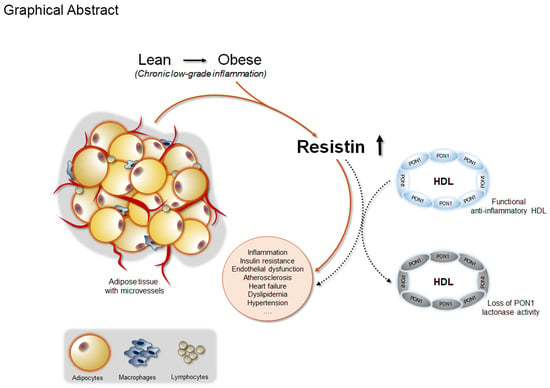
Crosstalk Between Adipokines and Paraoxonase 1: A New Potential Axis Linking Oxidative Stress and Inflammation
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Participants
2.2. Biochemical Assays
2.3. Adipokines Assessment
2.4. PON1 Activities
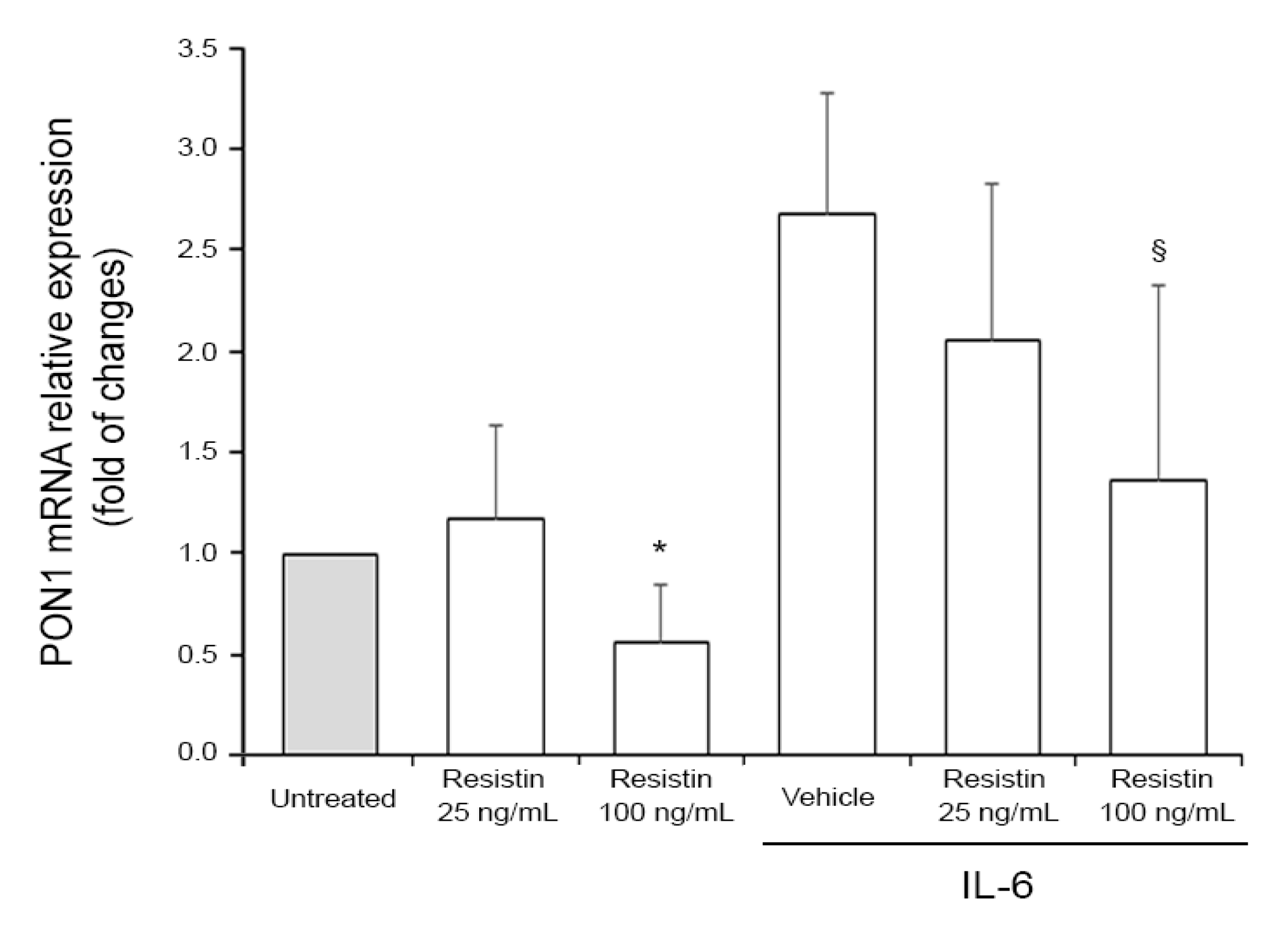
2.5. Cell Treatments and RT-PCR Analysis
2.6. Statistical Analysis
3. Results
Associations Between PON1 Activities and Adipokines: Study Population and In Vitro Assessment
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Furlong, C.E.; Marsillach, J.; Jarvik, G.P.; Costa, L.G. Paraoxonases-1, -2 and -3: What are their functions? Chem. Biol. Interact. 2016, 259, 51–62. [Google Scholar] [CrossRef] [PubMed]
- Cervellati, C.; Valacchi, G.; Tisato, V.; Zuliani, G.; Marsillach, J. Evaluating the link between Paraoxonase-1 levels and Alzheimer’s disease development. Minerva Med. 2019, 110, 238–250. [Google Scholar] [CrossRef] [PubMed]
- Levy, D.; Reichert, C.O.; Bydlowski, S.P. Paraoxonases Activities and Polymorphisms in Elderly and Old-Age Diseases: An Overview. Antioxidants 2019, 8, 118. [Google Scholar] [CrossRef] [PubMed]
- Mackness, M.; Mackness, B. Human paraoxonase-1 (PON1): Gene structure and expression, promiscuous activities and multiple physiological roles. Gene 2015, 567, 12–21. [Google Scholar] [CrossRef] [PubMed]
- Mineo, C.; Shaul, P.W. PON-dering differences in HDL function in coronary artery disease. J. Clin. Invest. 2011, 121, 2545–2548. [Google Scholar] [CrossRef] [PubMed]
- Cervellati, C.; Vigna, G.B.; Trentini, A.; Sanz, J.M.; Zimetti, F.; Dalla Nora, E.; Morieri, M.L.; Zuliani, G.; Passaro, A. Paraoxonase-1 activities in individuals with different HDL circulating levels: Implication in reverse cholesterol transport and early vascular damage. Atherosclerosis 2019, 285, 64–70. [Google Scholar] [CrossRef] [PubMed]
- Camps, J.; Marsillach, J.; Joven, J. The paraoxonases: Role in human diseases and methodological difficulties in measurement. Crit. Rev. Clin. Lab. Sci. 2009, 46, 83–106. [Google Scholar] [CrossRef] [PubMed]
- Cervellati, C.; Trentini, A.; Romani, A.; Bellini, T.; Bosi, C.; Ortolani, B.; Zurlo, A.; Passaro, A.; Seripa, D.; Zuliani, G. Serum paraoxonase and arylesterase activities of paraoxonase-1 (PON-1), mild cognitive impairment, and 2-year conversion to dementia: A pilot study. J. Neurochem. 2015, 135, 395–401. [Google Scholar] [CrossRef] [PubMed]
- Ritchie, S.A.; Connell, J.M.C. The link between abdominal obesity, metabolic syndrome and cardiovascular disease. Nutr. Metab. Cardiovasc. Dis. 2007, 17, 319–326. [Google Scholar] [CrossRef]
- Keaney, J.F.; Larson, M.G.; Vasan, R.S.; Wilson, P.W.F.; Lipinska, I.; Corey, D.; Massaro, J.M.; Sutherland, P.; Vita, J.A.; Benjamin, E.J. Obesity and systemic oxidative stress: Clinical correlates of oxidative stress in the Framingham study. Arterioscler. Thromb. Vasc. Biol. 2003, 23, 434–439. [Google Scholar] [CrossRef]
- Valacchi, G.; Virgili, F.; Cervellati, C.; Pecorelli, A. OxInflammation: From Subclinical Condition to Pathological Biomarker. Front. Physiol. 2018, 9, 858. [Google Scholar] [CrossRef] [PubMed]
- Thomàs-Moyà, E.; Gómez-Pérez, Y.; Fiol, M.; Gianotti, M.; Lladó, I.; Proenza, A.M. Gender related differences in paraoxonase 1 response to high-fat diet-induced oxidative stress. Obesity (Silver Spring) 2008, 16, 2232–2238. [Google Scholar] [CrossRef] [PubMed]
- Woudberg, N.J.; Goedecke, J.H.; Blackhurst, D.; Frias, M.; James, R.; Opie, L.H.; Lecour, S. Association between ethnicity and obesity with high-density lipoprotein (HDL) function and subclass distribution. Lipids Health Dis. 2016, 15, 92. [Google Scholar] [CrossRef] [PubMed]
- Cervellati, C.; Bonaccorsi, G.; Trentini, A.; Valacchi, G.; Sanz, J.M.; Squerzanti, M.; Spagnolo, M.; Massari, L.; Crivellari, I.; Greco, P.; et al. Paraoxonase, arylesterase and lactonase activities of paraoxonase-1 (PON1) in obese and severely obese women. Scand. J. Clin. Lab. Invest. 2018, 78, 18–24. [Google Scholar] [CrossRef] [PubMed]
- Huen, K.; Harley, K.; Beckman, K.; Eskenazi, B.; Holland, N. Associations of PON1 and genetic ancestry with obesity in early childhood. PLoS ONE 2013, 8, e62565. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharyya, T.; Nicholls, S.J.; Topol, E.J.; Zhang, R.; Yang, X.; Schmitt, D.; Fu, X.; Shao, M.; Brennan, D.M.; Ellis, S.G.; et al. Relationship of paraoxonase 1 (PON1) gene polymorphisms and functional activity with systemic oxidative stress and cardiovascular risk. JAMA 2008, 299, 1265–1276. [Google Scholar] [CrossRef]
- Van Himbergen, T.M.; van der Schouw, Y.T.; Voorbij, H.A.M.; van Tits, L.J.H.; Stalenhoef, A.F.H.; Peeters, P.H.M.; Roest, M. Paraoxonase (PON1) and the risk for coronary heart disease and myocardial infarction in a general population of Dutch women. Atherosclerosis 2008, 199, 408–414. [Google Scholar] [CrossRef]
- Costa, L.G.; Vitalone, A.; Cole, T.B.; Furlong, C.E. Modulation of paraoxonase (PON1) activity. Biochem. Pharmacol. 2005, 69, 541–550. [Google Scholar] [CrossRef]
- Kwon, H.; Pessin, J.E. Adipokines mediate inflammation and insulin resistance. Front. Endocrinol. (Lausanne) 2013, 4, 71. [Google Scholar] [CrossRef]
- Fain, J.N.; Madan, A.K.; Hiler, M.L.; Cheema, P.; Bahouth, S.W. Comparison of the release of adipokines by adipose tissue, adipose tissue matrix, and adipocytes from visceral and subcutaneous abdominal adipose tissues of obese humans. Endocrinology 2004, 145, 2273–2282. [Google Scholar] [CrossRef]
- Ouchi, N.; Parker, J.L.; Lugus, J.J.; Walsh, K. Adipokines in inflammation and metabolic disease. Nat. Rev. Immunol. 2011, 11, 85–97. [Google Scholar] [CrossRef] [PubMed]
- Cervellati, C.; Bonaccorsi, G.; Bergamini, C.M.; Fila, E.; Greco, P.; Valacchi, G.; Massari, L.; Gonelli, A.; Tisato, V. Association between circulatory levels of adipokines and bone mineral density in postmenopausal women. Menopause 2016, 23, 984–992. [Google Scholar] [CrossRef] [PubMed]
- Cervellati, C.; Pansini, F.S.; Bonaccorsi, G.; Pascale, G.; Bagni, B.; Castaldini, C.; Ferrazini, S.; Ridolfi, F.; Pedriali, M.; Guariento, A.; et al. Body mass index is a major determinant of abdominal fat accumulation in pre-, peri- and post-menopausal women. Gynecol. Endocrinol. 2009, 25, 413–417. [Google Scholar] [CrossRef] [PubMed]
- Ley, C.J.; Lees, B.; Stevenson, J.C. Sex- and menopause-associated changes in body-fat distribution. Am. J. Clin. Nutr. 1992, 55, 950–954. [Google Scholar] [CrossRef] [PubMed]
- Cervellati, C.; Pansini, F.S.; Bonaccorsi, G.; Bergamini, C.M.; Patella, A.; Casali, F.; Fantini, G.F.; Pascale, G.; Castaldini, C.; Ferrazzini, S.; et al. 17β-estradiol levels and oxidative balance in a population of pre-, peri-, and post-menopausal women. Gynecol. Endocrinol. 2011, 27, 1028–1032. [Google Scholar] [CrossRef] [PubMed]
- Tisato, V.; Zauli, G.; Gianesini, S.; Menegatti, E.; Brunelli, L.; Manfredini, R.; Zamboni, P.; Secchiero, P. Modulation of Circulating Cytokine-Chemokine Profile in Patients Affected by Chronic Venous Insufficiency Undergoing Surgical Hemodynamic Correction. J. Immunol. Res. 2014, 2014. [Google Scholar] [CrossRef] [PubMed]
- Hayek, J.; Cervellati, C.; Crivellari, I.; Pecorelli, A.; Valacchi, G. Lactonase Activity and Lipoprotein-Phospholipase A2as Possible Novel Serum Biomarkers for the Differential Diagnosis of Autism Spectrum Disorders and Rett Syndrome: Results from a Pilot Study. Oxid. Med. Cell. Longev. 2017, 2017, 5694058. [Google Scholar] [CrossRef] [PubMed]
- Zauli, G.; Tisato, V.; Melloni, E.; Volpato, S.; Cervellati, C.; Bonaccorsi, G.; Radillo, O.; Marci, R.; Secchiero, P. Inverse correlation between circulating levels of TNF-related apoptosis-inducing ligand and 17β-estradiol. J. Clin. Endocrinol. Metab. 2014, 99, 659–664. [Google Scholar] [CrossRef] [PubMed]
- Ponce-Ruiz, N.; Murillo-González, F.E.; Rojas-García, A.E.; Mackness, M.; Bernal-Hernández, Y.Y.; Barrón-Vivanco, B.S.; González-Arias, C.A.; Medina-Díaz, I.M. Transcriptional regulation of human Paraoxonase 1 by nuclear receptors. Chem. Biol. Interact. 2017, 268, 77–84. [Google Scholar] [CrossRef] [PubMed]
- Kumon, Y.; Suehiro, T.; Ikeda, Y.; Hashimoto, K. Human paraoxonase-1 gene expression by HepG2 cells is downregulated by interleukin-1β and tumor necrosis factor-α, but is upregulated by interleukin-6. Life Sci. 2003, 73, 2807–2815. [Google Scholar] [CrossRef]
- Cheng, C.-C.; Hsueh, C.-M.; Chen, C.-Y.; Chen, T.-H.; Hsu, S.-L. Interleukin-6 upregulates paraoxonase 1 gene expression via an AKT/NF-κB-dependent pathway. Biochem. Biophys. Res. Commun. 2013, 437, 55–61. [Google Scholar] [CrossRef] [PubMed]
- Pecorelli, A.; Cervellati, C.; Hayek, J.; Valacchi, G. OxInflammation in Rett syndrome. Int. J. Biochem. Cell Biol. 2016, 81, 246–253. [Google Scholar] [CrossRef] [PubMed]
- Mackness, B.; Hine, D.; Liu, Y.; Mastorikou, M.; Mackness, M. Paraoxonase-1 inhibits oxidised LDL-induced MCP-1 production by endothelial cells. Biochem. Biophys. Res. Commun. 2004, 318, 680–683. [Google Scholar] [CrossRef] [PubMed]
- Castellazzi, M.; Trentini, A.; Romani, A.; Valacchi, G.; Bellini, T.; Bonaccorsi, G.; Fainardi, E.; Cavicchio, C.; Passaro, A.; Zuliani, G.; et al. Decreased arylesterase activity of paraoxonase-1 (PON-1) might be a common denominator of neuroinflammatory and neurodegenerative diseases. Int. J. Biochem. Cell Biol. 2016, 81, 356–363. [Google Scholar] [CrossRef] [PubMed]
- Patra, S.K.; Singh, K.; Singh, R. Paraoxonase 1: A better atherosclerotic risk predictor than HDL in type 2 diabetes mellitus. Diabetes Metab. Syndr. 2013, 7, 108–111. [Google Scholar] [CrossRef] [PubMed]
- Huen, K.; Richter, R.; Furlong, C.; Eskenazi, B.; Holland, N. Validation of PON1 enzyme activity assays for longitudinal studies. Clin. Chim. Acta 2009, 402, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Passaro, A.; Vigna, G.B.; Romani, A.; Sanz, J.M.; Cavicchio, C.; Bonaccorsi, G.; Valacchi, G.; Cervellati, C. Distribution of Paraoxonase-1 (PON-1) and Lipoprotein Phospholipase A2 (Lp-PLA2) across Lipoprotein Subclasses in Subjects with Type 2 Diabetes. Oxid. Med. Cell. Longev. 2018, 2018, 1752940. [Google Scholar] [CrossRef] [PubMed]
- Aharoni, S.; Aviram, M.; Fuhrman, B. Paraoxonase 1 (PON1) reduces macrophage inflammatory responses. Atherosclerosis 2013, 228, 353–361. [Google Scholar] [CrossRef]
- Fülöp, P.; Harangi, M.; Seres, I.; Paragh, G. Paraoxonase-1 and adipokines: Potential links between obesity and atherosclerosis. Chem. Biol. Interact. 2016, 259, 388–393. [Google Scholar] [CrossRef]
- Beltowski, J.; Jamroz-Wiśniewska, A.; Borkowska, E.; Wójcicka, G. Differential effect of antioxidant treatment on plasma and tissue paraoxonase activity in hyperleptinemic rats. Pharmacol. Res. 2005, 51, 523–532. [Google Scholar] [CrossRef]
- Varga, Z.; Paragh, G.; Seres, I.; Kakuk, G.; Karanyi, Z.; Karpati, I.; Matyus, J.; Csongradi, E.; Juhasz, A.; Balla, J.; et al. Hyperleptinemia Is Not Responsible for Decreased Paraoxonase Activity in Hemodialysis Patients. Nephron Clin. Pract. 2006, 103, c114–c120. [Google Scholar] [CrossRef] [PubMed]
- Bajnok, L.; Seres, I.; Varga, Z.; Jeges, S.; Peti, A.; Karanyi, Z.; Juhasz, A.; Csongradi, E.; Mezosi, E.; Nagy, E.V.; et al. Relationship of endogenous hyperleptinemia to serum paraoxonase 1, cholesteryl ester transfer protein, and lecithin cholesterol acyltransferase in obese individuals. Metabolism 2007, 56, 1542–1549. [Google Scholar] [CrossRef] [PubMed]
- Bajnok, L.; Csongradi, E.; Seres, I.; Varga, Z.; Jeges, S.; Peti, A.; Karanyi, Z.; Juhasz, A.; Mezosi, E.; Nagy, E.V.; et al. Relationship of adiponectin to serum paraoxonase 1. Atherosclerosis 2008, 197, 363–367. [Google Scholar] [CrossRef] [PubMed]
- Koncsos, P.; Seres, I.; Harangi, M.; Illyés, I.; Józsa, L.; Gönczi, F.; Bajnok, L.; Paragh, G. Human paraoxonase-1 activity in childhood obesity and its relation to leptin and adiponectin levels. Pediatr. Res. 2010, 67, 309–313. [Google Scholar] [CrossRef]
- Bajnok, L.; Seres, I.; Varga, Z.; Jeges, S.; Peti, A.; Karanyi, Z.; Juhasz, A.; Csongradi, E.; Mezosi, E.; Nagy, E.; et al. Relationship of Serum Resistin Level to Traits of Metabolic Syndrome and Serum Paraoxonase 1 Activity in a Population with a Broad Range of Body Mass Index. Exp. Clin. Endocrinol. Diabetes 2008, 116, 592–599. [Google Scholar] [CrossRef]
- Bednarska-Makaruk, M.; Graban, A.; Wiśniewska, A.; Łojkowska, W.; Bochyńska, A.; Gugała-Iwaniuk, M.; Sławińska, K.; Ługowska, A.; Ryglewicz, D.; Wehr, H. Association of adiponectin, leptin and resistin with inflammatory markers and obesity in dementia. Biogerontology 2017, 18, 561–580. [Google Scholar] [CrossRef]
- Fernández-Sánchez, A.; Madrigal-Santillán, E.; Bautista, M.; Esquivel-Soto, J.; Morales-González, Á.; Esquivel-Chirino, C.; Durante-Montiel, I.; Sánchez-Rivera, G.; Valadez-Vega, C.; Morales-González, J.A. Inflammation, oxidative stress, and obesity. Int. J. Mol. Sci. 2011, 12, 3117–3132. [Google Scholar] [CrossRef]
- Oh, K.W.; Lee, W.Y.; Rhee, E.J.; Baek, K.H.; Yoon, K.H.; Kang, M.I.; Yun, E.J.; Park, C.Y.; Ihm, S.H.; Choi, M.G.; et al. The relationship between serum resistin, leptin, adiponectin, ghrelin levels and bone mineral density in middle-aged men. Clin. Endocrinol. (Oxf) 2005, 63, 131–138. [Google Scholar] [CrossRef]
- Aggarwal, G.; Prajapati, R.; Tripathy, R.K.; Bajaj, P.; Iyengar, A.R.S.; Sangamwar, A.T.; Pande, A.H. Toward Understanding the Catalytic Mechanism of Human Paraoxonase 1: Site-Specific Mutagenesis at Position 192. PLoS ONE 2016, 11, e0147999. [Google Scholar] [CrossRef]
- Marsillach, J.; Camps, J.; Ferré, N.; Beltran, R.; Rull, A.; Mackness, B.; Mackness, M.; Joven, J. Paraoxonase-1 is related to inflammation, fibrosis and PPAR delta in experimental liver disease. BMC Gastroenterol. 2009, 9, 3. [Google Scholar] [CrossRef]
- Nguyen, S.D.; Hung, N.D.; Cheon-Ho, P.; Ree, K.M.; Dai-Eun, S. Oxidative inactivation of lactonase activity of purified human paraoxonase 1 (PON1). Biochim. Biophys. Acta 2009, 1790, 155–160. [Google Scholar] [CrossRef] [PubMed]
| Demographic and Clinical Parameters | Value |
|---|---|
| Number of subjects, n | 107 |
| Age, years | 57 ± 4 |
| Years since menopause, years | 4 (2–6) |
| Smoking, n (%) | 8 (7) |
| Hypertension, n (%) | 10 (9) |
| BMI, kg/m2 | 24 ± 3 |
| Waist circumference, cm | 82 ± 10 |
| Total cholesterol, mmol/L | 5.1 ± 1.3 |
| HDL-C, mmol/L | 1.8 ± 0.6 |
| Triglycerides, mmol/L | 0.9 ± 0.6 |
| LDL-C, mmol/L | 3.0 ± 1.3 |
| hs-CRP, mg/dL | 1.3 (0.5–3.0) |
| Adipokines | Arylesterase | Paraoxonase | Log10 Lactonase |
|---|---|---|---|
| IL-8 | 0.265 a | −0.014 | 0.086 |
| TNF-α | 0.001 | 0.090 | −0.024 |
| IL-6 | −0.120 | 0.034 | −0.039 |
| Resistin | 0.069 | −0.102 | −0.322 b |
| Adiponectin | 0.085 | −0.049 | −0.152 |
| Leptin | 0.017 | 0.164 | 0.070 |
| MCP-1 | 0.022 | 0.012 | −0.022 |
| HGF | −0.037 | 0.053 | −0.007 |
| Dependent Variable | Predictor | β (p Value) | βage/HDL-C adjusted (p Value) | βfully adjusted * (p Value) |
|---|---|---|---|---|
| Arylesterase | IL-8 | 0.265 a | 0.221 | 0.185 |
| Lactonase | Resistin | −0.326 b | −0.344 b | −0.346 b |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tisato, V.; Romani, A.; Tavanti, E.; Melloni, E.; Milani, D.; Bonaccorsi, G.; Sanz, J.M.; Gemmati, D.; Passaro, A.; Cervellati, C. Crosstalk Between Adipokines and Paraoxonase 1: A New Potential Axis Linking Oxidative Stress and Inflammation. Antioxidants 2019, 8, 287. https://doi.org/10.3390/antiox8080287
Tisato V, Romani A, Tavanti E, Melloni E, Milani D, Bonaccorsi G, Sanz JM, Gemmati D, Passaro A, Cervellati C. Crosstalk Between Adipokines and Paraoxonase 1: A New Potential Axis Linking Oxidative Stress and Inflammation. Antioxidants. 2019; 8(8):287. https://doi.org/10.3390/antiox8080287
Chicago/Turabian StyleTisato, Veronica, Arianna Romani, Elisa Tavanti, Elisabetta Melloni, Daniela Milani, Gloria Bonaccorsi, Juana M. Sanz, Donato Gemmati, Angelina Passaro, and Carlo Cervellati. 2019. "Crosstalk Between Adipokines and Paraoxonase 1: A New Potential Axis Linking Oxidative Stress and Inflammation" Antioxidants 8, no. 8: 287. https://doi.org/10.3390/antiox8080287
APA StyleTisato, V., Romani, A., Tavanti, E., Melloni, E., Milani, D., Bonaccorsi, G., Sanz, J. M., Gemmati, D., Passaro, A., & Cervellati, C. (2019). Crosstalk Between Adipokines and Paraoxonase 1: A New Potential Axis Linking Oxidative Stress and Inflammation. Antioxidants, 8(8), 287. https://doi.org/10.3390/antiox8080287