. 2018, 26, 518–528. [Google Scholar] [CrossRef]
- Edziri, H.; Jaziri, R.; Chehab, H.; Verschaeve, L.; Flamini, G.; Boujnah, D.; Hammami, M.; Aouni, M.; Mastouri, M. A comparative study on chemical composition, antibiofilm and biological activities of leaves extracts of four Tunisian olive cultivars. Heliyon 2019, 5, e01604. [Google Scholar] [CrossRef]
- Nassima, B.; Nassima, B.; Riadh, K. Antimicrobial and antibiofilm activities of phenolic compounds extracted from Populus nigra and Populus alba buds (Algeria). Braz. J. Pharm. Sci. 2019, 55, e18114. [Google Scholar] [CrossRef]
- Blando, F.; Russo, R.; Negro, C.; De Bellis, L.; Frassinetti, S. Antimicrobial and antibiofilm activity against Staphylococcus aureus of Opuntia ficus-indica (L.) Mill. cladode polyphenolic extracts. Antioxidants 2019, 8, 117. [Google Scholar] [CrossRef] [PubMed]
- Famuyide, I.M.; Aro, A.O.; Fasina, F.O.; Eloff, J.N.; McGaw, L.J. Antibacterial and antibiofilm activity of acetone leaf extracts of nine under-investigated south African Eugenia and Syzygium (Myrtaceae) species and their selectivity indices. BMC Complement. Altern. Med. 2019, 19, 141. [Google Scholar] [CrossRef] [PubMed]
- Radojević, I.D.; Branković, S.R.; Vasić, S.M.; Ćirić, A.R.; Topuzović, M.D.; Čomić, L.R. Phytochemical profiles, antioxidant and antimicrobial with antibiofilm activities of wild growing Potentilla visianii extracts. Nat. Prod. Commun. 2018, 13, 851–854. [Google Scholar] [CrossRef]
- Stefanović, O.; Ličina, B.; Vasić, S.; Radojević, I.; Čomić, L. Bioactive extracts of Gentiana asclepiadea: Antioxidant, antimicrobial, and antibiofilm activity. Bot. Serbica 2018, 42, 223–229. [Google Scholar]
- Muñoz-Cazares, N.; García-Contreras, R.; Pérez-López, M.; Castillo-Juárez, I. Phenolic compounds with anti-virulence properties. In Phenolic Compounds-Biological Activity; Marcos Soto-Hernandez, M., Palma-Tenango, M., Del Rosario Garcia-Mateos, M., Eds.; IntechOpen: London, UK, 2017; pp. 139–167. [Google Scholar]
- Fisher, E.L.; Otto, M.; Cheung, G.Y. Basis of virulence in enterotoxin-mediated staphylococcal food poisoning. Front. Microbiol. 2018, 9, 436. [Google Scholar] [CrossRef]
- Argudín, M.Á.; Mendoza, M.C.; Rodicio, M.R. Food poisoning and Staphylococcus aureus enterotoxins. Toxins 2010, 2, 1751–1773. [Google Scholar] [CrossRef]
- Albano, M.; Alves, F.C.B.; Andrade, B.F.M.T.; Barbosa, L.N.; Pereira, A.F.M.; Da Cunha, M.; Rall, V.L.M.; Júnior, A.F. Antibacterial and anti-staphylococcal enterotoxin activities of phenolic compounds. Innov. Food Sci. Emerg. Technol. 2016, 38, 83–90. [Google Scholar] [CrossRef]
- Rasooly, R.; Molnar, A.; Choi, H.-Y.; Do, P.; Racicot, K.; Apostolidis, E. In-vitro inhibition of staphylococcal pathogenesis by witch-hazel and green tea extracts. Antibiotics 2019, 8, 244. [Google Scholar] [CrossRef]
- Qiu, J.; Feng, H.; Xiang, H.; Wang, D.; Xia, L.; Jiang, Y.; Song, K.; Lu, J.; Yu, L.; Deng, X. Influence of subinhibitory concentrations of licochalcone A on the secretion of enterotoxins A and B by Staphylococcus aureus. FEMS Microbiol. Lett. 2010, 307, 135–141. [Google Scholar] [CrossRef]
- Souza, E.L.; Oliveira, C.E.V.; Stamford, T.L.M.; Conceição, M.L.; Gomes Neto, N.J. Influence of carvacrol and thymol on the physiological attributes, enterotoxin production and surface characteristics of Staphylococcus aureus strains isolated from foods. Braz. J. Microbiol. 2013, 44, 29–36. [Google Scholar] [CrossRef]
- Shimamura, Y.; Aoki, N.; Sugiyama, Y.; Tanaka, T.; Murata, M.; Masuda, S. Plant-derived polyphenols interact with staphylococcal enterotoxin A and inhibit toxin activity. PLoS ONE 2016, 11, e0157082. [Google Scholar] [CrossRef]
- Shimamura, Y.; Hirai, C.; Sugiyama, Y.; Shibata, M.; Ozaki, J.; Murata, M.; Ohashi, N.; Masuda, S. Inhibitory effects of food additives derived from polyphenols on staphylococcal enterotoxin A production and biofilm formation by Staphylococcus aureus. Biosci. Biotechnol. Biochem. 2017, 81, 2346–2352. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Zhu, A.; Tang, J.; Tang, C.; Chen, J. Comparative effects of food preservatives on the production of staphylococcal enterotoxin I from Staphylococcus aureus isolate. J. Food Qual. 2017, 2017, 9495314. [Google Scholar] [CrossRef]
- Rasooly, R.; Do, P.M.; Friedman, M. Inhibition of biological activity of staphylococcal enterotoxin A (SEA) by apple juice and apple polyphenols. J. Agric. Food Chem. 2010, 58, 5421–5426. [Google Scholar] [CrossRef] [PubMed]
- Braga, L.C.; Shupp, J.W.; Cummings, C.; Jett, M.; Takahashi, J.A.; Carmo, L.S.; Chartone-Souza, E.; Nascimento, A.M.A. Pomegranate extract inhibits Staphylococcus aureus growth and subsequent enterotoxin production. J. Ethnopharmacol. 2005, 96, 335–339. [Google Scholar] [CrossRef]
- Tranter, H.S.; Tassou, S.C.; Nychas, G.J. The effect of the olive phenolic compound, oleuropein, on growth and enterotoxin B production by Staphylococcus aureus. J. Appl. Bacteriol. 1993, 74, 253–259. [Google Scholar] [CrossRef]
- Reddy, S.; Taylor, M.; Zhao, M.; Cherubin, P.; Geden, S.; Ray, S.; Francis, D.; Teter, K. Grape extracts inhibit multiple events in the cell biology of cholera intoxication. PLoS ONE 2013, 8, e73390. [Google Scholar] [CrossRef]
- Cherubin, P.; Garcia, M.C.; Curtis, D.; Britt, C.B.; Craft, J.W., Jr.; Burress, H.; Berndt, C.; Reddy, S.; Guyette, J.; Zheng, T.; et al. Inhibition of cholera toxin and other AB toxins by polyphenolic compounds. PLoS ONE 2016, 11, e0166477. [Google Scholar] [CrossRef]
- Quiñones, B.; Massey, S.; Friedman, M.; Swimley, M.S.; Teter, K. Novel cell-based method to detect Shiga toxin 2 from Escherichia coli O157: H7 and inhibitors of toxin activity. Appl. Environ. Microbiol. 2009, 75, 1410–1416. [Google Scholar] [CrossRef] [PubMed]
| Foodborne Pathogen Bacteria | Toxin Production | Type of Disease | Main Food Sources of Infection | Reference |
|---|---|---|---|---|
| Bacillus cereus | Emetic toxin, diarrheal toxin | Emetic syndrome, diarrhea | Rice, pasta, noodles, pastry | [13] |
| Campylobacter coli, Campylobacter jejuni | Cytolethal distending toxin | Campylobacteriosis | Poultry products, unpasteurized milk | [14,15] |
| Clostridium botulinum | Botulinum toxin | Botulism | Improperly processed canned foods | [13] |
| Escherichia coli O157:H7 | Shiga-toxin | Hemorrhagic colitis | Ground meats, raw or under-pasteurized milk, sprouts | [13,15] |
| Listeria monocytogenes | Listeriolysin O | Listeriosis | Soft cheeses from unpasteurized milk, ready-to-eat products | [15,16] |
| Salmonella Typhi, Salmonella Typhimurium, Salmonella Enteritidis | Enterotoxin | Typhoid fever, salmonellosis (gastroenteritis) | Any type of food: meat, poultry, fish, milk, eggs, vegetables, water | [13,15] |
| Staphylococcus aureus | Heat stable enterotoxins | Gastrointestinal symptoms | Meat, dairy products, salads | [13] |
| Vibrio cholerae, Vibrio parahaemolyticus, Vibrio vulnificus | Cholera toxin | Cholera, gastroenteritis | Raw/undercooked shellfish, meat, contaminated water | [13] |
| Plant Materials | Type of Extraction | Target Organism | Antimicrobial Activity | Reference |
|---|---|---|---|---|
| Fruit samples | ||||
| Red wine grape pomace | 70% acetone/0.1% HCl/29.9% water (v/v/v) | Escherichia coli, Listeria innocua | Pinot Noir-pomace and skin MIC 1: E. coli, 3% and 6%; L. innocua, 2% and 7% | [37] |
| Merlot-pomace and skin MIC: E. coli, 9%; L. innocua, 8% | ||||
| Apple peel | Ethanol | Lactobacillus acidophilus, Lactobacillus bulgaricus, Lactobacillus plantarum, Lactobacillus rhamnosus, E. coli, Bacillus cereus, Staphylococcus aureus | Extract concentration: 2-20 µg/disk | [38] |
| B. cereus and E. coli: inhibition haloes of 6 to 14 mm | ||||
| Probiotic lactobacilli: no inhibition | ||||
| S. aureus: no inhibition | ||||
| Red and white grape pomaces | Acetone | Listeria monocytogenes, S. aureus, E. coli O157:H7, Salmonella Typhimurium | MIC (mg/mL): L. monocytogenes, 4.69–18.8; S. aureus, 40.6–250 | [39] |
| MBC 2 (mg/mL): L. monocytogenes, 9.38–37.5; S. aureus, > 250 | ||||
| E. coli: no inhibition | ||||
| S. Typhimurium: no inhibition | ||||
| Cinnamon bark and Ajowan fruit | Acetone, ethanol | Pseudomonas sp., Bacillus subtilis, E. coli, S. aureus | MIC values (μg/mL): | [40] |
| ethanol extract of cinnamon, 32–64; ethanol extract of Ajowan, 32–64; acetone extracts of cinnamon, 16–64; Acetone extract of Ajowan, 64–128 | ||||
| Blackberry and blueberry pomaces | 10% methanol, 10% ethanol | Campylobacter jejuni | MIC (mg/mL GAE): blackberry, 0.6; blueberry, 0.4 | [41] |
| MBC (mg/mL GAE): blackberry, 0.8; blueberry, 0.5 | ||||
| Blueberry puree | 75% ethanol | L. monocytogenes, Salmonella Enteritidis | MIC (mg/mL): L. monocytogenes, 300–750; S. Enteritidis, 400–1200 | [42] |
| Apple pomace | Ethyl acetate | S. aureus, E. coli | MIC (mg/mL): S. aureus, 1.25; E. coli, 2.50 | [43] |
| Black grape pomace, apple and pitahaya residues | 10% ethanol after enzyme-aided extraction | B. subtilis, B. cereus, L. monocytogenes, S. aureus, methicillin-resistant S. aureus, E. coli, S. Typhimurium, Pseudomonas putida, P. aeruginosa | MICs: from 12.5 to ≥ 100 mg/mL | [3] |
| Bayberry | Ethanol | S. aureus, L. innocua, β-hemolytic Streptococcus, S. Enteritidis, Salmonella typhi, Shigella dysenteriae | Diameter of inhibition (mm): | [44] |
| S. aureus, 22.9; L. innocua, 21.5; β-hemolytic Streptococcus, 22.7; S. Enteritidis, 20.1; S. typhi, 13.3; S. dysenteriae, 19.3 | ||||
| Grape pomace | 50% methanol, 50% ethanol | S. aureus, E. coli, P. aeruginosa, Candida albicans | Extract concentration: 1 mg/disk | [45] |
| Diameter of inhibition (mm): | ||||
| C. albicans, 12–13 | ||||
| S. aureus, E. coli, P. aeruginosa: no inhibition | ||||
| Grape residues | Ultrasound-assisted extraction, methanol:acetone: water:acetic acid (30:42:27.5:0.5) | Clostridium perfringens, B. cereus, L. monocytogenes, S. aureus, Sarcina lutea, Micrococcus flavus, E. coli, P. aeruginosa, S. Enteritidis, Shigella sonnei, Klebsiella pneumoniae, C. albicans | Extract concentration: 30 µg/disk | [46] |
| Diameter of inhibition (mm): | ||||
| C. perfringens, 15.9–17.7; B. cereus, 15.2–17.1; L. monocytogenes, 16.4–18.5; S. aureus,16.5–18.5; S. lutea, 17.3–19.7; M. flavus, 14.8-16.9; E. coli, 12.1–15.7; P. aeruginosa, 13.5–15.9; S. Enteritidis, 13–15.4; S. sonnei, 15.6–17.7; K. pneumoniae, 15–16.6; C. albicans, 13.1–15.5 | ||||
| Grape marc waste | Aqueous extraction and Amberlite FPX-66 purification | E. coli, S. aureus, C. albicans | MBC (%, w/v): E. coli, 2; S. aureus, 0.125; C. albicans, no effect | [47] |
| Apple phenolic fractions | Acetone:ethanol (1:3), solid phase extraction | L. monocytogenes, S. aureus, E. coli, S. Typhimurium | Extract concentration: 10–5000 µg/disk | [48] |
| Diameter of inhibition (mm): | ||||
| L. monocytogenes, 3.7–14.6; S. aureus, 10.9–17.6; E. coli, 7.5; S. Typhimurium, 4.5–7 | ||||
| Medicinal plants and herbs | ||||
| Punica granatum L. var. pleniflora flowers | Ethanol | S. aureus, B. cereus, L. monocytogenes, E. coli, S. dysenteriae, S. typhi | Extract concentration: 50 mg/well | [49] |
| Diameter of inhibition (mm): | ||||
| S. aureus, 32; B. cereus, 28; L. monocytogenes, 32; E. coli, 22; S. dysenteriae, 30; S. typhi, 27 | ||||
| Ziziphus and eucalyptus leaves | Aqueous and ethanol | B. subtilis, E. coli, S. aureus, P. aeruginosa, Streptococcus sp. | Extract concentration: 50–100 mg/mL | [50] |
| Diameter of inhibition (mm): | ||||
| B. subtilis, 11–19; E. coli, 10–16; S. aureus, 8–17; P. aeruginosa, 9–16; Streptococcus sp., 11–18 | ||||
| Marsilea minuta leaf | Methanol, hexane: methanol | B. subtilis, Enterococcus faecalis, K. pneumoniae, P. aeruginosa | MICs: from 125 to 250 µg/mL | [51] |
| Roselle, rosemary, clove and thyme | Aqueous and ethanol | B. cereus, S. aureus, E. coli, S. Enteritidis, Vibrio parahaemolyticus, P. aeruginosa, Candida albicans | MICs: from 0.313 to 20% (w/v) | [52] |
| Pelargonium sidoides DC. | Methanol (85%), acetone (80%) | C. perfringens, S. aureus, Shigella flexneri, E. coli O157, S. Typhimurium, C. albicans | Diameter of inhibition (mm): | [53] |
| C. perfringens, 8–35; S. aureus, 13–29.7; S. flexneri, 13-35.3; E. coli, 16–36; S. Typhimurium, 11.3–30; C. albicans, 12–30 | ||||
| 15 Mediterranean medicinal plants | Ethanol:water (80:20) | Camplyobacter coli, E. coli, Salmonella Infantis, B. cereus, L. monocytogenes, S. aureus | Lowest MIC values (mg/mL): | [54] |
| C. coli, 0.83 (e.g., bearberry); E. coli, 1.67 (bearberry); S. Infantis, 1.67 (bearberry); B. cereus, 1.67 (e.g., bearberry); L. monocytogenes, 1.67 (e.g., bearberry); S. aureus, 0.35 (bearberry) | ||||
| Ginger rhizomes | Aqueous, ethanol, n-hexane | K. pneumoniae, S. typhi, Shigella spp., P. aeruginosa, E. coli, S. aureus | Extract concentration: 10 µg/mL | [55] |
| Diameter of inhibition (mm): | ||||
| K. pneumoniae, 0.8–15.4; S. typhi, 13.2–16.2; Shigella spp., 12.3–17.7; P. aeruginosa, 12.6–16; E. coli, 14.7–17.2; S. aureus, 13.3–18.3 | ||||
| Ruta chalepensis | Methanol | S. aureus, E. coli, P. aeruginosa | Extract concentration: 10 mg/disk | [56] |
| Diameter of inhibition (mm): | ||||
| S. aureus, 12.3–16.3; E. coli, 13–17.3; P. aeruginosa, 7.7–17.7 | ||||
| Syzygium polyanthum L. leaves | Ethanol | E. coli O157:H7, K. pneumoniae, L. monocytogenes, Proteus mirabilis, P. aeruginosa, S. Typhimurium, S. aureus, Vibrio cholerae, V. parahaemolyticus | Extract concentration: 100 µg/disk | [57] |
| Diameter of inhibition (mm): | ||||
| E. coli, 7; K. pneumoniae, 9.3; L. monocytogenes, 9.6; P. mirabilis, 6.6; P. aeruginosa, 7; S. Typhimurium, 6.6; S. aureus, 9.3; V. cholerae, 8.3; V. parahaemolyticus, 6.6; | ||||
| MICs: from 0.63 to 1.25 mg/mL | ||||
| MBCs: from 0.63 to 2.5 mg/mL | ||||
| Compounds | Type of Solvent | Target Organism | Antimicrobial Activity | Reference |
|---|---|---|---|---|
| Coumarin, quercetin | Dissolved in dimethyl sulfoxide (DMSO) | Escherichia coli, Enterobacter aerogenes, Salmonella infantis, Salmonella Typhimurium | Coumarin: MIC 1, 0.625–5 mg/mL; MBC 2, ≥ 5 mg/mL | [67] |
| Quercetin: no effect | ||||
| Gallic acid, catechin | Dissolved in DMSO | E. coli | Inhibition haloes of 12 and 14 mm in the presence of 2.5 and 15 mg/well gallic acid and catechin, respectively. | [68] |
| Ellagic acid, quercetin-3-galactoside, chlorogenic acid, quercetin | Tryptic soy broth | Listeria monocytogenes, Salmonella Enteritidis | Effective concentrations: chlorogenic acid, 500 µg/mL; quercetin and quercetin-3-galactoside, 200 µg/mL; ellagic acid, 44 µg/mL | [42] |
| Phloridzin, phloretin | Ethanol | Staphylococcus aureus, E. coli | MIC: S. aureus 0.50 and 0.10 mg/mL, E. coli 1.50 and 0.75 mg/ml | [43] |
| Thymol | Dissolved in ethanol | L. monocytogenes | MIC: 2 mg/mL | [69] |
| 11 phenolic compounds | Dissolved in 10% ethanol | Bacillus subtilis, Bacillus cereus, L. monocytogenes, S. aureus, methicillin-resistant S. aureus, E. coli, S. Typhimurium, Pseudomonas putida, Pseudomonas aeruginosa | MICs: from 125 to ≥ 500 µg/mL | [3] |
| Cinnamic acid and resveratrol, 125 µg/mL; p-coumaric acid, 250 µg/mL; quercetin, 500 µg/mL against B. subtilis, B. cereus, respectively. Resveratrol, 250 and 500 µg/mL against P. aeruginosa and P. putida, respectively. | ||||
| 17 phenolic compounds | Dissolved in absolute ethanol | 19 S. aureus strains, including enterotoxin producers | Compound concentration: 200 µg/disk | [70] |
| Hydroquinone, thymol, carvacrol, butylated hydroxyanisole, octyl gallate, and tannic acid inhibited the growth of all strains tested. |
| Source/Residue | Solvent of Extraction | Target Biofilm | Percent Biofilm Inhibition | Reference |
|---|---|---|---|---|
| Black Cardamom (Amomum tsao-ko) extract | 80% ethanol | Staphylococcus aureus, Salmonella Typhimurium, Pseudomonas aeruginosa | 45.2–51.9% (4 mg/mL cc.) | [129] |
| Propolis and bud poplar resins | 85% ethanol | P. aeruginosa | 50–60% (100 µg/mL cc.) | [130] |
| Butia odorata extract | acetone | S. aureus | 99.9% (11.4–22.8 mg/mL cc.) | [131] |
| Onion extracts | methanol | P. aeruginosa, S. aureus | 27.3–61.5% (50 µg/mL cc.) | [132] |
| Olive leaves | methanol | P. aeruginosa, methicillin-resistant Staphylococcus aureus (MRSA), S. aureus, Bacillus subtilis, Escherichia coli, Enterococcus faecalis, Candida albicans | 29.3–98% (32-512 µg/mL cc.) | [133] |
| Populus nigra and Populus alba bud extracts | methanol | MRSA, S. aureus | >70% for P. nigra, >50% for P. alba | [134] |
| Opuntia ficus-indica cladodes | 80% methanol | S. aureus | 71–85% (1–1.5 mg/mL cc.) | [135] |
| Eugenia and Syzygium leaf extracts | acetone | P. aeruginosa, S. Typhimurium, S. aureus, E. faecalis, E. coli, Bacillus cereus | >50% for several samples (1 mg/mL cc.) | [136] |
| Potentilla visianii extracts | methanol, ethyl acetate | Salmonella enterica, E. coli, S. aureus, B. subtilis | >50% (1.1–10 mg/mL cc.) | [137] |
| Gentiana asclepiadea extracts | water, ethanol, acetone | S. aureus, P. aeruginosa, Proteus mirabilis | >50% (2.1–37 mg/mL cc.) | [138] |
| Phenolic Extract/Compound | Target Staphylococcal Enterotoxin | Anti-Enterotoxin Activity | Reference |
|---|---|---|---|
| Licochalcone A | Enterotoxins A (SEA) and B (SEB) | Effective concentration: 0.25 mg/mL | [144] |
| Secretion inhibition; Inhibition of regulatory gene (agrA) transcription | |||
| Carvacrol and thymol | Not specified | Total inhibition of secretion at 0.3 and 0.15 µL/mL concentrations | [145] |
| Cinnamaldehyde, citronellol, eugenol, geraniol and terpineol | SEA, SEB, Enterotoxins C (SEC) and D (SED) | Concentrations: 120–1300 µg/mL | [142] |
| SEA: eugenol, citronellol and geraniol reduced the production; SEB: terpineol and eugenol inhibited the production; SEC: most sensitive to the phenolics; SED: no inhibition | |||
| 16 phenolic compounds | SEA | Inhibition of SEA protein level (penta-galloyl-glucose, corilagin, punicalagin, castalagin and procyanidin B2 at 0.25 mg/mL) and activity (penta-galloyl-glucose, tannic acid, persimmon tannin, corilagin, punicalagin, eugeniin, sanguiin H-6, geraniin, pedunculagin and castalagin, 3–25 μg/mL), interaction with SEA (eugeniin, castalagin, punicalagin, pedunculagin, corilagin, geraniin, penta-galloyl-glucose and sanguiin H-6 at 0.25 mg/mL) | [146] |
| 14 phenolic food additives | SEA | SEA production decrease: Tannic acid AL, Purephenon 50 W and Polyphenon 70A at 0.25 mg/mL; Gravinol®-N, Blackcurrant polyphenol AC10 and Resveratrol-P5 at 1.0 mg/mL | [147] |
| Inhibition of sea gene expression (mg/mL): Tannic acid, 0.3; Gravinol®-N, 1; Blackcurrant polyphenol AC10, 1; Resveratrol-P5, 2 | |||
| Tea catechin | Enterotoxin I (SEI) | Inhibition of sei gene expression at 0.4 g/L concentration | [148] |
| Apple juice and apple polyphenols | SEA | Activity inhibition: | [149] |
| Red Delicious at 0.025% | |||
| Apple Poly phenol-rich extract at 0.06–0.3% | |||
| Witch-hazel and green tea extracts | SEA | Witch-hazel: inhibition of SEA production at 0.015 mg/mL GAE concentration | [143] |
| Green tea: no effect | |||
| Pomegranate extract | SEA | Inhibition of SEA production at 0.05% (v/v) concentration | [150] |
| Oleuropein | SEB | Inhibition of SEB production at > 0.2% (w/v) concentrations | [151] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Takó, M.; Kerekes, E.B.; Zambrano, C.; Kotogán, A.; Papp, T.; Krisch, J.; Vágvölgyi, C. Plant Phenolics and Phenolic-Enriched Extracts as Antimicrobial Agents against Food-Contaminating Microorganisms. Antioxidants 2020, 9, 165. https://doi.org/10.3390/antiox9020165
Takó M, Kerekes EB, Zambrano C, Kotogán A, Papp T, Krisch J, Vágvölgyi C. Plant Phenolics and Phenolic-Enriched Extracts as Antimicrobial Agents against Food-Contaminating Microorganisms. Antioxidants. 2020; 9(2):165. https://doi.org/10.3390/antiox9020165
Chicago/Turabian StyleTakó, Miklós, Erika Beáta Kerekes, Carolina Zambrano, Alexandra Kotogán, Tamás Papp, Judit Krisch, and Csaba Vágvölgyi. 2020. "Plant Phenolics and Phenolic-Enriched Extracts as Antimicrobial Agents against Food-Contaminating Microorganisms" Antioxidants 9, no. 2: 165. https://doi.org/10.3390/antiox9020165
APA StyleTakó, M., Kerekes, E. B., Zambrano, C., Kotogán, A., Papp, T., Krisch, J., & Vágvölgyi, C. (2020). Plant Phenolics and Phenolic-Enriched Extracts as Antimicrobial Agents against Food-Contaminating Microorganisms. Antioxidants, 9(2), 165. https://doi.org/10.3390/antiox9020165







