Antioxidants in Extra Virgin Olive Oil and Table Olives: Connections between Agriculture and Processing for Health Choices
Abstract
1. Introduction
2. Analytical Problems in the Phenol Evaluation
3. Antioxidants in EVOO Depending on the Extractive Technology
4. Antioxidants in Conventional and Organic EVOO
5. TO Technology
6. EVOO Antioxidants and the Cooking Methods
7. TO Antioxidants, Thermal Treatments and Shelf-Life
8. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hoffman, R.; Gerber, M. Food processing and the Mediterranean Diet. Nutrients 2015, 7, 7925–7964. [Google Scholar] [CrossRef]
- Battino, M.; Forbes-Hernández, T.Y.; Gasparrini, M.; Afrin, S.; Cianciosi, D.; Zhang, J.; Manna, P.P.; Reboredo-Rodríguez, P.; Varela Lopez, A.; Quiles, J.L.; et al. Relevance of functional foods in the Mediterranean diet: The role of olive oil, berries and honey in the prevention of cancer and cardiovascular diseases. Crit. Rev. Food Sci. Nutr. 2019, 59, 893–920. [Google Scholar] [CrossRef]
- Pintó, X.; Fanlo-Maresma, M.; Corbella, E.; Corbella, X.; Mitjavila, M.T.; Moreno, J.J.; Casas, R.; Estruch, R.; Corella, D.; Bulló, M.; et al. A Mediterranean Diet rich in extra-virgin olive oil is associated with a reduced prevalence of nonalcoholic fatty liver disease in older individuals at high cardiovascular risk. J. Nutr. 2019, 149, 1920–1929. [Google Scholar] [CrossRef]
- Salazar-Ordóñez, M.; Schuberth, F.; Cabrera, E.R.; Arriaza, M.; Rodríguez-Entrena, M. The effects of person-related and environmental factors on consumers’ decision-making in agri-food markets: The case of olive oils. Food Res. Int. 2018, 112, 412–424. [Google Scholar] [CrossRef]
- Hyötyläinen, T.; Bondia-Pons, I.; Orešič, M. Lipidomics in nutrition and food research. Mol. Nutr. Food Res. 2013, 57, 1306–1318. [Google Scholar] [CrossRef]
- Perona, J.S.; Vögler, O.; Sánchez-Domínguez, J.M.; Montero, E.; Escribá, P.V.; Ruiz-Gutierrez, V. Consumption of virgin olive oil influences membrane lipid composition and regulates intracellular signaling in elderly adults with type 2 diabetes mellitus. J. Gerontol. A Biol Sci Med. Sci. 2007, 62, 256–263. [Google Scholar] [CrossRef]
- Farré-Ribes, M.; Lozano-Cabedo, C.; Aguilar-Criado, E. The role of knowledge in constructing the quality of olive oil in Spain. Sustainability 2019, 11, 4029. [Google Scholar] [CrossRef]
- Salazar-Ordóñez, M.; Rodriguez Entrena, M.; Cabrera, E.R.; Henseler, J. Understanding product differentiation failures in agri-food markets: The role of product knowledge and brand credence in olive oil markets. Food Qual. Prefer. 2018, 68, 146–155. [Google Scholar] [CrossRef]
- Selvaggini, R.; Servili, M.; Urbani, S.; Esposto, S.; Taticchi, A.; Montedoro, G. Evaluation of phenolic compounds in virgin olive oil by direct injection in high-performance liquid chromatography with fluorometric detection. J. Agric. Food Chem. 2006, 54, 2832–2838. [Google Scholar] [CrossRef]
- Antonini, E.; Farina, A.; Scarpa, E.S.; Frati, A.; Ninfali, P. Quantity and quality of secoiridoids and lignans in extra virgin olive oils: The effect of two- and three-way decanters on Leccino and Raggiola olive cultivars. Int. J. Food Sci. Nutr. 2016, 67, 9–15. [Google Scholar] [CrossRef]
- Ninfali, P.; Bacchiocca, M.; Biagiotti, E.; Servili, M.; Montedoro, G. Validation of the oxygen radical absorbance capacity (ORAC) parameter as a new index of quality and stability of virgin olive oil. J. Am. Oil Chem. Soc. 2002, 79, 977–982. [Google Scholar] [CrossRef]
- Psomiadou, E.; Tsimidou, M.; Boskou, D. α-Tocopherol content of greek virgin olive oil. J. Agric. Food Chem. 2000, 48, 1770–1775. [Google Scholar] [CrossRef]
- Bianchi, G. Lipids and phenols in table olives. Eur. J. Lipid Sci. Technol. 2003, 105, 229–242. [Google Scholar] [CrossRef]
- Servili, M.; Selvaggini, R.; Esposto, S.; Taticchi, A.; Montedoro, G.; Morozzi, G. Health and sensory properties of virgin olive oil hydrophilic phenols: Agronomic and technological aspects of production that affect their occurrence in the oil. J. Chrom. A 2004, 1054, 113–127. [Google Scholar] [CrossRef]
- Angelino, D.; Gennari, L.; Blasa, M.; Selvaggini, R.; Urbani, S.; Esposto, S.; Servili, M.; Ninfali, P. Chemical and cellular antioxidant activity of phytochemicals purified from olive mill waste waters. J. Agric. Food Chem. 2011, 59, 2011–2018. [Google Scholar] [CrossRef]
- Esposto, S.; Veneziani, G.; Taticchi, A.; Selvaggini, R.; Urbani, S.; Di Maio, I.; Sordini, B.; Minnocci, A.; Sebastiani, L.; Servili, M. Flash thermal conditioning of olive pastes during the olive oil mechanical extraction process: Impact on the structural modifications of pastes and oil quality. J. Agric. Food Chem. 2013, 61, 4953–4960. [Google Scholar] [CrossRef]
- Trombetta, D.; Smeriglio, A.; Marcoccia, D.; Giofrè, S.V.; Toscano, G.; Mazzotti, F.; Giovanazzi, A.; Lorenzetti, S. Analytical evaluation and antioxidant properties of some secondary metabolites in Northern Italian mono- and multi-varietal extra virgin olive oils (EVOOs) from early and late harvested olives. Int. J. Mol. Sci. 2017, 18, 797. [Google Scholar] [CrossRef]
- Inarejos-García, A.M.; Gómez-Rico, A.; Desamparados Salvador, M.; Fregapane, G. Effect of preprocessing olive storage conditions on virgin olive oil quality and composition. J. Agric. Food Chem. 2010, 58, 4858–4865. [Google Scholar] [CrossRef]
- Cicerale, S.; Lucas, L.; Keast, R. Biological activities of phenolic compounds present in virgin olive oil. Int. J. Mol. Sci. 2010, 11, 458–479. [Google Scholar] [CrossRef]
- Rubio, L.; Valls, R.M.; Macia, A.; Pedret, A.; Giralt, M.; Romero, M.P.; de la Torre, R.; Covas, M.I.; Sola, R.; Motilva, M.J. Impact of olive oil phenolic concentration on human plasmatic phenolic metabolites. Food Chem. 2012, 135, 2922–2929. [Google Scholar] [CrossRef]
- Pitozzi, V.; Jacomelli, M.; Catelan, D.; Servili, M.; Taticchi, A.; Biggeri, A.; Dolara, P.; Giovannelli, L. Long-term dietary extra-virgin olive oil rich in polyphenols reverses age-related dysfunctions in motor coordination and contextual memory in mice: Role of oxidative stress. Rejuvenation Res. 2012, 15, 601–612. [Google Scholar] [CrossRef]
- Beauchamp, G.K.; Keast, R.S.J.; Morel, D.; Lin, J.M.; Pika, J.; Han, Q.; Lee, C.H.; Smith, A.B.; Breslin, P.A.S. Phytochemistry—Ibuprofen-like activity in extra-virgin olive oil. Nature 2005, 437, 45–46. [Google Scholar] [CrossRef]
- Servili, M.; Sordini, B.; Esposto, S.; Urbani, S.; Veneziani, G.; Di Maio, I.; Selvaggini, R.; Taticchi, A. Biological activities of phenolic compounds of extra virgin olive oil. Antioxidants 2014, 3, 1–23. [Google Scholar] [CrossRef]
- Alkhatib, A.; Tsang, C.; Tuomilehto, J. Olive oil nutraceuticals in the prevention and management of diabetes: From molecules to lifestyle. Int. J. Mol. Sci. 2018, 19, 2024. [Google Scholar] [CrossRef]
- Lanza, B. Nutritional and sensory quality of table olives. In Olive Germplasm—The Olive Cultivation, Table Olive and Olive Oil Industry in Italy; Muzzalupo, I., Ed.; InTech: Rijeka, Croatia, 2012; pp. 343–372. [Google Scholar]
- Bellumori, M.; Cecchi, L.; Innocenti, M.; Clodoveo, M.L.; Corbo, F.; Mulinacci, N. The EFSA health claim on olive oil polyphenols: Acid hydrolysis validation and total hydroxytyrosol and tyrosol determination in Italian virgin olive oils. Molecules 2019, 24, 2179. [Google Scholar] [CrossRef]
- Antonini, E.; Farina, A.; Leone, A.; Mazzara, E.; Urbani, S.; Selvaggini, R.; Servili, M.; Ninfali, P. Phenolic compounds and quality parameters of family farming versus protected designation of origin (PDO) extra-virgin olive oils. J. Food Compos. Anal. 2015, 43, 75–81. [Google Scholar] [CrossRef]
- Commission Regulation (EU) N. 432/2012 establishing a list of permitted health claims made on foods, other than those referring to the reduction of disease risk and to children’s development and health. Comm. Off. J. Eur. Union 2012, 136, 1–40. [Google Scholar]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventos, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-Ciocalteu reagent. Oxidants Antioxid. Part A 1999, 299, 152–178. [Google Scholar]
- IOC. Determination of Biophenols in Olive Oils by HPLC; COI/T.20/Doc No 29/Rev.1 2017; International Olive Council: Madrid, Spain, 2017. [Google Scholar]
- Romero, C.; Brenes, M. Analysis of total contents of hydroxytyrosol and tyrosol in olive oils. J. Agric. Food Chem. 2012, 60, 9017–9022. [Google Scholar] [CrossRef]
- Brenes, M.; García, A.; García, P.; Garrido, A. Acid hydrolysis of secoiridoid aglycons during storage of virgin olive oil. J. Agric. Food Chem. 2001, 49, 5609–5614. [Google Scholar] [CrossRef]
- Purcaro, G.; Codony, R.; Pizzale, L.; Mariani, C.; Conte, L. Evaluation of total hydroxytyrosol and tyrosol in extra virgin olive oils. Eur. J. Lipid Sci. Technol. 2014, 116, 805–811. [Google Scholar] [CrossRef]
- Servili, M.; Esposto, S.; Taticchi, A.; Urbani, S.; Di Maio, I.; Veneziani, G.; Selvaggini, R. New approaches to virgin olive oil quality, technology, and by-products valorization. Eur. J. Lipid Sci. Technol. 2015, 117, 1882–1892. [Google Scholar] [CrossRef]
- Bendini, A.; Cerretani, L.; Vecchi, S.; Carrasco-Pancorbo, A.; Lercker, G. Protective effects of extra virgin olive oil phenolics on oxidative stability in the presence or absence of copper ions. J. Agric. Food Chem. 2006, 54, 4880–4887. [Google Scholar] [CrossRef]
- Franco, M.N.; Galeano-Díaz, T.; López, O.; Fernández-Bolaños, J.G.; Sánchez, J.; De Miguel, C.; Gil, M.V.; Martín-Vertedor, D. Phenolic compounds and antioxidant capacity of virgin olive oil. Food Chem. 2014, 163, 289–298. [Google Scholar] [CrossRef]
- Samaniego Sánchez, C.; Troncoso González, A.M.; García-Parrilla, M.C.; López García de la Serrana, H.; López Martínez, M.C. Different radical scavenging tests in virgin olive oil and their relation to the total phenol content. Anal. Chim. Acta 2007, 593, 1103–1107. [Google Scholar] [CrossRef]
- Ma, L.; Liu, G.-G. Simultaneous analysis of malondialdehyde, 4-hydroxy-2-hexenal, and 4-hydroxy-2-nonenal in vegetable oil by reversed-phase high-performance liquid chromatography. J. Agric. Food Chem. 2017, 65, 11320–11328. [Google Scholar] [CrossRef]
- Angerosa, F.; Servili, M.; Selvaggini, R.; Taticchi, A.; Esposto, S.; Montedoro, G.F. Volatile compounds in virgin olive oil: Occurrence and their relationship with the quality. J. Chrom. A 2004, 1054, 17–31. [Google Scholar] [CrossRef]
- Taticchi, A.; Esposto, S.; Veneziani, G.; Urbani, S.; Selvaggini, R.; Servili, M. The influence of the malaxation temperature on the activity of polyphenoloxidase and peroxidase and on the phenolic composition of virgin olive oil. Food Chem. 2013, 136, 975–998. [Google Scholar] [CrossRef]
- Chiacchierini, E.; Mele, G.; Restuccia, D.; Vinci, G. Impact evaluation of innovative and sustainable extraction technologies on olive oil quality. Trends Food Sci. Technol. 2007, 18, 299–305. [Google Scholar] [CrossRef]
- Vierhuis, E.; Servili, M.; Baldioli, M.; Schols, H.A.; Voragen, A.G.J.; Montedoro, G.F. Effect of enzyme treatment during mechanical extraction of olive oil on phenolic compounds and polysaccharides. J. Agric. Food Chem. 2001, 49, 1218–1223. [Google Scholar] [CrossRef]
- Tovar, M.J.; Motilva, M.J.; Romero, M.P. Changes in the phenolic composition of virgin olive oil from young trees (Olea europaea L. cv. Arbequina) grown under linear irrigation strategies. J. Agric. Food Chem. 2001, 49, 5502–5508. [Google Scholar] [CrossRef]
- Gucci, R.; Caruso, G.; Gennai, C.; Esposto, S.; Urbani, S.; Servili, M. Fruit growth, yield and oil quality changes induced by deficit irrigation at different stages of olive fruit development. Agric. Water Manag. 2019, 212, 88–98. [Google Scholar] [CrossRef]
- Fernández-Escobar, R.; Beltrán, G.; Sánchez-Zamora, M.A.; García-Novelo, J.; Aguilera, M.P.; Uceda, M. Olive oil quality decreases with nitrogen over-fertilization. Hort. Sci. 2006, 41, 215–219. [Google Scholar] [CrossRef]
- Tognetti, R.; Morales-Sillero, A.; D’Andria, R.; Fernández, J.E.; Lavini, A.; Sebastiani, L.; Troncoso, A. Deficit irrigation and fertigation practices in olive growing: Convergences and divergences in two case studies. Plant Biosyst. 2008, 142, 138–148. [Google Scholar] [CrossRef]
- Simoes, P.; Pinheiro-Alves, C.; Cordeiro, A.M.; Marcelo, M.E. Effect of the nitrogen and potassium fertilization on fatty acids composition and oxidative stability for ‘Carrrasquenha’ cultivar olive oil at different harvest periods—Preliminary study. Acta Hort. 2002, 586, 337–340. [Google Scholar] [CrossRef]
- Gutiérrez, F.; Arnaud, T.; Albi, M.A. Influence of ecological cultivation on virgin olive oil quality. JAOCS 1999, 76, 617–621. [Google Scholar] [CrossRef]
- Ninfali, P.; Bacchiocca, M.; Biagiotti, E.; Esposto, S.; Servili, M.; Rosati, A.; Montedoro, G. A 3-year study on quality, nutritional and organoleptic evaluation of organic and conventional extra-virgin olive oils. J. Am. Oil Chem. Soc. 2008, 85, 151–158. [Google Scholar] [CrossRef]
- Rosati, A.; Cafiero, C.; Paoletti, A.; Alfei, B.; Caporali, S.; Casciani, L.; Valentini, M. Effect of agronomical practices on carpology, fruit and oil composition, and oil sensory properties, in olive (Olea europaea L.). Food Chem. 2014, 159, 236–243. [Google Scholar] [CrossRef]
- López-Yerena, A.; Lozano-Castellón, J.; Olmo-Cunillera, A.; Tresserra-Rimbau, A.; Quifer-Rada, P.; Jiménez, B.; Pérez, M.; Vallverdú-Queralt, A. Effects of organic and conventional growing systems on the phenolic profile of extra-virgin olive oil. Molecules 2019, 24, 1986. [Google Scholar]
- Proietti, P.; Nasini, L.; Famiani, F.; Guelfi, P.; Standardi, A. Influence of light availability on fruit and oil characteristics in Olea europea L. Acta Hort. 2012, 949, 243–250. [Google Scholar] [CrossRef]
- Uccella, N. Olive biophenols: Biomolecular characterization, distribution and phytoalexin histochemical localization in the drupes. Trends Food Sci. Technol. 2001, 11, 315–327. [Google Scholar] [CrossRef]
- Lanza, B.; Di Serio, M.G.; Iannucci, E.; Russi, F.; Marfisi, P. Nutritional, textural and sensorial characterization of Italian table olives (Olea europaea L. cv. “Intosso d’Abruzzo”). Int. J. Food Sci. Technol. 2010, 45, 67–74. [Google Scholar] [CrossRef]
- Lanza, B.; Russo, A.; Di Serio, M.G.; Benincasa, C.; Russi, F.; Mucciarella, M.R.; Perri, E. The effect of lime-and-ash debittering and the fermentation with and without starter on the composition in sugars and phenols in table olives. Riv. Ital. Sostanze Grasse 2013, 3, 189–196. [Google Scholar]
- Lanza, B.; Di Serio, M.G.; Russi, F.; Di Giacinto, L. Evaluation of the nutritional value of oven-dried table olives (cv. Majatica) processed by the Ferrandina style. Riv. Ital. Sostanze Grasse 2014, 91, 117–127. [Google Scholar]
- Marsilio, V.; Campestre, C.; Lanza, B. Phenolic compounds change during California-style ripe olives processing. Food Chem. 2001, 74, 55–60. [Google Scholar] [CrossRef]
- Ramírez, E.; García-García, P.; De Castro, A.; Romero, C.; Brenes, M. Debittering of black dry-salted olives. Eur. J. Lipid Sci. Technol. 2013, 115, 1319–1324. [Google Scholar] [CrossRef]
- IOC. Trade Standards Applying to Table Olives; COI/OT/NC No. 1; International Olive Council: Madrid, Spain, 2004. [Google Scholar]
- Servili, M.; Minnocci, A.; Veneziani, G.; Taticchi, A.; Urbani, S.; Esposto, S.; Sebastiani, L.; Valmorri, S.; Corsetti, A. Compositional and tissue modifications induced by natural fermentation process in table olives. J. Agric. Food Chem. 2008, 56, 6389–6396. [Google Scholar] [CrossRef]
- Servili, M.; Urbani, S.; Esposto, S.; Taticchi, A.; Veneziani, G.; Di Maio, I.; Sordini, B.; Selvaggini, R.; Corsetti, A. Standardizzazione del processo di deamarizzazione biologica per il miglioramento della qualità delle olive da tavola di varietà italiane. In Sistemi di Controllo del Rispetto delle Norme di Autenticità e Qualità Dell’olio di Oliva e delle Olive da Tavola; Tipografia Grafica G&G: Marino, Italy, 2012; pp. 109–121. [Google Scholar]
- Boskou, G. Antioxidant capacity and phenolic profile of table olives from the Greek market. In Olive and Olive Oil in Health and Disease Prevention; Elsevier: Amsterdam, The Netherlands, 2010; pp. 925–934. [Google Scholar]
- Ben Othman, N.; Roblain, D.; Chammen, N.; Thonart, P.; Hamdi, M. Antioxidant phenolic compounds loss during the fermentation of Chetoui olives. Food Chem. 2009, 116, 662–669. [Google Scholar] [CrossRef]
- Marsilio, V.; Seghetti, L.; Iannucci, E.; Russi, F.; Lanza, B.; Felicioni, M. Use of a lactic acid bacteria starter culture during green olive (Olea europaea L cv Ascolana tenera) processing. J. Sci. Food Agric. 2005, 85, 1084–1090. [Google Scholar] [CrossRef]
- Ambra, R.; Natella, F.; Bello, C.; Lucchetti, S.; Forte, V.; Pastore, G. Phenolics fate in table olives (Olea europaea L. cv. Nocellara del Belice) debittered using the Spanish and Castelvetrano methods. Food Res. Int. 2017, 100, 369–376. [Google Scholar] [CrossRef]
- Perpetuini, G.; Caruso, G.; Urbani, S.; Schirone, M.; Esposto, S.; Ciarrocchi, A.; Prete, R.; Garcia-Gonzalez, N.; Battistelli, N.; Gucci, R.; et al. Changes in polyphenolic concentrations of table olives (cv. Itrana) produced under different irrigation regimes during spontaneous or inoculated fermentation. Front. Microbiol. 2018, 9, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Serreli, G.; Incani, A.; Atzeri, A.; Angioni, A.; Campus, M.; Cauli, E.; Zurru, R.; Deiana, M. Antioxidant effect of natural table olives phenolic extract against oxidative stress and membrane damage in enterocyte-like cells. J. Food Sci. 2017, 82, 380–385. [Google Scholar] [CrossRef] [PubMed]
- Malheiro, R.; Sousa, A.; Casal, S.; Pereira, J.A. Cultivar effect on the phenolic composition and antioxidant potential of stoned table olives. Food Chem. Toxicol. 2011, 49, 450–457. [Google Scholar] [CrossRef]
- Durante, M.; Tufariello, M.; Tommasi, L.; Lenucci, M.S.; Bleve, G.; Mita, G. Evaluation of bioactive compounds in black table olives fermented with selected microbial starters. J. Sci. Food Agric. 2018, 98, 96–103. [Google Scholar] [CrossRef]
- Regulation (EC) N. 1924/2006 of the European Parliament and of the Council of 20 December 2006 on nutrition and health claims made on foods. Off. J. Eur. Union 2006, 404, 9–25. [Google Scholar]
- Directive 90/496/EEC of 24 September 1990 on nutrition labelling for foodstuffs. Off. J. Eur. Union Commun. 1990, 276, 40–44.
- Directive 2008/100/EC of 28 October 2008 amending Council Directive 90/496/EEC on nutrition labelling for foodstuffs as regards recommended daily allowances, energy conversion factors and definitions. Off. J. Eur. Union 2008, 285, 9–12.
- Jimenez, A.; Rodriguez, R.; Fernandez-Caro, I.; Guillen, R.; Fernandez-Bolanos, J.; Heredia, A. Dietary fibre content of table olives processed under different European styles: Study of physico-chemical characteristics. J. Sci. Food Agric. 2000, 80, 1903–1908. [Google Scholar] [CrossRef]
- Perez Jimenez, J.; Serrano, J.; Tabellero, M.; Arranz, S.; Díaz-Rubio, M.E.; García-Diz, L.; Goñi, I.; Saura-Calixto, F. Effects of grape antioxidant dietary fiber in cardiovascular disease risk factors. Nutrition 2008, 24, 646–653. [Google Scholar] [CrossRef]
- Domínguez-Avila, J.A.; Wall-Medrano, A.; Velderrain-Rodríguez, G.R.; Chen, C.Y.O.; Salazar-López, N.J.; Robles-Sánchez, M.; González-Aguilar, G.A. Gastrointestinal interactions, absorption, splanchnic metabolism and pharmacokinetics of orally ingested phenolic compounds. Food Funct. 2017, 8, 15–38. [Google Scholar] [CrossRef]
- D’Antuono, I.; Antonella Garbetta, A.; Ciasca, B.; Linsalata, V.; Minervini, F.; Lattanzio, V.M.T.; Logrieco, A.F.; Cardinali, A. Biophenols from table olive cv Bella di Cerignola: Chemical characterization, bioaccessibility, and intestinal absorption. J. Agric. Food Chem. 2016, 64, 5671–5678. [Google Scholar] [CrossRef]
- Gouvinhas, I.; Machado, N.; Sobreira, C.; Domínguez-Perles, R.; Gomes, S.; Rosa, E.; Barros, A.I.R.N.A. Critical review on the significance of olive phytochemicals in plant physiology and human health. Molecules 2017, 22, 986. [Google Scholar] [CrossRef]
- Daskalaki, D.; Kefi, G.; Kotsiou, K.; Tasioula-Margari, M. Evaluation of phenolic compounds degradation in virgin olive oil during storage and heating. J. Food Nutr. Res. 2009, 48, 31–41. [Google Scholar]
- Carrasco-Pancorbo, A.; Cerretani, L.; Bendini, A.; Segura-Carretero, A.; Del Carlo, M.; Gallina-Toschi, T.; Lercker, G.; Compagnone, D.; Fernández-Gutiérrez, A. Evaluation of the antioxidant capacity of individual phenolic compounds in virgin olive oil. J. Agric. Food Chem. 2005, 53, 8918–8925. [Google Scholar] [CrossRef]
- Silva, L.; Garcia, B.; Paiva-Martins, F. Oxidative stability of olive oil and its polyphenolic compounds after boiling vegetable process. LWT-Food Sci. Technol. 2010, 43, 1336–1344. [Google Scholar] [CrossRef]
- Gómez-Alonso, S.; Fregapane, G.; Salvador, M.D.; Gordon, M.H. Changes in phenolic composition and antioxidant activity of virgin olive oil during frying. J. Agric. Food Chem. 2003, 51, 667–672. [Google Scholar] [CrossRef]
- Ricci, A.; Antonini, E.; Ninfali, P. Homenade tomato souce in the Mediterranean Diet: A rich source of antioxidants. Ital. J. Food Sci. 2018, 30. [Google Scholar] [CrossRef]
- Codex Alimentarius Commission (FAO/WHO). Codex Standard for Table Olives (Codex Stan 66-1981); FAO/WHO: Rome, Italy, 2013. [Google Scholar]
- Lanza, B.; Di Serio, M.G.; Giansante, L.; Di Loreto, G.; Russi, F.; Di Giacinto, L. Effect of pasteurization and storage on quality characteristics of table olives preserved in olive oil. Int. J. Food Sci. Technol. 2013, 48, 2630–2637. [Google Scholar] [CrossRef]
- Charoenprasert, S.; Mitchell, A. Factors influencing phenolic compounds in table olives (Olea europaea). J. Agric. Food Chem. 2012, 60, 7081–7095. [Google Scholar] [CrossRef]
- Saponari, M.; Boscia, D.; Altamura, G.; D’Attoma, G.; Cavalieri, V.; Loconsole, G.; Zicca, G.S.; Dongiovanni, C.; Palmisano, F.; Susca, L.; et al. Pilot project on Xylella fastidiosa to reduce risk assessment uncertainties. EFSA 2016, EN-1013, 60. [Google Scholar] [CrossRef]
- Giampetruzzi, A.; Morelli, M.; Saponari, M.; Loconsole, G.; Chiumenti, M.; Boscia, D.; Savino, V.N.; Martelli, G.P.; Saldarelli, P. Transcriptome profiling of two olive cultivars in response to infection by the CoDiRO strain of Xylella fastidiosa subsp. pauca. BMC Genom. 2016, 17, 475. [Google Scholar] [CrossRef]
- Mazzanti, L.; Battino, M.; Nanetti, L.; Raffaelli, F.; Alidori, A.; Sforza, G.; Carle, F.; Quagliarini, V.; Cester, N.; Vignini, A. Effect of 1-year dietary supplementation with vitaminized olive oil on markers of bone turnover and oxidative stress in healthy post-menopausal women. Endocrine 2015, 50, 326–334. [Google Scholar] [CrossRef]
- Romani, A.; Pinelli, P.; Ieri, F.; Bernini, R. Sustainability, innovation, and green chemistry in the production and valorization of phenolic extracts from Olea europaea L. Sustainability 2016, 8, 1002. [Google Scholar] [CrossRef]
- Romani, A.; Ieri, F.; Urciuoli, S.; Noce, A.; Marrone, G.; Nediani, C.; Bernini, R. Health effects of phenolic compounds found in extra-virgin olive oil, by-products, and leaf of Olea europaea L. Nutrients 2019, 11, 1776. [Google Scholar] [CrossRef]
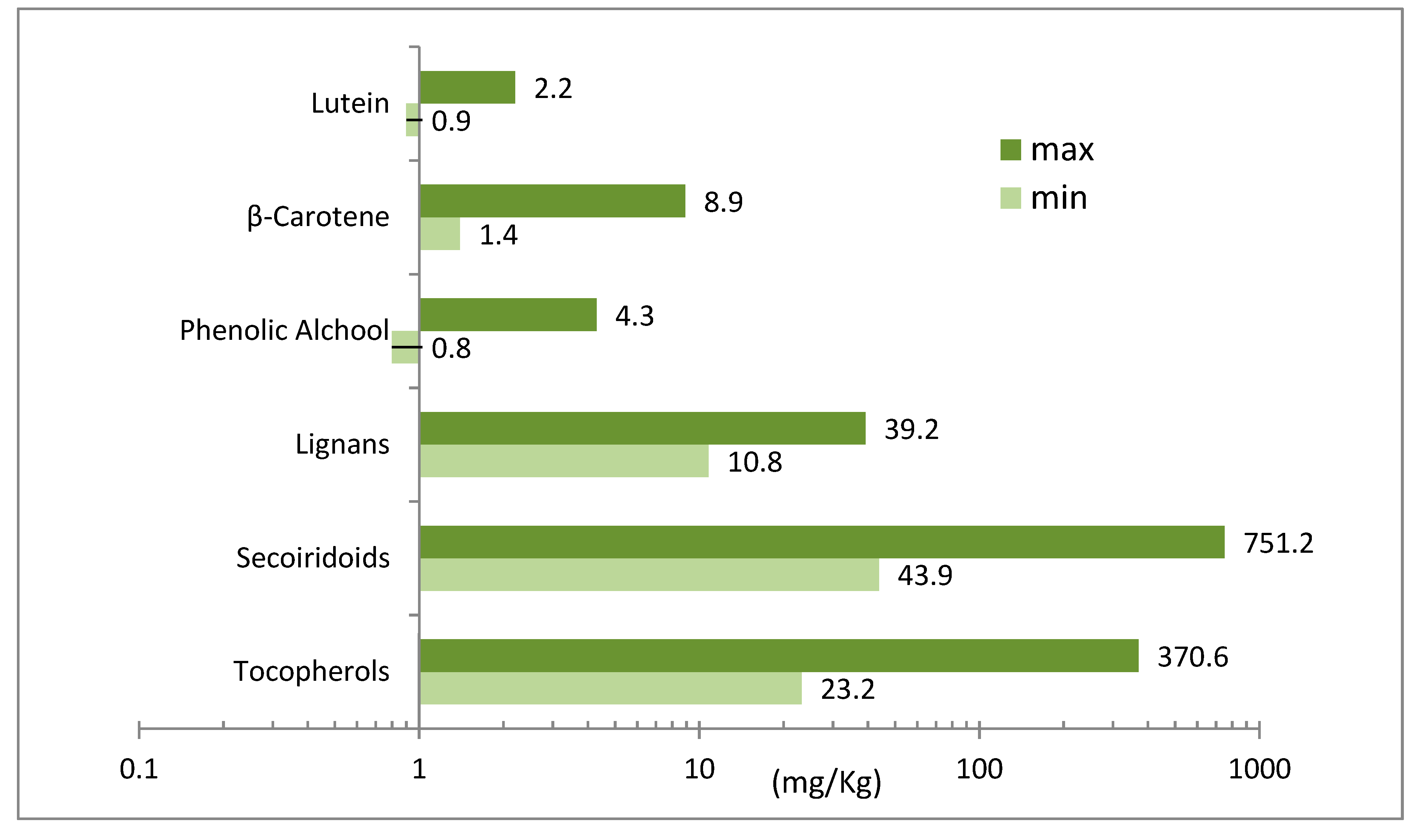
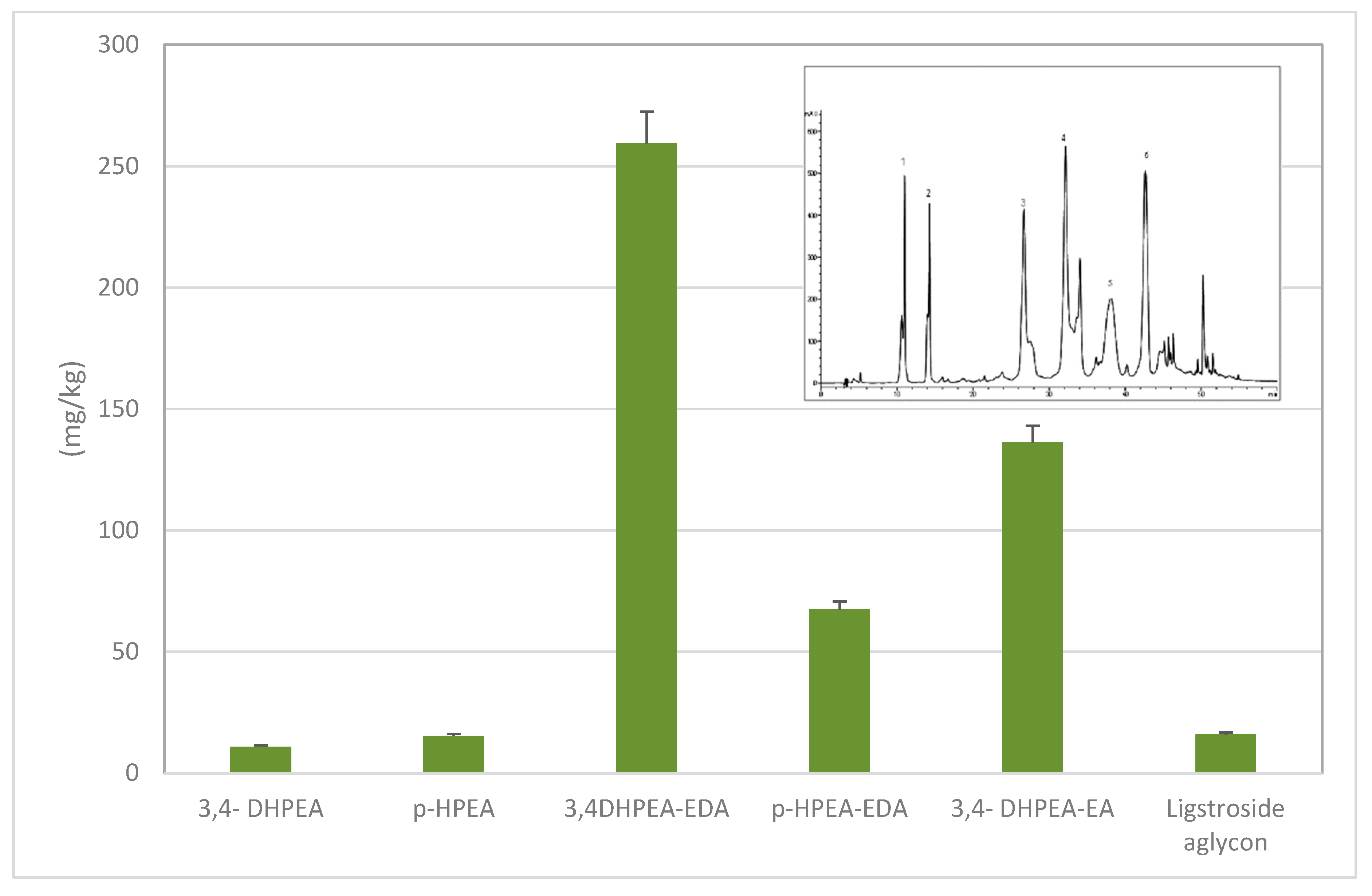
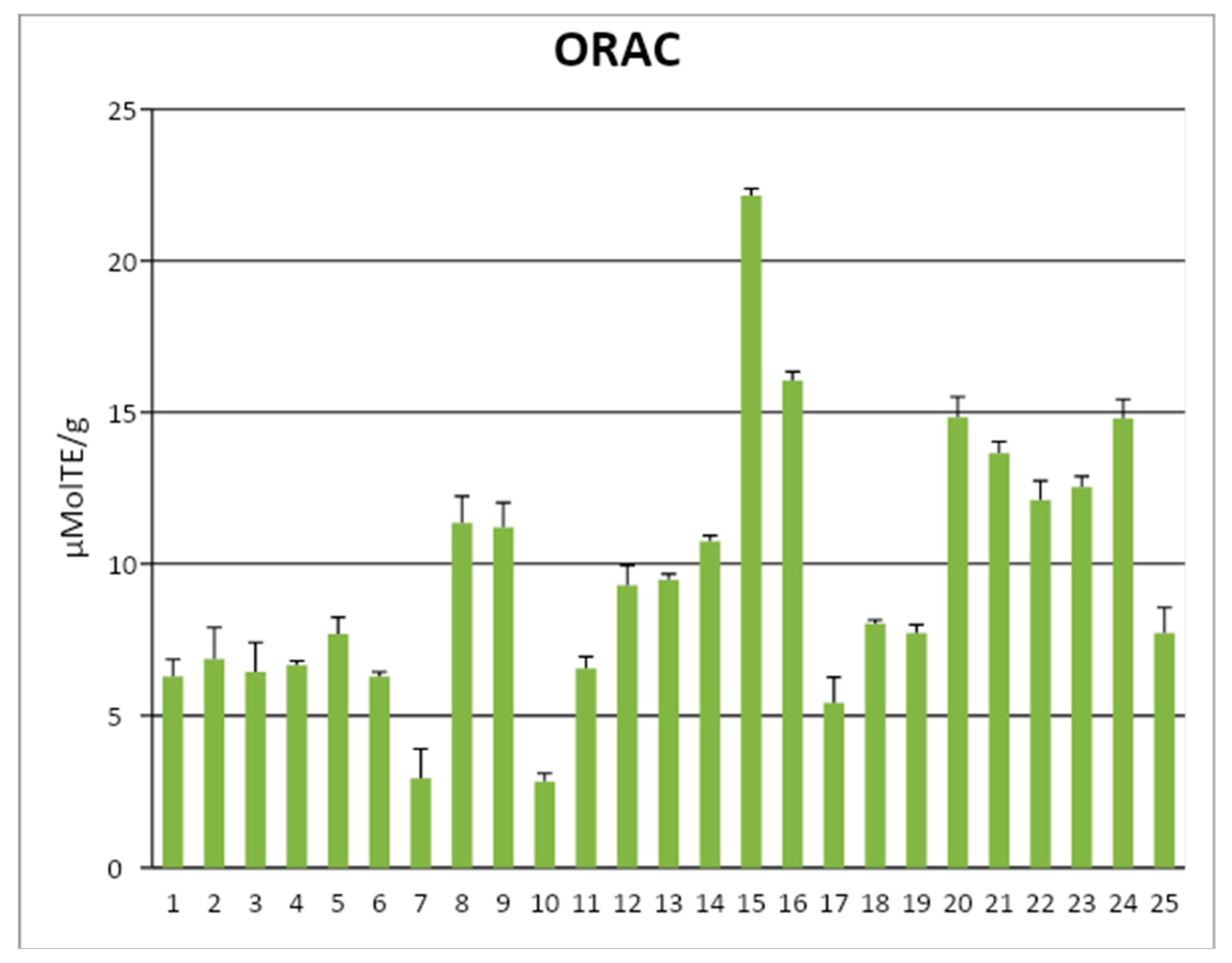


| Phenols | Frantoio | Leccino | ||||
|---|---|---|---|---|---|---|
| Raw | Spontaneous | Inoculated | Raw | Spontaneous | Inoculated | |
| Hydroxytyrosol | 293 | 357 | 490 | 567 | 693 | 1169 |
| Tyrosol | 123 | 121 | 112 | 165 | 163 | 158 |
| Oleuropein | 505 | 460 | nd | 1451 | 1377 | 230 |
| Demethyloleuropein | 1115 | 991 | 215 | 904 | 803 | 156 |
| Verbascoside | 886 | 787 | 663 | 2297 | 2040 | 773 |
| 3,4-DHPEA-EDA | 2644 | 2576 | 1536 | 5840 | 5691 | 2936 |
| p-HPEA-EDA | 23 | 21 | 8 | 106 | 103 | nd |
| Antioxidant Molecules | Max Value | Cultivar | Technological Process | Reference |
|---|---|---|---|---|
| Phenolic alcohols | ||||
| Hydrotyrosol | 2119 ** | Coratina | Greek inoculated | [60] |
| Tyrosol | 245 ** | Coratina | Greek | [60] |
| Phenolic acids | ||||
| Caffeic acid | 318 * | Peranzana | Greek | [61] |
| p-Cumaric acid | 10 ** | Crete | Greek | [62] |
| Ferulic acid | 3 * | Chetoui | Greek inoculated | [63] |
| p-Hydroxybenzoic acid | 10 ** | Crete | Greek | [62] |
| p-Hydroxyphenylacetic acid | 60 ** | Tsakistes | Greek | [62] |
| 3,4-Dihydroxyphenylacetic acid | 100 ** | Tsakistes | Greek | [62] |
| Protocatechuic acid | 70 ** | Crete | Greek | [62] |
| Syringic acid | 4 * | Chetoui | Greek inoculated | [63] |
| Vanillic acid | 26 ** | Ascolana tenera | Greek | [64] |
| Secoiridoids and derivatives | ||||
| Oleuropein | 3403 * | Nocellara del Belice | Spanish | [65] |
| Demethyloleuropein | 2013 ** | Coratina | Greek | [60] |
| Ligstroside | 418 * | Nocellara del Belice | Spanish | [65] |
| Oleoside 11-methylester | 279 * | Nocellara del Belice | Spanish | [65] |
| 3,4-DHPEA-EDA | 8987 ** | Coratina | Greek | [60] |
| p-HPEA-EDA | 103 ** | Leccino | Greek | [60] |
| Hydroxycinnamic acid derivatives | ||||
| Verbascoside | 2093 ** | Coratina | Greek | [60] |
| Isoverbascoside | 4164 * | Peranzana | Greek | [61] |
| Lignans | ||||
| 1-Acetoxypinoresinol | 39 * | Nocellara del Belice | Spanish | [65] |
| Pinoresinol | 2 ** | Itrana | Greek inoculated | [66] |
| Flavonoids | ||||
| Luteolin | 801 ** | Mele | Lime and ash | [55] |
| Luteolin-7-O-glucoside (cynaroside) | 30 * | Nocellara del Belice | Castelvetrano | [65] |
| Apigenin | 23 ** | Tonda di Cagliari | Greek | [67] |
| Apigenin-7-O-glucoside | 3 ** | Verdeal Trasmontana | Alcaparra stoned | [68] |
| Quercetin-3-rutinoside (rutin) | 46 ** | Cellina di Nardò | Greek inoculated | [69] |
| Cyanidin-3-rutinoside | 35 ** | Cellina di Nardò | Greek inoculated | [69] |
| Tocopherols | ||||
| α-Tocopherol | 59 ** | Conservolea | Greek inoculated | [69] |
| Triterpenic acids | ||||
| Maslinic acid | 2133 ** | Conservolea | Greek inoculated | [69] |
| Oleanolic acid | 1333 ** | Kalamata | Greek inoculated | [69] |
| Phytosterols | ||||
| Total sterols | 1863 *** | Majatica | Oven-dried | [56] |
| Vitamin | Quantity |
|---|---|
| Vitamin C (mg/kg) | <1 |
| Provitamin A carotenoids | |
| β-criptoxantin (mg/kg) | <0.1 |
| 13-cis- β-carotene (mg/kg) | 0.1 |
| All-trans- α-carotene (mg/kg) | <0.1 |
| All-trans- β-carotene (mg/kg) | 0.7 |
| 9-cis- β-carotene (mg/kg) | <0.1 |
| Retinol equivalents (μg/100g) | 12 |
| Vitamin E (mg/100g) | 6.44 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lanza, B.; Ninfali, P. Antioxidants in Extra Virgin Olive Oil and Table Olives: Connections between Agriculture and Processing for Health Choices. Antioxidants 2020, 9, 41. https://doi.org/10.3390/antiox9010041
Lanza B, Ninfali P. Antioxidants in Extra Virgin Olive Oil and Table Olives: Connections between Agriculture and Processing for Health Choices. Antioxidants. 2020; 9(1):41. https://doi.org/10.3390/antiox9010041
Chicago/Turabian StyleLanza, Barbara, and Paolino Ninfali. 2020. "Antioxidants in Extra Virgin Olive Oil and Table Olives: Connections between Agriculture and Processing for Health Choices" Antioxidants 9, no. 1: 41. https://doi.org/10.3390/antiox9010041
APA StyleLanza, B., & Ninfali, P. (2020). Antioxidants in Extra Virgin Olive Oil and Table Olives: Connections between Agriculture and Processing for Health Choices. Antioxidants, 9(1), 41. https://doi.org/10.3390/antiox9010041






