Natural Antioxidant and Anti-Inflammatory Compounds in Foodstuff or Medicinal Herbs Inducing Heme Oxygenase-1 Expression
Abstract
1. Introduction
2. Beneficial Effects of Heme Oxygenase-1 Induction as Preventive Measures against Diseases
2.1. Obesity
2.2. Hypertension
2.3. Atherosclerosis
2.4. Parkinson’s Disease
2.5. Hepatic Fibrosis
3. HO-1 Signaling Pathway
3.1. Upstream Kinases: ERK, JNK, p38 and Akt
3.2. Transcription Factors: Nrf2, NF-κB and AP-1
3.3. Transcription Repressor: Bach1
3.4. Translational Repressor: miRNAs
3.5. Enzymatic Activity of HO-1
4. Phenolics
4.1. Flavonoid
4.2. Non-Flavonoid Phenolics
5. Terpenoids and Steroids
5.1. Monoterpenes
5.2. Sesquiiterpenes
5.3. Diterpenes
5.4. Titerpenes
5.5. Steroids
5.6. Other Natural Products from Mevalonate Pathway
6. Lipids
7. Lignan
8. Amino Acid Derivatives
9. Alkaloids and Nitrogen-Containing Natural Products
10. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Tenhunen, R.; Marver, H.S.; Schmid, R. The enzymatic conversion of heme to bilirubin by microsomal heme oxygenase. Proc. Natl. Acad. Sci. USA 1968, 61, 748–755. [Google Scholar] [CrossRef] [PubMed]
- Funes, S.C.; Rios, M.; Fernandez-Fierro, A.; Covian, C.; Bueno, S.M.; Riedel, C.A.; Mackern-Oberti, J.P.; Kalergis, A.M. Naturally Derived Heme-Oxygenase 1 Inducers and Their Therapeutic Application to Immune-Mediated Diseases. Front. Immunol. 2020, 11, 1467. [Google Scholar] [CrossRef] [PubMed]
- Choi, A.M.; Alam, J. Heme oxygenase-1: Function, regulation, and implication of a novel stress-inducible protein in oxidant-induced lung injury. Am. J. Respir. Cell. Mol. Biol. 1996, 15, 9–19. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, S.; Omata, Y.; Sakamoto, H.; Higashimoto, Y.; Hara, T.; Sagara, Y.; Noguchi, M. Characterization of rat heme oxygenase-3 gene. Implication of processed pseudogenes derived from heme oxygenase-2 gene. Gene 2004, 336, 241–250. [Google Scholar] [CrossRef]
- Nitti, M.; Piras, S.; Brondolo, L.; Marinari, U.M.; Pronzato, M.A.; Furfaro, A.L. Heme Oxygenase 1 in the Nervous System: Does It Favor Neuronal Cell Survival or Induce Neurodegeneration? Int. J. Mol. Sci. 2018, 19, 2260. [Google Scholar] [CrossRef]
- Maines, M.D.; Trakshel, G.M.; Kutty, R.K. Characterization of two constitutive forms of rat liver microsomal heme oxygenase. Only one molecular species of the enzyme is inducible. J. Biol. Chem. 1986, 261, 411–419. [Google Scholar]
- Prawan, A.; Kundu, J.K.; Surh, Y.J. Molecular basis of heme oxygenase-1 induction: Implications for chemoprevention and chemoprotection. Antioxid. Redox Signal. 2005, 7, 1688–1703. [Google Scholar] [CrossRef]
- Li, M.; Kim, D.H.; Tsenovoy, P.L.; Peterson, S.J.; Rezzani, R.; Rodella, L.F.; Aronow, W.S.; Ikehara, S.; Abraham, N.G. Treatment of obese diabetic mice with a heme oxygenase inducer reduces visceral and subcutaneous adiposity, increases adiponectin levels, and improves insulin sensitivity and glucose tolerance. Diabetes 2008, 57, 1526–1535. [Google Scholar] [CrossRef]
- Minamino, T.; Christou, H.; Hsieh, C.M.; Liu, Y.; Dhawan, V.; Abraham, N.G.; Perrella, M.A.; Mitsialis, S.A.; Kourembanas, S. Targeted expression of heme oxygenase-1 prevents the pulmonary inflammatory and vascular responses to hypoxia. Proc. Natl. Acad. Sci. USA 2001, 98, 8798–8803. [Google Scholar] [CrossRef]
- Juan, S.H.; Lee, T.S.; Tseng, K.W.; Liou, J.Y.; Shyue, S.K.; Wu, K.K.; Chau, L.Y. Adenovirus-mediated heme oxygenase-1 gene transfer inhibits the development of atherosclerosis in apolipoprotein E-deficient mice. Circulation 2001, 104, 1519–1525. [Google Scholar] [CrossRef]
- Hung, S.Y.; Liou, H.C.; Kang, K.H.; Wu, R.M.; Wen, C.C.; Fu, W.M. Overexpression of heme oxygenase-1 protects dopaminergic neurons against 1-methyl-4-phenylpyridinium-induced neurotoxicity. Mol. Pharmacol. 2008, 74, 1564–1575. [Google Scholar] [CrossRef] [PubMed]
- Sodhi, K.; Puri, N.; Favero, G.; Stevens, S.; Meadows, C.; Abraham, N.G.; Rezzani, R.; Ansinelli, H.; Lebovics, E.; Shapiro, J.I. Fructose Mediated Non-Alcoholic Fatty Liver Is Attenuated by HO-1-SIRT1 Module in Murine Hepatocytes and Mice Fed a High Fructose Diet. PLoS ONE 2015, 10, e0128648. [Google Scholar] [CrossRef] [PubMed]
- Waza, A.A.; Hamid, Z.; Ali, S.; Bhat, S.A.; Bhat, M.A. A review on heme oxygenase-1 induction: Is it a necessary evil. Inflamm. Res. 2018, 67, 579–588. [Google Scholar] [CrossRef] [PubMed]
- Sporn, M.B.; Suh, N. Chemoprevention: An essential approach to controlling cancer. Nat. Rev. Cancer 2002, 2, 537–543. [Google Scholar] [CrossRef]
- Kang, N.J.; Shin, S.H.; Lee, H.J.; Lee, K.W. Polyphenols as small molecular inhibitors of signaling cascades in carcinogenesis. Pharmacol. Ther. 2011, 130, 310–324. [Google Scholar] [CrossRef]
- Li Volti, G.; Sacerdoti, D.; Di Giacomo, C.; Barcellona, M.L.; Scacco, A.; Murabito, P.; Biondi, A.; Basile, F.; Gazzolo, D.; Abella, R.; et al. Natural heme oxygenase-1 inducers in hepatobiliary function. World J. Gastroenterol. 2008, 14, 6122–6132. [Google Scholar] [CrossRef]
- Barbagallo, I.; Galvano, F.; Frigiola, A.; Cappello, F.; Riccioni, G.; Murabito, P.; D’Orazio, N.; Torella, M.; Gazzolo, D.; Li Volti, G. Potential therapeutic effects of natural heme oxygenase-1 inducers in cardiovascular diseases. Antioxid. Redox Signal. 2013, 18, 507–521. [Google Scholar] [CrossRef]
- Ferrandiz, M.L.; Devesa, I. Inducers of heme oxygenase-1. Curr. Pharm. Des. 2008, 14, 473–486. [Google Scholar] [CrossRef]
- Couillard, C.; Mauriege, P.; Imbeault, P.; Prud’homme, D.; Nadeau, A.; Tremblay, A.; Bouchard, C.; Despres, J.P. Hyperleptinemia is more closely associated with adipose cell hypertrophy than with adipose tissue hyperplasia. Int. J. Obes. Relat. Metab. Disord. 2000, 24, 782–788. [Google Scholar] [CrossRef]
- Ogden, C.L.; Fryar, C.D.; Martin, C.B.; Freedman, D.S.; Carroll, M.D.; Gu, Q.; Hales, C.M. Trends in Obesity Prevalence by Race and Hispanic Origin-1999–2000 to 2017–2018. JAMA 2020. [Google Scholar] [CrossRef]
- Hall, J.E. The kidney, hypertension, and obesity. Hypertension 2003, 41, 625–633. [Google Scholar] [CrossRef] [PubMed]
- Al-Goblan, A.S.; Al-Alfi, M.A.; Khan, M.Z. Mechanism linking diabetes mellitus and obesity. Diabetes Metab. Syndr. Obes. 2014, 7, 587–591. [Google Scholar] [CrossRef] [PubMed]
- Drummond, G.S.; Baum, J.; Greenberg, M.; Lewis, D.; Abraham, N.G. HO-1 overexpression and underexpression: Clinical implications. Arch. Biochem. Biophys. 2019, 673, 108073. [Google Scholar] [CrossRef] [PubMed]
- Ndisang, J.F.; Jadhav, A.; Mishra, M. The heme oxygenase system suppresses perirenal visceral adiposity, abates renal inflammation and ameliorates diabetic nephropathy in Zucker diabetic fatty rats. PLoS ONE 2014, 9, e87936. [Google Scholar] [CrossRef] [PubMed]
- Sowers, J.R.; Epstein, M.; Frohlich, E.D. Diabetes, hypertension, and cardiovascular disease: An update. Hypertension 2001, 37, 1053–1059. [Google Scholar] [CrossRef] [PubMed]
- O’Donnell, C.J.; Kannel, W.B. Epidemiologic appraisal of hypertension as a coronary risk factor in the elderly. Am. J. Geriatr. Cardiol. 2002, 11, 86–92. [Google Scholar] [CrossRef] [PubMed]
- Katholi, R.E.; Couri, D.M. Left ventricular hypertrophy: Major risk factor in patients with hypertension: Update and practical clinical applications. Int. J. Hypertens 2011, 2011, 495349. [Google Scholar] [CrossRef]
- Shimzu, K.; Takahashi, T.; Iwasaki, T.; Shimizu, H.; Inoue, K.; Morimatsu, H.; Omori, E.; Matsumi, M.; Akagi, R.; Morita, K. Hemin treatment abrogates monocrotaline-induced pulmonary hypertension. Med. Chem. 2008, 4, 572–576. [Google Scholar] [CrossRef]
- Burgess, A.; Li, M.; Vanella, L.; Kim, D.H.; Rezzani, R.; Rodella, L.; Sodhi, K.; Canestraro, M.; Martasek, P.; Peterson, S.J.; et al. Adipocyte heme oxygenase-1 induction attenuates metabolic syndrome in both male and female obese mice. Hypertension 2010, 56, 1124–1130. [Google Scholar] [CrossRef]
- Filipovic, N.; Rosic, M.; Tanaskovic, I.; Milosevic, Z.; Nikolic, D.; Zdravkovic, N.; Peulic, A.; Kojic, M.R.; Fotiadis, D.I.; Parodi, O. ARTreat Project: Three-dimensional numerical simulation of plaque formation and development in the arteries. IEEE Trans. Inf. Technol. Biomed. 2012, 16, 272–278. [Google Scholar] [CrossRef]
- Kishimoto, Y.; Kondo, K.; Momiyama, Y. The Protective Role of Heme Oxygenase-1 in Atherosclerotic Diseases. Int. J. Mol. Sci. 2019, 20, 3628. [Google Scholar] [CrossRef] [PubMed]
- Radhakrishnan, N.; Yadav, S.P.; Sachdeva, A.; Pruthi, P.K.; Sawhney, S.; Piplani, T.; Wada, T.; Yachie, A. Human heme oxygenase-1 deficiency presenting with hemolysis, nephritis, and asplenia. J. Pediatr. Hematol. Oncol. 2011, 33, 74–78. [Google Scholar] [CrossRef] [PubMed]
- Yachie, A.; Niida, Y.; Wada, T.; Igarashi, N.; Kaneda, H.; Toma, T.; Ohta, K.; Kasahara, Y.; Koizumi, S. Oxidative stress causes enhanced endothelial cell injury in human heme oxygenase-1 deficiency. J. Clin. Investig. 1999, 103, 129–135. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, K.; Sugawara, D.; Wang, X.; Suzuki, K.; Itabe, H.; Maruyama, Y.; Lusis, A.J. Heme oxygenase-1 inhibits atherosclerotic lesion formation in ldl-receptor knockout mice. Circ. Res. 2001, 88, 506–512. [Google Scholar] [CrossRef]
- Ishikawa, K.; Sugawara, D.; Goto, J.; Watanabe, Y.; Kawamura, K.; Shiomi, M.; Itabe, H.; Maruyama, Y. Heme oxygenase-1 inhibits atherogenesis in Watanabe heritable hyperlipidemic rabbits. Circulation 2001, 104, 1831–1836. [Google Scholar] [CrossRef]
- Poss, K.D.; Tonegawa, S. Reduced stress defense in heme oxygenase 1-deficient cells. Proc. Natl. Acad. Sci. USA 1997, 94, 10925–10930. [Google Scholar] [CrossRef]
- Poss, K.D.; Tonegawa, S. Heme oxygenase 1 is required for mammalian iron reutilization. Proc. Natl. Acad. Sci. USA 1997, 94, 10919–10924. [Google Scholar] [CrossRef]
- Ishikawa, K.; Navab, M.; Lusis, A.J. Vasculitis, Atherosclerosis, and Altered HDL Composition in Heme-Oxygenase-1-Knockout Mice. Int. J. Hypertens 2012, 2012, 948203. [Google Scholar] [CrossRef]
- Teismann, P.; Tieu, K.; Choi, D.K.; Wu, D.C.; Naini, A.; Hunot, S.; Vila, M.; Jackson-Lewis, V.; Przedborski, S. Cyclooxygenase-2 is instrumental in Parkinson’s disease neurodegeneration. Proc. Natl. Acad. Sci. USA 2003, 100, 5473–5478. [Google Scholar] [CrossRef]
- Colombrita, C.; Calabrese, V.; Stella, A.M.; Mattei, F.; Alkon, D.L.; Scapagnini, G. Regional rat brain distribution of heme oxygenase-1 and manganese superoxide dismutase mRNA: Relevance of redox homeostasis in the aging processes. Exp. Biol. Med. 2003, 228, 517–524. [Google Scholar] [CrossRef]
- More, S.; Choi, D.K. Neuroprotective Role of Atractylenolide-I in an In Vitro and In Vivo Model of Parkinson’s Disease. Nutrients 2017, 9, 451. [Google Scholar] [CrossRef]
- Masaki, Y.; Izumi, Y.; Matsumura, A.; Akaike, A.; Kume, T. Protective effect of Nrf2-ARE activator isolated from green perilla leaves on dopaminergic neuronal loss in a Parkinson’s disease model. Eur. J. Pharmacol. 2017, 798, 26–34. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Tang, Y.; Yan, F.; Zhu, J.; Li, J. Potent inhibition of TGF-beta signaling pathway regulator Abl: Potential therapeutics for hepatic fibrosis. J. Recept. Signal. Transduct. Res. 2015, 35, 410–419. [Google Scholar] [CrossRef] [PubMed]
- Lim, Y.S.; Kim, W.R. The global impact of hepatic fibrosis and end-stage liver disease. Clin. Liver Dis. 2008, 12, 733–746. [Google Scholar] [CrossRef] [PubMed]
- Bauer, M.; Bauer, I. Heme oxygenase-1: Redox regulation and role in the hepatic response to oxidative stress. Antioxid. Redox Signal. 2002, 4, 749–758. [Google Scholar] [CrossRef] [PubMed]
- Pratt, R.; Lakhani, H.V.; Zehra, M.; Desauguste, R.; Pillai, S.S.; Sodhi, K. Mechanistic Insight of Na/K-ATPase Signaling and HO-1 into Models of Obesity and Nonalcoholic Steatohepatitis. Int. J. Mol. Sci. 2019, 21, 87. [Google Scholar] [CrossRef]
- Barikbin, R.; Neureiter, D.; Wirth, J.; Erhardt, A.; Schwinge, D.; Kluwe, J.; Schramm, C.; Tiegs, G.; Sass, G. Induction of heme oxygenase 1 prevents progression of liver fibrosis in Mdr2 knockout mice. Hepatology 2012, 55, 553–562. [Google Scholar] [CrossRef]
- Mebratu, Y.; Tesfaigzi, Y. How ERK1/2 activation controls cell proliferation and cell death: Is subcellular localization the answer? Cell Cycle 2009, 8, 1168–1175. [Google Scholar] [CrossRef]
- Li, Z.; Theus, M.H.; Wei, L. Role of ERK 1/2 signaling in neuronal differentiation of cultured embryonic stem cells. Dev. Growth Differ. 2006, 48, 513–523. [Google Scholar] [CrossRef]
- Traore, K.; Sharma, R.; Thimmulappa, R.K.; Watson, W.H.; Biswal, S.; Trush, M.A. Redox-regulation of Erk1/2-directed phosphatase by reactive oxygen species: Role in signaling TPA-induced growth arrest in ML-1 cells. J. Cell Physiol. 2008, 216, 276–285. [Google Scholar] [CrossRef]
- Chen, K.; Maines, M.D. Nitric oxide induces heme oxygenase-1 via mitogen-activated protein kinases ERK and p38. Cell Mol. Biol. 2000, 46, 609–617. [Google Scholar] [PubMed]
- Wu, C.C.; Hsu, M.C.; Hsieh, C.W.; Lin, J.B.; Lai, P.H.; Wung, B.S. Upregulation of heme oxygenase-1 by Epigallocatechin-3-gallate via the phosphatidylinositol 3-kinase/Akt and ERK pathways. Life Sci. 2006, 78, 2889–2897. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.C.; Lii, C.K.; Lin, A.H.; Yeh, Y.W.; Yao, H.T.; Li, C.C.; Liu, K.L.; Chen, H.W. Induction of glutathione synthesis and heme oxygenase 1 by the flavonoids butein and phloretin is mediated through the ERK/Nrf2 pathway and protects against oxidative stress. Free Radic. Biol. Med. 2011, 51, 2073–2081. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.Q.; Ding, J.; Xiao, Z.H.; Liu, C.M. Puerarin ameliorates carbon tetrachloride-induced oxidative DNA damage and inflammation in mouse kidney through ERK/Nrf2/ARE pathway. Food Chem. Toxicol. 2014, 71, 264–271. [Google Scholar] [CrossRef]
- Feng, J.; Zhang, P.; Chen, X.; He, G. PI3K and ERK/Nrf2 pathways are involved in oleanolic acid-induced heme oxygenase-1 expression in rat vascular smooth muscle cells. J. Cell Biochem. 2011, 112, 1524–1531. [Google Scholar] [CrossRef] [PubMed]
- Yarza, R.; Vela, S.; Solas, M.; Ramirez, M.J. c-Jun N-terminal Kinase (JNK) Signaling as a Therapeutic Target for Alzheimer’s Disease. Front. Pharmacol. 2015, 6, 321. [Google Scholar] [CrossRef]
- Kietzmann, T.; Samoylenko, A.; Immenschuh, S. Transcriptional regulation of heme oxygenase-1 gene expression by MAP kinases of the JNK and p38 pathways in primary cultures of rat hepatocytes. J. Biol. Chem. 2003, 278, 17927–17936. [Google Scholar] [CrossRef]
- Xiao, Q.; Piao, R.; Wang, H.; Li, C.; Song, L. Orientin-mediated Nrf2/HO-1 signal alleviates H2O2-induced oxidative damage via induction of JNK and PI3K/AKT activation. Int. J. Biol. Macromol. 2018, 118, 747–755. [Google Scholar] [CrossRef]
- Zhang, H.; Liu, X.; Zhou, S.; Jia, Y.; Li, Y.; Song, Y.; Wang, J.; Wu, H. SP600125 suppresses Keap1 expression and results in NRF2-mediated prevention of diabetic nephropathy. J. Mol. Endocrinol. 2018, 60, 145–157. [Google Scholar] [CrossRef]
- Tsai, K.H.; Wang, W.J.; Lin, C.W.; Pai, P.; Lai, T.Y.; Tsai, C.Y.; Kuo, W.W. NADPH oxidase-derived superoxide anion-induced apoptosis is mediated via the JNK-dependent activation of NF-kappaB in cardiomyocytes exposed to high glucose. J. Cell Physiol. 2012, 227, 1347–1357. [Google Scholar] [CrossRef]
- Guan, J.; Wu, X.; Arons, E.; Christou, H. The p38 mitogen-activated protein kinase pathway is involved in the regulation of heme oxygenase-1 by acidic extracellular pH in aortic smooth muscle cells. J. Cell Biochem. 2008, 105, 1298–1306. [Google Scholar] [CrossRef]
- Carchman, E.H.; Rao, J.; Loughran, P.A.; Rosengart, M.R.; Zuckerbraun, B.S. Heme oxygenase-1-mediated autophagy protects against hepatocyte cell death and hepatic injury from infection/sepsis in mice. Hepatology 2011, 53, 2053–2062. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.E.; Jeong, S.I.; Yang, H.; Park, C.S.; Jin, Y.H.; Park, Y.S. Fisetin induces Nrf2-mediated HO-1 expression through PKC-delta and p38 in human umbilical vein endothelial cells. J. Cell Biochem. 2011, 112, 2352–2360. [Google Scholar] [CrossRef]
- Zhou, M.M.; Zhang, W.Y.; Li, R.J.; Guo, C.; Wei, S.S.; Tian, X.M.; Luo, J.; Kong, L.Y. Anti-inflammatory activity of Khayandirobilide A from Khaya senegalensis via NF-kappaB, AP-1 and p38 MAPK/Nrf2/HO-1 signaling pathways in lipopolysaccharide-stimulated RAW 264.7 and BV-2 cells. Phytomedicine 2018, 42, 152–163. [Google Scholar] [CrossRef]
- Downward, J. Mechanisms and consequences of activation of protein kinase B/Akt. Curr. Opin. Cell Biol. 1998, 10, 262–267. [Google Scholar] [CrossRef]
- Songyang, Z.; Baltimore, D.; Cantley, L.C.; Kaplan, D.R.; Franke, T.F. Interleukin 3-dependent survival by the Akt protein kinase. Proc. Natl. Acad. Sci. USA 1997, 94, 11345–11350. [Google Scholar] [CrossRef]
- Mo, L.; Yang, C.; Gu, M.; Zheng, D.; Lin, L.; Wang, X.; Lan, A.; Hu, F.; Feng, J. PI3K/Akt signaling pathway-induced heme oxygenase-1 upregulation mediates the adaptive cytoprotection of hydrogen peroxide preconditioning against oxidative injury in PC12 cells. Int. J. Mol. Med. 2012, 30, 314–320. [Google Scholar] [CrossRef][Green Version]
- Kwon, M.Y.; Park, E.; Lee, S.J.; Chung, S.W. Heme oxygenase-1 accelerates erastin-induced ferroptotic cell death. Oncotarget 2015, 6, 24393–24403. [Google Scholar] [CrossRef]
- Hassannia, B.; Wiernicki, B.; Ingold, I.; Qu, F.; Van Herck, S.; Tyurina, Y.Y.; Bayir, H.; Abhari, B.A.; Angeli, J.P.F.; Choi, S.M.; et al. Nano-targeted induction of dual ferroptotic mechanisms eradicates high-risk neuroblastoma. J. Clin. Investig. 2018, 128, 3341–3355. [Google Scholar] [CrossRef]
- Chang, L.C.; Chiang, S.K.; Chen, S.E.; Yu, Y.L.; Chou, R.H.; Chang, W.C. Heme oxygenase-1 mediates BAY 11-7085 induced ferroptosis. Cancer Lett. 2018, 416, 124–137. [Google Scholar] [CrossRef]
- Vitek, L.; Schwertner, H.A. The heme catabolic pathway and its protective effects on oxidative stress-mediated diseases. Adv. Clin. Chem. 2007, 43, 1–57. [Google Scholar] [CrossRef] [PubMed]
- Abraham, N.G.; Kappas, A. Pharmacological and clinical aspects of heme oxygenase. Pharmacol. Rev. 2008, 60, 79–127. [Google Scholar] [CrossRef] [PubMed]
- Johnson, J.A.; Johnson, D.A.; Kraft, A.D.; Calkins, M.J.; Jakel, R.J.; Vargas, M.R.; Chen, P.C. The Nrf2-ARE pathway: An indicator and modulator of oxidative stress in neurodegeneration. Ann. N. Y. Acad. Sci. 2008, 1147, 61–69. [Google Scholar] [CrossRef]
- Canning, P.; Sorrell, F.J.; Bullock, A.N. Structural basis of Keap1 interactions with Nrf2. Free Radic. Biol. Med. 2015, 88, 101–107. [Google Scholar] [CrossRef] [PubMed]
- Ishii, T.; Itoh, K.; Takahashi, S.; Sato, H.; Yanagawa, T.; Katoh, Y.; Bannai, S.; Yamamoto, M. Transcription factor Nrf2 coordinately regulates a group of oxidative stress-inducible genes in macrophages. J. Biol. Chem. 2000, 275, 16023–16029. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, P.; Basu, A.; Datta, D.; Gasser, M.; Waaga-Gasser, A.M.; Pal, S. The heme oxygenase-1 protein is overexpressed in human renal cancer cells following activation of the Ras-Raf-ERK pathway and mediates anti-apoptotic signal. J. Biol. Chem. 2011, 286, 33580–33590. [Google Scholar] [CrossRef] [PubMed]
- Wijayanti, N.; Huber, S.; Samoylenko, A.; Kietzmann, T.; Immenschuh, S. Role of NF-kappaB and p38 MAP kinase signaling pathways in the lipopolysaccharide-dependent activation of heme oxygenase-1 gene expression. Antioxid. Redox Signal. 2004, 6, 802–810. [Google Scholar] [CrossRef]
- Guijarro-Munoz, I.; Compte, M.; Alvarez-Cienfuegos, A.; Alvarez-Vallina, L.; Sanz, L. Lipopolysaccharide activates Toll-like receptor 4 (TLR4)-mediated NF-kappaB signaling pathway and proinflammatory response in human pericytes. J. Biol. Chem. 2014, 289, 2457–2468. [Google Scholar] [CrossRef]
- Karki, R.; Igwe, O.J. Toll-like receptor 4-mediated nuclear factor kappa B activation is essential for sensing exogenous oxidants to propagate and maintain oxidative/nitrosative cellular stress. PLoS ONE 2013, 8, e73840. [Google Scholar] [CrossRef]
- Camhi, S.L.; Alam, J.; Otterbein, L.; Sylvester, S.L.; Choi, A.M. Induction of heme oxygenase-1 gene expression by lipopolysaccharide is mediated by AP-1 activation. Am. J. Respir. Cell Mol. Biol. 1995, 13, 387–398. [Google Scholar] [CrossRef]
- Alam, J.; Cook, J.L. Transcriptional regulation of the heme oxygenase-1 gene via the stress response element pathway. Curr. Pharm. Des. 2003, 9, 2499–2511. [Google Scholar] [CrossRef]
- Sun, J.; Hoshino, H.; Takaku, K.; Nakajima, O.; Muto, A.; Suzuki, H.; Tashiro, S.; Takahashi, S.; Shibahara, S.; Alam, J.; et al. Hemoprotein Bach1 regulates enhancer availability of heme oxygenase-1 gene. EMBO J. 2002, 21, 5216–5224. [Google Scholar] [CrossRef]
- Zhang, X.; Guo, J.; Wei, X.; Niu, C.; Jia, M.; Li, Q.; Meng, D. Bach1: Function, Regulation, and Involvement in Disease. Oxid. Med. Cell Longev. 2018, 2018, 1347969. [Google Scholar] [CrossRef]
- Mito, S.; Ozono, R.; Oshima, T.; Yano, Y.; Watari, Y.; Yamamoto, Y.; Brydun, A.; Igarashi, K.; Yoshizumi, M. Myocardial protection against pressure overload in mice lacking Bach1, a transcriptional repressor of heme oxygenase-1. Hypertension 2008, 51, 1570–1577. [Google Scholar] [CrossRef]
- Hama, M.; Kirino, Y.; Takeno, M.; Takase, K.; Miyazaki, T.; Yoshimi, R.; Ueda, A.; Itoh-Nakadai, A.; Muto, A.; Igarashi, K.; et al. Bach1 regulates osteoclastogenesis in a mouse model via both heme oxygenase 1-dependent and heme oxygenase 1-independent pathways. Arthritis Rheum. 2012, 64, 1518–1528. [Google Scholar] [CrossRef] [PubMed]
- Pu, M.; Li, C.; Qi, X.; Chen, J.; Wang, Y.; Gao, L.; Miao, L.; Ren, J. MiR-1254 suppresses HO-1 expression through seed region-dependent silencing and non-seed interaction with TFAP2A transcript to attenuate NSCLC growth. PLoS Genet. 2017, 13, e1006896. [Google Scholar] [CrossRef] [PubMed]
- Beckman, J.D.; Chen, C.; Nguyen, J.; Thayanithy, V.; Subramanian, S.; Steer, C.J.; Vercellotti, G.M. Regulation of heme oxygenase-1 protein expression by miR-377 in combination with miR-217. J. Biol. Chem. 2011, 286, 3194–3202. [Google Scholar] [CrossRef] [PubMed]
- Xiao, S.; Wang, X.; Ni, H.; Li, N.; Zhang, A.; Liu, H.; Pu, F.; Xu, L.; Gao, J.; Zhao, Q.; et al. MicroRNA miR-24-3p promotes porcine reproductive and respiratory syndrome virus replication through suppression of heme oxygenase-1 expression. J. Virol. 2015, 89, 4494–4503. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Shan, Y.; Zheng, J.; Lambrecht, R.W.; Bonkovsky, H.L. Reciprocal effects of micro-RNA-122 on expression of heme oxygenase-1 and hepatitis C virus genes in human hepatocytes. Gastroenterology 2007, 133, 1166–1174. [Google Scholar] [CrossRef]
- Piras, S.; Furfaro, A.L.; Caggiano, R.; Brondolo, L.; Garibaldi, S.; Ivaldo, C.; Marinari, U.M.; Pronzato, M.A.; Faraonio, R.; Nitti, M. microRNA-494 Favors HO-1 Expression in Neuroblastoma Cells Exposed to Oxidative Stress in a Bach1-Independent Way. Front. Oncol. 2018, 8, 199. [Google Scholar] [CrossRef]
- Chiang, S.K.; Chen, S.E.; Chang, L.C. A Dual Role of Heme Oxygenase-1 in Cancer Cells. Int. J. Mol. Sci. 2018, 20, 39. [Google Scholar] [CrossRef] [PubMed]
- Durante, W. Targeting Heme Oxygenase-1 in the Arterial Response to Injury and Disease. Antioxidants 2020, 9, 829. [Google Scholar] [CrossRef] [PubMed]
- Kähkönen, M.P.; Hopia, A.I.; Vuorela, H.J.; Rauha, J.-P.; Pihlaja, K.; Kujala, T.S.; Heinonen, M. Antioxidant Activity of Plant Extracts Containing Phenolic Compounds. J. Agric. Food Chem. 1999, 47, 3954–3962. [Google Scholar] [CrossRef]
- Bendary, E.; Francis, R.R.; Ali, H.M.G.; Sarwat, M.I.; El Hady, S. Antioxidant and structure–activity relationships (SARs) of some phenolic and anilines compounds. Ann. Agric. Sci. 2013, 58, 173–181. [Google Scholar] [CrossRef]
- Li, Y.; Yao, J.; Han, C.; Yang, J.; Chaudhry, M.T.; Wang, S.; Liu, H.; Yin, Y. Quercetin, inflammation and immunity. Nutrients 2016, 8, 167. [Google Scholar] [CrossRef]
- Chen, J.-C.; Ho, F.-M.; Chao, P.-D.L.; Chen, C.-P.; Jeng, K.-C.G.; Hsu, H.-B.; Lee, S.-T.; Wu, W.T.; Lin, W.-W. Inhibition of iNOS gene expression by quercetin is mediated by the inhibition of IκB kinase, nuclear factor-kappa B and STAT1, and depends on heme oxygenase-1 induction in mouse BV-2 microglia. Eur. J. Pharm. 2005, 521, 9–20. [Google Scholar] [CrossRef]
- Lin, H.-C.; Cheng, T.-H.; Chen, Y.-C.; Juan, S.-H. Mechanism of heme oxygenase-1 gene induction by quercetin in rat aortic smooth muscle cells. Pharmacology 2004, 71, 107–112. [Google Scholar] [CrossRef]
- Chow, J.-M.; Shen, S.-C.; Huan, S.K.; Lin, H.-Y.; Chen, Y.-C. Quercetin, but not rutin and quercitrin, prevention of H2O2-induced apoptosis via anti-oxidant activity and heme oxygenase 1 gene expression in macrophages. Biochem. Pharm. 2005, 69, 1839–1851. [Google Scholar] [CrossRef]
- Liu, S.; Hou, W.; Yao, P.; Li, N.; Zhang, B.; Hao, L.; Nuessler, A.K.; Liu, L. Heme oxygenase-1 mediates the protective role of quercetin against ethanol-induced rat hepatocytes oxidative damage. Toxicol. In Vitro 2012, 26, 74–80. [Google Scholar] [CrossRef]
- Yao, P.; Nussler, A.; Liu, L.; Hao, L.; Song, F.; Schirmeier, A.; Nussler, N. Quercetin protects human hepatocytes from ethanol-derived oxidative stress by inducing heme oxygenase-1 via the MAPK/Nrf2 pathways. J. Hepatol. 2007, 47, 253–261. [Google Scholar] [CrossRef]
- Tang, Y.; Gao, C.; Shi, Y.; Zhu, L.; Hu, X.; Wang, D.; Lv, Y.; Yang, X.; Liu, L.; Yao, P. Quercetin attenuates ethanol-derived microsomal oxidative stress: Implication of haem oxygenase-1 induction. Food Chem. 2012, 132, 1769–1774. [Google Scholar] [CrossRef]
- Kim, C.-S.; Choi, H.-S.; Joe, Y.; Chung, H.T.; Yu, R. Induction of heme oxygenase-1 with dietary quercetin reduces obesity-induced hepatic inflammation through macrophage phenotype switching. Nutr. Res. Pract. 2016, 10, 623–628. [Google Scholar] [CrossRef]
- Yang, J.; Kim, C.-S.; Tu, T.H.; Kim, M.-S.; Goto, T.; Kawada, T.; Choi, M.-S.; Park, T.; Sung, M.-K.; Yun, J.W.; et al. Quercetin protects obesity-induced hypothalamic inflammation by reducing microglia-mediated inflammatory responses via HO-1 induction. Nutrients 2017, 9, 650. [Google Scholar] [CrossRef]
- Kim, Y.; Kim, C.-S.; Joe, Y.; Chung, H.T.; Ha, T.Y.; Yu, R. Quercetin Reduces Tumor Necrosis Factor Alpha-Induced Muscle Atrophy by Upregulation of Heme Oxygenase-1. J. Med. Food 2018, 21, 551–559. [Google Scholar] [CrossRef]
- Rayamajhi, N.; Kim, S.-K.; Go, H.; Joe, Y.; Callaway, Z.; Kang, J.-G.; Ryter, S.W.; Chung, H.T. Quercetin induces mitochondrial biogenesis through activation of HO-1 in HepG2 cells. Oxid. Med. Cell. Longev. 2013, 154211. [Google Scholar] [CrossRef]
- Luo, M.; Tian, R.; Yang, Z.; Peng, Y.-Y.; Lu, N. Quercetin suppressed NADPH oxidase-derived oxidative stress via heme oxygenase-1 induction in macrophages. Arch. Biochem. Biophys. 2019, 671, 69–76. [Google Scholar] [CrossRef]
- Tian, R.; Yang, Z.; Lu, N.; Peng, Y.-Y. Quercetin, but not rutin, attenuated hydrogen peroxide-induced cell damage via heme oxygenase-1 induction in endothelial cells. Arch. Biochem. Biophys. 2019. [Google Scholar] [CrossRef]
- Hayashi, Y.; Matsushima, M.; Nakamura, T.; Shibasaki, M.; Hashimoto, N.; Imaizumi, K.; Shimokata, K.; Hasegawa, Y.; Kawabe, T. Quercetin protects against pulmonary oxidant stress via heme oxygenase-1 induction in lung epithelial cells. Biochem. Biophys. Res. Commun. 2012, 417, 169–174. [Google Scholar] [CrossRef]
- Jin, J.Y.; Choi, E.Y.; Park, H.R.; Choi, J.I.; Choi, I.S.; Kim, S.J. Isorhamnetin inhibits Prevotella intermedia lipopolysaccharide-induced production of interleukin-6 in murine macrophages via anti-inflammatory heme oxygenase-1 induction and inhibition of nuclear factor-κB and signal transducer and activator of transcription 1 activation. J. Periodontal Res. 2013, 48, 687–695. [Google Scholar] [CrossRef]
- Luo, Y.; Sun, G.; Dong, X.; Wang, M.; Qin, M.; Yu, Y.; Sun, X. Isorhamnetin attenuates atherosclerosis by inhibiting macrophage apoptosis via PI3K/AKT activation and HO-1 induction. PLoS ONE 2015, 10, e0120259. [Google Scholar] [CrossRef]
- Choi, Y.H. The cytoprotective effect of isorhamnetin against oxidative stress is mediated by the upregulation of the Nrf2-dependent HO-1 expression in C2C12 myoblasts through scavenging reactive oxygen species and ERK inactivation. Gen. Physiol. Biophys. 2016, 35, 145–154. [Google Scholar] [CrossRef] [PubMed]
- Dilshara, M.G.; Jayasooriya, R.G.P.T.; Lee, S.; Choi, Y.H.; Kim, G.-Y. Morin downregulates nitric oxide and prostaglandin E2 production in LPS-stimulated BV2 microglial cells by suppressing NF-κB activity and activating HO-1 induction. Environ. Toxicol. Pharmacol. 2016, 44, 62–68. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Li, Z.; Shen, B.; Zhang, Q.; Feng, H. Protective effects of morin on lipopolysaccharide/D-galactosamine-induced acute liver injury by inhibiting TLR4/NF-κB and activating Nrf2/HO-1 signaling pathways. Int. Immunopharmacol. 2017, 45, 148–155. [Google Scholar] [CrossRef]
- Cho, B.O.; Yin, H.H.; Park, S.H.; Byun, E.B.; Ha, H.Y.; Jang, S.I. Anti-inflammatory activity of myricetin from Diospyros lotus through suppression of NF-κB and STAT1 activation and Nrf2-mediated HO-1 induction in lipopolysaccharide-stimulated RAW264.7 macrophages. Biosci. Biotechnol. Biochem. 2016, 80, 1520–1530. [Google Scholar] [CrossRef]
- Paredes-Gonzalez, X.; Fuentes, F.; Jeffery, S.; Saw, C.L.-L.; Shu, L.; Su, Z.-Y.; Kong, A.-N.T. Induction of NRF2-mediated gene expression by dietary phytochemical flavones apigenin and luteolin. Biopharm. Drug Dispos. 2015, 36, 440–451. [Google Scholar] [CrossRef]
- Park, E.J.; Kim, Y.M.; Kim, H.J.; Chang, K.C. Luteolin activates ERK1/2- and Ca2+-dependent HO-1 induction that reduces LPS-induced HMGB1, iNOS/NO, and COX-2 expression in RAW264.7 cells and mitigates acute lung injury of endotoxin mice. Inflam. Res. 2018, 67, 445–453. [Google Scholar] [CrossRef]
- Liu, C.-W.; Lin, H.-W.; Yang, D.-J.; Chen, S.-Y.; Tseng, J.-K.; Chang, T.-J.; Chang, Y.-Y. Luteolin inhibits viral-induced inflammatory response in RAW264.7 cells via suppression of STAT1/3 dependent NF-κB and activation of HO-1. Free Radical Biol. Med. 2016, 95, 180–189. [Google Scholar] [CrossRef]
- Alekhya Sita, G.J.; Gowthami, M.; Srikanth, G.; Krishna, M.M.; Rama Sireesha, K.; Sajjarao, M.; Nagarjuna, K.; Nagarjuna, M.; Chinnaboina, G.K.; Mishra, A.; et al. Protective role of luteolin against bisphenol A-induced renal toxicity through suppressing oxidative stress, inflammation, and upregulating Nrf2/ARE/HO-1 pathway. IUBMB Life 2019, 71, 1041–1047. [Google Scholar] [CrossRef]
- Hui, K.M.; Wang, X.H.; Xue, H. Interaction of flavones from the roots of Scutellaria baicalensis with the benzodiazepine site. Planta Med. 2000, 66, 91–93. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.-M.; Cheng, P.-Y.; Chim, L.-S.; Kung, C.-W.; Ka, S.-M.; Chung, M.-T.; Sheu, J.-R. Baicalein, an active component of Scutellaria baicalensis Georgi, improves cardiac contractile function in endotoxaemic rats via induction of heme oxygenase-1 and suppression of inflammatory responses. J. Ethnopharmacol. 2011, 135, 179–185. [Google Scholar] [CrossRef]
- Kwak, H.J.; Yang, D.; Hwang, Y.; Jun, H.-S.; Cheon, H.G. Baicalein protects rat insulinoma INS-1 cells from palmitate-induced lipotoxicity by inducing HO-1. PLoS ONE 2017, 12, e0176432. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.-S.; Lii, C.-K.; Lin, A.-H.; Yeh, Y.-W.; Yao, H.-T.; Li, C.-C.; Wang, T.-S.; Chen, H.-W. Protection by chrysin, apigenin, and luteolin against oxidative stress is mediated by the Nrf2-dependent up-regulation of heme oxygenase 1 and glutamate cysteine ligase in rat primary hepatocytes. Arch. Toxicol. 2013, 87, 167–178. [Google Scholar] [CrossRef]
- Namkoong, S.; Sung, J.; Yang, J.; Choi, Y.; Jeong, H.S.; Lee, J. Nobiletin Attenuates the Inflammatory Response Through Heme Oxygenase-1 Induction in the Crosstalk Between Adipocytes and Macrophages. J. Med. Food 2017, 20, 873–881. [Google Scholar] [CrossRef] [PubMed]
- Song, H.J.; Shin, C.Y.; Oh, T.Y.; Sohn, U.D. The protective effect of eupatilin on indomethacin-induced cell damage in cultured feline ileal smooth muscle cells: Involvement of HO-1 and ERK. J. Ethnopharm. 2008, 118, 94–101. [Google Scholar] [CrossRef] [PubMed]
- Kang, C.-H.; Kim, M.J.; Seo, M.J.; Choi, Y.H.; Jo, W.S.; Lee, K.-T.; Jeong, Y.K.; Kim, G.-Y. 5-Hydroxy-3,6,7,8,3’4’-hexamethoxyflavone inhibits nitric oxide production in lipopolysaccharide-stimulated BV2 microglia via NF-κB suppression and Nrf-2-dependent heme oxygenase-1 induction. Food Chem. Toxicol. 2013, 57, 119–125. [Google Scholar] [CrossRef]
- Li, B.; Lee, D.-S.; Jeong, G.-S.; Kim, Y.-C. Involvement of heme oxygenase-1 induction in the cytoprotective and immunomodulatory activities of 6,4’-dihydroxy-7-methoxyflavanone in murine hippocampal and microglia cells. Eur. J. Pharmacol. 2012, 674, 153–162. [Google Scholar] [CrossRef]
- Kou, X.; Shen, K.; An, Y.; Qi, S.; Dai, W.-X.; Yin, Z. Ampelopsin Inhibits H2O2-induced Apoptosis by ERK and Akt Signaling Pathways and Up-regulation of Heme Oxygenase-1. Phytother. Res. 2012, 26, 988–994. [Google Scholar] [CrossRef]
- Fan, R.; Pan, T.; Zhu, A.-L.; Zhang, M.-H. Anti-inflammatory and anti-arthritic properties of naringenin via attenuation of NF-κB and activation of the heme oxygenase (HO)-1/related factor 2 pathway. Pharmacol. Rep. 2017, 69, 1021–1029. [Google Scholar] [CrossRef]
- Li, K.K.; Shen, S.S.; Deng, X.; Shiu, H.T.; Siu, W.S.; Leung, P.C.; Ko, C.H.; Cheng, B.H. Dihydrofisetin exerts its anti-inflammatory effects associated with suppressing ERK/p38 MAPK and Heme Oxygenase-1 activation in lipopolysaccharide-stimulated RAW 264.7 macrophages and carrageenan-induced mice paw edema. Int. Immunopharmacol. 2018, 54, 366–374. [Google Scholar] [CrossRef]
- Kang, T.-H.; Jeong, S.-J.; Ko, W.-G.; Kim, N.-Y.; Lee, B.-H.; Inagaki, M.; Miyamoto, T.; Higuchi, R.; Kim, Y.-C. Cytotoxic lavandulyl flavanones from Sophora f lavescens. J. Nat. Prod. 2000, 63, 680–681. [Google Scholar] [CrossRef]
- Jeong, G.-S.; Li, B.; Lee, D.-S.; Byun, E.; An, R.-B.; Pae, H.-O.; Chung, H.-T.; Youn, K.-H.; Kim, Y.-C. Lavandulyl flavanones from Sophora flavescens protect mouse hippocampal cells against glutamate-induced neurotoxicity via the induction of heme oxygenase-1. Biol. Pharm. Bull. 2008, 31, 1964–1967. [Google Scholar] [CrossRef] [PubMed]
- Guo, C.; Yang, L.; Luo, J.; Zhang, C.; Xia, Y.; Ma, T.; Kong, L. Sophoraflavanone G from Sophora alopecuroides inhibits lipopolysaccharide-induced inflammation in RAW264.7 cells by targeting PI3K/Akt, JAK/STAT and Nrf2/HO-1 pathways. Int. Immunopharmacol. 2016, 38, 349–356. [Google Scholar] [CrossRef] [PubMed]
- Higdon, J.V.; Frei, B. Tea catechins and polyphenols: Health effects, metabolism, and antioxidant functions. Crit. Rev. Food Sci. Nutr. 2003, 43, 89–143. [Google Scholar] [CrossRef]
- Romeo, L.; Intrieri, M.; D’Agata, V.; Mangano, N.G.; Oriani, G.; Ontario, M.L.; Scapagnini, G. The major green tea polyphenol,(-)-epigallocatechin-3-gallate, induces heme oxygenase in rat neurons and acts as an effective neuroprotective agent against oxidative stress. J. Am. Coll. Nutr. 2009, 28, 492S–499S. [Google Scholar] [CrossRef] [PubMed]
- Saito, K.; Mori, S.; Date, F.; Ono, M. Epigallocatechin gallate inhibits oxidative stress-induced DNA damage and apoptosis in MRL-Faslpr mice with autoimmune sialadenitis via upregulation of heme oxygenase-1 and Bcl-2. Autoimmunity 2014, 47, 13–22. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Lee, J. Effect of (–)-epigallocatechin-3-gallate on anti-inflammatory response via heme oxygenase-1 induction during adipocyte–macrophage interactions. Food Sci. Biotech. 2016, 25, 1767–1773. [Google Scholar] [CrossRef]
- Gao, Z.; Han, Y.; Hu, Y.; Wu, X.; Wang, Y.; Zhang, X.; Fu, J.; Zou, X.; Zhang, J.; Chen, X. Targeting HO-1 by epigallocatechin-3-gallate reduces contrast-induced renal injury via anti-oxidative stress and anti-inflammation pathways. PLoS ONE 2016, 11, e0149032. [Google Scholar] [CrossRef]
- Pullikotil, P.; Chen, H.; Muniyappa, R.; Greenberg, C.C.; Yang, S.; Reiter, C.E.; Lee, J.-W.; Chung, J.H.; Quon, M.J. Epigallocatechin gallate induces expression of heme oxygenase-1 in endothelial cells via p38 MAPK and Nrf-2 that suppresses proinflammatory actions of TNF-α. J. Nutr. Biochem. 2012, 23, 1134–1145. [Google Scholar] [CrossRef]
- Di Carlo, G.; Mascolo, N.; Izzo, A.A.; Capasso, F. Flavonoids: Old and new aspects of a class of natural therapeutic drugs. Life Sci. 1999, 65, 337–353. [Google Scholar] [CrossRef]
- Nowakowska, Z. A review of anti-infective and anti-inflammatory chalcones. Eur. J. Med. Chem. 2007, 42, 125–137. [Google Scholar] [CrossRef]
- Lee, D.-S.; Li, B.; Kim, K.-S.; Jeong, G.-S.; Kim, E.-C.; Kim, Y.-C. Butein protects human dental pulp cells from hydrogen peroxide-induced oxidative toxicity via Nrf2 pathway-dependent heme oxygenase-1 expressions. Toxicol. In Vitro 2013, 27, 874–881. [Google Scholar] [CrossRef]
- Wang, Z.; Ka, S.-O.; Lee, Y.; Park, B.-H.; Bae, E.J. Butein induction of HO-1 by p38 MAPK/Nrf2 pathway in adipocytes attenuates high-fat diet induced adipose hypertrophy in mice. Eur. J. Pharm. 2017, 799, 201–210. [Google Scholar] [CrossRef]
- Shen, J.; Zhang, H.; Lin, H.; Su, H.; Xing, D.; Du, L. Brazilein protects the brain against focal cerebral ischemia reperfusion injury correlating to inflammatory response suppression. Eur. J. Pharm. 2007, 558, 88–95. [Google Scholar] [CrossRef]
- Yodsaoue, O.; Cheenpracha, S.; Karalai, C.; Ponglimanont, C.; Tewtrakul, S. Anti-allergic activity of principles from the roots and heartwood of caesalpinia sappan on antigen-induced β-hexosaminidase release. Phytother. Res. Int. J. Devoted Pharmacol. Toxicol. Eval. Nat. Prod. Deriv. 2009, 23, 1028–1031. [Google Scholar] [CrossRef]
- Kim, J.-H.; Choo, Y.-Y.; Tae, N.; Min, B.-S.; Lee, J.-H. The anti-inflammatory effect of 3-deoxysappanchalcone is mediated by inducing heme oxygenase-1 via activating the AKT/mTOR pathway in murine macrophages. Int. Immunopharmacol. 2014, 22, 420–426. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.-M.; Jeong, G.-S.; Lim, H.-D.; An, R.-B.; Kim, Y.-C.; Kim, E.-C. Isoliquiritigenin 2′-methyl ether induces growth inhibition and apoptosis in oral cancer cells via heme oxygenase-1. Toxicol. In Vitro 2010, 24, 776–782. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.-S.; Li, B.; Im, N.-K.; Kim, Y.-C.; Jeong, G.-S. 4, 2′, 5′-Trihydroxy-4′-methoxychalcone from Dalbergia odorifera exhibits anti-inflammatory properties by inducing heme oxygenase-1 in murine macrophages. Int. Immunopharmacol. 2013, 16, 114–121. [Google Scholar] [CrossRef]
- Kil, J.-S.; Son, Y.; Cheong, Y.-K.; Kim, N.-H.; Jeong, H.J.; Kwon, J.-W.; Lee, E.-J.; Kwon, T.-O.; Chung, H.-T.; Pae, H.-O. Okanin, a chalcone found in the genus Bidens, and 3-penten-2-one inhibit inducible nitric oxide synthase expression via heme oxygenase-1 induction in RAW264. 7 macrophages activated with lipopolysaccharide. J. Clin. Biochem. Nutr. 2011. [Google Scholar] [CrossRef]
- Li, Y.-Y.; Huang, S.-S.; Lee, M.-M.; Deng, J.-S.; Huang, G.-J. Anti-inflammatory activities of cardamonin from Alpinia katsumadai through heme oxygenase-1 induction and inhibition of NF-κB and MAPK signaling pathway in the carrageenan-induced paw edema. Int. Immunopharmacol. 2015, 25, 332–339. [Google Scholar] [CrossRef]
- Nikolic, D.; B Van Breemen, R. Analytical methods for quantitation of prenylated flavonoids from hops. Curr. Anal. Chem. 2013, 9, 71–85. [Google Scholar] [CrossRef]
- Lee, I.-S.; Lim, J.; Gal, J.; Kang, J.C.; Kim, H.J.; Kang, B.Y.; Choi, H.J. Anti-inflammatory activity of xanthohumol involves heme oxygenase-1 induction via NRF2-ARE signaling in microglial BV2 cells. Neurochem. Int. 2011, 58, 153–160. [Google Scholar] [CrossRef]
- Choi, B.-M.; Oh, G.-S.; Lee, J.W.; Mok, J.Y.; Kim, D.K.; Jeong, S.-I.; Jang, S.I. Prenylated chalcone from Sophora flavescens suppresses Th2 chemokine expression induced by cytokines via heme oxygenase-1 in human keratinocytes. Arch. Pharmacal Res. 2010, 33, 753–760. [Google Scholar] [CrossRef]
- Su, X.; Huang, Q.; Chen, J.; Wang, M.; Pan, H.; Wang, R.; Zhou, H.; Zhou, Z.; Liu, J.; Yang, F. Calycosin suppresses expression of pro-inflammatory cytokines via the activation of p62/Nrf2-linked heme oxygenase 1 in rheumatoid arthritis synovial fibroblasts. Pharmacol. Res. 2016, 113, 695–704. [Google Scholar] [CrossRef]
- Meng, X.-h.; Ni, C.; Zhu, L.; Shen, Y.-l.; Wang, L.-l.; Chen, Y. Puerarin protects against high glucose-induced acute vascular dysfunction: Role of heme oxygenase-1 in rat thoracic aorta. Vasc. Pharmacol. 2009, 50, 110–115. [Google Scholar] [CrossRef] [PubMed]
- Hwang, Y.P.; Jeong, H.G. Mechanism of phytoestrogen puerarin-mediated cytoprotection following oxidative injury: Estrogen receptor-dependent up-regulation of PI3K/Akt and HO-1. Toxicol. Appl. Pharmacol. 2008, 233, 371–381. [Google Scholar] [CrossRef]
- Lestari, M.L.; Indrayanto, G. Curcumin. In Profiles of Drug Substances, Excipients and Related Methodology; Elsevier: Amsterdam, The Netherlands, 2014; Volume 39, pp. 113–204. [Google Scholar]
- Reddy, R.C.; Vatsala, P.G.; Keshamouni, V.G.; Padmanaban, G.; Rangarajan, P.N. Curcumin for malaria therapy. Biochem. Biophys. Res. Commun. 2005, 326, 472–474. [Google Scholar] [CrossRef]
- Mahady, G.; Pendland, S.; Yun, G.; Lu, Z. Turmeric (Curcuma longa) and curcumin inhibit the growth of Helicobacter pylori, a group 1 carcinogen. Anticancer Res. 2002, 22, 4179–4181. [Google Scholar]
- Terry, C.M.; Clikeman, J.A.; Hoidal, J.R.; Callahan, K.S. Effect of tumor necrosis factor-α and interleukin-1α on heme oxygenase-1 expression in human endothelial cells. Am. J. Physiol. Heart Circ. Physiol. 1998, 274, H883–H891. [Google Scholar] [CrossRef]
- Motterlini, R.; Foresti, R.; Bassi, R.; Green, C.J. Curcumin, an antioxidant and anti-inflammatory agent, induces heme oxygenase-1 and protects endothelial cells against oxidative stress. Free Radic. Biol. Med. 2000, 28, 1303–1312. [Google Scholar] [CrossRef]
- Hill-Kapturczak, N.; Thamilselvan, V.; Liu, F.; Nick, H.S.; Agarwal, A. Mechanism of heme oxygenase-1 gene induction by curcumin in human renal proximal tubule cells. Am. J. Physiol. Ren. Physiol. 2001. [Google Scholar] [CrossRef]
- Balogun, E.; Hoque, M.; Gong, P.; Killeen, E.; Green, C.J.; Foresti, R.; Alam, J.; Motterlini, R. Curcumin activates the haem oxygenase-1 gene via regulation of Nrf2 and the antioxidant-responsive element. Biochem. J. 2003, 371, 887–895. [Google Scholar] [CrossRef]
- McNally, S.J.; Harrison, E.M.; Ross, J.A.; Garden, O.J.; Wigmore, S.J. Curcumin induces heme oxygenase 1 through generation of reactive oxygen species, p38 activation and phosphatase inhibition. Int. J. Mol. Med. 2007, 19, 165–172. [Google Scholar] [CrossRef]
- Aggarwal, B.B.; Sundaram, C.; Malani, N.; Ichikawa, H. Curcumin: The Indian solid gold. In The Molecular Targets and Therapeutic Uses of Curcumin in Health and Disease; Springer: Berlin/Heidelberg, Germany, 2007; pp. 1–75. [Google Scholar]
- Corson, T.W.; Crews, C.M. Molecular understanding and modern application of traditional medicines: Triumphs and trials. Cell 2007, 130, 769–774. [Google Scholar] [CrossRef]
- Selvam, R.; Subramanian, L.; Gayathri, R.; Angayarkanni, N. The anti-oxidant activity of turmeric (Curcuma longa). J. Ethnopharmacol. 1995, 47, 59–67. [Google Scholar] [CrossRef]
- Bao, W.; Li, K.; Rong, S.; Yao, P.; Hao, L.; Ying, C.; Zhang, X.; Nussler, A.; Liu, L. Curcumin alleviates ethanol-induced hepatocytes oxidative damage involving heme oxygenase-1 induction. J. Ethnopharmacol. 2010, 128, 549–553. [Google Scholar] [CrossRef]
- Chen, M.-H.; Lee, M.-Y.; Chuang, J.-J.; Li, Y.-Z.; Ning, S.-T.; Chen, J.-C.; Liu, Y.-W. Curcumin inhibits HCV replication by induction of heme oxygenase-1 and suppression of AKT. Int. J. Mol. Med. 2012, 30, 1021–1028. [Google Scholar] [CrossRef]
- Woo, J.M.; Shin, D.-Y.; Lee, S.J.; Joe, Y.; Zheng, M.; Yim, J.H.; Callaway, Z.; Chung, H.T. Curcumin protects retinal pigment epithelial cells against oxidative stress via induction of heme oxygenase-1 expression and reduction of reactive oxygen. Mol. Vis. 2012, 18, 901. [Google Scholar]
- Chen, D.; Wu, C.; Qiu, Y.-b.; Chu, Q.; Sun, X.-q.; Wang, X.; Chen, J.-l.; Lu, M.-d.; Chen, D.-z.; Pang, Q.-f. Curcumin ameliorates hepatic chronic inflammation induced by bile duct obstruction in mice through the activation of heme oxygenase-1. Int. Immunopharmacol. 2020, 78. [Google Scholar] [CrossRef]
- Arslan, E.; Orzan, E.; Santarelli, R. Global Problem of Drug-Induced Hearing Loss. Ann. N. Y. Acad. Sci. 1999, 884, 1–14. [Google Scholar] [CrossRef]
- Fetoni, A.R.; Eramo, S.L.; Paciello, F.; Rolesi, R.; Podda, M.V.; Troiani, D.; Paludetti, G. Curcuma longa (curcumin) decreases in vivo cisplatin-induced ototoxicity through heme oxygenase-1 induction. Otol. Neurotol. 2014, 35, e169–e177. [Google Scholar] [CrossRef]
- Zheng, K.-m.; Zhang, J.; Zhang, C.-l.; Zhang, Y.-w.; Chen, X.-c. Curcumin inhibits appoptosin-induced apoptosis via upregulating heme oxygenase-1 expression in SH-SY5Y cells. Acta Pharm. Sin. 2015, 36, 544–552. [Google Scholar] [CrossRef] [PubMed]
- Priyadarsini, K.I.; Maity, D.K.; Naik, G.; Kumar, M.S.; Unnikrishnan, M.; Satav, J.; Mohan, H. Role of phenolic OH and methylene hydrogen on the free radical reactions and antioxidant activity of curcumin. Free Radic. Biol. Med. 2003, 35, 475–484. [Google Scholar] [CrossRef]
- Juan, S.-H.; Cheng, T.-H.; Lin, H.-C.; Chu, Y.-L.; Lee, W.-S. Mechanism of concentration-dependent induction of heme oxygenase-1 by resveratrol in human aortic smooth muscle cells. Biochem. Pharmacol. 2005, 69, 41–48. [Google Scholar] [CrossRef]
- Lee, D.-S.; Cha, B.-Y.; Woo, J.-T.; Kim, Y.-C.; Jang, J.-H. Acerogenin a from Acer nikoense maxim prevents oxidative stress-induced neuronal cell death through Nrf2-mediated heme oxygenase-1 expression in mouse hippocampal HT22 cell line. Molecules 2015, 20, 12545–12557. [Google Scholar] [CrossRef] [PubMed]
- Bankova, V. Chemical diversity of propolis makes it a valuable source of new biologically active compounds. J. Apiproduct Apimedical Sci. 2009, 1, 23–28. [Google Scholar] [CrossRef]
- Murtaza, G.; Karim, S.; Akram, M.R.; Khan, S.A.; Azhar, S.; Mumtaz, A.; Bin Asad, M.H.H. Caffeic acid phenethyl ester and therapeutic potentials. Biomed Res. Int. 2014, 2014. [Google Scholar] [CrossRef] [PubMed]
- Scapagnini, G.; Foresti, R.; Calabrese, V.; Stella, A.G.; Green, C.; Motterlini, R. Caffeic acid phenethyl ester and curcumin: A novel class of heme oxygenase-1 inducers. Mol. Pharmacol. 2002, 61, 554–561. [Google Scholar] [CrossRef]
- Wang, X.; Stavchansky, S.; Zhao, B.; Bynum, J.A.; Kerwin, S.M.; Bowman, P.D. Cytoprotection of human endothelial cells from menadione cytotoxicity by caffeic acid phenethyl ester: The role of heme oxygenase-1. Eur. J. Pharmacol. 2008, 591, 28–35. [Google Scholar] [CrossRef]
- Morroni, F.; Sita, G.; Graziosi, A.; Turrini, E.; Fimognari, C.; Tarozzi, A.; Hrelia, P. Neuroprotective effect of caffeic acid phenethyl ester in a mouse model of Alzheimer’s disease involves Nrf2/HO-1 pathway. Aging Dis. 2018, 9, 605. [Google Scholar] [CrossRef]
- Kurauchi, Y.; Hisatsune, A.; Isohama, Y.; Mishima, S.; Katsuki, H. Caffeic acid phenethyl ester protects nigral dopaminergic neurons via dual mechanisms involving haem oxygenase-1 and brain-derived neurotrophic factor. Br. J. Pharmacol. 2012, 166, 1151–1168. [Google Scholar] [CrossRef]
- Sorrenti, V.; Raffaele, M.; Vanella, L.; Acquaviva, R.; Salerno, L.; Pittalà, V.; Intagliata, S.; Di Giacomo, C. Protective effects of caffeic acid phenethyl ester (CAPE) and novel cape analogue as inducers of heme oxygenase-1 in streptozotocin-induced type 1 diabetic rats. Int. J. Mol. Sci. 2019, 20, 2441. [Google Scholar] [CrossRef] [PubMed]
- Pittala, V.; Vanella, L.; Salerno, L.; Di Giacomo, C.; Acquaviva, R.; Raffaele, M.; Romeo, G.; N Modica, M.; Prezzavento, O.; Sorrenti, V. Novel caffeic acid phenethyl ester (Cape) analogues as inducers of heme oxygenase-1. Curr. Pharm. Des. 2017, 23, 2657–2664. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Stavchansky, S.; Kerwin, S.M.; Bowman, P.D. Structure–activity relationships in the cytoprotective effect of caffeic acid phenethyl ester (CAPE) and fluorinated derivatives: Effects on heme oxygenase-1 induction and antioxidant activities. Eur. J. Pharmacol. 2010, 635, 16–22. [Google Scholar] [CrossRef]
- Kweon, M.-H.; Jung, M.-J.; Sung, H.-C. Cytoprotective effects of heme oxygenase-1 induction by 3-O-caffeoyl-1-methylquinic acid. Free Radic. Biol. Med. 2004, 36, 40–52. [Google Scholar] [CrossRef]
- Jin, C.H.; Yang, H.S.; Choi, D.S.; Byun, M.W.; Kim, W.G.; Jeong, I.Y. Rosmarinic acid attenuated SIN-1-induced cytotoxicity in HepG2 cells through the HO-1 induction and radical scavenging activity. Food Sci. Biotechnol. 2013, 22, 549–556. [Google Scholar] [CrossRef]
- So, Y.; Lee, S.Y.; Han, A.-R.; Kim, J.-B.; Jeong, H.G.; Jin, C.H. Rosmarinic acid methyl ester inhibits LPS-induced NO production via suppression of MyD88-dependent and-independent pathways and induction of HO-1 in RAW 264.7 cells. Molecules 2016, 21, 1083. [Google Scholar] [CrossRef]
- Joe, Y.; Zheng, M.; Kim, H.J.; Kim, S.; Uddin, M.J.; Park, C.; Ryu, D.G.; Kang, S.S.; Ryoo, S.; Ryter, S.W.; et al. Salvianolic Acid B Exerts Vasoprotective Effects through the Modulation of Heme Oxygenase-1 and Arginase Activities. J. Pharmacol. Exp. Ther. 2012, 341, 850–858. [Google Scholar] [CrossRef]
- Hwa, J.S.; Jin, Y.C.; Lee, Y.S.; Ko, Y.S.; Kim, Y.M.; Shi, L.Y.; Kim, H.J.; Lee, J.H.; Ngoc, T.M.; Bae, K.H.; et al. 2-Methoxycinnamaldehyde from Cinnamomum cassia reduces rat myocardial ischemia and reperfusion injury in vivo due to HO-1 induction. J. Ethnopharmacol. 2012, 139, 605–615. [Google Scholar] [CrossRef]
- Martínez, L.; Ros, G.; Nieto, G. Hydroxytyrosol: Health Benefits and Use as Functional Ingredient in Meat. Medicines 2018, 5, 13. [Google Scholar] [CrossRef]
- Zrelli, H.; Matsuoka, M.; Kitazaki, S.; Araki, M.; Kusunoki, M.; Zarrouk, M.; Miyazaki, H. Hydroxytyrosol Induces Proliferation and Cytoprotection against Oxidative Injury in Vascular Endothelial Cells: Role of Nrf2 Activation and HO-1 Induction. J. Agric. Food Chem. 2011, 59, 4473–4482. [Google Scholar] [CrossRef]
- Zrelli, H.; Kusunoki, M.; Miyazaki, H. Role of Hydroxytyrosol-dependent Regulation of HO-1 Expression in Promoting Wound Healing of Vascular Endothelial Cells via Nrf2 De Novo Synthesis and Stabilization. Phytother. Res. 2015, 29, 1011–1018. [Google Scholar] [CrossRef]
- Sgarbossa, A.; Dal Bosco, M.; Pressi, G.; Cuzzocrea, S.; Dal Toso, R.; Menegazzi, M. Phenylpropanoid glycosides from plant cell cultures induce heme oxygenase 1 gene expression in a human keratinocyte cell line by affecting the balance of NRF2 and BACH1 transcription factors. Chem. Biol. Interact. 2012, 199, 87–95. [Google Scholar] [CrossRef]
- Das, A.B.; Goud, V.V.; Das, C. 9—Phenolic Compounds as Functional Ingredients in Beverages. In Value-Added Ingredients and Enrichments of Beverages; Grumezescu, A.M., Holban, A.M., Eds.; Academic Press: Cambridge, MA, USA, 2019; pp. 285–323. [Google Scholar] [CrossRef]
- Hisamoto, M.; Kikuzaki, H.; Nakatani, N. Constituents of the leaves of Peucedanum japonicum Thunb. and their biological activity. J. Agric. Food Chem. 2004, 52, 445–450. [Google Scholar] [CrossRef]
- Hisamoto, M.; Kikuzaki, H.; Ohigashi, H.; Nakatani, N. Antioxidant compounds from the leaves of Peucedanum japonicum Thunb. J. Agric. Food Chem. 2003, 51, 5255–5261. [Google Scholar] [CrossRef]
- Taira, J.; Ogi, T. Induction of antioxidant protein HO-1 through Nrf2-ARE signaling due to pteryxin in Peucedanum japonicum Thunb in RAW264. 7 macrophage cells. Antioxidants 2019, 8, 621. [Google Scholar] [CrossRef]
- Savouret, J.F.; Quesne, M. Resveratrol and cancer: A review. Biomed. Pharmacother. 2002, 56, 84–87. [Google Scholar] [CrossRef]
- Orallo, F. Comparative studies of the antioxidant effects of cis-and trans-resveratrol. Curr. Med. Chem. 2006, 13, 87–98. [Google Scholar] [CrossRef]
- Wadsworth, T.L.; Koop, D.R. Effects of the wine polyphenolics quercetin and resveratrol on pro-inflammatory cytokine expression in RAW 264.7 macrophages. Biochem. Pharmacol. 1999, 57, 941–949. [Google Scholar] [CrossRef]
- Frankel, D.; Mehindate, K.; Schipper, H.M. Role of heme oxygenase-1 in the regulation of manganese superoxide dismutase gene expression in oxidatively-challenged astroglia. J. Cell. Physiol. 2000, 185, 80–86. [Google Scholar] [CrossRef]
- Kim, J.W.; Lim, S.C.; Lee, M.Y.; Lee, J.W.; Oh, W.K.; Kim, S.K.; Kang, K.W. Inhibition of neointimal formation by trans-resveratrol: Role of phosphatidyl inositol 3-kinase-dependent Nrf2 activation in heme oxygenase-1 induction. Mol. Nutr. Food Res. 2010, 54, 1497–1505. [Google Scholar] [CrossRef]
- Zhuang, H.; KIM, Y.S.; Koehler, R.C.; Doré, S. Potential mechanism by which resveratrol, a red wine constituent, protects neurons. Ann. N. Y. Acad. Sci. 2003, 993, 276–286. [Google Scholar] [CrossRef] [PubMed]
- Lin, T.-K.; Chen, S.-D.; Chuang, Y.-C.; Lin, H.-Y.; Huang, C.-R.; Chuang, J.-H.; Wang, P.-W.; Huang, S.-T.; Tiao, M.-M.; Chen, J.-B. Resveratrol partially prevents rotenone-induced neurotoxicity in dopaminergic SH-SY5Y cells through induction of heme oxygenase-1 dependent autophagy. Int. J. Mol. Sci. 2014, 15, 1625–1646. [Google Scholar] [CrossRef] [PubMed]
- Gu, J.; Song, Z.-p.; Gui, D.-m.; Hu, W.; Chen, Y.-g.; Zhang, D.-d. Resveratrol attenuates doxorubicin-induced cardiomyocyte apoptosis in lymphoma nude mice by heme oxygenase-1 induction. Cardiovasc. Toxicol. 2012, 12, 341–349. [Google Scholar] [CrossRef]
- Wung, B.-S.; Hsu, M.-C.; Wu, C.-C.; Hsieh, C.-W. Piceatannol upregulates endothelial heme oxygenase-1 expression via novel protein kinase C and tyrosine kinase pathways. Pharmacol. Res. 2006, 53, 113–122. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Ko, W.; Chowdhury, A.; Li, B.; Kim, S.C.; Oh, H.; Kim, Y.C.; Woo, E.R.; Baek, N.I.; Lee, D.S. Brassicaphenanthrene A from Brassica rapa protects HT22 neuronal cells through the regulation of Nrf2-mediated heme oxygenase-1 expression. Mol. Med. Rep. 2020, 21, 493–500. [Google Scholar] [CrossRef]
- Lee, W.; Yang, S.; Lee, C.; Park, E.K.; Kim, K.-M.; Ku, S.-K.; Bae, J.-S. Aloin reduces inflammatory gene iNOS via inhibition activity and p-STAT-1 and NF-κB. Food Chem. Toxicol. 2019, 126, 67–71. [Google Scholar] [CrossRef]
- Shie, P.-H.; Wang, S.-Y.; Lay, H.-L.; Huang, G.-J. 4, 7-Dimethoxy-5-methyl-1, 3-benzodioxole from Antrodia camphorata inhibits LPS-induced inflammation via suppression of NF-κB and induction HO-1 in RAW264. 7 cells. Int. Immunopharmacol. 2016, 31, 186–194. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.-C.; Lee, D.-S.; Ko, W.; Kim, K.-W.; Kim, H.J.; Yoon, C.-S.; Oh, H.; Kim, Y.-C. Heme Oxygenase-1-Inducing Activity of 4-Methoxydalbergione and 4′-Hydroxy-4-methoxydalbergione from Dalbergia odorifera and Their Anti-inflammatory and Cytoprotective Effects in Murine Hippocampal and BV2 Microglial Cell Line and Primary Rat Microglial Cells. Neurotox. Res. 2018, 33, 337–352. [Google Scholar]
- Ye, M.; Zhang, L.; Yan, Y.; Lin, H. Punicalagin protects H9c2 cardiomyocytes from doxorubicin-induced toxicity through activation of Nrf2/HO-1 signaling. Biosci. Rep. 2019, 39. [Google Scholar] [CrossRef]
- Clementi, M.E.; Sampaolese, B.; Sciandra, F.; Tringali, G. Punicalagin Protects Human Retinal Pigment Epithelium Cells from Ultraviolet Radiation-Induced Oxidative Damage by Activating Nrf2/HO-1 Signaling Pathway and Reducing Apoptosis. Antioxidants 2020, 9, 473. [Google Scholar] [CrossRef]
- Chen, H.; Li, H.; Cao, F.; Zhen, L.; Bai, J.; Yuan, S.; Mei, Y. 1,2,3,4,6-penta-O-galloyl-β-D-glucose protects PC12 Cells from MPP+-mediated cell death by inducing heme oxygenase-1 in an ERK- and Akt-dependent manner. J. Huazhong Univ. Sci. Technol. Med. Sci. 2012, 32, 737–745. [Google Scholar] [CrossRef]
- Gschwendt, M.; Muller, H.; Kielbassa, K.; Zang, R.; Kittstein, W.; Rincke, G.; Marks, F. Rottlerin, a novel protein kinase inhibitor. Biochem. Biophys. Res. Commun. 1994, 199, 93–98. [Google Scholar] [CrossRef]
- Park, E.J.; Lim, J.H.; Nam, S.I.; Park, J.W.; Kwon, T.K. Rottlerin induces heme oxygenase-1 (HO-1) up-regulation through reactive oxygen species (ROS) dependent and PKC δ-independent pathway in human colon cancer HT29 cells. Biochimie 2010, 92, 110–115. [Google Scholar] [CrossRef]
- Chen, L.; Teng, H.; Zhang, K.Y.; Skalicka-Wozniak, K.; Georgiev, M.I.; Xiao, J. Agrimonolide and desmethylagrimonolide induced HO-1 expression in HepG2 cells through Nrf2-transduction and p38 inactivation. Front. Pharmacol. 2016, 7, 513. [Google Scholar] [CrossRef]
- Chen, W.-C.; Wang, S.-Y.; Chiu, C.-C.; Tseng, C.-K.; Lin, C.-K.; Wang, H.-C.; Lee, J.-C. Lucidone suppresses hepatitis C virus replication by Nrf2-mediated heme oxygenase-1 induction. Antimicrob. Agents Chemother. 2013, 57, 1180–1191. [Google Scholar] [CrossRef]
- Kim, K.C.; Kang, K.A.; Zhang, R.; Piao, M.J.; Kim, G.Y.; Kang, M.Y.; Lee, S.J.; Lee, N.H.; Surh, Y.-J.; Hyun, J.W. Up-regulation of Nrf2-mediated heme oxygenase-1 expression by eckol, a phlorotannin compound, through activation of Erk and PI3K/Akt. Int. J. Biochem. Cell Biol. 2010, 42, 297–305. [Google Scholar] [CrossRef]
- Lee, S.; Seo, J.; Ryoo, S.; Cuong, T.D.; Min, B.-S.; Lee, J.-H. Malabaricone C inhibits PDGF-induced proliferation and migration of aortic smooth muscle cells through induction of heme oxygenase-1. J. Cell. Biochem. 2012, 113, 2866–2876. [Google Scholar] [CrossRef]
- Jiang, G.; Hu, Y.; Liu, L.; Cai, J.; Peng, C.; Li, Q. Gastrodin protects against MPP+-induced oxidative stress by up regulates heme oxygenase-1 expression through p38 MAPK/Nrf2 pathway in human dopaminergic cells. Neurochem. Int. 2014, 75, 79–88. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Yuan, B.; Huang, H.; Qu, S.; Yang, S.; Zeng, Z. Gastrodin induced HO-1 and Nrf2 up-regulation to alleviate H2O2-induced oxidative stress in mouse liver sinusoidal endothelial cells through p38 MAPK phosphorylation. Braz. J. Med. Biol. Res. 2018, 51, e7439. [Google Scholar] [CrossRef] [PubMed]
- Farkhondeh, T.; Samarghandian, S.; Borji, A. An overview on cardioprotective and anti-diabetic effects of thymoquinone. Asian Pac. J. Trop. Med. 2017, 10, 849–854. [Google Scholar] [CrossRef]
- Kundu, J.; Kim, D.-H.; Kundu, J.K.; Chun, K.-S. Thymoquinone induces heme oxygenase-1 expression in HaCaT cells via Nrf2/ARE activation: Akt and AMPKα as upstream targets. Food Chem. Toxicol. 2014, 65, 18–26. [Google Scholar] [CrossRef] [PubMed]
- Moon, M.K.; Choi, B.-M.; Oh, G.-S.; Pae, H.-O.; Kim, J.-D.; Oh, H.; Oh, C.-S.; Kim, D.-H.; Rho, Y.-D.; Shin, M.-K.; et al. Catalposide protects Neuro 2A cells from hydrogen peroxide-induced cytotoxicity via the expression of heme oxygenase-1. Toxicol. Lett. 2003, 145, 46–54. [Google Scholar] [CrossRef]
- Bae, G.-S.; Kim, D.-G.; Jo, I.-J.; Choi, S.-B.; Kim, M.-J.; Shin, J.Y.; Kim, D.-U.; Song, H.-J.; Joo, M.; Park, S.-J. Heme oxygenase-1 induced by desoxo-narchinol-A attenuated the severity of acute pancreatitis via blockade of neutrophil infiltration. Int. Immunopharmacol. 2019, 69, 225–234. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.-W.; Yoon, C.-S.; Kim, Y.-C.; Oh, H. Desoxo-narchinol A and Narchinol B Isolated from Nardostachys jatamansi Exert Anti-neuroinflammatory Effects by Up-regulating of Nuclear Transcription Factor Erythroid-2-Related Factor 2/Heme Oxygenase-1 Signaling. Neurotoxic. Res. 2019, 35, 230–243. [Google Scholar] [CrossRef]
- Duñg, N.X.; Chińh, T.D.; Rañg, D.D.; Leclercq, P.A. The constituents of the rhizome oil of Zingiber zerumbet (L.) Sm. from Vietnam. J. Essent. Oil Res. 1993, 5, 553–555. [Google Scholar] [CrossRef]
- Leung, W.-S.; Yang, M.-L.; Lee, S.-S.; Kuo, C.-W.; Ho, Y.-C.; Huang-Liu, R.; Lin, H.-W.; Kuan, Y.-H. Protective effect of zerumbone reduces lipopolysaccharide-induced acute lung injury via antioxidative enzymes and Nrf2/HO-1 pathway. Int. Immunopharmacol. 2017, 46, 194–200. [Google Scholar] [CrossRef]
- Shin, J.-W.; Ohnishi, K.; Murakami, A.; Lee, J.-S.; Kundu, J.K.; Na, H.-K.; Ohigashi, H.; Surh, Y.-J. Zerumbone induces heme oxygenase-1 expression in mouse skin and cultured murine epidermal cells through activation of Nrf2. Cancer Prev. Res. 2011, 4, 860–870. [Google Scholar] [CrossRef]
- Tsoyi, K.; Jang, H.J.; Lee, Y.S.; Kim, Y.M.; Kim, H.J.; Seo, H.G.; Lee, J.H.; Kwak, J.H.; Lee, D.-U.; Chang, K.C. (+)-Nootkatone and (+)-valencene from rhizomes of Cyperus rotundus increase survival rates in septic mice due to heme oxygenase-1 induction. J. Ethnopharmacol. 2011, 137, 1311–1317. [Google Scholar] [CrossRef]
- Lee, D.-S.; Choi, H.-G.; Wan Woo, K.; Kang, D.-G.; Lee, H.-S.; Oh, H.; Ro Lee, K.; Kim, Y.-C. Pulchellamin G, an amino acid-sesquiterpene lactone, from Saussurea pulchella suppresses lipopolysaccharide-induced inflammatory responses via heme oxygenase-1 expression in murine peritoneal macrophages. Eur. J. Pharmacol. 2013, 715, 123–132. [Google Scholar] [CrossRef]
- Jeong, G.-S.; Pae, H.-O.; Jeong, S.-O.; Kim, Y.-C.; Kwon, T.-O.; Lee, H.S.; Kim, N.-S.; Park, S.D.; Chung, H.-T. The α-methylene-γ-butyrolactone moiety in dehydrocostus lactone is responsible for cytoprotective heme oxygenase-1 expression through activation of the nuclear factor E2-related factor 2 in HepG2 cells. Eur. J. Pharmacol. 2007, 565, 37–44. [Google Scholar] [CrossRef]
- Park, E.J.; Park, S.W.; Kim, H.J.; Kwak, J.-H.; Lee, D.-U.; Chang, K.C. Dehydrocostuslactone inhibits LPS-induced inflammation by p38MAPK-dependent induction of hemeoxygenase-1 in vitro and improves survival of mice in CLP-induced sepsis in vivo. Int. Immunopharmacol. 2014, 22, 332–340. [Google Scholar] [CrossRef] [PubMed]
- Kim, N.; Hwangbo, C.; Lee, S.; Lee, J.-H. Eupatolide Inhibits PDGF-induced Proliferation and Migration of Aortic Smooth Muscle Cells Through ROS-dependent Heme Oxygenase-1 Induction. Phytother. Res. 2013, 27, 1700–1707. [Google Scholar] [CrossRef] [PubMed]
- Shen, X.-Y.; Qin, D.-P.; Zhou, H.; Luo, J.-F.; Yao, Y.-D.; Lio, C.-K.; Li, H.-B.; Dai, Y.; Yu, Y.; Yao, X.-S. Nardochinoids a–c, three dimeric sesquiterpenoids with specific fused-ring skeletons from nardostachys chinensis. Org. Lett. 2018, 20, 5813–5816. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.-D.; Shen, X.-Y.; Machado, J.; Luo, J.-F.; Dai, Y.; Lio, C.-K.; Yu, Y.; Xie, Y.; Luo, P.; Liu, J.-X.; et al. Nardochinoid B inhibited the activation of RAW264.7 macrophages stimulated by lipopolysaccharide through activating the Nrf2/HO-1 pathway. Molecules 2019, 24, 2482. [Google Scholar] [CrossRef] [PubMed]
- Muroi, H.; Kubo, I. Antibacterial activity of anacardic acid and totarol, alone and in combination with methicillin, against methicillinresistant Staphylococcus aureus. J. Appl. Bacteriol. 1996, 80, 387–394. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Xu, X.; Chang, S.; Wang, Y.; Xu, Y.; Ran, S.; Huang, Z.; Li, P.; Li, J.; Zhang, L.; et al. Totarol prevents neuronal injury in vitro and ameliorates brain ischemic stroke: Potential roles of Akt activation and HO-1 induction. Toxicol. Appl. Pharmacol. 2015, 289, 142–154. [Google Scholar] [CrossRef]
- Martin, D.; Rojo, A.I.; Salinas, M.; Diaz, R.; Gallardo, G.; Alam, J.; Ruiz de Galarreta, C.M.; Cuadrado, A. Regulation of Heme Oxygenase-1 Expression through the Phosphatidylinositol 3-Kinase/Akt Pathway and the Nrf2 Transcription Factor in Response to the Antioxidant Phytochemical Carnosol. J. Biol. Chem. 2004, 279, 8919–8929. [Google Scholar] [CrossRef]
- Campbell, N.K.; Fitzgerald, H.K.; Fletcher, J.M.; Dunne, A. Plant-derived polyphenols modulate human dendritic cell metabolism and immune function via AMPK-dependent induction of heme oxygenase-1. Front. Immunol. 2019, 10, 345. [Google Scholar] [CrossRef]
- Ha, D.T.; Phuong, T.T.; Oh, J.; Bae, K.H.; Nguyen, D.T.; Na, M. Palbinone from Paeonia suffruticosa Protects Hepatic Cells via Up-regulation of Heme Oxygenase-1. Phytother. Res. 2014, 28, 308–311. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, J.; Jia, W.; Zhao, A.; Li, T. Distinct immunosuppressive effect by Isodon serra extracts. Int. Immunopharmacol. 2005, 5, 1957–1965. [Google Scholar] [CrossRef]
- Hu, A.P.; Du, J.M.; Li, J.Y.; Liu, J.W. Oridonin promotes CD4+/CD25+ Treg differentiation, modulates Th1/Th2 balance and induces HO-1 in rat splenic lymphocytes. Inflamm. Res. 2008, 57, 163–170. [Google Scholar] [CrossRef] [PubMed]
- Mishra, S.K.; Sangwan, N.S.; Sangwan, R.S. Phcog Rev.: Plant Review Andrographis paniculata (Kalmegh): A Review. Pharmacogn. Rev. 2007, 1, 283–298. [Google Scholar]
- Yu, A.-L.; Lu, C.-Y.; Wang, T.-S.; Tsai, C.-W.; Liu, K.-L.; Cheng, Y.-P.; Chang, H.C.; Lii, C.-K.; Chen, H.-W. Induction of Heme Oxygenase 1 and Inhibition of Tumor Necrosis Factor α-Induced Intercellular Adhesion Molecule Expression by Andrographolide in EA.hy926 Cells. J. Agric. Food Chem. 2010, 58, 7641–7648. [Google Scholar] [CrossRef] [PubMed]
- Uto, T.; Qin, G.-W.; Morinaga, O.; Shoyama, Y. 17-Hydroxy-jolkinolide B, a diterpenoid from Euphorbia fischeriana, inhibits inflammatory mediators but activates heme oxygenase-1 expression in lipopolysaccharide-stimulated murine macrophages. Int. Immunopharmacol. 2012, 12, 101–109. [Google Scholar] [CrossRef]
- Yan, S.C.; Wang, Y.J.; Li, Y.J.; Cai, W.Y.; Weng, X.G.; Li, Q.; Chen, Y.; Yang, Q.; Zhu, X.X. Dihydroartemisinin regulates the Th/Treg balance by inducing activated CD4+ T cell apoptosis via heme oxygenase-1 induction in mouse models of inflammatory bowel disease. Molecules 2019, 24, 2475. [Google Scholar] [CrossRef]
- Wang, Y.; Li, L.; Wang, Y.; Zhu, X.; Jiang, M.; Song, E.; Song, Y. New application of the commercial sweetener rebaudioside a as a hepatoprotective candidate: Induction of the Nrf2 signaling pathway. Eur. J. Pharmacol. 2018, 822, 128–137. [Google Scholar] [CrossRef]
- Setty, A.R.; Sigal, L.H. Herbal medications commonly used in the practice of rheumatology: Mechanisms of action, efficacy, and side effects. Semin. Arthritis Rheum. 2005, 34, 773–784. [Google Scholar] [CrossRef]
- Yu, X.; Tao, W.; Jiang, F.; Li, C.; Lin, J.; Liu, C. Celastrol Attenuates Hypertension-Induced Inflammation and Oxidative Stress in Vascular Smooth Muscle Cells via Induction of Heme Oxygenase-1. Am. J. Hypertens. 2010, 23, 895–903. [Google Scholar] [CrossRef]
- Youn, G.S.; Kwon, D.-J.; Ju, S.M.; Rhim, H.; Bae, Y.S.; Choi, S.Y.; Park, J. Celastrol ameliorates HIV-1 Tat-induced inflammatory responses via NF-kappaB and AP-1 inhibition and heme oxygenase-1 induction in astrocytes. Toxicol. Appl. Pharmacol. 2014, 280, 42–52. [Google Scholar] [CrossRef]
- Tseng, C.-K.; Hsu, S.-P.; Lin, C.-K.; Wu, Y.-H.; Lee, J.-C.; Young, K.-C. Celastrol inhibits hepatitis C virus replication by upregulating heme oxygenase-1 via the JNK MAPK/Nrf2 pathway in human hepatoma cells. Antiviral Res. 2017, 146, 191–200. [Google Scholar] [CrossRef]
- Luo, D.; Guo, Y.; Cheng, Y.; Zhao, J.; Wang, Y.; Rong, J. Natural product celastrol suppressed macrophage M1 polarization against inflammation in diet-induced obese mice via regulating Nrf2/HO-1, MAP kinase and NF-κB pathways. Aging 2017, 9, 2069–2082. [Google Scholar] [CrossRef] [PubMed]
- Jeong, G.-S.; An, R.-B.; Pae, H.-O.; Oh, G.-S.; Chung, H.-T.; Kim, Y.-C. Heme oxygenase-1 inducing constituent of Prunella vulgaris in HepG2 cells. Biol. Pharm. Bull. 2008, 31, 531–533. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Barker, E.C.; Gatbonton-Schwager, T.N.; Han, Y.; Clay, J.E.; Letterio, J.J.; Tochtrop, G.P. Bryonolic Acid: A Large-Scale Isolation and Evaluation of Heme Oxygenase 1 Expression in Activated Macrophages. J. Nat. Prod. 2010, 73, 1064–1068. [Google Scholar] [CrossRef] [PubMed]
- Tung, N.T.; Cuong, T.D.; Hung, T.M.; Lee, J.H.; Woo, M.H.; Choi, J.S.; Kim, J.; Ryu, S.H.; Min, B.S. Inhibitory effect on NO production of triterpenes from the fruiting bodies of Ganoderma lucidum. Bioorg. Med. Chem. Lett. 2013, 23, 1428–1432. [Google Scholar] [CrossRef]
- Choi, S.; Nguyen, V.T.; Tae, N.; Lee, S.; Ryoo, S.; Min, B.-S.; Lee, J.-H. Anti-inflammatory and heme oxygenase-1 inducing activities of lanostane triterpenes isolated from mushroom Ganoderma lucidum in RAW264.7 cells. Toxicol. Appl. Pharmacol. 2014, 280, 434–442. [Google Scholar] [CrossRef]
- Kim, Y.M.; Kim, H.J.; Chang, K.C. Glycyrrhizin reduces HMGB1 secretion in lipopolysaccharide-activated RAW 264.7 cells and endotoxemic mice by p38/Nrf2-dependent induction of HO-1. Int. Immunopharmacol. 2015, 26, 112–118. [Google Scholar] [CrossRef]
- Mou, K.; Pan, W.; Han, D.; Wen, X.; Cao, F.; Miao, Y.; Li, P. Glycyrrhizin protects human melanocytes from H2O2-induced oxidative damage via the Nrf2-dependent induction of HO-1. Int. J. Mol. Med. 2019, 44, 253–261. [Google Scholar] [CrossRef]
- Bian, X.; Liu, X.; Liu, J.; Zhao, Y.; Li, H.; Zhang, L.; Li, P.; Gao, Y. Hepatoprotective effect of chiisanoside from Acanthopanax sessiliflorus against LPS/D-GalN-induced acute liver injury by inhibiting NF-κB and activating Nrf2/HO-1 signaling pathways. J. Sci. Food Agric. 2019, 99, 3283–3290. [Google Scholar] [CrossRef] [PubMed]
- Heyninck, K.; Sabbe, L.; Chirumamilla, C.S.; Szarc vel Szic, K.; Vander Veken, P.; Lemmens, K.J.A.; Lahtela-Kakkonen, M.; Naulaerts, S.; Op de Beeck, K.; Laukens, K.; et al. Withaferin A induces heme oxygenase (HO-1) expression in endothelial cells via activation of the Keap1/Nrf2 pathway. Biochem. Pharmacol. 2016, 109, 48–61. [Google Scholar] [CrossRef] [PubMed]
- Shi, W.; Wang, Y.; Li, J.; Zhang, H.; Ding, L. Investigation of ginsenosides in different parts and ages of Panax ginseng. Food Chem. 2007, 102, 664–668. [Google Scholar] [CrossRef]
- Chang-Xiao, L.; Pei-Gen, X. Recent advances on ginseng research in China. J. Ethnopharmacol. 1992, 36, 27–38. [Google Scholar] [CrossRef]
- Chen, R.J.; Chung, T.-y.; Li, F.-y.; Lin, N.-h.; TzEN, J.T. Effect of sugar positions in ginsenosides and their inhibitory potency on Na+/K+-ATPase activity. Acta Pharmacol. Sin. 2009, 30, 61–69. [Google Scholar] [CrossRef] [PubMed]
- Hwang, Y.P.; Jeong, H.G. Ginsenoside Rb1 protects against 6-hydroxydopamine-induced oxidative stress by increasing heme oxygenase-1 expression through an estrogen receptor-related PI3K/Akt/Nrf2-dependent pathway in human dopaminergic cells. Toxicol. Appl. Pharmacol. 2010, 242, 18–28. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Choi, S.Y.; Kim, K.-T.; Rhee, Y.K.; Hur, J. Ginsenoside Rg18 suppresses lipopolysaccharide-induced neuroinflammation in BV2 microglia and amyloid-β-induced oxidative stress in SH-SY5Y neurons via nuclear factor erythroid 2-related factor 2/heme oxygenase-1 induction. J. Funct. Foods 2017, 31, 71–78. [Google Scholar] [CrossRef]
- Yan, T.; Yu, X.; Sun, X.; Meng, D.; Jia, J.-m. A new steroidal saponin, furotrilliumoside from Trillium tschonoskii inhibits lipopolysaccharide-induced inflammation in Raw264.7 cells by targeting PI3K/Akt, MARK and Nrf2/HO-1 pathways. Fitoterapia 2016, 115, 37–45. [Google Scholar] [CrossRef] [PubMed]
- Higuera-Ciapara, I.; Felix-Valenzuela, L.; Goycoolea, F. Astaxanthin: A review of its chemistry and applications. Crit. Rev. Food Sci. Nutr. 2006, 46, 185–196. [Google Scholar] [CrossRef]
- Ma, H.; Chen, S.; Xiong, H.; Wang, M.; Hang, W.; Zhu, X.; Zheng, Y.; Ge, B.; Li, R.; Cui, H. Astaxanthin from Haematococcus pluvialis ameliorates the chemotherapeutic drug (doxorubicin) induced liver injury through the Keap1/Nrf2/HO-1 pathway in mice. Food Funct. 2020, 11, 4659–4671. [Google Scholar] [CrossRef]
- Yan, N.; Liu, Y.; Gong, D.; Du, Y.; Zhang, H.; Zhang, Z. Solanesol: A review of its resources, derivatives, bioactivities, medicinal applications, and biosynthesis. Phytochem. Rev. 2015, 14, 403–417. [Google Scholar] [CrossRef]
- Yao, X.; Lu, B.; Lu, C.; Bai, Q.; Yan, D.; Wu, Y.; Hong, Z.; Xu, H. Solanesol induces the expression of heme oxygenase-1 via p38 and Akt and suppresses the production of proinflammatory cytokines in RAW264.7 cells. Food Funct. 2017, 8, 132–141. [Google Scholar] [CrossRef]
- Lee, S.E.; Kim, G.-D.; Yang, H.; Son, G.W.; Park, H.R.; Cho, J.-J.; Ahn, H.-J.; Park, C.-S.; Park, Y.S. Effects of Eicosapentaenoic Acid on the Cytoprotection Through Nrf2-Mediated Heme Oxygenase-1 in Human Endothelial Cells. J. Cardiovasc. Pharmacol. 2015, 66, 108–117. [Google Scholar] [CrossRef]
- Cui, Z.-G.; Ogawa, R.; Tsuneyama, K.; Yan, G.; Tao, L.; Shimomura, A.; Inadera, H. Insight into the molecular mechanism of heme oxygenase-1 induction by docosahexaenoic acid in U937 cells. Chem. Biol. Interact. 2015, 238, 180–188. [Google Scholar] [CrossRef] [PubMed]
- Hou, C.-C.; Huang, C.-C.; Shyur, L.-F. Echinacea Alkamides Prevent Lipopolysaccharide/d-Galactosamine- Induced Acute Hepatic Injury through JNK Pathway-Mediated HO-1 Expression. J. Agric. Food Chem. 2011, 59, 11966–11974. [Google Scholar] [CrossRef]
- Park, S.Y.; Seetharaman, R.; Ko, M.J.; Kim, D.Y.; Kim, T.H.; Yoon, M.K.; Kwak, J.H.; Lee, S.J.; Bae, Y.S.; Choi, Y.W. Ethyl linoleate from garlic attenuates lipopolysaccharide-induced pro-inflammatory cytokine production by inducing heme oxygenase-1 in RAW264.7 cells. Int. Immunopharmacol. 2014, 19, 253–261. [Google Scholar] [CrossRef] [PubMed]
- Fukuda, N.; Zhang, L.; Kodama, M.; Sakono, M.; Ide, T.; Yamamoto, K.; Sugano, M. Effect of dietary sesamin on metabolic fate of an exogenous linolelaidic acid in perfused rat liver. J. Nutr. Sci. Vitaminol. 1999, 45, 437–448. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hamada, N.; Tanaka, A.; Fujita, Y.; Itoh, T.; Ono, Y.; Kitagawa, Y.; Tomimori, N.; Kiso, Y.; Akao, Y.; Nozawa, Y.; et al. Involvement of heme oxygenase-1 induction via Nrf2/ARE activation in protection against H2O2-induced PC12 cell death by a metabolite of sesamin contained in sesame seeds. Bioorg. Med. Chem. 2011, 19, 1959–1965. [Google Scholar] [CrossRef]
- Banerjee, P.; Basu, A.; Arbiser, J.L.; Pal, S. The natural product honokiol inhibits calcineurin inhibitor-induced and Ras-mediated tumor promoting pathways. Cancer Lett. 2013, 338, 292–299. [Google Scholar] [CrossRef]
- Lu, S.-H.; Hsu, W.-L.; Chen, T.-H.; Chou, T.-C. Activation of Nrf2/HO-1signaling pathway involves the anti-inflammatory activity of magnolol in Porphyromonas gingivalis lipopolysaccharide-stimulated mouse RAW 264.7 macrophages. Int. Immunopharmacol. 2015, 29, 770–778. [Google Scholar] [CrossRef]
- Sung, S.H.; Huh, M.S.; Kim, Y.C. New tetrahydrofuran-type sesquilignans of Saururus chinensis root. Chem. Pharm. Bull. 2001, 49, 1192–1194. [Google Scholar] [CrossRef]
- Li, B.; Lee, Y.J.; Kim, Y.C.; Yoon, J.J.; Lee, S.M.; Lee, Y.P.; Kang, D.G.; Lee, H.S. Sauchinone from Saururus chinensis protects vascular inflammation by heme oxygenase-1 induction in human umbilical vein endothelial cells. Phytomedicine 2014, 21, 101–108. [Google Scholar] [CrossRef]
- Meng, X.; Kim, I.; Jeong, Y.J.; Cho, Y.M.; Kang, S.C. Anti-inflammatory effects of Saururus chinensis aerial parts in murine macrophages via induction of heme oxygenase-1. Exp. Biol. Med. 2016, 241, 461–701. [Google Scholar] [CrossRef]
- Ryu, H.S.; Lee, H.K.; Kim, J.S.; Kim, Y.G.; Pyo, M.; Yun, J.; Hwang, B.Y.; Hong, J.T.; Kim, Y.; Han, S.-B. Saucerneol D inhibits dendritic cell activation by inducing heme oxygenase-1, but not by directly inhibiting toll-like receptor 4 signaling. J. Ethnopharmacol. 2015, 166, 92–101. [Google Scholar] [CrossRef] [PubMed]
- Willför, S.M.; Ahotupa, M.O.; Hemming, J.E.; Reunanen, M.H.; Eklund, P.C.; Sjöholm, R.E.; Eckerman, C.S.; Pohjamo, S.P.; Holmbom, B.R. Antioxidant activity of knotwood extractives and phenolic compounds of selected tree species. J. Agric. Food Chem. 2003, 51, 7600–7606. [Google Scholar] [CrossRef] [PubMed]
- Bajpai, V.K.; Alam, M.B.; Quan, K.T.; Kwon, K.-R.; Ju, M.-K.; Choi, H.-J.; Lee, J.S.; Yoon, J.-I.; Majumder, R.; Rather, I.A. Antioxidant efficacy and the upregulation of Nrf2-mediated HO-1 expression by (+)-lariciresinol, a lignan isolated from Rubia philippinensis, through the activation of p38. Sci. Rep. 2017, 7, 46035. [Google Scholar] [CrossRef] [PubMed]
- Erbil, Y.; Doğru-Abbasoğlu, S.; Yanık, B.T.; Alış, H.; Olgaç, V.; Toker, G.A. The effect of heme oxygenase-1 induction by glutamine on TNBS-induced colitis. Int. J. Colorectal Dis. 2007, 22, 591–599. [Google Scholar]
- Auld, F.; Maschauer, E.L.; Morrison, I.; Skene, D.J.; Riha, R.L. Evidence for the efficacy of melatonin in the treatment of primary adult sleep disorders. Sleep Med. Rev. 2017, 34, 10–22. [Google Scholar] [CrossRef]
- Reiter, R.J. Pineal melatonin: Cell biology of its synthesis and of its physiological interactions. Endocr. Rev. 1991, 12, 151–180. [Google Scholar] [CrossRef]
- Arnao, M.B.; Hernández-Ruiz, J. Functions of melatonin in plants: A review. J. Pineal Res. 2015, 59, 133–150. [Google Scholar] [CrossRef]
- Butterfield, D.A. β-Amyloid-associated free radical oxidative stress and neurotoxicity: Implications for Alzheimer’s disease. Chem. Res. Toxicol. 1997, 10, 495–506. [Google Scholar] [CrossRef]
- Markesbery, W.R. Oxidative stress hypothesis in Alzheimer’s disease. Free Radic. Biol. Med. 1997, 23, 134–147. [Google Scholar] [CrossRef]
- Clapp-Lilly, K.L.; Smith, M.A.; Perry, G.; Harris, P.L.; Zhu, X.; Duffy, L.K. Melatonin acts as antioxidant and pro-oxidant in an organotypic slice culture model of Alzheimer’s disease. Neuroreport 2001, 12, 1277–1280. [Google Scholar] [CrossRef]
- Kwon, K.J.; Kim, J.N.; Kim, M.K.; Lee, J.; Ignarro, L.J.; Kim, H.J.; Shin, C.Y.; Han, S.H. Melatonin synergistically increases resveratrol-induced heme oxygenase-1 expression through the inhibition of ubiquitin-dependent proteasome pathway: A possible role in neuroprotection. J. Pineal Res. 2011, 50, 110–123. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.W.; Lee, S.M. Melatonin inhibits type 1 interferon signaling of toll-like receptor 4 via heme oxygenase-1 induction in hepatic ischemia/reperfusion. J. Pineal Res. 2012, 53, 67–76. [Google Scholar] [CrossRef] [PubMed]
- Park, J.M.; Han, Y.M.; Kangwan, N.; Lee, S.Y.; Jung, M.K.; Kim, E.H.; Hahm, K.B. S-allyl cysteine alleviates nonsteroidal anti-inflammatory drug-induced gastric mucosal damages by increasing cyclooxygenase-2 inhibition, heme oxygenase-1 induction, and histone deacetylation inhibition. J. Gastroenterol. Hepatol. 2014, 29, 80–92. [Google Scholar] [CrossRef]
- Choi, B.-M.; Kim, S.-M.; Park, T.-K.; Li, G.; Hong, S.-J.; Park, R.; Chung, H.-T.; Kim, B.-R. Piperine protects cisplatin-induced apoptosis via heme oxygenase-1 induction in auditory cells. J. Nutr. Biochem. 2007, 18, 615–622. [Google Scholar] [CrossRef] [PubMed]
- Yamasaki, H. Pharmacology of sinomenine, an anti-rheumatic alkaloid from Sinomenium acutum. Acta Medica Okayama 1976, 30. [Google Scholar]
- Song, S.; Shen, X.; Tang, Y.; Wang, Z.; Guo, W.; Ding, G.; Wang, Q.; Fu, Z. Sinomenine pretreatment attenuates cold ischemia/reperfusion injury in rats: The role of heme oxygenase-1. Int. Immunopharmacol. 2010, 10, 679–684. [Google Scholar] [CrossRef] [PubMed]
- Ha, Y.M.; Kim, M.Y.; Park, M.K.; Lee, Y.S.; Kim, Y.M.; Kim, H.J.; Lee, J.H.; Chang, K.C. Higenamine reduces HMGB1 during hypoxia-induced brain injury by induction of heme oxygenase-1 through PI3K/Akt/Nrf-2 signal pathways. Apoptosis 2012, 17, 463–474. [Google Scholar] [CrossRef]
- Zhang, Z.; Li, M.; Wang, Y.; Wu, J.; Li, J. Higenamine promotes M2 macrophage activation and reduces Hmgb1 production through HO-1 induction in a murine model of spinal cord injury. Int. Immunopharmacol. 2014, 23, 681–687. [Google Scholar] [CrossRef]
- Zhou, L.; Du, L.; Chen, X.; Li, X.; Li, Z.; Wen, Y.; Li, Z.; He, X.; Wei, Y.; Zhao, X. The antitumor and antimetastatic effects of N-trimethyl chitosan-encapsulated camptothecin on ovarian cancer with minimal side effects. Oncol. Rep. 2010, 24, 941–948. [Google Scholar]
- Stewart, A.F.; Schütz, G. Camptothecin-induced in vivo topoisomerase I cleavages in the transcriptionally active tyrosine aminotransferase gene. Cell 1987, 50, 1109–1117. [Google Scholar] [CrossRef]
- Jayasooriya, R.G.P.T.; Park, S.R.; Choi, Y.H.; Hyun, J.-W.; Chang, W.-Y.; Kim, G.-Y. Camptothecin suppresses expression of matrix metalloproteinase-9 and vascular endothelial growth factor in DU145 cells through PI3K/Akt-mediated inhibition of NF-κB activity and Nrf2-dependent induction of HO-1 expression. Environ. Toxicol. Pharmacol. 2015, 39, 1189–1198. [Google Scholar] [CrossRef] [PubMed]
- Mahmoud, A.M.; Germoush, M.O.; Soliman, A.S. Berberine attenuates isoniazid-induced hepatotoxicity by modulating peroxisome proliferator-activated receptor gamma, oxidative stress and inflammation. Int. J. Pharmacol. 2014, 10, 451–460. [Google Scholar] [CrossRef]
- Germoush, M.O.; Mahmoud, A.M. Berberine mitigates cyclophosphamide-induced hepatotoxicity by modulating antioxidant status and inflammatory cytokines. J. Cancer Res. Clin. Oncol. 2014, 140, 1103–1109. [Google Scholar] [CrossRef] [PubMed]
- Mahmoud, A.M.; Abdel-Rahman, M.M.; Bastawy, N.A.; Eissa, H.M. Modulatory effect of berberine on adipose tissue PPARγ, adipocytokines and oxidative stress in high fat diet/streptozotocin-induced diabetic rats. J. Appl. Pharm. Sci 2017, 7. [Google Scholar]
- Mahmoud, A.M.; Hozayen, W.G.; Ramadan, S.M. Berberine ameliorates methotrexate-induced liver injury by activating Nrf2/HO-1 pathway and PPARγ, and suppressing oxidative stress and apoptosis in rats. Biomed. Pharmacother. 2017, 94, 280–291. [Google Scholar] [CrossRef]
- Reyes-Escogido, M.D.L.; Gonzalez-Mondragon, E.G.; Vazquez-Tzompantzi, E. Chemical and pharmacological aspects of capsaicin. Molecules 2011, 16, 1253–1270. [Google Scholar] [CrossRef]
- Joung, E.-J.; Li, M.-H.; Lee, H.G.; Somparn, N.; Jung, Y.S.; Na, H.-K.; Kim, S.-H.; Cha, Y.-N.; Surh, Y.-J. Capsaicin Induces Heme Oxygenase-1 Expression in HepG2 Cells Via Activation of PI3K-Nrf2 Signaling: NAD(P)H:Quinone Oxidoreductase as a Potential Target. Antioxid. Redox Signal. 2007, 9, 2087–2098. [Google Scholar] [CrossRef]
- Kim, Y.; Lee, J. Anti-Inflammatory Activity of Capsaicin and Dihydrocapsaicin through Heme Oxygenase-1 Induction in Raw264.7 Macrophages. J. Food Biochem. 2014, 38, 381–387. [Google Scholar] [CrossRef]
- Zhang, H.-F.; Shi, L.-J.; Song, G.-Y.; Cai, Z.-G.; Wang, C.; An, R.-J. Protective effects of matrine against progression of high-fructose diet-induced steatohepatitis by enhancing antioxidant and anti-inflammatory defences involving Nrf2 translocation. Food Chem. Toxicol. 2013, 55, 70–77. [Google Scholar] [CrossRef]
- Zhang, B.; Liu, Z.-Y.; Li, Y.-Y.; Luo, Y.; Liu, M.-L.; Dong, H.-Y.; Wang, Y.-X.; Liu, Y.; Zhao, P.-T.; Jin, F.-G. Antiinflammatory effects of matrine in LPS-induced acute lung injury in mice. Eur. J. Pharm. Sci. 2011, 44, 573–579. [Google Scholar] [CrossRef]
- Liu, Y.; Xu, Y.; Ji, W.; Li, X.; Sun, B.; Gao, Q.; Su, C. Anti-tumor activities of matrine and oxymatrine: Literature review. Tumor Biol. 2014, 35, 5111–5119. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zhang, X.; Ma, K.; Zhang, R.; Hou, P.; Sun, B.; Yuan, S.; Wang, Z.; Liu, Z. Matrine alleviates early brain injury after experimental subarachnoid hemorrhage in rats: Possible involvement of PI3K/Akt-mediated NF-κB inhibition and Keap1/Nrf2-dependent HO-1 inductionn. Cell Mol. Biol. 2016, 62, 38–44. [Google Scholar] [PubMed]
- Gai, G.-z.; Jin, S.-j.; Wang, B.; Li, Y.; Li, C. The efficacy of Cordyceps militaris capsules in treatment of chronic bronchitis in comparison with Jinshuibao capsules. Chin. New Drugs J. 2004, 13, 169–170. [Google Scholar]
- Zeng, Y.; Lian, S.; Li, D.; Lin, X.; Chen, B.; Wei, H.; Yang, T. Anti-hepatocarcinoma effect of cordycepin against NDEA-induced hepatocellular carcinomas via the PI3K/Akt/mTOR and Nrf2/HO-1/NF-κB pathway in mice. Biomed. Pharm. 2017, 95, 1868–1875. [Google Scholar] [CrossRef] [PubMed]
- Rukachaisirikul, T.; Prabpai, S.; Champung, P.; Suksamrarn, A. Chabamide, a novel piperine dimer from stems of Piper chaba. Planta Med. 2002, 68, 853–855. [Google Scholar] [CrossRef] [PubMed]
- Ngo, Q.T.; Tran, P.T.; Tran, M.H.; Kim, J.A.; Rho, S.S.; Lim, C.H.; Kim, J.C.; Woo, M.H.; Choi, J.S.; Lee, J.H. Alkaloids from Piper nigrum exhibit antiinflammatory activity via activating the Nrf2/HO1 pathway. Phytother. Res. 2017, 31, 663–670. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.-N.; Jin, H.-O.; Park, J.-A.; Kim, J.-H.; Kim, J.-Y.; Kim, B.; Kim, W.; Hong, S.-E.; Lee, Y.-H.; Chang, Y.H. Heme oxygenase-1 determines the differential response of breast cancer and normal cells to piperlongumine. Mol. Cells 2015, 38, 327. [Google Scholar] [CrossRef] [PubMed]
- Lu, W.; Shi, L.; Gao, J.; Zhu, H.; Hua, Y.; Cai, J.; Wu, X.; Wan, C.; Zhao, W.; Zhang, B. Piperlongumine Inhibits Zika Virus Replication In vitro and Promotes Up-Regulation of HO-1 Expression, Suggesting An Implication of Oxidative Stress. Virol. Sin. 2020, 1–11. [Google Scholar]
- Liu, S.; Yang, T.; Ming, T.W.; Gaun, T.K.W.; Zhou, T.; Wang, S.; Ye, B. Isosteroid alkaloids from Fritillaria cirrhosa bulbus as inhibitors of cigarette smoke-induced oxidative stress. Fitoterapia 2020, 140, 104434. [Google Scholar] [CrossRef]
- Xu, J.; Zhao, W.; Pan, L.; Zhang, A.; Chen, Q.; Xu, K.; Lu, H.; Chen, Y. Peimine, a main active ingredient of Fritillaria, exhibits anti-inflammatory and pain suppression properties at the cellular level. Fitoterapia 2016, 111, 1–6. [Google Scholar] [CrossRef]
- Luo, Z.; Zheng, B.; Jiang, B.; Xue, X.; Xue, E.; Zhou, Y. Peiminine inhibits the IL-1β induced inflammatory response in mouse articular chondrocytes and ameliorates murine osteoarthritis. Food Funct. 2019, 10, 2198–2208. [Google Scholar] [CrossRef] [PubMed]
- Duvigneau, J.C.; Esterbauer, H.; Kozlov, A.V. Role of heme oxygenase as a modulator of heme-mediated pathways. Antioxidants 2019, 8, 475. [Google Scholar] [CrossRef] [PubMed]
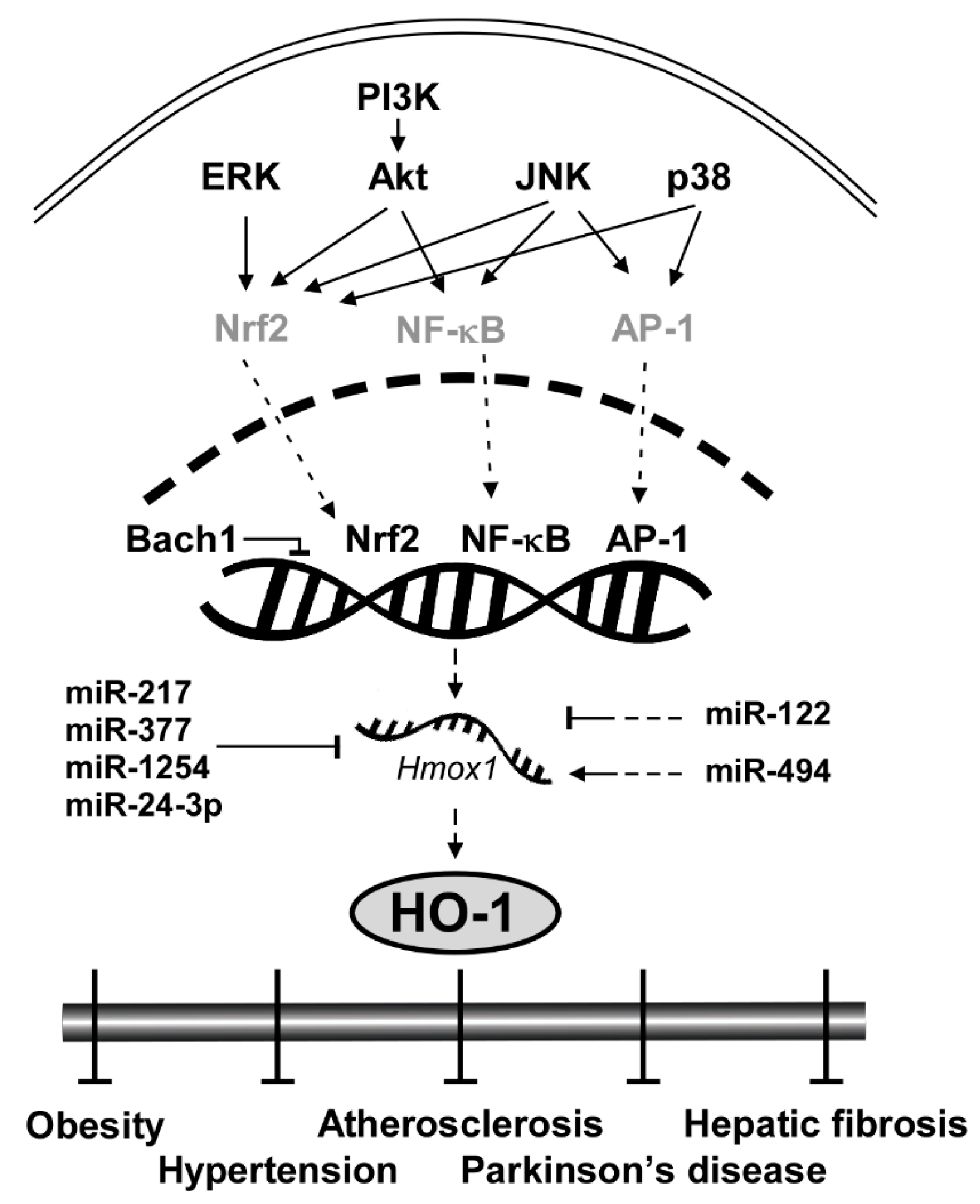
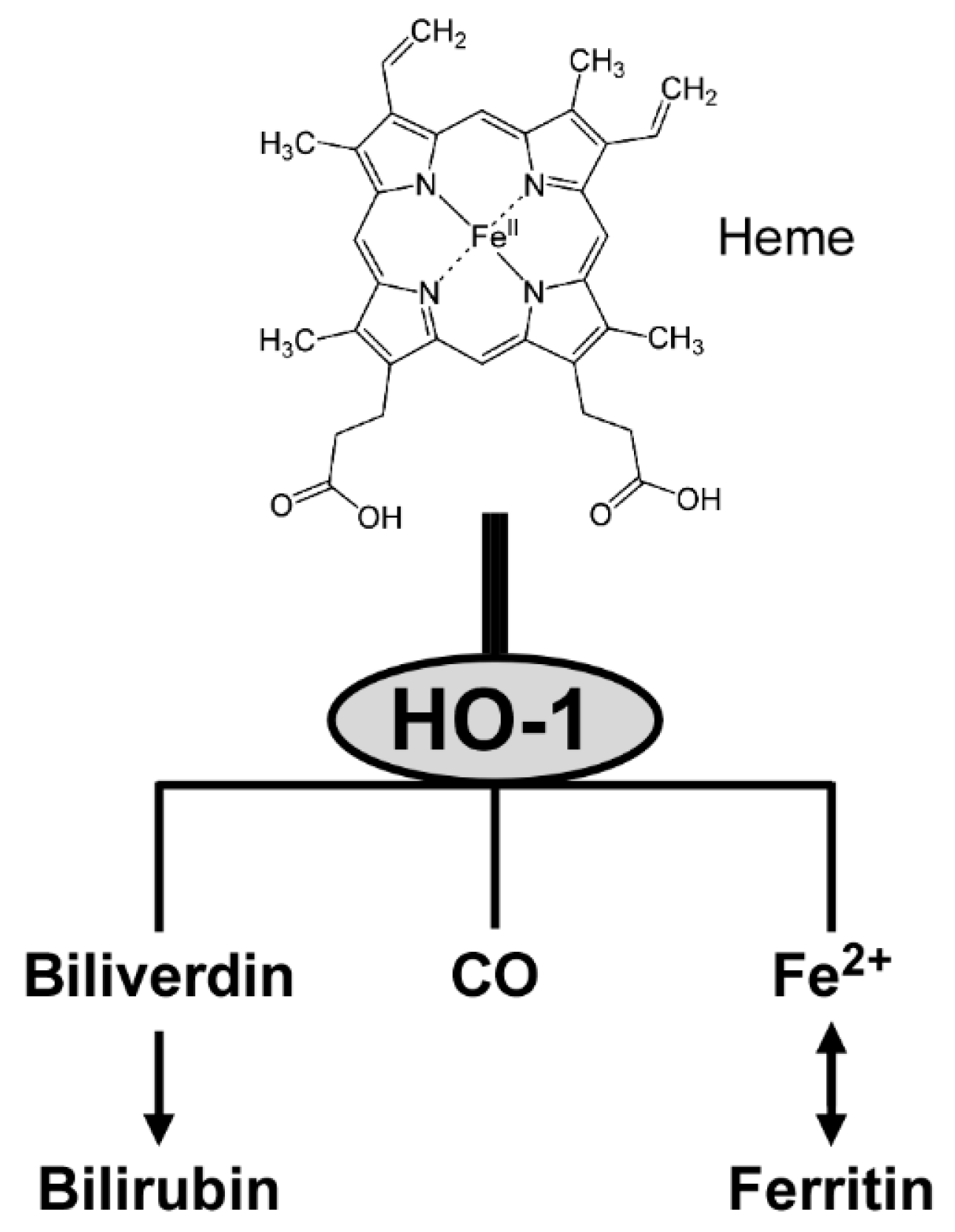

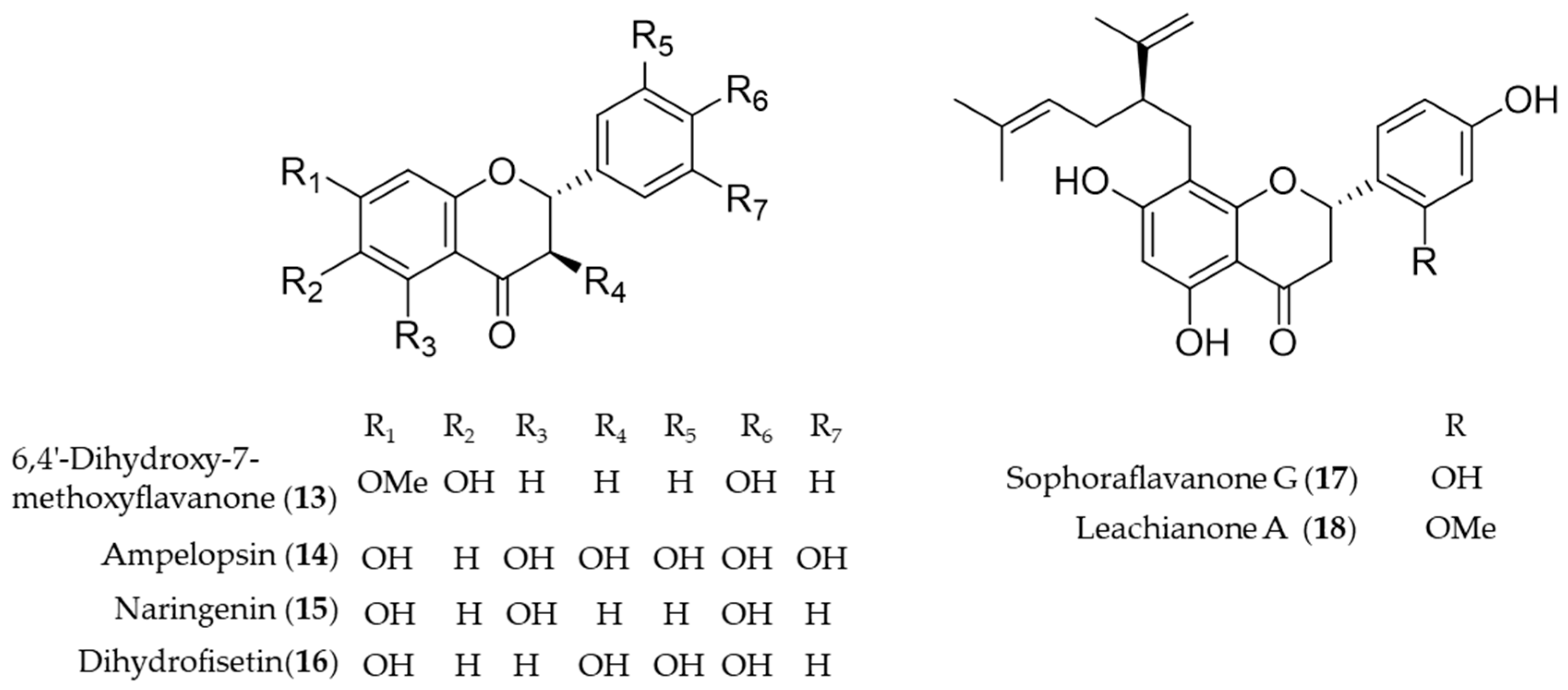

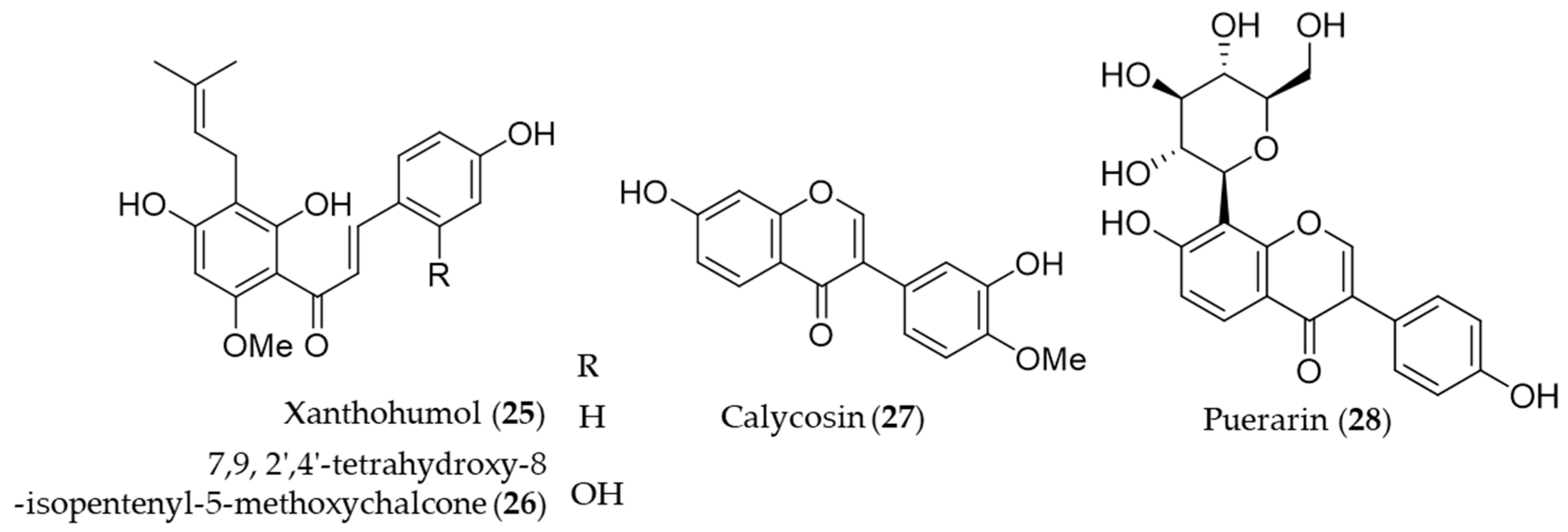
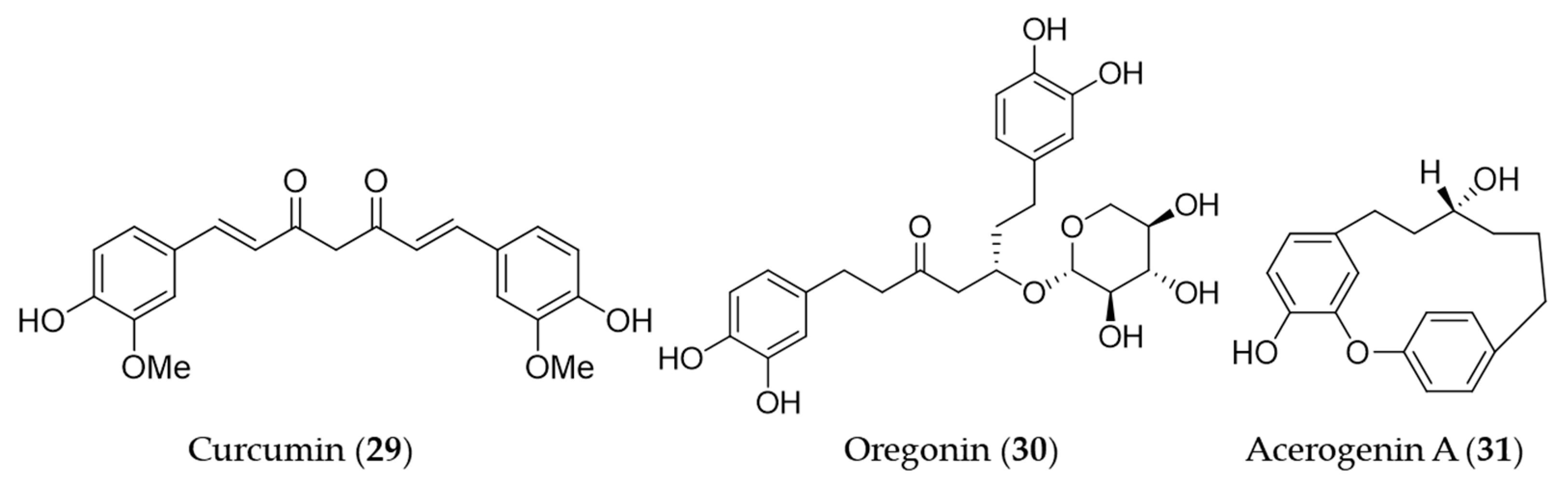
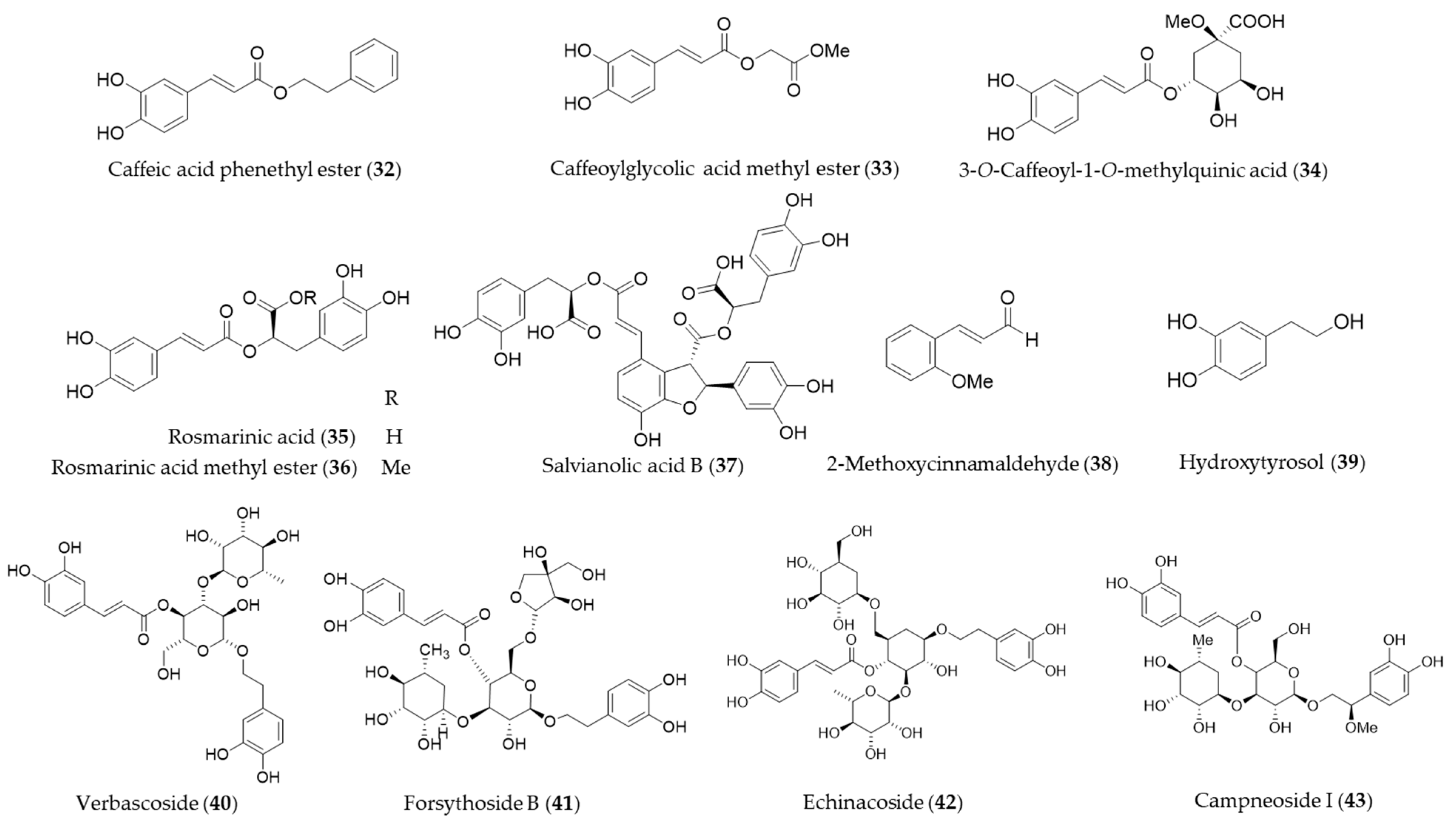
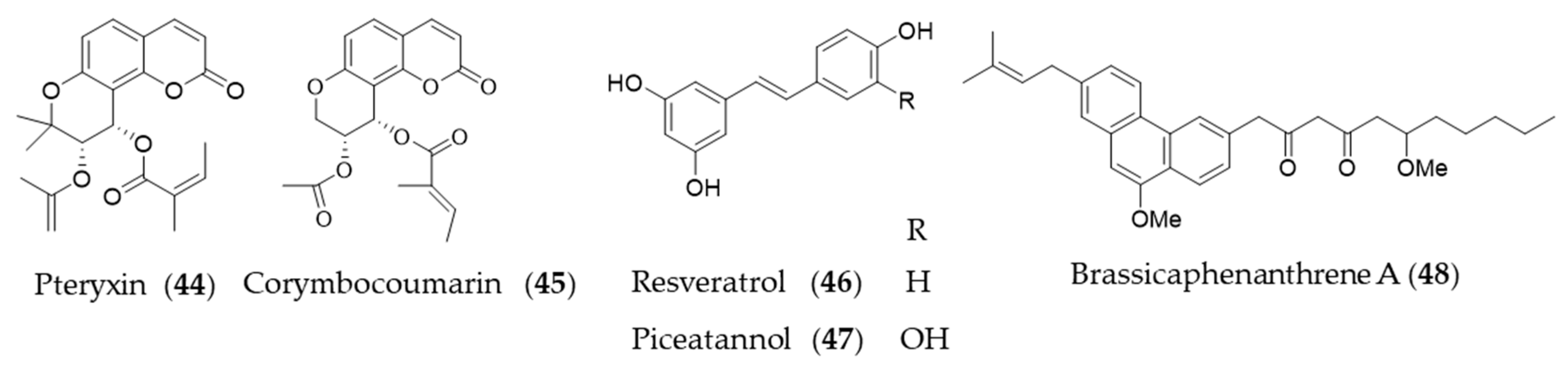
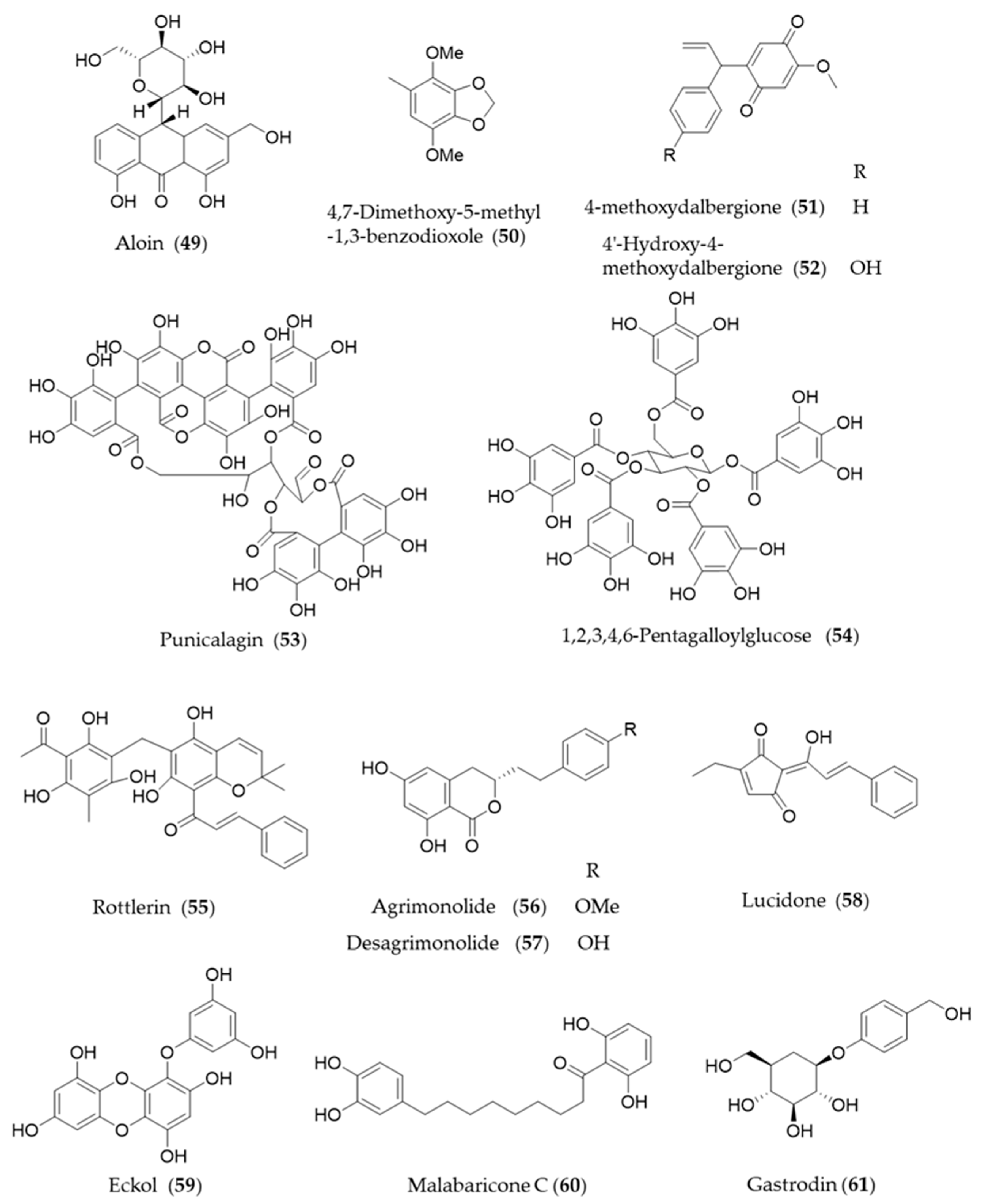
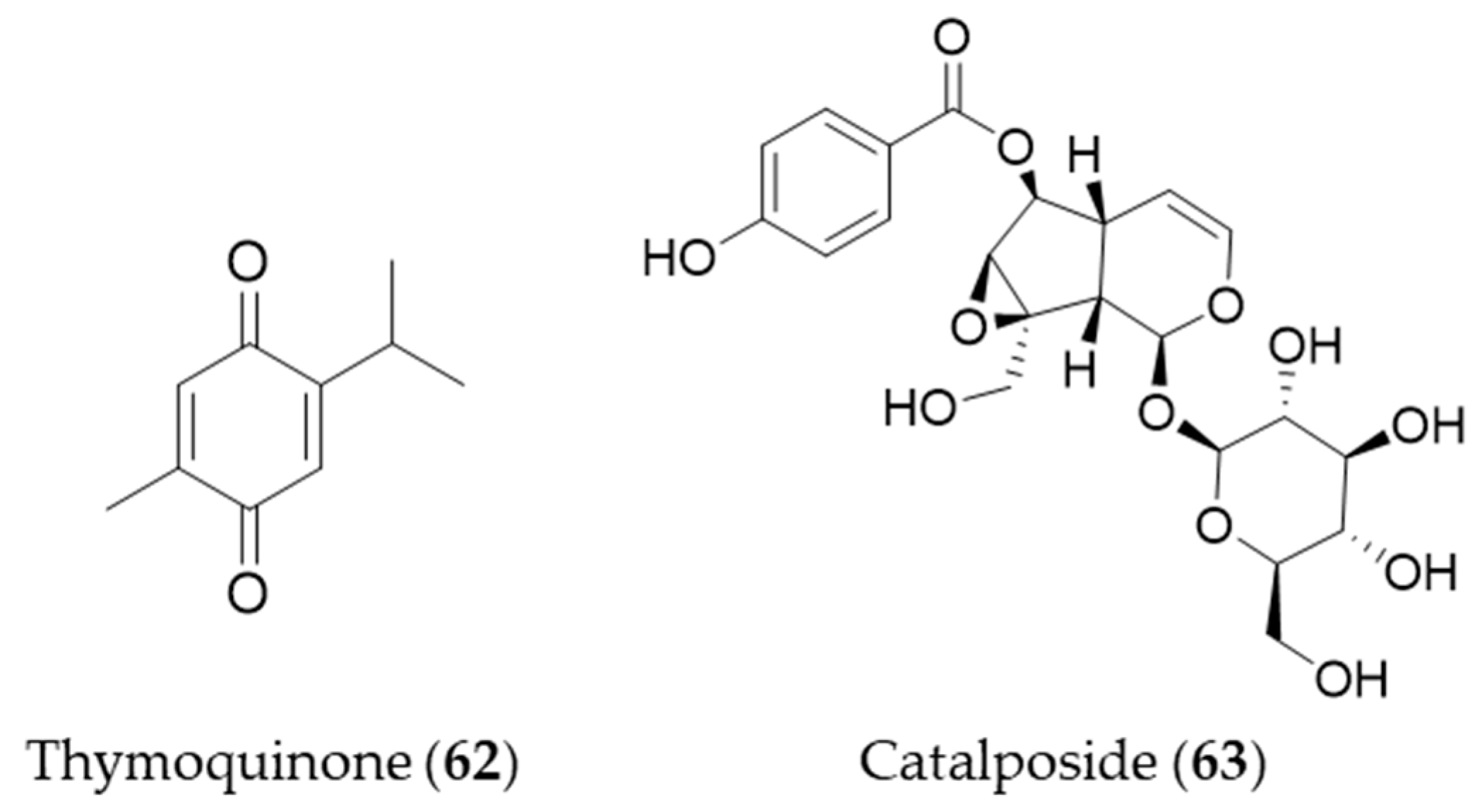
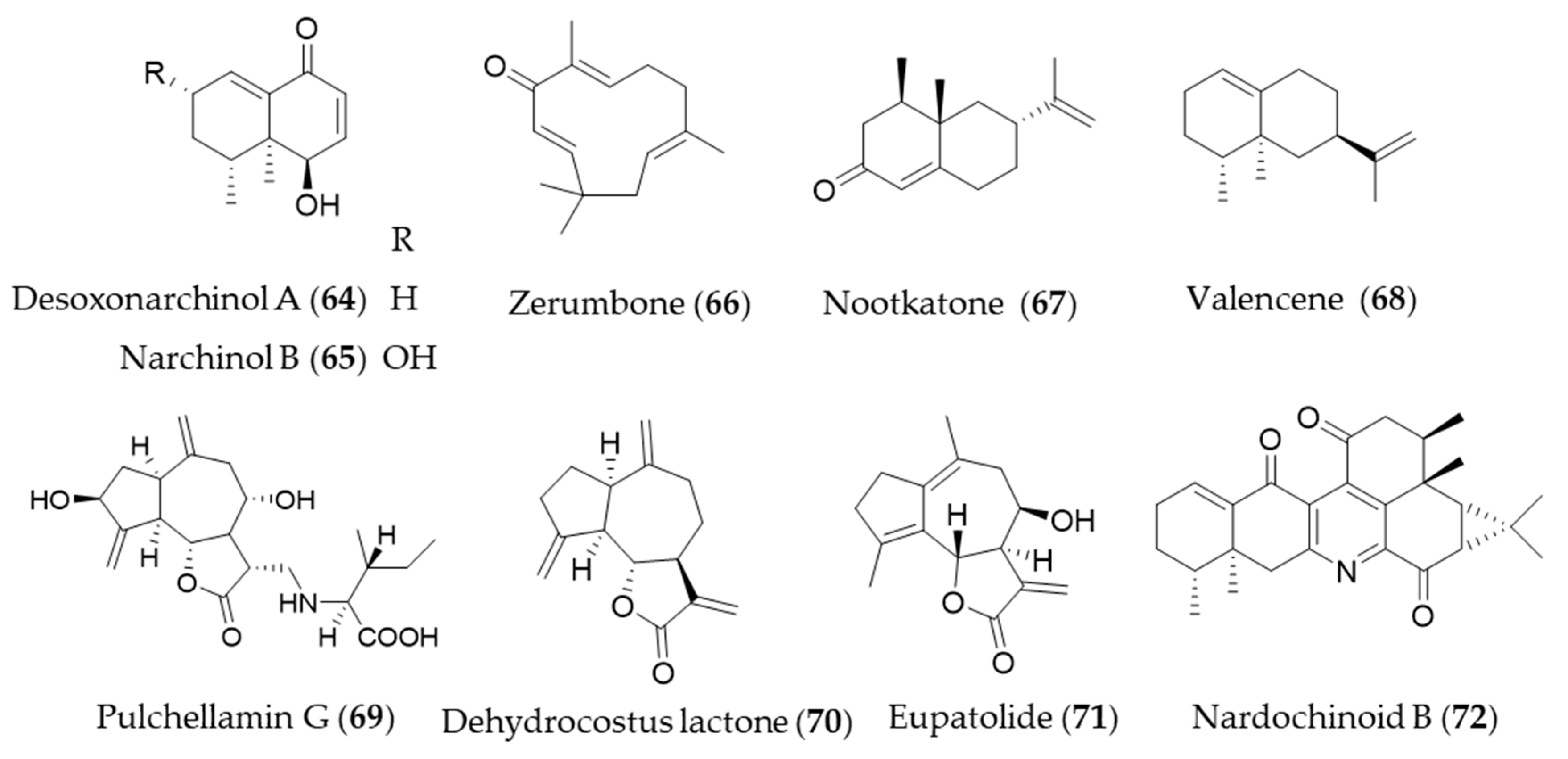
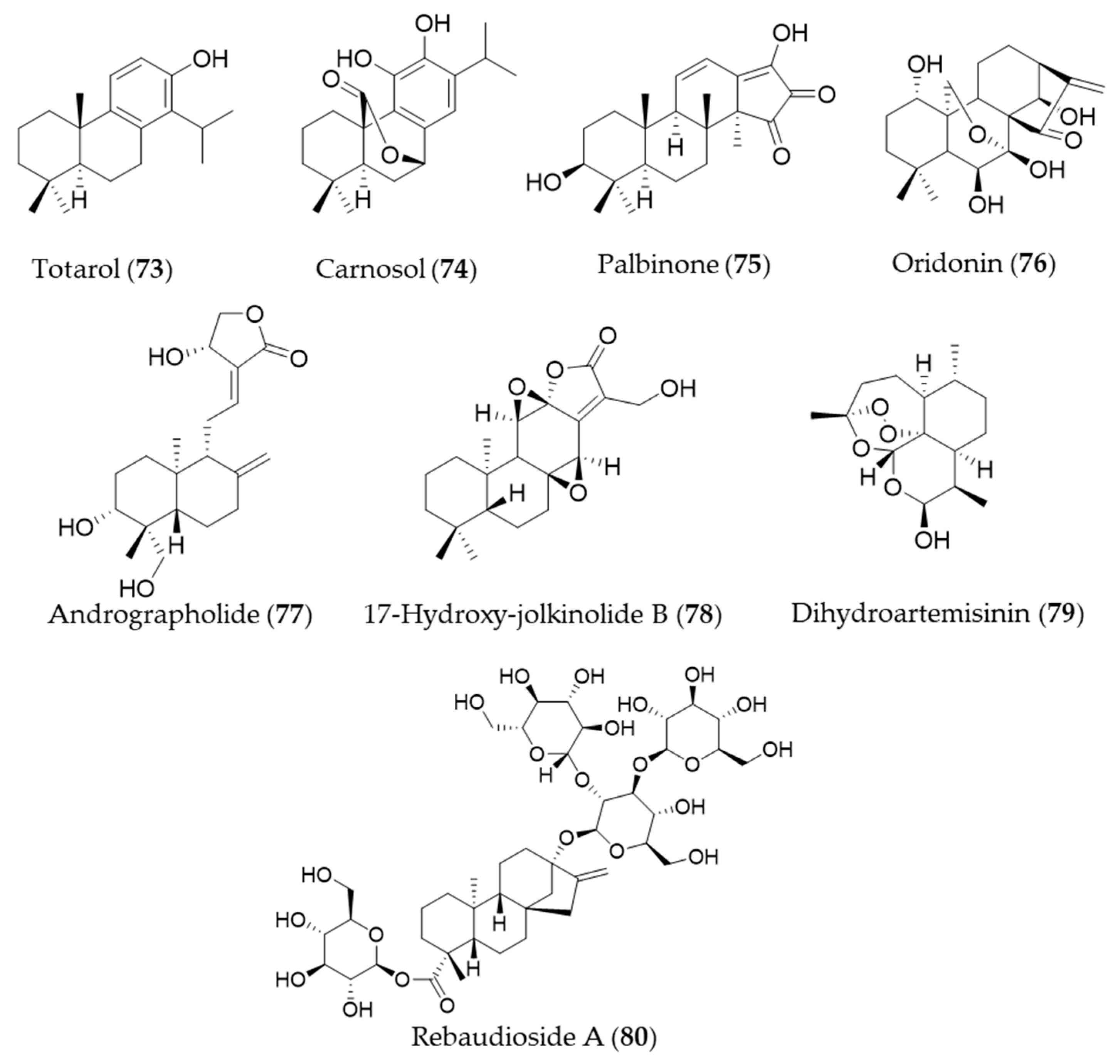
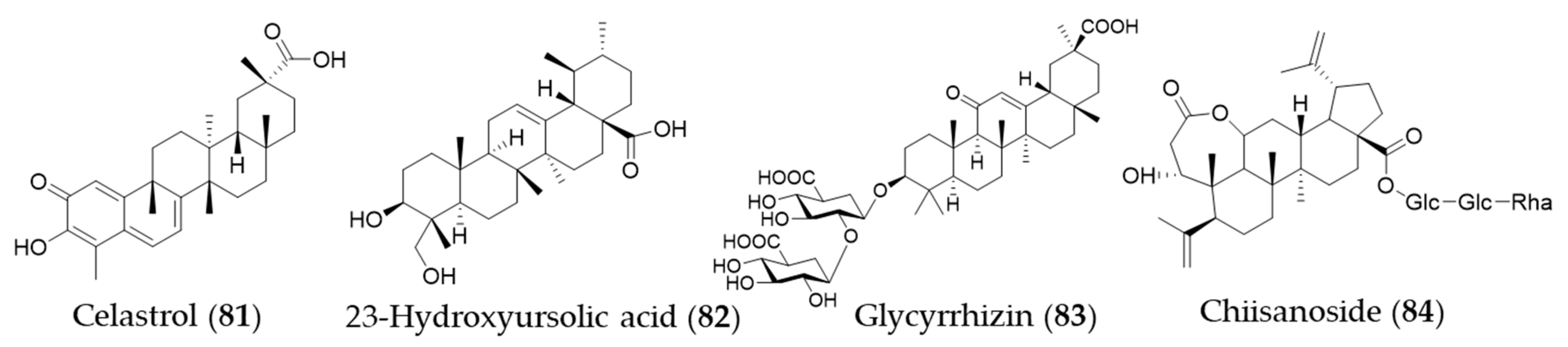
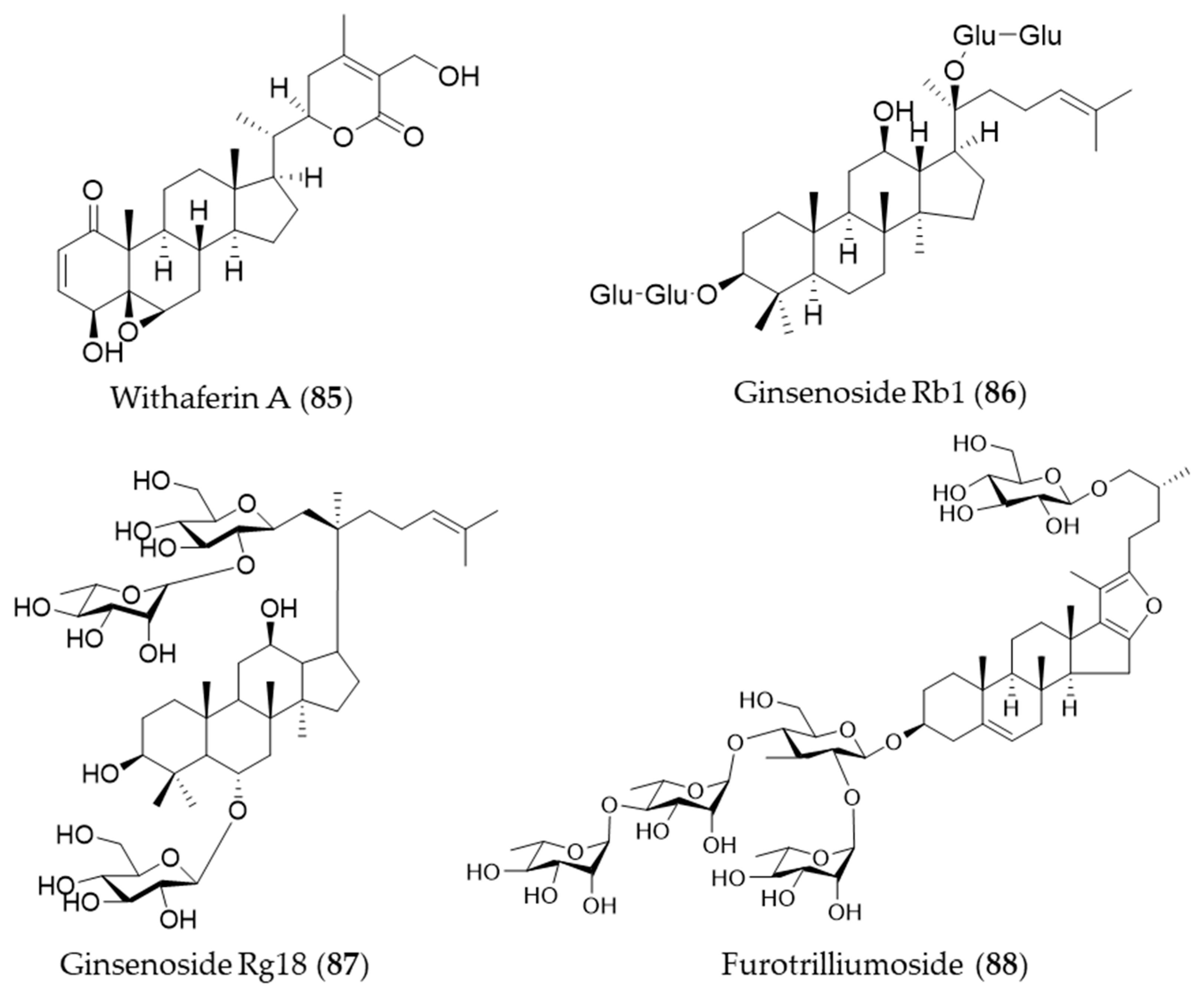

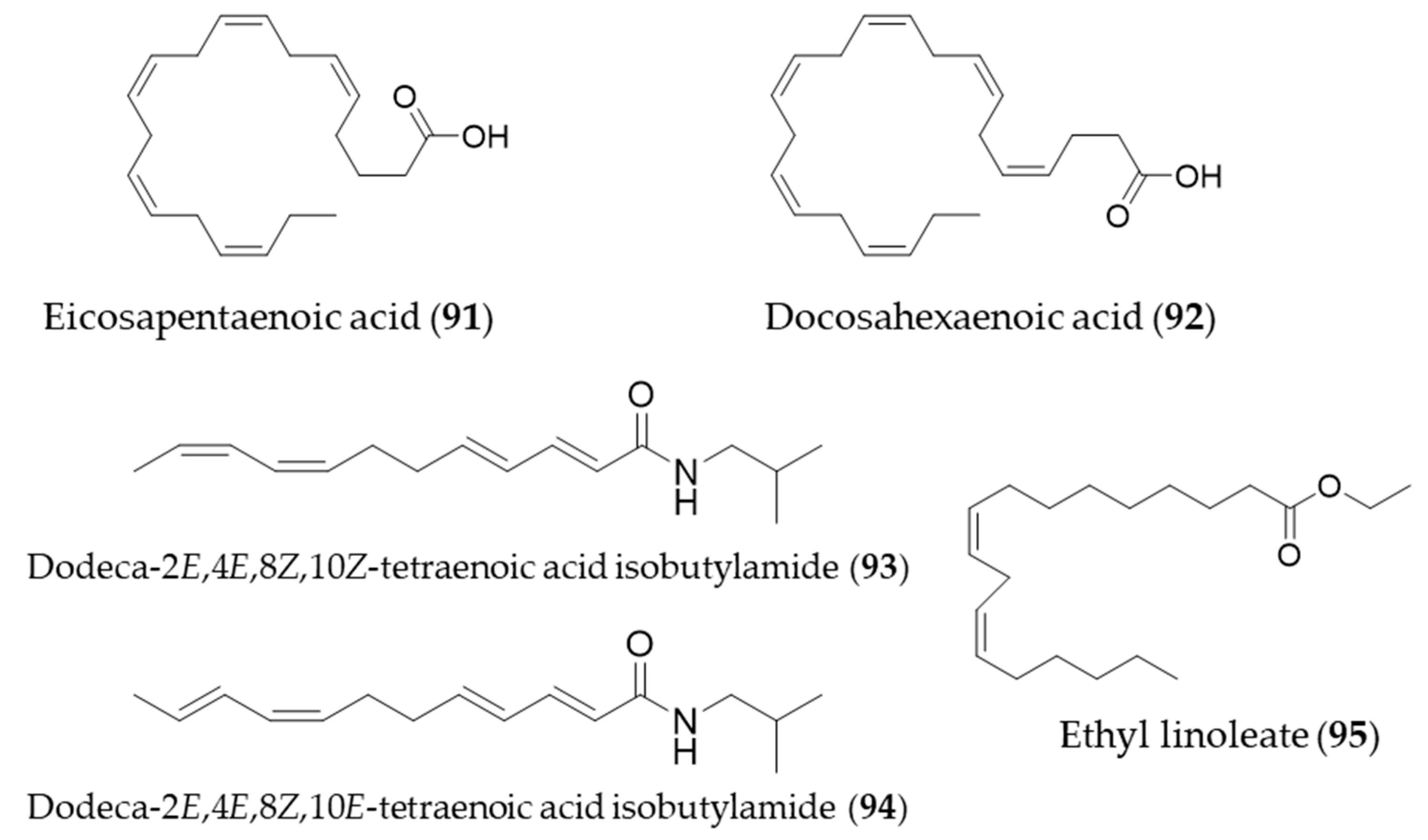
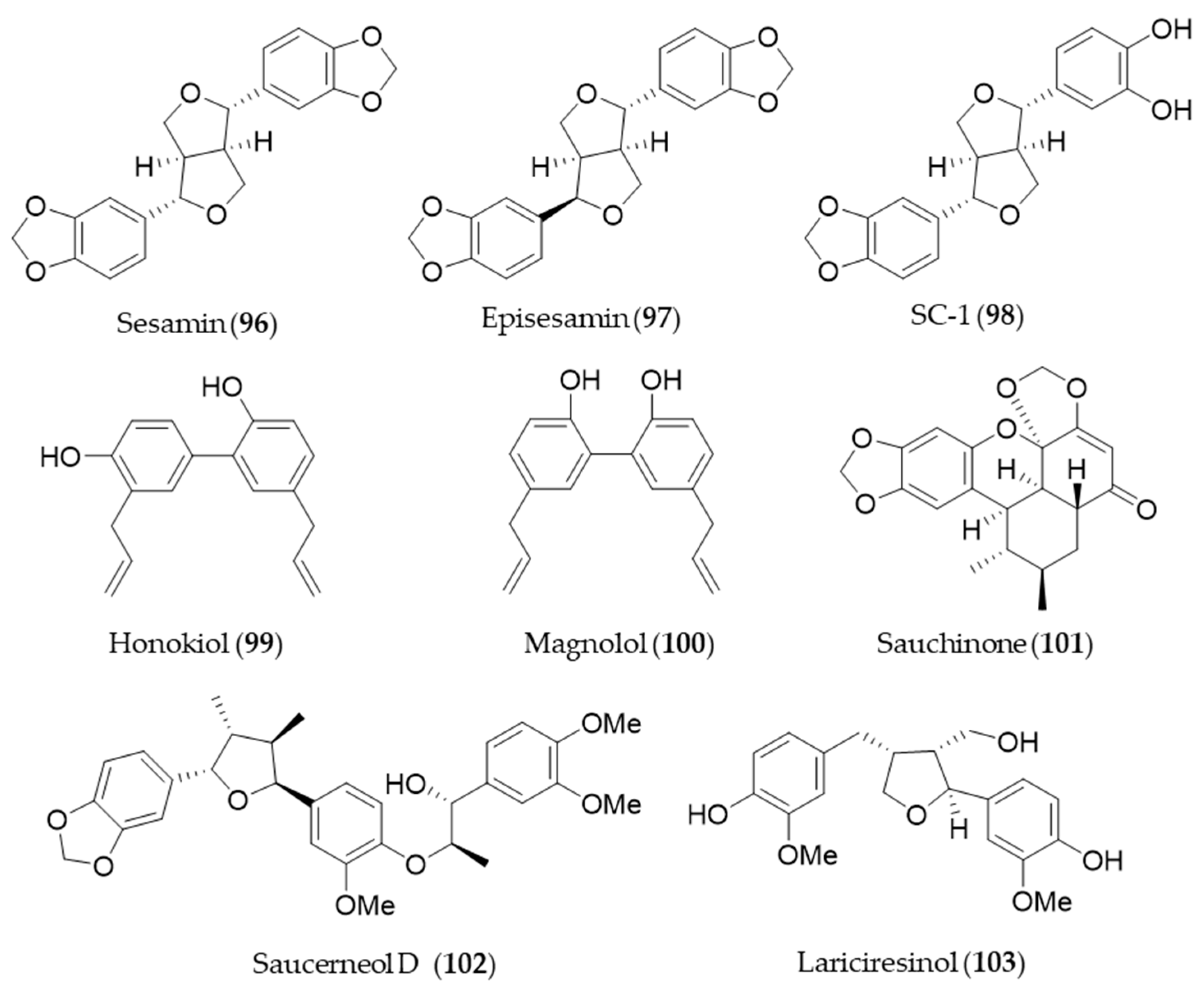
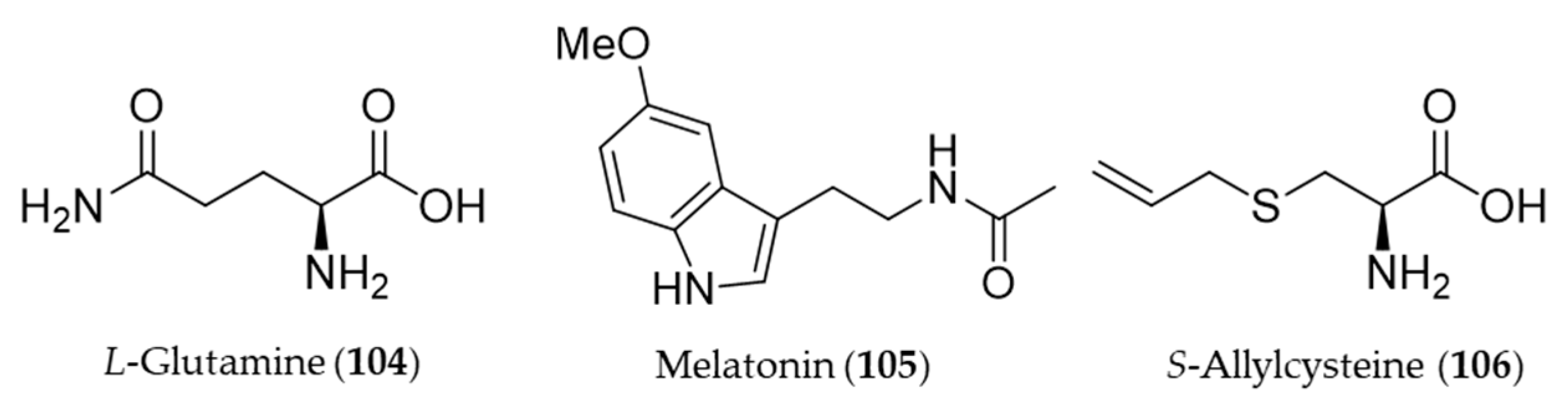
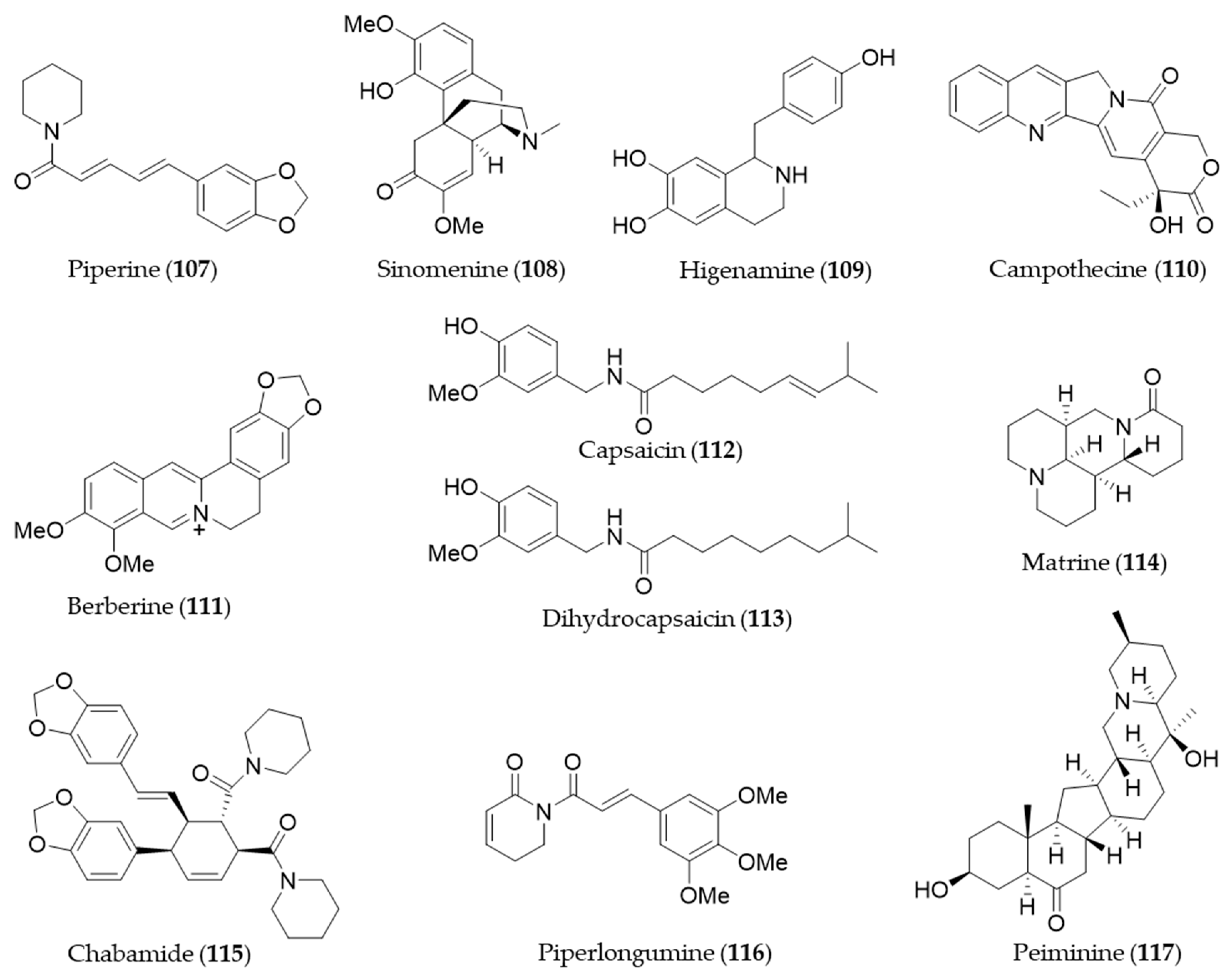
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hahn, D.; Shin, S.H.; Bae, J.-S. Natural Antioxidant and Anti-Inflammatory Compounds in Foodstuff or Medicinal Herbs Inducing Heme Oxygenase-1 Expression. Antioxidants 2020, 9, 1191. https://doi.org/10.3390/antiox9121191
Hahn D, Shin SH, Bae J-S. Natural Antioxidant and Anti-Inflammatory Compounds in Foodstuff or Medicinal Herbs Inducing Heme Oxygenase-1 Expression. Antioxidants. 2020; 9(12):1191. https://doi.org/10.3390/antiox9121191
Chicago/Turabian StyleHahn, Dongyup, Seung Ho Shin, and Jong-Sup Bae. 2020. "Natural Antioxidant and Anti-Inflammatory Compounds in Foodstuff or Medicinal Herbs Inducing Heme Oxygenase-1 Expression" Antioxidants 9, no. 12: 1191. https://doi.org/10.3390/antiox9121191
APA StyleHahn, D., Shin, S. H., & Bae, J.-S. (2020). Natural Antioxidant and Anti-Inflammatory Compounds in Foodstuff or Medicinal Herbs Inducing Heme Oxygenase-1 Expression. Antioxidants, 9(12), 1191. https://doi.org/10.3390/antiox9121191






