Spotlight on a New Heme Oxygenase Pathway: Testosterone-Induced Shifts in Cardiac Oxidant/Antioxidant Status
Abstract
1. Introduction
2. Materials and Methods
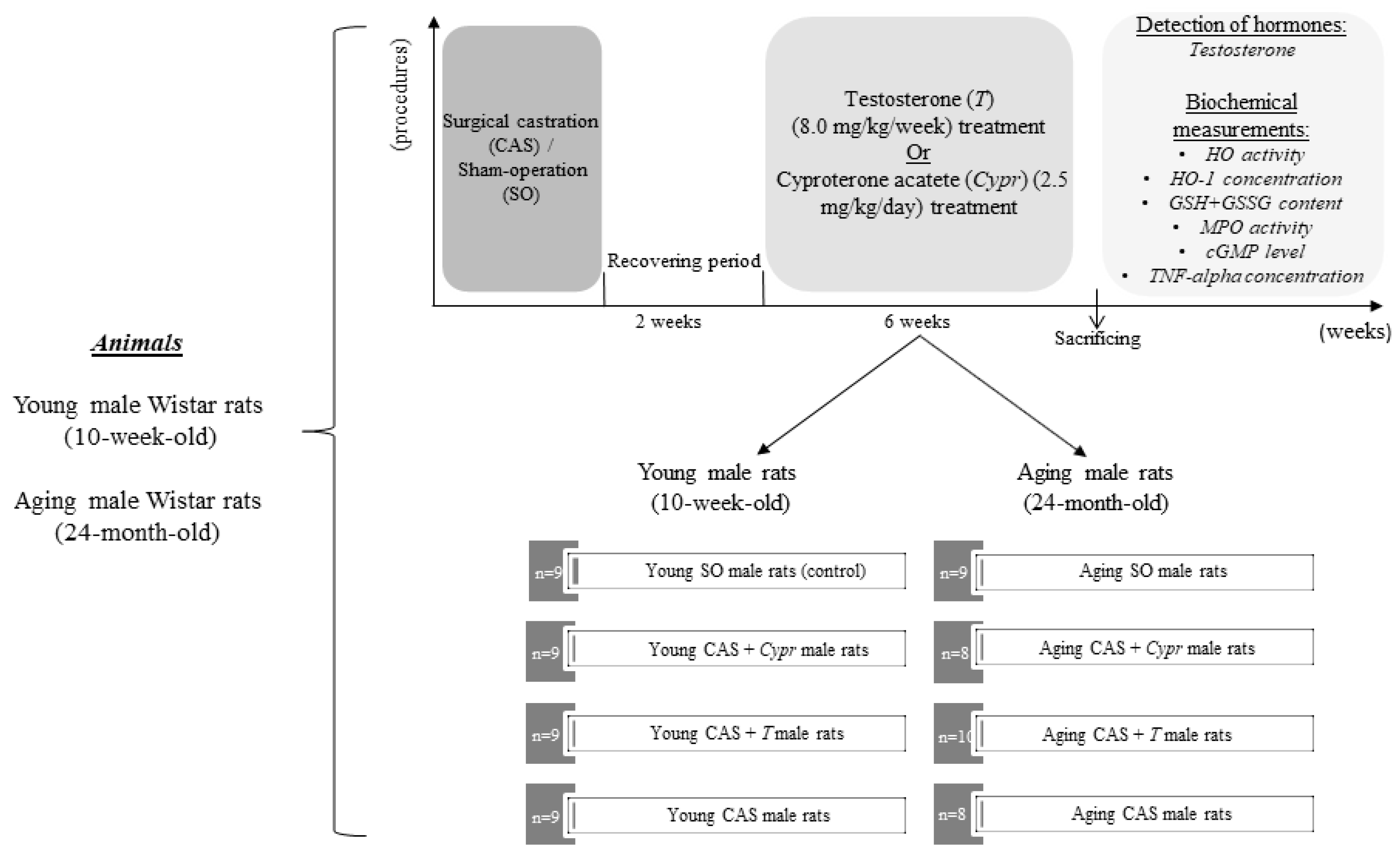
2.1. Experimental Protocol
2.2. Measurement of Serum Testosterone, GOT, GPT, Cholesterol and Triglyceride Levels
2.3. Measurement of HO Activity
2.4. Measurement of Cardiac GSH + GSSG Content
2.5. Determination of Cardiac HO-1, TNF-Alpha and cGMP Concentrations
2.6. Measurement of MPO Activity
2.7. Protein Determination
2.8. Statistical Analysis
3. Results
3.1. Changes in Serum Testosterone
3.2. Measurement of Cardiac HO Activity and HO-1 Concentration
3.3. Determination of Cardiac GSH + GSSG Content
3.4. Evaluation of Cardiac cGMP Level
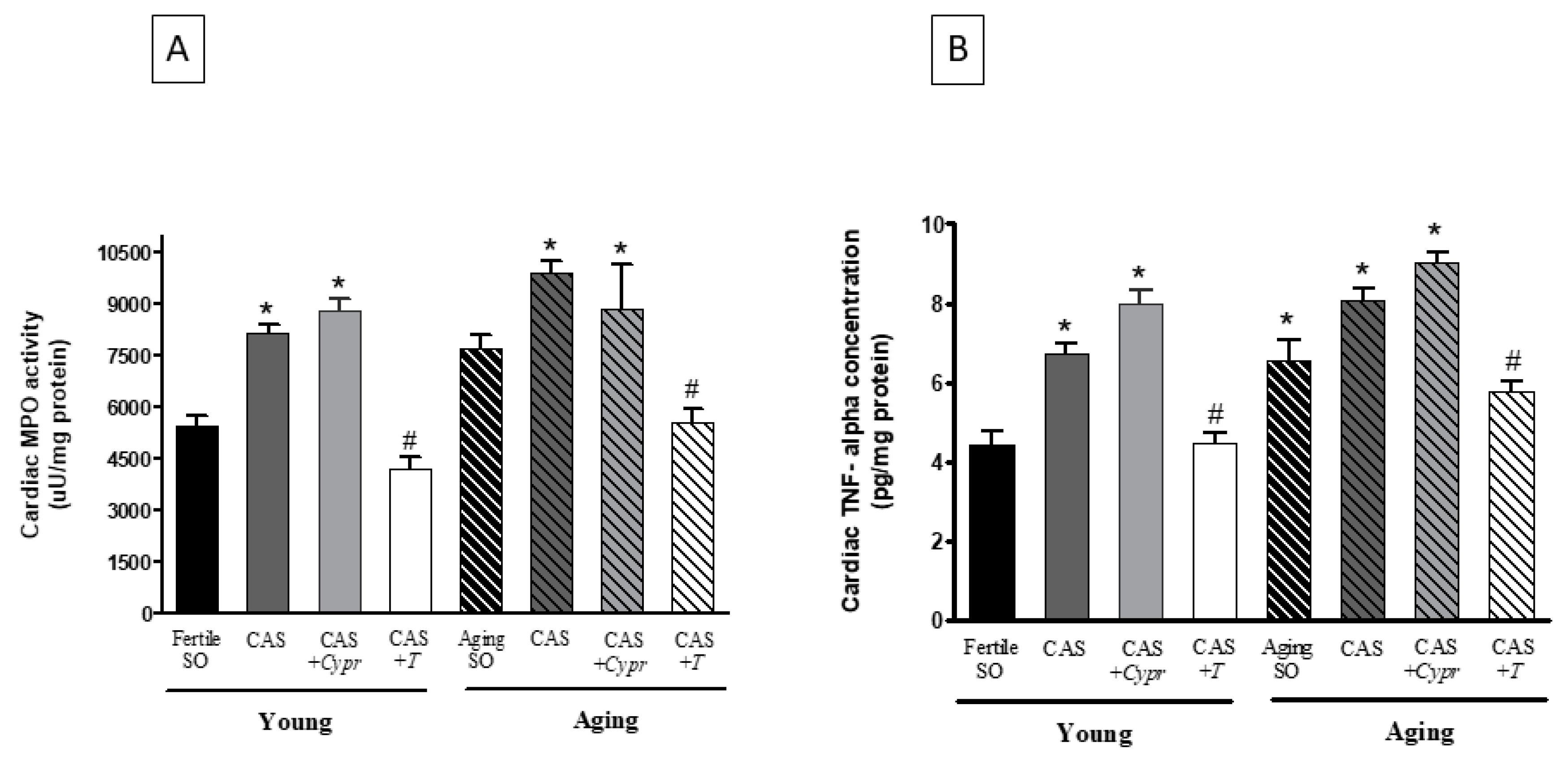
3.5. Cardiac MPO Activity
3.6. Cardiac TNF-α Concentration
3.7. Serum GOT, GPT, Cholesterol, and Triglyceride Concentrations
4. Discussion
Author Contributions
Funding
Conflicts of Interest
References
- Muraleedharan, V.; Jones, T.H. Testosterone and the metabolic syndrome. Ther. Adv. Endocrinol. Metab. 2010, 1, 207–223. [Google Scholar] [CrossRef] [PubMed]
- Herring, M.J.; Oskui, P.M.; Hale, S.L.; Kloner, R.A. Testosterone and the cardiovascular system: A comprehensive review of the basic science literature. J. Am. Heart Assoc. 2013, 2, e000271. [Google Scholar] [CrossRef] [PubMed]
- Lopes, R.A.; Neves, K.B.; Carneiro, F.S.; Tostes, R.C. Testosterone and vascular function in aging. Front Physiol 2012. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Vanhoutte, P.M.; Leung, S.W. Vascular nitric oxide: Beyond eNOS. J. Pharmacol. Sci. 2015, 129, 83–94. [Google Scholar] [CrossRef] [PubMed]
- Chignalia, A.Z.; Schuldt, E.Z.; Camargo, L.L.; Montezano, A.C.; Callera, G.E.; Laurindo, F.R.; Lopes, L.R.; Avellar, M.C.; Carvalho, M.H.; Fortes, Z.B.; et al. Testosterone induces vascular smooth muscle cell migration by NADPH oxidase and c-Src-dependent pathways. Hypertension 2012, 59, 1263–1271. [Google Scholar] [CrossRef] [PubMed]
- Lopes, R.A.; Neves, K.B.; Pestana, C.R.; Queiroz, A.L.; Zanotto, C.Z.; Chignalia, A.Z.; Valim, Y.M.; Silveira, L.R.; Curti, C.; Tostes, R.C. Testosterone induces apoptosis in vascular smooth muscle cells via extrinsic apoptotic pathway with mitochondria-generated reactive oxygen species involvement. Am. J. Physiol. Circ. Physiol. 2014, 306, H1485–H1494. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Wu, S.; Ruan, Y.; Hong, L.; Xing, X.; Lai, W. Testosterone suppresses oxidative stress via androgen receptor-independent pathway in murine cardiomyocytes. Mol. Med. Rep. 2011, 4, 1183–1188. [Google Scholar]
- Drummond, H.A.; Mitchell, Z.L.; Abraham, N.G.; Stec, D.E. Targeting Heme Oxygenase-1 in Cardiovascular and Kidney Disease. Antioxidants 2019, 8, 181. [Google Scholar] [CrossRef]
- Haines, D.D.; Lekli, I.; Teissier, P.; Bak, I.; Tosaki, A. Role of haeme oxygenase-1 in resolution of oxidative stress-related pathologies: Focus on cardiovascular, lung, neurological and kidney disorders. Acta Physiol (Oxf.) 2012, 204, 487–501. [Google Scholar] [CrossRef]
- Stocker, R.; Perrella, M.A. Heme oxygenase-1: A novel drug target for atherosclerotic diseases? Circulation 2006, 114, 2178–2189. [Google Scholar] [CrossRef]
- Sadowska-Krepa, E.; Klapcinska, B.; Jagsz, S.; Sobczak, A.; Chrapusta, S.J.; Chalimoniuk, M.; Grieb, P.; Poprzecki, S.; Langfort, J. High-dose testosterone propionate treatment reverses the effects of endurance training on myocardial antioxidant defenses in adolescent male rats. Cardiovasc Toxicol. 2011, 11, 118–127. [Google Scholar] [CrossRef]
- dos Santos, R.L.; da Silva, F.B.; Ribeiro, R.F.; Stefanon, I., Jr. Sex hormones in the cardiovascular system. Horm. Mol. Biol. Clin. Investig. 2014, 18, 89–103. [Google Scholar] [CrossRef]
- Posa, A.; Szabo, R.; Csonka, A.; Veszelka, M.; Berko, A.M.; Barath, Z.; Menesi, R.; Pavo, I.; Gyongyosi, M.; Laszlo, F.; et al. Endogenous Estrogen-Mediated Heme Oxygenase Regulation in Experimental Menopause. Oxid. Med. Cell. Longev. 2015, 2015, 429713. [Google Scholar] [CrossRef]
- Szabo, R.; Karacsonyi, Z.; Borzsei, D.; Juhasz, B.; Al-Awar, A.; Torok, S.; Berko, A.M.; Takacs, I.; Kupai, K.; Varga, C.; et al. Role of Exercise-Induced Cardiac Remodeling in Ovariectomized Female Rats. Oxid. Med. Cell. Longev. 2018, 2018, 6709742. [Google Scholar] [CrossRef] [PubMed]
- Goodale, T.; Sadhu, A.; Petak, S.; Robbins, R. Testosterone and the Heart. Methodist Debakey Cardiovasc. J. 2017, 13, 68–72. [Google Scholar] [CrossRef] [PubMed]
- Darbandi, M.; Darbandi, S.; Agarwal, A.; Sengupta, P.; Durairajanayagam, D.; Henkel, R.; Sadeghi, M.R. Reactive oxygen species and male reproductive hormones. Reprod. Biol. Endocrinol. 2018, 16, 87. [Google Scholar] [CrossRef] [PubMed]
- Posa, A.; Kupai, K.; Menesi, R.; Szalai, Z.; Szabo, R.; Pinter, Z.; Palfi, G.; Gyongyosi, M.; Berko, A.; Pavo, I.; et al. Sexual dimorphism of cardiovascular ischemia susceptibility is mediated by heme oxygenase. Oxid. Med. Cell. Longev. 2013, 2013, 521563. [Google Scholar] [CrossRef]
- Barta, T.; Tosaki, A.; Haines, D.; Balla, G.; Lekli, I.; Tosaki, A. Endothelin-1-induced hypertrophic alterations and heme oxygenase-1 expression in cardiomyoblasts are counteracted by beta estradiol: In vitro and in vivo studies. Naunyn Schmiedebergs Arch Pharmacol. 2018, 391, 371–383. [Google Scholar] [CrossRef]
- Juhasz, B.; Varga, B.; Czompa, A.; Bak, I.; Lekli, I.; Gesztelyi, R.; Zsuga, J.; Kemeny-Beke, A.; Antal, M.; Szendrei, L.; et al. Postischemic cardiac recovery in heme oxygenase-1 transgenic ischemic/reperfused mouse myocardium. J. Cell. Mol. Med. 2011, 15, 1973–1982. [Google Scholar] [CrossRef]
- Juhasz, B.; Kertesz, A.; Balla, J.; Balla, G.; Szabo, Z.; Bombicz, M.; Priksz, D.; Gesztelyi, R.; Varga, B.; Haines, D.D.; et al. Cardioprotective effects of sour cherry seed extract (SCSE) on the hypercholesterolemic rabbit heart. Curr. Pharm. Des. 2013, 19, 6896–6905. [Google Scholar] [CrossRef]
- Demirbag, R.; Yilmaz, R.; Erel, O. The association of total antioxidant capacity with sex hormones. Scand. Cardiovasc. J. 2005, 39, 172–176. [Google Scholar] [CrossRef]
- Liguori, I.; Russo, G.; Curcio, F.; Bulli, G.; Aran, L.; Della-Morte, D.; Gargiulo, G.; Testa, G.; Cacciatore, F.; Bonaduce, D.; et al. Oxidative stress, aging and diseases. Clin. Interv. Aging 2018, 13, 757–772. [Google Scholar] [CrossRef]
- Posa, A.; Szabo, R.; Kupai, K.; Berko, A.M.; Veszelka, M.; Szucs, G.; Borzsei, D.; Gyongyosi, M.; Pavo, I.; Deim, Z.; et al. Cardioprotective Effect of Selective Estrogen Receptor Modulator Raloxifene Are Mediated by Heme Oxygenase in Estrogen-Deficient Rat. Oxid. Med. Cell. Longev. 2017, 2017, 2176749. [Google Scholar] [CrossRef]
- Klapcinska, B.; Jagsz, S.; Sadowska-Krepa, E.; Gorski, J.; Kempa, K.; Langfort, J. Effects of castration and testosterone replacement on the antioxidant defense system in rat left ventricle. J. Physiol. Sci. 2008, 58, 173–177. [Google Scholar] [CrossRef]
- Militaru, C.; Donoiu, I.; Dracea, O.; Ionescu, D.D. Serum testosterone and short-term mortality in men with acute myocardial infarction. Cardiol. J. 2010, 17, 249–253. [Google Scholar]
- Malkin, C.J.; Pugh, P.J.; Morris, P.D.; Asif, S.; Jones, T.H.; Channer, K.S. Low serum testosterone and increased mortality in men with coronary heart disease. Heart 2010, 96, 1821–1825. [Google Scholar] [CrossRef]
- Muraleedharan, V.; Marsh, H.; Kapoor, D.; Channer, K.S.; Jones, T.H. Testosterone deficiency is associated with increased risk of mortality and testosterone replacement improves survival in men with type 2 diabetes. Eur. J. Endocrinol. 2013, 169, 725–733. [Google Scholar] [CrossRef]
- Mancini, A.; Leone, E.; Festa, R.; Grande, G.; Silvestrini, A.; de Marinis, L.; Pontecorvi, A.; Maira, G.; Littarru, G.P.; Meucci, E. Effects of testosterone on antioxidant systems in male secondary hypogonadism. J. Androl. 2008, 29, 622–629. [Google Scholar] [CrossRef]
- Golia, E.; Limongelli, G.; Natale, F.; Fimiani, F.; Maddaloni, V.; Pariggiano, I.; Bianchi, R.; Crisci, M.; D’Acierno, L.; Giordano, R.; et al. Inflammation and cardiovascular disease: From pathogenesis to therapeutic target. Curr. Atheroscler. Rep. 2014, 16, 435. [Google Scholar] [CrossRef]
- Bianchi, V.E. The Anti-Inflammatory Effects of Testosterone. J. Endocr. Soc. 2019, 3, 91–107. [Google Scholar] [CrossRef]
- Haring, R.; Baumeister, S.E.; Volzke, H.; Dorr, M.; Kocher, T.; Nauck, M.; Wallaschofski, H. Prospective inverse associations of sex hormone concentrations in men with biomarkers of inflammation and oxidative stress. J. Androl. 2012, 33, 944–950. [Google Scholar] [CrossRef]
- Kapturczak, M.H.; Wasserfall, C.; Brusko, T.; Campbell-Thompson, M.; Ellis, T.M.; Atkinson, M.A.; Agarwal, A. Heme oxygenase-1 modulates early inflammatory responses: Evidence from the heme oxygenase-1-deficient mouse. Am. J. Pathol. 2004, 165, 1045–1053. [Google Scholar] [CrossRef]
- Lucas-Herald, A.K.; Alves-Lopes, R.; Montezano, A.C.; Ahmed, S.F.; Touyz, R.M. Genomic and non-genomic effects of androgens in the cardiovascular system: Clinical implications. Clin. Sci. 2017, 131, 1405–1418. [Google Scholar] [CrossRef]
- Cai, J.J.; Wen, J.; Jiang, W.H.; Lin, J.; Hong, Y.; Zhu, Y.S. Androgen actions on endothelium functions and cardiovascular diseases. J. Geriatr. Cardiol. 2016, 13, 183–196. [Google Scholar]
- Paine, A.; Eiz-Vesper, B.; Blasczyk, R.; Immenschuh, S. Signaling to heme oxygenase-1 and its anti-inflammatory therapeutic potential. Biochem. Pharmacol. 2010, 80, 1895–1903. [Google Scholar] [CrossRef]
- Rizzo, N.O.; Maloney, E.; Pham, M.; Luttrell, I.; Wessells, H.; Tateya, S.; Daum, G.; Handa, P.; Schwartz, M.W.; Kim, F. Reduced NO-cGMP signaling contributes to vascular inflammation and insulin resistance induced by high-fat feeding. Arterioscler. Thromb. Vasc. Biol. 2010, 30, 758–765. [Google Scholar] [CrossRef]
- Rouver, W.N.; Delgado, N.T.; Menezes, J.B.; Santos, R.L.; Moyses, M.R. Testosterone Replacement Therapy Prevents Alterations of Coronary Vascular Reactivity Caused by Hormone Deficiency Induced by Castration. PLoS ONE 2015, 10, e0137111. [Google Scholar] [CrossRef][Green Version]


| Parameters | Young | Aging | ||||||
|---|---|---|---|---|---|---|---|---|
| Fertile | CAS | CAS + Cypr | CAS + T | SO | CAS | CAS + Cypr | CAS + T | |
| HO activity | 8 | 8 | 9 | 8 | 9 | 8 | 7 | 9 |
| HO-1 concentration | 5 | 6 | 4 | 5 | 5 | 5 | 5 | 5 |
| GSH + GSSG content | 9 | 9 | 8 | 9 | 8 | 8 | 8 | 9 |
| cGMP level | 6 | 5 | 4 | 5 | 8 | 6 | 5 | 9 |
| MPO activity | 8 | 8 | 8 | 8 | 7 | 8 | 7 | 10 |
| TNF-alpha concentration | 8 | 9 | 8 | 7 | 7 | 8 | 8 | 9 |
| Testosterone level | 5 | 6 | 4 | 7 | 7 | 6 | 7 | 8 |
| GOT level | 7 | 6 | 4 | 7 | 8 | 7 | 7 | 7 |
| GPT level | 7 | 4 | 4 | 7 | 8 | 4 | 6 | 8 |
| Cholesterol level | 7 | 6 | 4 | 7 | 8 | 7 | 6 | 7 |
| Triglyceride level | 7 | 5 | 4 | 5 | 8 | 6 | 6 | 6 |
| Young | Aging | |||||||
|---|---|---|---|---|---|---|---|---|
| Fertile | CAS | CSA + Cypr | CAS + T | SO | CAS | CAS + Cypr | CAS + T | |
| Testosterone (ng/dl) | 237.40 ± 36.58 | 0 ± 0 * | 0 ± 0 * | 149.86 ± 21.19 # | 95.90 ± 12.26 * | 0 ± 0 * | 0 ± 0 * | 364.50 ± 50.19 *,# |
| GOT (U/l) | 94.57 ± 3.02 | 106.67 ± 11.85 | 142.50 ± 23.50 | 105.14 ± 3.91 | 184.75 ± 18.72 * | 163.86 ± 6.11 * | 163.00 ± 10.64 * | 145.29 ± 13.03 |
| GPT (U/l) | 56.00 ± 1.60 | 5.00 ± 1.22 | 77.00 ± 6.23 | 59.57 ± 1.85 | 94.00 ± 10.10 * | 88.25 ± 3.66 * | 90.67 ± 6.38 * | 79.13 ± 5.11 |
| Cholesterol (mmol/l) | 2.20 ± 0.13 | 2.78 ± 0.13 | 3.09 ± 0.12 | 2.29 ± 0.08 | 3.86 ± 0.44 | 4.65 ± 0.63 * | 4.81 ± 0.86 * | 4.46 ± 0.51 * |
| Triglyceride (mmol/l) | 0.92 ± 0.13 | 1.01 ± 0.06 | 2.84 ± 0.39 * | 1.28 ± 0.13 | 1.62 ± 0.31 | 1.69 ± 0.27 | 1.75 ± 0.30 | 1.33 ± 0.15 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szabó, R.; Börzsei, D.; Kupai, K.; Hoffmann, A.; Gesztelyi, R.; Magyariné Berkó, A.; Varga, C.; Pósa, A. Spotlight on a New Heme Oxygenase Pathway: Testosterone-Induced Shifts in Cardiac Oxidant/Antioxidant Status. Antioxidants 2019, 8, 288. https://doi.org/10.3390/antiox8080288
Szabó R, Börzsei D, Kupai K, Hoffmann A, Gesztelyi R, Magyariné Berkó A, Varga C, Pósa A. Spotlight on a New Heme Oxygenase Pathway: Testosterone-Induced Shifts in Cardiac Oxidant/Antioxidant Status. Antioxidants. 2019; 8(8):288. https://doi.org/10.3390/antiox8080288
Chicago/Turabian StyleSzabó, Renáta, Denise Börzsei, Krisztina Kupai, Alexandra Hoffmann, Rudolf Gesztelyi, Anikó Magyariné Berkó, Csaba Varga, and Anikó Pósa. 2019. "Spotlight on a New Heme Oxygenase Pathway: Testosterone-Induced Shifts in Cardiac Oxidant/Antioxidant Status" Antioxidants 8, no. 8: 288. https://doi.org/10.3390/antiox8080288
APA StyleSzabó, R., Börzsei, D., Kupai, K., Hoffmann, A., Gesztelyi, R., Magyariné Berkó, A., Varga, C., & Pósa, A. (2019). Spotlight on a New Heme Oxygenase Pathway: Testosterone-Induced Shifts in Cardiac Oxidant/Antioxidant Status. Antioxidants, 8(8), 288. https://doi.org/10.3390/antiox8080288








