Selenium Anticancer Properties and Impact on Cellular Redox Status
Abstract
1. Introduction
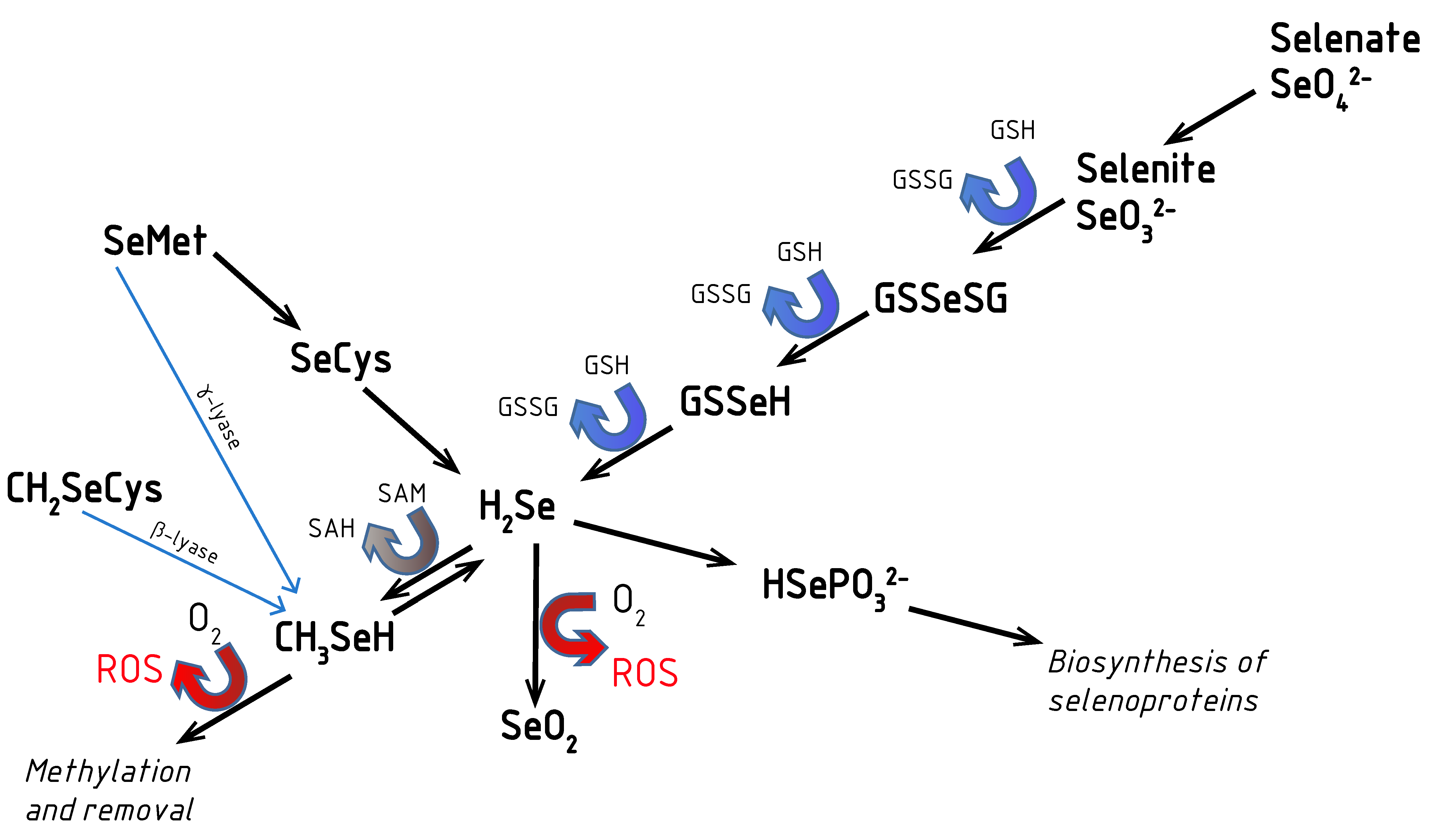
2. Aspects of Selenium Metabolism: Redox Activity of Se Compounds
3. Selenium Status and Its Biological Importance: Selenium in the Structure of Selenoproteins
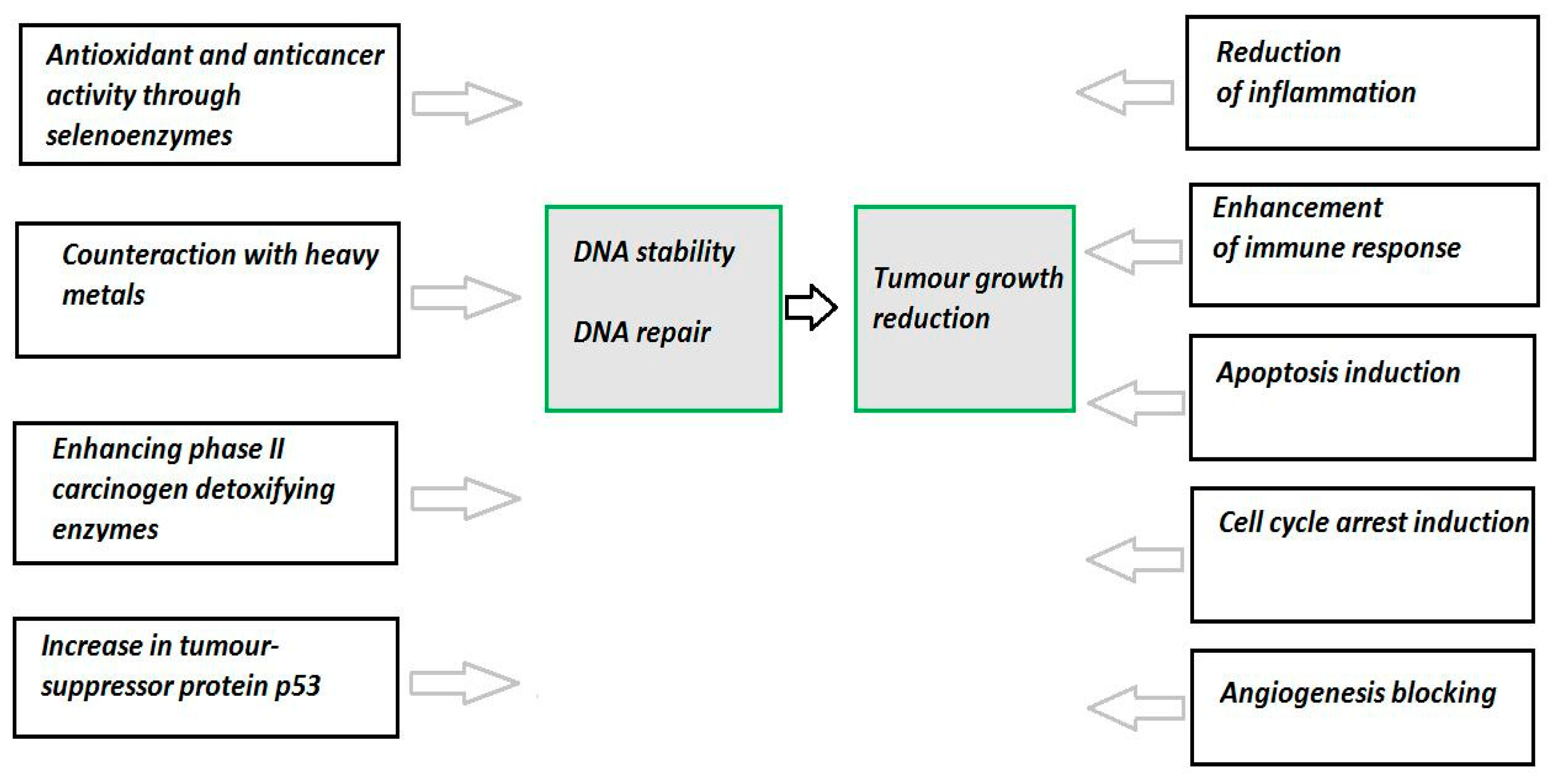
4. Selenium and Cancer
5. Chemotherapeutic Application of Redox Active Se Compounds
6. Conclusions
Author Contributions
Conflicts of Interest
References
- Dos Santos, M.; da Silva, F.M.; Muccillo-Baisch, A.L. Selenium content of Brazilian foods: A review of the literature values. J. Food Compos. Anal. 2017, 58, 10–15. [Google Scholar] [CrossRef]
- Saha, U.; Fayiga, A.; Sonon, L. Selenium in the soil-plant environment: A review. IJAAR 2017, 3, 1–18. [Google Scholar] [CrossRef]
- Kieliszek, M.; Błażejak, S. Current knowledge on the importance of selenium in food for living organisms: A review. Molecules 2016, 21, 609. [Google Scholar] [CrossRef] [PubMed]
- Duntas, L.H.; Benvenga, S. Selenium: An element for life. Endocrine 2015, 48, 756–775. [Google Scholar] [CrossRef] [PubMed]
- Pedrero, Z.; Madrid, Y. Novel approaches for selenium speciation in foodstuffs and biological specimens: A review. Anal. Chim. Acta 2009, 634, 135–152. [Google Scholar] [CrossRef]
- Beck, M.A.; Levander, O.A.; Handy, J. Selenium deficiency and viral infection. J. Nutr. 2003, 133, 1463–1467. [Google Scholar] [CrossRef]
- Kobayashi, Y.; Ogra, Y.; Ishiwata, K.; Takayama, H.; Aimi, N.; Suzuki, K.T. Selenosugars are key and urinary metabolites for selenium excretion within the required to low-toxic range. Proc. Natl. Acad. Sci. USA 2002, 99, 15932–15936. [Google Scholar] [CrossRef]
- Kim, H.W.; Ha, U.S.; Woo, J.C.; Kim, S.J.; Yoon, B.I.; Lee, S.J.; Cho, Y.H. Preventive effect of selenium on chronic bacterial prostatitis. J. Infect. Chemother. 2012, 18, 30–34. [Google Scholar] [CrossRef]
- Wang, C.; Wang, H.; Luo, J.; Hu, Y.; Wei, L.; Duan, M.; He, H. Selenium deficiency impairs host innate immune response and induces susceptibility to Listeria monocytogenes infection. BMC Immunol. 2009, 10, 55. [Google Scholar] [CrossRef]
- Navarro-Alarcon, M.; Cabrera-Vique, C. Selenium in food and the human body: A review. Sci. Total Environ. 2008, 400, 115–141. [Google Scholar] [CrossRef]
- Short, S.P.; Williams, C.S. Selenoproteins in tumorigenesis and cancer progression. Adv. Cancer Res. 2017, 136, 49–83. [Google Scholar] [CrossRef] [PubMed]
- Yang, R.; Liu, Y.; Zhou, Z. Selenium and selenoproteins, from structure, function to food resource and nutrition. Food Sci. Technol. Res. 2017, 23, 363–373. [Google Scholar] [CrossRef]
- Misra, S.; Boylan, M.; Selvam, A.; Spallholz, J.E.; Björnstedt, M. Redox-active selenium compounds—From toxicity and cell death to cancer treatment. Nutrients 2015, 7, 3536–3556. [Google Scholar] [CrossRef]
- Combs, G.F., Jr. Biomarkers of selenium status. Nutrients 2015, 7, 2209–2236. [Google Scholar] [CrossRef] [PubMed]
- Mehdi, Y.; Hornick, J.L.; Istasse, L.; Dufrasne, I. Selenium in the environment, metabolism and involvement in body functions. Molecules 2013, 18, 3292–3311. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, K.T.; Ogra, Y. Metabolic pathway for selenium in the body: Speciation by HPLC-ICP MS with enriched Se. Food Addit. Contamin. 2002, 19, 974–983. [Google Scholar] [CrossRef] [PubMed]
- Lyons, M.P.; Papazyan, T.T.; Surai, P.F. Selenium in food chain and animal nutrition: Lessons from nature—Review. AJAS 2007, 20, 1135–1155. [Google Scholar] [CrossRef]
- Zachara, B.A.; Pawluk, H.; Bloch-Boguslawska, E.; Sliwka, K.M.; Korenkiewicz, J.; Skok, Z.; Ryc, K. Tissue level, distribution and total body selenium content in healthy and diseased humans in Poland. Arch. Environ. Health 2001, 56, 461–466. [Google Scholar] [CrossRef]
- Drasch, G.; Mailander, S.; Schlosser, C. Content of non-mercury-associated selenium in human tissues. Biol. Trace Elem. Res. 2000, 77, 219–230. [Google Scholar] [CrossRef]
- Combs, G.F., Jr. Selenium in global food systems. Br. J. Nutr. 2001, 85, 517–547. [Google Scholar] [CrossRef]
- Rayman, M.P. The use of high-selenium yeast to raise selenium status: How does it measure up? Br. J. Nutr. 2004, 92, 557–573. [Google Scholar] [CrossRef] [PubMed]
- Battin, E.E.; Brumaghim, J.L. Metal specificity in DNA damage prevention by sulfur antioxidants. J. Inorg. Biochem. 2008, 102, 2036–2042. [Google Scholar] [CrossRef] [PubMed]
- Nilsonne, G.; Sun, X.; Nystrom, C.; Rundlof, A.K.; Potamitou Fernandes, A.; Bjornstedt, M.; Dobra, K. Selenite induces apoptosis in sarcomatoid malignant mesothelioma cells through oxidative stress. Free Radic. Biol. Med. 2006, 41, 874–885. [Google Scholar] [CrossRef] [PubMed]
- Ramoutar, R.R.; Brumaghim, J.L. Investigating the antioxidant properties of oxo-sulfur compounds on metal-mediated DNA damage. Main Group Chem. 2007, 6, 143–153. [Google Scholar] [CrossRef]
- Holmgren, A. Selenite in cancer therapy: A commentary on “Selenite induces apoptosis in sarcomatoid malignant mesothelioma cells through oxidative stress”. Free Radic. Biol. Med. 2006, 41, 862–865. [Google Scholar] [CrossRef]
- Zhou, N.; Xiao, H.; Li, T.K.; Nur-E-Kamal, A.; Liu, L.F. DNA damage-mediated apoptosis induced by selenium compounds. J. Biol. Chem. 2003, 278, 29532–29537. [Google Scholar] [CrossRef]
- Zimmerman, M.T.; Bayse, C.A.; Ramoutar, R.R.; Brumaghim, J.L. Sulfur and selenium antioxidants: Challenging radical scavenging mechanisms and developing structure-activity relationships based on metal binding. J. Inorg. Biochem. 2015, 145, 30–40. [Google Scholar] [CrossRef]
- Estevam, E.C.; Witek, K.; Faulstich, L.; Nasim, M.J.; Latacz, G.; Domínguez-Álvarez, E.; Kieć-Kononowicz, K.; Demasi, M.; Handzlik, J.; Jacob, C. Aspects of a distinct cytotoxicity of selenium salts and organic selenides in living cells with possible implications for drug design. Molecules 2015, 20, 13894–13912. [Google Scholar] [CrossRef]
- Wallenberg, M.; Misra, S.; Björnstedt, M. Selenium cytotoxicity in cancer. Basic Clin. Pharmacol. Toxicol. 2014, 114, 377–386. [Google Scholar] [CrossRef]
- Lavu, R.V.; Van De Wiele, T.; Pratti, V.L.; Tack, F.; Du Laing, G. Selenium bioaccessibility in stomach, small intestine and colon: Comparison between pure Se compounds, Se-enriched food crops and food supplements. Food Chem. 2016, 197, 382–387. [Google Scholar] [CrossRef]
- Zeng, H.; Wu, M.; Botnen, J.H. Methylselenol, a selenium metabolite, induces cell cycle arrest in G1 phase and apoptosis via the extracellular-regulated kinase 1/2 pathway and other cancer signaling genes. J. Nutr. 2009, 139, 1613–1618. [Google Scholar] [CrossRef] [PubMed]
- Stoffaneller, R.; Morse, N.L. A review of dietary selenium intake and selenium status in Europe and the Middle East. Nutrients 2015, 7, 1494–1537. [Google Scholar] [CrossRef] [PubMed]
- Labunsky, V.M.; Hatfield, D.L.; Gladyshev, V.N. Selenoproteins: Molecular pathways and physiological roles. Physiol. Rev. 2014, 94, 739–777. [Google Scholar] [CrossRef]
- Kasaikina, M.V.; Hatfield, D.L.; Gladyshev, V.N. Understanding selenoprotein function and regulation through the use of rodent models. Biochim. Biophys. Acta 2012, 1823, 1633–1642. [Google Scholar] [CrossRef]
- Pillai, R.; Uyehara-Lock, J.H.; Bellinger, F.P. Selenium and selenoprotein function in brain disorders. IUBMB Life 2014, 66, 229–239. [Google Scholar] [CrossRef] [PubMed]
- Rayman, M.P. Selenium in cancer prevention: A review of the evidence and mechanism of action. Proc. Nutr. Soc. 2005, 64, 527–542. [Google Scholar] [CrossRef] [PubMed]
- Kohrle, J.; Jakob, F.; Contempre, B.; Dumont, J.E. Selenium, the thyroid, and the endocrine system. Endocr. Rev. 2005, 26, 944–984. [Google Scholar] [CrossRef] [PubMed]
- Schomburg, L. Selenium, selenoproteins and the thyroid gland: Interactions in health and disease. Ant. Rev. Endocrinol. 2012, 8, 160–171. [Google Scholar] [CrossRef]
- Jonklaas, J.; Danielsen, M.; Wang, H. A pilot study of serum selenium, vitamin D, and thyrotropin concentrations in patients with thyroid cancer. Thyroid 2013, 23, 1079–1086. [Google Scholar] [CrossRef]
- Papp, L.V.; Lu, J.; Holmgren, A.; Khanna, K.K. From selenium to selenoproteins: Synthesis, identity, and their role in human health. Antioxid. Redox Sign. 2007, 9, 775–806. [Google Scholar] [CrossRef]
- Kipp, A.P.; Strohm, D.; Brigelius-Flohé, R.; Schomburg, L.; Bechthold, A.; Leschik-Bonnet, E.; Heseker, H. German Nutrition Society (DGE). Revised reference values for selenium intake. J. Trace Elem. Med. Biol. 2015, 32, 195–199. [Google Scholar] [CrossRef] [PubMed]
- Pappa, E.C.; Pappas, A.C.; Surai, P.F. Selenium content in selected foods from the Greek market and estimation of the daily intake. Sci. Total Environ. 2006, 372, 100–108. [Google Scholar] [CrossRef] [PubMed]
- Ren, B.; Liu, M.; Ni, J.; Tian, J. Role of Selenoprotein F in Protein Folding and Secretion: Potential Involvement in Human Disease. Nutrients 2018, 10, 1619. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Shi, Q.Q.; Ge, L.Y.; Zhuang, Q.F.; Xue, D.; Xu, H.Y.; He, X.Z. Selenoprotein M stimulates the proliferative and metastatic capacities of renal cell carcinoma through activating the PI3K/AKT/mTOR pathway. Cancer Med. 2019, 8, 4836–4844. [Google Scholar] [CrossRef] [PubMed]
- Duntas, L.H. The role of selenium in thyroid autoimmunity and cancer. Thyroid 2006, 16, 455–460. [Google Scholar] [CrossRef]
- Pang, K.L.; Chin, K.Y. Emerging anticancer potentials of selenium on osteosarcoma. Int. J. Mol. Sci. 2019, 20, 5318. [Google Scholar] [CrossRef]
- Evans, S.O.; Khairuddin, P.F.; Jameson, M.B. Optimising selenium for modulation of cancer treatments. Anticancer Res. 2017, 37, 6497–6509. [Google Scholar] [CrossRef]
- Tan, Q.; Li, J.; Yin, H.W.; Wang, L.H.; Tang, W.C.; Zhao, F.; Liu, X.M.; Zeng, H.H. Augmented antitumor effects of combination therapy of cisplatin with ethaselen as a novel thioredoxin reductase inhibitor on human A549 cell in vivo. Investig. New Drugs 2010, 28, 205–215. [Google Scholar] [CrossRef]
- Puspitasari, I.M.; Abdulah, R.; Yamazaki, C.; Kameo, S.; Nakano, T.; Koyama, H. Updates on clinical studies of selenium supplementation in radiotherapy. Radiat. Oncol. 2014, 9, 125. [Google Scholar] [CrossRef]
- Yakubov, E.; Buchfelder, M.; Eyupoglu, I.Y.; Savaskan, N.E. Selenium action in neuro-oncology. Biol. Trace Elem. Res. 2014, 161, 246–254. [Google Scholar] [CrossRef]
- Song, H.; Hur, I.; Park, H.J.; Nam, J.; Park, G.B.; Kong, K.H.; Hwang, Y.M.; Kim, Y.S.; Cho, D.H.; Lee, W.J.; et al. Selenium inhibits metastasis of murine melanoma cells through the induction of cell cycle arrest and cell death. Immune Netw. 2009, 9, 236–242. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.C.; Prabhu, K.S.; Das, A.; Mastro, A.M. Dietary selenium supplementation modifies breast tumor growth and metastasis. Int. J. Cancer 2013, 133, 2054–2064. [Google Scholar] [CrossRef] [PubMed]
- Davis, C.D.; Tsuji, P.A.; Milner, J.A. Selenoproteins and cancer prevention. Annu. Rev. Nutr. 2012, 32, 73–95. [Google Scholar] [CrossRef] [PubMed]
- Fairweather-Tait, S.J.; Collings, R.; Hurst, R. Selenium bioavailability: Current knowledge and future research requirements. Am. J. Clin. Nutr. 2010, 91, 1484–1491. [Google Scholar] [CrossRef]
- Ekoue, D.N.; Zaichick, S.; Valyi-Nagy, K.; Picklo, M.; Lacher, C.; Hoskins, K.; Warso, M.A.; Bonini, M.G.; Diamond, A.M. Selenium levels in human breast carcinoma tissue are associated with a common polymorphism in the gene for SELENOP (Selenoprotein P). J. Trace Elem. Med. Biol. 2017, 39, 227–233. [Google Scholar] [CrossRef]
- Shen, F.; Cai, W.S.; Li, J.L.; Feng, Z.; Cao, J.; Xu, B. The association between serum levels of selenium, copper, and magnesium with thyroid cancer: A meta-analysis. Biol. Trace Elem. Res. 2015, 167, 225–235. [Google Scholar] [CrossRef]
- Whanger, P.D. Selenium and its relationship to cancer: An update dagger. Br. J. Nutr. 2004, 91, 11–28. [Google Scholar] [CrossRef]
- El-Bayoumy, K. The protective role of selenium on genetic damage and on cancer. Mutat. Res. 2001, 475, 123–139. [Google Scholar] [CrossRef]
- Liu, W.; Zheng, W.J.; Zhang, Y.B.; Cao, W.Q.; Chen, T.F. Selenium nanoparticles as a carrier of 5-fluorouracil to achieve anticancer synergism. ACS Nano 2012, 6, 6578–6591. [Google Scholar] [CrossRef]
- Qin, S.; Huang, B.; Ma, J.; Wang, X.; Zhang, J.; Li, L.; Chen, F. Effects of selenium-chitosan on blood selenium concentration, antioxidation status, and cellular and humoral immunity in mice. Biol. Trace Elem. Res. 2015, 165, 145–152. [Google Scholar] [CrossRef]
- Farhat, Z.; Browne, R.W.; Bonner, M.R.; Tian, L.; Deng, F.; Swanson, M.; Mu, L. How do glutathione antioxidant enzymes and total antioxidant status respond to air pollution exposure? Environ. Int. 2018, 112, 287–293. [Google Scholar] [CrossRef] [PubMed]
- El-Demerdash, F.M. Antioxidant effect of vitamin E and selenium on lipid peroxidation, enzyme activities and biochemical parameters in rats exposed to aluminium. J. Trace Elem. Med. Biol. 2004, 18, 113–121. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.H.; Jeong, D. Bimodal actions of selenium essential for antioxidant and toxic pro-oxidant activities: The selenium paradox: A review. Mol. Med. Rep. 2012, 5, 299–304. [Google Scholar] [CrossRef] [PubMed]
- Björnstedt, M.; Fernandes, A.P. Selenium in the prevention of human cancers. EPMA J. 2010, 1, 389–395. [Google Scholar] [CrossRef] [PubMed]
- Yildiz, A.; Kaya, Y.; Tanriverdi, O. Effect of the interaction between selenium and zinc on DNA repair in association with cancer prevention. J. Cancer Prev. 2019, 24, 146–154. [Google Scholar] [CrossRef] [PubMed]
- Brozmanova, J.; Manikova, D.; Vlckova, V.; Chovanec, M. Selenium: A double-edged sword for defense and offence in cancer. Arch. Toxicol. 2010, 84, 919–938. [Google Scholar] [CrossRef] [PubMed]
- Drutel, A.; Archambeaud, F.; Caron, P. Selenium and the thyroid gland: More good news for clinicians. Clin. Endocrinol. 2013, 78, 155–164. [Google Scholar] [CrossRef]
- Chen, Y.C.; Prabhu, K.S.; Mastro, A.M. Is selenium a potential treatment for cancer metastasis? Nutrients 2013, 5, 1149–1168. [Google Scholar] [CrossRef]
- Wrobel, J.K.; Power, R.; Toborek, M. Biological activity of selenium: Revisited. IUBMB Life 2016, 68, 97–105. [Google Scholar] [CrossRef]
- Venza, M.; Visalli, M.; Beninati, C.; De Gaetano, G.V.; Teti, D.; Venza, I. Cellular mechanisms of oxidative stress and action in melanoma. Oxid. Med. Cell Longev. 2015, 2015, 481782. [Google Scholar] [CrossRef]
- Zachara, B.A. Selenium and selenium-dependent antioxidants in chronic kidney disease. Adv. Clin. Chem. 2015, 68, 131–151. [Google Scholar] [CrossRef] [PubMed]
- Gromadzinska, J.; Reszka, E.; Bruzelius, K.; Wasowicz, W.; Akesson, B. Selenium and cancer: Biomarkers of selenium status and molecular action of selenium supplements. Eur. J. Clin. Nutr. 2008, 47, 29–50. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, A.P.; Gandin, V. Selenium compounds as therapeutic agents in cancer. Biochim. Biophys. Acta 2015, 1850, 1642–1660. [Google Scholar] [CrossRef] [PubMed]
- Gandin, V.; Khalkar, P.; Braude, J.; Fernandes, A.P. Organic selenium compounds as potential chemotherapeutic agents for improved cancer treatment. Free Radic. Biol. Med. 2018, 127, 80–97. [Google Scholar] [CrossRef]
- Menon, S.; Ks, S.D.; Santhiya, R.; Rajeshkumar, S.; Kumar, V. Selenium nanoparticles: A potent chemotherapeutic agent and an elucidation of its mechanism. Colloids Surf. B 2018, 170, 280–292. [Google Scholar] [CrossRef]
- Yan, Q.; Chen, X.; Gong, H.; Qiu, P.; Xiao, X.; Dang, S.; Hong, A.; Ma, Y. Delivery of a TNF-α–derived peptide by nanoparticles enhances its antitumor activity by inducing cell-cycle arrest and caspase-dependent apoptosis. FASEB J. 2018, 32, 6948–6964. [Google Scholar] [CrossRef]
- Khurana, A.; Tekula, S.; Saifi, M.A.; Venkatesh, P.; Godugu, C. Therapeutic applications of selenium nanoparticles. Biomed. Pharmacother. 2019, 111, 802–812. [Google Scholar] [CrossRef]
- Tan, H.W.; Mo, H.Y.; Lau, A.T.Y.; Xu, Y.M. Selenium species: Current status and potentials in cancer prevention and therapy. Int. J. Mol. Sci. 2019, 20, 75. [Google Scholar] [CrossRef]
- Maiyo, F.; Singh, M. Selenium nanoparticles: Potential in cancer gene and drug delivery. Nanomedicine 2017, 12, 1075–1089. [Google Scholar] [CrossRef]
- Sadek, K.M.; Lebda, M.A.; Abouzed, T.K.; Nasr, S.M.; Shoukry, M. Neuro-and nephrotoxicity of subchronic cadmium chloride exposure and the potential chemoprotective effects of selenium nanoparticles. Metab. Brain Dis. 2017, 32, 1659–1673. [Google Scholar] [CrossRef]
| Selenoprotein | Location | Main Function | References |
|---|---|---|---|
| GPx1 | cell cytosol and mitochondria | reduces peroxides to water | [11] |
| GPx2 | gastrointestinal tract, liver | reduces free hydroperoxides of fatty acid and hydrogene peroxide | [12] |
| GPx3 | plasma and extracellular fluid, kidneys | perform antioxidant function in plasma | [11,12] |
| GPx4 | cell cytosol and cell membranes, testis | reduces lipid hydroperoxides, participates in ferroptosis (iron–based cell death) | [11,12] |
| TRx1 | cytosol of liver, kidney, bone, heart cells | reduces thioredoxins | [12] |
| TRx2 | mitochondria | reduces thioredoxins | [11] |
| TRx3 | testis | reduces thioredoxins | [11] |
| SelP | plasma, extracellular compartment | storage and transport of Se from liver to other tissues | [12] |
| SelF | endoplasmic reticulum of liver, prostate, T-cells | possesses oxidoreductase activity, regulates protein folding | [11] |
| SelS | endoplasmic reticulum | regulates cellular redox balance | [40] |
| SelM | endoplasmic reticulum | regulates protein folding | [11] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kuršvietienė, L.; Mongirdienė, A.; Bernatonienė, J.; Šulinskienė, J.; Stanevičienė, I. Selenium Anticancer Properties and Impact on Cellular Redox Status. Antioxidants 2020, 9, 80. https://doi.org/10.3390/antiox9010080
Kuršvietienė L, Mongirdienė A, Bernatonienė J, Šulinskienė J, Stanevičienė I. Selenium Anticancer Properties and Impact on Cellular Redox Status. Antioxidants. 2020; 9(1):80. https://doi.org/10.3390/antiox9010080
Chicago/Turabian StyleKuršvietienė, Lolita, Aušra Mongirdienė, Jurga Bernatonienė, Jurgita Šulinskienė, and Inga Stanevičienė. 2020. "Selenium Anticancer Properties and Impact on Cellular Redox Status" Antioxidants 9, no. 1: 80. https://doi.org/10.3390/antiox9010080
APA StyleKuršvietienė, L., Mongirdienė, A., Bernatonienė, J., Šulinskienė, J., & Stanevičienė, I. (2020). Selenium Anticancer Properties and Impact on Cellular Redox Status. Antioxidants, 9(1), 80. https://doi.org/10.3390/antiox9010080









