Myeloperoxidase Modulates Hydrogen Peroxide Mediated Cellular Damage in Murine Macrophages
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Myeloperoxidase (MPO) Modulates the Extent of Glucose/GO-Induced J774A.1 Cell Death
3.2. Effect of Supplementation with SCN− on Glucose/GO/MPO-Induced Cell Death.
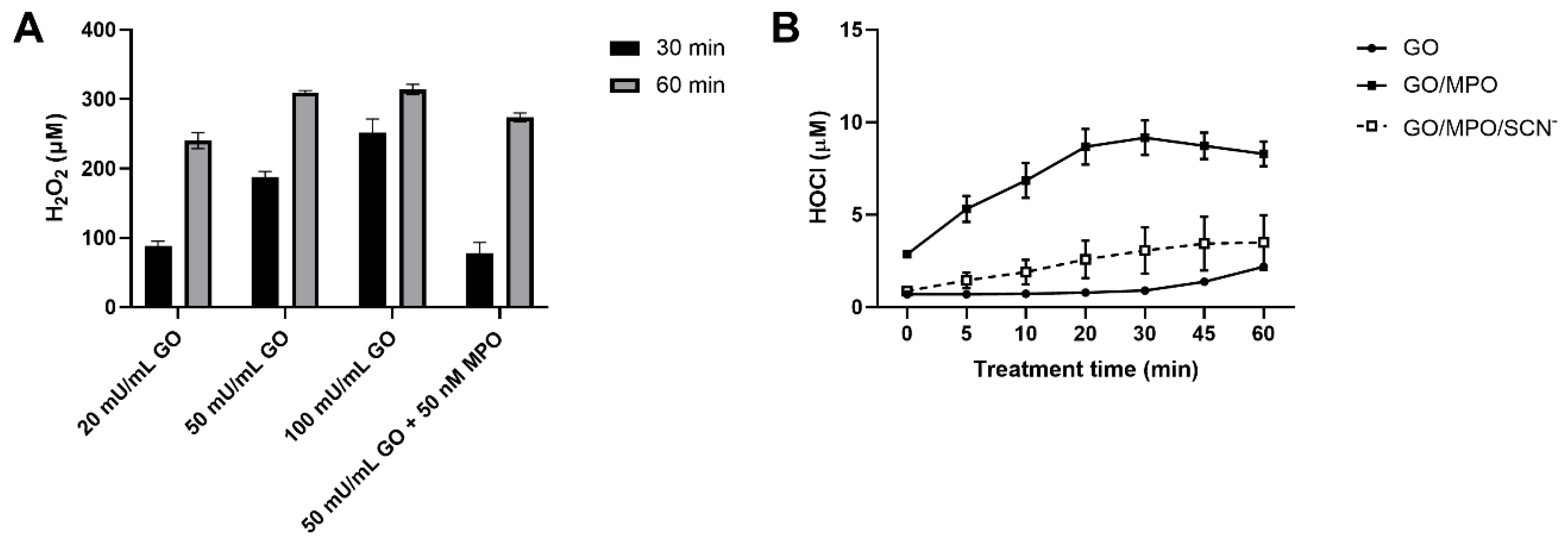
3.3. Production of H2O2 and HOCl by the Glucose/GO/MPO System
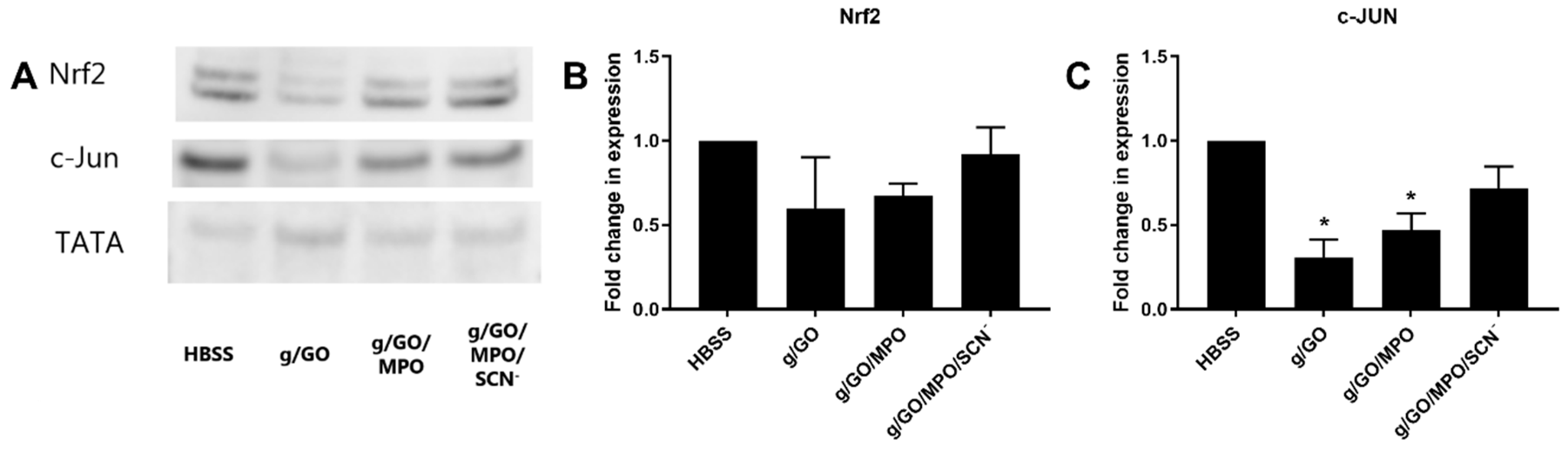
3.4. Pathways Involved in Alteration of H2O2-Induced Cell Damage by MPO.
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Abbreviations
References
- Klebanoff, S.J. Myeloperoxidase: Friend and foe. J. Leukoc. Biol. 2005, 77, 598–625. [Google Scholar] [CrossRef] [PubMed]
- Davies, M.J.; Hawkins, C.L. The role of myeloperoxidase in biomolecule modification, chronic inflammation, and disease. Antioxid. Redox Signal. 2020, 32, 957–981. [Google Scholar] [CrossRef]
- Daugherty, A.; Dunn, J.L.; Rateri, D.L.; Heinecke, J.W. Myeloperoxidase, a catalyst for lipoprotein oxidation, is expressed in human atherosclerotic lesions. J. Clin. Investig. 1994, 94, 437–444. [Google Scholar] [CrossRef] [PubMed]
- Davies, M.J.; Hawkins, C.L.; Pattison, D.I.; Rees, M.D. Mammalian heme peroxidases: From molecular mechanisms to health implications. Antioxid. Redox Signal. 2008, 10, 1199–1234. [Google Scholar] [CrossRef] [PubMed]
- Van Dalen, C.J.; Whitehouse, M.W.; Winterbourn, C.C.; Kettle, A.J. Thiocyanate and chloride as competing substrates for myeloperoxidase. Biochem. J. 1997, 327, 487–492. [Google Scholar] [CrossRef]
- Chandler, J.D.; Day, B.J. Biochemical mechanisms and therapeutic potential of pseudohalide thiocyanate in human health. Free Radic. Res. 2015, 49, 695–710. [Google Scholar] [CrossRef]
- Ashby, M.T.; Carlson, A.C.; Scott, M.J. Redox buffering of hypochlorous acid by thiocyanate in physiologic fluids. J. Am. Chem. Soc. 2004, 126, 15976–15977. [Google Scholar] [CrossRef]
- Pattison, D.I.; Davies, M.J.; Hawkins, C.L. Reactions and reactivity of myeloperoxidase-derived oxidants: Differential biological effects of hypochlorous and hypothiocyanous acids. Free Radic. Res. 2012, 46, 975–995. [Google Scholar] [CrossRef]
- Day, B.J. The science of licking your wounds: Function of oxidants in the innate immune system. Biochem. Pharmacol. 2019, 163, 451–457. [Google Scholar] [CrossRef]
- Hawkins, C.L. Hypochlorous acid-mediated modification of proteins and its consequences. Essays Biochem. 2020, 64, 75–86. [Google Scholar] [CrossRef]
- Rayner, B.S.; Love, D.T.; Hawkins, C.L. Comparative reactivity of myeloperoxidase-derived oxidants with mammalian cells. Free Radic. Biol. Med. 2014, 71, 240–255. [Google Scholar] [CrossRef] [PubMed]
- Barrett, T.J.; Pattison, D.I.; Leonard, S.E.; Carroll, K.S.; Davies, M.J.; Hawkins, C.L. Inactivation of thiol-dependent enzymes by hypothiocyanous acid: Role of sulfenyl thiocyanate and sulfenic acid intermediates. Free Radic. Biol. Med. 2012, 52, 1075–1085. [Google Scholar] [CrossRef] [PubMed]
- Skaff, O.; Pattison, D.I.; Davies, M.J. Hypothiocyanous acid reactivity with low-molecular-mass and protein thiols: Absolute rate constants and assessment of biological relevance. Biochem. J. 2009, 422, 111–117. [Google Scholar] [CrossRef] [PubMed]
- Chandler, J.D.; Nichols, D.P.; Nick, J.A.; Hondal, R.J.; Day, B.J. Selective metabolism of hypothiocyanous acid by mammalian thioredoxin reductase promotes lung innate immunity and antioxidant defense. J. Biol. Chem. 2013, 288, 18421–18428. [Google Scholar] [CrossRef]
- Love, D.T.; Guo, C.; Nikelshparg, E.I.; Brazhe, N.A.; Sosnovtseva, O.; Hawkins, C.L. The role of the myeloperoxidase-derived oxidant hypothiocyanous acid (HOSCN) in the induction of mitochondrial dysfunction in macrophages. Redox Biol. 2020, 36, 101602. [Google Scholar] [CrossRef]
- Lloyd, M.M.; Grima, M.A.; Rayner, B.S.; Hadfield, K.A.; Davies, M.J.; Hawkins, C.L. Comparative reactivity of the myeloperoxidase-derived oxidants hypochlorous acid and hypothiocyanous acid with human coronary artery endothelial cells. Free Radic. Biol. Med. 2013, 65, 1352–1362. [Google Scholar] [CrossRef]
- Lloyd, M.M.; Van Reyk, D.M.; Davies, M.J.; Hawkins, C.L. HOSCN is a more potent inducer of apoptosis and protein thiol depletion in murine macrophage cells than HOCl or HOBr. Biochem. J. 2008, 414, 271–280. [Google Scholar] [CrossRef]
- Pan, G.J.; Rayner, B.S.; Zhang, Y.; van Reyk, D.M.; Hawkins, C.L. A pivotal role for NF-kappaB in the macrophage inflammatory response to the myeloperoxidase oxidant hypothiocyanous acid. Arch. Biochem. Biophys. 2018, 642, 23–30. [Google Scholar] [CrossRef]
- Wagner, B.A.; Reszka, K.J.; McCormick, M.L.; Britigan, B.E.; Evig, C.B.; Burns, C.P. Role of thiocyanate, bromide and hypobromous acid in hydrogen peroxide-induced apoptosis. Free Radic. Res. 2004, 38, 167–175. [Google Scholar] [CrossRef]
- Xu, Y.; Szep, S.; Lu, Z. The antioxidant role of thiocyanate in the pathogenesis of cystic fibrosis and other inflammation-related diseases. Proc. Natl. Acad. Sci. USA 2009, 106, 20515–20519. [Google Scholar] [CrossRef]
- Vanichkitrungruang, S.; Chuang, C.Y.; Hawkins, C.L.; Davies, M.J. Myeloperoxidase-derived damage to human plasma fibronectin: Modulation by protein binding and thiocyanate ions (SCN−). Redox Biol. 2020, 36, 101641. [Google Scholar] [CrossRef]
- Cai, H.; Chuang, C.Y.; Hawkins, C.L.; Davies, M.J. Binding of myeloperoxidase to the extracellular matrix of smooth muscle cells and subsequent matrix modification. Sci. Rep. 2020, 10, 666. [Google Scholar] [CrossRef]
- Chandler, J.D.; Min, E.; Huang, J.; McElroy, C.S.; Dickerhof, N.; Mocatta, T.; Fletcher, A.A.; Evans, C.M.; Liang, L.; Patel, M.; et al. Antiinflammatory and antimicrobial effects of thiocyanate in a cystic fibrosis mouse model. Am. J. Respir. Cell. Mol. Biol. 2015, 53, 193–205. [Google Scholar] [CrossRef]
- Morgan, P.E.; Laura, R.P.; Maki, R.A.; Reynolds, W.F.; Davies, M.J. Thiocyanate supplementation decreases atherosclerotic plaque in mice expressing human myeloperoxidase. Free Radic. Res. 2015, 49, 743–749. [Google Scholar] [CrossRef] [PubMed]
- Zietzer, A.; Niepmann, S.T.; Camara, B.; Lenart, M.A.; Jansen, F.; Becher, M.U.; Andrie, R.; Nickenig, G.; Tiyerili, V. Sodium thiocyanate treatment attenuates atherosclerotic plaque formation and improves endothelial regeneration in mice. PLoS ONE 2019, 14, e0214476. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Burton, T.; Rayner, B.S.; San Gabriel, P.T.; Shi, H.; El Kazzi, M.; Wang, X.; Dennis, J.M.; Ahmad, G.; Schroder, A.L.; et al. The role of sodium thiocyanate supplementation during dextran sodium sulphate-stimulated experimental colitis. Arch. Biochem. Biophys. 2020, 692, 108490. [Google Scholar] [CrossRef] [PubMed]
- Guo, C.; Davies, M.J.; Hawkins, C.L. Role of thiocyanate in the modulation of myeloperoxidase-derived oxidant induced damage to macrophages. Redox Biol. 2020, 36, 101666. [Google Scholar] [CrossRef] [PubMed]
- Mutze, S.; Hebling, U.; Stremmel, W.; Wang, J.; Arnhold, J.; Pantopoulos, K.; Mueller, S. Myeloperoxidase-derived hypochlorous acid antagonizes the oxidative stress-mediated activation of iron regulatory protein 1. J. Biol. Chem. 2003, 278, 49662. [Google Scholar] [CrossRef]
- Fotakis, G.; Timbrell, J.A. In vitro cytotoxicity assays: Comparison of LDH, neutral red, MTT and protein assay in hepatoma cell lines following exposure to cadmium chloride. Toxicol. Lett. 2006, 160, 171–177. [Google Scholar] [CrossRef]
- Hawkins, C.L.; Morgan, P.E.; Davies, M.J. Quantification of protein modification by oxidants. Free Radic. Biol. Med. 2009, 46, 965–988. [Google Scholar] [CrossRef]
- Albrett, A.M.; Ashby, L.V.; Dickerhof, N.; Kettle, A.J.; Winterbourn, C.C. Heterogeneity of hypochlorous acid production in individual neutrophil phagosomes revealed by a rhodamine-based probe. J. Biol. Chem. 2018, 293, 15715–15724. [Google Scholar] [CrossRef] [PubMed]
- Morris, J.C. The acid ionization constant of HOCl from 5 °C to 35 °C. J. Phys. Chem. 1966, 70, 3798–3805. [Google Scholar] [CrossRef]
- Venugopal, R.; Jaiswal, A.K. Nrf2 and Nrf1 in association with Jun proteins regulate antioxidant response element-mediated expression and coordinated induction of genes encoding detoxifying enzymes. Oncogene 1998, 17, 3145–3156. [Google Scholar] [CrossRef] [PubMed]
- Levy, S.; Jaiswal, A.K.; Forman, H.J. The role of c-Jun phosphorylation in EpRE activation of phase II genes. Free Radic. Biol. Med. 2009, 47, 1172–1179. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kang, E.S.; Kim, G.H.; Woo, I.S.; Kim, H.J.; Eun, S.Y.; Ham, S.A.; Jin, H.; Kim, M.Y.; Park, M.H.; Kim, H.J.; et al. Down-regulation of aldose reductase renders J774A.1 cells more susceptible to acrolein- or hydrogen peroxide-induced cell death. Free Radic. Res. 2008, 42, 930–938. [Google Scholar] [CrossRef] [PubMed]
- Ashby, M.T.; Aneetha, H.; Carlson, A.C.; Scott, M.J.; Beal, J.L. Bioorganic chemistry of hypothiocyanite. Phosphorus Sulfur Silicon Relat. Elem. 2005, 180, 1369–1374. [Google Scholar] [CrossRef]
- Gorudko, I.V.; Sokolov, A.V.; Shamova, E.V.; Grigorieva, D.V.; Mironova, E.V.; Kudryavtsev, I.V.; Gusev, S.A.; Gusev, A.A.; Chekanov, A.V.; Vasilyev, V.B. Binding of human myeloperoxidase to red blood cells: Molecular targets and biophysical consequences at the plasma membrane level. Arch. Biochem. Biophys. 2016, 591, 87–97. [Google Scholar] [CrossRef] [PubMed]
- Klinke, A.; Nussbaum, C.; Kubala, L.; Friedrichs, K.; Rudolph, T.K.; Rudolph, V.; Paust, H.J.; Schroder, C.; Benten, D.; Lau, D.; et al. Myeloperoxidase attracts neutrophils by physical forces. Blood 2011, 117, 1350–1358. [Google Scholar] [CrossRef]
- Haegens, A.; Vernooy, J.H.; Heeringa, P.; Mossman, B.T.; Wouters, E.F. Myeloperoxidase modulates lung epithelial responses to pro-inflammatory agents. Eur. Respir. J. 2008, 31, 252–260. [Google Scholar] [CrossRef]
- Yogalingam, G.; Lee, A.R.; Mackenzie, D.S.; Maures, T.J.; Rafalko, A.; Prill, H.; Berguig, G.Y.; Hague, C.; Christianson, T.; Bell, S.M.; et al. Cellular uptake and delivery of myeloperoxidase to lysosomes promote lipofuscin degradation and lysosomal stress in retinal cells. J. Biol. Chem. 2017, 292, 4255–4265. [Google Scholar] [CrossRef]
- Lu, J.; Holmgren, A. The thioredoxin antioxidant system. Free Radic. Biol. Med. 2014, 66, 75–87. [Google Scholar] [CrossRef] [PubMed]
- Jeelani, R.; Chatzicharalampous, C.; Kohan-Ghadr, H.-R.; Bai, D.; Morris, R.T.; Sliskovic, I.; Awonuga, A.; Abu-Soud, H.M. Hypochlorous acid reversibly inhibits caspase-3: A potential regulator of apoptosis. Free Radic. Res. 2020, 54, 43–56. [Google Scholar] [CrossRef] [PubMed]
- Dinkova-Kostova, A.T.; Talalay, P. NAD(P)H:quinone acceptor oxidoreductase 1 (NQO1), a multifunctional antioxidant enzyme and exceptionally versatile cytoprotector. Arch. Biochem. Biophys. 2010, 501, 116–123. [Google Scholar] [CrossRef] [PubMed]
- Ross, D.; Siegel, D. Functions of NQO1 in Cellular Protection and CoQ10 Metabolism and its Potential Role as a Redox Sensitive Molecular Switch. Front. Physiol. 2017, 8, 595. [Google Scholar] [CrossRef]
- Woods, C.G.; Fu, J.; Xue, P.; Hou, Y.; Pluta, L.J.; Yang, L.; Zhang, Q.; Thomas, R.S.; Andersen, M.E.; Pi, J. Dose-dependent transitions in Nrf2-mediated adaptive response and related stress responses to hypochlorous acid in mouse macrophages. Toxicol. Appl. Pharmacol. 2009, 238, 27–36. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, C.; Sileikaite, I.; Davies, M.J.; Hawkins, C.L. Myeloperoxidase Modulates Hydrogen Peroxide Mediated Cellular Damage in Murine Macrophages. Antioxidants 2020, 9, 1255. https://doi.org/10.3390/antiox9121255
Guo C, Sileikaite I, Davies MJ, Hawkins CL. Myeloperoxidase Modulates Hydrogen Peroxide Mediated Cellular Damage in Murine Macrophages. Antioxidants. 2020; 9(12):1255. https://doi.org/10.3390/antiox9121255
Chicago/Turabian StyleGuo, Chaorui, Inga Sileikaite, Michael J. Davies, and Clare L. Hawkins. 2020. "Myeloperoxidase Modulates Hydrogen Peroxide Mediated Cellular Damage in Murine Macrophages" Antioxidants 9, no. 12: 1255. https://doi.org/10.3390/antiox9121255
APA StyleGuo, C., Sileikaite, I., Davies, M. J., & Hawkins, C. L. (2020). Myeloperoxidase Modulates Hydrogen Peroxide Mediated Cellular Damage in Murine Macrophages. Antioxidants, 9(12), 1255. https://doi.org/10.3390/antiox9121255






