Tackling Chronic Inflammation with Withanolide Phytochemicals—A Withaferin A Perspective
Abstract
1. Introduction
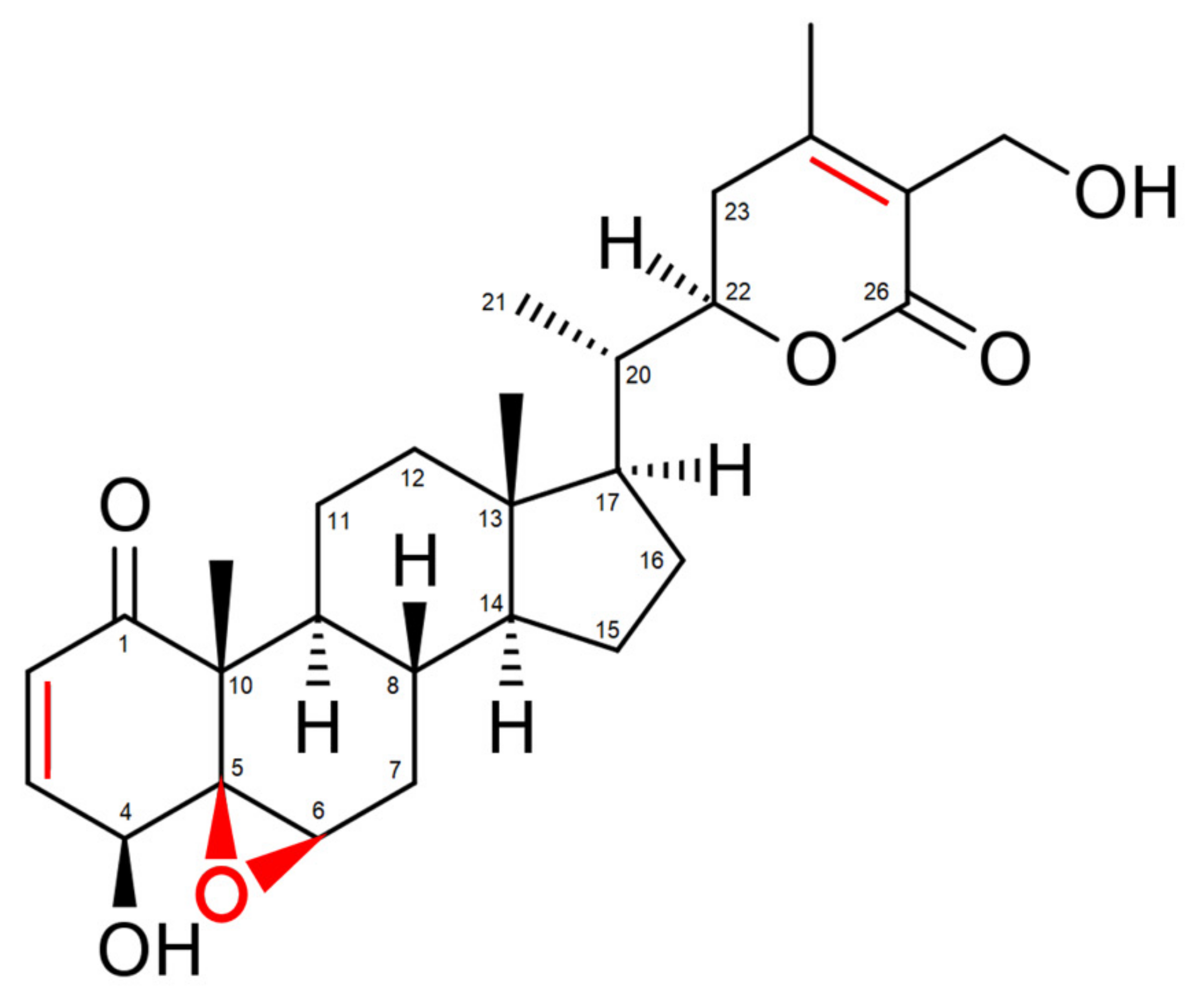
2. Withaferin A: The Major Bioactive Constituent Isolated from Withania somnifera
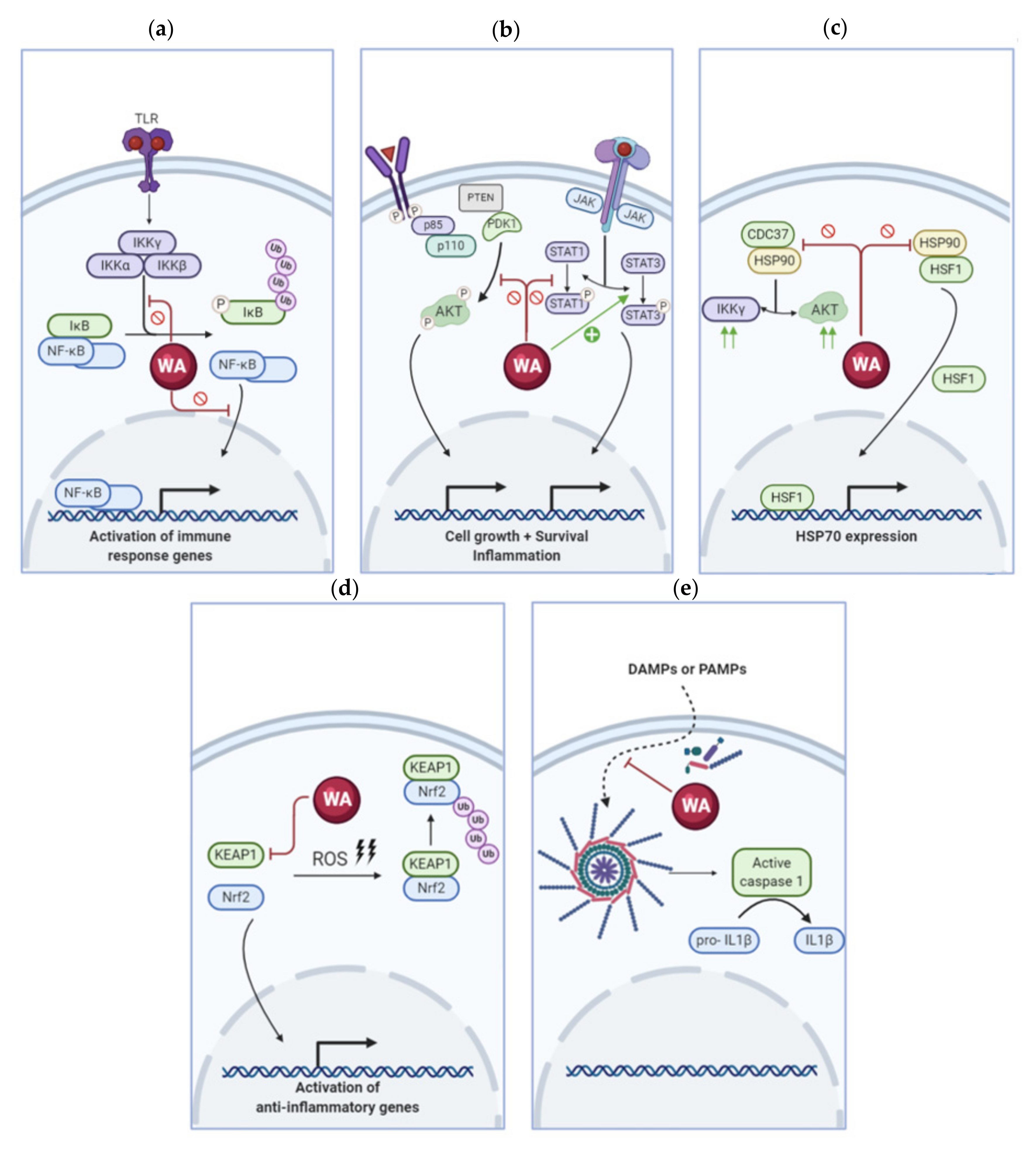
3. Molecular Targets of Withaferin A in the Inflammatory Response Pathway
3.1. Withaferin A Inhibits the NF-κB Pathway
3.2. Modulation of Kinase Activity by Withaferin A
| Chronic Disease | Animal Model | Dose WA (mg/kg) | Notable Changes Observed | Reference(s) |
|---|---|---|---|---|
| Amyotrophic lateral sclerosis | TDP-43 transgenic C57BL/6J mice | 3 mg/kg | Reduced inflammation Improved motor behavior | [51] |
| SOD1 transgenic C57BL/6J mice | 4 mg/kg | Extended survival Reduction of early neuronal injury response Reduced SOD1 misfolding Reduced neuroinflammatory signals | [52] | |
| Gliosis | Excitotoxic model of inner retinal injury in C57BL/6J mice | 2 mg/kg | Reduced inner retinal apoptosis Reduced levels of intermediate filaments | [53] |
| Parkinson’s disease | Age-mediated impairment of the dopamine system in Wistar Albino rats | 50 mg/kg | Improved motor behavior Resurge of dopamine in substantia nigra and striatum | [54,55] |
| Liver fibrosis | Bile duct ligation induced liver fibrosis in C57BL/6J mice | 1–3 mg/kg | Restoration of liver function and tissue architecture Reduction of collagen deposition Attenuated oxidative stress Reduced NF-κB expression | [44] |
| Obesity | High fat diet induced obesity in C57BL/6J mice | 1.25 mg/kg | Reduced obesity-associated abnormalities (hepatic steatosis) Increased leptin potency Improved hepatic insulin sensitivity Enhanced glucose tolerance and glucose homeostasis Reduction in pro-inflammatory cytokines Enhanced lipid and glucose metabolism | [56,57] |
| High fat diet induced obesity in C57BL/6J mice | 10 mg/kg | Reduced weight gain Lower epididymal and mesenteric fat pad mass Improved lipid profile Reduction in inflammatory cytokines | [58] | |
| Diabetes | Streptozotocin induced diabetes in Swiss albino mice | 10 mg/kg | Reduced diabetes incidence Reduced hyperglycemia Improved glucose clearance | [36] |
| Arthritis | Monosodium urate crystal- induced inflammation as Gouty arthritis model | 30 mg/kg | Reduced paw volume Reduced lipid peroxidation Reduced TNF-α levels Reduced levels of β-glucuronidase and lactate dehydrogenase | [59] |
| Intradermal induction of rheumatoid arthritis in Albino Wistar rats | 30 mg/kg free WA or 10 mg/kg liposomal WA | Reduced oxidative stress Reduced paw edema No bone erosion or cartilage degradation Increase in anti-inflammatory cytokines (IL-10) | [60] |
3.3. Withaferin A Regulates Heat Shock Proteins
3.4. Withaferin A Alters the Cellular Redox Balance through Nrf2 Regulation
3.5. Withaferin A Affects Inflammasome Activation
3.6. Other Inflammatory Factors Targeted by Withaferin A
4. Future Perspectives
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Chen, L.; Deng, H.; Cui, H.; Fang, J.; Zuo, Z.; Deng, J.; Li, Y.; Wang, X.; Zhao, L. Inflammatory responses and inflammation-associated diseases in organs. Oncotarget 2018, 9, 7204–7218. [Google Scholar] [CrossRef] [PubMed]
- Carrera Silva, E.A.; Chan, P.Y.; Joannas, L.; Errasti, A.E.; Gagliani, N.; Bosurgi, L.; Jabbour, M.; Perry, A.; Smith-Chakmakova, F.; Mucida, D.; et al. T cell-derived protein S engages TAM receptor signaling in dendritic cells to control the magnitude of the immune response. Immunity 2013, 39, 160–170. [Google Scholar] [CrossRef] [PubMed]
- Dalpke, A.; Heeg, K.; Bartz, H.; Baetz, A. Regulation of innate immunity by suppressor of cytokine signaling (SOCS) proteins. Immunobiology 2008, 213, 225–235. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.L.; Lv, J.J.; Lv, J.; Di, C.X.; Zhang, Y.J.; Zhou, T.; Liu, J.L.; Xia, Z.W. Heme oxygenase-1 directly binds STAT3 to control the generation of pathogenic Th17 cells during neutrophilic airway inflammation. Allergy 2017, 72, 1972–1987. [Google Scholar] [CrossRef]
- Bachmaier, K.; Krawczyk, C.; Kozieradzki, I.; Kong, Y.Y.; Sasaki, T.; Oliveira dos Santos, A.; Mariathasan, S.; Bouchard, D.; Wakeham, A.; Itie, A.; et al. Negative regulation of lymphocyte activation and autoimmunity by the molecular adaptor Cbl-b. Nature 2000, 403, 211–216. [Google Scholar] [CrossRef]
- Schmidt, C.Q.; Lambris, J.D.; Ricklin, D. Protection of host cells by complement regulators. Immunol. Rev. 2016, 274, 152–171. [Google Scholar] [CrossRef]
- Lawrence, T.; Gilroy, D.W. Chronic inflammation: A failure of resolution? Int. J. Exp. Pathol. 2007, 88, 85–94. [Google Scholar] [CrossRef]
- Fleit, H.B. Chronic Inflammation. In Pathobiology of Human Disease; McManus, L.M., Mitchell, R.N., Eds.; Academic Press: San Diego, CA, USA, 2014; pp. 300–314. [Google Scholar]
- Valgimigli, M.; Ceconi, C.; Malagutti, P.; Merli, E.; Soukhomovskaia, O.; Francolini, G.; Cicchitelli, G.; Olivares, A.; Parrinello, G.; Percoco, G.; et al. Tumor necrosis factor-alpha receptor 1 is a major predictor of mortality and new-onset heart failure in patients with acute myocardial infarction: The Cytokine-Activation and Long-Term Prognosis in Myocardial Infarction (C-ALPHA) study. Circulation 2005, 111, 863–870. [Google Scholar] [CrossRef]
- Libby, P. Inflammation and cardiovascular disease mechanisms. Am. J. Clin. Nutr. 2006, 83, 456S–460S. [Google Scholar] [CrossRef]
- Crook, M. Type 2 diabetes mellitus: A disease of the innate immune system? An update. Diabet. Med. 2004, 21, 203–207. [Google Scholar] [CrossRef]
- Festa, A.; D’Agostino, R., Jr.; Howard, G.; Mykkanen, L.; Tracy, R.P.; Haffner, S.M. Chronic subclinical inflammation as part of the insulin resistance syndrome: The Insulin Resistance Atherosclerosis Study (IRAS). Circulation 2000, 102, 42–47. [Google Scholar] [CrossRef] [PubMed]
- Wong, S.H.; Lord, J.M. Factors underlying chronic inflammation in rheumatoid arthritis. Arch. Immunol. Ther. Exp. Warsz. 2004, 52, 379–388. [Google Scholar] [PubMed]
- Woo, J.H.; Lee, J.H.; Kim, H.; Park, S.J.; Joe, E.H.; Jou, I. Control of Inflammatory Responses: A New Paradigm for the Treatment of Chronic Neuronal Diseases. Exp. Neurobiol. 2015, 24, 95–102. [Google Scholar] [CrossRef] [PubMed]
- Mantovani, A.; Allavena, P.; Sica, A.; Balkwill, F. Cancer-related inflammation. Nature 2008, 454, 436–444. [Google Scholar] [CrossRef] [PubMed]
- WHO. Preventing Chronic Diseases—A Vital Investment. Available online: https://www.who.int/chp/chronic_disease_report/en/ (accessed on 26 September 2020).
- Dinarello, C.A. Anti-inflammatory Agents: Present and Future. Cell 2010, 140, 935–950. [Google Scholar] [CrossRef]
- Pahwa, R.; Jialal, I. Chronic Inflammation; StatPearls Publishing: Treasure Island, FL, USA, 2019. [Google Scholar]
- Dunbar-Jacob, J.; Mortimer-Stephens, M.K. Treatment adherence in chronic disease. J. Clin. Epidemiol. 2001, 54 (Suppl. 1), S57–S60. [Google Scholar] [CrossRef]
- Liu, D.; Ahmet, A.; Ward, L.; Krishnamoorthy, P.; Mandelcorn, E.D.; Leigh, R.; Brown, J.P.; Cohen, A.; Kim, H. A practical guide to the monitoring and management of the complications of systemic corticosteroid therapy. Allergy Asthma Clin. Immunol. 2013, 9, 30. [Google Scholar] [CrossRef]
- Owona, B.A.; Abia, W.A.; Moundipa, P.F. Natural compounds flavonoids as modulators of inflammasomes in chronic diseases. Int. Immunopharmacol. 2020, 84, 106498. [Google Scholar] [CrossRef]
- Apetz, N.; Munch, G.; Govindaraghavan, S.; Gyengesi, E. Natural compounds and plant extracts as therapeutics against chronic inflammation in Alzheimer’s disease—A translational perspective. CNS Neurol. Disord. Drug. Targets 2014, 13, 1175–1191. [Google Scholar] [CrossRef]
- Kunnumakkara, A.B.; Bordoloi, D.; Padmavathi, G.; Monisha, J.; Roy, N.K.; Prasad, S.; Aggarwal, B.B. Curcumin, the golden nutraceutical: Multitargeting for multiple chronic diseases. Br. J. Pharmacol. 2017, 174, 1325–1348. [Google Scholar] [CrossRef]
- Tangney, C.C.; Rasmussen, H.E. Polyphenols, inflammation, and cardiovascular disease. Curr. Atheroscler. Rep. 2013, 15, 324. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, B.B.; Prasad, S.; Reuter, S.; Kannappan, R.; Yadev, V.R.; Park, B.; Kim, J.H.; Gupta, S.C.; Phromnoi, K.; Sundaram, C.; et al. Identification of novel anti-inflammatory agents from Ayurvedic medicine for prevention of chronic diseases: “reverse pharmacology” and “bedside to bench” approach. Curr. Drug Targets 2011, 12, 1595–1653. [Google Scholar] [CrossRef] [PubMed]
- Altman, R.D.; Marcussen, K.C. Effects of a ginger extract on knee pain in patients with osteoarthritis. Arthritis Rheum. 2001, 44, 2531–2538. [Google Scholar] [CrossRef]
- Jurenka, J.S. Anti-inflammatory properties of curcumin, a major constituent of Curcuma longa: A review of preclinical and clinical research. Altern. Med. Rev. 2009, 14, 141–153. [Google Scholar] [PubMed]
- Nunes, C.D.R.; Barreto Arantes, M.; Menezes de Faria Pereira, S.; Leandro da Cruz, L.; de Souza Passos, M.; Pereira de Moraes, L.; Vieira, I.J.C.; Barros de Oliveira, D. Plants as Sources of Anti-Inflammatory Agents. Molecules 2020, 25. [Google Scholar] [CrossRef]
- Vanden Berghe, W.; Sabbe, L.; Kaileh, M.; Haegeman, G.; Heyninck, K. Molecular insight in the multifunctional activities of Withaferin A. Biochem. Pharmacol. 2012, 84, 1282–1291. [Google Scholar] [CrossRef]
- Rastogi, R.P.M.B. Compendium of Indian Medicinal Plants; Central Drug Research Institute: New Delhi, India, 1998; p. 6. [Google Scholar]
- Sun, G.Y.; Li, R.; Cui, J.; Hannink, M.; Gu, Z.; Fritsche, K.L.; Lubahn, D.B.; Simonyi, A. Withania somnifera and Its Withanolides Attenuate Oxidative and Inflammatory Responses and Up-Regulate Antioxidant Responses in BV-2 Microglial Cells. Neuromol. Med. 2016, 18, 241–252. [Google Scholar] [CrossRef]
- Khedgikar, V.; Ahmad, N.; Kushwaha, P.; Gautam, J.; Nagar, G.K.; Singh, D.; Trivedi, P.K.; Mishra, P.R.; Sangwan, N.S.; Trivedi, R. Preventive effects of Withaferin A isolated from the leaves of an Indian medicinal plant Withania somnifera (L.): Comparisons with 17-β-estradiol and alendronate. Nutrition 2015, 31, 205–213. [Google Scholar] [CrossRef]
- Chandrasekaran, S.; Veronica, J.; Sundar, S.; Maurya, R. Alcoholic Fractions F5 and F6 from Withania somnifera Leaves Show a Potent Antileishmanial and Immunomodulatory Activities to Control Experimental Visceral Leishmaniasis. Front. Med. 2017, 4, 55. [Google Scholar] [CrossRef]
- Jayaprakasam, B.; Zhang, Y.; Seeram, N.P.; Nair, M.G. Growth inhibition of human tumor cell lines by withanolides from Withania somnifera leaves. Life Sci. 2003, 74, 125–132. [Google Scholar] [CrossRef]
- Maitra, R.; Porter, M.A.; Huang, S.; Gilmour, B.P. Inhibition of NF-kappaB by the natural product Withaferin A in cellular models of Cystic Fibrosis inflammation. J. Inflamm. Lond. Engl. 2009, 6, 15. [Google Scholar] [CrossRef] [PubMed]
- Tekula, S.; Khurana, A.; Anchi, P.; Godugu, C. Withaferin A attenuates multiple low doses of Streptozotocin (MLD-STZ) induced type 1 diabetes. Biomed. Pharmacother. 2018, 106, 1428–1440. [Google Scholar] [CrossRef] [PubMed]
- Bale, S.; Venkatesh, P.; Sunkoju, M.; Godugu, C. An Adaptogen: Withaferin A Ameliorates in Vitro and in Vivo Pulmonary Fibrosis by Modulating the Interplay of Fibrotic, Matricelluar Proteins, and Cytokines. Front. Pharmacol. 2018, 9, 248. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Zhang, L.; Joo, D.; Sun, S.C. NF-kappaB signaling in inflammation. Signal Transduct. Target Ther. 2017, 2. [Google Scholar] [CrossRef] [PubMed]
- Oeckinghaus, A.; Ghosh, S. The NF-kappaB family of transcription factors and its regulation. Cold Spring Harb. Perspect. Biol. 2009, 1, a000034. [Google Scholar] [CrossRef] [PubMed]
- White, P.T.; Subramanian, C.; Motiwala, H.F.; Cohen, M.S. Natural Withanolides in the Treatment of Chronic Diseases. Adv. Exp. Med. Biol. 2016, 928, 329–373. [Google Scholar] [PubMed]
- Grover, A.; Shandilya, A.; Punetha, A.; Bisaria, V.S.; Sundar, D. Inhibition of the NEMO/IKKbeta association complex formation, a novel mechanism associated with the NF-kappaB activation suppression by Withania somnifera’s key metabolite Withaferin A. BMC Genom. 2010, 11 (Suppl 4), S25. [Google Scholar] [CrossRef]
- Heyninck, K.; Lahtela-Kakkonen, M.; Van der Veken, P.; Haegeman, G.; Vanden Berghe, W. Withaferin A inhibits NF-kappaB activation by targeting cysteine 179 in IKKβ. Biochem. Pharmacol. 2014, 91, 501–509. [Google Scholar] [CrossRef]
- Kaileh, M.; Vanden Berghe, W.; Heyerick, A.; Horion, J.; Piette, J.; Libert, C.; De Keukeleire, D.; Essawi, T.; Haegeman, G. Withaferin A strongly elicits IkappaB kinase beta hyperphosphorylation concomitant with potent inhibition of its kinase activity. J. Biol. Chem. 2007, 282, 4253–4264. [Google Scholar] [CrossRef]
- Sayed, N.; Khurana, A.; Saifi, M.A.; Singh, M.; Godugu, C. Withaferin A reverses bile duct ligation-induced liver fibrosis by modulating extracellular matrix deposition: Role of LOXL2/Snail1, vimentin, and NFκB signaling. Biofactors 2019, 45, 959–974. [Google Scholar] [CrossRef]
- Bale, S.; Pulivendala, G.; Godugu, C. Withaferin A attenuates bleomycin-induced scleroderma by targeting FoxO3a and NF-κβ signaling: Connecting fibrosis and inflammation. Biofactors 2018, 44, 507–517. [Google Scholar] [CrossRef] [PubMed]
- Ashkenazi, S.; Plotnikov, A.; Bahat, A.; Ben-Zeev, E.; Warszawski, S.; Dikstein, R. A Novel Allosteric Mechanism of NF-κB Dimerization and DNA Binding Targeted by an Anti-Inflammatory Drug. Mol. Cell. Biol. 2016, 36, 1237–1247. [Google Scholar] [CrossRef] [PubMed]
- Purushotham, P.M.; Kim, J.M.; Jo, E.K.; Senthil, K. Withanolides against TLR4-Activated Innate Inflammatory Signalling Pathways: A Comparative Computational and Experimental Study. Phytother. Res. PTR 2017, 31, 152–163. [Google Scholar] [CrossRef] [PubMed]
- Atluri, V.S.R.; Tiwari, S.; Rodriguez, M.; Kaushik, A.; Yndart, A.; Kolishetti, N.; Yatham, M.; Nair, M. Inhibition of Amyloid-Beta Production, Associated Neuroinflammation, and Histone Deacetylase 2-Mediated Epigenetic Modifications Prevent Neuropathology in Alzheimer’s Disease in vitro Model. Front. Aging Neurosci. 2019, 11, 342. [Google Scholar] [CrossRef] [PubMed]
- Kontzias, A.; Laurence, A.; Gadina, M.; O’Shea, J.J. Kinase inhibitors in the treatment of immune-mediated disease. F1000 Med. Rep. 2012, 4, 5. [Google Scholar] [CrossRef] [PubMed]
- Patterson, H.; Nibbs, R.; McInnes, I.; Siebert, S. Protein kinase inhibitors in the treatment of inflammatory and autoimmune diseases. Clin. Exp. Immunol. 2014, 176, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Swarup, V.; Phaneuf, D.; Dupré, N.; Petri, S.; Strong, M.; Kriz, J.; Julien, J.P. Deregulation of TDP-43 in amyotrophic lateral sclerosis triggers nuclear factor κB-mediated pathogenic pathways. J. Exp. Med. 2011, 208, 2429–2447. [Google Scholar] [CrossRef]
- Patel, P.; Julien, J.P.; Kriz, J. Early-stage treatment with Withaferin A reduces levels of misfolded superoxide dismutase 1 and extends lifespan in a mouse model of amyotrophic lateral sclerosis. Neurotherapeutics 2015, 12, 217–233. [Google Scholar] [CrossRef]
- Livne-Bar, I.; Lam, S.; Chan, D.; Guo, X.; Askar, I.; Nahirnyj, A.; Flanagan, J.G.; Sivak, J.M. Pharmacologic inhibition of reactive gliosis blocks TNF-α-mediated neuronal apoptosis. Cell Death Dis. 2016, 7, e2386. [Google Scholar] [CrossRef]
- Raziya Banu, M.; Ibrahim, M.; Prabhu, K.; Rajasankar, S. Ameliorative Effect of Withaferin A on Ageing-Mediated Impairment in the Dopamine System and Its Associated Behavior of Wistar Albino Rat. Pharmacology 2019, 103, 114–119. [Google Scholar] [CrossRef]
- Raziya Banu, M.; Ibrahim, M.; Prabhu, K.; Rajasankar, S. Withaferin A Protects the Nigral Dopamine Neuron and Recovers Motor Activity in Aged Rats. Cells Tissues Organs 2019, 208, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Abu Bakar, M.H.; Azmi, M.N.; Shariff, K.A.; Tan, J.S. Withaferin A Protects Against High-Fat Diet-Induced Obesity Via Attenuation of Oxidative Stress, Inflammation, and Insulin Resistance. Appl. Biochem. Biotechnol. 2019, 188, 241–259. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Liu, J.; Feng, X.; Salazar Hernández, M.A.; Mucka, P.; Ibi, D.; Choi, J.W.; Ozcan, U. Withaferin A is a leptin sensitizer with strong antidiabetic properties in mice. Nat. Med. 2016, 22, 1023–1032. [Google Scholar] [CrossRef] [PubMed]
- Khalilpourfarshbafi, M.; Devi Murugan, D.; Abdul Sattar, M.Z.; Sucedaram, Y.; Abdullah, N.A. Withaferin A inhibits adipogenesis in 3T3-F442A cell line, improves insulin sensitivity and promotes weight loss in high fat diet-induced obese mice. PLoS ONE 2019, 14, e0218792. [Google Scholar] [CrossRef]
- Sabina, E.P.; Chandal, S.; Rasool, M.K. Inhibition of monosodium urate crystal-induced inflammation by Withaferin A. J. Pharm. Pharm. Sci. 2008, 11, 46–55. [Google Scholar]
- Sultana, F.; Neog, M.K.; Rasool, M. Withaferin A, a steroidal lactone encapsulated mannose decorated liposomes ameliorates rheumatoid arthritis by intriguing the macrophage repolarization in adjuvant-induced arthritic rats. Colloids Surf. B Biointerfaces 2017, 155, 349–365. [Google Scholar] [CrossRef]
- Mehta, M.; Gohil, D.; Khattry, N.; Kumar, R.; Sandur, S.; Sharma, D.; Checker, R.; Agarwal, B.; Jha, D.; Majumdar, A.; et al. Prevention of acute graft-versus-host-disease by Withaferin A via suppression of AKT/mTOR pathway. Int. Immunopharmacol. 2020, 84, 106575. [Google Scholar] [CrossRef]
- Noh, E.J.; Kang, M.J.; Jeong, Y.J.; Lee, J.Y.; Park, J.H.; Choi, H.J.; Oh, S.M.; Lee, K.B.; Kim, D.J.; Shin, J.A.; et al. Withaferin A inhibits inflammatory responses induced by Fusobacterium nucleatum and Aggregatibacter actinomycetemcomitans in macrophages. Mol. Med. Rep. 2016, 14, 983–988. [Google Scholar] [CrossRef][Green Version]
- Min, K.J.; Choi, K.; Kwon, T.K. Withaferin A down-regulates lipopolysaccharide-induced cyclooxygenase-2 expression and PGE2 production through the inhibition of STAT1/3 activation in microglial cells. Int. Immunopharmacol. 2011, 11, 1137–1142. [Google Scholar] [CrossRef]
- Zhao, H.M.; Gao, Z.W.; Xie, S.X.; Han, X.; Sun, Q.S. Withaferin A attenuates ovalbumin induced airway inflammation. Front. Biosci. 2019, 24, 576–596. [Google Scholar]
- Darnell, J.E., Jr.; Kerr, I.M.; Stark, G.R. Jak-STAT pathways and transcriptional activation in response to IFNs and other extracellular signaling proteins. Science 1994, 264, 1415–1421. [Google Scholar] [CrossRef] [PubMed]
- Hansen, D.V.; Hanson, J.E.; Sheng, M. Microglia in Alzheimer’s disease. J. Cell Biol. 2018, 217, 459–472. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Wang, F. Role of Neuroinflammation in Amyotrophic Lateral Sclerosis: Cellular Mechanisms and Therapeutic Implications. Front. Immunol. 2017, 8, 1005. [Google Scholar] [CrossRef] [PubMed]
- Neog, M.K.; Sultana, F.; Rasool, M. Targeting RAW 264.7 macrophages (M1 type) with Withaferin A decorated mannosylated liposomes induces repolarization via downregulation of NF-κB and controlled elevation of STAT-3. Int. Immunopharmacol. 2018, 61, 64–73. [Google Scholar] [CrossRef]
- Kim, G.; Kim, T.H.; Kang, M.J.; Choi, J.A.; Pack, D.Y.; Lee, I.R.; Kim, M.G.; Han, S.S.; Kim, B.Y.; Oh, S.M.; et al. Inhibitory effect of Withaferin A on Helicobacter pylori-induced IL-8 production and NF-κB activation in gastric epithelial cells. Mol. Med. Rep. 2016, 13, 967–972. [Google Scholar] [CrossRef][Green Version]
- Kuppusamy, P.; Nagalingam, A.; Muniraj, N.; Saxena, N.K.; Sharma, D. Concomitant activation of ETS-like transcription factor-1 and Death Receptor-5 via extracellular signal-regulated kinase in Withaferin A-mediated inhibition of hepatocarcinogenesis in mice. Sci. Rep. 2017, 7, 17943. [Google Scholar] [CrossRef]
- Yu, T.J.; Tang, J.Y.; Ou-Yang, F.; Wang, Y.Y.; Yuan, S.F.; Tseng, K.; Lin, L.C.; Chang, H.W. Low Concentration of Withaferin A Inhibits Oxidative Stress-Mediated Migration and Invasion in Oral Cancer Cells. Biomolecules 2020, 10. [Google Scholar] [CrossRef]
- Hahm, E.R.; Lee, J.; Singh, S.V. Role of mitogen-activated protein kinases and Mcl-1 in apoptosis induction by Withaferin A in human breast cancer cells. Mol. Carcinog. 2014, 53, 907–916. [Google Scholar] [CrossRef]
- Karnitz, L.M.; Felts, S.J. Cdc37 regulation of the kinome: When to hold ‘em and when to fold ‘em. Sci. STKE 2007, 2007, pe22. [Google Scholar] [CrossRef]
- Grover, A.; Shandilya, A.; Agrawal, V.; Pratik, P.; Bhasme, D.; Bisaria, V.S.; Sundar, D. Hsp90/Cdc37 chaperone/co-chaperone complex, a novel junction anticancer target elucidated by the mode of action of herbal drug Withaferin A. BMC Bioinform. 2011, 12 (Suppl 1), S30. [Google Scholar] [CrossRef]
- Yu, Y.; Hamza, A.; Zhang, T.; Gu, M.; Zou, P.; Newman, B.; Li, Y.; Gunatilaka, A.A.; Zhan, C.G.; Sun, D. Withaferin A targets heat shock protein 90 in pancreatic cancer cells. Biochem. Pharmacol. 2010, 79, 542–551. [Google Scholar] [CrossRef]
- Narayan, M.; Zhang, J.; Braswell, K.; Gibson, C.; Zitnyar, A.; Lee, D.C.; Varghese-Gupta, S.; Jinwal, U.K. Withaferin A Regulates LRRK2 Levels by Interfering with the Hsp90-Cdc37 Chaperone Complex. Curr. Aging Sci. 2015, 8, 259–265. [Google Scholar] [CrossRef] [PubMed]
- Wallings, R.L.; Tansey, M.G. LRRK2 regulation of immune-pathways and inflammatory disease. Biochem. Soc. Trans. 2019, 47, 1581–1595. [Google Scholar] [CrossRef]
- Schildt, A.; Walker, M.D.; Dinelle, K.; Miao, Q.; Schulzer, M.; O’Kusky, J.; Farrer, M.J.; Doudet, D.J.; Sossi, V. Single Inflammatory Trigger Leads to Neuroinflammation in LRRK2 Rodent Model without Degeneration of Dopaminergic Neurons. J. Parkinsons Dis. 2019, 9, 121–139. [Google Scholar] [CrossRef] [PubMed]
- Narayan, M.; Seeley, K.W.; Jinwal, U.K. Identification and quantitative analysis of cellular proteins affected by treatment with Withaferin A using a SILAC-based proteomics approach. J. Ethnopharmacol. 2015, 175, 86–92. [Google Scholar] [CrossRef] [PubMed]
- Dayalan Naidu, S.; Dinkova-Kostova, A.T. Regulation of the mammalian heat shock factor 1. FEBS J. 2017, 284, 1606–1627. [Google Scholar] [CrossRef]
- Zou, J.; Guo, Y.; Guettouche, T.; Smith, D.F.; Voellmy, R. Repression of heat shock transcription factor HSF1 activation by HSP90 (HSP90 complex) that forms a stress-sensitive complex with HSF1. Cell 1998, 94, 471–480. [Google Scholar] [CrossRef]
- Vihervaara, A.; Sistonen, L. HSF1 at a glance. J. Cell Sci. 2014, 127, 261–266. [Google Scholar] [CrossRef]
- Santagata, S.; Xu, Y.M.; Wijeratne, E.M.; Kontnik, R.; Rooney, C.; Perley, C.C.; Kwon, H.; Clardy, J.; Kesari, S.; Whitesell, L.; et al. Using the heat-shock response to discover anticancer compounds that target protein homeostasis. ACS Chem. Biol. 2012, 7, 340–349. [Google Scholar] [CrossRef]
- Kunwar, A.; Priyadarsini, K.I. Free radicals, oxidative stress and importance of antioxidants in human health. J. Med. Allied Sci. 2011, 1, 53–60. [Google Scholar]
- Solleiro-Villavicencio, H.; Rivas-Arancibia, S. Effect of Chronic Oxidative Stress on Neuroinflammatory Response Mediated by CD4(+)T Cells in Neurodegenerative Diseases. Front. Cell Neurosci. 2018, 12, 114. [Google Scholar] [CrossRef] [PubMed]
- Hardbower, D.M.; de Sablet, T.; Chaturvedi, R.; Wilson, K.T. Chronic inflammation and oxidative stress: The smoking gun for Helicobacter pylori-induced gastric cancer? Gut Microbes 2013, 4, 475–481. [Google Scholar] [CrossRef] [PubMed]
- Biswas, S.K. Does the Interdependence between Oxidative Stress and Inflammation Explain the Antioxidant Paradox? Oxid. Med. Cell Longev. 2016, 2016, 5698931. [Google Scholar] [CrossRef] [PubMed]
- Tabas, I.; Glass, C.K. Anti-inflammatory therapy in chronic disease: Challenges and opportunities. Science 2013, 339, 166–172. [Google Scholar] [CrossRef] [PubMed]
- Hassannia, B.; Logie, E.; Vandenabeele, P.; Vanden Berghe, T.; Vanden Berghe, W. Withaferin A: From ayurvedic folk medicine to preclinical anti-cancer drug. Biochem. Pharmacol. 2020, 173, 113602. [Google Scholar] [CrossRef]
- Hahm, E.R.; Moura, M.B.; Kelley, E.E.; Van Houten, B.; Shiva, S.; Singh, S.V. Withaferin A-Induced Apoptosis in Human Breast Cancer Cells Is Mediated by Reactive Oxygen Species. PLoS ONE 2011, 6. [Google Scholar] [CrossRef]
- Sehrawat, A.; Samanta, S.K.; Hahm, E.R.; St Croix, C.; Watkins, S.; Singh, S.V. Withaferin A-mediated apoptosis in breast cancer cells is associated with alterations in mitochondrial dynamics. Mitochondrion 2019, 47, 282–293. [Google Scholar] [CrossRef]
- Guo, R.; Gan, L.; Lau, W.B.; Yan, Z.; Xie, D.; Gao, E.; Christopher, T.A.; Lopez, B.L.; Ma, X.; Wang, Y. Withaferin A Prevents Myocardial Ischemia/Reperfusion Injury by Upregulating AMP-Activated Protein Kinase-Dependent B-Cell Lymphoma2 Signaling. Circ. J. Off. J. Jpn. Circ. Soc. 2019, 83, 1726–1736. [Google Scholar] [CrossRef]
- Batumalaie, K.; Amin, M.A.; Murugan, D.D.; Sattar, M.Z.; Abdullah, N.A. Withaferin A protects against palmitic acid-induced endothelial insulin resistance and dysfunction through suppression of oxidative stress and inflammation. Sci. Rep. 2016, 6, 27236. [Google Scholar] [CrossRef]
- Ahmed, S.M.; Luo, L.; Namani, A.; Wang, X.J.; Tang, X. Nrf2 signaling pathway: Pivotal roles in inflammation. Biochim. Biophys. Acta Mol. Basis. Dis. 2017, 1863, 585–597. [Google Scholar] [CrossRef]
- Hybertson, B.M.; Gao, B.; Bose, S.; McCord, J.M. Phytochemical Combination PB125 Activates the Nrf2 Pathway and Induces Cellular Protection against Oxidative Injury. Antioxidants 2019, 8. [Google Scholar] [CrossRef] [PubMed]
- Heyninck, K.; Sabbe, L.; Chirumamilla, C.S.; Szarc Vel Szic, K.; Vander Veken, P.; Lemmens, K.J.A.; Lahtela-Kakkonen, M.; Naulaerts, S.; Op de Beeck, K.; Laukens, K.; et al. Withaferin A induces heme oxygenase (HO-1) expression in endothelial cells via activation of the Keap1/Nrf2 pathway. Biochem. Pharmacol. 2016, 109, 48–61. [Google Scholar] [CrossRef] [PubMed]
- Kanak, M.A.; Shahbazov, R.; Yoshimatsu, G.; Levy, M.F.; Lawrence, M.C.; Naziruddin, B. A small molecule inhibitor of NFκB blocks ER stress and the NLRP3 inflammasome and prevents progression of pancreatitis. J. Gastroenterol. 2017, 52, 352–365. [Google Scholar] [CrossRef] [PubMed]
- Patel, D.P.; Yan, T.; Kim, D.; Dias, H.B.; Krausz, K.W.; Kimura, S.; Gonzalez, F.J. Withaferin A Improves Nonalcoholic Steatohepatitis in Mice. J. Pharmacol. Exp. Ther. 2019, 371, 360–374. [Google Scholar] [CrossRef] [PubMed]
- Gu, J.; Chen, C.; Wang, J.; Chen, T.; Yao, W.; Yan, T.; Liu, Z. Withaferin A Exerts Preventive Effect on Liver Fibrosis through Oxidative Stress Inhibition in a Sirtuin 3-Dependent Manner. Oxid. Med. Cell Longev. 2020, 2020, 2452848. [Google Scholar] [CrossRef]
- Giralt, A.; Villarroya, F. SIRT3, a pivotal actor in mitochondrial functions: Metabolism, cell death and aging. Biochem. J. 2012, 444, 1–10. [Google Scholar] [CrossRef]
- Ahn, B.H.; Kim, H.S.; Song, S.; Lee, I.H.; Liu, J.; Vassilopoulos, A.; Deng, C.X.; Finkel, T. A role for the mitochondrial deacetylase Sirt3 in regulating energy homeostasis. Proc. Natl. Acad. Sci. USA 2008, 105, 14447–14452. [Google Scholar] [CrossRef]
- Sundaresan, N.R.; Gupta, M.; Kim, G.; Rajamohan, S.B.; Isbatan, A.; Gupta, M.P. Sirt3 blocks the cardiac hypertrophic response by augmenting Foxo3a-dependent antioxidant defense mechanisms in mice. J. Clin. Investig. 2009, 119, 2758–2771. [Google Scholar] [CrossRef]
- Tyagi, A.; Nguyen, C.U.; Chong, T.; Michel, C.R.; Fritz, K.S.; Reisdorph, N.; Knaub, L.; Reusch, J.E.B.; Pugazhenthi, S. SIRT3 deficiency-induced mitochondrial dysfunction and inflammasome formation in the brain. Sci. Rep. 2018, 8, 17547. [Google Scholar] [CrossRef]
- Xu, H.; Hertzel, A.V.; Steen, K.A.; Bernlohr, D.A. Loss of Fatty Acid Binding Protein 4/aP2 Reduces Macrophage Inflammation Through Activation of SIRT3. Mol. Endocrinol. 2016, 30, 325–334. [Google Scholar] [CrossRef]
- Kurundkar, D.; Kurundkar, A.R.; Bone, N.B.; Becker, E.J., Jr.; Liu, W.; Chacko, B.; Darley-Usmar, V.; Zmijewski, J.W.; Thannickal, V.J. SIRT3 diminishes inflammation and mitigates endotoxin-induced acute lung injury. JCI Insight 2019, 4. [Google Scholar] [CrossRef] [PubMed]
- Vedi, M.; Sabina, E.P. Assessment of hepatoprotective and nephroprotective potential of Withaferin A on bromobenzene-induced injury in Swiss albino mice: Possible involvement of mitochondrial dysfunction and inflammation. Cell Biol. Toxicol. 2016, 32, 373–390. [Google Scholar] [CrossRef] [PubMed]
- Peddakkulappagari, C.S.; Saifi, M.A.; Khurana, A.; Anchi, P.; Singh, M.; Godugu, C. Withaferin A ameliorates renal injury due to its potent effect on inflammatory signaling. Biofactors 2019, 45, 750–762. [Google Scholar] [CrossRef] [PubMed]
- Jadeja, R.N.; Urrunaga, N.H.; Dash, S.; Khurana, S.; Saxena, N.K. Withaferin A Reduces Acetaminophen-Induced Liver Injury in Mice. Biochem. Pharmacol. 2015, 97, 122–132. [Google Scholar] [CrossRef]
- Dixon, S.J.; Lemberg, K.M.; Lamprecht, M.R.; Skouta, R.; Zaitsev, E.M.; Gleason, C.E.; Patel, D.N.; Bauer, A.J.; Cantley, A.M.; Yang, W.S.; et al. Ferroptosis: An iron-dependent form of nonapoptotic cell death. Cell 2012, 149, 1060–1072. [Google Scholar] [CrossRef]
- Sun, Y.; Chen, P.; Zhai, B.; Zhang, M.; Xiang, Y.; Fang, J.; Xu, S.; Gao, Y.; Chen, X.; Sui, X.; et al. The emerging role of ferroptosis in inflammation. Biomed. Pharmacother. 2020, 127, 110108. [Google Scholar] [CrossRef]
- Hassannia, B.; Wiernicki, B.; Ingold, I.; Qu, F.; Van Herck, S.; Tyurina, Y.Y.; Bayır, H.; Abhari, B.A.; Angeli, J.P.F.; Choi, S.M.; et al. Nano-targeted induction of dual ferroptotic mechanisms eradicates high-risk neuroblastoma. J. Clin. Investig. 2018, 128, 3341–3355. [Google Scholar] [CrossRef]
- Ngoungoure, F.P.; Owona, B.A. Withaferin A modulates AIM2 inflammasome and caspase-1 expression in THP-1 polarized macrophages. Exp. Cell Res. 2019, 383, 111564. [Google Scholar] [CrossRef]
- Kim, J.E.; Lee, J.Y.; Kang, M.J.; Jeong, Y.J.; Choi, J.A.; Oh, S.M.; Lee, K.B.; Park, J.H. Withaferin A Inhibits Helicobacter pylori-induced Production of IL-1β in Dendritic Cells by Regulating NF-κB and NLRP3 Inflammasome Activation. Immune Netw. 2015, 15, 269–277. [Google Scholar] [CrossRef]
- Guo, H.; Callaway, J.B.; Ting, J.P. Inflammasomes: Mechanism of action, role in disease, and therapeutics. Nat. Med. 2015, 21, 677–687. [Google Scholar] [CrossRef]
- Place, D.E.; Kanneganti, T.D. Recent advances in inflammasome biology. Curr. Opin. Immunol. 2018, 50, 32–38. [Google Scholar] [CrossRef] [PubMed]
- Dubey, S.; Yoon, H.; Cohen, M.S.; Nagarkatti, P.; Nagarkatti, M.; Karan, D. Withaferin A Associated Differential Regulation of Inflammatory Cytokines. Front. Immunol. 2018, 9, 195. [Google Scholar] [CrossRef] [PubMed]
- Tiruveedi, V.L.; Bale, S.; Khurana, A.; Godugu, C. Withaferin A, a novel compound of Indian ginseng (Withania somnifera), ameliorates Cerulein-induced acute pancreatitis: Possible role of oxidative stress and inflammation. Phytother. Res. PTR 2018, 32, 2586–2596. [Google Scholar] [CrossRef] [PubMed]
- Lamkanfi, M.; Dixit, V.M. Mechanisms and functions of inflammasomes. Cell 2014, 157, 1013–1022. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Zhang, S.; Xiao, Y.; Zhang, W.; Wu, S.; Qin, T.; Yue, Y.; Qian, W.; Li, L. NLRP3 Inflammasome and Inflammatory Diseases. Oxid. Med. Cell Longev. 2020, 2020, 4063562. [Google Scholar] [CrossRef]
- Tyagi, S.; Gupta, P.; Saini, A.S.; Kaushal, C.; Sharma, S. The peroxisome proliferator-activated receptor: A family of nuclear receptors role in various diseases. J. Adv. Pharm. Technol. Res. 2011, 2, 236–240. [Google Scholar] [CrossRef]
- Konstantinopoulos, P.A.; Vandoros, G.P.; Sotiropoulou-Bonikou, G.; Kominea, A.; Papavassiliou, A.G. NF-kappaB/PPAR gamma and/or AP-1/PPAR gamma ‘on/off’ switches and induction of CBP in colon adenocarcinomas: Correlation with COX-2 expression. Int. J. Color. Dis. 2007, 22, 57–68. [Google Scholar] [CrossRef]
- Scirpo, R.; Fiorotto, R.; Villani, A.; Amenduni, M.; Spirli, C.; Strazzabosco, M. Stimulation of nuclear receptor peroxisome proliferator-activated receptor-gamma limits NF-kappaB-dependent inflammation in mouse cystic fibrosis biliary epithelium. Hepatology 2015, 62, 1551–1562. [Google Scholar] [CrossRef]
- Remels, A.H.; Langen, R.C.; Gosker, H.R.; Russell, A.P.; Spaapen, F.; Voncken, J.W.; Schrauwen, P.; Schols, A.M. PPARgamma inhibits NF-kappaB-dependent transcriptional activation in skeletal muscle. Am. J. Physiol. Endocrinol. Metab. 2009, 297, E174–E183. [Google Scholar] [CrossRef]
- Kytikova, O.Y.; Perelman, J.M.; Novgorodtseva, T.P.; Denisenko, Y.K.; Kolosov, V.P.; Antonyuk, M.V.; Gvozdenko, T.A. Peroxisome Proliferator-Activated Receptors as a Therapeutic Target in Asthma. PPAR Res. 2020, 2020, 8906968. [Google Scholar] [CrossRef]
- Lima Ede, A.L.M.; Marques, C.D.; Duarte, A.L.; Pita Ida, R.; Pita, M.G. Peroxisome proliferator-activated receptor agonists (PPARs): A promising prospect in the treatment of psoriasis and psoriatic arthritis. An. Bras. Dermatol. 2013, 88, 1029–1035. [Google Scholar] [CrossRef] [PubMed]
- Park, H.J.; Rayalam, S.; Della-Fera, M.A.; Ambati, S.; Yang, J.Y.; Baile, C.A. Withaferin A induces apoptosis and inhibits adipogenesis in 3T3-L1 adipocytes. Biofactors 2008, 33, 137–148. [Google Scholar] [CrossRef] [PubMed]
- Omary, M.B.; Coulombe, P.A.; McLean, W.H. Intermediate filament proteins and their associated diseases. N. Engl. J. Med. 2004, 351, 2087–2100. [Google Scholar] [CrossRef] [PubMed]
- Park, J.E.; Kim, H.T.; Lee, S.; Lee, Y.S.; Choi, U.K.; Kang, J.H.; Choi, S.Y.; Kang, T.C.; Choi, M.S.; Kwon, O.S. Differential expression of intermediate filaments in the process of developing hepatic steatosis. Proteomics 2011, 11, 2777–2789. [Google Scholar] [CrossRef]
- Bargagna-Mohan, P.; Paranthan, R.R.; Hamza, A.; Dimova, N.; Trucchi, B.; Srinivasan, C.; Elliott, G.I.; Zhan, C.G.; Lau, D.L.; Zhu, H.; et al. Withaferin A targets intermediate filaments glial fibrillary acidic protein and vimentin in a model of retinal gliosis. J. Biol. Chem. 2010, 285, 7657–7669. [Google Scholar] [CrossRef]
- Bargagna-Mohan, P.; Hamza, A.; Kim, Y.E.; Khuan Abby Ho, Y.; Mor-Vaknin, N.; Wendschlag, N.; Liu, J.; Evans, R.M.; Markovitz, D.M.; Zhan, C.G.; et al. The tumor inhibitor and antiangiogenic agent Withaferin A targets the intermediate filament protein vimentin. Chem. Biol. 2007, 14, 623–634. [Google Scholar] [CrossRef]
- Dom, M.; Offner, F.; Vanden Berghe, W.; Van Ostade, X. Proteomic characterization of Withaferin A-targeted protein networks for the treatment of monoclonal myeloma gammopathies. J. Proteom. 2018, 179, 17–29. [Google Scholar] [CrossRef]
- Bargagna-Mohan, P.; Paranthan, R.R.; Hamza, A.; Zhan, C.G.; Lee, D.M.; Kim, K.B.; Lau, D.L.; Srinivasan, C.; Nakayama, K.; Nakayama, K.I.; et al. Corneal antifibrotic switch identified in genetic and pharmacological deficiency of vimentin. J. Biol. Chem. 2012, 287, 989–1006. [Google Scholar] [CrossRef]
- Chen, Z.; Bozec, A.; Ramming, A.; Schett, G. Anti-inflammatory and immune-regulatory cytokines in rheumatoid arthritis. Nat. Rev. Rheumatol. 2019, 15, 9–17. [Google Scholar] [CrossRef]
- Cicchese, J.M.; Evans, S.; Hult, C.; Joslyn, L.R.; Wessler, T.; Millar, J.A.; Marino, S.; Cilfone, N.A.; Mattila, J.T.; Linderman, J.J.; et al. Dynamic balance of pro- and anti-inflammatory signals controls disease and limits pathology. Immunol. Rev. 2018, 285, 147–167. [Google Scholar] [CrossRef]
- Keane, M.P.; Strieter, R.M. The importance of balanced pro-inflammatory and anti-inflammatory mechanisms in diffuse lung disease. Respir. Res. 2002, 3, 5. [Google Scholar] [CrossRef] [PubMed]
- Pires, N.; Gota, V.; Gulia, A.; Hingorani, L.; Agarwal, M.; Puri, A. Safety and pharmacokinetics of Withaferin A in advanced stage high grade osteosarcoma: A phase I trial. J. Ayurveda Integr. Med. 2020, 11, 68–72. [Google Scholar] [CrossRef] [PubMed]
- Patil, D.; Gautam, M.; Mishra, S.; Karupothula, S.; Gairola, S.; Jadhav, S.; Pawar, S.; Patwardhan, B. Determination of Withaferin A and Withanolide A in mice plasma using high-performance liquid chromatography-tandem mass spectrometry: Application to pharmacokinetics after oral administration of Withania somnifera aqueous extract. J. Pharm. Biomed. Anal. 2013, 80, 203–212. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Zhao, J.; Bai, J.; Gao, K.; Cui, D.; Chen, Y.; Song, Y.; Jia, Y.; Wen, A. Liquid chromatography-tandem mass spectrometry to assess the pharmacokinetics and tissue distribution of Withaferin A in rats. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2019, 1122–1123, 90–95. [Google Scholar] [CrossRef] [PubMed]
- Dai, T.; Jiang, W.; Guo, Z.; Wang, Z.; Huang, M.; Zhong, G.; Liang, C.; Pei, X.; Dai, R. Studies on oral bioavailability and first–pass metabolism of Withaferin A in rats using LC-MS/MS and Q-TRAP. Biomed. Chromatogr. 2019, 33, e4573. [Google Scholar] [CrossRef] [PubMed]
- Gupta, R.C.; Bansal, S.S.; Aqil, F.; Jeyabalan, J.; Cao, P.; Kausar, H.; Russell, G.K.; Munagala, R.; Ravoori, S.; Vadhanam, M.V. Controlled-release systemic delivery—A new concept in cancer chemoprevention. Carcinogenesis 2012, 33, 1608–1615. [Google Scholar] [CrossRef]
- Alnuqaydan, A.M.; Rah, B.; Almutary, A.G.; Chauhan, S.S. Synergistic antitumor effect of 5-fluorouracil and Withaferin A induces endoplasmic reticulum stress-mediated autophagy and apoptosis in colorectal cancer cells. Am. J. Cancer Res. 2020, 10, 799–815. [Google Scholar]
- Kyakulaga, A.H.; Aqil, F.; Munagala, R.; Gupta, R.C. Synergistic combinations of paclitaxel and Withaferin A against human non-small cell lung cancer cells. Oncotarget 2020, 11, 1399–1416. [Google Scholar] [CrossRef]
- Chopra, A.; Saluja, M.; Tillu, G.; Venugopalan, A.; Narsimulu, G.; Sarmukaddam, S.; Patwardhan, B. Evaluating higher doses of Shunthi-Guduchi formulations for safety in treatment of osteoarthritis knees: A Government of India NMITLI arthritis project. J. Ayurveda Integr. Med. 2012, 3, 38–44. [Google Scholar] [CrossRef]
- Chengappa, K.N.R.; Brar, J.S.; Gannon, J.M.; Schlicht, P.J. Adjunctive Use of a Standardized Extract of Withania somnifera (Ashwagandha) to Treat Symptom Exacerbation in Schizophrenia: A Randomized, Double-Blind, Placebo-Controlled Study. J. Clin. Psychiatry 2018, 79, 1–9. [Google Scholar] [CrossRef]
- Lee, I.C.; Choi, B.Y. Withaferin A—A Natural Anticancer Agent with Pleitropic Mechanisms of Action. Int. J. Mol. Sci. 2016, 17. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Logie, E.; Vanden Berghe, W. Tackling Chronic Inflammation with Withanolide Phytochemicals—A Withaferin A Perspective. Antioxidants 2020, 9, 1107. https://doi.org/10.3390/antiox9111107
Logie E, Vanden Berghe W. Tackling Chronic Inflammation with Withanolide Phytochemicals—A Withaferin A Perspective. Antioxidants. 2020; 9(11):1107. https://doi.org/10.3390/antiox9111107
Chicago/Turabian StyleLogie, Emilie, and Wim Vanden Berghe. 2020. "Tackling Chronic Inflammation with Withanolide Phytochemicals—A Withaferin A Perspective" Antioxidants 9, no. 11: 1107. https://doi.org/10.3390/antiox9111107
APA StyleLogie, E., & Vanden Berghe, W. (2020). Tackling Chronic Inflammation with Withanolide Phytochemicals—A Withaferin A Perspective. Antioxidants, 9(11), 1107. https://doi.org/10.3390/antiox9111107





