Vitamin C Recycling Regulates Neurite Growth in Neurospheres Differentiated In Vitro
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Primary and Cell Line Culture
2.3. Cell Treatments, Viability and AA Concentration
2.4. Immunocytochemistry and Neurite Quantification
2.5. Uptake Analysis
2.6. Reverse Transcription Polymerase Chain Reaction
2.7. Western Blot Analysis
2.8. Immunodetection of Carboxymethyllysine (CML) and Carbonylated Proteins
2.9. Flow Cytometry
2.10. Statistical Analysis
3. Results
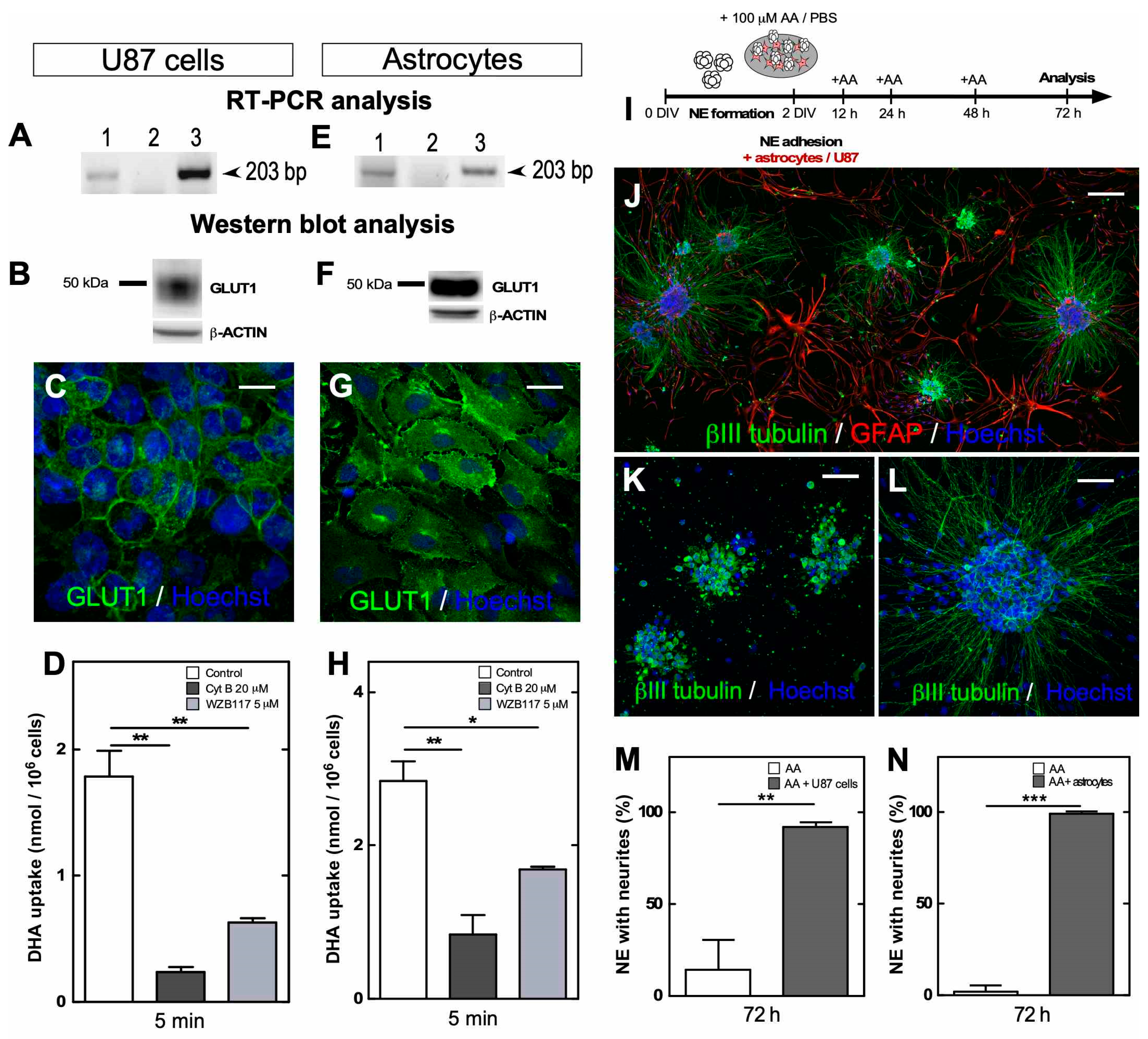
3.1. NEs Adhered In Vitro Were Mainly Formed by Immature Neurons Positive for βIII Tubulin and SVCT2
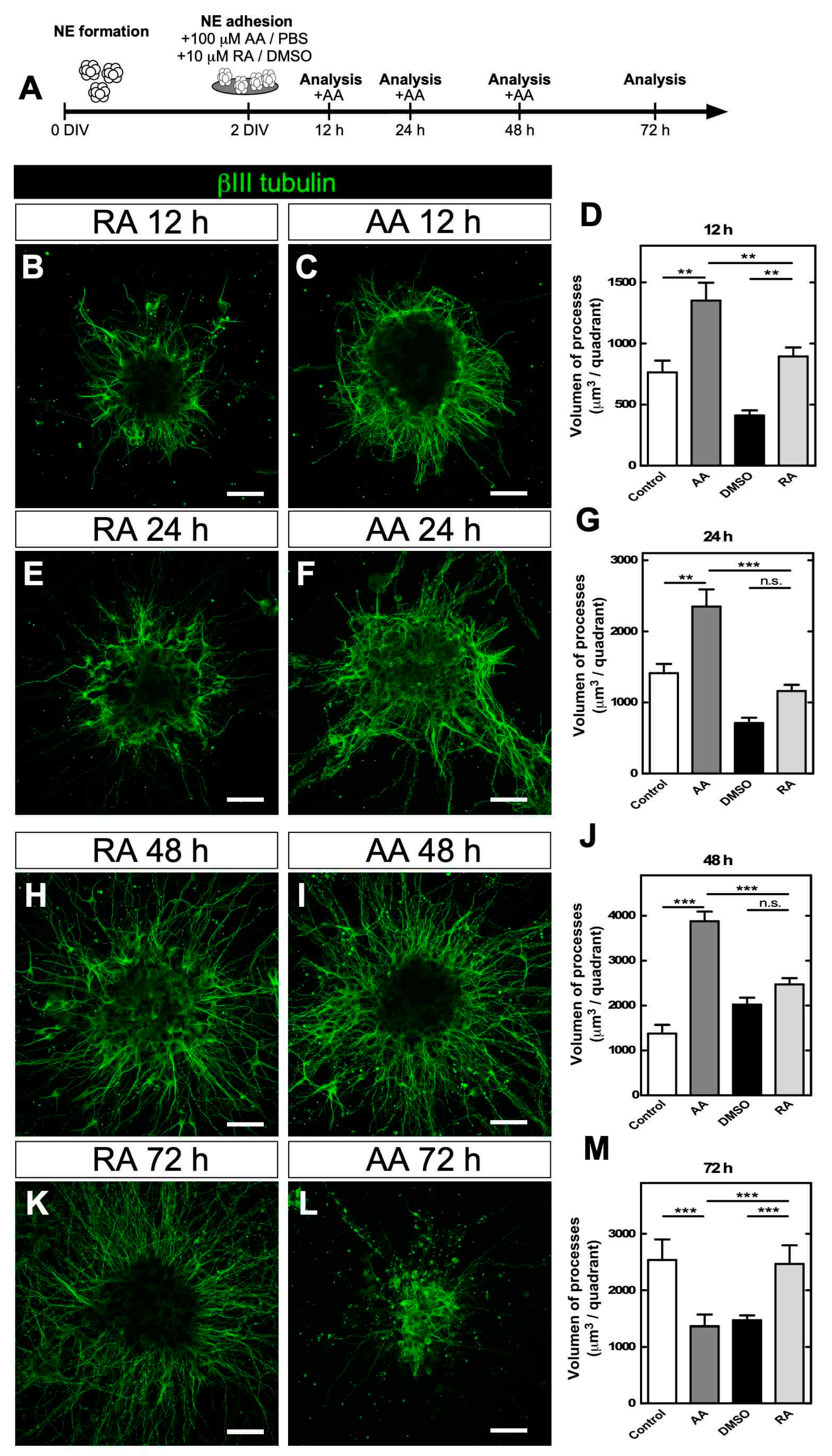
3.2. Prolonged AA Neurosphere Treatment Induced the Loss of Cellular Neurites
3.3. Vitamin C Recycling In Vitro Recovers Neuritic Morphology after Prolonged Treatment with AA
3.4. The Gradual Accumulation of DHA Impacts Redox Balance and Induces Protein Modifications in Adhered NEs
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Barnes, A.P.; Polleux, F. Establishment of axon-dendrite polarity in developing neurons. Annu. Rev. Neurosci. 2009, 32, 347–381. [Google Scholar] [CrossRef] [PubMed]
- Takano, T.; Xu, C.; Funahashi, Y.; Namba, T.; Kaibuchi, K. Neuronal polarization. Development 2015, 142, 2088–2093. [Google Scholar] [CrossRef] [PubMed]
- Oga, T.; Elston, G.N.; Fujita, I. Postnatal Dendritic Growth and Spinogenesis of Layer-V Pyramidal Cells Differ between Visual, Inferotemporal, and Prefrontal Cortex of the Macaque Monkey. Front. Neurosci. 2017, 11, 118. [Google Scholar] [CrossRef] [PubMed]
- Ge, W.P.; Miyawaki, A.; Gage, F.H.; Jan, Y.N.; Jan, L.Y. Local generation of glia is a major astrocyte source in postnatal cortex. Nature 2012, 484, 376–380. [Google Scholar] [CrossRef]
- Tabata, H. Diverse subtypes of astrocytes and their development during corticogenesis. Front. Neurosci. 2015, 9, 114. [Google Scholar] [CrossRef]
- Freeman, M.R. Specification and morphogenesis of astrocytes. Science 2010, 330, 774–778. [Google Scholar] [CrossRef]
- Farhy-Tselnicker, I.; Allen, N.J. Astrocytes, neurons, synapses: A tripartite view on cortical circuit development. Neural Dev. 2018, 13, 7. [Google Scholar] [CrossRef]
- Nualart, F.J.; Rivas, C.I.; Montecinos, V.P.; Godoy, A.S.; Guaiquil, V.H.; Golde, D.W.; Vera, J.C. Recycling of vitamin C by a bystander effect. J. Biol. Chem. 2003, 278, 10128–10133. [Google Scholar] [CrossRef]
- Nualart, F.; Salazar, K.; Oyarce, K.; Cisternas, P.; Jara, N.; Silva-Alvarez, C.; Pastor, P.; Martinez, F.; Garcia, A.; Garcia-Robles Mde, L.; et al. Typical and atypical stem cells in the brain, vitamin C effect and neuropathology. Biol. Res. 2012, 45, 243–256. [Google Scholar] [CrossRef]
- Yun, J.; Mullarky, E.; Lu, C.; Bosch, K.N.; Kavalier, A.; Rivera, K.; Roper, J.; Chio, I.I.C.; Giannopoulou, E.G.; Rago, C.; et al. Vitamin C selectively kills KRAS and BRAF mutant colorectal cancer cells by targeting GAPDH. Science 2015, 350, 1391–1396. [Google Scholar] [CrossRef]
- Castro, M.; Caprile, T.; Astuya, A.; Millan, C.; Reinicke, K.; Vera, J.C.; Vasquez, O.; Aguayo, L.G.; Nualart, F. High-affinity sodium-vitamin C co-transporters (SVCT) expression in embryonic mouse neurons. J. Neurochem. 2001, 78, 815–823. [Google Scholar] [CrossRef] [PubMed]
- Oyarce, K.; Silva-Alvarez, C.; Ferrada, L.; Martinez, F.; Salazar, K.; Nualart, F. SVCT2 Is Expressed by Cerebellar Precursor Cells, Which Differentiate into Neurons in Response to Ascorbic Acid. Mol. Neurobiol. 2018, 55, 1136–1149. [Google Scholar] [CrossRef] [PubMed]
- Tsukaguchi, H.; Tokui, T.; Mackenzie, B.; Berger, U.V.; Chen, X.Z.; Wang, Y.; Brubaker, R.F.; Hediger, M.A. A family of mammalian Na+-dependent L-ascorbic acid transporters. Nature 1999, 399, 70–75. [Google Scholar] [CrossRef]
- Portugal, C.C.; Socodato, R.; Canedo, T.; Silva, C.M.; Martins, T.; Coreixas, V.S.; Loiola, E.C.; Gess, B.; Rohr, D.; Santiago, A.R.; et al. Caveolin-1-mediated internalization of the vitamin C transporter SVCT2 in microglia triggers an inflammatory phenotype. Sci. Signal. 2017, 10, eaal2005. [Google Scholar] [CrossRef] [PubMed]
- Gess, B.; Rohr, D.; Fledrich, R.; Sereda, M.W.; Kleffner, I.; Humberg, A.; Nowitzki, J.; Strecker, J.K.; Halfter, H.; Young, P. Sodium-dependent vitamin C transporter 2 deficiency causes hypomyelination and extracellular matrix defects in the peripheral nervous system. J. Neurosci. Off. J. Soc. Neurosci. 2011, 31, 17180–17192. [Google Scholar] [CrossRef]
- Salazar, K.; Cerda, G.; Martinez, F.; Sarmiento, J.M.; Gonzalez, C.; Rodriguez, F.; Garcia-Robles, M.; Tapia, J.C.; Cifuentes, M.; Nualart, F. SVCT2 transporter expression is post-natally induced in cortical neurons and its function is regulated by its short isoform. J. Neurochem. 2014, 130, 693–706. [Google Scholar] [CrossRef]
- Salazar, K.; Martinez, M.; Ulloa, V.; Bertinat, R.; Martinez, F.; Jara, N.; Espinoza, F.; Bongarzone, E.R.; Nualart, F. SVCT2 Overexpression in Neuroblastoma Cells Induces Cellular Branching that is Associated with ERK Signaling. Mol. Neurobiol. 2016, 53, 6668–6679. [Google Scholar] [CrossRef]
- Cisternas, P.; Silva-Alvarez, C.; Martinez, F.; Fernandez, E.; Ferrada, L.; Oyarce, K.; Salazar, K.; Bolanos, J.P.; Nualart, F. The oxidized form of vitamin C, dehydroascorbic acid, regulates neuronal energy metabolism. J. Neurochem. 2014, 129, 663–671. [Google Scholar] [CrossRef]
- Garcia-Krauss, A.; Ferrada, L.; Astuya, A.; Salazar, K.; Cisternas, P.; Martinez, F.; Ramirez, E.; Nualart, F. Dehydroascorbic Acid Promotes Cell Death in Neurons Under Oxidative Stress: A Protective Role for Astrocytes. Mol. Neurobiol. 2016, 53, 5847–5863. [Google Scholar] [CrossRef]
- Nualart, F.; Mack, L.; Garcia, A.; Cisternas, P.; Bongarzone, E.R.; Heitzer, M.; Jara, N.; Martinez, F.; Ferrada, L.; Espinoza, F.; et al. Vitamin C Transporters, Recycling and the Bystander Effect in the Nervous System: SVCT2 versus Gluts. J. Stem Cell Res. Ther. 2014, 4, 209. [Google Scholar] [CrossRef]
- Rice, M.E. Ascorbate regulation and its neuroprotective role in the brain. Trends Neurosci. 2000, 23, 209–216. [Google Scholar] [CrossRef]
- Silva-Alvarez, C.; Salazar, K.; Cisternas, P.; Martinez, F.; Liour, S.; Jara, N.; Bertinat, R.; Nualart, F. Apical Polarization of SVCT2 in Apical Radial Glial Cells and Progenitors During Brain Development. Mol. Neurobiol. 2017, 54, 5449–5467. [Google Scholar] [CrossRef] [PubMed]
- Jones-Villeneuve, E.M.; McBurney, M.W.; Rogers, K.A.; Kalnins, V.I. Retinoic acid induces embryonal carcinoma cells to differentiate into neurons and glial cells. J. Cell Biol. 1982, 94, 253–262. [Google Scholar] [CrossRef] [PubMed]
- Smith, P.K.; Krohn, R.I.; Hermanson, G.T.; Mallia, A.K.; Gartner, F.H.; Provenzano, M.D.; Fujimoto, E.K.; Goeke, N.M.; Olson, B.J.; Klenk, D.C. Measurement of protein using bicinchoninic acid. Anal. Biochem. 1985, 150, 76–85. [Google Scholar] [CrossRef]
- Lee, J.Y.; Chang, M.Y.; Park, C.H.; Kim, H.Y.; Kim, J.H.; Son, H.; Lee, Y.S.; Lee, S.H. Ascorbate-induced differentiation of embryonic cortical precursors into neurons and astrocytes. J. Neurosci. Res. 2003, 73, 156–165. [Google Scholar] [CrossRef]
- Ojelabi, O.A.; Lloyd, K.P.; Simon, A.H.; De Zutter, J.K.; Carruthers, A. WZB117 (2-Fluoro-6-(m-hydroxybenzoyloxy) Phenyl m-Hydroxybenzoate) Inhibits GLUT1-mediated Sugar Transport by Binding Reversibly at the Exofacial Sugar Binding Site. J. Biol. Chem. 2016, 291, 26762–26772. [Google Scholar] [CrossRef]
- Scheffler, J.; Bork, K.; Bezold, V.; Rosenstock, P.; Gnanapragassam, V.S.; Horstkorte, R. Ascorbic acid leads to glycation and interferes with neurite outgrowth. Exp. Gerontol. 2019, 117, 25–30. [Google Scholar] [CrossRef]
- Pastor, P.; Cisternas, P.; Salazar, K.; Silva-Alvarez, C.; Oyarce, K.; Jara, N.; Espinoza, F.; Martinez, A.D.; Nualart, F. SVCT2 vitamin C transporter expression in progenitor cells of the postnatal neurogenic niche. Front. Cell. Neurosci. 2013, 7, 119. [Google Scholar] [CrossRef]
- Shin, D.M.; Ahn, J.I.; Lee, K.H.; Lee, Y.S.; Lee, Y.S. Ascorbic acid responsive genes during neuronal differentiation of embryonic stem cells. Neuroreport 2004, 15, 1959–1963. [Google Scholar] [CrossRef]
- Yan, J.; Studer, L.; McKay, R.D. Ascorbic acid increases the yield of dopaminergic neurons derived from basic fibroblast growth factor expanded mesencephalic precursors. J. Neurochem. 2001, 76, 307–311. [Google Scholar] [CrossRef]
- He, X.B.; Kim, M.; Kim, S.Y.; Yi, S.H.; Rhee, Y.H.; Kim, T.; Lee, E.H.; Park, C.H.; Dixit, S.; Harrison, F.E.; et al. Vitamin C facilitates dopamine neuron differentiation in fetal midbrain through TET1- and JMJD3-dependent epigenetic control manner. Stem Cells 2015, 33, 1320–1332. [Google Scholar] [CrossRef] [PubMed]
- Wulansari, N.; Kim, E.H.; Sulistio, Y.A.; Rhee, Y.H.; Song, J.J.; Lee, S.H. Vitamin C-Induced Epigenetic Modifications in Donor NSCs Establish Midbrain Marker Expressions Critical for Cell-Based Therapy in Parkinson’s Disease. Stem Cell Rep. 2017, 9, 1192–1206. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Cai, J.; Li, W.; Su, H.; Qin, D.; Yang, J.; Zhu, F.; Xu, J.; He, W.; Guo, X.; Labuda, K.; et al. Generation of human induced pluripotent stem cells from umbilical cord matrix and amniotic membrane mesenchymal cells. J. Biol. Chem. 2010, 285, 11227–11234. [Google Scholar] [CrossRef] [PubMed]
- Nitti, M.; Furfaro, A.L.; Cevasco, C.; Traverso, N.; Marinari, U.M.; Pronzato, M.A.; Domenicotti, C. PKC delta and NADPH oxidase in retinoic acid-induced neuroblastoma cell differentiation. Cell. Signal. 2010, 22, 828–835. [Google Scholar] [CrossRef] [PubMed]
- Siegenthaler, J.A.; Ashique, A.M.; Zarbalis, K.; Patterson, K.P.; Hecht, J.H.; Kane, M.A.; Folias, A.E.; Choe, Y.; May, S.R.; Kume, T.; et al. Retinoic acid from the meninges regulates cortical neuron generation. Cell 2009, 139, 597–609. [Google Scholar] [CrossRef] [PubMed]
- Kratzing, C.C.; Kelly, J.D.; Kratzing, J.E. Ascorbic acid in fetal rat brain. J. Neurochem. 1985, 44, 1623–1624. [Google Scholar] [CrossRef]
- Wu, H.; Wu, Y.; Ai, Z.; Yang, L.; Gao, Y.; Du, J.; Guo, Z.; Zhang, Y. Vitamin C enhances Nanog expression via activation of the JAK/STAT signaling pathway. Stem Cells 2014, 32, 166–176. [Google Scholar] [CrossRef]
- Ferrada, L.; Barahona, M.J.; Salazar, K.; Vandenabeele, P.; Nualart, F. Vitamin C controls neuronal necroptosis under oxidative stress. Redox Biol. 2020, 29, 101408. [Google Scholar] [CrossRef]
- Himmelreich, U.; Drew, K.N.; Serianni, A.S.; Kuchel, P.W. 13C NMR studies of vitamin C transport and its redox cycling in human erythrocytes. Biochemistry 1998, 37, 7578–7588. [Google Scholar] [CrossRef]
- Smuda, M.; Glomb, M.A. Maillard degradation pathways of vitamin C. Angew. Chem. 2013, 52, 4887–4891. [Google Scholar] [CrossRef]
- Fan, X.; Reneker, L.W.; Obrenovich, M.E.; Strauch, C.; Cheng, R.; Jarvis, S.M.; Ortwerth, B.J.; Monnier, V.M. Vitamin C mediates chemical aging of lens crystallins by the Maillard reaction in a humanized mouse model. Proc. Natl. Acad. Sci. USA 2006, 103, 16912–16917. [Google Scholar] [CrossRef] [PubMed]
- Regulus, P.; Desilets, J.F.; Klarskov, K.; Wagner, J.R. Characterization and detection in cells of a novel adduct derived from the conjugation of glutathione and dehydroascorbate. Free Radic. Biol. Med. 2010, 49, 984–991. [Google Scholar] [CrossRef] [PubMed]
- Ahuie Kouakou, G.; Gagnon, H.; Lacasse, V.; Wagner, J.R.; Naylor, S.; Klarskov, K. Dehydroascorbic acid S-Thiolation of peptides and proteins: Role of homocysteine and glutathione. Free Radic. Biol. Med. 2019, 141, 233–243. [Google Scholar] [CrossRef] [PubMed]
- Wilson, C.; Gonzalez-Billault, C. Regulation of cytoskeletal dynamics by redox signaling and oxidative stress: Implications for neuronal development and trafficking. Front. Cell. Neurosci. 2015, 9, 381. [Google Scholar] [CrossRef]
- Dalle-Donne, I.; Giustarini, D.; Rossi, R.; Colombo, R.; Milzani, A. Reversible S-glutathionylation of Cys 374 regulates actin filament formation by inducing structural changes in the actin molecule. Free Radic. Biol. Med. 2003, 34, 23–32. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Espinoza, F.; Magdalena, R.; Saldivia, N.; Jara, N.; Martínez, F.; Ferrada, L.; Salazar, K.; Ávila, F.; Nualart, F. Vitamin C Recycling Regulates Neurite Growth in Neurospheres Differentiated In Vitro. Antioxidants 2020, 9, 1276. https://doi.org/10.3390/antiox9121276
Espinoza F, Magdalena R, Saldivia N, Jara N, Martínez F, Ferrada L, Salazar K, Ávila F, Nualart F. Vitamin C Recycling Regulates Neurite Growth in Neurospheres Differentiated In Vitro. Antioxidants. 2020; 9(12):1276. https://doi.org/10.3390/antiox9121276
Chicago/Turabian StyleEspinoza, Francisca, Rocío Magdalena, Natalia Saldivia, Nery Jara, Fernando Martínez, Luciano Ferrada, Katterine Salazar, Felipe Ávila, and Francisco Nualart. 2020. "Vitamin C Recycling Regulates Neurite Growth in Neurospheres Differentiated In Vitro" Antioxidants 9, no. 12: 1276. https://doi.org/10.3390/antiox9121276
APA StyleEspinoza, F., Magdalena, R., Saldivia, N., Jara, N., Martínez, F., Ferrada, L., Salazar, K., Ávila, F., & Nualart, F. (2020). Vitamin C Recycling Regulates Neurite Growth in Neurospheres Differentiated In Vitro. Antioxidants, 9(12), 1276. https://doi.org/10.3390/antiox9121276




