Nanoantioxidants: Recent Trends in Antioxidant Delivery Applications
Abstract
1. Introduction
2. Antioxidant Functionalized Nanoparticles
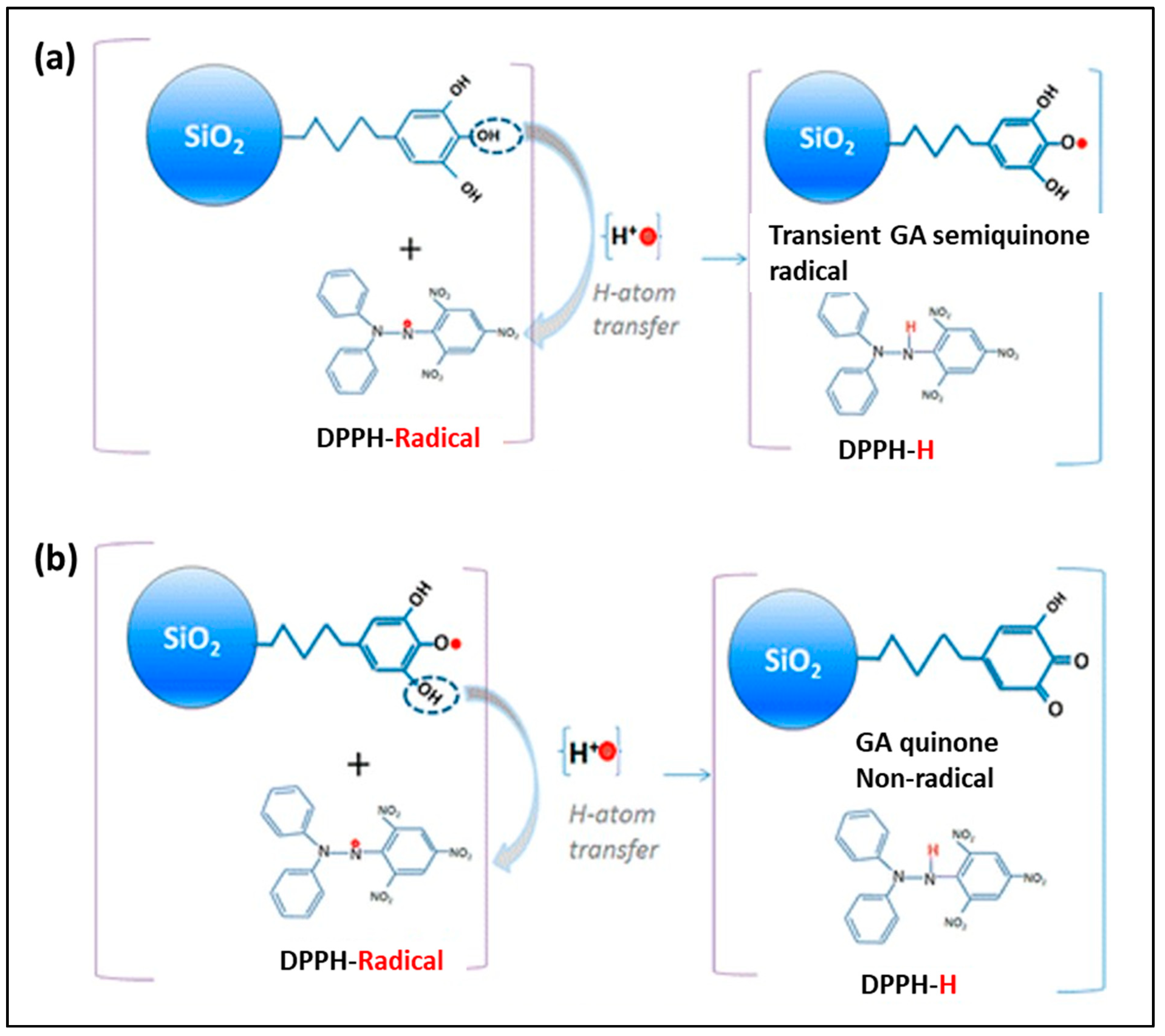
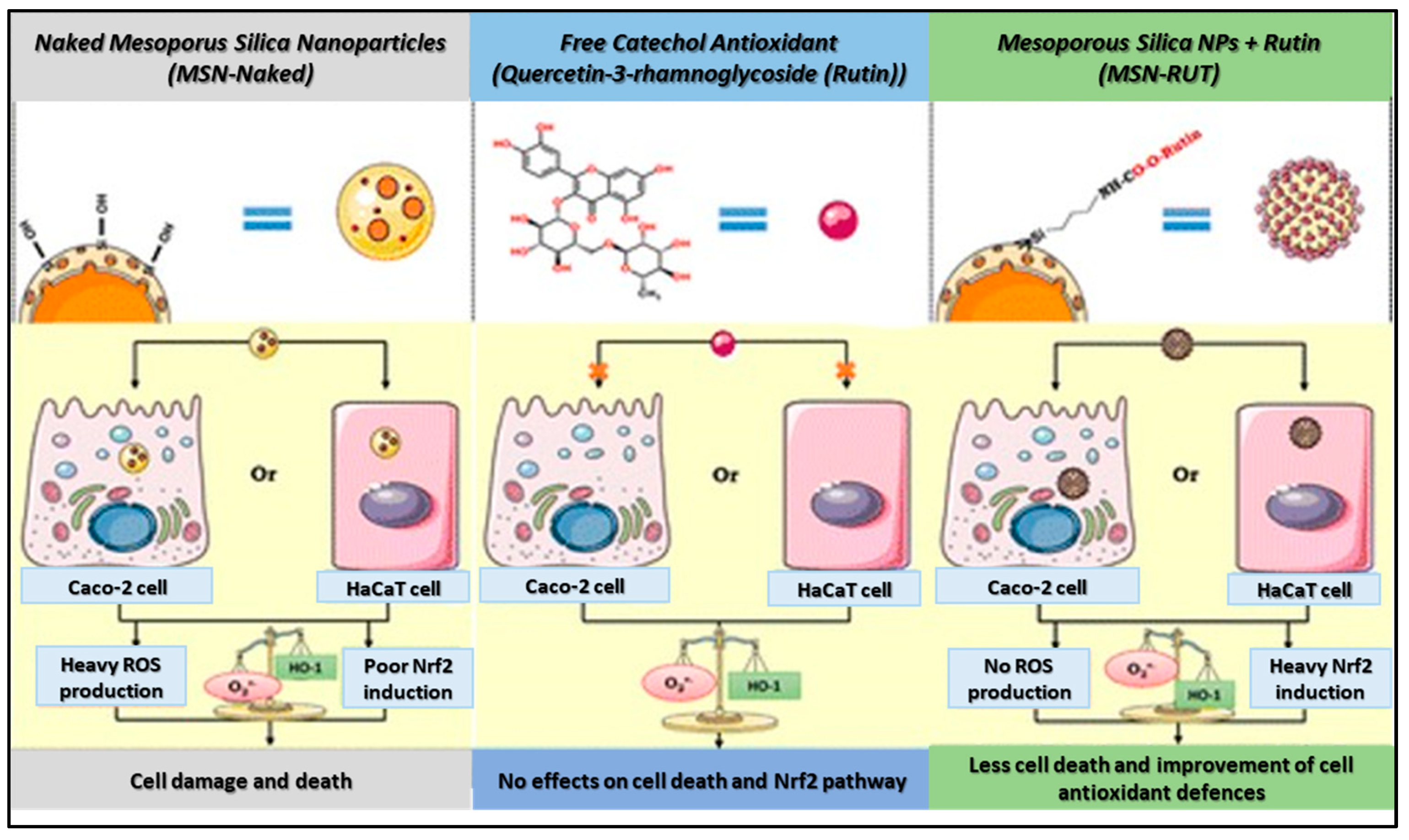
2.1. SiO2 Nanoantioxidant
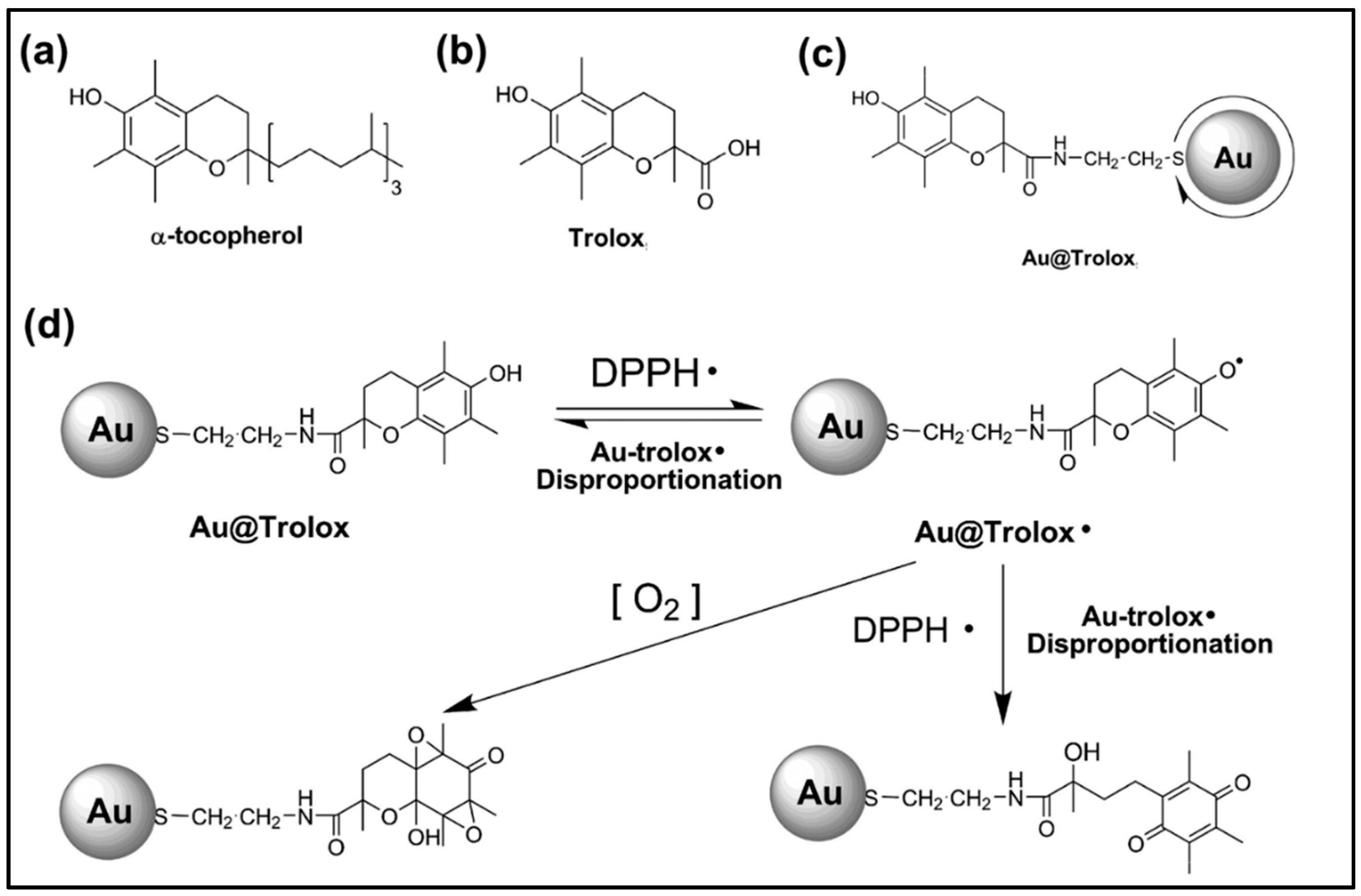
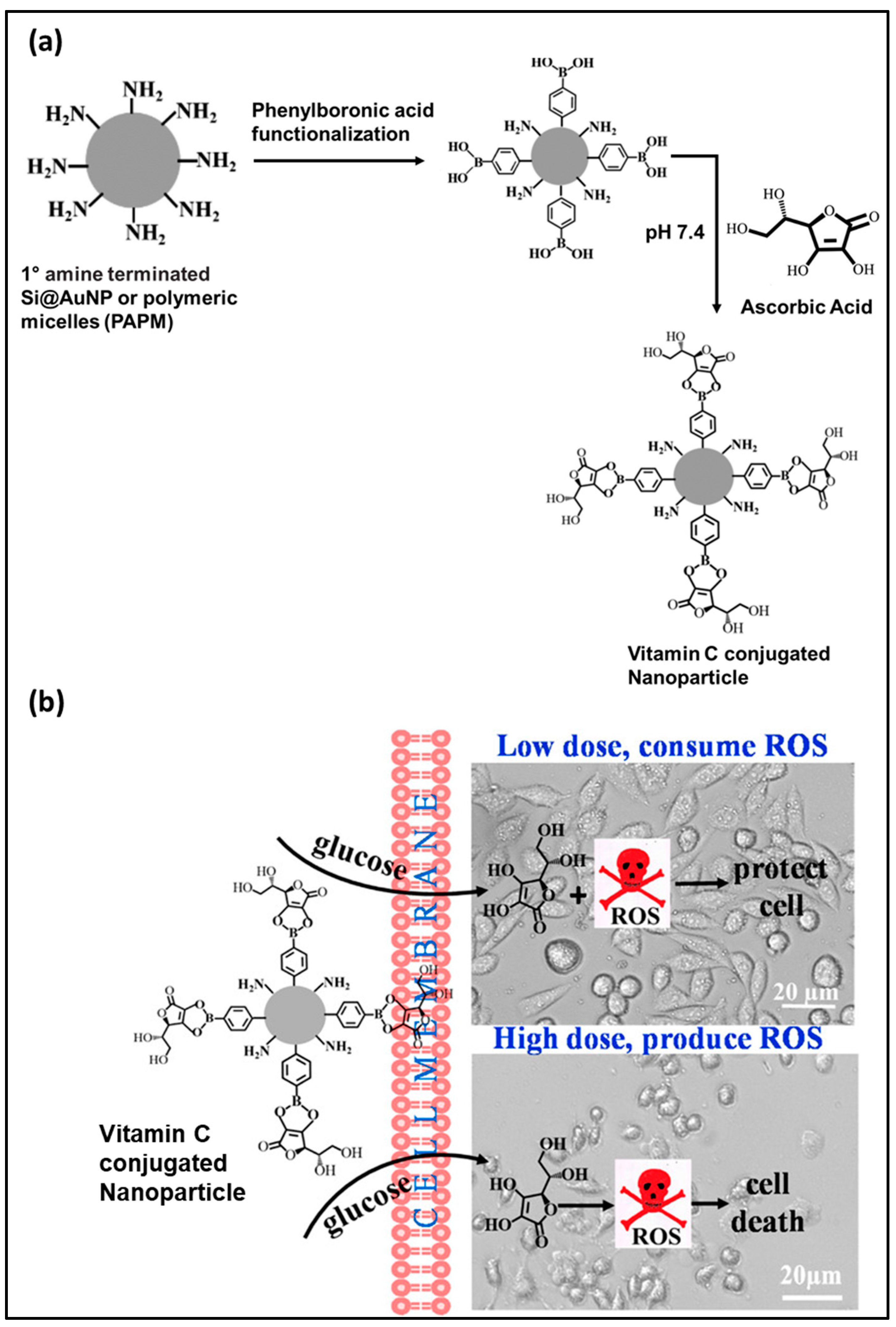
2.2. AuNPs Nanoantioxidant
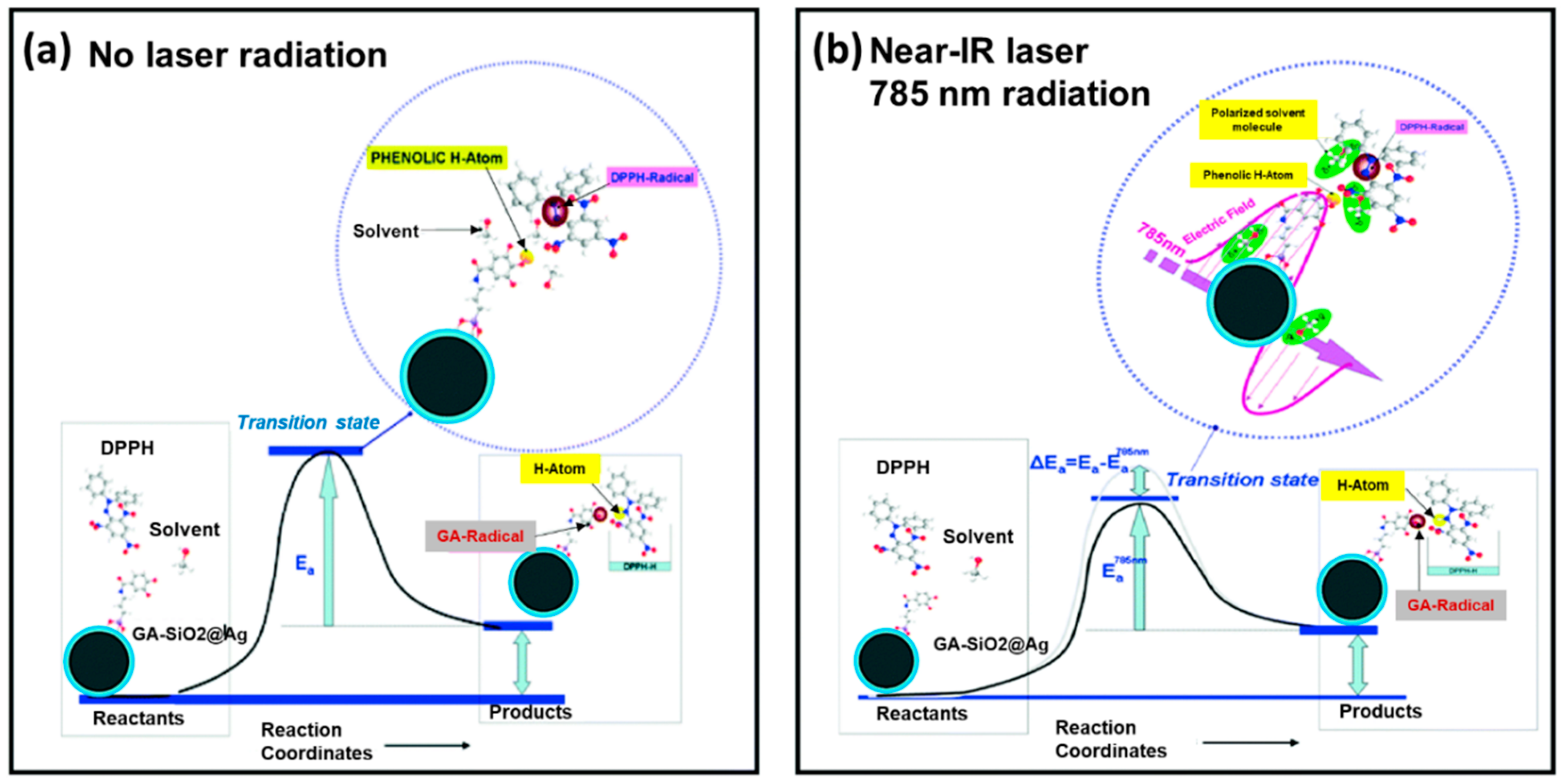
2.3. Silver Nanoparticles (AgNPs) Nanoantioxidant
2.4. Iron-Oxide Magnetic Nanoparticles (Fe2O3NPs) Nanoantioxidant
2.5. Cerium Oxide Nanoparticles Nanoantioxidant
2.6. Dual Nanoparticles Nanoantioxidant
2.7. Polymeric Nanoantioxidant
3. Nanogel Entrapped Antioxidant
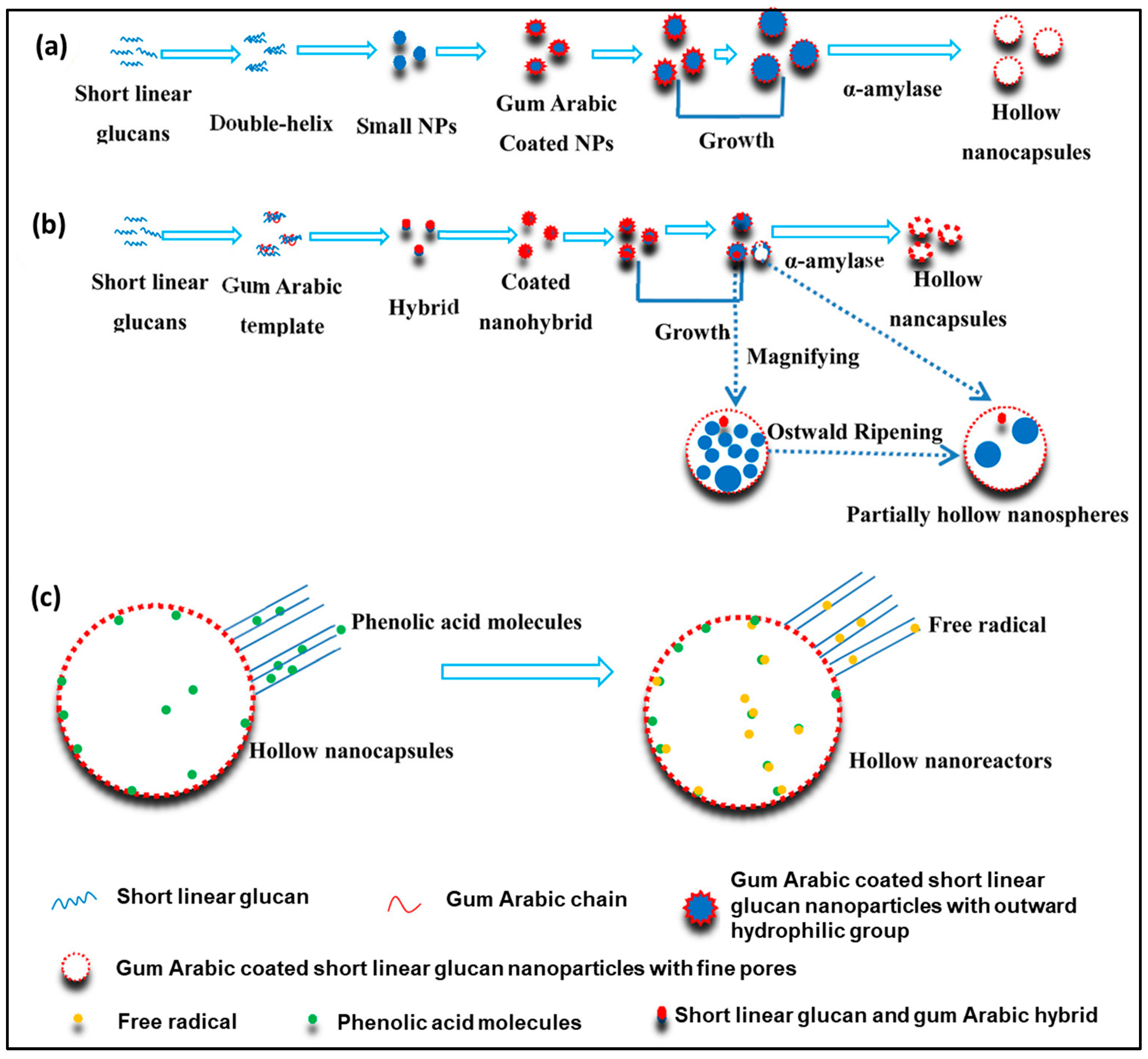
4. Hollow Nanosphere Tagged Nanoantioxidant
5. Nanoparticles Mediated Antioxidant Encapsulation and Delivery
5.1. Polymeric Encapsulation and Delivery
5.1.1. Poly-d,l-lactide-Based Nanoparticles
5.1.2. PLGA-Based Nanoparticles
5.1.3. Poly(ε-caprolactone)
5.1.4. Poly(β-amino esters)
5.1.5. Polyanhydride Nanoparticles
5.1.6. Prodrug Approaches
5.2. Polysaccharide-Based Nanoparticles
5.2.1. Chitosan Originated Nanocarrier
5.2.2. Starch Nanoparticles
5.2.3. Alginate
5.3. Protein-Based Nanoparticles
5.3.1. Albumin
5.3.2. Gelatin
5.3.3. Other Protein-Based Nanoparticles
5.4. Calcium Phosphate Nanoparticle
6. Conclusions and Future Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Lobo, V.; Patil, A.; Phatak, A.; Chandra, N. Free radicals, antioxidants and functional foods: Impact on human health. Pharmacogn. Rev. 2010, 4, 118. [Google Scholar] [CrossRef] [PubMed]
- Gupta, P.; Authimoolam, S.P.; Hilt, J.Z.; Dziubla, T.D. Quercetin conjugated poly (β-amino esters) nanogels for the treatment of cellular oxidative stress. Acta Biomater. 2015, 27, 194–204. [Google Scholar] [CrossRef] [PubMed]
- Sharpe, E.; Andreescu, D.; Andreescu, S. Artificial nanoparticle antioxidants. In Oxidative Stress: Diagnostics, Prevention, and Therapy; ACS Publications: Washington, DC, USA, 2011; pp. 235–253. [Google Scholar]
- Halliwell, B. Antioxidants in human health and disease. Annu. Rev. Nutr. 1996, 16, 33–50. [Google Scholar] [CrossRef] [PubMed]
- Wattamwar, P.P.; Mo, Y.; Wan, R.; Palli, R.; Zhang, Q.; Dziubla, T.D. Antioxidant activity of degradable polymer poly (trolox ester) to suppress oxidative stress injury in the cells. Adv. Funct. Mater. 2010, 20, 147–154. [Google Scholar] [CrossRef]
- Koltover, V. Antioxidant biomedicine: From free radical chemistry to systems biology mechanisms. Russ. Chem. Bull. 2010, 59, 37–42. [Google Scholar] [CrossRef]
- Simic, M.G. Mechanisms of inhibition of free-radical processes in mutagenesis and carcinogenesis. Mut. Res./Fundam. Mol. Mech. Mutagen. 1988, 202, 377–386. [Google Scholar] [CrossRef]
- Pellegrini, N.; Serafini, M.; Colombi, B.; Del Rio, D.; Salvatore, S.; Bianchi, M.; Brighenti, F. Total antioxidant capacity of plant foods, beverages and oils consumed in Italy assessed by three different in vitro assays. J. Nutr. 2003, 133, 2812–2819. [Google Scholar] [CrossRef]
- Khan, M.I.; Giridhar, P. Dietary antioxidants: The insurer of health. Everyman’s Sci. 2011, 46, 214–218. [Google Scholar]
- Medhe, S.; Bansal, P.; Srivastava, M.M. Enhanced antioxidant activity of gold nanoparticle embedded 3, 6-dihydroxyflavone: A combinational study. Appl. Nanosci. 2014, 4, 153–161. [Google Scholar] [CrossRef]
- Fleuriet, A.; Macheix, J.-J. Phenolic acids in fruits and vegetables. Flavonoids Health Dis. 2003, 1, 17–20. [Google Scholar]
- Lim, Y.; Lim, T.; Tee, J. Antioxidant properties of several tropical fruits: A comparative study. Food Chem. 2007, 103, 1003–1008. [Google Scholar] [CrossRef]
- Anagnostopoulou, M.A.; Kefalas, P.; Papageorgiou, V.P.; Assimopoulou, A.N.; Boskou, D. Radical scavenging activity of various extracts and fractions of sweet orange peel (Citrus sinensis). Food Chem. 2006, 94, 19–25. [Google Scholar] [CrossRef]
- Li, L.; Ng, T.; Gao, W.; Li, W.; Fu, M.; Niu, S.; Zhao, L.; Chen, R.; Liu, F. Antioxidant activity of gallic acid from rose flowers in senescence accelerated mice. Life Sci. 2005, 77, 230–240. [Google Scholar] [CrossRef] [PubMed]
- Tułodziecka, A.; Szydłowska-Czerniak, A. Determination of Total antioxidant capacity of rapeseed and its by-products by a novel cerium oxide nanoparticle-based spectrophotometric method. Food Anal. Methods 2016, 9, 3053–3062. [Google Scholar] [CrossRef]
- Verma, A.K. Anti-oxidant activities of biopolymeric nanoparticles: Boon or bane! J. Pharm. Res. 2014, 8, 871–876. [Google Scholar]
- Pratt, D.E.; Hudson, B.J. Natural antioxidants not exploited commercially. In Food Antioxidants; Springer: Berlin/Heidelberg, Germany, 1990; pp. 171–191. [Google Scholar]
- Yehye, W.A.; Abdul Rahman, N.; A Alhadi, A.; Khaledi, H.; Ng, S.W.; Ariffin, A. Butylated hydroxytoluene analogs: Synthesis and evaluation of their multipotent antioxidant activities. Molecules 2012, 17, 7645–7665. [Google Scholar] [CrossRef]
- Yusof, F.; Ismail, N.A.S. Antioxidants effects of platinum nanoparticles: A potential alternative treatment to lung diseases. J. Appl. Pharm. Sci. 2015, 5, 140–145. [Google Scholar] [CrossRef]
- Watanabe, A.; Kajita, M.; Kim, J.; Kanayama, A.; Takahashi, K.; Mashino, T.; Miyamoto, Y. In vitro free radical scavenging activity of platinum nanoparticles. Nanotechnology 2009, 20, 455105. [Google Scholar] [CrossRef]
- Liu, Y.; Ai, K.; Ji, X.; Askhatova, D.; Du, R.; Lu, L.; Shi, J. Comprehensive insights into the multi-antioxidative mechanisms of melanin nanoparticles and their application to protect brain from injury in ischemic stroke. J. Am. Chem. Soc. 2017, 139, 856–862. [Google Scholar] [CrossRef]
- Patlolla, A.K.; Hackett, D.; Tchounwou, P.B. Genotoxicity study of silver nanoparticles in bone marrow cells of Sprague–Dawley rats. Food Chem. Toxicol. 2015, 85, 52–60. [Google Scholar] [CrossRef]
- Jadhav, M.S.; Kulkarni, S.; Raikar, P.; Barretto, D.A.; Vootla, S.K.; Raikar, U. Green biosynthesis of CuO & Ag–CuO nanoparticles from Malus domestica leaf extract and evaluation of antibacterial, antioxidant and DNA cleavage activities. New J. Chem. 2018, 42, 204–213. [Google Scholar]
- Saikia, J.P.; Paul, S.; Konwar, B.K.; Samdarshi, S.K. Nickel oxide nanoparticles: A novel antioxidant. Coll. Surf. B Biointerfaces 2010, 78, 146–148. [Google Scholar] [CrossRef] [PubMed]
- Kajita, M.; Hikosaka, K.; Iitsuka, M.; Kanayama, A.; Toshima, N.; Miyamoto, Y. Platinum nanoparticle is a useful scavenger of superoxide anion and hydrogen peroxide. Free Radic. Res. 2007, 41, 615–626. [Google Scholar] [CrossRef] [PubMed]
- Vilas, V.; Philip, D.; Mathew, J. Biosynthesis of Au and Au/Ag alloy nanoparticles using Coleus aromaticus essential oil and evaluation of their catalytic, antibacterial and antiradical activities. J. Mol. Liq. 2016, 221, 179–189. [Google Scholar] [CrossRef]
- Shah, S.T.; A Yehya, W.; Saad, O.; Simarani, K.; Chowdhury, Z.; A Alhadi, A.; Al-Ani, L.A. Surface functionalization of iron oxide nanoparticles with gallic acid as potential antioxidant and antimicrobial agents. Nanomaterials 2017, 7, 306. [Google Scholar] [CrossRef]
- Elsaesser, A.; Howard, C.V. Toxicology of nanoparticles. Adv. Drug Deliv. Rev. 2012, 64, 129–137. [Google Scholar] [CrossRef]
- Bhattacherjee, A.; Dhara, K.; Chakraborti, A.S. Argpyrimidine-tagged rutin-encapsulated biocompatible (ethylene glycol dimers) nanoparticles: Synthesis, characterization and evaluation for targeted drug delivery. Int. J. Pharm. 2016, 509, 507–517. [Google Scholar] [CrossRef]
- Deligiannakis, Y.; Sotiriou, G.A.; Pratsinis, S.E. Antioxidant and antiradical SiO2 nanoparticles covalently functionalized with gallic acid. ACS Appl. Mater. Interfaces 2012, 4, 6609–6617. [Google Scholar] [CrossRef]
- Viglianisi, C.; Di Pilla, V.; Menichetti, S.; Rotello, V.M.; Candiani, G.; Malloggi, C.; Amorati, R. Linking an α-tocopherol derivative to Cobalt (0) nanomagnets: Magnetically Responsive antioxidants with superior radical trapping activity and reduced cytotoxicity. Chem. A Eur. J. 2014, 20, 6857–6860. [Google Scholar] [CrossRef]
- Chakraborty, A.; Jana, N.R. Vitamin C-conjugated nanoparticle protects cells from oxidative stress at low doses but induces oxidative stress and cell death at high doses. ACS Appl. Mater. Interfaces 2017, 9, 41807–41817. [Google Scholar] [CrossRef]
- Keles, E.; Song, Y.; Du, D.; Dong, W.-J.; Lin, Y. Recent progress in nanomaterials for gene delivery applications. Biomater. Sci. 2016, 4, 1291–1309. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Liu, R.; Ji, W.; Li, Y.; Liu, L.; Zhang, X. Delivery systems for theranostics in neurodegenerative diseases. Nano Res. 2018, 11, 5535–5555. [Google Scholar] [CrossRef]
- Sandhir, R.; Yadav, A.; Sunkaria, A.; Singhal, N. Nano-antioxidants: An emerging strategy for intervention against neurodegenerative conditions. Neurochem. Int. 2015, 89, 209–226. [Google Scholar] [CrossRef] [PubMed]
- Suarez, S.; Almutairi, A.; Christman, K. Micro-and nanoparticles for treating cardiovascular disease. Biomater. Sci. 2015, 3, 564–580. [Google Scholar] [CrossRef] [PubMed]
- Yoshitomi, T.; Nagasaki, Y. Development of silica-containing redox nanoparticles for medical applications. Biomater. Sci. 2015, 3, 810–815. [Google Scholar] [CrossRef]
- Ramesh, S.; Grijalva, M.; Debut, A.; Beatriz, G.; Albericio, F.; Cumbal, L.H. Peptides conjugated to silver nanoparticles in biomedicine—A “value-added” phenomenon. Biomater. Sci. 2016, 4, 1713–1725. [Google Scholar] [CrossRef]
- Eftekhari, A.; Dizaj, S.M.; Chodari, L.; Sunar, S.; Hasanzadeh, A.; Ahmadian, E.; Hasanzadeh, M. The promising future of nano-antioxidant therapy against environmental pollutants induced-toxicities. Biomed. Pharmacother. 2018, 103, 1018–1027. [Google Scholar] [CrossRef]
- Liberman, A.; Mendez, N.; Trogler, W.C.; Kummel, A.C. Synthesis and surface functionalization of silica nanoparticles for nanomedicine. Surf. Sci. Rep. 2014, 69, 132–158. [Google Scholar] [CrossRef]
- Arriagada, F.; Correa, O.; Günther, G.; Nonell, S.; Mura, F.; Olea-Azar, C.; Morales, J. Morin flavonoid adsorbed on mesoporous silica, a novel antioxidant nanomaterial. PLoS ONE 2016, 11, e0164507. [Google Scholar] [CrossRef]
- Sahiner, N.; Sagbas, S.; Aktas, N. Preparation and characterization of monodisperse, mesoporous natural poly (tannic acid)–silica nanoparticle composites with antioxidant properties. Microporous Mesoporous Mater. 2016, 226, 316–324. [Google Scholar] [CrossRef]
- Ebabe Elle, R.; Rahmani, S.; Lauret, C.L.; Morena, M.; Bidel, L.P.R.G.; Boulahtouf, A.; Balaguer, P.; Cristol, J.-P.; Durand, J.-O.; Charnay, C. Functionalized mesoporous silica nanoparticle with antioxidants as a new carrier that generates lower oxidative stress impact on cells. Mol. Pharm. 2016, 13, 2647–2660. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.S.; Vishakante, G.D.; Siddaramaiah, H. Gold nanoparticles: A paradigm shift in biomedical applications. Adv. Coll. Interface Sci. 2013, 199, 44–58. [Google Scholar] [CrossRef] [PubMed]
- Esumi, K.; Takei, N.; Yoshimura, T. Antioxidant-potentiality of gold–chitosan nanocomposites. Coll. Surf. B Biointerfaces 2003, 32, 117–123. [Google Scholar] [CrossRef]
- Du, L.; Suo, S.; Wang, G.; Jia, H.; Liu, K.J.; Zhao, B.; Liu, Y. Mechanism and cellular kinetic studies of the enhancement of antioxidant activity by using surface-functionalized gold nanoparticles. Chem. A Eur. J. 2013, 19, 1281–1287. [Google Scholar] [CrossRef]
- Bumbudsanpharoke, N.; Choi, J.; Park, I.; Ko, S. Facile biosynthesis and antioxidant property of nanogold-cellulose fiber composite. J. Nanomater. 2015, 16, 195. [Google Scholar] [CrossRef]
- Nie, Z.; Liu, K.J.; Zhong, C.-J.; Wang, L.-F.; Yang, Y.; Tian, Q.; Liu, Y. Enhanced radical scavenging activity by antioxidant-functionalized gold nanoparticles: A novel inspiration for development of new artificial antioxidants. Free Radic. Biol. Med. 2007, 43, 1243–1254. [Google Scholar] [CrossRef]
- Vilas, V.; Philip, D.; Mathew, J. Essential oil mediated synthesis of silver nanocrystals for environmental, anti-microbial and antioxidant applications. Mater. Sci. Eng. C 2016, 61, 429–436. [Google Scholar] [CrossRef]
- Marulasiddeshwara, M.; Dakshayani, S.; Kumar, M.S.; Chethana, R.; Kumar, P.R.; Devaraja, S. Facile-one pot-green synthesis, antibacterial, antifungal, antioxidant and antiplatelet activities of lignin capped silver nanoparticles: A promising therapeutic agent. Mater. Sci. Eng. C 2017, 81, 182–190. [Google Scholar] [CrossRef]
- Sriranjani, R.; Srinithya, B.; Vellingiri, V.; Brindha, P.; Anthony, S.P.; Sivasubramanian, A.; Muthuraman, M.S. Silver nanoparticle synthesis using Clerodendrum phlomidis leaf extract and preliminary investigation of its antioxidant and anticancer activities. J. Mol. Liq. 2016, 220, 926–930. [Google Scholar] [CrossRef]
- Kalaiyarasan, T.; Bharti, V.K.; Chaurasia, O. One pot green preparation of Seabuckthorn silver nanoparticles (SBT@ AgNPs) featuring high stability and longevity, antibacterial, antioxidant potential: A nano disinfectant future perspective. RSC Adv. 2017, 7, 51130–51141. [Google Scholar] [CrossRef]
- Teerasong, S.; Jinnarak, A.; Chaneam, S.; Wilairat, P.; Nacapricha, D. Poly (vinyl alcohol) capped silver nanoparticles for antioxidant assay based on seed-mediated nanoparticle growth. Talanta 2017, 170, 193–198. [Google Scholar] [CrossRef] [PubMed]
- Alves, A.d.C.S.; Mainardes, R.M.; Khalil, N.M. Nanoencapsulation of gallic acid and evaluation of its cytotoxicity and antioxidant activity. Mater. Sci. Eng. C 2016, 60, 126–134. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, K.; Gogoi, B.; Buragohain, A.; Deb, P. Fe2O3/C nanocomposites having distinctive antioxidant activity and hemolysis prevention efficiency. Mater. Sci. Eng. C 2014, 42, 595–600. [Google Scholar] [CrossRef] [PubMed]
- Santiago-Rodríguez, L.; Lafontaine, M.M.; Castro, C.; Méndez-Vega, J.; Latorre-Esteves, M.; Juan, E.J.; Mora, E.; Torres-Lugo, M.; Rinaldi, C. Synthesis, stability, cellular uptake, and blood circulation time of carboxymethyl-inulin coated magnetic nanoparticles. J. Mater. Chem. B 2013, 1, 2807–2817. [Google Scholar] [CrossRef]
- Szekeres, M.; Illés, E.; Janko, C.; Farkas, K.; Tóth, I.Y.; Nesztor, D.; Zupkó, I.; Földesi, I.; Alexiou, C.; Tombácz, E. Hemocompatibility and biomedical potential of poly (gallic acid) coated iron oxide nanoparticles for theranostic use. J. Nanomed. Nanotechnol. 2015, 6, 252. [Google Scholar]
- Song, W.; Muthana, M.; Mukherjee, J.; Falconer, R.J.; Biggs, C.A.; Zhao, X. Magnetic-silk core–shell nanoparticles as potential carriers for targeted delivery of curcumin into human breast cancer cells. ACS Biomater. Sci. Eng. 2017, 3, 1027–1038. [Google Scholar] [CrossRef]
- Dorniani, D.; Hussein, M.Z.B.; Kura, A.U.; Fakurazi, S.; Shaari, A.H.; Ahmad, Z. Preparation of Fe3O4 magnetic nanoparticles coated with gallic acid for drug delivery. Int. J. Nanomed. 2012, 7, 5745. [Google Scholar] [CrossRef]
- Nelson, B.C.; Johnson, M.E.; Walker, M.L.; Riley, K.R.; Sims, C.M. Antioxidant cerium oxide nanoparticles in biology and medicine. Antioxidants 2016, 5, 15. [Google Scholar] [CrossRef]
- Kalashnikova, I.; Mazar, J.; Neal, C.J.; Rosado, A.L.; Das, S.; Westmoreland, T.J.; Seal, S. Nanoparticle delivery of curcumin induces cellular hypoxia and ROS-mediated apoptosis via modulation of Bcl-2/Bax in human neuroblastoma. Nanoscale 2017, 9, 10375–10387. [Google Scholar] [CrossRef]
- Mittal, A.K.; Kumar, S.; Banerjee, U.C. Quercetin and gallic acid mediated synthesis of bimetallic (silver and selenium) nanoparticles and their antitumor and antimicrobial potential. J. Coll Interface Sci. 2014, 431, 194–199. [Google Scholar] [CrossRef]
- Sotiriou, G.A.; Blattmann, C.O.; Deligiannakis, Y. Nanoantioxidant-driven plasmon enhanced proton-coupled electron transfer. Nanoscale 2016, 8, 796–803. [Google Scholar] [CrossRef] [PubMed]
- Bitencourt, P.E.; Ferreira, L.M.; Cargnelutti, L.O.; Denardi, L.; Boligon, A.; Fleck, M.; Brandão, R.; Athayde, M.L.; Cruz, L.; Zanette, R.A. A new biodegradable polymeric nanoparticle formulation containing Syzygium cumini: Phytochemical profile, antioxidant and antifungal activity and in vivo toxicity. Ind. Crops Prod. 2016, 83, 400–407. [Google Scholar] [CrossRef]
- Tsai, Y.-H.; Yang, Y.-N.; Ho, Y.-C.; Tsai, M.-L.; Mi, F.-L. Drug release and antioxidant/antibacterial activities of silymarin-zein nanoparticle/bacterial cellulose nanofiber composite films. Carbohydr. Polym. 2018, 180, 286–296. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Tang, H.; Lu, M.; Gao, C.; Wang, F.; Ding, Y.; Zhang, S.; Yang, M. Preparation of nanosilica-immobilized antioxidant and the antioxidative behavior in low density polyethylene. Polym. Degrad. Stab. 2017, 135, 1–7. [Google Scholar] [CrossRef]
- Kotcherlakota, R.; Barui, A.K.; Prashar, S.; Fajardo, M.; Briones, D.; Rodríguez-Diéguez, A.; Patra, C.R.; Gómez-Ruiz, S. Curcumin loaded mesoporous silica: An effective drug delivery system for cancer treatment. Biomater. Sci. 2016, 4, 448–459. [Google Scholar] [CrossRef]
- Kang, D.-W.; Kim, C.K.; Jeong, H.-G.; Soh, M.; Kim, T.; Choi, I.-Y.; Ki, S.-K.; Yang, W.; Hyeon, T.; Lee, S.-H. Biocompatible custom ceria nanoparticles against reactive oxygen species resolve acute inflammatory reaction after intracerebral hemorrhage. Nano Res. 2017, 10, 2743–2760. [Google Scholar] [CrossRef]
- Joseph, A.; Wood, T.; Chen, C.-C.; Corry, K.; Snyder, J.M.; Juul, S.E.; Parikh, P.; Nance, E. Curcumin-loaded polymeric nanoparticles for neuroprotection in neonatal rats with hypoxic-ischemic encephalopathy. Nano Res. 2018, 11, 5670–5688. [Google Scholar] [CrossRef]
- Wattamwar, P.P.; Biswal, D.; Cochran, D.B.; Lyvers, A.C.; Eitel, R.E.; Anderson, K.W.; Hilt, J.Z.; Dziubla, T.D. Synthesis and characterization of poly(antioxidant β-amino esters) for controlled release of polyphenolic antioxidants. Acta Biomater. 2012, 8, 2529–2537. [Google Scholar] [CrossRef]
- Li, X.; Li, M.; Liu, J.; Ji, N.; Liang, C.; Sun, Q.; Xiong, L. Preparation of Hollow Biopolymer Nanospheres Employing Starch Nanoparticle Templates for Enhancement of Phenolic Acid Antioxidant Activities. J. Agric. Food Chem. 2017, 65, 3868–3882. [Google Scholar] [CrossRef]
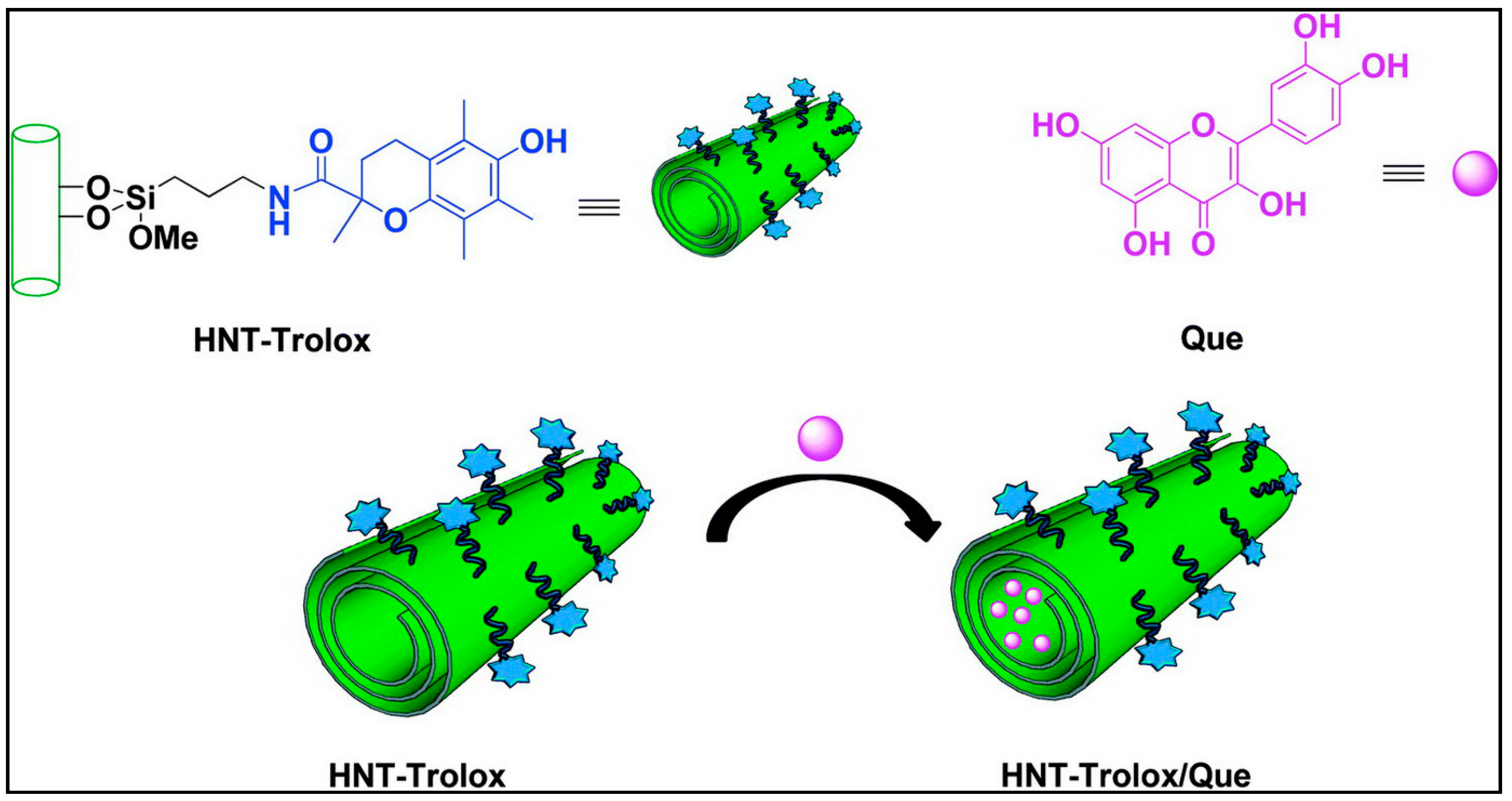
- Massaro, M.; Riela, S.; Guernelli, S.; Parisi, F.; Lazzara, G.; Baschieri, A.; Valgimigli, L.; Amorati, R. A synergic nanoantioxidant based on covalently modified halloysite–trolox nanotubes with intra-lumen loaded quercetin. J. Mater. Chem. B 2016, 4, 2229–2241. [Google Scholar] [CrossRef]
- Bakowska, A.; Kucharska, A.Z.; Oszmiański, J. The effects of heating, UV irradiation, and storage on stability of the anthocyanin–polyphenol copigment complex. Food Chem. 2003, 81, 349–355. [Google Scholar] [CrossRef]
- Liu, C.; Ge, S.; Yang, J.; Xu, Y.; Zhao, M.; Xiong, L.; Sun, Q. Adsorption mechanism of polyphenols onto starch nanoparticles and enhanced antioxidant activity under adverse conditions. J. Funct. Foods 2016, 26, 632–644. [Google Scholar] [CrossRef]
- Müller, R.H.; MaÈder, K.; Gohla, S. Solid lipid nanoparticles (SLN) for controlled drug delivery–a review of the state of the art. Eur. J. Pharm. Biopharm. 2000, 50, 161–177. [Google Scholar] [CrossRef]
- Du, L.; Li, J.; Chen, C.; Liu, Y. Nanocarrier: A potential tool for future antioxidant therapy. Free Radic. Res. 2014, 48, 1061–1069. [Google Scholar] [CrossRef] [PubMed]
- Hans, M.; Lowman, A. Biodegradable nanoparticles for drug delivery and targeting. Curr. Opin. Solid State Mater. Sci. 2002, 6, 319–327. [Google Scholar] [CrossRef]
- Panyam, J.; Labhasetwar, V. Biodegradable nanoparticles for drug and gene delivery to cells and tissue. Adv. Drug Deliv. Rev. 2003, 55, 329–347. [Google Scholar] [CrossRef]
- Karewicz, A.; Bielska, D.; Gzyl-Malcher, B.; Kepczynski, M.; Lach, R.; Nowakowska, M. Interaction of curcumin with lipid monolayers and liposomal bilayers. Coll. Surf. B Biointerfaces 2011, 88, 231–239. [Google Scholar] [CrossRef]
- Roussaki, M.; Gaitanarou, A.; Diamanti, P.C.; Vouyiouka, S.; Papaspyrides, C.; Kefalas, P.; Detsi, A. Encapsulation of the natural antioxidant aureusidin in biodegradable PLA nanoparticles. Polym. Degrad. Stab. 2014, 108, 182–187. [Google Scholar] [CrossRef]
- Antônio, E.; Junior, O.d.R.A.; de Araújo, I.S.; Khalil, N.M.; Mainardes, R.M. Poly (lactic acid) nanoparticles loaded with ursolic acid: Characterization and in vitro evaluation of radical scavenging activity and cytotoxicity. Mater. Sci. Eng. C 2017, 71, 156–166. [Google Scholar] [CrossRef]
- Kumari, A.; Yadav, S.K.; Pakade, Y.B.; Singh, B.; Yadav, S.C. Development of biodegradable nanoparticles for delivery of quercetin. Coll. Surf. B Biointerfaces 2010, 80, 184–192. [Google Scholar] [CrossRef]
- Anwer, M.K.; Al-Mansoor, M.A.; Jamil, S.; Al-Shdefat, R.; Ansari, M.N.; Shakeel, F. Development and evaluation of PLGA polymer based nanoparticles of quercetin. Int. J. Biol. Macromol. 2016, 92, 213–219. [Google Scholar] [CrossRef] [PubMed]
- Betbeder, D.; Lipka, E.; Howsam, M.; Carpentier, R. Evolution of availability of curcumin inside poly-lactic-co-glycolic acid nanoparticles: Impact on antioxidant and antinitrosant properties. Int. J. Nanomed. 2015, 10, 5355. [Google Scholar]
- Muwaffak, Z.; Goyanes, A.; Clark, V.; Basit, A.W.; Hilton, S.T.; Gaisford, S. Patient-specific 3D scanned and 3D printed antimicrobial polycaprolactone wound dressings. Int. J. Pharm. 2017, 527, 161–170. [Google Scholar] [CrossRef]
- Oliveira, A.I.; Pinho, C.; Fonte, P.; Sarmento, B.; Dias, A.C. Development, characterization, antioxidant and hepatoprotective properties of poly (ε-caprolactone) nanoparticles loaded with a neuroprotective fraction of Hypericum perforatum. Int. J. Biol. Macromol. 2018, 110, 185–196. [Google Scholar] [CrossRef] [PubMed]
- Perni, S.; Prokopovich, P. Poly-beta-amino-esters nano-vehicles based drug delivery system for cartilage. Nanomed. Nanotechnol. Biol. Med. 2017, 13, 539–548. [Google Scholar] [CrossRef] [PubMed]
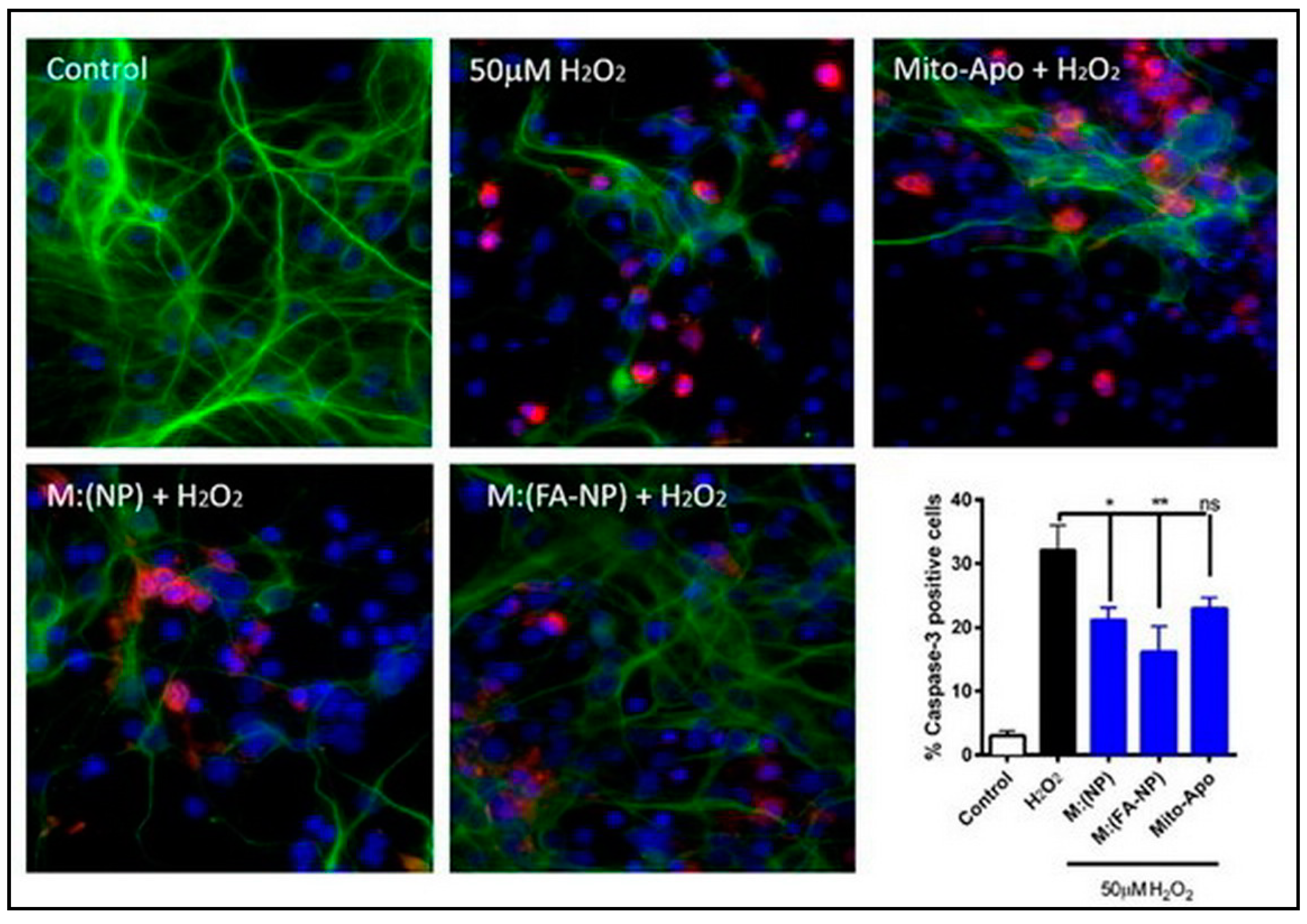
- Brenza, T.M.; Ghaisas, S.; Ramirez, J.E.V.; Harischandra, D.; Anantharam, V.; Kalyanaraman, B.; Kanthasamy, A.G.; Narasimhan, B. Neuronal protection against oxidative insult by polyanhydride nanoparticle-based mitochondria-targeted antioxidant therapy. Nanomed. Nanotechnol. Biol. Med. 2017, 13, 809–820. [Google Scholar] [CrossRef]
- Kwon, J.; Kim, J.; Park, S.; Khang, G.; Kang, P.M.; Lee, D. Inflammation-responsive antioxidant nanoparticles based on a polymeric prodrug of vanillin. Biomacromolecules 2013, 14, 1618–1626. [Google Scholar] [CrossRef]
- Vila, A.; Sanchez, A.; Tobıo, M.; Calvo, P.; Alonso, M. Design of biodegradable particles for protein delivery. J. Control. Release 2002, 78, 15–24. [Google Scholar] [CrossRef]
- Nayak, D.; Minz, A.P.; Ashe, S.; Rauta, P.R.; Kumari, M.; Chopra, P.; Nayak, B. Synergistic combination of antioxidants, silver nanoparticles and chitosan in a nanoparticle based formulation: Characterization and cytotoxic effect on MCF-7 breast cancer cell lines. J. Coll. Interface Sci. 2016, 470, 142–152. [Google Scholar] [CrossRef]
- Shah, B.R.; Zhang, C.; Li, Y.; Li, B. Bioaccessibility and antioxidant activity of curcumin after encapsulated by nano and Pickering emulsion based on chitosan-tripolyphosphate nanoparticles. Food Res. Int. 2016, 89, 399–407. [Google Scholar] [CrossRef]
- Pu, H.-L.; Chiang, W.-L.; Maiti, B.; Liao, Z.-X.; Ho, Y.-C.; Shim, M.S.; Chuang, E.-Y.; Xia, Y.; Sung, H.-W. Nanoparticles with dual responses to oxidative stress and reduced pH for drug release and anti-inflammatory applications. ACS Nano 2014, 8, 1213–1221. [Google Scholar] [CrossRef] [PubMed]
- Kaur, R.; Rajput, R.; Nag, P.; Kumar, S.; Singh, M. Synthesis, characterization and evaluation of antioxidant properties of catechin hydrate nanoparticles. J. Drug Deliv. Sci. Technol. 2017, 39, 398–407. [Google Scholar] [CrossRef]
- Nallamuthu, I.; Devi, A.; Khanum, F. Chlorogenic acid loaded chitosan nanoparticles with sustained release property, retained antioxidant activity and enhanced bioavailability. Asian J. Pharm. Sci. 2015, 10, 203–211. [Google Scholar] [CrossRef]
- Wang, Q.; Zhao, Y.; Guan, L.; Zhang, Y.; Dang, Q.; Dong, P.; Li, J.; Liang, X. Preparation of astaxanthin-loaded DNA/chitosan nanoparticles for improved cellular uptake and antioxidation capability. Food Chem. 2017, 227, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Draget, K.I.; Smidsrød, O.; Skjåk-Bræk, G. Alginates from algae. Biopolym. Online 2005, 6. [Google Scholar] [CrossRef]
- Krasaekoopt, W.; Bhandari, B.; Deeth, H. Evaluation of encapsulation techniques of probiotics for yoghurt. Int. Dairy J. 2003, 13, 3–13. [Google Scholar] [CrossRef]
- De Santis, S.; Diociaiuti, M.; Cametti, C.; Masci, G. Hyaluronic acid and alginate covalent nanogels by template cross-linking in polyion complex micelle nanoreactors. Carbohydr. Polym. 2014, 101, 96–103. [Google Scholar] [CrossRef]
- Debele, T.A.; Mekuria, S.L.; Tsai, H.-C. Polysaccharide based nanogels in the drug delivery system: Application as the carrier of pharmaceutical agents. Mater. Sci. Eng. C 2016, 68, 964–981. [Google Scholar] [CrossRef]
- Das, R.K.; Kasoju, N.; Bora, U. Encapsulation of curcumin in alginate-chitosan-pluronic composite nanoparticles for delivery to cancer cells. Nanomed. Nanotechnol. Biol. Med. 2010, 6, 153–160. [Google Scholar] [CrossRef]
- Venkatesan, J.; Anil, S.; Kim, S.-K.; Shim, M.S. Seaweed polysaccharide-based nanoparticles: Preparation and applications for drug delivery. Polymers 2016, 8, 30. [Google Scholar] [CrossRef]
- Aluani, D.; Tzankova, V.; Kondeva-Burdina, M.; Yordanov, Y.; Nikolova, E.; Odzhakov, F.; Apostolov, A.; Markova, T.; Yoncheva, K. Evaluation of biocompatibility and antioxidant efficiency of chitosan-alginate nanoparticles loaded with quercetin. Int. J. Biol. Macromol. 2017, 103, 771–782. [Google Scholar] [CrossRef] [PubMed]
- Patil, G.V. Biopolymer albumin for diagnosis and in drug delivery. Drug Dev. Res. 2003, 58, 219–247. [Google Scholar] [CrossRef]
- Irache, J.; Merodio, M.; Arnedo, A.; Camapanero, M.; Mirshahi, M.; Espuelas, S. Albumin nanoparticles for the intravitreal delivery of anticytomegaloviral drugs. Mini Rev. Med. Chem. 2005, 5, 293–305. [Google Scholar] [CrossRef] [PubMed]
- Weber, C.; Coester, C.; Kreuter, J.; Langer, K. Desolvation process and surface characterisation of protein nanoparticles. Int. J. Pharm. 2000, 194, 91–102. [Google Scholar] [CrossRef]
- Fasano, M.; Curry, S.; Terreno, E.; Galliano, M.; Fanali, G.; Narciso, P.; Notari, S.; Ascenzi, P. The extraordinary ligand binding properties of human serum albumin. IUBMB Life 2005, 57, 787–796. [Google Scholar] [CrossRef]
- Kratz, F. Albumin as a drug carrier: Design of prodrugs, drug conjugates and nanoparticles. J. Control. Release 2008, 132, 171–183. [Google Scholar] [CrossRef]
- Tantra, R.; Tompkins, J.; Quincey, P. Characterisation of the de-agglomeration effects of bovine serum albumin on nanoparticles in aqueous suspension. Coll. Surf. B Biointerfaces 2010, 75, 275–281. [Google Scholar] [CrossRef]
- Wongsasulak, S.; Patapeejumruswong, M.; Weiss, J.; Supaphol, P.; Yoovidhya, T. Electrospinning of food-grade nanofibers from cellulose acetate and egg albumen blends. J. Food Eng. 2010, 98, 370–376. [Google Scholar] [CrossRef]
- Merodio, M.; Arnedo, A.; Renedo, M.J.; Irache, J.M. Ganciclovir-loaded albumin nanoparticles: Characterization and in vitro release properties. Eur. J. Pharm. Sci. 2001, 12, 251–259. [Google Scholar] [CrossRef]
- Meziani, M.J.; Sun, Y.-P. Protein-conjugated nanoparticles from rapid expansion of supercritical fluid solution into aqueous solution. J. Am. Chem. Soc. 2003, 125, 8015–8018. [Google Scholar] [CrossRef]
- Elzoghby, A.O.; Samy, W.M.; Elgindy, N.A. Protein-based nanocarriers as promising drug and gene delivery systems. J. Control. Release 2012, 161, 38–49. [Google Scholar] [CrossRef]
- Jahanshahi, M.; Babaei, Z. Protein nanoparticle: A unique system as drug delivery vehicles. Afr. J. Biotechnol. 2008, 7, 4926–4936. [Google Scholar]
- Muro, S.; Garnacho, C.; Champion, J.A.; Leferovich, J.; Gajewski, C.; Schuchman, E.H.; Mitragotri, S.; Muzykantov, V.R. Control of endothelial targeting and intracellular delivery of therapeutic enzymes by modulating the size and shape of ICAM-1-targeted carriers. Mol. Ther. 2008, 16, 1450–1458. [Google Scholar] [CrossRef] [PubMed]
- Xu, R.; Fisher, M.; Juliano, R. Targeted albumin-based nanoparticles for delivery of amphipathic drugs. Bioconjug. Chem. 2011, 22, 870–878. [Google Scholar] [CrossRef]
- Singh, H.D.; Wang, G.; Uludağ, H.; Unsworth, L.D. Poly-L-lysine-coated albumin nanoparticles: Stability, mechanism for increasing in vitro enzymatic resilience, and siRNA release characteristics. Acta Biomater. 2010, 6, 4277–4284. [Google Scholar] [CrossRef] [PubMed]
- Fang, R.; Hao, R.; Wu, X.; Li, Q.; Leng, X.; Jing, H. Bovine serum albumin nanoparticle promotes the stability of quercetin in simulated intestinal fluid. J. Agric. Food Chem. 2011, 59, 6292–6298. [Google Scholar] [CrossRef]
- Ninan, G.; Jose, J.; Abubacker, Z. Preparation and characterization of gelatin extracted from the skins of rohu (Labeo rohita) and common carp (Cyprinus carpio). J. Food Process. Preserv. 2011, 35, 143–162. [Google Scholar] [CrossRef]
- Sahoo, N.; Sahoo, R.K.; Biswas, N.; Guha, A.; Kuotsu, K. Recent advancement of gelatin nanoparticles in drug and vaccine delivery. Int. J. Biol. Macromol. 2015, 81, 317–331. [Google Scholar] [CrossRef]
- Elzoghby, A.O. Gelatin-based nanoparticles as drug and gene delivery systems: Reviewing three decades of research. J. Control. Release 2013, 172, 1075–1091. [Google Scholar] [CrossRef]
- Shutava, T.G.; Balkundi, S.S.; Vangala, P.; Steffan, J.J.; Bigelow, R.L.; Cardelli, J.A.; O’Neal, D.P.; Lvov, Y.M. Layer-by-layer-coated gelatin nanoparticles as a vehicle for delivery of natural polyphenols. ACS Nano 2009, 3, 1877–1885. [Google Scholar] [CrossRef]
- Numata, K.; Kaplan, D.L. Silk-based delivery systems of bioactive molecules. Adv. Drug Deliv. Rev. 2010, 62, 1497–1508. [Google Scholar] [CrossRef] [PubMed]
- Gupta, V.; Aseh, A.; Ríos, C.N.; Aggarwal, B.B.; Mathur, A.B. Fabrication and characterization of silk fibroin-derived curcumin nanoparticles for cancer therapy. Int. J. Nanomed. 2009, 4, 115. [Google Scholar] [CrossRef] [PubMed]
- Teng, Z.; Luo, Y.; Wang, Q. Nanoparticles synthesized from soy protein: Preparation, characterization, and application for nutraceutical encapsulation. J. Agric. Food Chem. 2012, 60, 2712–2720. [Google Scholar] [CrossRef] [PubMed]
- Lohcharoenkal, W.; Wang, L.; Chen, Y.C.; Rojanasakul, Y. Protein nanoparticles as drug delivery carriers for cancer therapy. Biomed Res. Int. 2014, 2014. [Google Scholar] [CrossRef]
- Li, B.; Du, W.; Jin, J.; Du, Q. Preservation of (−)-epigallocatechin-3-gallate antioxidant properties loaded in heat treated β-lactoglobulin nanoparticles. J. Agric. Food Chem. 2012, 60, 3477–3484. [Google Scholar] [CrossRef]
- ABD EL-SALAM, M.H.; EL-SHIBINY, S. Formation and potential uses of milk proteins as nano delivery vehicles for nutraceuticals: A review. Int. J. Dairy Technol. 2012, 65, 13–21. [Google Scholar] [CrossRef]
- Pan, K.; Luo, Y.; Gan, Y.; Baek, S.J.; Zhong, Q. pH-driven encapsulation of curcumin in self-assembled casein nanoparticles for enhanced dispersibility and bioactivity. Soft Matter 2014, 10, 6820–6830. [Google Scholar] [CrossRef]
- Pan, K.; Zhong, Q.; Baek, S.J. Enhanced dispersibility and bioactivity of curcumin by encapsulation in casein nanocapsules. J. Agric. Food Chem. 2013, 61, 6036–6043. [Google Scholar] [CrossRef]
- Patra, M.; Mukherjee, R.; Banik, M.; Dutta, D.; Begum, N.A.; Basu, T. Calcium phosphate-quercetin nanocomposite (CPQN): A multi-functional nanoparticle having pH indicating, highly fluorescent and anti-oxidant properties. Coll. Surf. B Biointerfaces 2017, 154, 63–73. [Google Scholar] [CrossRef]
- Takahashi, M.; Uechi, S.; Takara, K.; Asikin, Y.; Wada, K. Evaluation of an oral carrier system in rats: Bioavailability and antioxidant properties of liposome-encapsulated curcumin. J. Agric. Food Chem. 2009, 57, 9141–9146. [Google Scholar] [CrossRef]
- Rashidinejad, A.; Birch, E.J.; Sun-Waterhouse, D.; Everett, D.W. Delivery of green tea catechin and epigallocatechin gallate in liposomes incorporated into low-fat hard cheese. Food Chem. 2014, 156, 176–183. [Google Scholar] [CrossRef] [PubMed]
- Fang, J.-Y.; Hwang, T.-L.; Huang, Y.-L.; Fang, C.-L. Enhancement of the transdermal delivery of catechins by liposomes incorporating anionic surfactants and ethanol. Int. J. Pharm. 2006, 310, 131–138. [Google Scholar] [CrossRef] [PubMed]
- Furukawa, R.; Yamada, Y.; Takenaga, M.; Igarashi, R.; Harashima, H. Octaarginine-modified liposomes enhance the anti-oxidant effect of Lecithinized superoxide dismutase by increasing its cellular uptake. Biochem. Biophys. Res. Commun. 2011, 404, 796–801. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.-H.; Yen, F.-L.; Lin, L.-T.; Tsai, T.-R.; Lin, C.-C.; Cham, T.-M. Preparation, physicochemical characterization, and antioxidant effects of quercetin nanoparticles. Int. J. Pharm. 2008, 346, 160–168. [Google Scholar] [CrossRef]
- Yen, F.-L.; Wu, T.-H.; Tzeng, C.-W.; Lin, L.-T.; Lin, C.-C. Curcumin nanoparticles improve the physicochemical properties of curcumin and effectively enhance its antioxidant and antihepatoma activities. J. Agric. Food Chem. 2010, 58, 7376–7382. [Google Scholar] [CrossRef] [PubMed]
- Peres, I.; Rocha, S.; Gomes, J.; Morais, S.; Pereira, M.C.; Coelho, M. Preservation of catechin antioxidant properties loaded in carbohydrate nanoparticles. Carbohydr. Polym. 2011, 86, 147–153. [Google Scholar] [CrossRef]
- Behl, G.; Sharma, M.; Sikka, M.; Dahiya, S.; Chhikara, A.; Chopra, M. Gallic acid loaded disulfide cross-linked biocompatible polymeric nanogels as controlled release system: Synthesis, characterization, and antioxidant activity. J. Biomater. Sci. Polym. Ed. 2013, 24, 865–881. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Yi, J.; Zhang, Y.; Yokoyama, W. Fabrication of curcumin-loaded bovine serum albumin (BSA)-dextran nanoparticles and the cellular antioxidant activity. Food Chem. 2018, 239, 1210–1218. [Google Scholar] [CrossRef]
| Nanoparticles | Antioxidants and Functionalization Strategy | Remarkable Features | Ref. |
|---|---|---|---|
| SiO2NPs | GA; covalent grafting | Fast HAT reactions toward DPPH radicals | [30] |
| MSN | morin (2′,3,4′,5,7-pentahydroxyflavone); surface functionalization | Potent HO• scavenger and 1O2 quencher | [41] |
| MSN | Poly Tannic acid; crossing linking | Efficient antioxidant activity | [42] |
| MSN | Caffeic acid and rutin; covalent grafting | Antiradical functions, cellular toxicity alleviation and effective against oxidative stress | [43] |
| SiO2NPs | 3,5-di-tert-butyl-4-hydroxybenzoic acid; grafting | Improved thermal oxidative stability of LDPE composite | [66] |
| MSN | Curcumin; loaded | Exhibited higher cellular uptake and inhibition of cancer cell viability | [67] |
| PEG coated AuNPs | Salvianic acid; Surface functionalization | Enhanced antioxidant and ROS scavenging in living cells | [46] |
| AuNPs | Trolox; Self-assembly | Enhanced antioxidant activity | [48] |
| AuNPs | 3,6-dihydroxyflavone, lutein and selenium methyl selenocysteine; embedded | Enhanced antioxidant activity | [10] |
| AgNPs | Lignin; capped | Potent antioxidant; antifungal and antibacterial agents against human pathogens S. aureus, E. coli, and A. niger | [50] |
| Fe2O3NPs | GA; surface functionalization | Magnetically separable; greater antioxidant activity; outstanding antibacterial and antifungal activity | [27] |
| Fe2O3NPs | Carboxymethyl-inulin; coated | non-cytotoxic to the immortalized human cancer cell lines | [56] |
| Fe2O3NPs | Carbon; coated | Potential antioxidant, exhibited compatibility with the peripheral blood mononuclear cells | [55] |
| Fe2O3NPs | Poly GA, coated | Significantly reduce the oxidative stress; biocompatible and bioactive | [57] |
| Magnetic-silk core-shell nanoparticle | Curcumin, loaded | Greater cellular uptake and cytotoxicity in human breast cancer cell line | [58] |
| Ceria nanoparticles | Dextran coated and curcumin loaded | Anti-cancer properties | [61] |
| Ceria nanoparticles | Phospholipid-PEG; coated | Biocompatible; reduce oxidative stress, cytotoxicity, and effective agent for intracerebral hemorrhage patient | [68] |
| PLGA-PEG | Curcumin; loaded | Ensures neuroprotection in neonatal with hypoxic-ischemic encephalopathy | [69] |
| Ag-Se bimetal | Quercetin and GA | Antioxidant, antimicrobial and antitumor potentials | [62] |
| Nanoparticle Carrier | Antioxidant | Nanoantioxidant Fabrication Method | Particle Size (nm) | Superiority | Ref. |
|---|---|---|---|---|---|
| Chitosan nanoformulations-AgNPs | Ascorbic acid, α-tochopherol, and catechol | Ionotropic gelation | Encapsulation efficiency: 76% Targeted delivery and sustained release to breast cancer cell, hemocompatible | [91] | |
| CS-TPP stabilized nano and pickering emulsion | Curcumin | Ionic gelation | - | Radical scavenging activity | [92] |
| PPADT encapsulated NPCS linked Cy3 nanoparticles | Curcumin | Responsive to both oxidative stress and reduced pH in inflammatory milieu To monitor in vitro drug release behavior | [93] | ||
| Tripolyphosphate and chitosan | CH | Ionic gelation | 68.76 ± 1.72 | Higher and prolonged antioxidant and radical scavenging activity against (DPPH, NO, H2O2) | [94] |
| Chitosan | CGA | Ionic gelation | ~250 | Encapsulation efficiency: 59% Sustained release over a period of 100 h. Less cytotoxic | [95] |
| Chitosan/DNA | Astaxanthin | Chemical reaction, Vacuum-evaporation | 92 ± 1 | Prompt cellular uptake by Caco-2 cell Improved cellular viability and ROS scavenging activity (2 fold more than free astazanthin) | [96] |
| BSA | Quercetin | hydrophobic interaction | <10 | Promotes stability of encapsulated quercetin while maintaining its antioxidant activity | [118] |
| Silk fibroin and chitosan polymer | Curcumin | capillary-microdot technique | <100 | Higher efficiency against breast cancer cell potential to treat in vivo breast tumors by local, sustained, and long-term therapeutic delivery | [124] |
| Liposomes | Curcumin | mechanochemical method with a microfluidizer | 263 ± 86.0 | 68.0% encapsulation efficiency Increased plasma antioxidant activity Enhanced bioavailability | [132] |
| Egg yolk phosphatidyl choline/dihexyl phosphate/cholesterol liposomal bilayer | Curcumin | Film evaporation method | 64.24 ± 0.57 to 80.64 ± 0.84 | Increase the nanocarrier stability | [79] |
| Soy lecithin liposome | Green tea catechin and epigallocatechin gallate (EGCG) | Water-oil-water emulsion | 139 ± 4 to 173 ± 5 | Encapsulation efficiency is more than 70% To make antioxidant rich functional food To protect and deliver antioxidant to gut | [133] |
| Liposomes with deoxycholic acid and dicetyl phosphate | Catechin ((+)-catechin, (−)-epicatechin, and (−)-EGCG) | 378.2 ± 10.9 | Encapsulation efficiency: 93.0 ± 0.1% Enhanced catechin delivery Limited skin disruption Good stability | [134] | |
| Octaarginine-modified liposomes | Superoxide dismutase | Lipid film hydration method | 170 ± 7 | Fast cellular uptake and efficient cytosolic delivery of SOD. Increased scavenging efficiency of intracellular O2− | [135] |
| Eudragit E and PVA | Quercetin | Nanoprecipitation technique | <85 | High encapsulation (99%) 74-fold higher drug delivery than pure drug Greater antioxidant activity | [136] |
| Polyvinylpyrrolidone | Curcumin | Nanoprecipitation technique | 142.90 ± 3.12 | Encapsulation efficiency (99.93 ± 0.01%) Enhanced antioxidant, drug release and antihepatoma activity | [137] |
| Gum arabic–maltodextrin | Epigallocatechin gallate | Spray drying | 400 | Highly efficient for encapsulation (96%) Integrity maintained with preserving antioxidant properties | [138] |
| Poly(ethylene glycol)-based nanogels | GA | Aqueous inverse miniemulsion using atom transfer radical polymerization | 227 ± 51.78 to 573.3 ± 207.2 | Encapsulation efficiency: 60–70% Guided controlled drug release, retained antioxidant property and biocompatible to HeLa cell lines. | [139] |
| Polyanhydride nanoparticles | Apocyanin | Anti-solvent nano-encapsulation method | 324 to 346 | [88] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khalil, I.; Yehye, W.A.; Etxeberria, A.E.; Alhadi, A.A.; Dezfooli, S.M.; Julkapli, N.B.M.; Basirun, W.J.; Seyfoddin, A. Nanoantioxidants: Recent Trends in Antioxidant Delivery Applications. Antioxidants 2020, 9, 24. https://doi.org/10.3390/antiox9010024
Khalil I, Yehye WA, Etxeberria AE, Alhadi AA, Dezfooli SM, Julkapli NBM, Basirun WJ, Seyfoddin A. Nanoantioxidants: Recent Trends in Antioxidant Delivery Applications. Antioxidants. 2020; 9(1):24. https://doi.org/10.3390/antiox9010024
Chicago/Turabian StyleKhalil, Ibrahim, Wageeh A. Yehye, Alaitz Etxabide Etxeberria, Abeer A. Alhadi, Seyedehsara Masoomi Dezfooli, Nurhidayatullaili Binti Muhd Julkapli, Wan Jefrey Basirun, and Ali Seyfoddin. 2020. "Nanoantioxidants: Recent Trends in Antioxidant Delivery Applications" Antioxidants 9, no. 1: 24. https://doi.org/10.3390/antiox9010024
APA StyleKhalil, I., Yehye, W. A., Etxeberria, A. E., Alhadi, A. A., Dezfooli, S. M., Julkapli, N. B. M., Basirun, W. J., & Seyfoddin, A. (2020). Nanoantioxidants: Recent Trends in Antioxidant Delivery Applications. Antioxidants, 9(1), 24. https://doi.org/10.3390/antiox9010024







