Resveratrol Improves Heart Function by Moderating Inflammatory Processes in Patients with Systolic Heart Failure
Abstract
1. Introduction
2. Materials and Methods
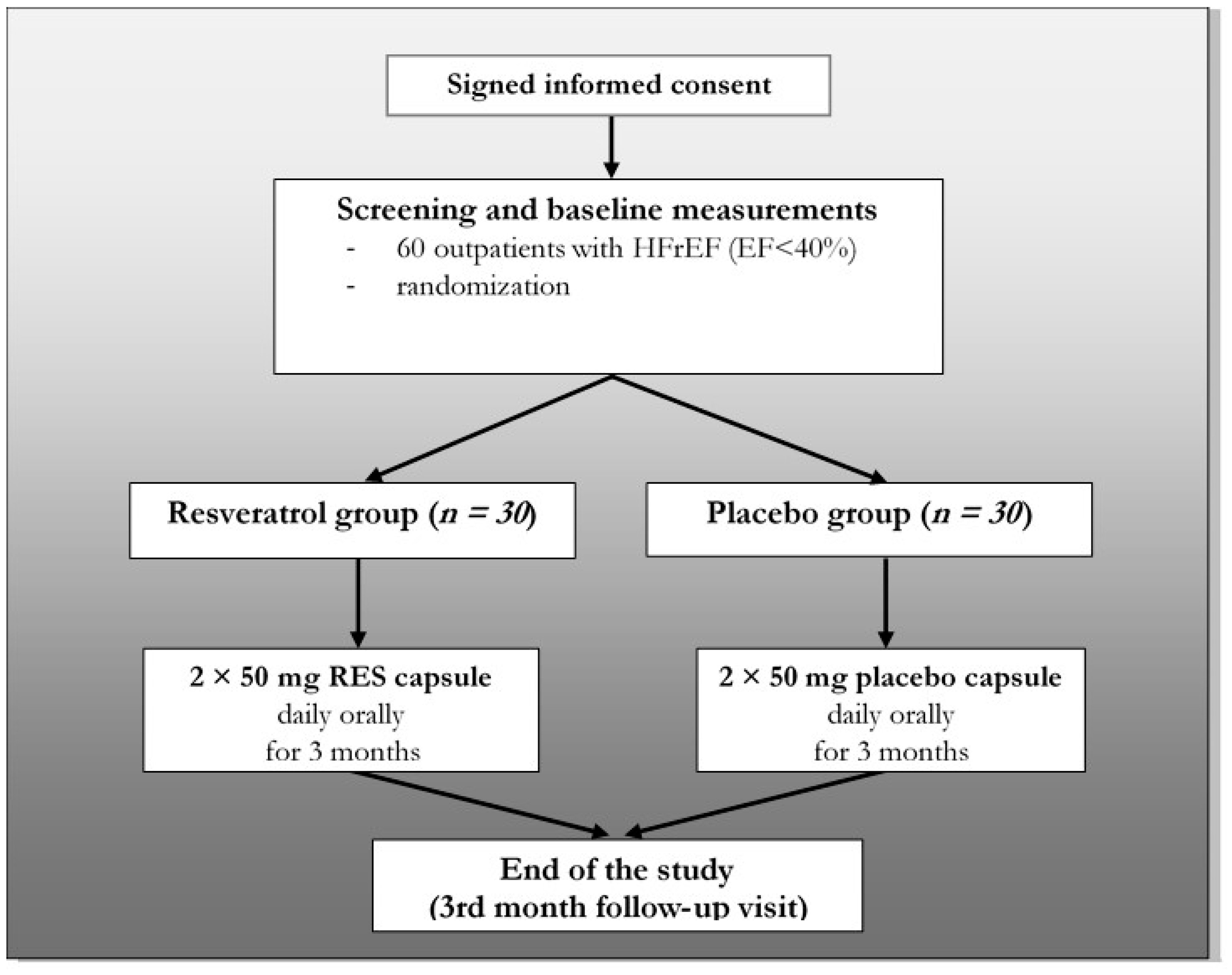
2.1. Study Design
2.2. Eligibility
2.3. Statistical Analysis
2.4. Blood Pressure and Body Weight Measurement
2.5. Laboratory Test
2.6. Echocardiography
2.7. Six-Minute Walk Test (6MWT)
2.8. Spirometry
2.9. Quality of Life Questionnaire (QoL Test)
2.10. RNA Isolation, RNA-Seq Library Preparation and Sequencing
2.11. RNA-Sequencing Data Processing and Analysis
3. Results
3.1. The Effect of Resveratrol on Laboratory Parameters
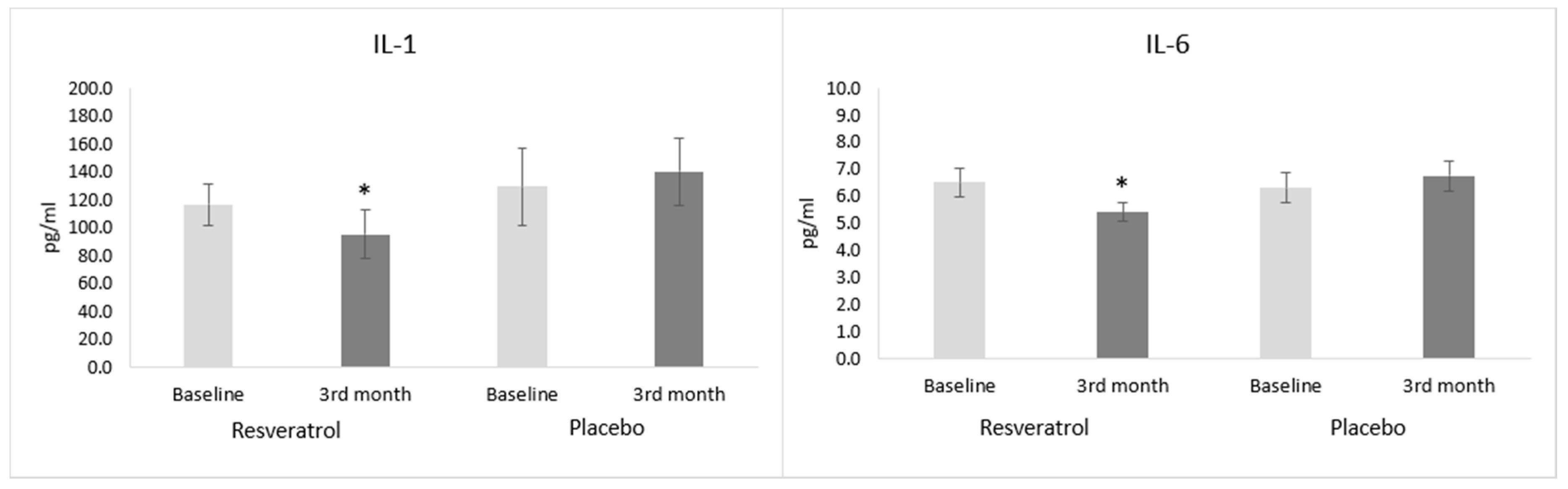
3.2. Inflammatory Parameters
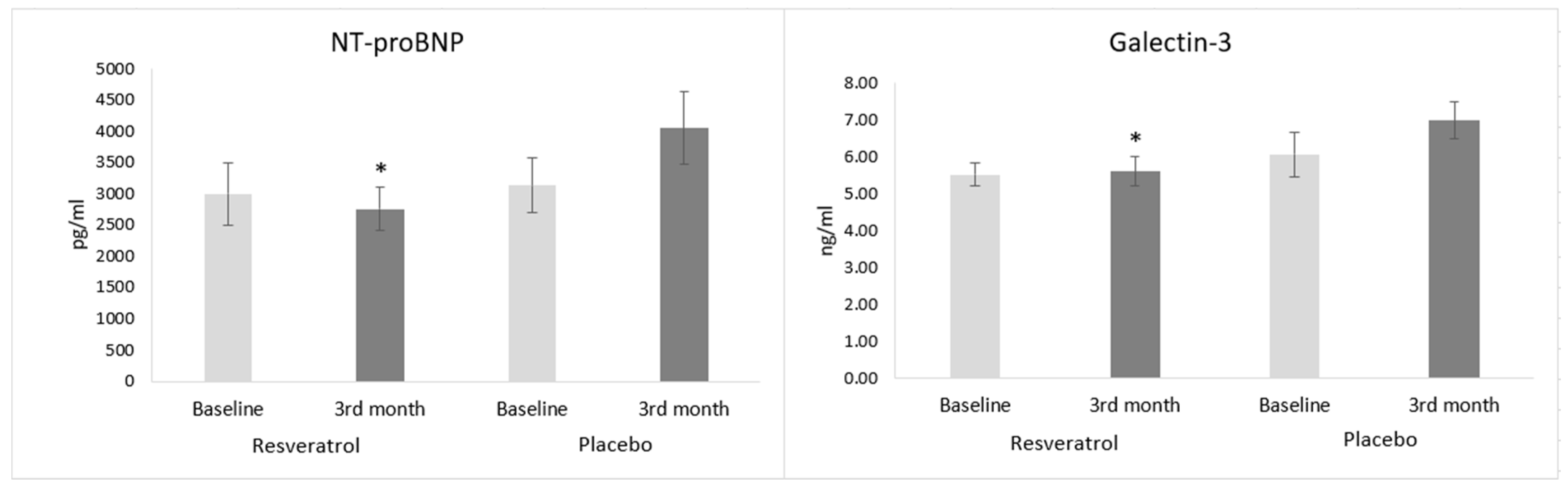
3.3. Biomarkers of Heart Failure
3.4. BMI, General Hemodynamic and ECG Parameters
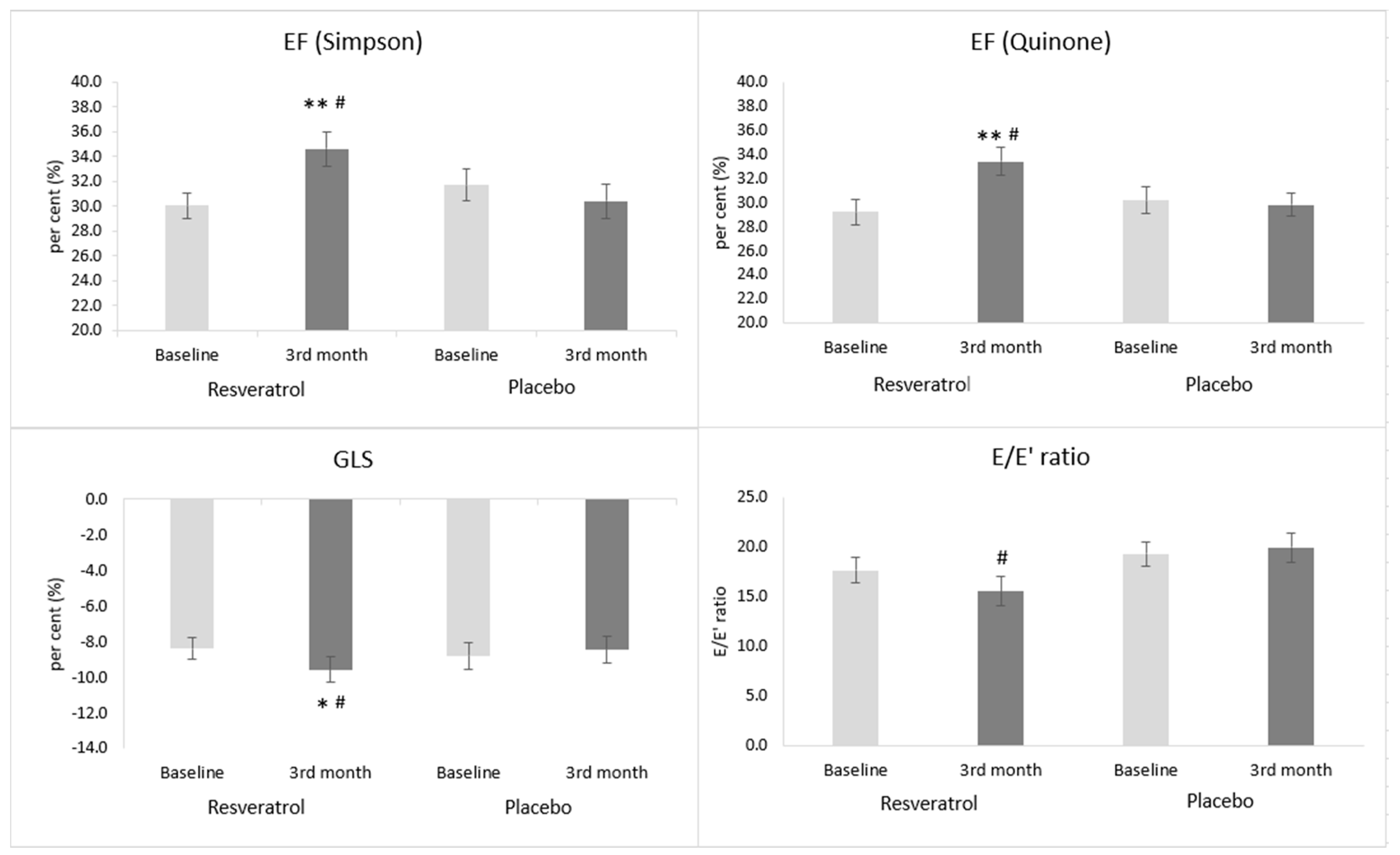
3.5. The Effect of Resveratrol on Echocardiographic Parameters
3.6. The Effect of Resveratrol on Exercise Capacity
3.7. The Effect of Resveratrol on the Respiratory Parameters
3.8. The Effect of Resveratrol on the Quality of Life in Patients with Systolic Heart Failure
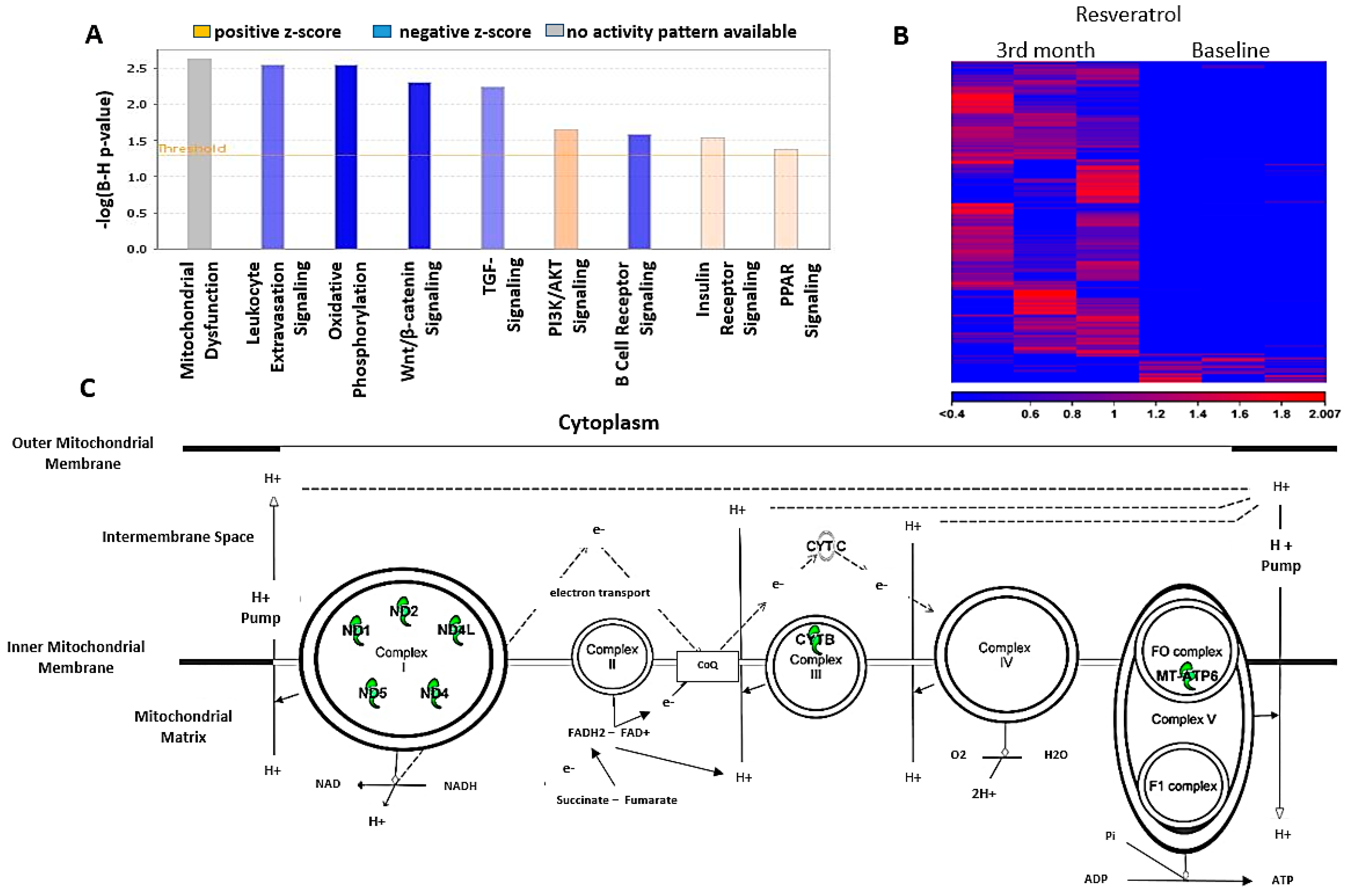
3.9. Sequence Analysis and Differential Expression
4. Discussion
5. Conclusions
6. Limitations of the Study
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ponikowski, P.; Voors, A.A.; Anker, S.D.; Bueno, H.; Cleland, J.G.F.; Coats, A.J.S.; Falk, V.; Gonzalez-Juanatey, J.R.; Harjola, V.; Jankowska, E.A. ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Eur. Heart J. 2016, 37, 2129–2200. [Google Scholar] [CrossRef] [PubMed]
- Greenberg, B.H. A Treatment Approach for Patients with Chronic Systolic Heart Failure. Rev. Cardiovasc. Med. 2016, 17, S22–S29. [Google Scholar] [PubMed]
- Mosterd, A.; Hoes, A.W. Clinical epidemiology of heart failure. Heart 2007, 93, 1137–1146. [Google Scholar] [CrossRef] [PubMed]
- Ziaeian, B.; Fonarow, G.C. Epidemiology and aetiology of heart failure. Nat. Rev. Cardiol. 2016, 13, 368–378. [Google Scholar] [CrossRef]
- Swedberg, K.; Komajda, M.; Böhm, M.; Borer, J.S.; Ford, I.; Dubost-Brama, A.; Lerebours, G.; Tavazzi, L. Ivabradine and outcomes in chronic heart failure (SHIFT): A randomised placebo-controlled study. Lancet 2010, 376, 875–885. [Google Scholar] [CrossRef]
- McMurray, J.J.; Packer, M.; Desai, A.S.; Gong, J.; Lefkowitz, M.P.; Rizkala, A.R.; Rouleau, J.L.; Shi, V.C.; Solomon, S.D.; Swedberg, K.; et al. PARADIGM-HF Investigators and Committees. Angiotensin-neprilysin inhibition versus enalapril in heart failure. N. Engl. J. Med. 2014, 371, 993–1004. [Google Scholar] [CrossRef]
- Sauer, A.J.; Cole, R.; Jensen, B.C.; Pal, J.; Sharma, N.; Yehya, A.; Vader, J. Practical guidance on the use of sacubitril/valsartan for heart failure. Heart Fail. Rev. 2019, 24, 167–176. [Google Scholar] [CrossRef]
- Bui, A.L.; Horwich, T.B.; Fonarow, G.C. Epidemiology and risk profile of heart failure. Nat. Rev. Cardiol. 2011, 8, 30–41. [Google Scholar] [CrossRef]
- Kim, D.H.; Chien, F.J.; Eisen, H.J. Pharmacologic Management for Heart Failure and Emerging Therapies. Curr. Cardiol. Rep. 2017, 19, 94. [Google Scholar] [CrossRef]
- Patel, C.; Deoghare, S. Heart failure: Novel therapeutic approaches. J. Postgrad. Med. 2015, 61, 101–108. [Google Scholar] [CrossRef]
- Tsutsui, H.; Kinugawa, S.; Matsushimam, S. Oxidative stress and heart failure. Am. J. Physiol. Heart Circ. Physiol. 2011, 301, H2181–H2190. [Google Scholar] [CrossRef]
- Oyewole, A.O.; Birch-Machin, M.A. Mitochondria-targeted antioxidants. FASEB J. 2015, 12, 4766–4771. [Google Scholar] [CrossRef] [PubMed]
- Raj, P.; Lieben Louis, X.; Thandapilly, S.J.; Movahed, A.; Zieroth, S.; Netticadan, T. Potential of resveratrol in the treatment of heart failure. Life Sci. 2014, 95, 63–71. [Google Scholar] [CrossRef] [PubMed]
- Gupta, P.K.; DiPette, D.J.; Supowit, S.C. Protective effect of resveratrol against pressure overload-induced heart failure. Food Sci. Nutr. 2014, 2, 218–229. [Google Scholar] [CrossRef] [PubMed]
- Wojciechowski, P.; Juric, D.; Louis, X.L.; Thandapilly, S.J.; Yu, L.; Taylor, C.; Netticadan, T. Resveratrol arrests and regresses the development of pressure over load-but not volume overload-induced cardiac hypertrophy in rats. J. Nutr. 2010, 140, 962–968. [Google Scholar] [CrossRef] [PubMed]
- Danz, E.D.; Skramsted, J.; Henry, N.; Bennett, J.A.; Keller, R.S. Resveratrol prevents doxorubicin cardiotoxicity through mitochondrial stabilization and the Sirt1pathway. Free Radic. Biol. Med. 2009, 46, 1589–1597. [Google Scholar] [CrossRef] [PubMed]
- Xia, N.; Daiber, A.; Förstermann, U.; Li, H. Antioxidant effects of resveratrol in the cardiovascular system. Br. J. Pharmacol. 2017, 174, 1633–1646. [Google Scholar] [CrossRef]
- Lagouge, M.; Argmann, C.; Gerhart-Hines, Z.; Meziane, H.; Lerin, C.; Daussin, F.; Messadeq, N.; Milne, J.; Lambert, P.; Elliott, P.; et al. Resveratrol improves mitochondrial function and protects against metabolic disease by activating SIRT1 and PGC-1alpha. Cell 2006, 127, 1109–1122. [Google Scholar] [CrossRef]
- Gu, X.S.; Wang, Z.B.; Ye, Z.; Lei, J.P.; Li, L.; Su, D.F.; Zheng, X. Resveratrol, an activator of SIRT1, upregulates AMPK and improves cardiac function in heart failure. Genet. Mol. Res. 2014, 13, 323–335. [Google Scholar] [CrossRef]
- Dyck, G.J.B.; Raj, P.; Zieroth, S.; Dyck, J.R.B.; Ezekowitz, J.A. The Effects of Resveratrol in Patients with Cardiovascular Disease and Heart Failure: A Narrative Review. Int. J. Mol. Sci. 2019, 20, 904. [Google Scholar] [CrossRef]
- Xia, N.; Forstermann, U.; Li, H. Effects of resveratrol on eNOS in the endothelium and the perivascular adipose tissue. Ann. N. Y. Acad. Sci. 2017, 1403, 132–141. [Google Scholar] [CrossRef] [PubMed]
- Schwager, J.; Richard, N.; Widmer, F.; Raederstorff, D. Resveratrol distinctively modulates the inflammatory profiles of immune and endothelial cells. BMC Complement. Altern. Med. 2017, 17, 309. [Google Scholar] [CrossRef] [PubMed]
- Carrizzo, A.; Puca, A.; Damato, A.; Marino, M.; Franco, E.; Pompeo, F.; Traficante, A.; Civitillo, F.; Santini, L.; Trimarco, V.; et al. Resveratrol improves vascular function in patients with hypertension and dyslipidemia by modulating NO metabolism. Hypertension 2013, 62, 359–366. [Google Scholar] [CrossRef] [PubMed]
- Simental-Mendía, L.E.; Guerrero-Romero, F. Effect of resveratrol supplementation on lipid profile in subjects with dyslipidemia: A randomized double-blind, placebo-controlled trial. Nutrition 2019, 58, 7–10. [Google Scholar] [CrossRef] [PubMed]
- Riba, A.; Deres, L.; Sumegi, B.; Toth, K.; Szabados, E.; Halmosi, R. Cardioprotective Effect of Resveratrol in a Postinfarction Heart Failure Model. Oxid. Med. Cell Longev. 2017, 2017, 6819281. [Google Scholar] [CrossRef]
- Sung, M.M.; Dyck, J.R.B. Therapeutic potential of resveratrol in heart failure. Ann. N. Y. Acad. Sci. 2015, 1348, 32–45. [Google Scholar] [CrossRef] [PubMed]
- Williams, B.; Mancia, G.; Spiering, W.; Rosei, E.A.; Azizi, M.; Burnier, M.; Clement, D.L.; Coca, A.; de Simone, G.; Dominiczak, A.; et al. 2018 ESC/ESH Guidelines for the management of arterial hypertension The Task Force for the management of arterial hypertension of the European Society of Cardiology (ESC) and the European Society of Hypertension (ESH). Eur. Heart J. 2018, 39, 3021–3104. [Google Scholar] [CrossRef]
- Crapo, R.O.; Casaburi, R.; Coates, A.L. ATS statement: Guidelines for the six-minute walk test. Am. J. Respir. Crit. Care Med. 2002, 166, 111–117. [Google Scholar]
- Johnson, B.D.; Beck, K.C.; Olson, L.J.; O’Malley, K.A.; Allison, T.G.; Squires, R.W.; Gau, G.T. Ventilatory constraints during exercise in patients with chronic heart failure. Chest 2000, 117, 321–332. [Google Scholar] [CrossRef]
- Dyer, M.T.D.; Goldsmith, K.A.; Sharples, L.S.; Buxton, M.J. A review of health utilities using the EQ-5D in studies of cardiovascular disease. Health Qual. Life Outcomes 2010, 8, 13. [Google Scholar] [CrossRef]
- Karlsson, J.; Emgard, M.; Brundin, P.; Burkitt, M.J. Trans-Resveratrol protects embryonic mesencephalic cells from tertButyl hydroperoxide: Electron paramagnetic resonance spin trapping evidence for a radical scavenging mechanism. J. Neurochem. 2000, 75, 141–150. [Google Scholar] [CrossRef] [PubMed]
- Spanier, G.; Xu, H.; Xia, N.; Tobias, S.; Deng, S.; Wojnowski, L.; Forstermann, U.; Li, H. Resveratrol reduces endothelial oxidative stress by modulating the gene expression of superoxide dismutase 1 (SOD1), glutathione peroxidase 1 (GPX1) and NADPH oxidase subunit (NOX4). J. Physiol. Pharmacol. 2009, 60, 111–116. [Google Scholar]
- Thirunavukkarasu, M.; Penumathsa, S.V.; Koneru, S.; Juhasz, B.; Zhan, L.; Otani, H.; Bagchi, D.; Das, D.K.; Maulik, N. Resveratrol alleviates cardiac dysfunction in streptozotocin-induced diabetes: Role of nitric oxide, thioredoxin and heme oxygenase. Free Radic. Biol. Med. 2007, 43, 720–729. [Google Scholar] [CrossRef]
- Yildiz, F.; Terzi, A.; Coban, S.; Celik, H.; Aksoy, N.; Bitiren, M.; Cakir, H.; Ozdogan, M.K. Protective effects of resveratrol on small intestines against intestinal ischemia-reperfusion injury in rats. J. Gastroenterol. Hepatol. 2009, 24, 1781–1785. [Google Scholar] [CrossRef]
- Sharma, S.; Chopra, K.; Kulkarni, S.K.; Agrewala, J.N. Resveratrol and curcumin suppress immune response through CD28/CTLA-4 and CD80 co-stimulatory pathway. Clin. Exp. Immunol. 2007, 147, 155–163. [Google Scholar] [CrossRef] [PubMed]
- Shen, M.Y.; Hsiao, G.; Liu, C.L.; Fong, T.H.; Lin, K.H.; Chou, D.S.; Sheu, J.R. Inhibitory mechanisms of resveratrol in platelet activation: Pivotal roles of p38 MAPK and NO/cyclic GMP. Br. J. Haematol. 2007, 139, 475–485. [Google Scholar] [CrossRef] [PubMed]
- Cho, I.J.; Ahn, J.Y.; Kim, S.; Choi, M.S.; Ha, T.Y. Resveratrol attenuates the expression of HMG-CoA reductase mRNA in hamsters. Biochem. Biophys. Res. Commun. 2008, 367, 190–194. [Google Scholar] [CrossRef]
- Baur, J.A.; Pearson, K.J.; Price, N.L.; Jamieson, H.A.; Lerin, C.; Kalra, A.; Prabhu, V.V.; Allard, J.S.; Lopez-Lluch, G.; Lewis, K.; et al. Resveratrol improves health and survival of mice on a high-calorie diet. Nature 2006, 444, 337–342. [Google Scholar] [CrossRef]
- Lin, Y.; Zhu, M.; Su, Z. The pursuit of balance: An overview of covariate-adaptive randomization techniques in clinical trials. Contemp. Clin. Trials 2015, 45, 21–25. [Google Scholar] [CrossRef]
- Wilson, D.J.; North, N.; Wilson, R.A. Comparison of Left Ventricular Ejection Fraction Calculation Methods. Echocardiography 1998, 15, 709–712. [Google Scholar] [CrossRef]
- Cameli, M.; Mondillo, S.; Righini, F.M.; Lisi, M.; Dokollari, A.; Lindqvist, P.; Maccherini, M.; Henein, M. Left Ventricular Deformation and Myocardial Fibrosis in Patients with Advanced Heart Failure Requiring Transplantation. J. Card. Fail. 2016, 22, 901–907. [Google Scholar] [CrossRef] [PubMed]
- Qin, F.; Siwik, D.A.; Luptak, I.; Hou, X.; Wang, L.; Higuchi, A.; Weisbrod, R.M.; Ouchi, N.; Tu, V.H.; Calamaras, T.D.; et al. The polyphenols resveratrol and S17834 prevent the structural and functional sequelae of diet-induced metabolic heart disease in mice. Circulation 2012, 125, 1757–1764. [Google Scholar] [CrossRef] [PubMed]
- Sulaiman, M.; Matta, M.J.; Sunderesan, N.R.; Gupta, M.P.; Periasamy, M.; Gupta, M. Resveratrol, an activator of SIRT1, upregulates sarcoplasmic calcium ATPase and improves cardiac function in diabetic cardiomyopathy. Am. J. Physiol. Heart Circ. Physiol. 2010, 298, H833–H843. [Google Scholar] [CrossRef] [PubMed]
- Matsumura, N.; Takahara, S.; Maayah, Z.H.; Parajuli, N.; Byrne, N.J.; Shoieb, S.M.; Soltys, C.M.; Beker, D.L.; Masson, G.; El-Kadi, A.O.S.; et al. Resveratrol improves cardiac function and exercise performance in MI-induced heart failure through the inhibition of cardiotoxic HETE metabolites. J. Mol. Cell Cardiol. 2018, 125, 162–173. [Google Scholar] [CrossRef] [PubMed]
- Magyar, K.; Halmosi, R.; Palfi, A.; Feher, G.; Czop, L.; Fulop, A.; Battyany, I.; Sumegi, B.; Toth, K.; Szabados, E. Cardioprotection by resveratrol: A human clinical trial in patients with stable coronary artery disease. Clin. Hemorheol. Microcirc. 2012, 50, 179–187. [Google Scholar] [CrossRef]
- Hart, N.; Sarga, L.; Csende, Z.; Koltai, E.; Koch, L.G.; Britton, S.L.; Davies, K.J.; Kouretas, D.; Wessner, B.; Radak, Z. Resveratrol enhances exercise training responses in rats selectively bred for high running performance. Food Chem. Toxicol. 2018, 61, 53–59. [Google Scholar] [CrossRef]
- Voduc, N.; la Porte, C.; Tessier, C.; Mallick, R.; Cameron, D.W. Effect of resveratrol on exercise capacity: A randomized placebo-controlled crossover pilot study. Appl. Physiol. Nutr. Metab. 2014, 39, 1183–1187. [Google Scholar] [CrossRef]
- Gliemann, L.; Schmidt, J.F.; Olesen, J.; Biensø, R.S.; Peronard, S.L.; Grandjean, S.U.; Mortensen, S.P.; Nyberg, M.; Bangsbo, J.; Pilegaard, H.; et al. Resveratrol blunts the positive effects of exercise training on cardiovascular health in aged men. J. Physiol. 2013, 591, 5047–5059. [Google Scholar] [CrossRef]
- Amin, H.Z.; Amin, L.Z.; Wijaya, I.P. Galectin-3: A novel biomarker for the prognosis of heart failure. Clujul. Med. 2017, 90, 129–132. [Google Scholar] [CrossRef]
- Brinchmann, M.F.; Patel, D.M.; Iversen, M.H. The Role of Galectins as Modulators of Metabolism and Inflammation. Mediators Inflamm. 2018, 2018, 9186940. [Google Scholar] [CrossRef]
- Militaru, C.; Donoiu, I.; Craciun, A.; Scorei, I.D.; Bulearca, A.M.; Scorei, R.I. Oral resveratrol and calcium fructoborate supplementation in subjects with stable angina pectoris: Effects on lipid profiles, inflammation markers, and quality of life. Nutrition 2013, 29, 178–183. [Google Scholar] [CrossRef] [PubMed]
- Gaggin, H.K.; Januzzi, J.L. Biomarkers and diagnostics in heart failure. Biochim. Biophys. Acta 2013, 1832, 2442–2450. [Google Scholar] [CrossRef] [PubMed]
- Chase, S.C.; Fermoyle, C.C.; Wheatley, C.M.; Schaefer, J.J.; Olson, L.J.; Johnson, B.D. The effect of diuresis on extravascular lung water and pulmonary function in acute decompensated heart failure. ESC Heart Fail. 2018, 5, 364–371. [Google Scholar] [CrossRef] [PubMed]
- Gehlbach, B.K.; Geppert, E. The pulmonary manifestations of left heart failure. Chest 2004, 125, 669–682. [Google Scholar] [CrossRef] [PubMed]
- Malaguarnera, L. Influence of resveratrol on the immune response. Nutrients 2019, 11, 946. [Google Scholar] [CrossRef] [PubMed]
- Yan, A.T.; Yan, R.T.; Cushman, M.; Redheuil, A.; Tracy, R.P.; Arnett, D.K.; Rosen, B.D.; McClelland, R.L.; Bluemke, D.A.; Lima, J.A.C. Relationship of interleukin-6 with regional and global left-ventricular function in asymptomatic individuals without clinical cardiovascular disease: Insights from the Multi-Ethnic Study of Atherosclerosis. Eur. Heart J. 2010, 31, 875–882. [Google Scholar] [CrossRef]
- Mann, D.L. Innate immunity and the failing heart: The cytokine hypothesis revisited. Circ. Res. 2015, 116, 1254–1268. [Google Scholar] [CrossRef]
| Inclusion Criteria | Exclusion Criteria |
|---|---|
|
|
|
|
|
|
|
|
|
|
| |
| |
| |
| |
| |
|
| Baseline Characteristics | Resveratrol (n = 30) | Placebo (n = 30) |
|---|---|---|
| Age (year) | 65.87 ± 1.91 | 67.53 ± 2.14 |
| Male | 22 (73%) | 21 (70%) |
| Ejection fraction (%) | 30.06 ± 1.04 | 31.70 ± 1.27 |
| NT-proBNP (pg/mL) | 2998 ± 507 | 3139 ± 446 |
| Serum creatinine (μmol/L) | 99.77 ± 4.42 | 104.47 ± 4.82 |
| Systolic BP (Hgmm) | 132.47 ± 3.41 | 128.77 ± 3.80 |
| Diastolic BP (Hgmm) | 79.10 ± 2.33 | 80.13 ± 2.66 |
| Heart rate (beat/min) | 72.20 ± 2.75 | 76.93 ± 2.50 |
| Etiological factors | ||
| Ischemic heart disease | 17 (56.7%) | 17 (56.7%) |
| Non-ischemic (alcohol, chemotherapy, myocarditis) | 13 (43.3%) | 13 (43.3%) |
| Risk factors, comorbidities | ||
| Hypertension | 22 (73%) | 23 (76%) |
| Diabetes | 13 (43%) | 14 (46%) |
| Smoking | 11 (36%) | 8 (27%) |
| Pulmonary diseases (asthma, COPD) | 7 (23%) | 8 (27%) |
| BMI (kg/m2) | 29.3 ± 0.9 | 30.4 ± 1.3 |
| Target heart rate (<70/min) | 23 (76.7%) | 20 (66.7%) |
| Atrial fibrillation | 7 (23%) | 10 (33.3%) |
| Concomitant treatment | ||
| ACE-inhibitor/ARB | 28 (93%) | 29 (97%) |
| Beta-blocker | 29 (97%) | 28 (93%) |
| MRA | 23 (76.7%) | 21 (70%) |
| Ivabradine | 6 (20%) | 6 (20%) |
| Diuretics | ||
| Loop diuretics (furosemide, etacrynic acid) | 27 (90%) | 28 (93%) |
| Thiazide or thiazide-like diuretics (hypothiazide, indapamide, etc.) | 8 (27%) | 9 (30%) |
| Device therapy | ||
| CRT-P/D | 9 (30%) | 7 (23.2%) |
| ICD | 4 (13%) | 3 (10%) |
| Examinations | Randomization | 1-Month | 3-Month |
|---|---|---|---|
| Physical examination | ✓ | ✓ | ✓ |
| Blood pressure monitoring | ✓ | ✓ | ✓ |
| 12-lead ECG | ✓ | ✓ | ✓ |
| Lab test | ✓ | ✓ | ✓ |
| NT-proBNP level | ✓ | ✓ | |
| mRNA analysis | ✓ | ✓ | |
| Echocardiography | ✓ | ✓ | |
| 6MWT | ✓ | ✓ | |
| Quality of life test (EQ-5D) | ✓ | ✓ | |
| Spirometry | ✓ | ✓ |
| Lab Parameters | Baseline | 3rd Month | ||
|---|---|---|---|---|
| Resveratrol | Placebo | Resveratrol | Placebo | |
| HgbA1c (%) | 6.37 ± 0.18 | 6.70 ± 0.24 | 6.38 ± 0.20 | 6.67 ± 0.23 |
| BUN (mmol/L) | 7.75 ± 0.45 | 8.80 ± 0.84 | 8.26 ± 0.44 | 8.75 ± 0.93 |
| Se creatinine (μmol/L) | 99.77 ± 4.42 | 104.47 ± 4.82 | 102.19 ± 5.71 | 109.31 ± 5.92 |
| TC (mmol/L) | 4.74 ± 0.22 # | 4.15 ± 0.23 | 4.52 ± 0.24 * # | 4.04 ± 0.28 |
| HDL-C (mmol/L) | 1.33 ± 0.06 | 1.29 ± 0.08 | 1.27 ± 0.07 * | 1.25 ± 0.06 |
| LDL-C (mmol/L) | 3.09 ± 0.23 # | 2.51 ± 0.21 | 2.79 ± 0.23 * # | 2.41 ± 0.23 |
| TG (mmol/L) | 1.91 ± 0.20 # | 1.61 ± 0.17 | 1.93 ± 0.21 # | 1.57 ± 0.22 |
| ALT (IU/L) | 25.87 ± 1.71 | 28.86 ± 1.58 | 27.33 ± 1.80 | 30.62 ± 2.39 |
| AST (IU/L) | 22.73 ± 2.35 | 28.07 ± 1.99 | 25.27 ± 3.42 | 26.93 ± 1.99 |
| cTroponin T (ng/L) | 23.26 ± 3.13 | 23.60 ± 2.56 | 22.25 ± 2.71 | 25.52 ± 2.91 |
| Se albumin (g/L) | 44.20 ± 0.56 | 44.82 ± 0.53 | 45.45 ± 0.58 | 46.05 ± 0.51 |
| Se Fe (μmol/L) | 15.11 ± 1.10 | 14.27 ± 1.13 | 15.54 ± 1.08 | 14.83 ± 1.02 |
| Transf. sat. (%) | 21.59 ± 0.84 | 20.08 ± 1.58 | 21.57 ± 1.60 | 20.37 ± 1.39 |
| Se ferritin (μg/L) | 151.1 ± 19.60 | 148.2 ± 18.46 | 138.5 ± 20.5 | 133.6 ± 15.85 |
| CRP (mg/L) | 4.66 ± 0.50 | 5.33 ± 0.83 | 4.23 ± 0.67 | 4.94 ± 0.73 |
| WBC (G/L) | 7.41 ± 0.33 | 7.73 ± 0.37 | 7.04 ± 0.29 | 7.78 ± 0.35 |
| Hct (%) | 42.00 ± 1.02 | 41.71 ± 0.90 | 41.69 ± 1.06 | 41.89 ± 0.94 |
| PLT (G/L) | 219.3 ± 15.38 | 217.3 ± 13.40 | 207.7 ± 11.3 | 206.6 ± 10.90 |
| Parameters | Baseline | 3rd Month | ||
|---|---|---|---|---|
| Resveratrol | Placebo | Resveratrol | Placebo | |
| BW (kg) | 85.53 ± 3.09 | 89.97 ± 5.06 | 84.77 ± 3.19 | 90.80 ± 4.77 |
| BMI (kg/m2) | 29.31 ± 0.99 | 31.04 ± 1.46 | 29.06 ± 1.01 | 31.34 ± 1.39 |
| Systolic BP (Hgmm) | 132.5 ± 3.41 | 128.8 ± 3.80 | 131.6 ± 3.21 | 125.8 ± 2.65 |
| Diastolic BP (Hgmm) | 79.10 ± 2.33 | 80.13 ± 2.66 | 77.83 ± 1.82 | 76.30 ± 2.24 |
| HR (beat/min) | 72.20 ± 2.75 | 76.93 ± 2.50 | 70.04 ± 2.87 | 70.73 ± 2.26 |
| PQ (ms) | 164.1 ± 5.37 | 187.9 ± 5.67 | 162.1 ± 6.28 | 189.9 ± 5.68 |
| QRS (ms) | 127.8 ± 5.35 | 137.3 ± 6.19 | 136.5 ± 5.68 | 132.9 ± 5.84 |
| QTc (ms) | 463.7 ± 5.03 | 476.9 ± 6.65 | 469.9 ± 5.99 | 471.2 ± 5.29 |
| Echocardiographic Parameters | Baseline | 3rd Month | ||
|---|---|---|---|---|
| Resveratrol | Placebo | Resveratrol | Placebo | |
| EF (Quinone, %) | 29.19 ± 1.04 | 30.16 ± 1.10 | 33.40 ± 1.20 ** # | 29.79 ± 0.95 |
| EF (Simson, %) | 30.06 ± 1.04 | 31.70 ± 1.27 | 34.60 ± 1.38 ** # | 30.41 ± 1.36 |
| LVIDd (mm) | 65.5 ± 1.55 | 62.83 ± 1.12 | 65.09 ± 1.44 | 62.62 ± 1.12 |
| LVIDs (mm) | 54.87 ± 1.67 | 51.90 ± 1.06 | 52.88 ± 1.62 | 51.67 ± 1.06 |
| LVEDV (mL) | 182.4 ± 11.73 | 157.3 ± 7.92 | 176.7 ± 10.05 | 160.1 ± 8.01 |
| LVESV (mL) | 129.0 ± 9.37 | 106.7 ± 5.80 | 116.3 ± 8.00 ** | 111.4 ± 5.60 |
| SV (mL) | 52.85 ± 2.82 | 50.69 ± 3.38 | 60.46 ± 3.13 ** # | 48.56 ± 3.84 |
| SW (mm) | 11.47 ± 0.28 | 11.83 ± 0.31 | 11.43 ± 0.29 | 11.83 ± 0.31 |
| PW (mm) | 11.03 ± 0.17 | 11.17 ± 0.20 | 11.20 ± 0.17 | 11.14 ± 0.21 |
| GLS (%) | −8.40 ± 0.62 | −8.80 ± 0.75 | −9.58 ± 0.73 * # | −8.45 ± 0.75 |
| E (m/s) | 0.89 ± 0.04 | 0.98 ± 0.05 | 0.80 ± 0.05 * # | 1.00 ± 0.05 |
| A (m/s) | 0.78 ± 0.04 | 0.71 ± 0.05 | 0.78 ± 0.04 | 0.76 ± 0.06 |
| E’ | 0.05 ± 0.002 | 0.05 ± 0.003 | 0.06 ± 0.003 | 0.05 ± 0.003 |
| E/A ratio | 1.26 ± 0.12 | 1.57 ± 0.20 | 1.18 ± 0.15 # | 1.54 ± 0.19 |
| E/E’ ratio | 17.65 ± 1.31 | 19.32 ± 1.21 | 15.55 ± 1.43 # | 19.94 ± 1.43 |
| Echocardiographic Parameters | Baseline | 3rd Month | ||
|---|---|---|---|---|
| Resveratrol | Placebo | Resveratrol | Placebo | |
| RVIDd-mid (mm) | 27.86 ± 1.14 | 23.90 ± 1.02 | 25.09 ± 1.09 | 26.96 ± 0.98 * |
| RVIDd-basal (mm) | 37.43 ± 1.43 | 34.14 ± 1.10 | 34.83 ± 1.12 * | 37.03 ± 1.17 * |
| RVIDs (mm) | 30.34 ± 1.21 | 29.43 ± 1.26 | 28.00 ± 1.05 * | 29.86 ± 1.13 |
| TAPSE (mm) | 16.24 ± 0.74 | 16.58 ± 0.74 | 17.96 ± 0.82 # | 15.76 ± 0.63 |
| LA long axis (mm) | 64.03 ± 1.68 | 64.21 ± 1.25 | 61.79 ± 1.72 * # | 64.17 ± 1.14 |
| LA short axis (mm) | 46.14 ± 0.98 | 45.38 ± 0.93 | 45.29 ± 1.03 | 45.45 ± 0.99 |
| LA area (cm2) | 27.11 ± 1.33 | 27.95 ± 1.10 | 25.99 ± 1.35 | 28.05 ± 1.10 |
| LAEDV (mL) | 59.96 ± 5.24 | 63.68 ± 4.21 | 50.74 ± 3.14 # | 66.24 ± 4.79 |
| LAESV (mL) | 87.52 ± 5.37 | 87.95 ± 4.69 | 78.12 ± 3.99 * # | 88.00 ± 4.92 |
| RA long axis (mm) | 58.34 ± 2.13 | 58.59 ± 1.63 | 56.72 ± 2.28 | 60.10 ± 1.65 |
| RA short axis (mm) | 41.34 ± 1.21 | 41.00 ± 1.11 | 39.86 ± 1.37 | 40.31 ± 1.04 |
| RA area (cm2) | 21.20 ± 2.12 | 21.28 ± 1.30 | 20.08 ± 2.09 | 21.56 ± 1.33 |
| RAEDV (mL) | 42.87 ± 4.44 | 44.24 ± 5.18 | 34.50 ± 3.47 * # | 43.14 ± 4.68 |
| RAESV (mL) | 54.63 ± 5.15 | 60.23 ± 5.53 | 49.32 ± 3.50 # | 63.04 ± 5.64 |
| IVC (mm) | 16.08 ± 1.01 | 17.38 ± 0.97 | 15.24 ± 0.89 # | 18.79 ± 0.84 |
| Spirometric Parameters | Baseline | 3rd Month | ||
|---|---|---|---|---|
| Resveratrol | Placebo | Resveratrol | Placebo | |
| FVC (%) | 75.38 ± 3.31 | 74.41 ± 3.75 | 79.27 ± 3.07 * | 76.91 ± 4.08 |
| FEV1 (%) | 64.67 ± 3.22 | 65.10 ± 3.67 | 67.38 ± 3.00 | 65.90 ± 3.98 |
| FEV1/FVC ratio (%) | 89.79 ± 1.84 | 94.63 ± 2.18 | 89.29 ± 2.33 | 93.74 ± 2.80 |
| PEF (%) | 56.50 ± 3.01 | 55.91 ± 3.67 | 57.13 ± 3.28 | 59.09 ± 3.89 |
| FEF 25-75 (%) | 55.92 ± 3.79 | 55.50 ± 3.80 | 55.81 ± 3.98 | 58.38 ± 4.82 |
| MEF75 (%) | 58.50 ± 3.60 | 56.68 ± 3.50 | 60.85 ± 3.99 | 60.90 ± 4.33 |
| MEF50 (%) | 55.65 ± 4.10 | 52.95 ± 3.83 | 55.35 ± 3.77 | 57.14 ± 4.09 |
| MEF25 (%) | 52.35 ± 4.30 | 55.95 ± 5.16 | 53.23 ± 4.73 | 56.21 ± 4.94 |
| IVC (%) | 83.35 ± 3.65 | 81.32 ± 3.60 | 90.27 ± 3.39 * | 86.36 ± 3.91 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gal, R.; Deres, L.; Horvath, O.; Eros, K.; Sandor, B.; Urban, P.; Soos, S.; Marton, Z.; Sumegi, B.; Toth, K.; et al. Resveratrol Improves Heart Function by Moderating Inflammatory Processes in Patients with Systolic Heart Failure. Antioxidants 2020, 9, 1108. https://doi.org/10.3390/antiox9111108
Gal R, Deres L, Horvath O, Eros K, Sandor B, Urban P, Soos S, Marton Z, Sumegi B, Toth K, et al. Resveratrol Improves Heart Function by Moderating Inflammatory Processes in Patients with Systolic Heart Failure. Antioxidants. 2020; 9(11):1108. https://doi.org/10.3390/antiox9111108
Chicago/Turabian StyleGal, Roland, Laszlo Deres, Orsolya Horvath, Krisztian Eros, Barbara Sandor, Peter Urban, Szilvia Soos, Zsolt Marton, Balazs Sumegi, Kalman Toth, and et al. 2020. "Resveratrol Improves Heart Function by Moderating Inflammatory Processes in Patients with Systolic Heart Failure" Antioxidants 9, no. 11: 1108. https://doi.org/10.3390/antiox9111108
APA StyleGal, R., Deres, L., Horvath, O., Eros, K., Sandor, B., Urban, P., Soos, S., Marton, Z., Sumegi, B., Toth, K., Habon, T., & Halmosi, R. (2020). Resveratrol Improves Heart Function by Moderating Inflammatory Processes in Patients with Systolic Heart Failure. Antioxidants, 9(11), 1108. https://doi.org/10.3390/antiox9111108





