Hydroxycinnamyl Derived BODIPY as a Lipophilic Fluorescence Probe for Peroxyl Radicals
Abstract
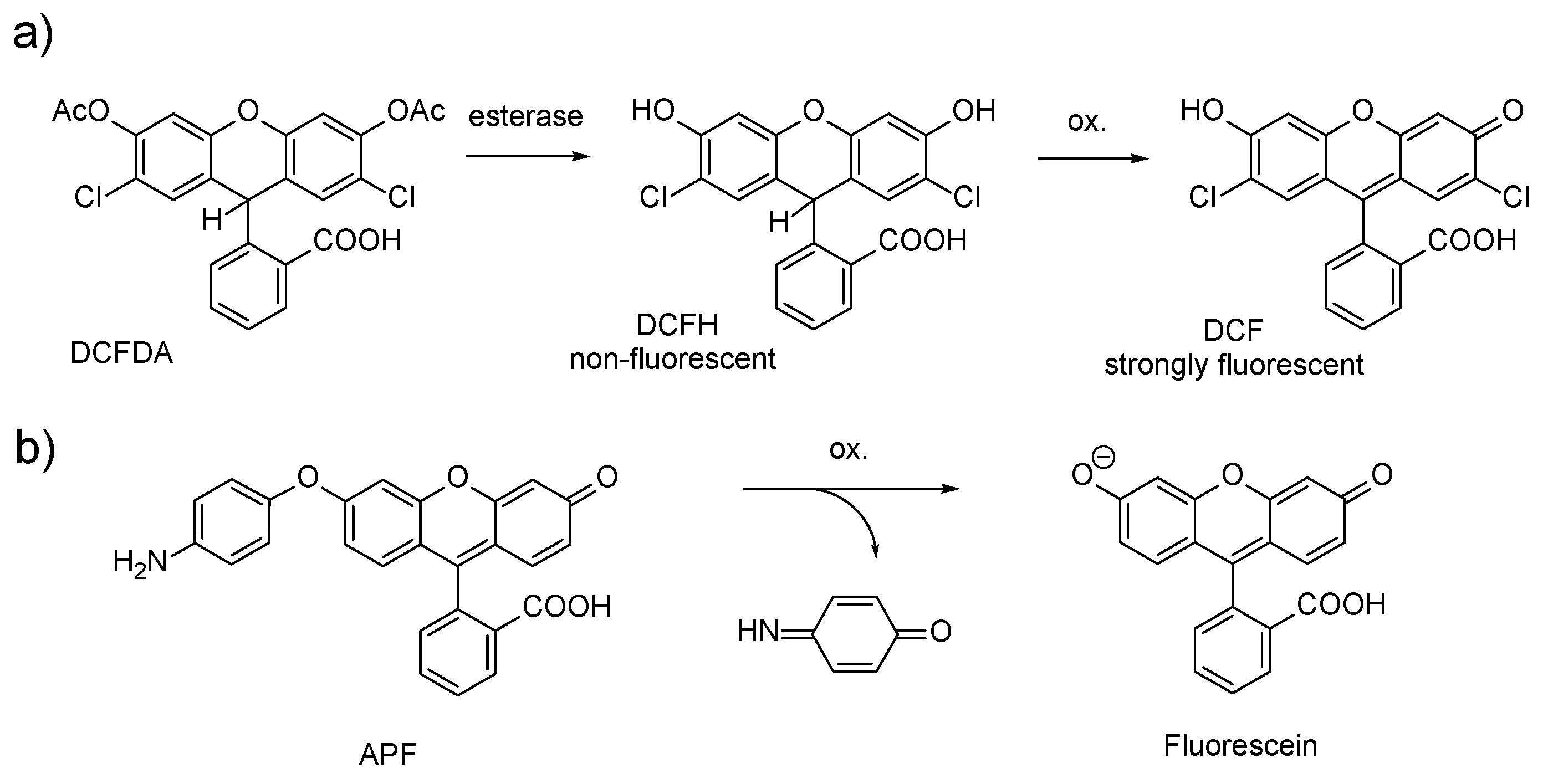
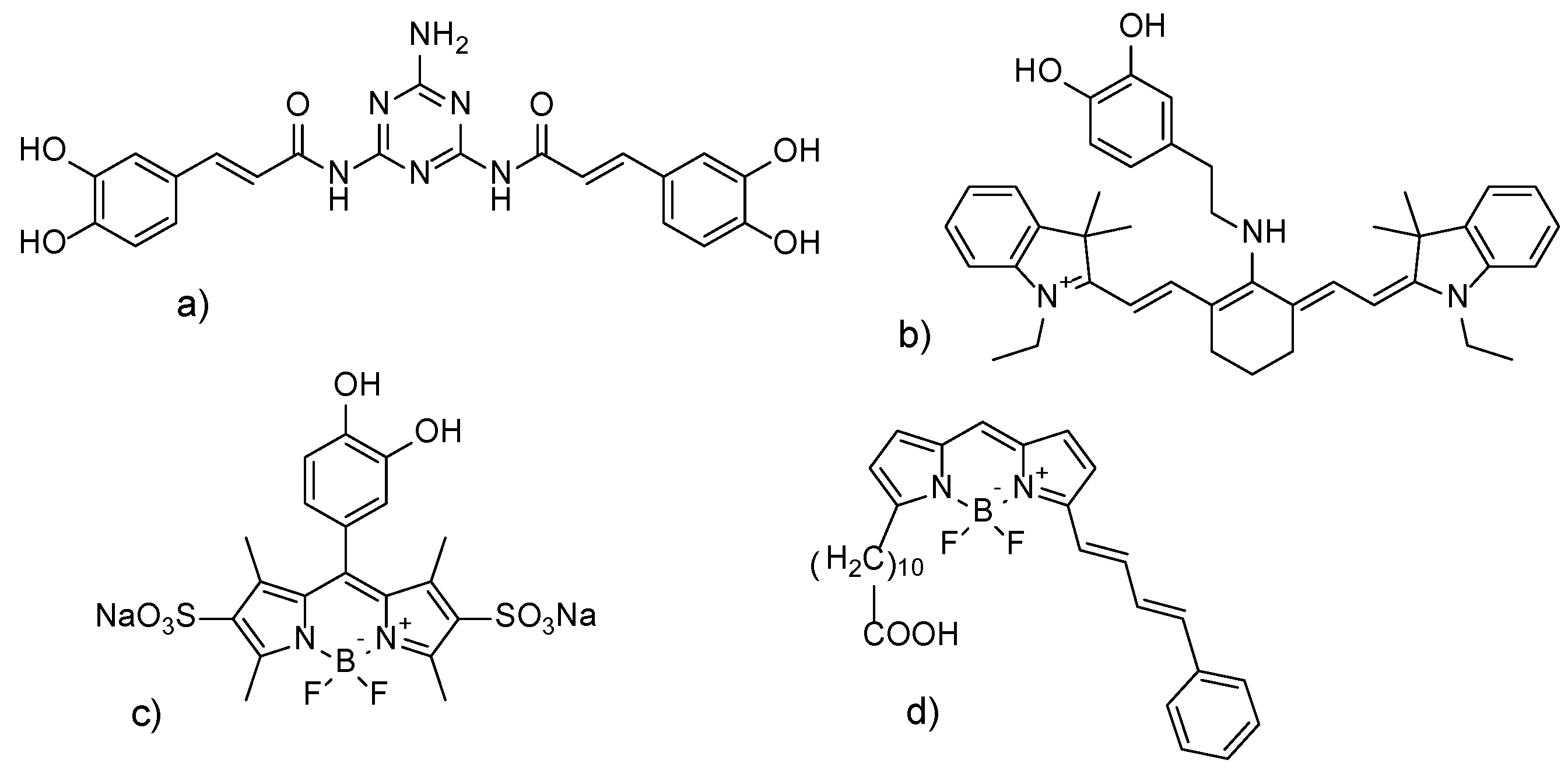
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. General Information
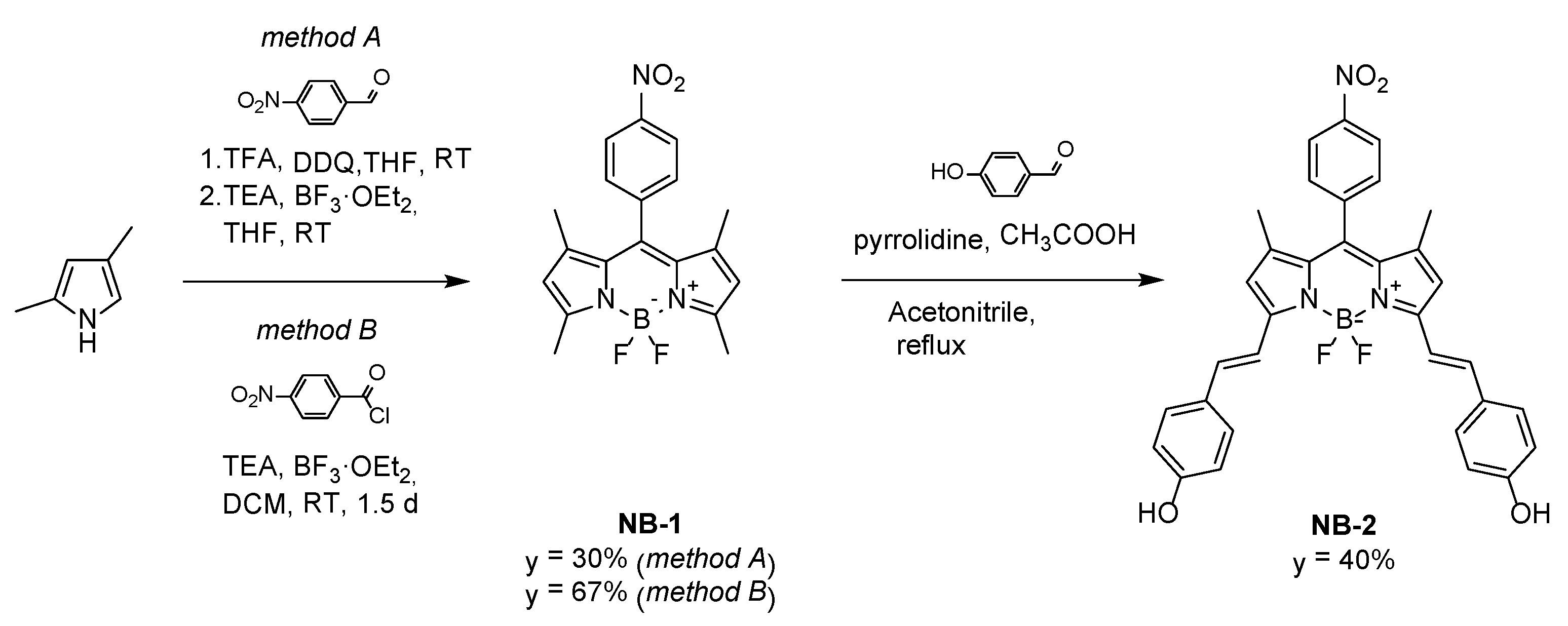
2.3. Synthesis Procedures
2.4. Preparation of Micelles
2.5. Methodology of Autoxidation Measurements
2.6. UV-vis Studies of Stability and Reactivity in Methanol and in Micelles
2.7. Spectrofluorometric Measurements
2.8. Photolysis of AIBN
3. Results and Discussion
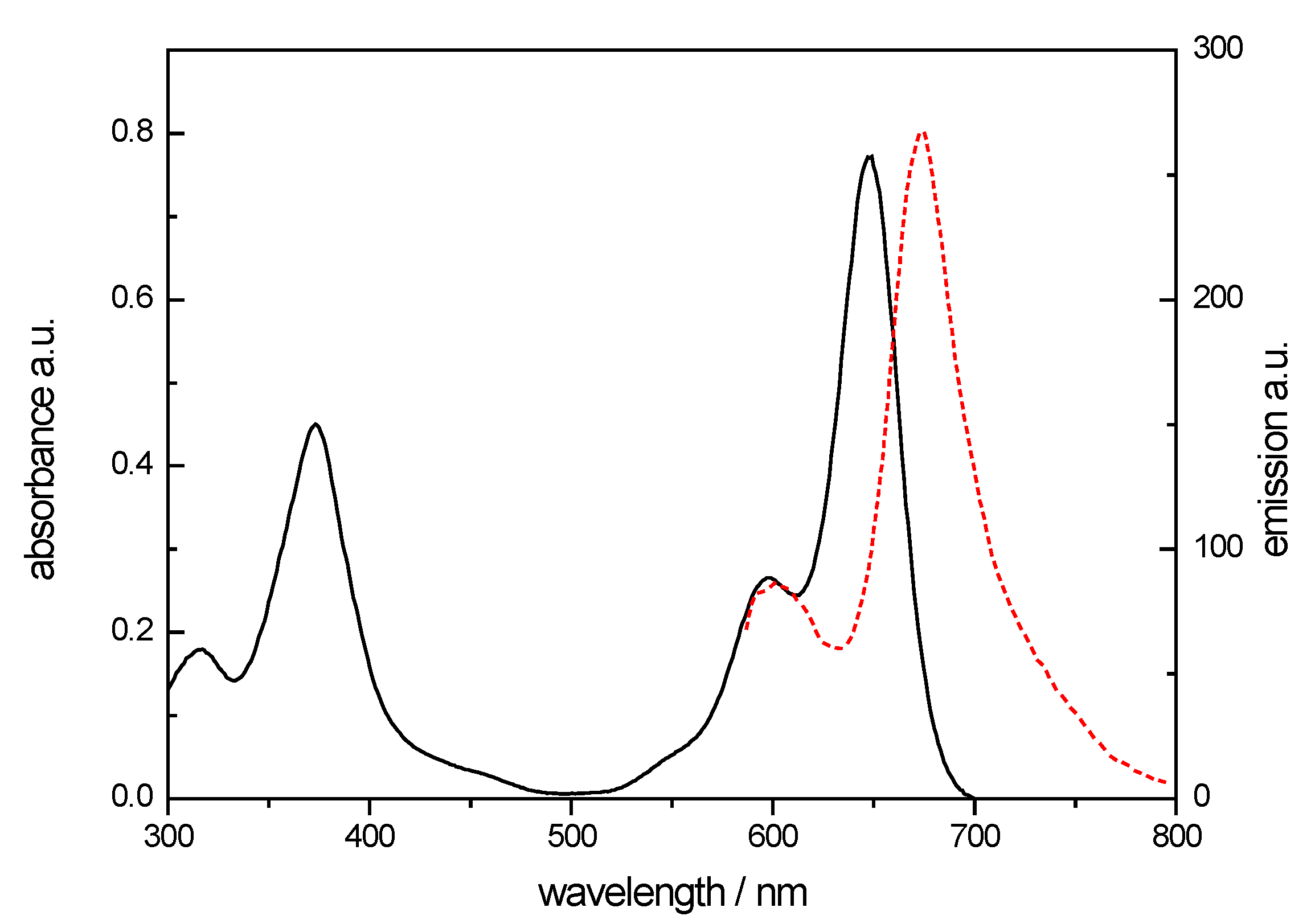
3.1. Synthesis and Spectral Chracteristics of NB-2
3.2. Kinetic Parameters of Reaction of NB-2 with Peroxyl Radicals
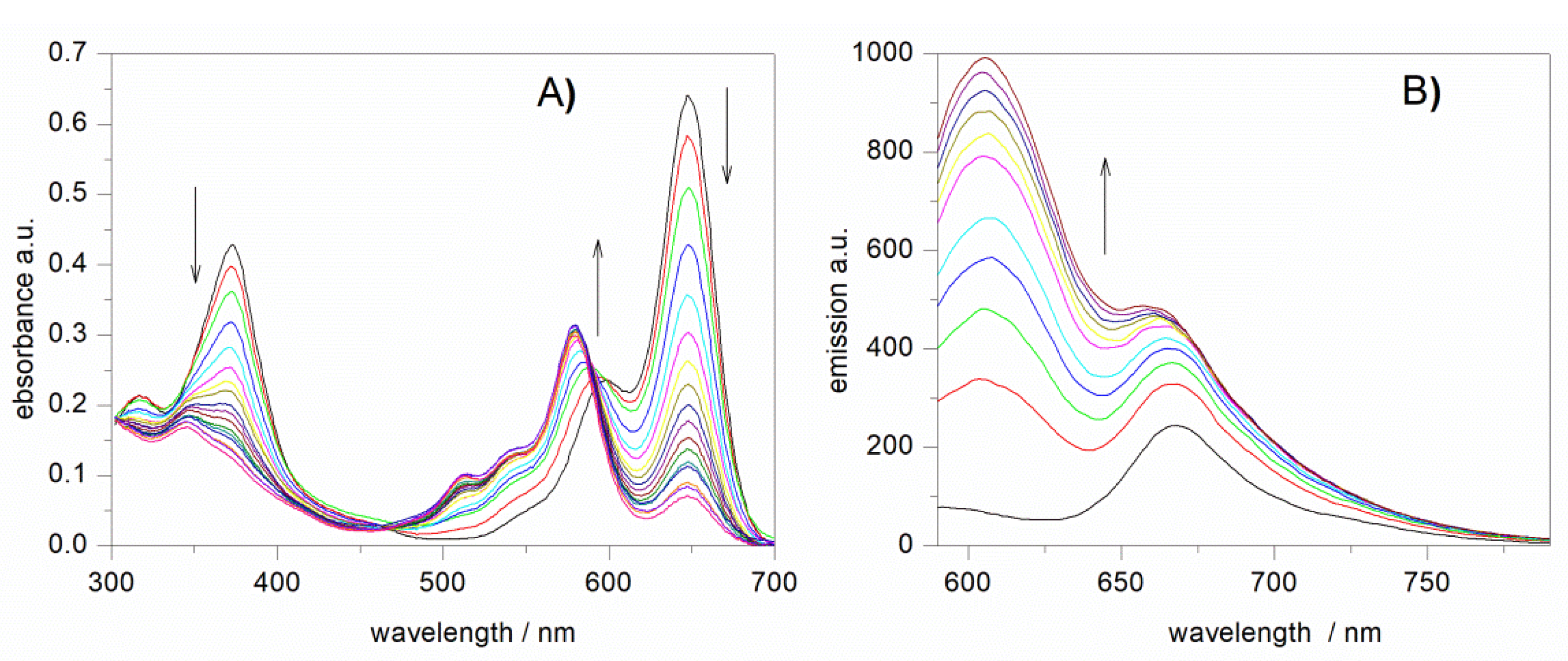
3.3. UV-vis and Spectrofluorimetric Study of the Reaction of NB-2 with Peroxyl Radicals in Methanol
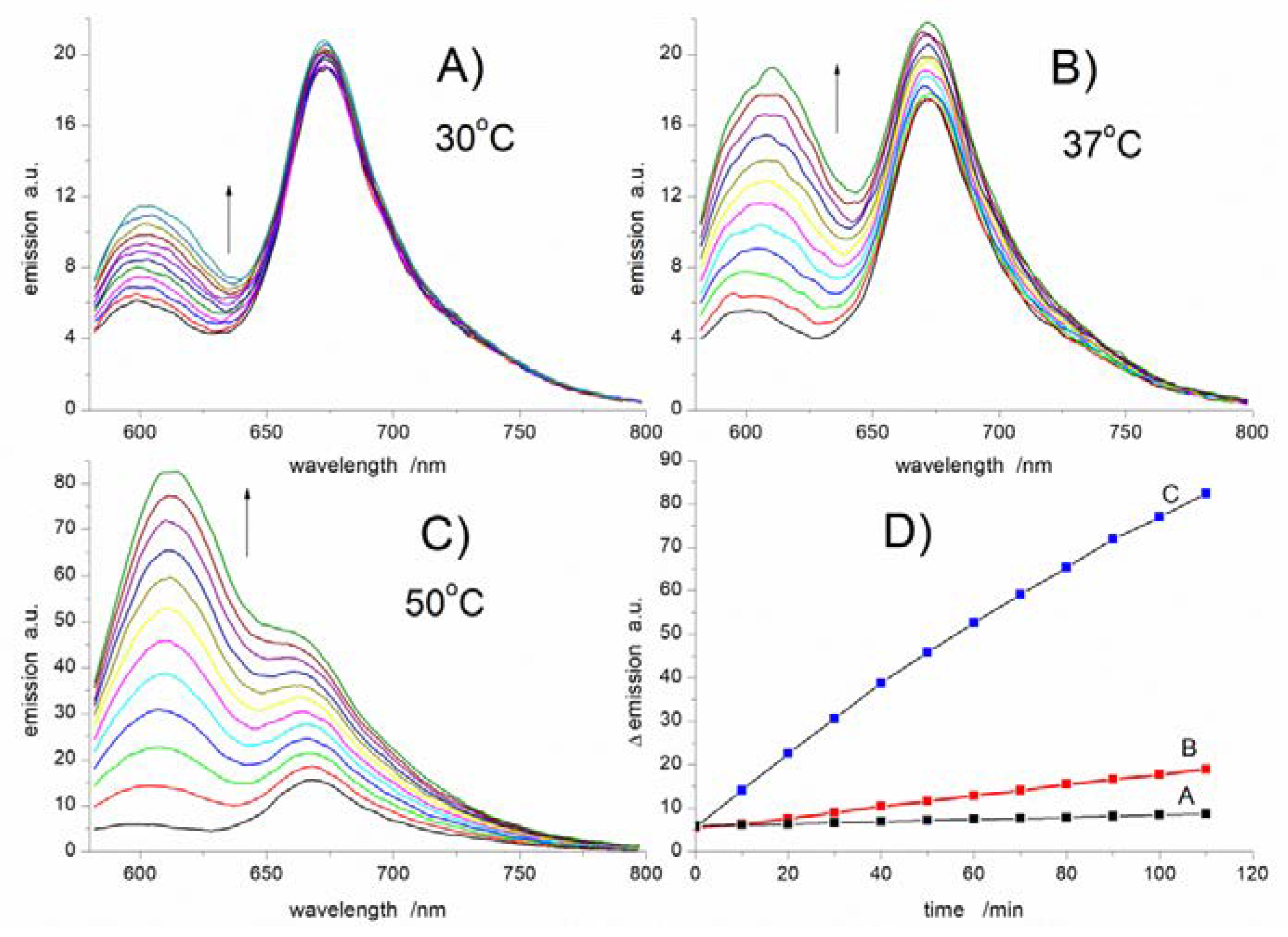
3.4. UV-vis and Spectrofluorimetric Study of the Reaction of NB-2 with Peroxyl Radicals in Micelles
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| ABAP | 2,2′-azobis(2-amidinopropane) |
| AIBN | α,α′-azobisisobutyronitrile |
| BODIPY | dipyrrometheneboron difluoride |
| CBA | chain-breaking antioxidant |
| DCM | dichloromethane |
| DDQ | 2,3-Dichloro-5,6-dicyano-1,4-benzoquinone |
| DMPC | 1,2-dimyristoyl-sn-glycero-3-phosphocholine |
| FP | fluorescent probe(s) |
| LinMe | methyl linoleate |
| LH | lipid molecule |
| PMHC | 2,2,5,7,8-pentamethylchroman-6-ol |
| PUFA | polyunsaturated fatty acids |
| R• | alkyl radical |
| LOO• | peroxyl radical |
| ROS | Reactive Oxygen Species |
| TEA | triethylamine |
| TFA | trifluoroacetic acid |
| THF | tetrahydrofuran |
| TLC | thin-layer chromatoraphy |
| Triton X-100 | polyethylene glycol p-(1,1,3,3-tetramethylbutyl)-phenyl ether |
References
- Russell, E.G.; Cotter, T.G. New Insight into the Role of Reactive Oxygen Species (ROS) in Cellular Signal-Transduction Processes. In International Review of Cell and Molecular Biology; Academic Press: Cambridge, MA, USA, 2015; Volume 319, pp. 221–254. [Google Scholar]
- Muller, F.L.; Lustgarten, M.S.; Jang, Y.; Richardson, A.; Van Remmen, H. Trends in oxidative aging theories. Free Radic. Biol. Med. 2007, 43, 477–503. [Google Scholar] [CrossRef]
- Liou, G.Y.; Storz, P. Reactive oxygen species in cancer. Free Radic. Res. 2010, 44, 479–496. [Google Scholar] [CrossRef]
- Aitken, R.J.; De Iuliis, G.N.; Gibb, Z.; Baker, M.A. The simmet lecture: New horizons on an old landscape—Oxidative stress, DNA damage and apoptosis in the male germ line. Reprod. Domest. Anim. 2012, 47, 7–14. [Google Scholar] [CrossRef]
- Liu, Y.W.; Sakaeda, T.; Takara, K.; Nakamura, T.; Ohmoto, N.; Komoto, C.; Kobayashi, H.; Yagami, T.; Okamura, N.; Okumura, K. Effects of reactive oxygen species on cell proliferation and death in HeLa cells and its MDR1-overexpressing derivative cell line. Biol. Pharm. Bull. 2003, 26, 278–281. [Google Scholar] [CrossRef] [PubMed]
- Grisham, M.B.; Jourd’heuil, D.; Wink, D.A. Chronic inflammation and reactive oxygen and nitrogen metabolism—Implications in DNA damage and mutagenesis. Aliment. Pharmacol. Ther. Suppl. 2000, 14, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Nunomura, A.; Moreira, P.I.; Takeda, A.; Smith, M.A.; Perry, G. Oxidative RNA damage and neurodegeneration. Curr. Med. Chem. 2007, 14, 2968–2975. [Google Scholar] [CrossRef] [PubMed]
- Curtin, J.F.; Donovan, M.; Cotter, T.G. Regulation and measurement of oxidative stress in apoptosis. J. Immunol. Methods 2002, 265, 49–72. [Google Scholar] [CrossRef]
- Szatrowski, T.P.; Nathan, C.F. Production of Large Amounts of Hydrogen Peroxide by Human Tumor Cells. Cancer Res. 1991, 51, 794–798. [Google Scholar]
- Gaschler, M.M.; Stockwell, B.R. Lipid peroxidation in cell death. Biochem. Biophys. Res. Commun. 2017, 482, 419–425. [Google Scholar] [CrossRef]
- Ghezzi, P.; Jaquet, V.; Marcucci, F.; Schmidt, H.H.H.W. The oxidative stress theory of disease: Levels of evidence and epistemological aspects. Br. J. Pharmacol. 2017, 174, 1784–1796. [Google Scholar] [CrossRef]
- Kaur, A. Fluorescent Tools for Imaging Oxidative Stress in Biology; Springer: Cham, Switzerland, 2018. [Google Scholar]
- Djamali, A.; Sadowski, E.A.; Muehrer, R.J.; Reese, S.; Smavatkul, C.; Vidyasagar, A.; Fain, S.B.; Lipscomb, R.C.; Hullett, D.H.; Samaniego-Picota, M.; et al. BOLD-MRI assessment of intrarenal oxygenation and oxidative stress in patients with chronic kidney allograft dysfunction. Am. J. Physiol. Ren. Physiol. 2007, 292, F513–F522. [Google Scholar] [CrossRef] [PubMed]
- Noseworthy, M.D.; Bray, T.M. Effect of oxidative stress on brain damage detected by MRI and in vivo 31P-NMR. Free Radic. Biol. Med. 1998, 24, 942–951. [Google Scholar] [CrossRef]
- Lü, R. Reaction-based small-molecule fluorescent probes for dynamic detection of ROS and transient redox changes in living cells and small animals. J. Mol. Cell. Cardiol. 2017, 110, 96–108. [Google Scholar] [CrossRef] [PubMed]
- Andina, D.; Leroux, J.C.; Luciani, P. Ratiometric Fluorescent Probes for the Detection of Reactive Oxygen Species. Chem. A Eur. J. 2017, 23, 13549–13573. [Google Scholar] [CrossRef]
- Pawley, J.B. Handbook of Biological Confocal Microscopy, 3rd ed.; Springer: Boston, MA, USA, 2006; pp. 1–985. [Google Scholar] [CrossRef]
- Wang, H.; Joseph, J.A. Quantifying cellular oxidative stress by dichlorofluorescein assay using microplate reader. Free Radic. Biol. Med. 1999, 27, 612–616. [Google Scholar] [CrossRef]
- Gomes, A.; Fernandes, E.; Lima, J.L. Fluorescence probes used for detection of reactive oxygen species. J. Biochem. Biophys. Methods 2005, 65, 45–80. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Li, P.; Yang, F.; Hu, X.; Sun, C.; Zhang, W.; Chen, D.; Tang, B. Dynamic and reversible fluorescence imaging of superoxide anion fluctuations in live cells and in vivo. J. Am. Chem. Soc. 2013, 135, 14956–14959. [Google Scholar] [CrossRef] [PubMed]
- Yu, F.; Song, P.; Li, P.; Wang, B.; Han, K. Development of reversible fluorescence probes based on redox oxoammonium cation for hypobromous acid detection in living cells. Chem. Commun. 2012, 48, 7735–7737. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Kim, Y. A water-soluble sulfonate-BODIPY based fluorescent probe for selective detection of HOCl/OCl− in aqueous media. Analyst 2014, 139, 2986–2989. [Google Scholar] [CrossRef] [PubMed]
- Drummen, G.P.C.; van Liebergen, L.C.M.; Op den Kamp, J.A.F.; Post, J.A. C11-BODIPY581/591, an oxidation-sensitive fluorescent lipid peroxidation probe: (Micro) spectroscopic characterization and validation of methodology. Free Radic. Biol. Med. 2002, 33, 473–490. [Google Scholar] [CrossRef]
- Durantini, A.M.; Greene, L.E.; Lincoln, R.; Martínez, S.R.; Cosa, G. Reactive Oxygen Species Mediated Activation of a Dormant Singlet Oxygen Photosensitizer: From Autocatalytic Singlet Oxygen Amplification to Chemicontrolled Photodynamic Therapy. J. Am. Chem. Soc. 2016, 138, 1215–1225. [Google Scholar] [CrossRef] [PubMed]
- Oleynik, P.; Ishihara, Y.; Cosa, G. Design and synthesis of a BODIPY-α-tocopherol adduct for use as an off/on fluorescent antioxidant indicator. J. Am. Chem. Soc. 2007, 129, 1842–1843. [Google Scholar] [CrossRef] [PubMed]
- Krumova, K.; Oleynik, P.; Karam, P.; Cosa, G. Phenol-based lipophilic fluorescent antioxidant indicators: A rational approach. J. Org. Chem. 2009, 74, 3641–3651. [Google Scholar] [CrossRef]
- Greene, L.E.; Lincoln, R.; Cosa, G. Tuning Photoinduced Electron Transfer Efficiency of Fluorogenic BODIPY-α-Tocopherol Analogues. Photochem. Photobiol. 2019, 95, 192–201. [Google Scholar] [CrossRef] [PubMed]
- Krumova, K.; Cosa, G. Fluorogenic Probes for Imaging Reactive Oxygen Species. In Photochemistry; The Royal Society of Chemistry: Cambridge, UK, 2013; Volume 41, pp. 279–301. [Google Scholar] [CrossRef]
- Pérez-Herrero, E.; Fernández-Medarde, A. Advanced targeted therapies in cancer: Drug nanocarriers, the future of chemotherapy. Eur. J. Pharm. Biopharm. 2015, 93, 52–79. [Google Scholar] [CrossRef] [PubMed]
- Jodko-Piorecka, K.; Litwinienko, G. Antioxidant activity of dopamine and L-DOPA in lipid micelles and their cooperation with an analogue of alpha-tocopherol. Free Radic. Biol. Med. 2015, 83, 1–11. [Google Scholar] [CrossRef]
- Konopko, A.; Kusio, J.; Litwinienko, G. Antioxidant Activity of Metal Nanoparticles Coated with Tocopherol-Like Residues—The Importance of Studies in Homo- and Heterogeneous Systems. Antioxidants 2020, 9, 5. [Google Scholar] [CrossRef]
- Van Wenum, E.; Jurczakowski, R.; Litwinienko, G. Media Effects on the Mechanism of Antioxidant Action of Silybin and 2,3-Dehydrosilybin: Role of the Enol Group. J. Org. Chem. 2013, 78, 9102–9112. [Google Scholar] [CrossRef]
- Fedeli, S.; Paoli, P.; Brandi, A.; Venturini, L.; Giambastiani, G.; Tuci, G.; Cicchi, S. Azido-Substituted BODIPY Dyes for the Production of Fluorescent Carbon Nanotubes. Chem. A Eur. J. 2015, 21, 15349–15353. [Google Scholar] [CrossRef]
- Foti, M.C.; Amorati, R. Non-phenolic radical-trapping antioxidants. J. Pharm. Pharmacol. 2009, 61, 1435–1448. [Google Scholar] [CrossRef]
- Barclay, L.R.C.; Baskin, K.A.; Dakin, K.A.; Locke, S.J.; Vinqvist, M.R. The Antioxidant Activities of Phenolic Antioxidants in Free-Radical Peroxidation of Phospholipid-Membranes. Can. J. Chem. 1990, 68, 2258–2269. [Google Scholar] [CrossRef]
- Denisov, E.T.; Afanas’ev, I.B. Oxidation and Antioxidants in Organic Chemistry and Biology; CRC Press: Boca Raton, FL, USA; Taylor & Francis Group: Abingdon-on-Thames, UK, 2005. [Google Scholar]
- Van Hook, J.P.; Tobolsky, A.V. The Thermal Decomposition of 2,2′-Azo-bis-isobutyronitrile. J. Am. Chem. Soc. 1958, 80, 779–782. [Google Scholar] [CrossRef]
- Denisov, E.T.; Denisova, T.G.; Pokidova, T.S. Handbook of Free Radical Initiators; John Wiley & Sons: Hoboken, NJ, USA, 2003. [Google Scholar]
- Koroleva, O.; Torkova, A.; Nikolaev, I.; Khrameeva, E.; Fedorova, T.; Tsentalovich, M.; Amarowicz, R. Evaluation of the Antiradical Properties of Phenolic Acids. Int. J. Mol. Sci. 2014, 15, 16351–16380. [Google Scholar] [CrossRef] [PubMed]
- Naguib, Y.M.A. A Fluorometric Method for Measurement of Peroxyl Radical Scavenging Activities of Lipophilic Antioxidants. Anal. Biochem. 1998, 265, 290–298. [Google Scholar] [CrossRef] [PubMed]

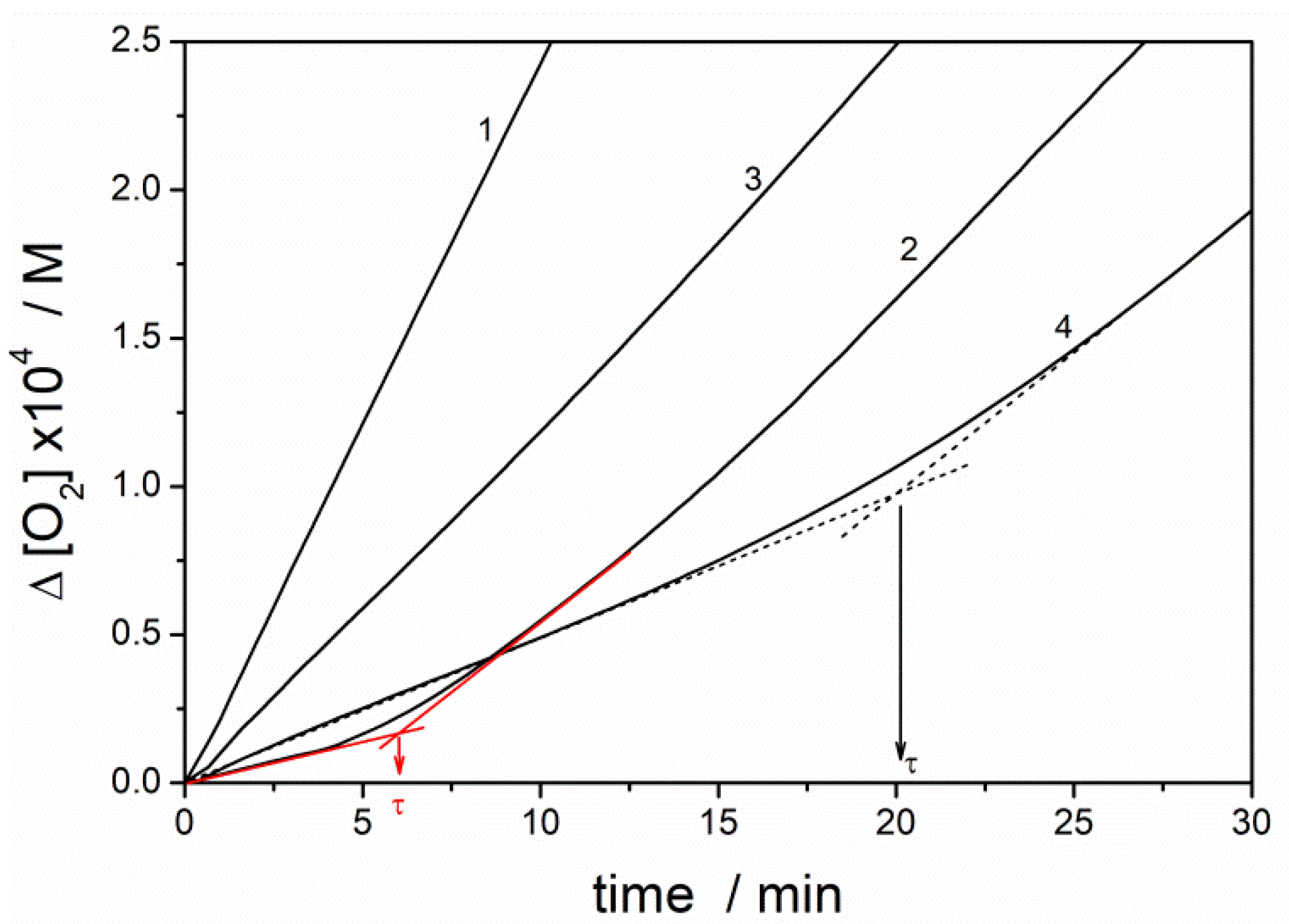


| Experimental System | τ/min | Ria/nMs−1 | Rinh/nMs−1 | Rox/Rinhb | 10−3 × kinh/M−1·s−1 |
|---|---|---|---|---|---|
| PMHC | 6.0 ± 0.6 | 4.3 ± 0.3 | 37 ± 13 | 11.6 | 12.1 ± 3.0 |
| NB-1 | - c | 4.3 ± 0.3 | 220 ± 15 c | 2.0 | - |
| NB-2 | 20.2 ± 0.8 | 4.4 ± 0.4 | 90 ± 9 | 4.8 | 1.0 ± 0.1 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kusio, J.; Sitkowska, K.; Konopko, A.; Litwinienko, G. Hydroxycinnamyl Derived BODIPY as a Lipophilic Fluorescence Probe for Peroxyl Radicals. Antioxidants 2020, 9, 88. https://doi.org/10.3390/antiox9010088
Kusio J, Sitkowska K, Konopko A, Litwinienko G. Hydroxycinnamyl Derived BODIPY as a Lipophilic Fluorescence Probe for Peroxyl Radicals. Antioxidants. 2020; 9(1):88. https://doi.org/10.3390/antiox9010088
Chicago/Turabian StyleKusio, Jaroslaw, Kaja Sitkowska, Adrian Konopko, and Grzegorz Litwinienko. 2020. "Hydroxycinnamyl Derived BODIPY as a Lipophilic Fluorescence Probe for Peroxyl Radicals" Antioxidants 9, no. 1: 88. https://doi.org/10.3390/antiox9010088
APA StyleKusio, J., Sitkowska, K., Konopko, A., & Litwinienko, G. (2020). Hydroxycinnamyl Derived BODIPY as a Lipophilic Fluorescence Probe for Peroxyl Radicals. Antioxidants, 9(1), 88. https://doi.org/10.3390/antiox9010088





