In Vitro Digestion of Human Milk: Influence of the Lactation Stage on the Micellar Carotenoids Content
Abstract
:1. Introduction
2. Materials and Methods
2.1. Subjects
2.2. Reagents
2.3. Measures
2.3.1. Milk Collection
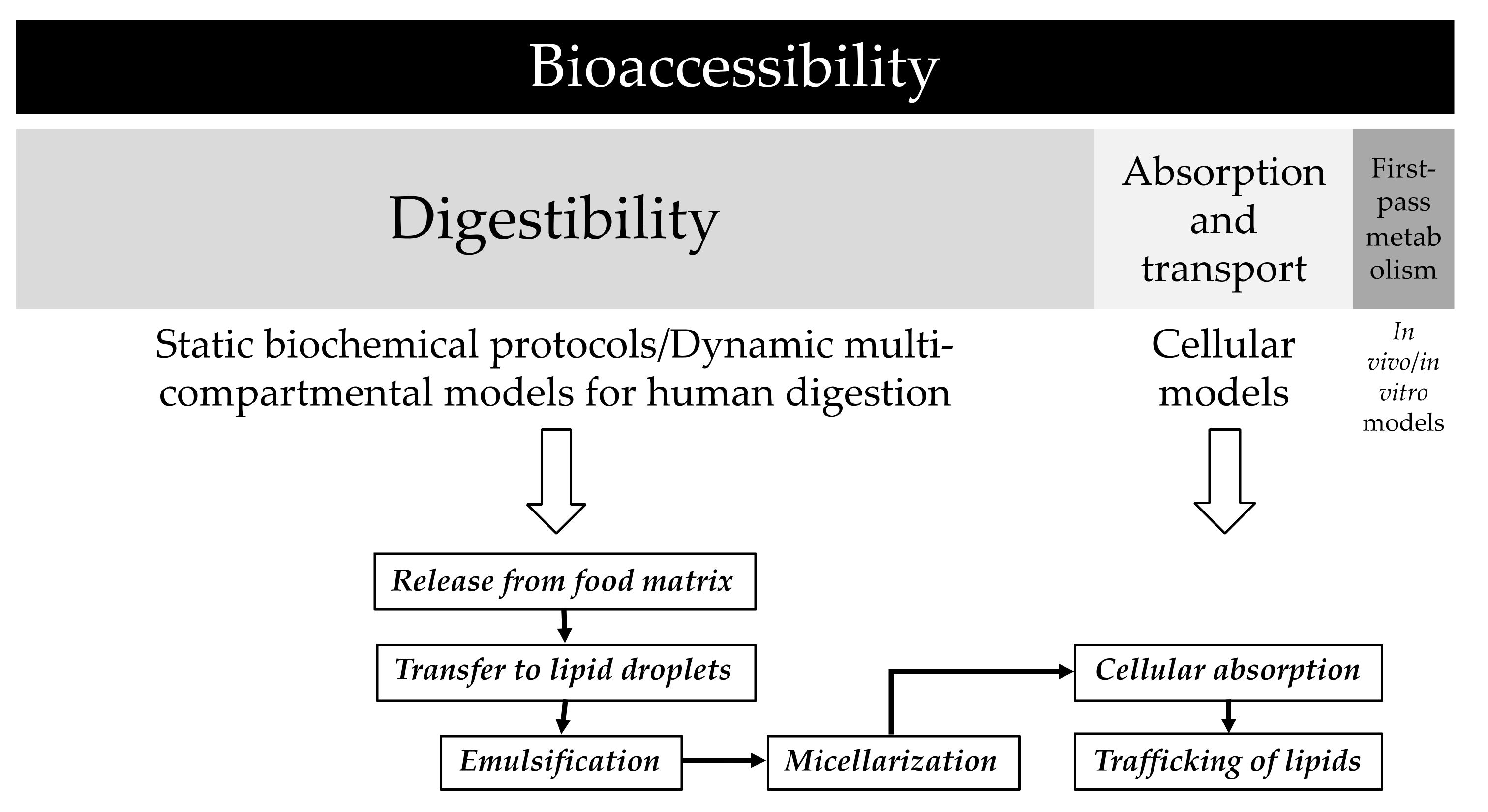
2.3.2. In Vitro Digestion of Human Milk
2.3.3. Extraction of the Carotenoid Fraction
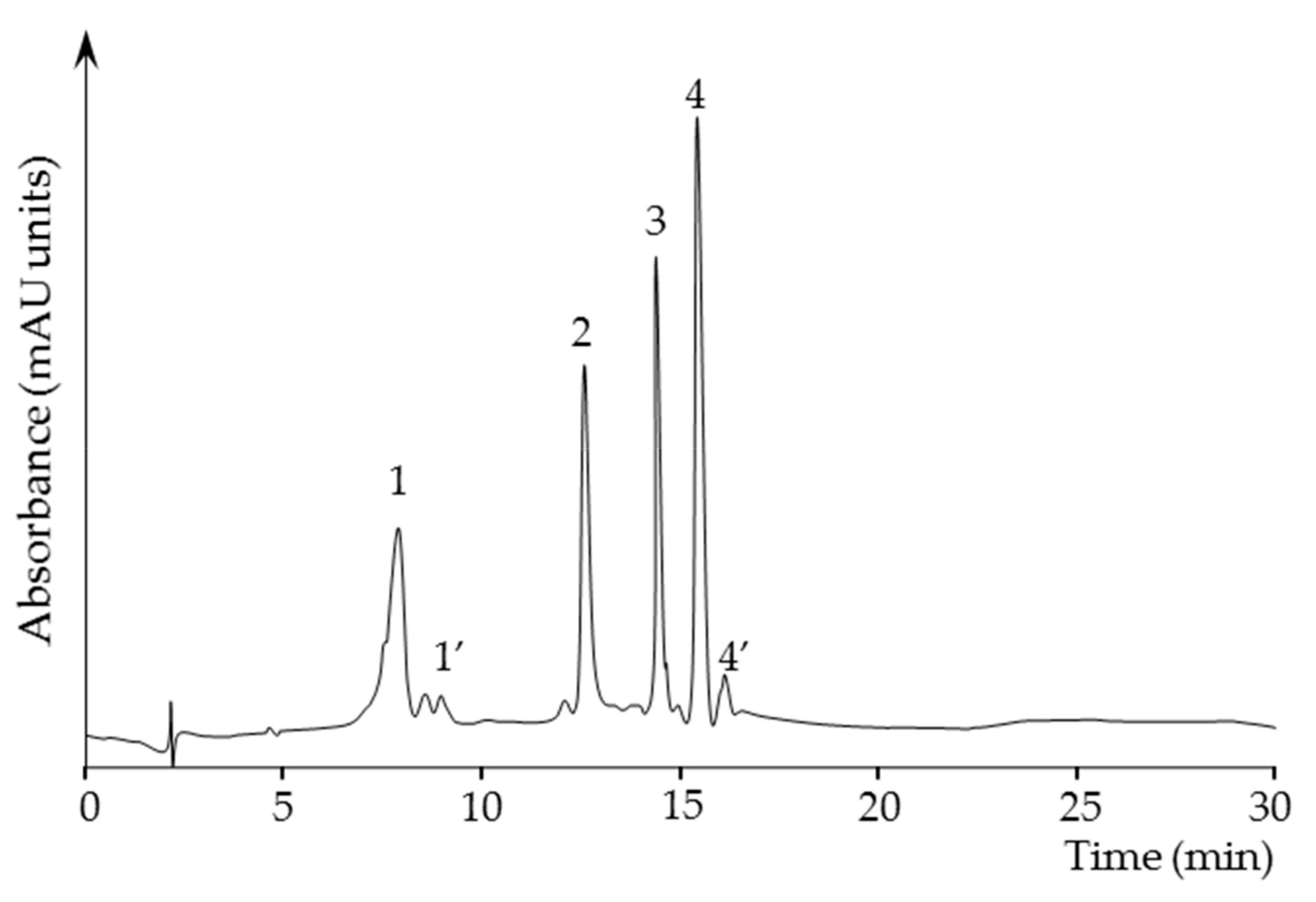
2.3.4. Quantification of Carotenoids in the Carotenoid Extracts
2.3.5. Determination of the Lipid Content
2.4. Ethics Approval
2.5. Statistical Analysis and Calculation
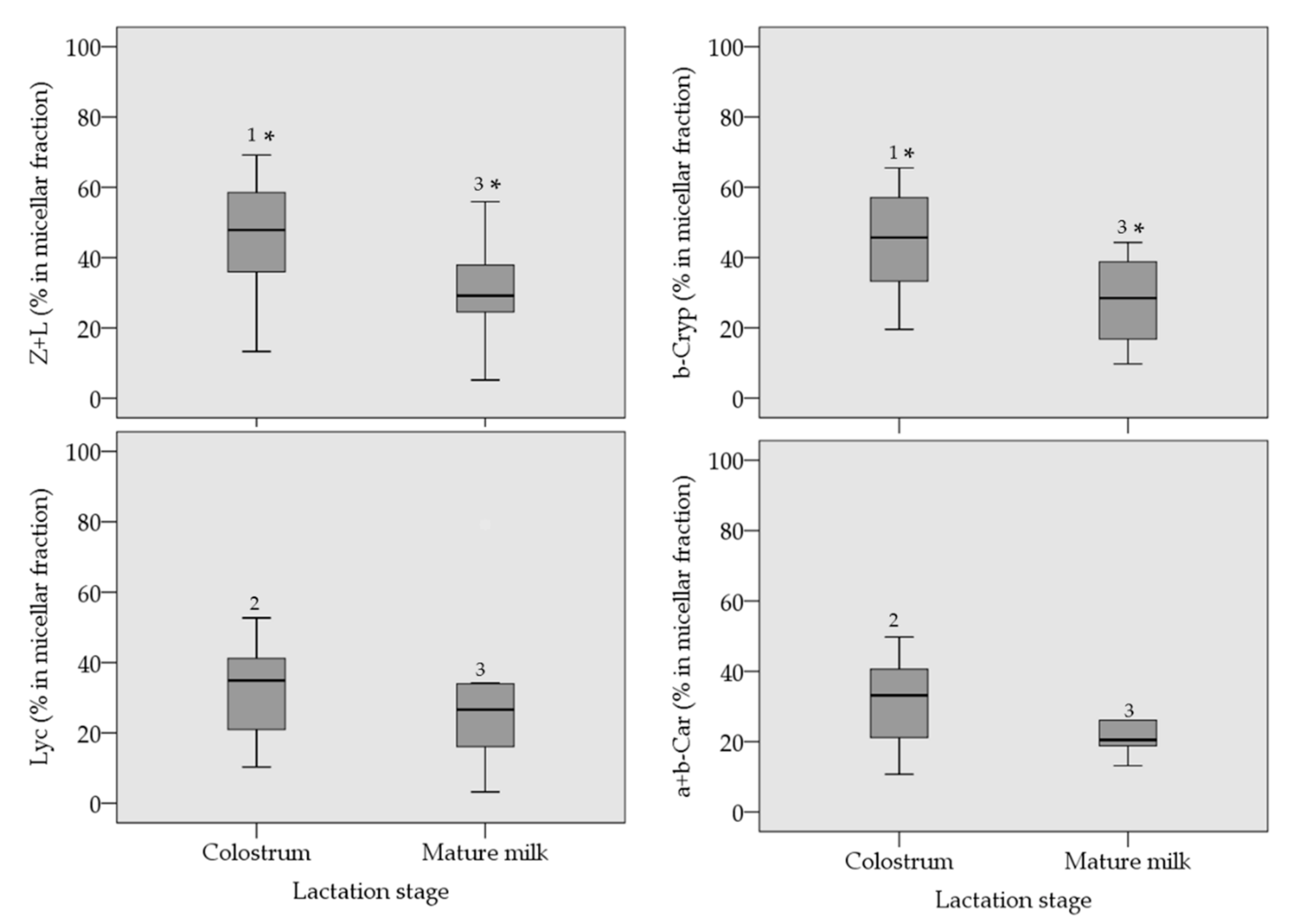
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Britton, G. General overview of carotenoid biosynthesis. In Carotenoid Esters in Foods: Physical, Chemical and Biological Properties, 1st ed.; Mercadante, A.Z., Ed.; The Royal Society of Chemistry: London, UK, 2019; pp. 109–136. [Google Scholar]
- Liaaen-Jensen, S. Basic Carotenoid Chemistry. In Carotenoids in Health and Disease, 1st ed.; Krinsky, N.I., Mayne, S.T., Sies, H., Eds.; Marcel Dekker: New York, NY, USA, 2004; pp. 1–30. [Google Scholar]
- Bauernfeind, J.C. Carotenoid vitamin A precursors and analogs in foods and feeds. J. Agric. Food Chem. 1972, 20, 456–473. [Google Scholar] [CrossRef] [PubMed]
- Bendich, A. Non-provitamin A activity of carotenoids: Immunoenhancement. Trends Food Sci. Technol. 1991, 2, 127–130. [Google Scholar] [CrossRef]
- Stahl, W.; Sies, H. Bioactivity and protective effects of natural carotenoids. Biochim. Biophys. Acta 2005, 1740, 101–107. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tapiero, H.; Townsend, D.M.; Tew, K.D. The role of carotenoids in the prevention of human pathologies. Biomed. Pharmacother. 2004, 58, 100–110. [Google Scholar] [CrossRef] [PubMed]
- Arathi, B.P.; Sowmya, P.R.R.; Vijay, K.; Baskaran, V.; Lakshminarayana, R. Metabolomics of carotenoids: The challenges and prospects. A review. Trends Food Sci. Technol. 2015, 45, 105–117. [Google Scholar] [CrossRef]
- Cooper, D.A. Carotenoids in health and disease: Recent evaluations, research recommendations and the consumer. J. Nutr. 2004, 134, 221S–224S. [Google Scholar] [CrossRef] [PubMed]
- Giovannucci, E.; Ascherio, A.; Rimm, E.B.; Stampfer, M.J.; Colditz, G.A.; Willett, W.C. Intake of carotenoids and retinol in relation to risk of prostate cancer. J. Natl. Cancer Inst. 1995, 87, 1767–1776. [Google Scholar] [CrossRef] [PubMed]
- Hu, F.; Wang, Y.B.; Zhang, W.; Liang, J.; Lin, C.; Li, D.; Wang, F.; Pang, D.; Zhao, Y. Carotenoids and breast cancer risk: A meta-analysis and meta regression. Breast Cancer Res. Treat. 2012, 131, 239–253. [Google Scholar] [CrossRef]
- World Cancer Research Fund. Continuous Update Project. Cancer Prevention Recommendations. Available online: https://wcrf.org/int/research-we-fund/continuous-update-project-cup (accessed on 25 June 2019).
- Wang, Y.; Chung, S.J.; McCullough, M.L.; Song, W.O.; Fernandez, M.L.; Koo, S.I.; Chun, O.K. Dietary carotenoids are associated with cardiovascular disease risk biomarkers mediated by serum carotenoid concentrations. J. Nutr. 2014, 144, 1067–1074. [Google Scholar] [CrossRef]
- Stoltzfus, R.J.; Hakima, M.; Miller, K.W.; Rasmussen, K.M.; Dawiesah, S.I.; Habicht, J.P.; Dibley, M.J. High dose vitamin A supplementation of breast-feeding Indonesian mothers: Effects on the vitamin A status of mother and infant. J. Nutr. 1993, 123, 666–675. [Google Scholar] [CrossRef]
- Castenmiller, J.J.M.; West, C.E. Bioavailability and bioconversion of carotenoids. Annu. Rev. Nutr. 1998, 18, 19–38. [Google Scholar] [CrossRef] [PubMed]
- Walker, A. Breast milk as the gold standard for protective nutrients. J. Pediatr. 2010, 156, S3–S7. [Google Scholar] [CrossRef] [PubMed]
- American Academy of Pediatrics. Breastfeeding and the use of human milk. American Academy of pediatrics policy statement. Pediatrics 2005, 115, 496–506. [Google Scholar] [CrossRef] [PubMed]
- Horta, B.L.; Bahl, R.; Martines, J.C.; Victora, C.G. Evidence on the Long-Term Effects of Breastfeeding. Systematic Reviews and Meta-Analyses; World Health Organization: Geneva, Switzerland, 2007; ISBN 978 92 4 159523 0. [Google Scholar]
- Ip, S.; Chung, M.; Raman, G.; Chew, P.; Magula, N.; Devine, D.; Trikalinos, T.; Lau, J. Breastfeeding and Maternal and Infant Health Outcomes in Developed Countries; Evidence Report/Technology Assessment No. 153; Agency for Healthcare Research and Quality: Rockville, MD, USA, 2007.
- Picciano, M.F. Nutrient composition of human milk. Pediatr. Clin. N. Am. 2001, 48, 53–67. [Google Scholar] [CrossRef]
- Ballard, O.; Morrow, A. Human milk composition: Nutrients and bioactive factors. Pediatr. Clin. N. Am. 2013, 60, 49–74. [Google Scholar] [CrossRef]
- Picciano, M.F. Pregnancy and lactation: Physiological adjustments, nutritional requirements and the role of dietary supplemnts. J. Nutr. 2003, 133, 1997S–2002S. [Google Scholar] [CrossRef]
- Singer, D.; Muhlfeld, C. Perinatal adaptation in mammals: The impact of metabolic rate. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2007, 148, 780–784. [Google Scholar] [CrossRef]
- Franco, M.C.; Kawamoto, E.M.; Gorjao, R.; Rastelli, V.M.; Curi, R.; Scavone, C.; Sawaya, A.L.; Fortes, Z.B.; Sesso, R. Biomarkers of oxidative stress and antioxidant status in children born small for gestational age: Evidence of lipid peroxidation. Pediatr. Res. 2007, 62, 204–208. [Google Scholar] [CrossRef]
- Pérez-Gálvez, A.; Jarén-Galán, M.; Garrido-Fernández, J.; Calvo, M.V.; Visioli, F.; Fontecha, J. Activities, bioavailability, and metabolism of lipids from structural membranes and oils: Promising research on mild cognitive impairment. Pharmacol. Res. 2008, 134, 299–304. [Google Scholar] [CrossRef]
- Pérez-Gálvez, A. In vitro digestion protocols: The benchmark for estimation of in vivo data. In Carotenoid Esters in Foods: Physical, Chemical and Biological Properties, 1st ed.; Mercadante, A.Z., Ed.; The Royal Society of Chemistry: London, UK, 2019; pp. 421–458. [Google Scholar]
- Reboul, E.; Richelle, M.; Perrot, E.; Desmoulins-Malezet, C.; Pirisi, V.; Borel, P. Bioaccessibility of carotenoids and vitamin E form their main dietary sources. J. Agric. Food Chem. 2006, 54, 8749–8755. [Google Scholar] [CrossRef]
- Werner, S.; Böhm, V. Bioaccessibility of carotenoids and vitamin E from pasta: Evaluation of an in vitro digestion model. J. Agric. Food Chem. 2011, 59, 1163–1170. [Google Scholar] [CrossRef] [PubMed]
- Carbonell-Capella, J.M.; Buniowska, M.; Barba, F.J.; Esteve, M.J.; Frígola, A. Analytical methods for determining bioavailability and bioaccessibility of bioactive compounds from fruits and vegetables: A review. Compr. Rev. Food Sci. Food Saf. 2014, 13, 155–171. [Google Scholar] [CrossRef]
- Rodrigues, D.B.; Mariutti, L.R.B.; Mercadante, A.Z. An in vitro digestion method adapted for carotenoids and carotenoid esters: Moving forward towards standardization. Food Funct. 2016, 7, 4992–5001. [Google Scholar] [CrossRef] [PubMed]
- Estévez-Santiago, R.; Olmedilla-Alonso, B.; Fernández-Jalao, I. Bioaccessibility of provitamin A carotenoids from fruits: Application of a standardised static in vitro digestion method. Food Funct. 2016, 7, 1354–1366. [Google Scholar] [CrossRef] [PubMed]
- Schweiggert, R.M.; Carle, R. Carotenoid deposition in plant and animal foods and its impact on bioavailability. Crit. Rev. Food Sci. Nutr. 2017, 57, 1807–1830. [Google Scholar] [CrossRef] [PubMed]
- Ménard, O.; Bourlieu, C.; De Oliveira, S.C.; Dellarosa, N.; Laghi, L.; Carrière, F.; Capozzi, F.; Dupont, D.; Deglaire, A. A first step towards a consensus static in vitro model for simulating full-term infant digestion. Food Chem. 2018, 240, 338–345. [Google Scholar] [CrossRef]
- Ye, A.; Cui, J.; Singh, H. Effect of fat globule membrane on in vitro digestion of milk fat globules with pancreatic lipase. Int. Dairy. J. 2010, 20, 822–829. [Google Scholar] [CrossRef]
- Berton, A.; Rouvellac, S.; Robert, B.; Rousseau, F.; Lopez, C.; Crenon, I. Effect of the size and interface composition of milk fat globules on their in vitro digestion by the human pancreatic lipase: Native versus homogenized fat globules. Food Hydrocoll. 2012, 29, 124–134. [Google Scholar] [CrossRef]
- Hernández-Alvarez, E.; Blanco-Navarro, I.; Pérez-Sacristán, B.; Sánchez-Siles, L.M.; Granado-Lorencio, F. In vitro digestion-assisted development of a β-cryptoxanthin-rich functional beverage; in vivo validation using systemic response and faecal content. Food Chem. 2016, 208, 18–25. [Google Scholar] [CrossRef]
- Hodgkinson, A.J.; Wallace, O.A.M.; Boggs, I.; Broadhurst, M.; Prosser, C.G. Gastric digestion of cow and goat milk: Impact of infant and young child in vitro digestion conditions. Food Chem. 2018, 245, 275–281. [Google Scholar] [CrossRef]
- Ríos, J.J.; Xavier, A.A.O.; Díaz-Salido, E.; Arenilla-Vélez, I.; Jarén-Galán, M.; Garrido-Fernández, J.; Aguayo-Maldonado, J.; Pérez-Gálvez, A. Xanthophyll esters are found in human colostrum. Mol. Nutr. Food Res. 2017, 61, 1700296. [Google Scholar] [CrossRef]
- Garrett, D.A.; Failla, M.L.; Sarama, R.J. Development of an in vitro digestion method to assess carotenoid bioavailability from meals. J. Agric. Food Chem. 1999, 47, 4301–4309. [Google Scholar] [CrossRef]
- Xavier, A.A.O.; Mercadante, A.Z.; Garrido-Fernández, J.; Pérez-Gálvez, A. Fat content affects bioaccessibility and efficiency of enzymatic hydrolysis of lutein esters added to milk and yogurt. Food Res. Int. 2014, 65, 171–176. [Google Scholar] [CrossRef] [Green Version]
- Britton, G. UV/visible spectroscopy. In Carotenoids: Spectroscopy, 1st ed.; Britton, G., Liaaen-Jensen, S., Pfander, H., Eds.; Birkhauser: Basel, Switzerland, 1995; Volume 1B, pp. 13–62. [Google Scholar]
- Jensen, R.G.; Lammi-Keefe, C.J.; Koletzko, B. Representative sampling of human milk and the extraction of fat for analysis of environmental lipophilic contaminants. Toxicol. Environ. Chem. 1997, 62, 229–247. [Google Scholar] [CrossRef]
- Garrett, D.A.; Failla, M.L.; Sarama, R.J. Estimation of carotenoid bioavailability from fresh stir-fried vegetables using an in vitro digestion/Caco-2 cell culture model. J. Nutr. Biochem. 2000, 11, 574–580. [Google Scholar] [CrossRef]
- Lipkie, T.E.; Banavara, D.; Shah, B.; Morrow, A.L.; McMahon, R.J.; Jouni, Z.E.; Ferruzzi, M.G. Caco-2 accumulation of lutein is greater from human milk than from infant formula despite similar bioaccessibility. Mol. Nutr. Food Res. 2014, 58, 2014–2022. [Google Scholar] [CrossRef]
- Minekus, M.; Alminger, M.; Alvito, P.; Ballance, S.; Bohn, T.; Bourlieu, C.; Carrière, F.; Boutrou, R.; Corredig, M.; Dupont, D.; et al. A standardized static in vitro digestion method suitable for food – an international consensus. Food Funct. 2014, 5, 1113–1124. [Google Scholar] [CrossRef]
- Bettler, J.; Zimmer, J.P.; Neuringer, M.; DeRusso, P.A. Serum lutein concentrations in healthy term infants fed human milk or infant formula with lutein. Eur. J. Nutr. 2010, 49, 45–51. [Google Scholar] [CrossRef]
- Sommerburg, O.; Meissner, K.; Nelle, M.; Lenhartz, H.; Leichsenring, M. Carotenoid supply in breast-fed and formula-fed neonates. Eur. J. Pediatr. 2000, 159, 86–90. [Google Scholar] [CrossRef]
- McManaman, J.L.; Neville, M.C. Mammary physiology and milk secretion. Adv. Drug. Deliv. Rev. 2003, 55, 629–641. [Google Scholar] [CrossRef]
- Reinhardt, T.A.; Lippolis, J.D. Developmental changes in the milk fat globule membrane proteome during the transition from colostrum to milk. J. Dairy Sci. 2008, 91, 2307–2318. [Google Scholar] [CrossRef]
- Abrahamse, E.; Minekus, M.; van Aken, G.A.; van de Heijning, B.; Knol, J.; Bartke, N.; Oozeer, R.; van der Beek, E.M.; Ludwig, T. Development of the digestive system-experimental challenges and approaches of infant lipid digestion. Food Dig. 2012, 3, 63–77. [Google Scholar] [CrossRef] [PubMed]
| Carotenoid | 25th Percentile | Median 1 | 75th Percentile |
|---|---|---|---|
| Colostrum 2 | |||
| Zeaxanthin + lutein 3 | 190 | 349 | 664 |
| β-cryptoxanthin | 195 | 285 | 464 |
| lycopene | 114 | 224 | 371 |
| α+β-carotene 4 | 133 | 241 | 265 |
| Mature Milk 5 | |||
| Zeaxanthin + lutein 3 | 26.7 | 43.9 | 67.0 |
| β-cryptoxanthin | 9.51 | 37.6 | 62.7 |
| lycopene | 6.79 | 11.6 | 19.8 |
| α+β-carotene 4 | 6.45 | 41.8 | 51.5 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xavier, A.A.O.; Garrido-López, J.E.; Aguayo-Maldonado, J.; Garrido-Fernández, J.; Fontecha, J.; Pérez-Gálvez, A. In Vitro Digestion of Human Milk: Influence of the Lactation Stage on the Micellar Carotenoids Content. Antioxidants 2019, 8, 291. https://doi.org/10.3390/antiox8080291
Xavier AAO, Garrido-López JE, Aguayo-Maldonado J, Garrido-Fernández J, Fontecha J, Pérez-Gálvez A. In Vitro Digestion of Human Milk: Influence of the Lactation Stage on the Micellar Carotenoids Content. Antioxidants. 2019; 8(8):291. https://doi.org/10.3390/antiox8080291
Chicago/Turabian StyleXavier, Ana A. O., Juan E. Garrido-López, Josefa Aguayo-Maldonado, Juan Garrido-Fernández, Javier Fontecha, and Antonio Pérez-Gálvez. 2019. "In Vitro Digestion of Human Milk: Influence of the Lactation Stage on the Micellar Carotenoids Content" Antioxidants 8, no. 8: 291. https://doi.org/10.3390/antiox8080291





