Investigating the Potential of Conjugated Selenium Redox Folic Acid as a Treatment for Triple Negative Breast Cancer
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
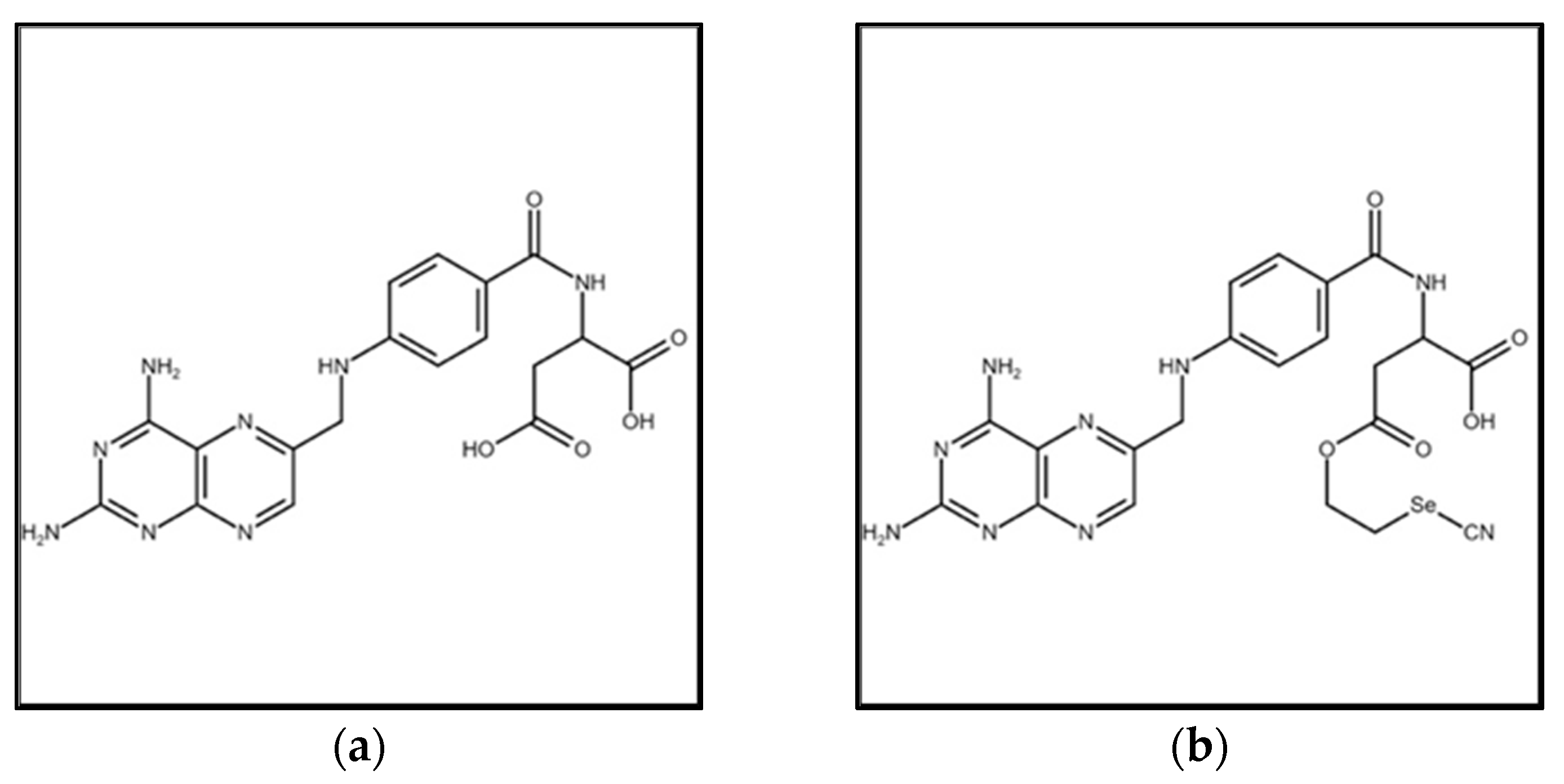
2.2. Synthesis of Selenofolate
2.3. Detection of Superoxide Generation In Vitro by Folic Acid and Selenofolate
2.4. Cell Culture
2.5. Western Analysis of FRA Protein Expression
2.6. Folic Acid and Selenofolate Treatments
2.7. Photographic Assessment of Control and Treated Cell Morphology
2.8. Cell Viability Analyzed by Trypan Blue Exclusion
2.9. Cell Viability as Measured by the MTT Assay
2.10. Detection of Intracellular Superoxide Generation with Dihydroethidium
2.11. MitoTracker® Red and Annexin V Staining for Apoptosis
2.12. Statistical Analyses
3. Results
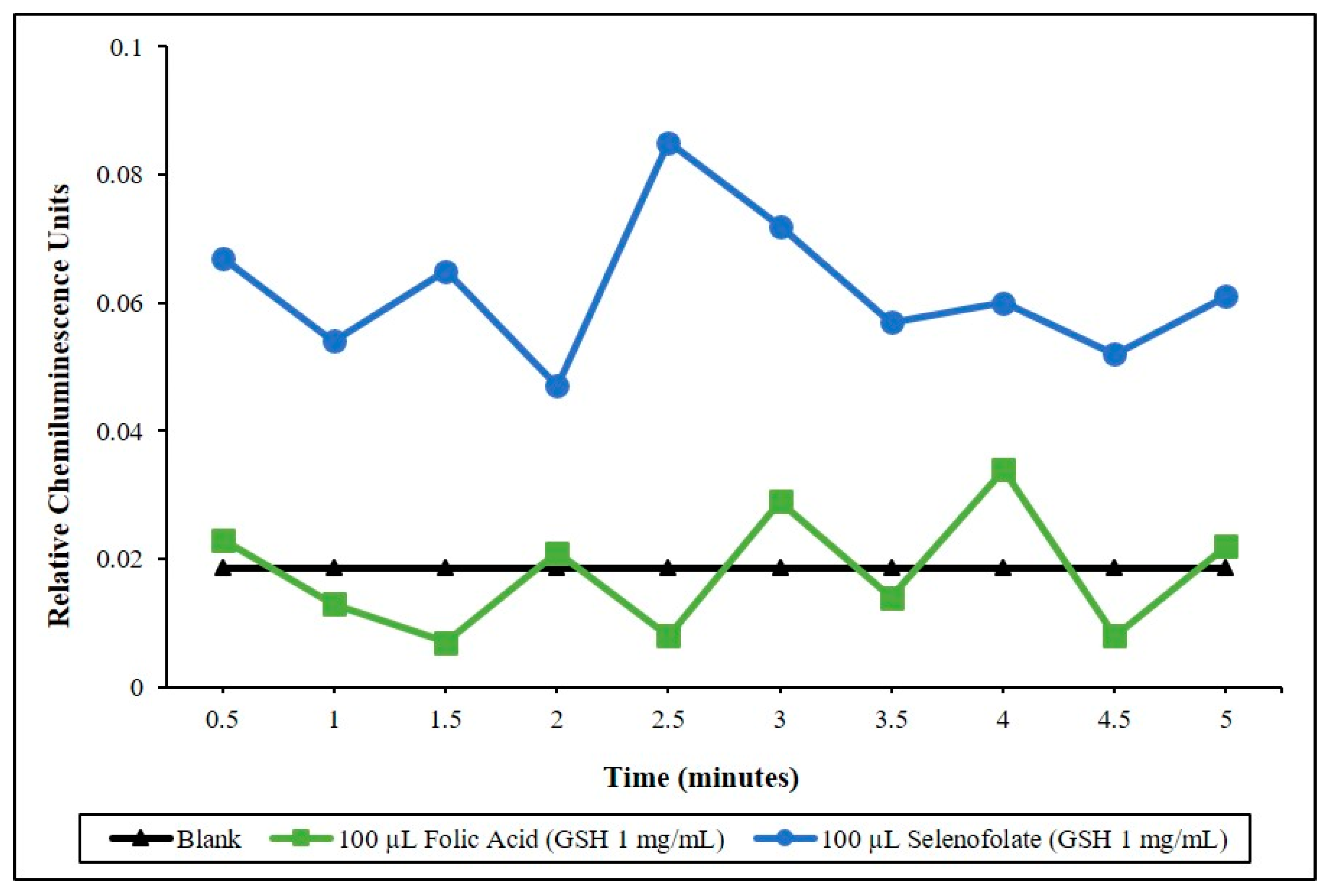
3.1. Determining the Redox Potential of Selenofolate
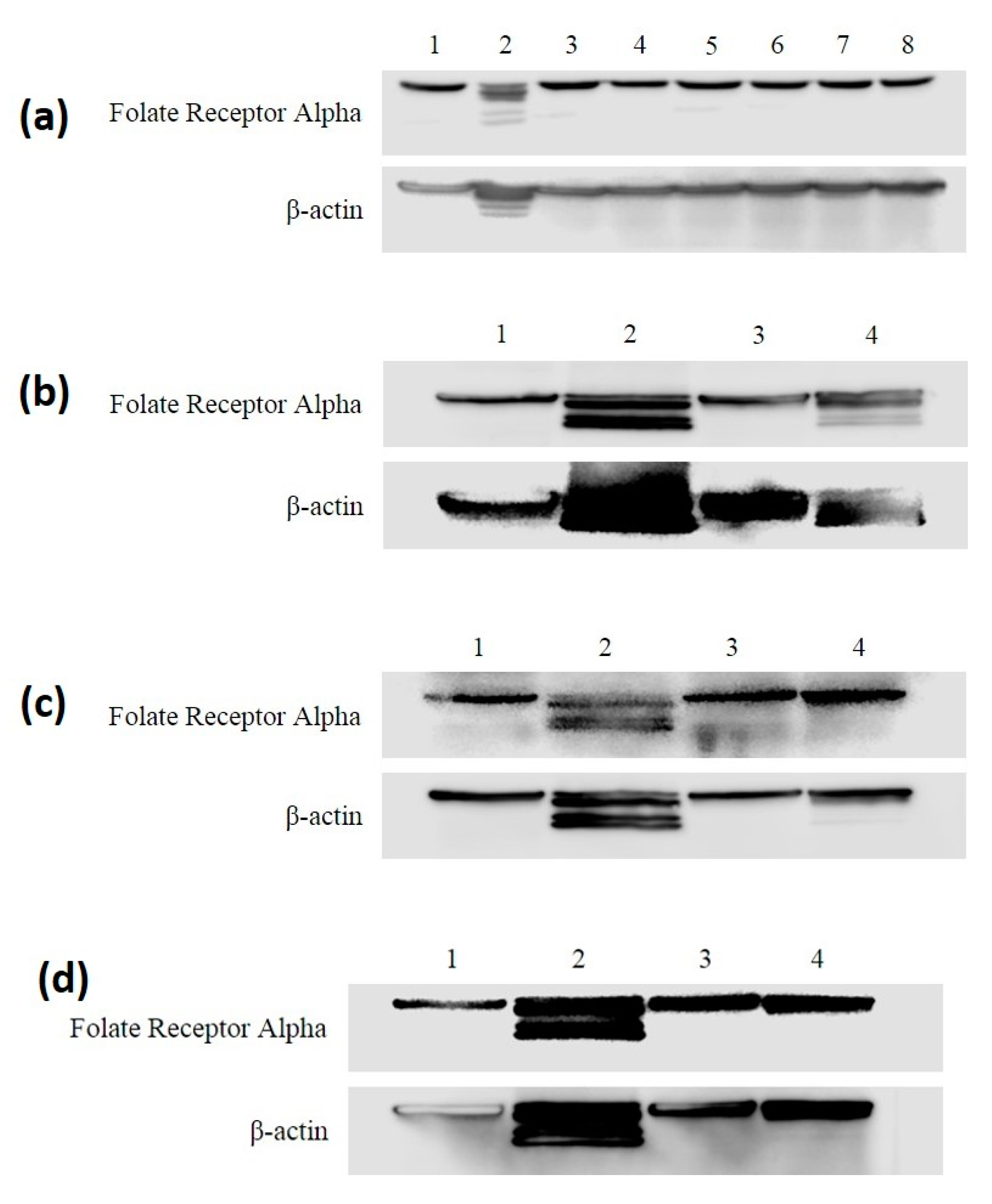
3.2. FRA Expression in MDA-MB-468 and HME50-5E Cells through All Treatments
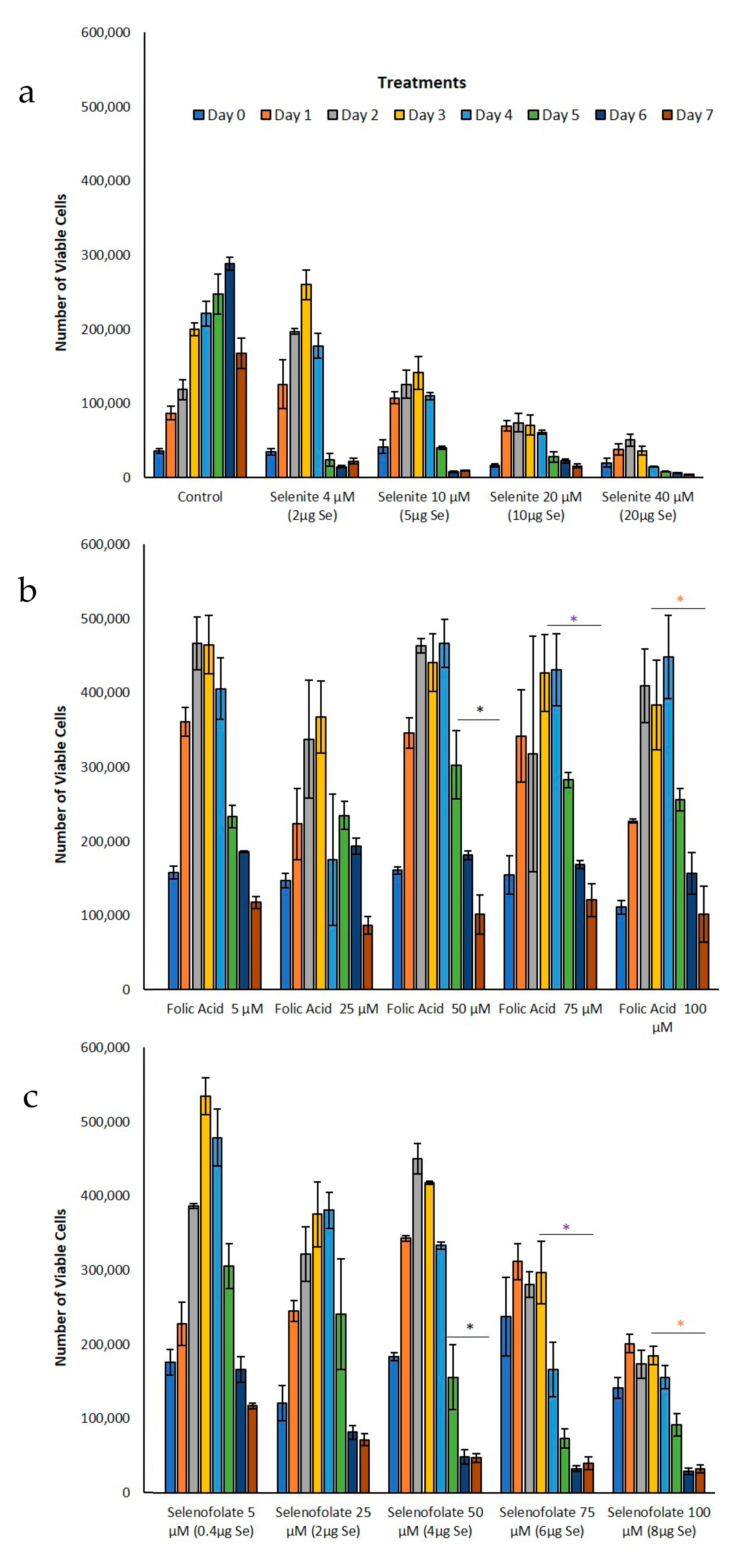
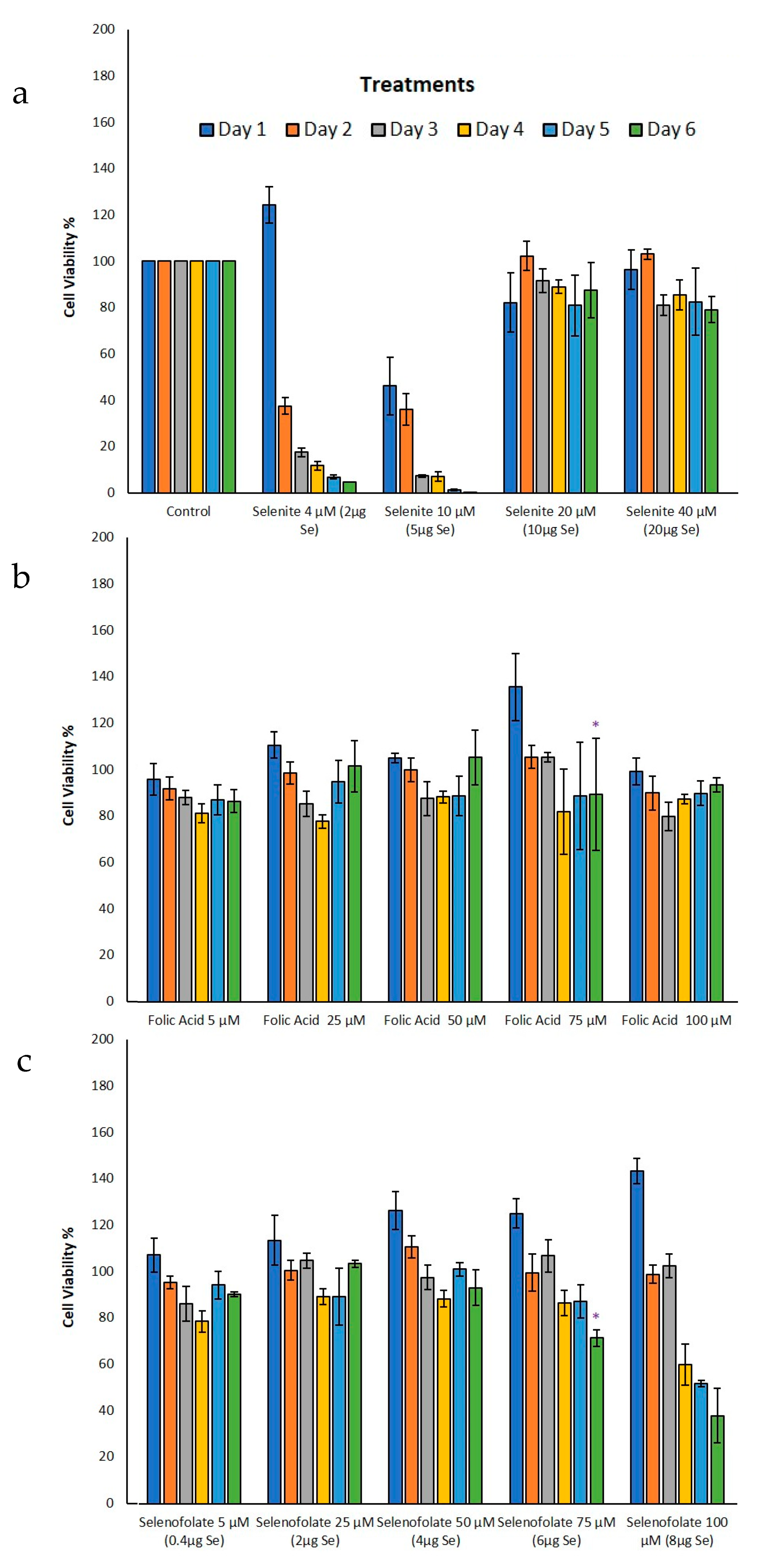
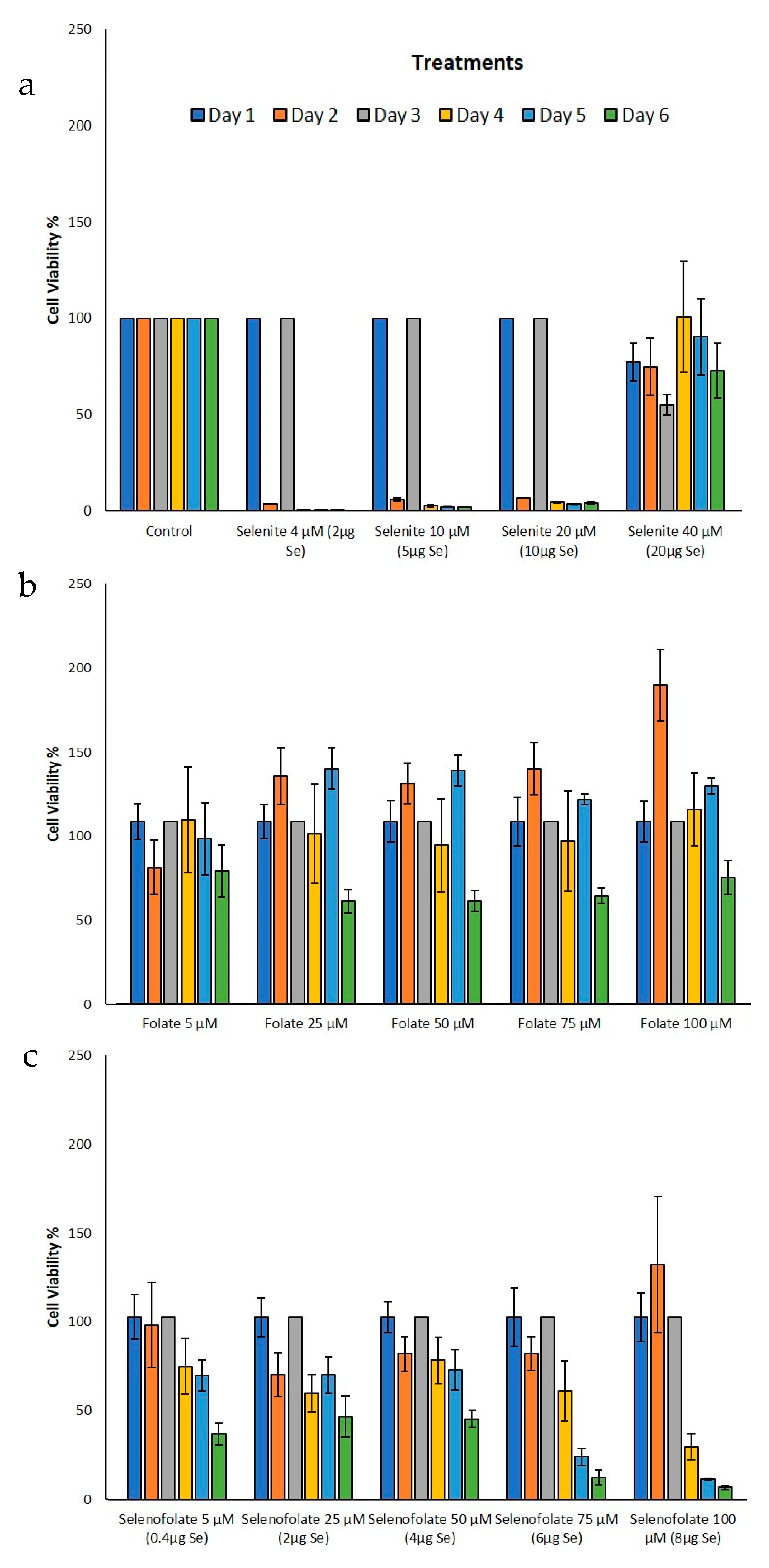
3.3. Analysis of Dosage/Time Dependent Cell Viability by Trypan Blue Exclusion
3.4. Analysis of Cell Metabolism/Viability by MTT
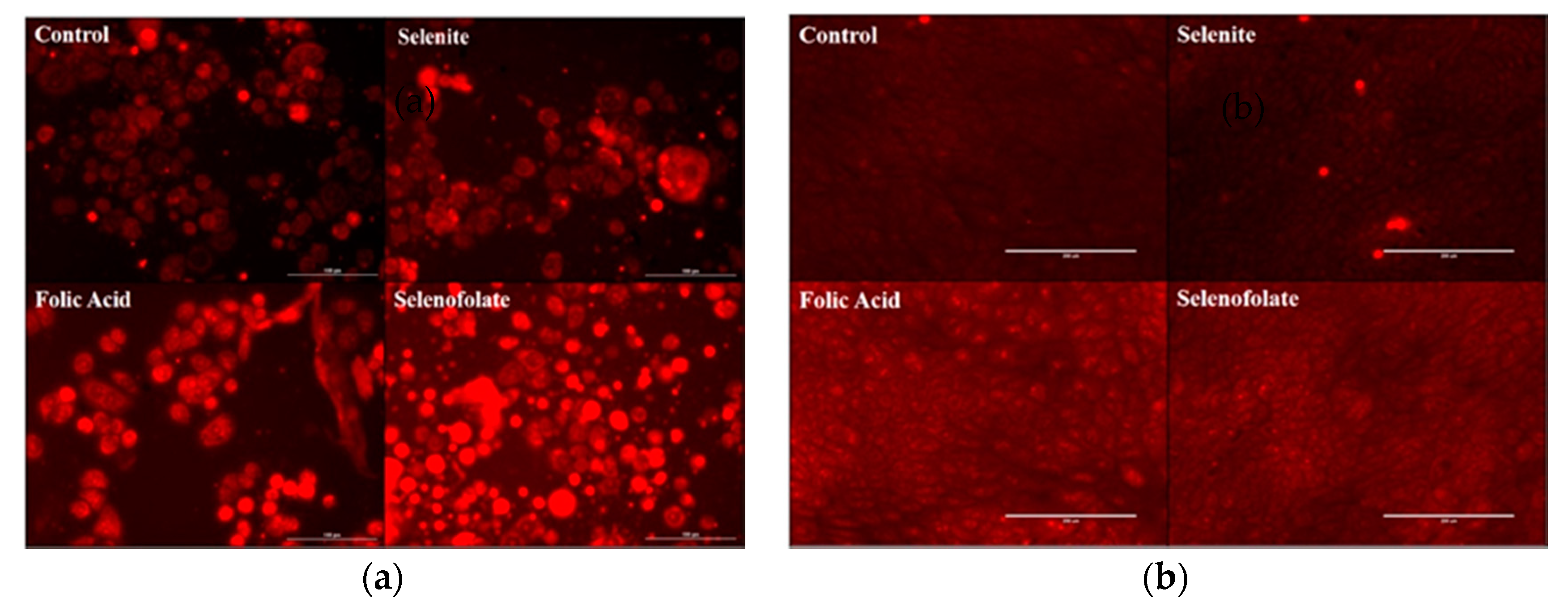
3.5. Generation of Intracellular Superoxide in Treated Cells
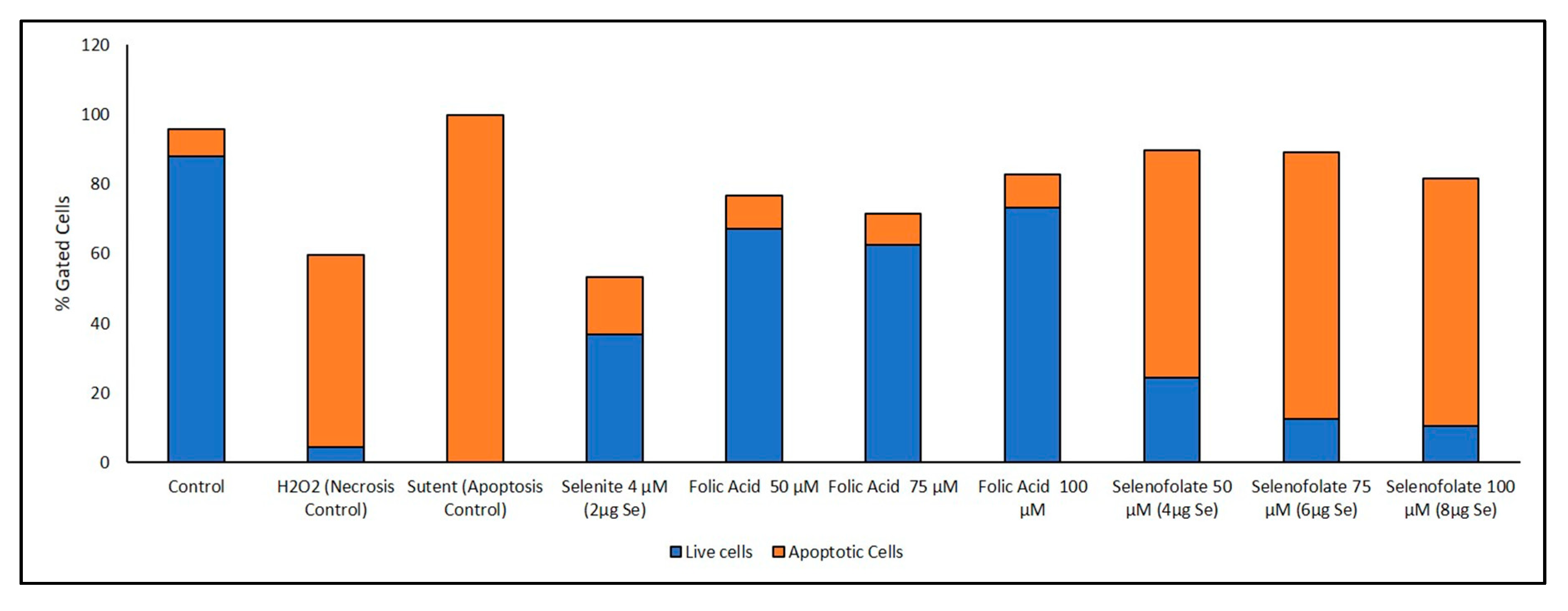
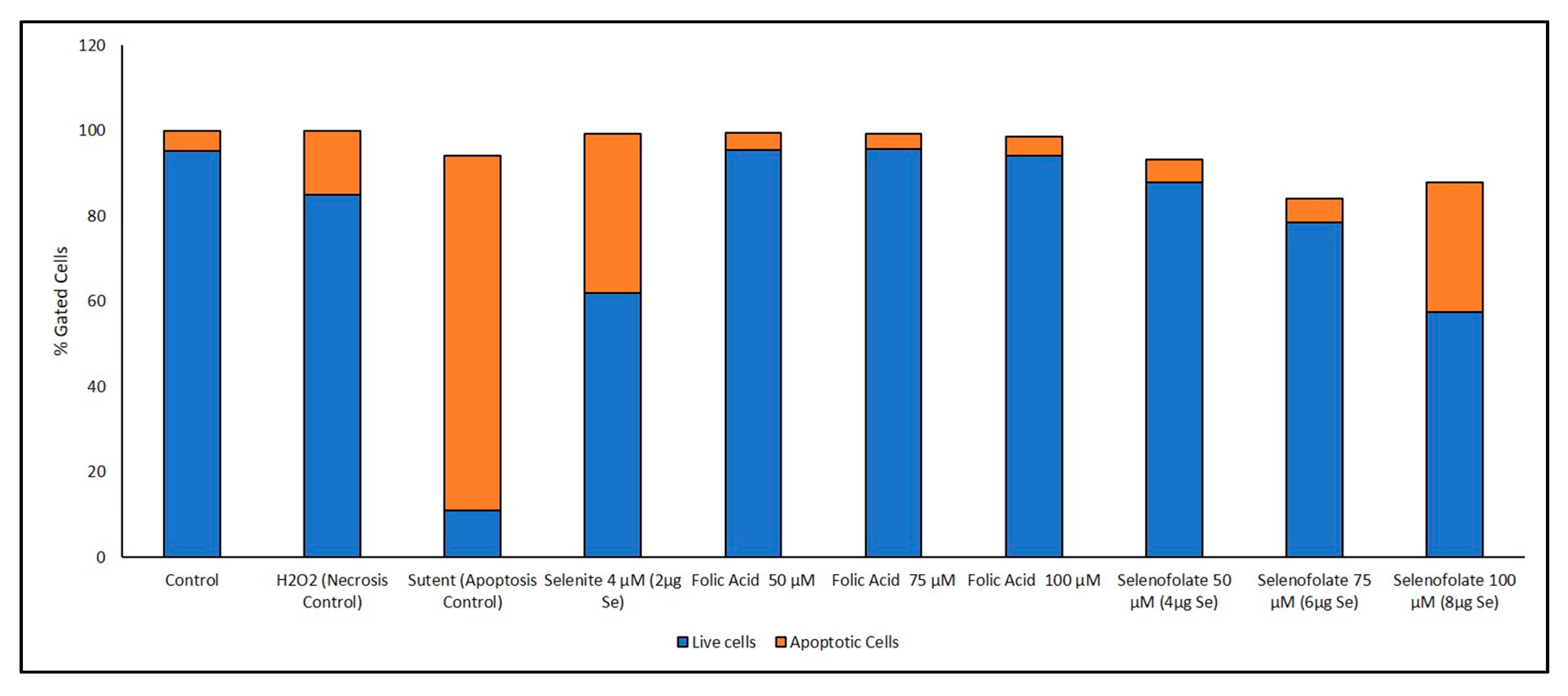
3.6. Analysis of Apoptosis-Induced Cell Death after Treatment with Selenofolate
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bailey, L.B.; Gregory, J.F. Folate metabolism and requirements. J. Nutr. 1999, 129, 779–782. [Google Scholar] [CrossRef] [PubMed]
- NIH U.S. National Library of Medicine. FOLR1 Gene Folate Receptor 1. Available online: https://ghr.nlm.nih.gov/gene/FOLR1 (accessed on 18 November 2017).
- Elnakat, H.; Ratnam, M. Distribution, functionality and gene regulation of folate receptor isoforms: Implications in targeted therapy. Adv. Drug Deliv. Rev. 2004, 56, 1067–1084. [Google Scholar] [CrossRef] [PubMed]
- Kelemen, L.E. The role of folate receptor α in cancer development, progression and treatment: Cause, consequence or innocent bystander? Int. J. Cancer 2006, 119, 243–250. [Google Scholar] [CrossRef] [PubMed]
- Antony, A.C. Folate receptors. Annu. Rev. Nutr. 1996, 16, 501–521. [Google Scholar] [CrossRef] [PubMed]
- Coney, L.R.; Tomassetti, A.; Carayannopoulos, L.; Frasca, V.; Kamen, B.A.; Colnaghi, M.I.; Zurawski, V.R. Cloning of a tumor-associated antigen: MOv18 and MOv19 antibodies recognize a folate-binding protein. Cancer Res. 1991, 51, 6125–6132. [Google Scholar] [PubMed]
- Shen, F.; Ross, J.F.; Wang, X.; Ratnam, M. Identification of a novel folate receptor, a truncated receptor, and receptor type. beta. in hematopoietic cells: cDNA cloning, expression, immunoreactivity, and tissue specificity. Biochemistry 1994, 33, 1209–1215. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, D.K.; White, A.J.; Weil, S.C.; Phillips, M.; Coleman, R.L. Farletuzumab (a monoclonal antibody against folate receptor alpha) in relapsed platinum-sensitive ovarian cancer. Gynecol. Oncol. 2013, 129, 452–458. [Google Scholar] [CrossRef]
- Nakashima-Matsushita, N.; Homma, T.; Yu, S.; Matsuda, T.; Sunahara, N.; Nakamura, T.; Tsukano, M.; Ratnam, M.; Matsuyama, T. Selective expression of folate receptor β and its possible role in methotrexate transport in synovial macrophages from patients with rheumatoid arthritis. Arthritis Rheumatol. 1999, 42, 1609–1616. [Google Scholar] [CrossRef]
- Turk, M.J.; Breur, G.J.; Widmer, W.R.; Paulos, C.M.; Xu, L.C.; Grote, L.A.; Low, P.S. Folate-targeted imaging of activated macrophages in rats with adjuvant-induced arthritis. Arthritis Rheumatol. 2002, 46, 1947–1955. [Google Scholar] [CrossRef]
- O’Shannessy, D.J.; Yu, G.; Smale, R.; Fu, Y.-S.; Singhal, S.; Thiel, R.P.; Somers, E.B.; Vachani, A. Folate receptor alpha expression in lung cancer: Diagnostic and prognostic significance. Oncotarget 2012, 3, 414–425. [Google Scholar] [CrossRef]
- Weitman, S.D.; Lark, R.H.; Coney, L.R.; Fort, D.W.; Frasca, V.; Zurawski, V.R.; Kamen, B.A. Distribution of the folate receptor GP38 in normal and malignant cell lines and tissues. Cancer Res. 1992, 52, 3396–3401. [Google Scholar] [PubMed]
- Weitman, S.D.; Weinberg, A.G.; Coney, L.R.; Zurawski, V.R.; Jennings, D.S.; Kamen, B.A. Cellular localization of the folate receptor: Potential role in drug toxicity and folate homeostasis. Cancer Res. 1992, 52, 6708–6711. [Google Scholar] [PubMed]
- O’Shannessy, D.J.; Somers, E.B.; Albone, E.; Cheng, X.; Park, Y.C.; Tomkowicz, B.E.; Hamuro, Y.; Kohl, T.O.; Forsyth, T.M.; Smale, R. Characterization of the human folate receptor alpha via novel antibody-based probes. Oncotarget 2011, 2, 1227–1243. [Google Scholar] [CrossRef] [PubMed]
- Van Dam, G.M.; Themelis, G.; Crane, L.M.; Harlaar, N.J.; Pleijhuis, R.G.; Kelder, W.; Sarantopoulos, A.; De Jong, J.S.; Arts, H.J.; Van Der Zee, A.G. Intraoperative tumor-specific fluorescence imaging in ovarian cancer by folate receptor-α targeting: First in-human results. Nat. Med. 2011, 17, 1315. [Google Scholar] [CrossRef]
- Sega, E.I.; Low, P.S. Tumor detection using folate receptor-targeted imaging agents. Cancer Metastasis Rev. 2008, 27, 655. [Google Scholar] [CrossRef]
- Serpe, L.; Gallicchio, M.; Canaparo, R.; Dosio, F. Targeted treatment of folate receptor-positive platinum-resistant ovarian cancer and companion diagnostics, with specific focus on vintafolide and etarfolatide. Pharm. Pers. Med. 2014, 7, 31–42. [Google Scholar]
- Brian, B.; Haines, J.O.N.; Marlene, C.H.; Christopher, W.; Tammie, C.Y.; Tianxiao, S.; Kristen, L.; Picard, T.Z.; Emmett, V.S.; Isabelle, D. Preclinical activity of Vintafolide/MK-8109 monotherapy and in combination with standard of care therapy in triple-negative breast cancer models. In Proceedings of the 105th Annual Meeting of the American Association for Cancer Research, San Diego, CA, USA, 5–9 April 2014. [Google Scholar]
- Inc, M. Vintafolide Fails Phase III PROCEED Trial for Ovarian Cancer. Available online: https://www.epgonline.org/global/news/vintafolide-fails-phase-iii-proceed-trial-for-ovarian-cancer-merck-inc-html (accessed on 3 March 2018).
- Goswami, D. Cytotoxic Effects of Selenium Conjugated Transferrins on Leukemia Cell Lines. Doctoral Dissertation, Texas Tech University, Lubbock, TX, USA, 2014. [Google Scholar]
- Khandelwal, S.; Boylan, M.; Spallholz, J.; Gollahon, L. Cytotoxicity of Selenium Immunoconjugates against Triple Negative Breast Cancer Cells. Int. J. Mol. Sci. 2018, 19, 3352. [Google Scholar] [CrossRef]
- Bapat, P. Cytotoxic Effects of Selenium Conjugated Trastuzumab on HER2+ Breast Cancer Cell Lines. Doctoral Dissertation, Texas Tech University, Lubbock, TX, USA, 2015. [Google Scholar]
- Seko, Y.; Saito, Y.; Kitahara, J.; Imura, N. Active oxygen generation by the reaction of selenite with reduced glutathione in vitro. In Selenium in Biology and Medicine; Springer: Berlin/Heidelberg, Germany, 1989; pp. 70–73. [Google Scholar]
- Chaudiere, J.; Courtin, O.; Leclaire, J. Glutathione oxidase activity of selenocystamine: A mechanistic study. Arch. Biochem. Biophys. 1992, 296, 328–336. [Google Scholar] [CrossRef]
- Shay, J.W.; Tomlinson, G.; Piatyszek, M.A.; Gollahon, L.S. Spontaneous in vitro immortalization of breast epithelial cells from a patient with Li-Fraumeni Syndrome. Mol. Cell Biol. 1995, 15, 425–432. [Google Scholar] [CrossRef][Green Version]
- Assaraf, Y.G.; Leamon, C.P.; Reddy, J.A. The folate receptor as a rational therapeutic target for personalized cancer treatment. Drug Resist. Updates 2014, 17, 89–95. [Google Scholar] [CrossRef]
- Vergote, I.; Leamon, C.P. Vintafolide: A novel targeted therapy for the treatment of folate receptor expressing tumors. Ther. Adv. Med. Oncol. 2015, 7, 206–218. [Google Scholar] [CrossRef] [PubMed]
- Hartmann, L.C.; Keeney, G.L.; Lingle, W.L.; Christianson, T.J.; Varghese, B.; Hillman, D.; Oberg, A.L.; Low, P.S. Folate receptor overexpression is associated with poor outcome in breast cancer. Int. J. Cancer 2007, 121, 938–942. [Google Scholar] [CrossRef] [PubMed]
- Brodin, O.; Eksborg, S.; Wallenberg, M.; Asker-Hagelberg, C.; Larsen, E.H.; Mohlkert, D.; Lenneby-Helleday, C.; Jacobsson, H.; Linder, S.; Misra, S. Pharmacokinetics and toxicity of sodium selenite in the treatment of patients with carcinoma in a phase I clinical trial: The SECAR study. Nutrients 2015, 7, 4978–4994. [Google Scholar] [CrossRef] [PubMed]
- Sinha, R.; Unni, E.; Ganther, H.E.; Medina, D. Methylseleninic acid, a potent growth inhibitor of synchronized mouse mammary epithelial tumor cells in vitro. Biochem. Pharmacol. 2001, 61, 311–317. [Google Scholar] [CrossRef]
- Ip, C. Lessons from basic research in selenium and cancer prevention. J. Nutr. 1998, 128, 1845–1854. [Google Scholar] [CrossRef]
- Yan, L.; Yee, J.A.; Boylan, M.; Spallholz, J.E. Effect of selenium compounds and thiols on human mammary tumor cells. Biol. Trace Elem. Res. 1991, 30, 145–162. [Google Scholar] [CrossRef]
- Lin, Y.; Spallholz, J.E. Generation of reactive oxygen species from the reaction of selenium compounds with thiols and mammary tumor cells. Biochem. Pharmacol. 1993, 45, 429–437. [Google Scholar] [CrossRef]
- Li, J.; Zuo, L.; Shen, T.; Xu, C.-M.; Zhang, Z.-N. Induction of apoptosis by sodium selenite in human acute promyelocytic leukemia NB4 cells: Involvement of oxidative stress and mitochondria. J. Trace Elem. Med. Biol. 2003, 17, 19–26. [Google Scholar] [CrossRef]
- Zou, Y.; Niu, P.; Yang, J.; Yuan, J.; Wu, T.; Chen, X. The JNK signaling pathway is involved in sodium-selenite-induced apoptosis mediated by reactive oxygen in HepG2 cells. Cancer Biol. Ther. 2008, 7, 689–696. [Google Scholar] [CrossRef]
- Nilsonne, G.; Sun, X.; Nyström, C.; Rundlöf, A.-K.; Fernandes, A.P.; Björnstedt, M.; Dobra, K. Selenite induces apoptosis in sarcomatoid malignant mesothelioma cells through oxidative stress. Free Radic. Biol. Med. 2006, 41, 874–885. [Google Scholar] [CrossRef]
- Orrenius, S. Mitochondrial regulation of apoptotic cell death. Toxicol. Lett. 2004, 149, 19–23. [Google Scholar] [CrossRef] [PubMed]
- Elmore, S. Apoptosis: A review of programmed cell death. Toxicol. Pathol. 2007, 35, 495–516. [Google Scholar] [CrossRef] [PubMed]
- Chinchar, E.; Makey, K.L.; Gibson, J.; Chen, F.; Cole, S.A.; Megason, G.C.; Vijayakumar, S.; Miele, L.; Gu, J.-W. Sunitinib significantly suppresses the proliferation, migration, apoptosis resistance, tumor angiogenesis and growth of triple-negative breast cancers but increases breast cancer stem cells. Vasc. Cell 2014, 6, 12. [Google Scholar] [CrossRef] [PubMed]
- Mgbonyebi, O.P.; Russo, J.; Russo, I.H. Roscovitine induces cell death and morphological changes indicative of apoptosis in MDA-MB-231 breast cancer cells. Cancer Res. 1999, 59, 1903–1910. [Google Scholar] [PubMed]
- Ly, J.D.; Grubb, D.; Lawen, A. The mitochondrial membrane potential (Δψm) in apoptosis; an update. Apoptosis 2003, 8, 115–128. [Google Scholar] [CrossRef] [PubMed]
- Sanmartín, C.; Plano, D.; Sharma, A.K.; Palop, J.A. Selenium compounds, apoptosis and other types of cell death: An overview for cancer therapy. Int. J. Mol. Sci. 2012, 13, 9649–9672. [Google Scholar] [CrossRef]
- Sinha, R.; El-Bayoumy, K. Apoptosis is a critical cellular event in cancer chemoprevention and chemotherapy by selenium compounds. Curr. Cancer Drug Targets 2004, 4, 13–28. [Google Scholar] [CrossRef]
- Toffoli, G.; Cernigoi, C.; Russo, A.; Gallo, A.; Bagnoli, M.; Boiocchi, M. Overexpression of folate binding protein in ovarian cancers. Int. J. Cancer 1997, 74, 193–198. [Google Scholar] [CrossRef]
- Nunez, M.I.; Behrens, C.; Woods, D.M.; Lin, H.; Suraokar, M.; Kadara, H.; Hofstetter, W.; Kalhor, N.; Lee, J.J.; Franklin, W. High expression of folate receptor alpha in lung cancer correlates with adenocarcinoma histology and mutation. J. Thorac. Oncol. 2012, 7, 833–840. [Google Scholar] [CrossRef]
- Müller, C.; Schibli, R. Folic acid conjugates for nuclear imaging of folate receptor–positive cancer. J. Nucl. Med. 2011, 52, 1–4. [Google Scholar] [CrossRef]
- Leamon, C.P.; Reddy, J.A. Folate-targeted chemotherapy. Adv. Drug Deliv. Rev. 2004, 56, 1127–1141. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Xu, H.; Huang, K. Mitochondrial permeabiltiy transition and cytochrome c release induced by selenite. J. Inorg. Biochem. 2002, 90, 43–50. [Google Scholar] [CrossRef]


| Number of Days | Experiment | Treatments | p Value | Significance |
|---|---|---|---|---|
| Day 3 | Trypan Blue (Figure 5b,c) | Folic acid 100 µM vs. Selenofolate 100 µM | 0.015 | * |
| Day 4 | Trypan Blue (Figure 5b,c) | Folic acid 50 µM vs. Selenofolate 50 µM | 0.008 | ** |
| Folic acid 75 µM vs. Selenofolate 75 µM | 0.006 | ** | ||
| Folic acid 100 µM vs. Selenofolate 100 µM | 0.004 | ** | ||
| Day 5 | Trypan Blue (Figure 5a–c) | Control vs. Selenofolate 75 µM | 0.020 | * |
| Control vs. Selenofolate 100 µM | 0.035 | * | ||
| Folic acid 50 µM vs. Selenofolate 50 µM | 0.041 | * | ||
| Folic acid 75 µM vs. Selenofolate 75 µM | 0.001 | *** | ||
| Folic acid 100 µM vs. Selenofolate 100 µM | 0.001 | *** | ||
| Day 3 | MTT (Figure 7b,c) | Folic acid 75 µM vs. Selenofolate 75 µM | 0.024 | * |
| Day 4 | MTT (Figure 7a,c) | Control vs. Selenofolate 100 µM | 0.022 | * |
| Day 3 | Annexin V (Early/Late Apoptosis) (Figure 10) | Folic acid 50 µM vs. Selenofolate 50 µM (early) | 0.001 | *** |
| Folic acid 75 µM vs. Selenofolate 75 µM (early) | 0.001 | *** | ||
| Folic acid 100 µM vs. Selenofolate 100 µM (early) | 0.002 | ** | ||
| Control vs. Selenofolate 50 µM (early) | 0.001 | *** | ||
| Control vs. Selenofolate 75 µM (early) | 0.002 | ** | ||
| Control vs. Selenofolate 100 µM (early) | 0.004 | ** | ||
| Folic acid 50 µM vs. Selenofolate 50 µM (late) | 0.004 | ** | ||
| Folic acid 75 µM vs. Selenofolate 75 µM (late) | 0.001 | *** | ||
| Folic acid 100 µM vs. Selenofolate 100 µM (late) | 0.001 | *** | ||
| Control vs. Selenofolate 50 µM (late) | 0.003 | ** | ||
| Control vs. Selenofolate 75 µM (late) | 0.001 | *** | ||
| Control vs. Selenofolate 100 µM (late) | 0.001 | *** |
| Number of Days | Experiment | Treatments | p Value | Significance |
|---|---|---|---|---|
| Day 3 | Trypan Blue (Figure 6b,c) | Folic acid 50 µM vs. Selenofolate 50 µM | 0.004 | ** |
| Folic acid 75 µM vs. Selenofolate 75 µM | 0.002 | ** | ||
| Day 4 | Trypan Blue (Figure 6b,c) | Folic acid 50 µM vs. Selenofolate 50 µM | 0.002 | ** |
| Folic acid 75 µM vs. Selenofolate 75 µM | 0.001 | *** | ||
| Folic acid 100 µM vs. Selenofolate 100 µM | 0.001 | *** | ||
| Day 4 | MTT (Figure 8b,c) | Folic acid 100 µM vs. Selenofolate 100 µM | 0.010 | * |
| Day 5 | MTT (Figure 8b,c) | Folic acid 50 µM vs. Selenofolate 50 µM | 0.007 | ** |
| Folic acid 75 µM vs. Selenofolate 75 µM | 0.001 | *** | ||
| Folic acid 100 µM vs. Selenofolate 100 µM | 0.001 | *** |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khandelwal, S.; Boylan, M.; Kirsch, G.; Spallholz, J.E.; Gollahon, L.S. Investigating the Potential of Conjugated Selenium Redox Folic Acid as a Treatment for Triple Negative Breast Cancer. Antioxidants 2020, 9, 138. https://doi.org/10.3390/antiox9020138
Khandelwal S, Boylan M, Kirsch G, Spallholz JE, Gollahon LS. Investigating the Potential of Conjugated Selenium Redox Folic Acid as a Treatment for Triple Negative Breast Cancer. Antioxidants. 2020; 9(2):138. https://doi.org/10.3390/antiox9020138
Chicago/Turabian StyleKhandelwal, Soni, Mallory Boylan, Gilbert Kirsch, Julian E. Spallholz, and Lauren S. Gollahon. 2020. "Investigating the Potential of Conjugated Selenium Redox Folic Acid as a Treatment for Triple Negative Breast Cancer" Antioxidants 9, no. 2: 138. https://doi.org/10.3390/antiox9020138
APA StyleKhandelwal, S., Boylan, M., Kirsch, G., Spallholz, J. E., & Gollahon, L. S. (2020). Investigating the Potential of Conjugated Selenium Redox Folic Acid as a Treatment for Triple Negative Breast Cancer. Antioxidants, 9(2), 138. https://doi.org/10.3390/antiox9020138






