Protective Effects of Sulforaphane on Exercise-Induced Organ Damage via Inducing Antioxidant Defense Responses
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
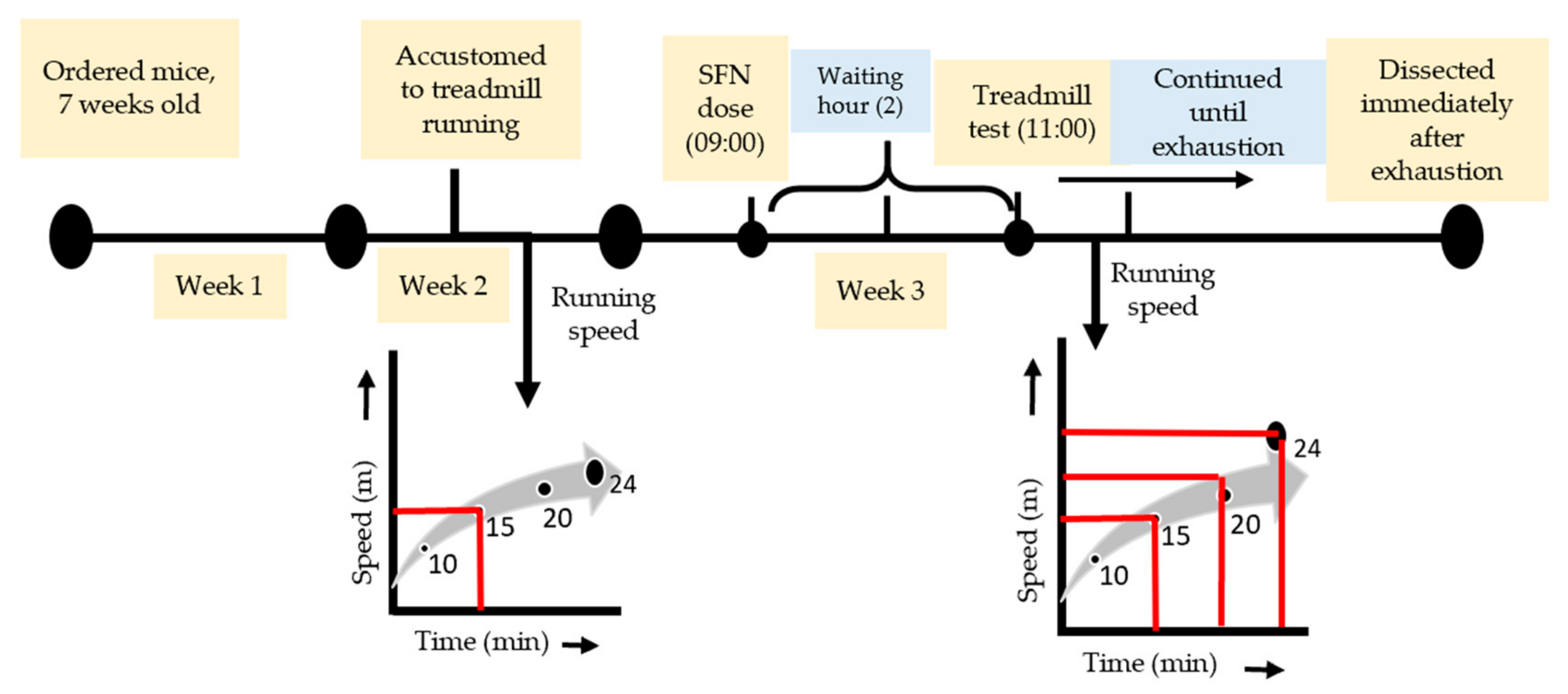
2.2. Exhaustive Exercise Protocol
2.3. Sample Collection
2.4. Plasma Biochemical Parameters
2.5. Real-Time (RT) Quantitative Polymerase Chain Reaction (qPCR)
2.6. Statistical Analysis
3. Results
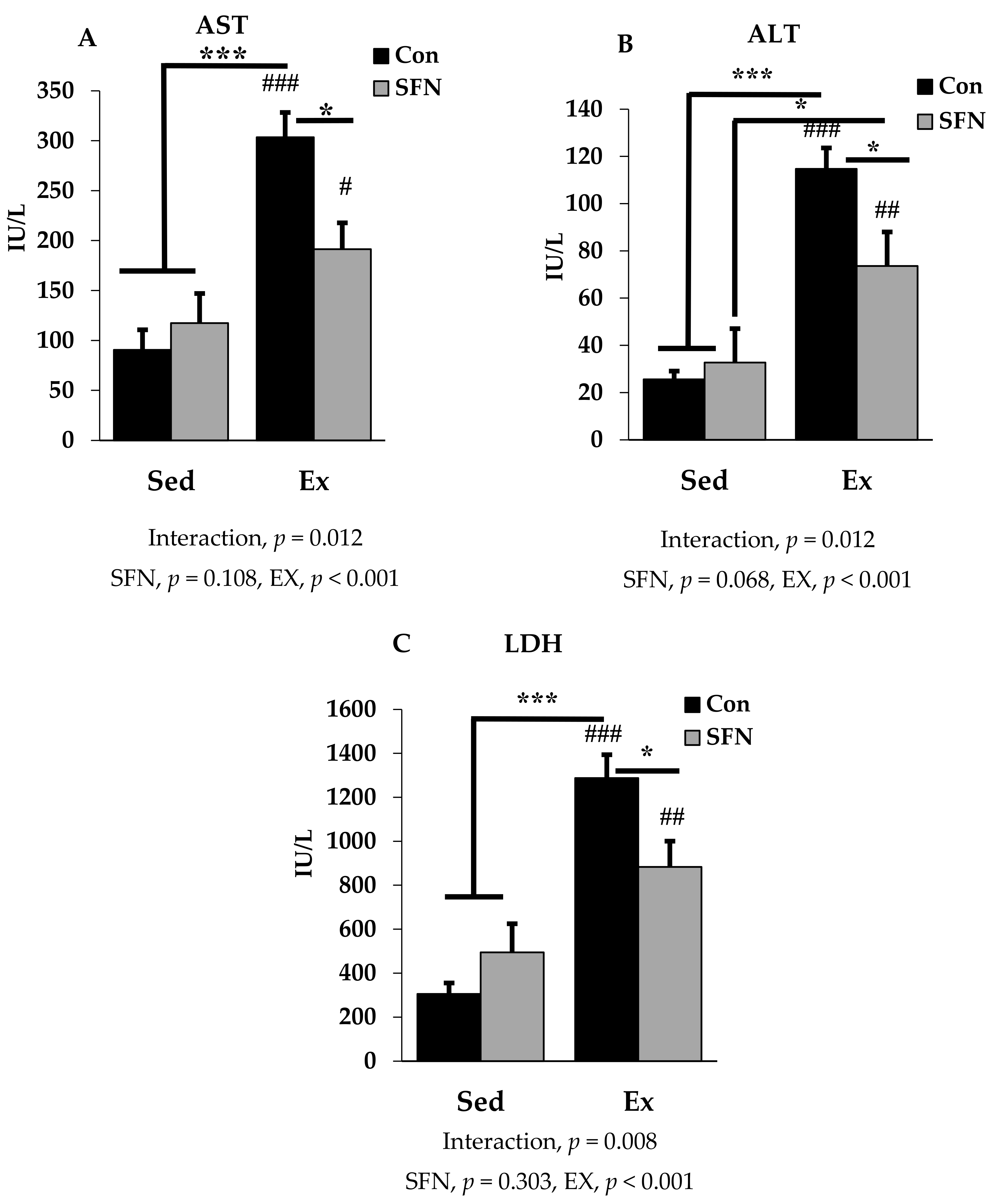
3.1. Effect of SFN on Plasma Levels of Exercise-Induced AST, ALT, and LDH
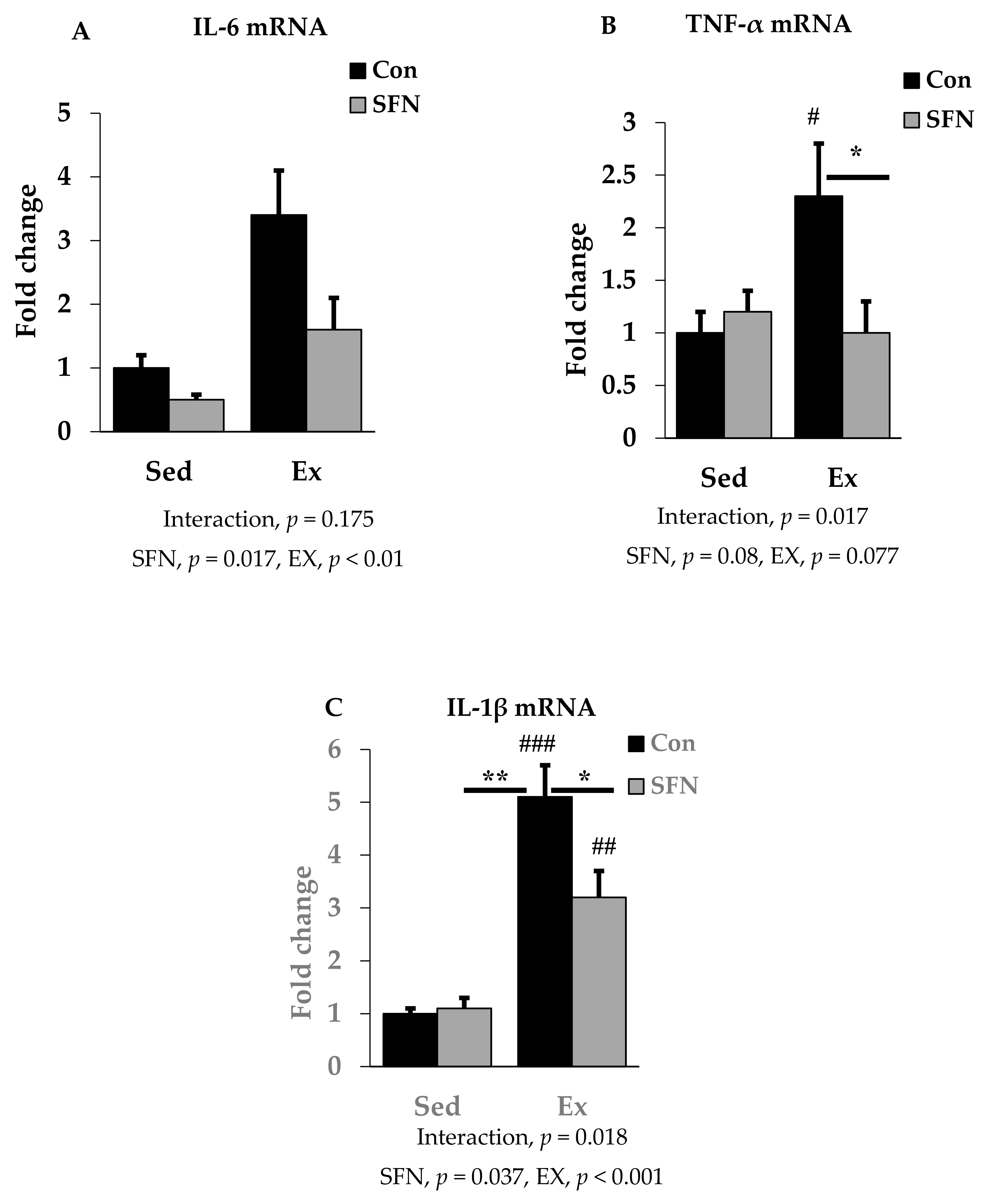
3.2. Effect of SFN on Exercise-Induced Pro-Inflammatory Cytokine Expression
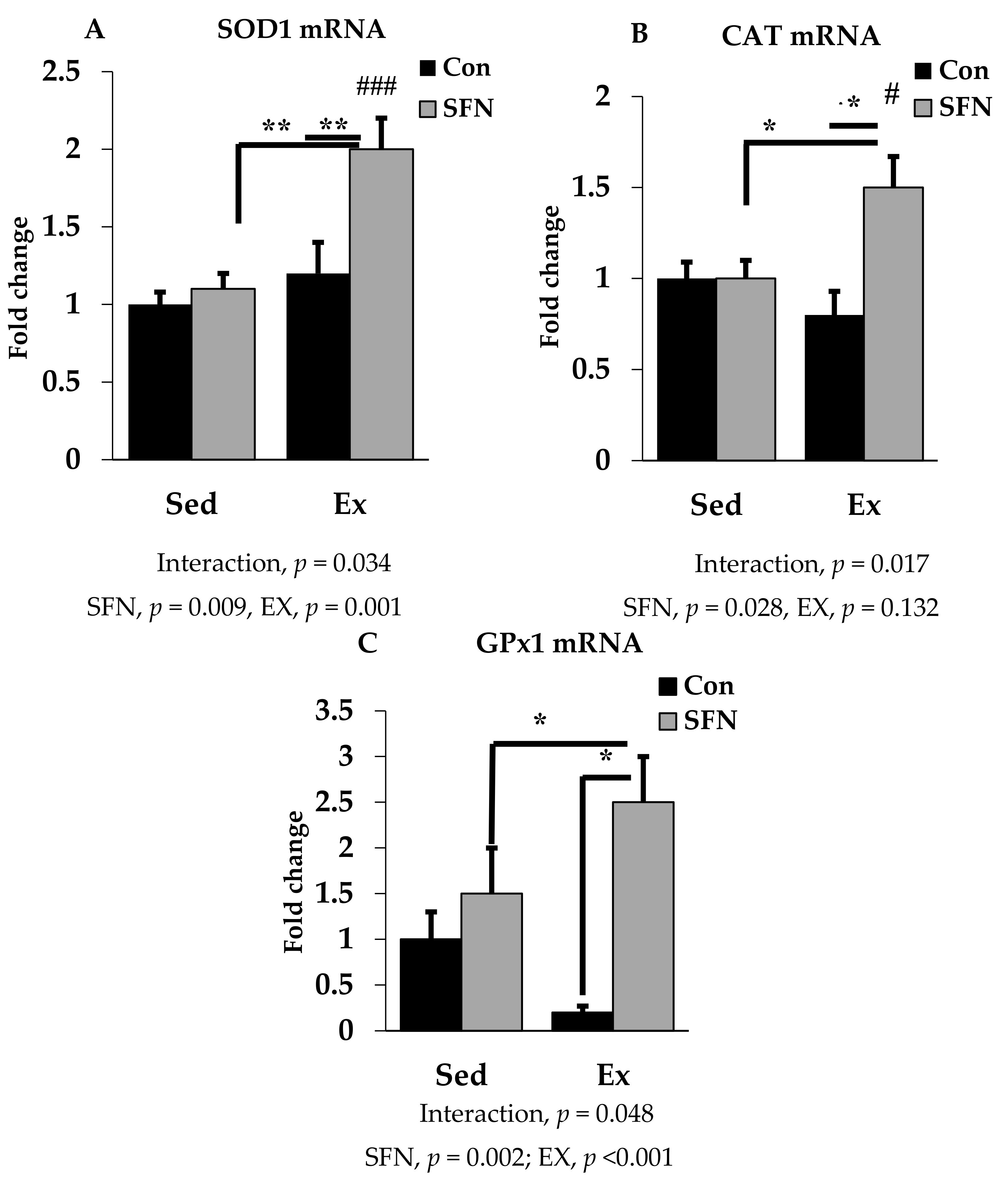
3.3. Effect of SFN on Antioxidant Defense System Gene Expression
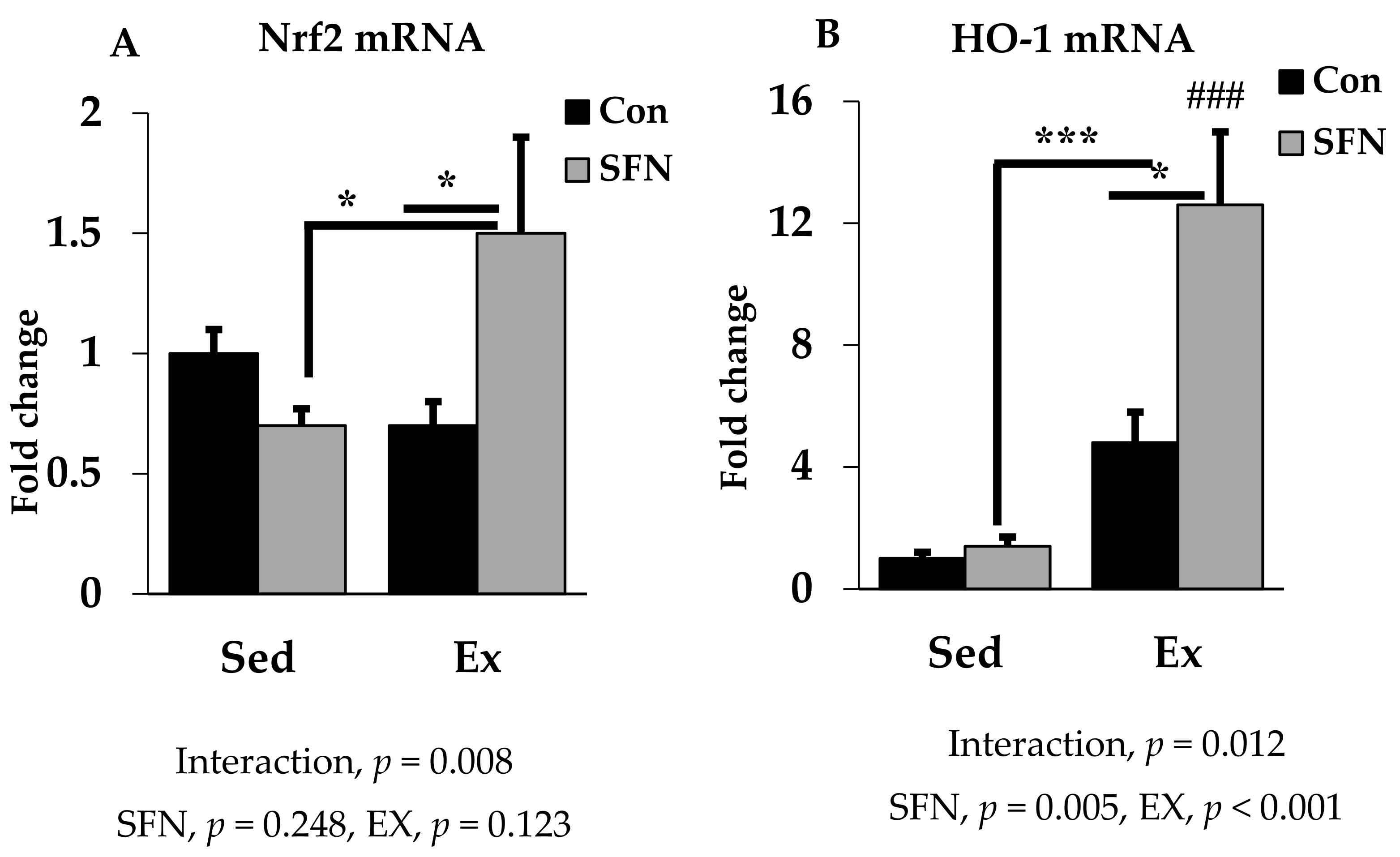
3.4. Effect of SFN on Nrf2/HO-1 Expression
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Suzuki, K. Chronic inflammation as an immunological abnormality and effectiveness of exercise. Biomolecules 2019, 9, 223. [Google Scholar] [CrossRef] [PubMed]
- Bouchard, C.; Depres, J.P.; Tremblay, A. Exercise and obesity. Obes. Res. 1993, 1, 133–147. [Google Scholar] [CrossRef] [PubMed]
- Thomas, D.; Elliott, E.J.; Naughton, G.A. Exercise for type 2 diabetes mellitus. Cochrane Database Syst. Rev. 2006, 19, CD002968. [Google Scholar] [CrossRef] [PubMed]
- Painter, P.L.; Hector, L.; Ray, K.; Lynes, L.; Paul, S.M.; Dodd, M.; Tomlanovich, S.L.; Ascher, N.L. Effects of exercise training on coronary heart disease risk factors in renal transplant recipients. Am. J. Kidney Dis. 2003, 42, 362–369. [Google Scholar] [CrossRef]
- Newton, R.U.; Galvao, D.A. Exercise in prevention and management of cancer. Curr. Treat. Options Oncol. 2008, 9, 135–146. [Google Scholar] [CrossRef]
- Pedersen, B.K.; Saltin, B. Exercise as medicine–evidence for prescribing exercise as therapy in 26 different chronic diseases. Scand. J. Med. Sci. Sports 2015, 25, 1–72. [Google Scholar] [CrossRef]
- Wenger, H.A.; Bell, G.J. The interactions of intensity, frequency and duration of exercise training in altering cardiorespiratory fitness. Sports Med. 1986, 3, 346–356. [Google Scholar] [CrossRef]
- Lambert, M.I.; Borresen, J. Measuring training load in sports. Int. J. Sports Physiol. Perform. 2010, 5, 406–411. [Google Scholar] [CrossRef]
- Cooper, C.; Vollaard, N.B.; Choueiri, T.; Wilson, M. Exercise, free radicals and oxidative stress. Biochem. Soc. Trans. 2002, 30, 280–285. [Google Scholar] [CrossRef]
- Baldwin, K.; Fitts, R.H.; Booth, F.; Winder, W.; Holloszy, J. Depletion of muscle and liver glycogen during exercise. Pflügers. Arch. 1975, 354, 203–212. [Google Scholar] [CrossRef]
- Yoon, M.Y.; Kim, S.N.; Kim, Y.C. Potentiation of acetaminophen hepatotoxicity by acute physical exercise in rats. Res. Commun. Mol. Pathol. Pharm. 1997, 96, 35–44. [Google Scholar]
- Sanchez-Valle, V.C.; Chavez-Tapia, N.; Uribe, M.; Mendez-Sanchez, N. Role of oxidative stress and molecular changes in liver fibrosis: A review. Curr. Med. Chem. 2012, 19, 4850–4860. [Google Scholar]
- Suzuki, K.; Totsuka, M.; Nakaji, S.; Yamada, M.; Kudoh, S.; Liu, Q.; Sugawara, K.; Yamaya, K.; Sato, K. Endurance exercise causes interaction among stress hormones, cytokines, neutrophil dynamics, and muscle damage. J. Appl. Physiol. 1999, 87, 1360–1367. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, K.; Nakaji, S.; Yamada, M.; Liu, Q.; Kurakake, S.; Okamura, N.; Kumae, T.; Umeda, T.; Sugawara, K. Impact of a competitive marathon race on systemic cytokine and neutrophil responses. Med. Sci. Sports Exerc. 2003, 35, 348–355. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, K. Cytokine response to exercise and its modulation. Antioxidants 2018, 7, 17. [Google Scholar] [CrossRef]
- Huang, Q.; Ma, S.; Tominaga, T.; Suzuki, K.; Liu, C. An 8-Week, Low carbohydrate, high fat, ketogenic diet enhanced exhaustive exercise capacity in mice Part 2: Effect on fatigue recovery, post-exercise biomarkers and anti-oxidation capacity. Nutrients 2018, 10, 1339. [Google Scholar] [CrossRef]
- Lim, C.L.; Suzuki, K. Systemic inflammation mediates the effects of endotoxemia in the mechanisms of heat stroke. Biol. Med. 2017, 9, 376. [Google Scholar] [CrossRef]
- Hung, Y.-L.; Suzuki, K. The pattern recognition receptors and lipopolysaccharides (LPS)-induced systemic inflammation. Int. J. Res. Stud. Med. Health Sci. 2017, 2, 1–7. [Google Scholar]
- Ruhee, R.T.; Ma, S.; Suzuki, K. Sulforaphane protects cells against lipopolysaccharide-stimulated inflammation in murine macrophages. Antioxidants 2019, 8, 577. [Google Scholar] [CrossRef]
- Mittler, R. Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci. 2002, 7, 405–410. [Google Scholar] [CrossRef]
- Batchu, S.; Chaudhary, K.; Wiebe, G.; Seubert, J. Bioactive food as dietary interventions for cardiovascular disease: Bioactive foods in chronic disease states. In Bioactive Compounds in Heart Disease, 1st ed; Elsevier Inc.: San Diego, CA, USA, 2013; p. 431. [Google Scholar]
- Rao, B.N. Bioactive phytochemicals in Indian foods and their potential in health promotion and disease prevention. Asia Pac. J. Clin. Nutr. 2003, 12, 9–22. [Google Scholar]
- Tang, L.; Paonessa, J.D.; Zhang, Y.; Ambrosone, C.B.; McCann, S.E. Total isothiocyanate yield from raw cruciferous vegetables commonly consumed in the United States. J. Funct. Foods 2013, 5, 1996–2001. [Google Scholar] [CrossRef] [PubMed]
- Jeffery, E.H.; Stewart, K.E. Upregulation of quinone reductase by glucosinolate hydrolysis products from dietary broccoli. In Methods in Enzymology; Elsevier: Amsterdam, The Netherlands, 2004; Volume 382, pp. 457–469. [Google Scholar]
- Angelino, D.; Dosz, E.B.; Sun, J.; Hoeflinger, J.L.; Van Tassell, M.L.; Chen, P.; Harnly, J.M.; Miller, M.J.; Jeffery, E.H. Myrosinase-dependent and–independent formation and control of isothiocyanate products of glucosinolate hydrolysis. Front. Plant Sci. 2015, 6, 831. [Google Scholar] [CrossRef] [PubMed]
- Sheweita, S.; Tilmisany, A. Cancer and phase II drug-metabolizing enzymes. Curr. Drug Metab. 2003, 4, 45–58. [Google Scholar] [CrossRef]
- Zhang, Y. Phase II Enzymes. Encycl. Cancer 2011, 2011, 2853–2855. [Google Scholar]
- Dhakshinamoorthy, S.; Jaiswal, A.K. Functional characterization and role of INrf2 in antioxidant response element-mediated expression and antioxidant induction of NAD(P)H:quinone oxidoreductase1 gene. Oncogene 2001, 20, 3906. [Google Scholar] [CrossRef] [PubMed]
- Stefanson, A.; Bakovic, M. Dietary regulation of Keap1/Nrf2/ARE pathway: focus on plant-derived compounds and trace minerals. Nutrients 2014, 6, 3777–3801. [Google Scholar] [CrossRef] [PubMed]
- Velichkova, M.; Hasson, T. Keap1 regulates the oxidation-sensitive shuttling of Nrf2 into and out of the nucleus via a Crm1-dependent nuclear export mechanism. Mol. Cell. Biol. 2005, 25, 4501–4513. [Google Scholar] [CrossRef]
- Osburn, W.O.; Wakabayashi, N.; Misra, V.; Nilles, T.; Biswal, S.; Trush, M.A.; Kensler, T.W. Nrf2 regulates an adaptive response protecting against oxidative damage following diquat-mediated formation of superoxide anion. Arch. Biochem. Biophys. 2006, 454, 7–15. [Google Scholar] [CrossRef]
- Kobayashi, A.; Kang, M.-I.; Watai, Y.; Tong, K.I.; Shibata, T.; Uchida, K.; Yamamoto, M. Oxidative and electrophilic stresses activate Nrf2 through inhibition of ubiquitination activity of Keap1. Mol. Cell. Biol. 2006, 26, 221–229. [Google Scholar] [CrossRef]
- Itoh, K.; Tong, K.I.; Yamamoto, M. Molecular mechanism activating Nrf2-Keap1 pathway in regulation of adaptive response to electrophiles. Free Radic. Biol. Med. 2004, 36, 1208–1213. [Google Scholar] [CrossRef]
- Ighodaro, O.; Akinloye, O. First line defence antioxidants-superoxide dismutase (SOD), catalase (CAT) and glutathione peroxidase (GPX): Their fundamental role in the entire antioxidant defence grid. Alexan. J. Med. 2018, 54, 287–293. [Google Scholar] [CrossRef]
- Halliwell, B.; Gutteridge, J. Antioxidant defences synthesized in vivo. In Free Radicals in Biology and Medicine; Oxford University Press Inc.: Oxford, UK, 2015; pp. 77–151. [Google Scholar]
- Gathwala, G.; Aggarwal, R. Selenium supplementation for the preterm Indian neonate. Indian J. Public Health 2016, 60, 142. [Google Scholar] [CrossRef] [PubMed]
- Jones, S.B.; Brooks, J.D. Modest induction of phase 2 enzyme activity in the F-344 rat prostate. Bmc Cancer 2006, 6, 62. [Google Scholar] [CrossRef]
- Watson, R.R.; Preedy, V.R. Dietary bioactive functional polyphenols in chronic lung diseases. In Bioactive Food as Dietary Interventions for Liver and Gastrointestinal Disease; Academic Press: Cambridge, MA, USA, 2012; pp. 513–525. [Google Scholar]
- Yada, K.; Suzuki, K.; Oginome, N.; Ma, S.; Fukuda, Y.; Iida, A.; Radak, Z. Single Dose administration of taheebo polyphenol enhances endurance capacity in mice. Sci. Rep. 2018, 8, 14625. [Google Scholar] [CrossRef]
- Yada, K.; Roberts, L.A.; Oginome, N.; Suzuki, K. Effect of Acacia Polyphenol Supplementation on Exercise-Induced Oxidative Stress in Mice Liver and Skeletal Muscle. Antioxidants 2019, 9. [Google Scholar] [CrossRef]
- Todd, J.J. Lactate: Valuable for physical performance and maintenance of brain function during exercise. Biosci. Horiz. 2014, 7, 1–7. [Google Scholar] [CrossRef]
- Radak, Z.; Suzuki, K.; Higuchi, M.; Balogh, L.; Boldogh, I.; Koltai, E. Physical exercise, reactive oxygen species and neuroprotection. Free Radic. Biol. Med. 2016, 98, 187–196. [Google Scholar] [CrossRef]
- Powers, S.K.; Ji, L.L.; Leeuwenburgh, C. Exercise training-induced alterations in skeletal muscle antioxidant capacity: A brief review. Med. Sci. Sports Exerc. 1999, 31, 987–997. [Google Scholar] [CrossRef]
- Packer, L.; Gohil, K.; DeLumen, B.; Terblanche, S. A comparative study on the effects of ascorbic acid deficiency and supplementation on endurance and mitochondrial oxidative capacities in various tissues of the guinea pig. Comp. Biochem. Physiol. B 1986, 83, 235–240. [Google Scholar] [CrossRef]
- Kanter, M.; Nolte, L.; Holloszy, J. Effects of an antioxidant vitamin mixture on lipid peroxidation at rest and postexercise. J. Appl. Physiol. 1993, 74, 965–969. [Google Scholar] [CrossRef]
- Gohil, K.; Packer, L.; De Lumen, B.; Brooks, G.; Terblanche, S. Vitamin E deficiency and vitamin C supplements: exercise and mitochondrial oxidation. J. Appl. Physiol. 1986, 60, 1986–1991. [Google Scholar] [CrossRef] [PubMed]
- Sumida, S.; Tanaka, K.; Kitao, H.; Nakadomo, F. Exercise-induced lipid peroxidation and leakage of enzymes before and after vitamin E supplementation. Int. J. Biochem. 1989, 21, 835–838. [Google Scholar] [PubMed]
- Juge, N.; Mithen, R.; Traka, M. Molecular basis for chemoprevention by sulforaphane: a comprehensive review. Cell. Mol. Life Sci. 2007, 64, 1105. [Google Scholar] [CrossRef] [PubMed]
- Noakes, T.D. Effect of exercise on serum enzyme activities in humans. Sports Med. 1987, 4, 245–267. [Google Scholar] [CrossRef]
- Ma, S.; Suzuki, K. Keto-adaptation and endurance exercise capacity, fatigue recovery, and exercise-induced muscle and organ damage prevention: A narrative review. Sports 2019, 7, 40. [Google Scholar] [CrossRef]
- Choudhary, A.K.; Devi, R.S. Serum biochemical responses under oxidative stress of aspartame in wistar albino rats. Asian Pac. J. Trop. Dis. 2014, 4, S403–S410. [Google Scholar] [CrossRef]
- Ma, S.; Huang, Q.; Yada, K.; Liu, C.; Suzuki, K. An 8-week ketogenic low carbohydrate, high fat diet enhanced exhaustive exercise capacity in mice. Nutrients 2018, 10, 673. [Google Scholar] [CrossRef]
- Zhao, H.-D.; Zhang, F.; Shen, G.; Li, Y.-B.; Li, Y.-H.; Jing, H.-R.; Ma, L.-F.; Yao, J.-H.; Tian, X.-F. Sulforaphane protects liver injury induced by intestinal ischemia reperfusion through Nrf2-ARE pathway. World J. Gastroenterol. 2010, 16, 3002. [Google Scholar] [CrossRef]
- Liu, W.; XU, Z.-f.; GUO, M.-x.; LI, H.-p.; YANG, T.-y.; FENG, S.; XU, B.; DENG, Y. Activation of Nrf2 pathway by sulforaphane against mercury-induced liver oxidative damage in rats. J. Env. Health 2016, 1, 8. [Google Scholar]
- Lee, C.; Yang, S.; Lee, B.-S.; Jeong, S.Y.; Kim, K.-M.; Ku, S.-K.; Bae, J.-S. Hepatic protective effects of sulforaphane through the modulation of inflammatory pathways. J. Asian Nat. Prod. Res. 2019, 1, 1–11. [Google Scholar] [CrossRef]
- Jurisic, V.; Radenkovic, S.; Konjevic, G. The actual role of LDH as tumor marker, biochemical and clinical aspects. In Advances in Cancer Biomarkers; Springer Nature Switzerland AG: Basel, Switzerland, 2015; Volume 867, pp. 115–124. [Google Scholar]
- Bergstrom, P.; Andersson, H.C.; Gao, Y.; Karlsson, J.-O.; Nodin, C.; Anderson, M.F.; Nilsson, M.; Hammarsten, O. Repeated transient sulforaphane stimulation in astrocytes leads to prolonged Nrf2-mediated gene expression and protection from superoxide-induced damage. Neuropharmacology 2011, 60, 343–353. [Google Scholar] [CrossRef] [PubMed]
- Khan, H.A.; Alhomida, A.S.; Sobki, S.H.; Habib, S.S.; Al Aseri, Z.; Khan, A.A.; Al Moghairi, A. Serum markers of tissue damage and oxidative stress in patients with acute myocardial infarction. Biomed. Res. 2013, 24, 15–20. [Google Scholar]
- Feagins, L.A.; Flores, A.; Arriens, C.; Park, C.; Crook, T.; Reimold, A.; Brown, G. Nonalcoholic fatty liver disease: A potential consequence of tumor necrosis factor-inhibitor therapy. Eur. J. Gastroenterol. Hepatol. 2015, 27, 1154. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Wang, N.; Ye, X.; Li, H.; Feng, Y.; Cheung, F.; Nagamatsu, T. Hepatoprotective effect and its possible mechanism of Coptidis rhizoma aqueous extract on carbon tetrachloride-induced chronic liver hepatotoxicity in rats. J. Ethnopharmacol. 2011, 138, 683–690. [Google Scholar] [CrossRef]
- Scheller, J.; Chalaris, A.; Schmidt-Arras, D.; Rose-John, S. The pro-and anti-inflammatory properties of the cytokine interleukin-6. Biochim. Biophys. Acta. 2011, 1813, 878–888. [Google Scholar] [CrossRef]
- Tilg, H. Cytokines and liver diseases. Can. J. Gastroenterol. 2001, 15, 661–668. [Google Scholar] [CrossRef]
- Lacour, S.; Gautier, J.-C.; Pallardy, M.; Roberts, R. Cytokines as potential biomarkers of liver toxicity. Cancer Biomark. 2005, 1, 29–39. [Google Scholar] [CrossRef]
- Ji, L.; Gomez-Cabrera, M.; Steinhafel, N.; Vina, J. Acute exercise activates nuclear factor (NF)-κB signaling pathway in rat skeletal muscle. Faseb J. 2004, 18, 1499–1506. [Google Scholar] [CrossRef]
- Mittal, M.; Siddiqui, M.R.; Tran, K.; Reddy, S.P.; Malik, A.B. Reactive oxygen species in inflammation and tissue injury. Antioxid. Redox Signal. 2014, 20, 1126–1167. [Google Scholar] [CrossRef]
- Vassilakopoulos, T.; Karatza, M.-H.; Katsaounou, P.; Kollintza, A.; Zakynthinos, S.; Roussos, C. Antioxidants attenuate the plasma cytokine response to exercise in humans. J. Appl. Physiol. 2003, 94, 1025–1032. [Google Scholar] [CrossRef]
- Palmer, H.J.; Paulson, K.E. Reactive oxygen species and antioxidants in signal transduction and gene expression. Nutr. Rev. 1997, 55, 353–361. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.-C.; Li, S.-J.; Yang, C.-L.; Xue, R.-L.; Xi, Y.-Y.; Wang, L.; Zhao, Q.-L.; Li, D.-J. Sulforaphane attenuates muscle inflammation in dystrophin-deficient Mdx mice via Nrf2-mediated inhibition of NF-κB signaling pathway. J. Biol. Chem. 2015, 2900, 17784–17795. [Google Scholar] [CrossRef] [PubMed]
- Townsend, B.E.; Johnson, R.W. Sulforaphane reduces lipopolysaccharide-induced proinflammatory markers in hippocampus and liver but does not improve sickness behavior. Nutr. Neurosci. 2017, 20, 195–202. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Cha, Y.-N.; Surh, Y.-J. A protective role of nuclear factor-erythroid 2-related factor-2 (Nrf2) in inflammatory disorders. Mutat. Res. 2010, 690, 12–23. [Google Scholar] [CrossRef]
- Peake, J.; Suzuki, K. Neutrophil activation, antioxidant supplements and exercise-induced oxidative stress. Exerc. Immunol. Rev. 2004, 10, 129–141. [Google Scholar]
- Taysi, S.; Oztasan, N.; Efe, H.; Polat, M.; Gumustekin, K.; Siktar, E.; Canakci, E.; Akcay, F.; Dane, S.; Gul, M. Endurance training attenuates the oxidative stress due to acute exhaustive exercise in rat liver. Acta Physiol. Hung. 2008, 95, 337–347. [Google Scholar] [CrossRef]
- Korivi, M.; Hou, C.-W.; Huang, C.-Y.; Lee, S.-D.; Hsu, M.-F.; Yu, S.-H.; Chen, C.-Y.; Liu, Y.-Y.; Kuo, C.-H. Ginsenoside-Rg1 protects the liver against exhaustive exercise-induced oxidative stress in rats. Evid-Based Complement. Altern. Med. 2012, 2012, 932165. [Google Scholar] [CrossRef]
- Polavarapu, R.; Spitz, D.R.; Sim, J.E.; Follansbee, M.H.; Oberley, L.W.; Rahemtulla, A.; Nanji, A.A. Increased lipid peroxidation and impaired antioxidant enzyme function is associated with pathological liver injury in experimental alcoholic liver disease in rats fed diets high in corn oil and fish oil. Hepatology 1998, 27, 1317–1323. [Google Scholar] [CrossRef]
- Chen, X.; Liu, J.; Chen, S.Y. Sulforaphane protects against ethanol-induced oxidative stress and apoptosis in neural crest cells by the induction of Nrf2-mediated antioxidant response. Br. J. Pharm. 2013, 169, 437–448. [Google Scholar] [CrossRef]
- Huang, C.-C.; Lin, T.-J.; Lu, Y.-F.; Chen, C.-C.; Huang, C.-Y.; Lin, W.-T. Protective effects of L-arginine supplementation against exhaustive exercise-induced oxidative stress in young rat tissues. Chin. J. Physiol. 2009, 52, 306–315. [Google Scholar] [CrossRef]
- Sen, C.K. Antioxidants in exercise nutrition. Sports Med. 2001, 31, 891–908. [Google Scholar] [CrossRef] [PubMed]
- Oh, S.; Komine, S.; Warabi, E.; Akiyama, K.; Ishii, A.; Ishige, K.; Mizokami, Y.; Kuga, K.; Horie, M.; Miwa, Y. Nuclear factor (erythroid derived 2)-like 2 activation increases exercise endurance capacity via redox modulation in skeletal muscles. Sci. Rep. 2017, 7, 12902. [Google Scholar] [CrossRef] [PubMed]
- Malaguti, M.; Angeloni, C.; Garatachea, N.; Baldini, M.; Leoncini, E.; Collado, P.S.; Teti, G.; Falconi, M.; Gonzalez-Gallego, J.; Hrelia, S. Sulforaphane treatment protects skeletal muscle against damage induced by exhaustive exercise in rats. J. Appl. Physiol. 2009, 107, 1028–1036. [Google Scholar] [CrossRef] [PubMed]
- Cardenia, V.; Rodriguez-Estrada, M.T.; Lorenzini, A.; Bandini, E.; Angeloni, C.; Hrelia, S.; Malaguti, M. Effect of broccoli extract enriched diet on liver cholesterol oxidation in rats subjected to exhaustive exercise. J. Steroid Biochem. Mol. Biol. 2017, 169, 137–144. [Google Scholar] [CrossRef]
- Itoh, K.; Chiba, T.; Takahashi, S.; Ishii, T.; Igarashi, K.; Katoh, Y.; Oyake, T.; Hayashi, N.; Satoh, K.; Hatayama, I. An Nrf2/small Maf heterodimer mediates the induction of phase II detoxifying enzyme genes through antioxidant response elements. Biochem. Biophys. Res. Commun. 1997, 236, 313–322. [Google Scholar] [CrossRef]
- Kalayarasan, S.; Prabhu, P.N.; Sriram, N.; Manikandan, R.; Arumugam, M.; Sudhandiran, G. Diallyl sulfide enhances antioxidants and inhibits inflammation through the activation of Nrf2 against gentamicin-induced nephrotoxicity in Wistar rats. Eur. J. Pharm. 2009, 606, 162–171. [Google Scholar] [CrossRef]
- Lin, W.; Wu, R.T.; Wu, T.; Khor, T.-O.; Wang, H.; Kong, A.-N. Sulforaphane suppressed LPS-induced inflammation in mouse peritoneal macrophages through Nrf2 dependent pathway. Biochem. Pharm. 2008, 76, 967–973. [Google Scholar] [CrossRef]
- Hu, R.; Hebbar, V.; Kim, B.-R.; Chen, C.; Winnik, B.; Buckley, B.; Soteropoulos, P.; Tolias, P.; Hart, R.P.; Kong, A.-N.T. In vivo pharmacokinetics and regulation of gene expression profiles by isothiocyanate sulforaphane in the rat. J. Pharm. Exp. 2004, 310, 263–271. [Google Scholar] [CrossRef]
- University, O.S. L.P.I. Isothiocyanates. Available online: https://lpi.oregonstate.edu/mic/dietary-factors/phytochemicals/isothiocyanates (accessed on 27 November 2019).
| Target Gene | Accession Number (Size, bp) | Forward (5′ → 3′) | Reverse (5′ → 3′) |
|---|---|---|---|
| 18S | NM_011296 (127) | TTCTGGCCAACGGTCTAGACAAC | CCAGTGGTCTTGGTGTGCTGA |
| IL-6 | NM_031168 (76) | TAGTCCTTCCTACCCCAATTTCC | TTGGTCCTTAGCCACTCCTTC |
| TNF-α | NM_013693.1 (102) | CCTCCCTCTCATCAGTTCTA | ACTTGGTGGTTTGCTACGAC |
| IL-1β | NM_008361.4 (183) | GGGCCTCAAAGGAAAGAATC | TTGCTTGGGATCCACACTCT |
| SOD1 | GI:56270594 (1107) | GAGACCTGGGCAATGTGACT | GTTTACTGCGCAATCCCAAT |
| CAT | XM_028766902 (100) | CAGAGAGCGGATTCCTGAGAGA | CTTTGCCTTGGAGTATCTGGTGAT |
| GPx | NM_001329527.1 (751) | AGTACGGATTCCACGTTTGA | GGAACTTCTCAAAGTTCCAG |
| Nrf2 | NM_010902(100) | GAGTCGCTTGCCCTGGATATC | TCATGGCTGCCTCCAGAGAA |
| HO-1 | NM_010442 (175) | CACGCATATACCCGCTACCT | CCAGAGTGTTCATTCGAGCA |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ruhee, R.T.; Ma, S.; Suzuki, K. Protective Effects of Sulforaphane on Exercise-Induced Organ Damage via Inducing Antioxidant Defense Responses. Antioxidants 2020, 9, 136. https://doi.org/10.3390/antiox9020136
Ruhee RT, Ma S, Suzuki K. Protective Effects of Sulforaphane on Exercise-Induced Organ Damage via Inducing Antioxidant Defense Responses. Antioxidants. 2020; 9(2):136. https://doi.org/10.3390/antiox9020136
Chicago/Turabian StyleRuhee, Ruheea Taskin, Sihui Ma, and Katsuhiko Suzuki. 2020. "Protective Effects of Sulforaphane on Exercise-Induced Organ Damage via Inducing Antioxidant Defense Responses" Antioxidants 9, no. 2: 136. https://doi.org/10.3390/antiox9020136
APA StyleRuhee, R. T., Ma, S., & Suzuki, K. (2020). Protective Effects of Sulforaphane on Exercise-Induced Organ Damage via Inducing Antioxidant Defense Responses. Antioxidants, 9(2), 136. https://doi.org/10.3390/antiox9020136







