Diazoxide and Exercise Enhance Muscle Contraction during Obesity by Decreasing ROS Levels, Lipid Peroxidation, and Improving Glutathione Redox Status
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Animals and Groups
2.2. Muscles Dissection
2.3. Isometric Tension Measurements
2.4. Reactive Oxygen Species Analysis
2.5. Lipid Peroxidation Measurement
2.6. Glutathione Redox State Measurement
2.7. Data Analysis
3. Results
3.1. Effect of Diazoxide and Exercise on the Physiological Parameters of Obese Rats
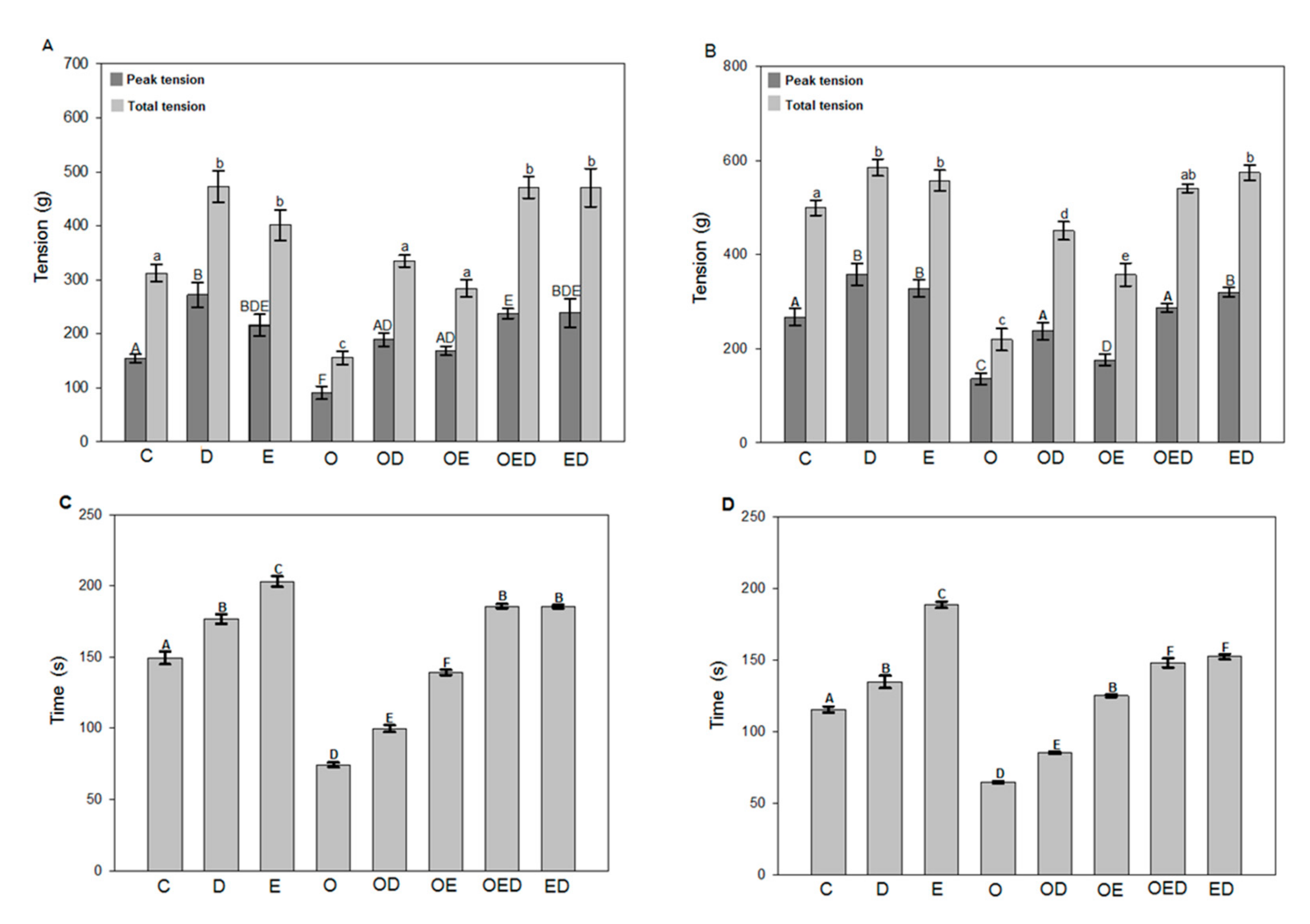
3.2. Effect of Diazoxide and Exercise on Maximum and Total Tension and Time of Resistance to Fatigue of Slow and Fast Skeletal Muscle of Obese Rats
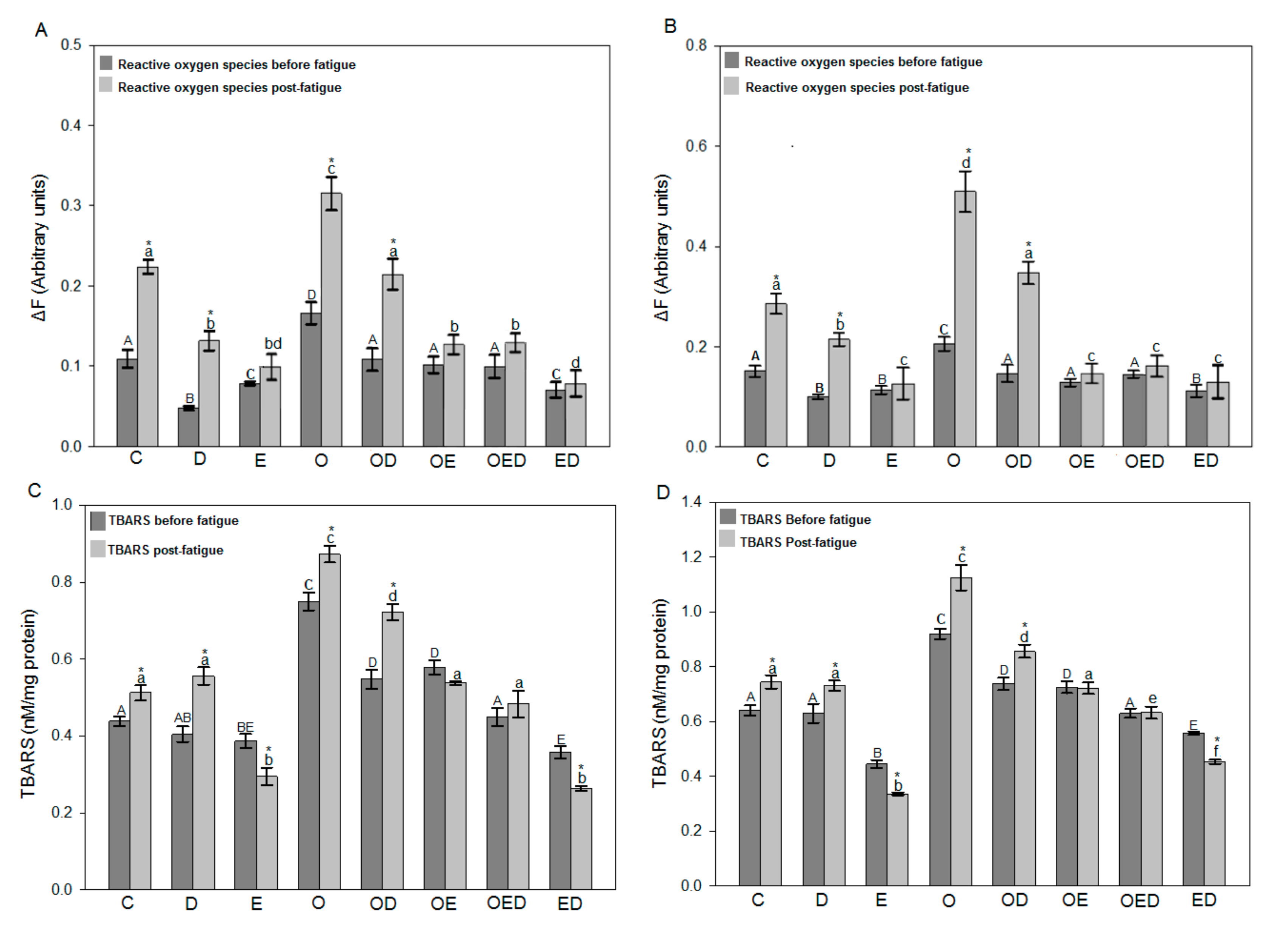
3.3. Diazoxide and Exercise Decrease ROS Levels and Lipid Peroxidation in Slow and Fast Skeletal Muscle of Obese Rats
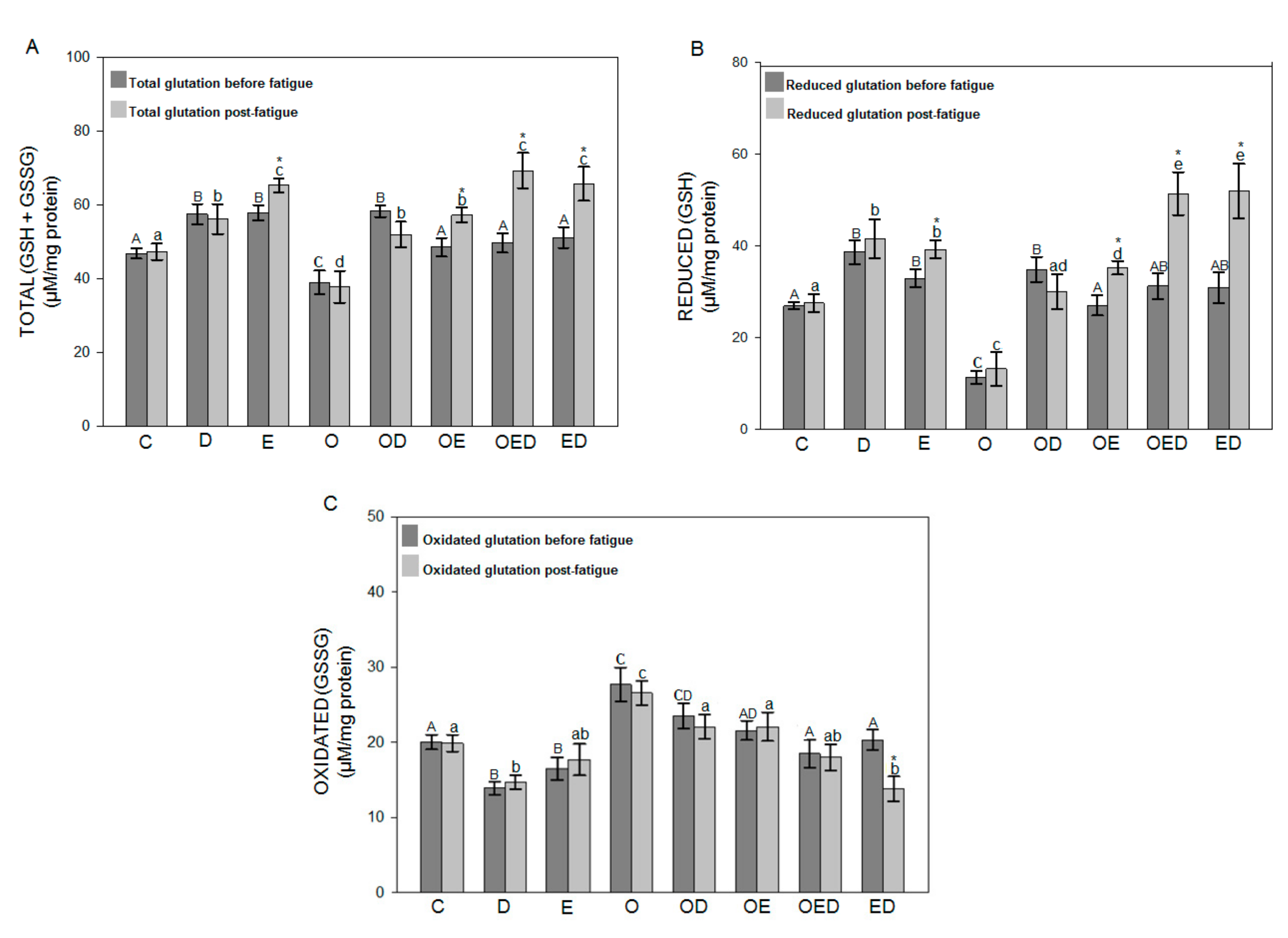
3.4. Effect of Diazoxide and Exercise on Glutathione Redox Status in Slow and Fast Skeletal Muscle of Obese Rats
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Pompeani, N.; Rybalka, E.; Latchman, H.; Murphy, R.M.; Croft, K.D.; Hayes, A. Skeletal muscle atrophy in sedentary Zucker obese rats is not caused by calpain-mediated muscle damage or lipid peroxidation induced by oxidative stress. J. Negat. Results Biomed. 2014, 13, 1–11. [Google Scholar] [CrossRef][Green Version]
- Abrigo, J.; Rivera, J.C.; Aravena, J.; Cabrera, D.; Simon, F.; Ezquer, F.; Ezquer, M.; Cabello-Verrugio, C. High Fat Diet-Induced Skeletal Muscle Wasting Is Decreased by Mesenchymal Stem Cells Administration: Implications on Oxidative Stress, Ubiquitin Proteasome Pathway Activation, and Myonuclear Apoptosis. Oxidative Med. Cell. Longev. 2016, 2016, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Huenchullan, S.F.; Maharjan, B.R.; Williams, P.F.; Tam, C.S.; McLennan, S.V.; Twigg, S.M. Differential metabolic effects of constant moderate versus high intensity interval training in high-fat fed mice: Possible role of muscle adiponectin. Physiol. Rep. 2018, 6, e13599. [Google Scholar] [CrossRef] [PubMed]
- Espinosa, A.; Henríquez-Olguín, C.; Jaimovich, E. Reactive oxygen species and calcium signals in skeletal muscle: A crosstalk involved in both normal signaling and disease. Cell Calcium 2016, 60, 172–179. [Google Scholar] [CrossRef] [PubMed]
- Bae, J.Y.; Shin, K.O.; Woo, J.; Woo, S.H.; Jang, K.S.; Lee, Y.H.; Kang, S. Exercise and dietary change ameliorate high fat diet induced obesity and insulin resistance via mTOR signaling pathway. J. Exerc. Nutr. Biochem. 2016, 20, 28–33. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.J.; Files, D.C.; Zhang, T.; Wang, Z.-M.; Messi, M.L.; Gregory, H.; Stone, J.; Lyles, M.F.; Dhar, S.; Marsh, A.P.; et al. Intramyocellular Lipid and Impaired Myofiber Contraction in Normal Weight and Obese Older Adults. J. Gerontol. A Boil. Sci. Med. Sci. 2015, 71, 557–564. [Google Scholar] [CrossRef] [PubMed]
- Heo, J.-W.; No, M.-H.; Park, D.-H.; Kang, J.-H.; Seo, D.Y.; Han, J.; Neufer, P.D.; Kwak, H.-B. Effects of exercise on obesity-induced mitochondrial dysfunction in skeletal muscle. Korean J. Physiol. Pharmacol. 2017, 21, 567–577. [Google Scholar] [CrossRef]
- Scott, K.; Benkhalti, M.; Calvert, N.D.; Paquette, M.; Zhen, L.; Harper, M.-E.; Al-Dirbashi, O.Y.; Renaud, J. KATP channel deficiency in mouse FDB causes an impairment of energy metabolism during fatigue. Am. J. Physiol. Physiol. 2016, 311, C559–C571. [Google Scholar] [CrossRef]
- Hibino, H.; Inanobe, A.; Furutani, K.; Murakami, S.; Findlay, I.; Kurachi, Y. Inwardly Rectifying Potassium Channels: Their Structure, Function, and Physiological Roles. Physiol. Rev. 2010, 90, 291–366. [Google Scholar] [CrossRef]
- Szabo, I.; Zoratti, M. Mitochondrial Channels: Ion Fluxes and More. Physiol. Rev. 2014, 94, 519–608. [Google Scholar] [CrossRef]
- Constant-Urban, C.C.; Charif, M.M.; Goffin, E.; Van Heugen, J.-C.; Elmoualij, B.B.; Chiap, P.; Mouithys-Mickalad, A.; Serteyn, D.D.; Lebrun, P.; Pirotte, B.; et al. Triphenylphosphonium salts of 1,2,4-benzothiadiazine 1,1-dioxides related to diazoxide targeting mitochondrial ATP-sensitive potassium channels. Bioorganic Med. Chem. Lett. 2013, 23, 5878–5881. [Google Scholar] [CrossRef] [PubMed]
- Garlid, A.O.; Jabůrek, M.; Jacobs, J.P.; Garlid, K.D. Mitochondrial reactive oxygen species: Which ROS signals cardioprotection? Am. J. Physiol. Circ. Physiol. 2013, 305, H960–H968. [Google Scholar] [CrossRef] [PubMed]
- Kraljević, J.; Hoydal, M.A.; Ljubkovic, M.; Moreira, J.B.N.; Jørgensen, K.; Ness, H.O.; Bækkerud, F.H.; Dujić, Ž; Wisløff, U.; Marinovic, J. Role of KATP Channels in Beneficial Effects of Exercise in Ischemic Heart Failure. Med. Sci. Sports Exerc. 2015, 47, 2504–2512. [Google Scholar] [CrossRef]
- Andrade, F.; Trujillo, X.; Sánchez-Pastor, E.; Montoya-Pérez, R.; Saavedra-Molina, A.; Ortiz-Mesina, M.; Huerta, M. Glibenclamide increases post-fatigue tension in slow skeletal muscle fibers of the chicken. J. Comp. Physiol. B 2011, 181, 403–412. [Google Scholar] [CrossRef] [PubMed]
- Cahoon, N.J.; Naparus, A.; Ashrafpour, H.; Hofer, S.; Huang, N.; Lipa, J.E.; Forrest, C.R.; Pang, C.Y. Pharmacologic Prophylactic Treatment for Perioperative Protection of Skeletal Muscle from Ischemia-Reperfusion Injury in Reconstructive Surgery. Plast. Reconstr. Surg. 2013, 131, 473–485. [Google Scholar] [CrossRef]
- Alemzadeh, R.; Tushaus, K.M. Modulation of Adipoinsular Axis in Prediabetic Zucker Diabetic Fatty Rats by Diazoxide. Endocrinology 2004, 145, 5476–5484. [Google Scholar] [CrossRef]
- Alemzadeh, R.; Karlstad, M.D.; Tushaus, K.; Buchholz, M. Diazoxide enhances basal metabolic rate and fat oxidation in obese Zucker rats. Metabolism 2008, 57, 1597–1607. [Google Scholar] [CrossRef]
- García, M.C.; Hernández, A.; Sánchez, J.A. Role of mitochondrial ATP-sensitive potassium channels on fatigue in mouse muscle fibers. Biochem. Biophys. Res. Commun. 2009, 385, 28–32. [Google Scholar] [CrossRef]
- Montoya-Perez, R.; Sánchez-Duarte, E.; Trujillo, X.; Huerta, M.; Ortiz-Mesina, M.; Cortés-Rojo, C.; Manzo-Ávalos, S.; Saavedra-Molina, A. Mitochondrial KATP channels in skeletal muscle: Are protein kinases C and G, and nitric oxide synthase involved in the fatigue process? Open Access Anim. Physiol. 2012, 4, 21–28. [Google Scholar] [CrossRef]
- Sánchez-Duarte, E.; Trujillo, X.; Cortés-Rojo, C.; Saavedra-Molina, A.; Camargo, G.; Hernández, L.; Huerta, M.; Montoya-Pérez, R. Nicorandil improves post-fatigue tension in slow skeletal muscle fibers by modulating glutathione redox state. J. Bioenerg. Biomembr. 2017, 49, 159–170. [Google Scholar] [CrossRef]
- Pattanakuhar, S.; Pongchaidecha, A.; Chattipakorn, N.; Chattipakorn, S.C. The effect of exercise on skeletal muscle fibre type distribution in obesity: From cellular levels to clinical application. Obes. Res. Clin. Pr. 2017, 11, 112–132. [Google Scholar] [CrossRef] [PubMed]
- Cartee, G.D.; Arias, E.B.; Yu, C.S.; Pataky, M.W. Novel single skeletal muscle fiber analysis reveals a fiber type-selective effect of acute exercise on glucose uptake. Am. J. Physiol. Metab. 2016, 311, E818–E824. [Google Scholar] [CrossRef] [PubMed]
- Ji, L.L.; Gomez-Cabrera, M.; Vina, J. Exercise and Hormesis: Activation of Cellular Antioxidant Signaling Pathway. Ann. N. Y. Acad. Sci. 2006, 1067, 425–435. [Google Scholar] [CrossRef] [PubMed]
- Lambertucci, R.H.; Levada-Pires, A.C.; Rossoni, L.V.; Curi, R.; Pithon-Curi, T.C. Effects of aerobic exercise training on antioxidant enzyme activities and mRNA levels in soleus muscle from young and aged rats. Mech. Ageing Dev. 2007, 128, 267–275. [Google Scholar] [CrossRef]
- Gornall, A.G.; Baradawill, C.J.; David, M.M. Determination of serum proteins by means of the biuret reaction. J. Biol. Chem. 1949, 177, 751. [Google Scholar]
- Ortiz-Avila, O.; Gallegos-Corona, M.A.; Sánchez-Briones, L.A.; Calderón-Cortés, E.; Montoya-Pérez, R.; Rodríguez-Orozco, A.R.; Campos-Garciía, J.; Saavedra-Molina, A.; Mejía-Zepeda, R.; Cortés-Rojo, C. Protective effects of dietary avocado oil on impaired electron transport chain function and exacerbated oxidative stress in liver mitochondria from diabetic rats. J. Bioenerg. Biomembr. 2015, 47, 337–353. [Google Scholar] [CrossRef]
- Cortés-Rojo, C.; Calderón-Cortés, E.; Clemente-Guerrero, M.; Estrada-Villagómez, M.; Manzo-Ávalos, S.; Mejía-Zepeda, R.; Boldogh, I.; Saavedra-Molina, A. Elucidation of the effects of lipoperoxidation on the mitochondrial electron transport chain using yeast mitochondria with manipulated fatty acid content. J. Bioenerg. Biomembr. 2009, 41, 15–28. [Google Scholar] [CrossRef]
- Akerboom, T.P.; Sies, H. Assay of glutathione, glutathione disulfide, and glutathione mixed disulfides in biological samples. Immobil. Enzym. 1981, 77, 373–382. [Google Scholar] [CrossRef]
- Ortiz-Avila, O.; Sámano-García, C.A.; Calderón-Cortés, E.; Pérez-Hernández, I.H.; Mejía-Zepeda, R.; Rodríguez-Orozco, A.R.; Saavedra-Molina, A.; Cortés-Rojo, C. Dietary avocado oil supplementation attenuates the alterations induced by type I diabetes and oxidative stress in electron transfer at the complex II-complex III segment of the electron transport chain in rat kidney mitochondria. J. Bioenerg. Biomembr. 2013, 45, 271–287. [Google Scholar] [CrossRef]
- Trewin, A.J.; Levinger, I.; Parker, L.; Shaw, C.S.; Serpiello, F.R.; Anderson, M.J.; McConell, G.K.; Hare, D.L.; Stepto, N.K. Acute exercise alters skeletal muscle mitochondrial respiration and H2O2 emission in response to hyperinsulinemic-euglycemic clamp in middle-aged obese men. PLoS ONE 2017, 12, e0188421. [Google Scholar] [CrossRef]
- World Health Organization (WHO). Available online: https://www.who.int/es/news-room/fact-sheets/detail/obesity-and-overweight (accessed on 5 May 2020).
- Lu, Y.; Li, H.; Shen, S.-W.; Shen, Z.-H.; Xu, M.; Yang, C.-J.; Li, F.; Feng, Y.-B.; Yun, J.-T.; Wang, L.; et al. Swimming exercise increases serum irisin level and reduces body fat mass in high-fat-diet fed Wistar rats. Lipids Health Dis. 2016, 15, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Botezelli, J.D.; Coope, A.; Ghezzi, A.C.; Cambri, L.T.; Moura, L.P.; Scariot, P.P.M.; Gaspar, R.S.; Mekary, R.A.; Ropelle, E.R.; Pauli, J.R. Strength Training Prevents Hyperinsulinemia, Insulin Resistance, and Inflammation Independent of Weight Loss in Fructose-Fed Animals. Sci. Rep. 2016, 6, 31106. [Google Scholar] [CrossRef]
- Brown, D.A.; Chicco, A.J.; Jew, K.N.; Johnson, M.S.; Lynch, J.M.; Watson, P.A.; Moore, R.L. Cardioprotection afforded by chronic exercise is mediated by the sarcolemmal, and not the mitochondrial, isoform of the KATPchannel in the rat. J. Physiol. 2005, 569, 913–924. [Google Scholar] [CrossRef] [PubMed]
- Debska, G.; Kicinska, A.; Skalska, J.; Szewczyk, A.; May, R.; E Elger, C.; Kunz, W.S. Opening of potassium channels modulates mitochondrial function in rat skeletal muscle. Biochim. Biophys. Acta Bioenerg. 2002, 1556, 97–105. [Google Scholar] [CrossRef]
- Andrukhiv, A.; Costa, A.D.; West, I.C.; Garlid, K.D. Opening mitoKATP increases superoxide generation from complex I of the electron transport chain. Am. J. Physiol. Heart Circ. Physiol. 2006, 291, H2067–H2074. [Google Scholar] [CrossRef] [PubMed]
- Costa, A.D.; Quinlan, C.L.; Andrukhiv, A.; West, I.C.; Jabůrek, M.; Garlid, K.D. The direct physiological effects of mitoKATP opening on heart mitocondria. Am. J. Physiol. Heart Circ. Physiol. 2006, 290, H406–H415. [Google Scholar] [CrossRef] [PubMed]
- Zingman, L.V.; Zhu, Z.; Sierra, A.; Stepniak, E.; Burnett, C.M.-L.; Maksymov, G.; Anderson, M.E.; Coetzee, W.A.; Hodgson-Zingman, D.M. Exercise-induced expression of cardiac ATP-sensitive potassium channels promotes action potential shortening and energy conservation. J. Mol. Cell. Cardiol. 2011, 51, 72–81. [Google Scholar] [CrossRef] [PubMed]
- Montoya-Pérez, R.; Saavedra-Molina, A.; Trujillo, X.; Huerta, M.; Andrade, F.; Sánchez-Pastor, E.; Ortiz, M. Inhibition of oxygen consumption in skeletal muscle-derived mitochondria by pinacidil, diazoxide, and glibenclamide, but not by 5-hydroxydecanoate. J. Bioenerg. Biomembr. 2010, 42, 21–27. [Google Scholar] [CrossRef]
- Fernández, J.M.; Da Silva-Grigoletto, M.E.; Tunez-Fiñaca, I. Estrés oxidativo inducido por el ejercicio. Rev. Andal. Med. Deporte. 2009, 2, 19–34. [Google Scholar]
- Ferranti, R.; Da Silva, M.M.; Kowaltowski, A.J. Mitochondrial ATP-sensitive K+ channel opening decreases reactive oxygen species generation. FEBS Lett. 2003, 536, 51–55. [Google Scholar] [CrossRef]
- Moghtadaei, M.; Habibey, R.; Ajami, M.; Soleimani, M.; Ebrahimi, S.A.; Pazoki-Toroudi, H. Skeletal muscle post-conditioning by diazoxide, anti-oxidative and anti-apoptotic mechanisms. Mol. Biol. Rep. 2012, 39, 11093–11103. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Duarte, E.; Cortés-Rojo, C.; Sánchez-Briones, L.A.; Campos-García, J.; Saavedra-Molina, A.; Delgado-Enciso, I.; A López-Lemus, U.; Montoya-Pérez, R. Nicorandil Affects Mitochondrial Respiratory Chain Function by Increasing Complex III Activity and ROS Production in Skeletal Muscle Mitochondria. J. Membr. Biol. 2020, 253, 309–318. [Google Scholar] [CrossRef] [PubMed]
- McGarrah, R.W.; Slentz, C.A.; Kraus, W.E. The Effect of Vigorous- Versus Moderate-Intensity Aerobic Exercise on Insulin Action. Curr. Cardiol. Rep. 2016, 18, 117. [Google Scholar] [CrossRef] [PubMed]
- Beckendorf, L.; Linke, W.A. Emerging importance of oxidative stress in regulating striated muscle elasticity. J. Muscle Res. Cell Motil. 2015, 36, 25–36. [Google Scholar] [CrossRef] [PubMed]
- Farahini, H.; Habibey, R.; Ajami, M.; Davoodi, S.H.; Azad, N.; Soleimani, M.; Tavakkoli-Hosseini, M.; Pazoki-Toroudi, H. Late anti-apoptotic effect of K(ATP) channel opening in skeletal muscle. Clin. Exp. Pharmacol. Physiol. 2012, 39, 909–916. [Google Scholar] [CrossRef]
- Marotte, C.; Zeni, S.N. Reactive oxygen species on bone cells activity. Acta Bioquím. Clín. Lat. 2013, 47, 661–674. [Google Scholar]

| Groups | Diet | Diazoxide 35 mg/kg | Exercise Moderate Intensity |
|---|---|---|---|
| Control (C) | Standard rodent chow® | no | no |
| Diazoxide (D) | Standard rodent chow® | yes | no |
| Exercise (E) | Standard rodent chow® | no | yes |
| Obese (O) | High fat diet | no | no |
| Obese diazoxide (OD) | High fat diet | yes | no |
| Obese exercise (OE) | High fat diet | no | yes |
| Obese exercise diazoxide (OED) | High fat diet | yes | yes |
| Exercise diazoxide (ED) | Standard rodent chow® | yes | yes |
| Groups | E, ED | OE, OED |
|---|---|---|
| Week 1 | 10 m/min (10 min) | 10 m/min (10 min) |
| Week 2 | 10 m/min (10 min) 16 m/min (5 min) | 10 m/min (10 min) 16 m/min (5 min) |
| Week 3 | 10 m/min (5 min) 16 m/min (5 min) 22 m/min (5 min) | 10 m/min (5 min) 16 m/min (10 min) |
| Week 4 | 10 m/min (5 min) 16 m/min (5 min) 22 m/min (5 min) | 10 m/min (5 min) 16 m/min (10 min) |
| Week 5 | 10 m/min (5 min) 16 m/min (5 min) 22 m/min (10 min) | 10 m/min (5 min) 16 m/min (15 min) |
| Groups | Bodyweight (g) | Glucose |
|---|---|---|
| C | 323.80 ± 2.80 g a | 77 ± 1.13 mg/dL a |
| D | 322.12 ± 2.27 g a | 85 ± 1.56 mg/dL b |
| E | 324.28 ± 5.37 g a | 70 ± 1.47 mg/dL c |
| O | 552.57 ± 3.61 g b | 98 ± 1.25 mg/dL d |
| OD | 451.16 ± 19.34 g c | 84 ± 1.65 mg/dL b |
| OE | 455.19 ± 21.66 g c | 77 ± 1.51 mg/dL a |
| OED | 427.16 ± 14.89 g c | 72 ± 1.84 mg/dL ac |
| ED | 354.33 ± 2.12 g a | 75 ± 2.80 mg/dL ac |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gómez-Barroso, M.; Moreno-Calderón, K.M.; Sánchez-Duarte, E.; Cortés-Rojo, C.; Saavedra-Molina, A.; Rodríguez-Orozco, A.R.; Montoya-Pérez, R. Diazoxide and Exercise Enhance Muscle Contraction during Obesity by Decreasing ROS Levels, Lipid Peroxidation, and Improving Glutathione Redox Status. Antioxidants 2020, 9, 1232. https://doi.org/10.3390/antiox9121232
Gómez-Barroso M, Moreno-Calderón KM, Sánchez-Duarte E, Cortés-Rojo C, Saavedra-Molina A, Rodríguez-Orozco AR, Montoya-Pérez R. Diazoxide and Exercise Enhance Muscle Contraction during Obesity by Decreasing ROS Levels, Lipid Peroxidation, and Improving Glutathione Redox Status. Antioxidants. 2020; 9(12):1232. https://doi.org/10.3390/antiox9121232
Chicago/Turabian StyleGómez-Barroso, Mariana, Koré M. Moreno-Calderón, Elizabeth Sánchez-Duarte, Christian Cortés-Rojo, Alfredo Saavedra-Molina, Alain R. Rodríguez-Orozco, and Rocío Montoya-Pérez. 2020. "Diazoxide and Exercise Enhance Muscle Contraction during Obesity by Decreasing ROS Levels, Lipid Peroxidation, and Improving Glutathione Redox Status" Antioxidants 9, no. 12: 1232. https://doi.org/10.3390/antiox9121232
APA StyleGómez-Barroso, M., Moreno-Calderón, K. M., Sánchez-Duarte, E., Cortés-Rojo, C., Saavedra-Molina, A., Rodríguez-Orozco, A. R., & Montoya-Pérez, R. (2020). Diazoxide and Exercise Enhance Muscle Contraction during Obesity by Decreasing ROS Levels, Lipid Peroxidation, and Improving Glutathione Redox Status. Antioxidants, 9(12), 1232. https://doi.org/10.3390/antiox9121232






