Therapeutic Perspective of Vitamin C and Its Derivatives
Abstract
:1. Introduction: Vitamin C and Its Antioxidant and Antitumor Activity
2. Antioxidant Properties of l-Ascorbic Acid Derivatives
2.1. Lipophilic ASA Derivatives with Modified Hydroxyl Groups
2.2. Glucoside ASA Derivatives
2.3. Conjugates of Vitamin C and E
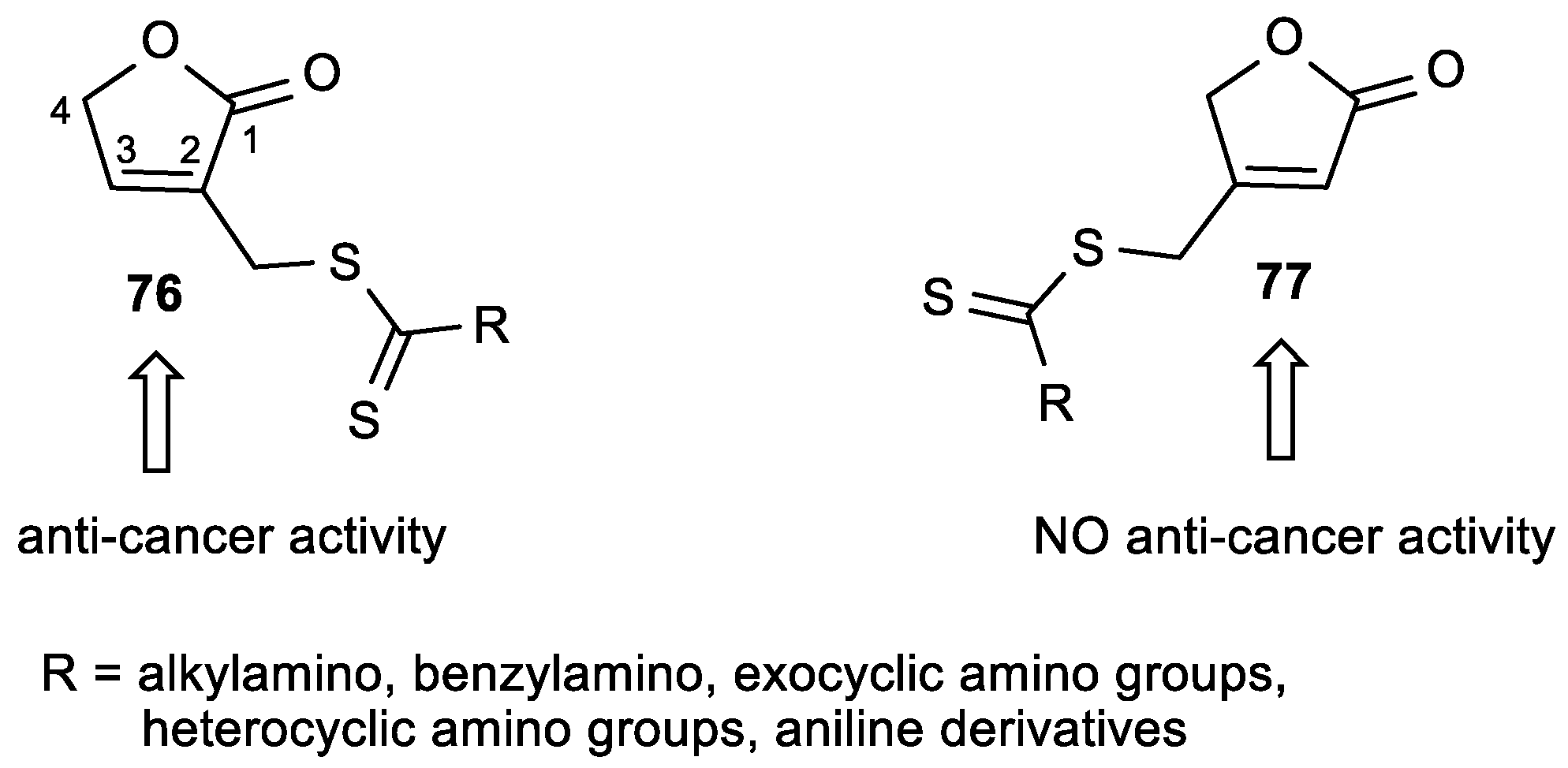
2.4. Butenolide Derivatives
3. Antitumor and Antiviral Activities of l-Ascorbic Acid Derivatives
3.1. Lipophilic ASA Derivatives with Modified Hydroxyl Groups
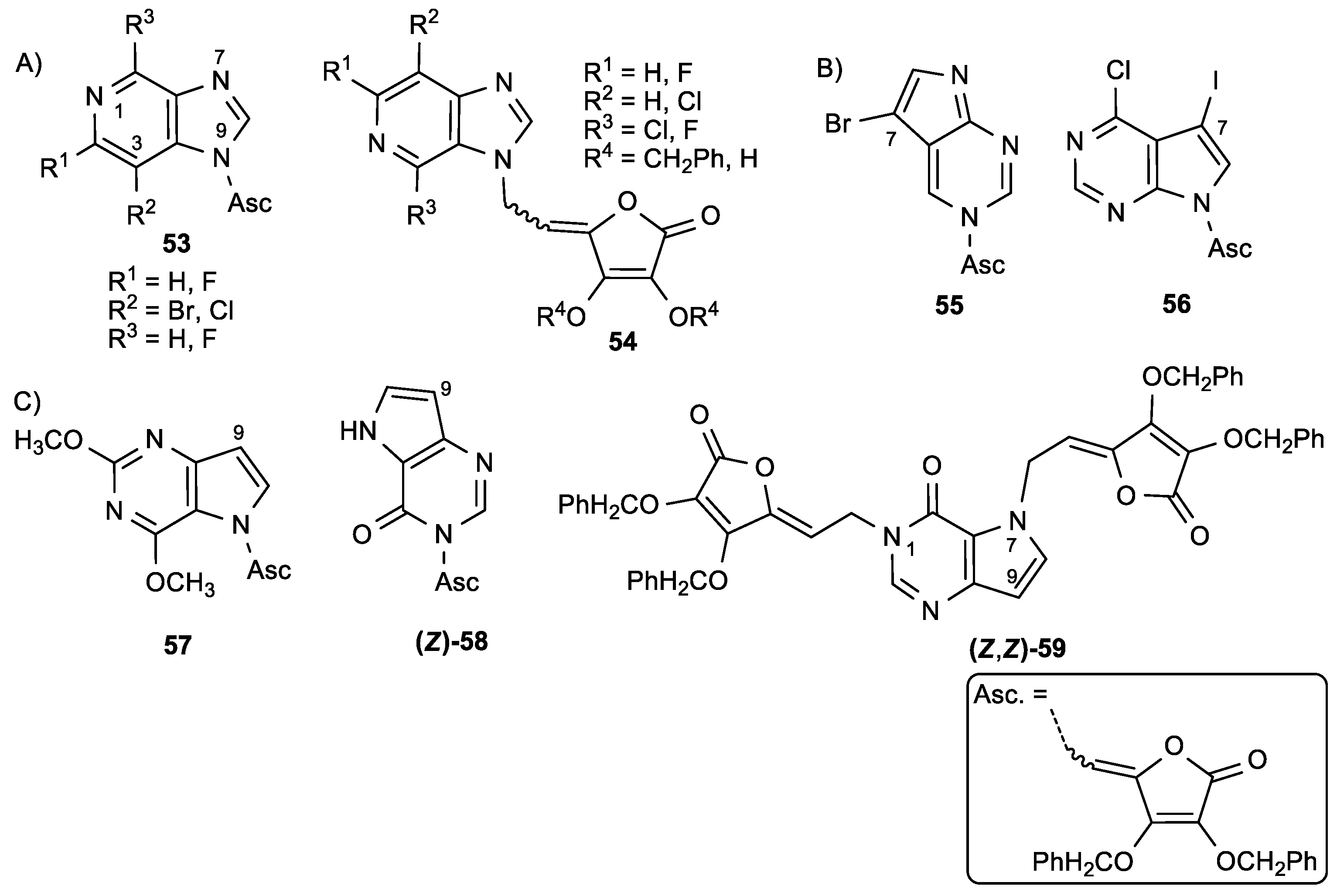
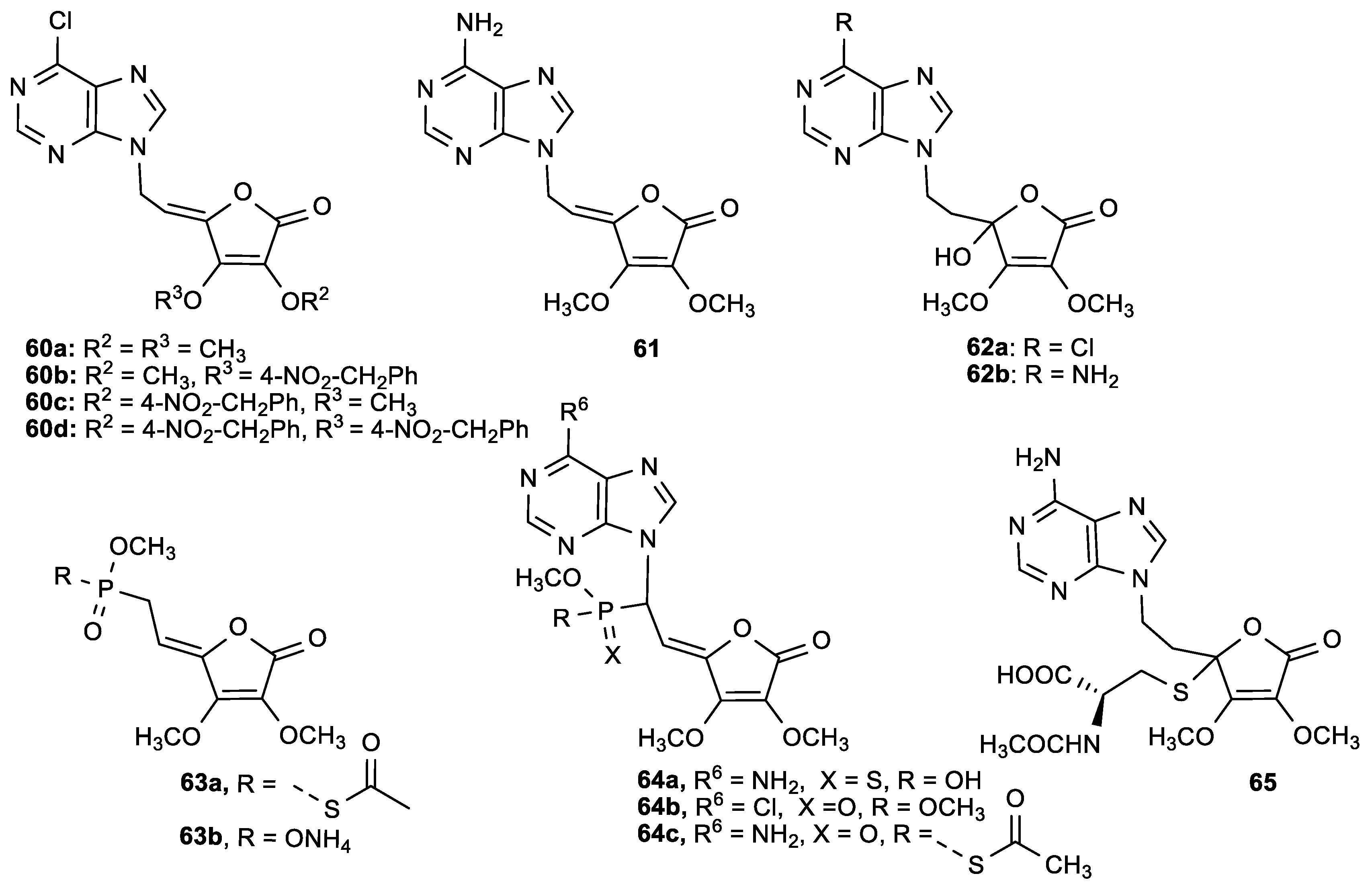
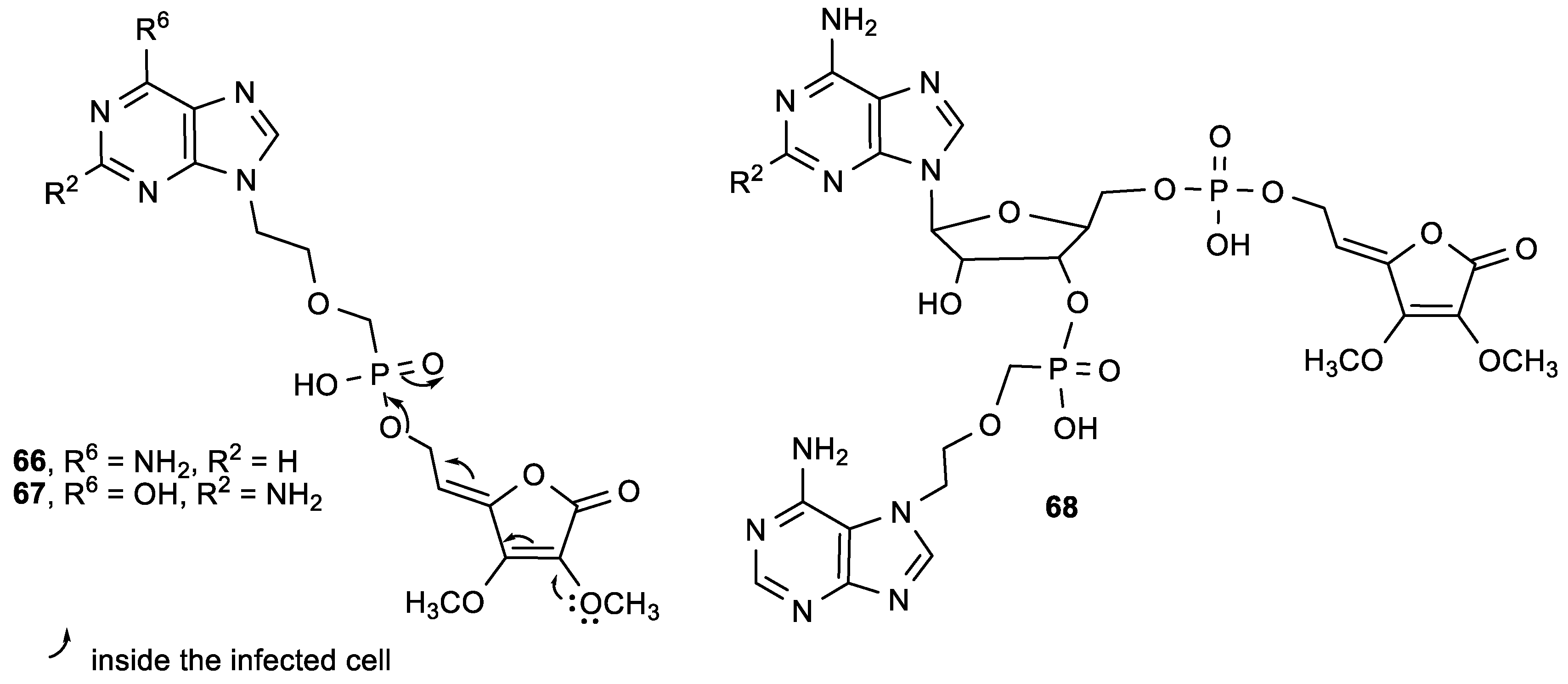
3.2. Conjugates of ASA Derivative and Pyrimidine and Purine Base
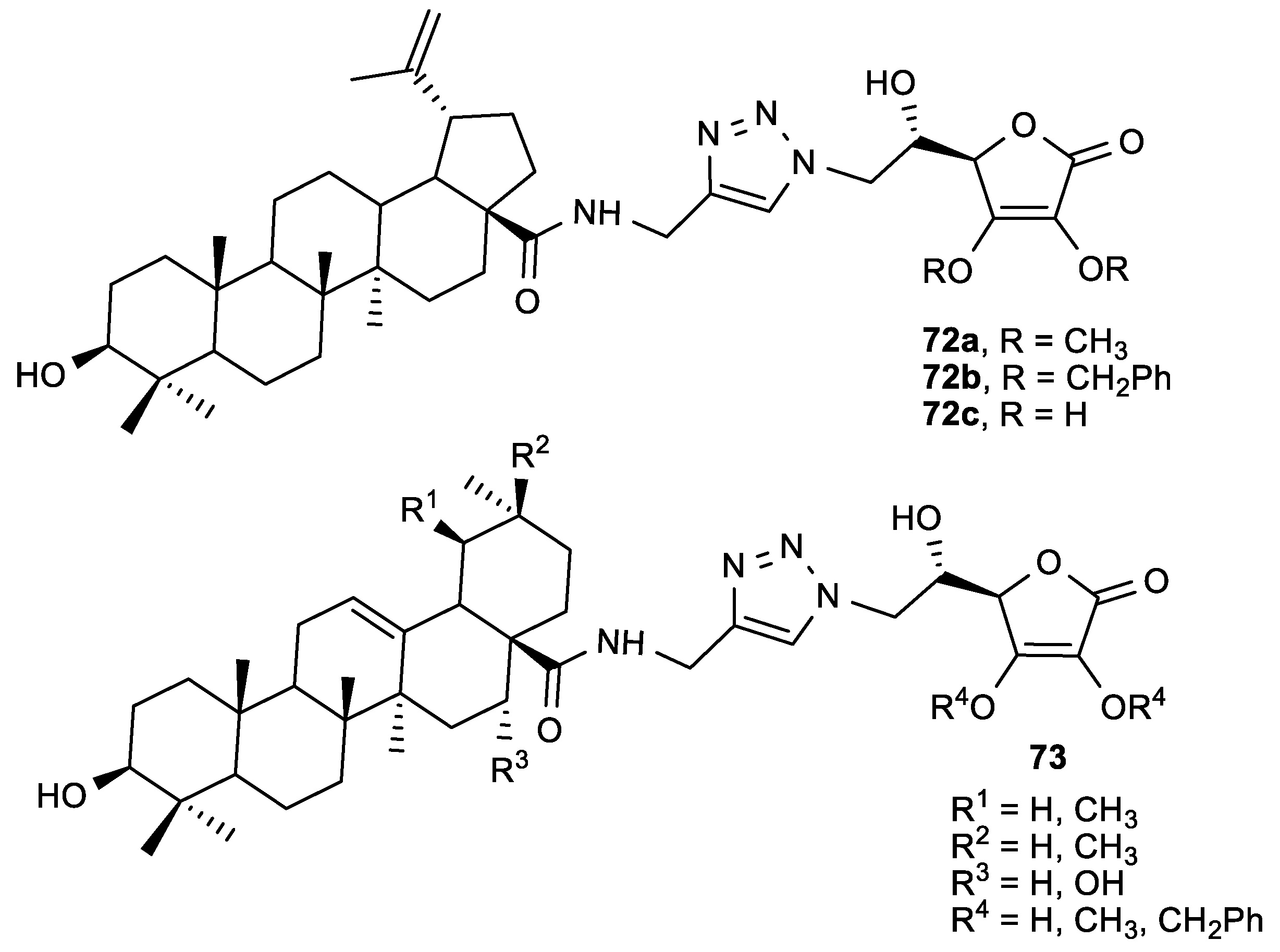
3.3. Conjugates of ASA Derivative and Triazole and Imidazole Moiety
3.4. Conjugates of ASA Derivative and Triterpene
3.5. Butenolide Derivatives
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Svirbely, J.L.; Szent-Györgyi, A. The chemical nature of vitamin C. Biochem. J. 1932, 26, 865–870. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ryan, M.J.; Dudash, H.J.; Docherty, M.; Geronilla, K.B.; Baker, B.A.; Haff, G.G.; Cutlip, R.G.; Alway, S.E. Vitamin E and C supplementation reduces oxidative stress, improves antioxidant enzymes and positive muscle work in chronically loaded muscles of aged rats. Exp. Gerontol. 2010, 45, 882–895. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Monacelli, F.; Acquarone, E.; Giannotti, C.; Borghi, R.; Nencioni, A. Vitamin C, Aging and Alzheimer’s Disease. Nutrients 2017, 9, 670. [Google Scholar] [CrossRef] [PubMed]
- Fang, Y.-Z.; Yang, S.; Wu, G. Free radicals, antioxidants, and nutrition. Nutrition 2002, 18, 872–879. [Google Scholar] [CrossRef]
- Ames, B.N.; Shigenaga, M.K.; Hagen, T.M. Oxidants, antioxidants, and the degenerative diseases of aging. Proc. Natl. Acad. Sci. USA 1993, 90, 7915–7922. [Google Scholar] [CrossRef]
- Burger, M.; Steinitz, A.; Geurts, J.; Pippenger, B.; Schaefer, D.; Martin, I.; Barbero, A.; Pelttari, K. Ascorbic Acid Attenuates Senescence of Human Osteoarthritic Osteoblasts. Int. J. Mol. Sci. 2017, 18, 2517. [Google Scholar] [CrossRef]
- Padayatty, S.J.; Katz, A.; Wang, Y.; Eck, P.; Kwon, O.; Lee, J.H.; Chen, S.; Corpe, C.; Levine, M.; Dutta, A.; et al. Vitamin C as an Antioxidant: Evaluation of Its Role in Disease Prevention. J. Am. Coll. Nutr. 2003, 22, 18–35. [Google Scholar] [CrossRef]
- Padayatty, S.J.; Sun, H.; Wang, Y.; Riordan, H.D.; Hewitt, S.M.; Katz, A.; Wesley, R.A.; Levine, M. Vitamin C Pharmacokinetics: Implications for Oral and Intravenous Use. Ann. Intern. Med. 2004, 140, 533–537. [Google Scholar] [CrossRef]
- Chen, Q.; Espey, M.G.; Sun, A.Y.; Lee, J.-H.; Krishna, M.C.; Shacter, E.; Choyke, P.L.; Pooput, C.; Kirk, K.L.; Buettner, G.R.; et al. Ascorbate in pharmacologic concentrations selectively generates ascorbate radical and hydrogen peroxide in extracellular fluid in vivo. Proc. Natl. Acad. Sci. USA 2007, 104, 8749–8754. [Google Scholar] [CrossRef]
- Chen, Q.; Espey, M.G.; Sun, A.Y.; Pooput, C.; Kirk, K.L.; Krishna, M.C.; Khosh, D.B.; Drisko, J.; Levine, M. Pharmacologic doses of ascorbate act as a prooxidant and decrease growth of aggressive tumor xenografts in mice. Proc. Natl. Acad. Sci. USA 2008, 105, 11105–11109. [Google Scholar] [CrossRef] [Green Version]
- Fenton, H.J.H. LXXIII.—Oxidation of tartaric acid in presence of iron. J. Chem. Soc. Trans. 1894, 65, 899–910. [Google Scholar] [CrossRef]
- Walling, C. Fenton’s reagent revisited. Acc. Chem. Res. 1975, 8, 125–131. [Google Scholar] [CrossRef]
- Buettner, G.R.; Jurkiewicz, B.A. Catalytic metals, ascorbate and free radicals: Combinations to avoid. Radiat. Res. 1996, 145, 532–541. [Google Scholar] [CrossRef] [PubMed]
- Potters, G.; De Gara, L.; Asard, H.; Horemans, N. Ascorbate and glutathione: Guardians of the cell cycle, partners in crime? Plant. Physiol. Biochem. 2002, 40, 537–548. [Google Scholar] [CrossRef]
- Halliwell, B. The wanderings of a free radical. Free Radic. Biol. Med. 2009, 46, 531–542. [Google Scholar] [CrossRef] [PubMed]
- Foyer, C.H.; Noctor, G. Ascorbate and Glutathione: The Heart of the Redox Hub. Plant. Physiol. 2011, 155, 2–18. [Google Scholar] [CrossRef] [Green Version]
- Thiele, N.A.; McGowan, J.; Sloan, K.B. 2-o-Acyl-3-o-(1-acyloxyalkyl) prodrugs of 5,6-isopropylidene-l-ascorbic acid and l-ascorbic acid: Antioxidant activity and ability to permeate silicone membranes. Pharmaceutics 2016, 8, 22. [Google Scholar] [CrossRef]
- Du, J.; Cullen, J.J.; Buettner, G.R. Ascorbic acid: Chemistry, biology and the treatment of cancer. Biochim. Biophys. Acta 2012, 1826, 443–457. [Google Scholar] [CrossRef] [Green Version]
- Buettner, G.R.; Jurkiewicz, B.A. Ascorbate free radical as a marker of oxidative stress: An EPR study. Free Radic. Biol. Med. 1993, 14, 49–55. [Google Scholar] [CrossRef]
- Corpe, C.P.; Lee, J.H.; Kwon, O.; Eck, P.; Narayanan, J.; Kirk, K.L.; Levine, M. 6-Bromo-6-deoxy-L-ascorbic acid: An ascorbate analog specific for Na+-dependent vitamin C transporter but not glucose transporter pathways. J. Biol. Chem. 2005, 280, 5211–5220. [Google Scholar] [CrossRef]
- Azzolini, C.; Fiorani, M.; Cerioni, L.; Guidarelli, A.; Cantoni, O. Sodium-dependent transport of ascorbic acid in U937 cell mitochondria. IUBMB Life 2013, 65, 149–153. [Google Scholar] [CrossRef]
- Pires, A.S.; Marques, C.R.; Encarnação, J.C.; Abrantes, A.M.; Mamede, A.C.; Laranjo, M.; Gonçalves, A.C.; Sarmento-Ribeiro, A.B.; Botelho, M.F. Ascorbic acid and colon cancer: An oxidative stimulus to cell death depending on cell profile. Eur. J. Cell Biol. 2016, 95, 208–218. [Google Scholar] [CrossRef]
- Chow, C.-W.; Herrera Abreu, M.T.; Suzuki, T.; Downey, G.P. Oxidative Stress and Acute Lung Injury. Am. J. Respir. Cell Mol. Biol. 2003, 29, 427–431. [Google Scholar] [CrossRef]
- Galley, H.F.; Davies, M.J.; Webster, N.R. Ascorbyl radical formation in patients with sepsis: Effect of ascorbate loading. Free Radic. Biol. Med. 1996, 20, 139–143. [Google Scholar] [CrossRef]
- Borrelli, E.; Roux-Lombard, P.; Grau, G.E.; Girardin, E.; Ricou, B.; Dayer, J.; Suter, P.M. Plasma concentrations of cytokines, their soluble receptors, and antioxidant vitamins can predict the development of multiple organ failure in patients at risk. Crit. Care Med. 1996, 24, 392–397. [Google Scholar] [CrossRef]
- Marik, P.E. Patterns of Death in Patients with Sepsis and the Use of Hydrocortisone, Ascorbic Acid, and Thiamine to Prevent These Deaths. Surg. Infect. 2018, 19, 812–820. [Google Scholar] [CrossRef]
- Zhang, M.; Jativa, D.F. Vitamin C supplementation in the critically ill: A systematic review and meta-analysis. SAGE Open Med. 2018, 6, 1–12. [Google Scholar] [CrossRef]
- Fowler, A.A., III; Kim, C.; Lepler, L.; Malhotra, R.; Debesa, O.; Natarajan, R.; Fisher, B.J.; Syed, A.; DeWilde, C.; Priday, A.; et al. Intravenous vitamin C as adjunctive therapy for enterovirus/rhinovirus induced acute respiratory distress syndrome. World J. Crit. Care Med. 2017, 6, 85. [Google Scholar] [CrossRef]
- Bharara, A.; Grossman, C.; Grinnan, D.; Syed, A.; Fisher, B.; DeWilde, C.; Natarajan, R.; Fowler, A.A. Intravenous Vitamin C Administered as Adjunctive Therapy for Recurrent Acute Respiratory Distress Syndrome. Case Rep. Crit. Care 2016, 2016, 1–4. [Google Scholar] [CrossRef] [Green Version]
- Kim, C.; Debesa, O.; Nicolato, P.; Fisher, B.; Natarajan, R.; Alsbury Fowler, A.; Fowler, A.A. Vitamin C Infusion for Gastric Acid Aspiration-Induced Acute Respiratory Distress Syndrome (ARDS). Pulm Res. Respir. Med. Open J. 2017, 4, 33–37. [Google Scholar] [CrossRef]
- Pauling, L. Respect for vitamin C. Science 1991, 254, 1712. [Google Scholar]
- Cameron, E.; Pauling, L. Supplemental ascorbate in the supportive treatment of cancer: Prolongation of survival times in terminal human cancer. Proc. Natl. Acad. Sci. USA 1976, 73, 3685–3689. [Google Scholar] [CrossRef]
- Cameron, E.; Pauling, L. Supplemental ascorbate in the supportive treatment of cancer: Reevaluation of prolongation of survival times in terminal human cancer. Proc. Natl. Acad. Sci. USA 1978, 75, 4538–4542. [Google Scholar] [CrossRef] [Green Version]
- Creagan, E.T.; Moertel, C.G.; O’Fallon, J.R.; Schutt, A.J.; O’Connell, M.J.; Rubin, J.; Frytak, S. Failure of High-Dose Vitamin C (Ascorbic Acid) Therapy to Benefit Patients with Advanced Cancer. N. Engl. J. Med. 1979, 301, 687–690. [Google Scholar] [CrossRef]
- Moertel, C.G.; Fleming, T.R.; Creagan, E.T.; Rubin, J.; O’Connell, M.J.; Ames, M.M. High-Dose Vitamin C versus Placebo in the Treatment of Patients with Advanced Cancer Who Have Had No Prior Chemotherapy. N. Engl. J. Med. 1985, 312, 137–141. [Google Scholar] [CrossRef]
- Nauman, G.; Gray, J.; Parkinson, R.; Levine, M.; Paller, C.; Nauman, G.; Gray, J.C.; Parkinson, R.; Levine, M.; Paller, C.J. Systematic Review of Intravenous Ascorbate in Cancer Clinical Trials. Antioxidants 2018, 7, 89. [Google Scholar] [CrossRef]
- Fritz, H.; Flower, G.; Weeks, L.; Cooley, K.; Callachan, M.; McGowan, J.; Skidmore, B.; Kirchner, L.; Seely, D. Intravenous vitamin C and cancer: A systematic review. Integr. Cancer Ther. 2014, 13, 280–300. [Google Scholar] [CrossRef]
- Levine, M.; Conry-Cantilena, C.; Wang, Y.; Welch, R.W.; Washko, P.W.; Dhariwal, K.R.; Park, J.B.; Lazarev, A.; Graumlich, J.F.; King, J.; et al. Vitamin C pharmacokinetics in healthy volunteers: Evidence for a recommended dietary allowance. Proc. Natl. Acad. Sci. USA 1996, 93, 3704–3709. [Google Scholar] [CrossRef]
- Stephenson, C.M.; Levin, R.D.; Spector, T.; Lis, C.G. Phase I clinical trial to evaluate the safety, tolerability, and pharmacokinetics of high-dose intravenous ascorbic acid in patients with advanced cancer. Cancer Chemother. Pharmacol. 2013, 72, 139–146. [Google Scholar] [CrossRef] [Green Version]
- Monti, D.A.; Mitchell, E.; Bazzan, A.J.; Littman, S.; Zabrecky, G.; Yeo, C.J.; Pillai, M.V.; Newberg, A.B.; Deshmukh, S.; Levine, M. Phase I Evaluation of Intravenous Ascorbic Acid in Combination with Gemcitabine and Erlotinib in Patients with Metastatic Pancreatic Cancer. PLoS ONE 2012, 7, e29794. [Google Scholar] [CrossRef]
- Welsh, J.L.; Wagner, B.A.; van’t Erve, T.J.; Zehr, P.S.; Berg, D.J.; Halfdanarson, T.R.; Yee, N.S.; Bodeker, K.L.; Du, J.; Roberts, L.J.; et al. Pharmacological ascorbate with gemcitabine for the control of metastatic and node-positive pancreatic cancer (PACMAN): Results from a phase I clinical trial. Cancer Chemother. Pharmacol. 2013, 71, 765–775. [Google Scholar] [CrossRef]
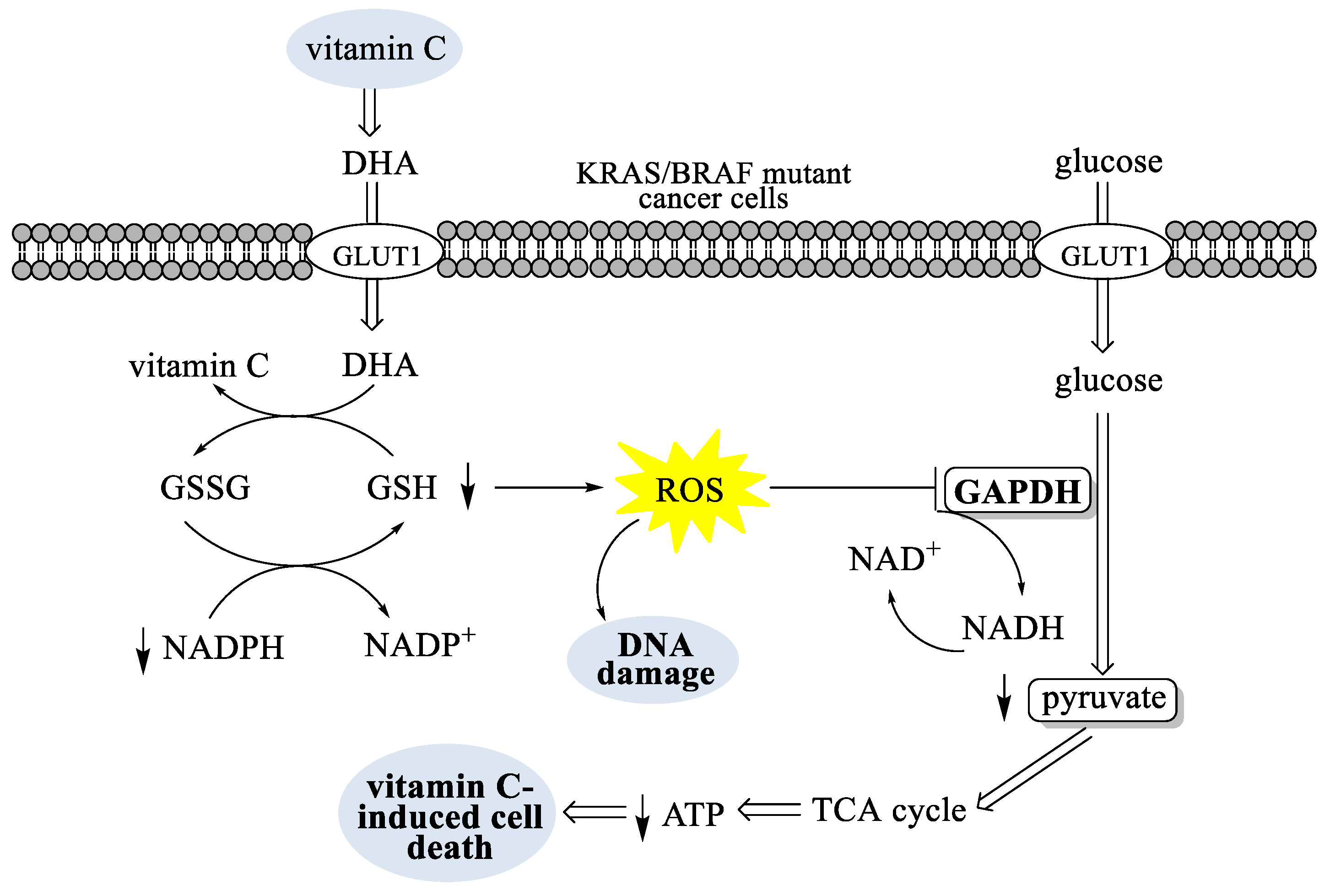
- Yun, J.; Mullarky, E.; Lu, C.; Bosch, K.N.; Kavalier, A.; Rivera, K.; Roper, J.; Chio, I.I.C.; Giannopoulou, E.G.; Rago, C.; et al. Vitamin C selectively kills KRAS and BRAF mutant colorectal cancer cells by targeting GAPDH. Science 2015, 350, 1391–1396. [Google Scholar] [CrossRef]
- Kaiser, J. Vitamin C could target some common cancers. Science 2015, 350, 619. [Google Scholar] [CrossRef]
- Drisko, J.A.; Serrano, O.K.; Spruce, L.R.; Chen, Q.; Levine, M. Treatment of pancreatic cancer with intravenous vitamin C. Anticancer. Drugs 2018, 29, 373–379. [Google Scholar] [CrossRef]
- Cieslak, J.A.; Cullen, J.J. Treatment of Pancreatic Cancer with Pharmacological Ascorbate. Curr. Pharm. Biotechnol. 2015, 16, 759–770. [Google Scholar] [CrossRef]
- Hong, S.W.; Jin, D.H.; Hahm, E.S.; Yim, S.H.; Lim, J.S.; Kim, K.I.; Yang, Y.; Lee, S.S.; Kang, J.S.; Lee, W.J.; et al. Ascorbate (vitamin C) induces cell death through the apoptosis-inducing factor in human breast cancer cells. Oncol. Rep. 2007, 18, 811–815. [Google Scholar] [CrossRef] [Green Version]
- Uetaki, M.; Tabata, S.; Nakasuka, F.; Soga, T.; Tomita, M. Metabolomic alterations in human cancer cells by Vitamin C-induced oxidative stress. Sci. Rep. 2015, 5, 1–9. [Google Scholar] [CrossRef]
- Kim, T.J.; Byun, J.S.; Kwon, H.S.; Kim, D.Y. Cellular toxicity driven by high-dose vitamin C on normal and cancer stem cells. Biochem. Biophys. Res. Commun. 2018, 497, 347–353. [Google Scholar] [CrossRef]
- Blaszczak, W.; Barczak, W.; Masternak, J.; Kopczyński, P.; Zhitkovich, A.; Rubiś, B. Vitamin C as a Modulator of the Response to Cancer Therapy. Molecules 2019, 24, 453. [Google Scholar] [CrossRef]
- Vissers, M.C.M.; Das, A.B. Potential Mechanisms of Action for Vitamin C in Cancer: Reviewing the Evidence. Front. Physiol. 2018, 9, 809. [Google Scholar] [CrossRef] [Green Version]
- Knowles, H.J.; Raval, R.R.; Harris, A.L.; Ratcliffe, P.J. Effect of ascorbate on the activity of hypoxia-inducible factor in cancer cells. Cancer Res. 2003, 63, 1764–1768. [Google Scholar] [PubMed]
- Kawada, H.; Kaneko, M.; Sawanobori, M.; Uno, T.; Matsuzawa, H.; Nakamura, Y.; Matsushita, H.; Ando, K. High Concentrations of L-Ascorbic Acid Specifically Inhibit the Growth of Human Leukemic Cells via Downregulation of HIF-1α Transcription. PLoS ONE 2013, 8, e62717. [Google Scholar] [CrossRef] [PubMed]
- Miles, S.L.; Fischer, A.P.; Joshi, S.J.; Niles, R.M. Ascorbic acid and ascorbate-2-phosphate decrease HIF activity and malignant properties of human melanoma cells. BMC Cancer 2015, 15, 867. [Google Scholar] [CrossRef] [PubMed]
- Fischer, A.P.; Miles, S.L.; Venturelli, S.; Sinnberg, T.W.; Niessner, H.; Busch, C.; Osipyants, A.I.; Poloznikov, A.A.; Smirnova, N.A.; Hushpulian, D.M.; et al. Pharmacologic ascorbate (P-AscH−) suppresses hypoxia-inducible Factor-1α (HIF-1α) in pancreatic adenocarcinoma. Free Radic. Biol. Med. 2018, 8, 1–10. [Google Scholar]
- Kuiper, C.; Vissers, M.C.M. Ascorbate as a co-factor for fe- and 2-oxoglutarate dependent dioxygenases: Physiological activity in tumor growth and progression. Front. Oncol. 2014, 4, 359. [Google Scholar] [CrossRef] [PubMed]
- Kuiper, C.; Dachs, G.U.; Currie, M.J.; Vissers, M.C.M. Intracellular ascorbate enhances hypoxia-inducible factor (HIF)-hydroxylase activity and preferentially suppresses the HIF-1 transcriptional response. Free Radic. Biol. Med. 2014, 69, 308–317. [Google Scholar] [CrossRef] [PubMed]
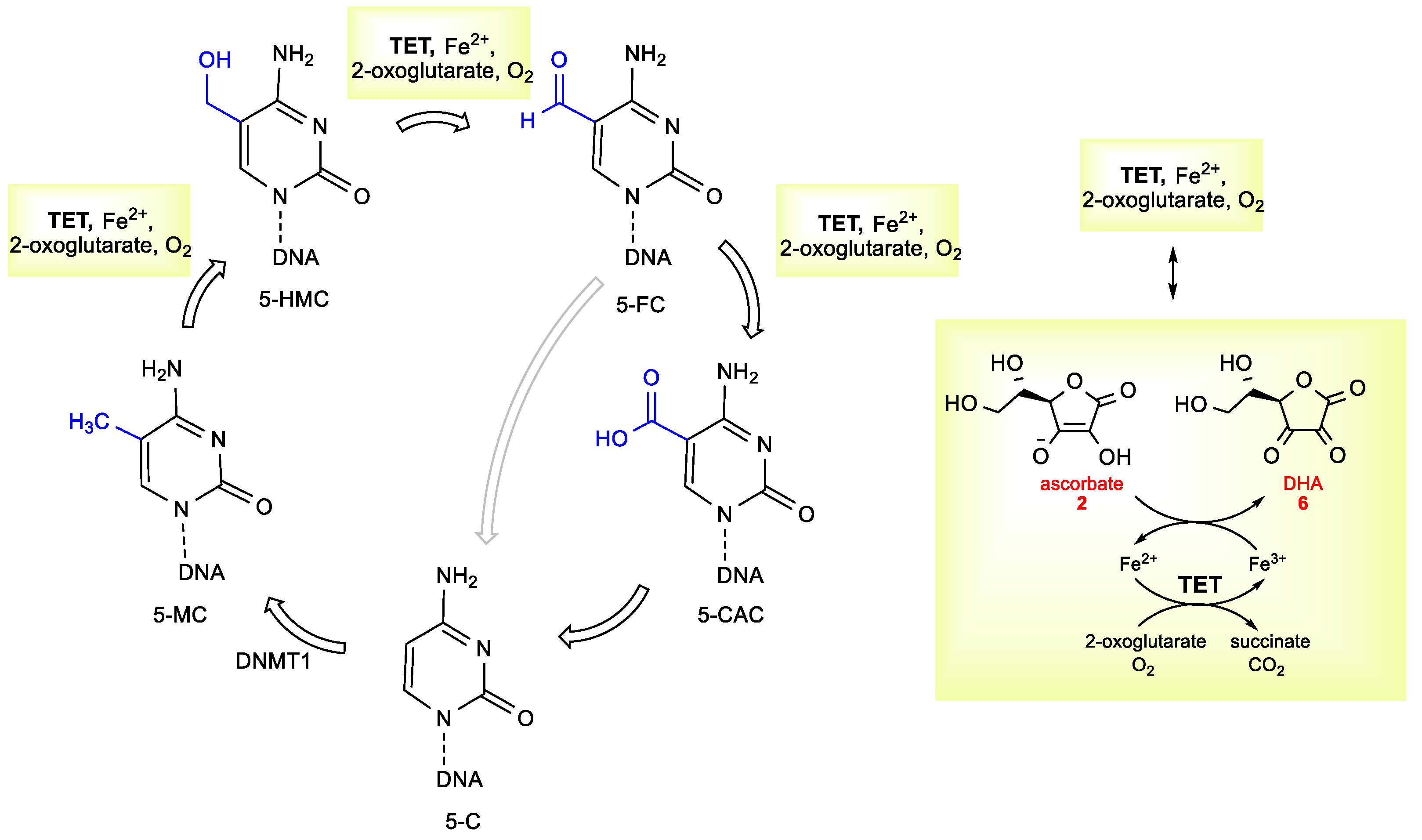
- Monfort, A.; Wutz, A. Breathing-in epigenetic change with vitamin C. EMBO Rep. 2013, 14, 337–346. [Google Scholar] [CrossRef]
- Gustafson, C.B.; Yang, C.; Dickson, K.M.; Shao, H.; Van Booven, D.; Harbour, J.W.; Liu, Z.-J.; Wang, G. Epigenetic reprogramming of melanoma cells by vitamin C treatment. Clin. Epigenetics 2015, 7, 51. [Google Scholar] [CrossRef]
- Harris, H.R.; Orsini, N.; Wolk, A. Vitamin C and survival among women with breast cancer: A Meta-analysis. Eur. J. Cancer 2014, 50, 1223–1231. [Google Scholar] [CrossRef]
- Young, J.I.; Züchner, S.; Wang, G. Regulation of the Epigenome by Vitamin C. Annu. Rev. Nutr. 2015, 35, 545–564. [Google Scholar] [CrossRef] [Green Version]
- Minor, E.A.; Court, B.L.; Young, J.I.; Wang, G. Ascorbate induces ten-eleven translocation (Tet) methylcytosine dioxygenase-mediated generation of 5-hydroxymethylcytosine. J. Biol. Chem. 2013, 288, 13669–13674. [Google Scholar] [CrossRef]
- Ferrarelli, L.K. Epigenetic Regulation by Vitamin C. Sci. Signal. 2013, 6, ec113. [Google Scholar] [CrossRef]
- Blaschke, K.; Ebata, K.T.; Karimi, M.M.; Zepeda-Martínez, J.A.; Goyal, P.; Mahapatra, S.; Tam, A.; Laird, D.J.; Hirst, M.; Rao, A.; et al. Vitamin C induces Tet-dependent DNA demethylation and a blastocyst-like state in ES cells. Nature 2013, 500, 222–226. [Google Scholar] [CrossRef]
- Weber, V.; Coudert, P.; Rubat, C.; Duroux, E.; Leal, F.; Couqulet, J.; Pharmacologie, L. De Antioxidant Properties of Novel Lipophilic Ascorbic Acid Analogues. J. Pharm. Pharmacol. 2000, 2, 523–530. [Google Scholar] [CrossRef]
- Tripathi, R.; Singh, B.; Bisht, S.S.; Pandey, J. L-ascorbic acid in organic synthesis: An overview. Curr. Org. Chem. 2009, 13, 99–122. [Google Scholar] [CrossRef]
- Liu, P.-Y.; Jiang, N.; Zhang, J.; Wei, X.; Lin, H.-H.; Yu, X.-Q. The oxidative damage of plasmid DNA by ascorbic acid derivatives in vitro: The first research on the relationship between the structure of ascorbic acid and the oxidative damage of plasmid DNA. Chem. Biodivers. 2006, 3, 958–966. [Google Scholar] [CrossRef]
- Tai, A.; Goto, S.; Ishiguro, Y.; Suzuki, K.; Nitoda, T.; Yamamoto, I. Permeation and metabolism of a series of novel lipophilic ascorbic acid derivatives, 6-O-acyl-2-O-α-D-glucopyranosyl-L-ascorbic acids with a branched-acyl chain, in a human living skin equivalent model. Bioorg. Med. Chem. Lett. 2004, 14, 623–627. [Google Scholar] [CrossRef]
- Nihro, Y.; Miyataka, H.; Sudo, T.; Matsumoto, H.; Satoh, T. 3-O-Alkylascorbic acids as free-radical quenchers: Synthesis and inhibitory effect on lipid peroxidation. J. Med. Chem. 1991, 34, 2152–2157. [Google Scholar] [CrossRef]
- Terao, S.; Shimamoto, N.; Hirata, M. Studies on Scavengers of Active Oxygen Species. 1. Synthesis and Biological Activity of 2-0-Alkylascorbic Acids. J. Med. Chem. 1988, 31, 793–798. [Google Scholar]
- Buettner, G.R. The Pecking Order of Free Radicals and Antioxidants: Lipid Peroxidation, α-Tocopherol, and Ascorbate. Arch. Biochem. Biophys. 1993, 300, 535–543. [Google Scholar] [CrossRef]
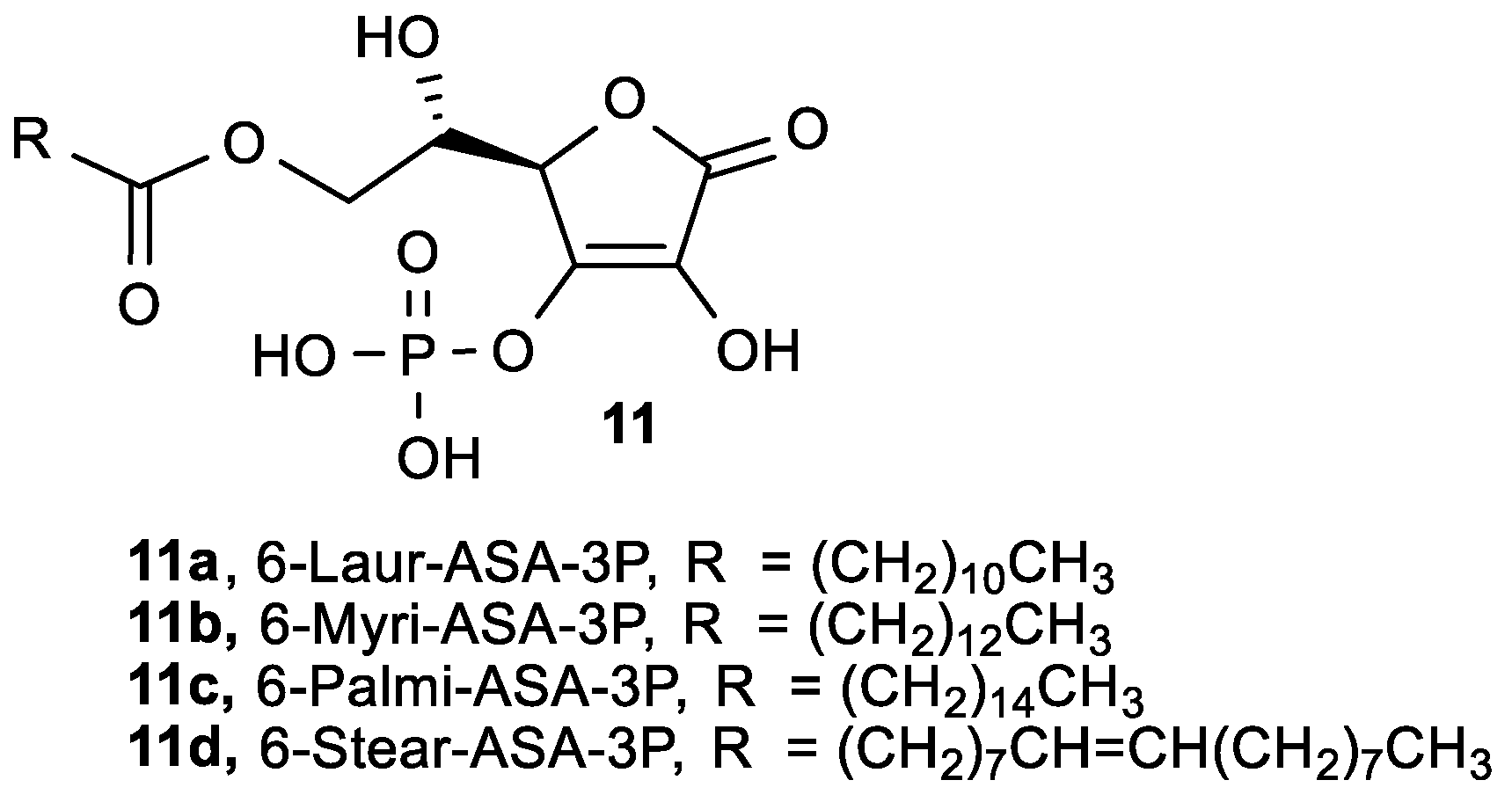
- Tao, Z.; Ren, Y.; Tong, W.; Wei, D. Synthesis of 6-O-acyl-L-ascorbic acid-2-O-phosphates and study of their antioxidant effects in 95-D cells. Pharmacol. Rep. 2005, 57, 77–83. [Google Scholar]
- Liu, J.; Zhang, X.; Yang, F.; Li, T.; Wei, D.; Ren, Y. Antimetastatic effect of a lipophilic ascorbic acid derivative with antioxidation through inhibition of tumor invasion. Cancer Chemother. Pharmacol. 2006, 57, 584–590. [Google Scholar] [CrossRef]
- Du, C.B.; Liu, J.W.; Su, W.; Ren, Y.H.; Wei, D.Z. The protective effect of ascorbic acid derivative on PC12 cells: Involvement of its ROS scavenging ability. Life Sci. 2003, 74, 771–780. [Google Scholar] [CrossRef]
- Fan, S.; Wei, D.; Liu, J.; Xin, X.; Tong, W.; Miwa, N. Ascorbic acid-2-o-phosphate-6-o-palmitate protecting the human umbilical cord vein endothelial cells against hydrogen peroxide and tert-butyl hydroperoxide induced cytotoxicity. Biomed. Pharmacother. 2004, 58, 205–211. [Google Scholar] [CrossRef]
- Lopez, E.; del Carmen Ortega-Liébana, M.; Salido, S.; Salido, G.M.; Altarejos, J.; Rosado, J.A.; Redondo, P.C. Evaluation of the antiaggregant activity of ascorbyl phenolic esters with antioxidant properties. J. Physiol. Biochem. 2015, 71, 415–434. [Google Scholar] [CrossRef]
- Kato, H.; Kino, T.; Yamamoto, F.; Kaneshiro, T.; Mukai, T.; Maeda, M. Ascorbate analogs for use in medical imaging: Synthesis and radical scavenging activity of 5-O-(4′-iodobenzyl)-L-ascorbic acid. Chem. Pharm. Bull. 2007, 55, 1700–1703. [Google Scholar] [CrossRef]
- Fujinami, Y.; Tai, A.; Yamamoto, I. Radical scavenging activity against 1,1-diphenyl-2-picrylhydrazyl of ascorbic acid 2-glucoside (AA-2G) and 6-acyl-AA-2G. Chem. Pharm. Bull. 2001, 49, 642–644. [Google Scholar] [CrossRef]
- Takebayashi, J.; Asano, R.; Nakae, Y.; Saito, M.; GOHDA, E.; Yamamoto, I.; Tai, A. 2-O-alfa-D-Glucopyranosyl-L-ascorbic Acid Scavenges 1,1-Diphenyl-2-picrylhydrazyl Radicals via a Covalent Adduct Formation. Biosci. Biotechnol. Biochem. 2007, 71, 754–760. [Google Scholar] [CrossRef]
- Ichiyama, K.; Mitsuzumi, H.; Zhong, M.; Tai, A.; Tsuchioka, A.; Kawai, S.; Yamamoto, I.; Gohda, E. Promotion of IL-4- and IL-5-dependent differentiation of anti-μ-primed B cells by ascorbic acid 2-glucoside. Immunol. Lett. 2009, 122, 219–226. [Google Scholar] [CrossRef]
- Wu, Y.L.; Gohda, E.; Iwao, M.; Matsunaga, T.; Nagao, T.; Takebe, T.; Yamamoto, I. Stimulation of hepatocyte growth factor production by ascorbic acid and its sTable 2-glucoside. Growth Horm. IGF Res. 1998, 8, 421–428. [Google Scholar] [CrossRef]
- Miyai, E.; Yanagida, M.; Akiyama, J.; Yamamoto, I. Ascorbic acid 2-O-alpha-glucoside, a sTable form of ascorbic acid, rescues human keratinocyte cell line, SCC, from cytotoxicity of ultraviolet light B. Biol. Pharm. Bull. 1996, 19, 984–987. [Google Scholar] [CrossRef]
- Yamamoto, I.; Muto, N.; Murakami, K.; Akiyama, J.-I. Collagen Synthesis in Human Skin Fibroblasts is Stimulated by a STable Form of Ascorbate, 2-O-α-D-Glucopyranosyl-L-Ascorbic Acid. J. Nutr. 1992, 122, 871–877. [Google Scholar] [CrossRef]
- Kumano, Y.; Sakamoto, T.; Egawa, M.; Iwai, I.; Tanaka, M.; Yamamoto, I. In vitro and in vivo prolonged biological activities of novel vitamin C derivative, 2-O-alpha-D-glucopyranosyl-L-ascorbic acid (AA-2G), in cosmetic fields. J. Nutr. Sci. Vitaminol. 1998, 44, 345–359. [Google Scholar] [CrossRef]
- Hanada, Y.; Iomori, A.; Ishii, R.; Gohda, E.; Tai, A. Protection of free radical-induced cytotoxicity by 2-O-α-d-glucopyranosyl-l-ascorbic acid in human dermal fibroblasts. Biosci. Biotechnol. Biochem. 2014, 78, 301–306. [Google Scholar] [CrossRef]
- Taniguchi, M.; Arai, N.; Kohno, K.; Ushio, S.; Fukuda, S. Anti-oxidative and anti-aging activities of 2-O-α-glucopyranosyl-L- ascorbic acid on human dermal fibroblasts. Eur. J. Pharmacol. 2012, 674, 126–131. [Google Scholar] [CrossRef]
- Xiao, L.; Tsutsui, T.; Miwa, N. The lipophilic vitamin C derivative, 6-o-palmitoylascorbate, protects human lymphocytes, preferentially over ascorbate, against X-ray-induced DNA damage, lipid peroxidation, and protein carbonylation. Mol. Cell. Biochem. 2014, 394, 247–259. [Google Scholar] [CrossRef]
- Mohamed, R.; Tarannum, S.; Yariswamy, M.; Vivek, H.K.; Siddesha, J.M.; Angaswamy, N.; Vishwanath, B.S. Ascorbic acid 6-palmitate: A potent inhibitor of human and soybean lipoxygenase-dependent lipid peroxidation. J. Pharm. Pharmacol. 2014, 66, 769–778. [Google Scholar] [CrossRef]
- Tai, A.; Kawasaki, D.; Sasaki, K.; Gohda, E.; Yamamoto, I. Synthesis and characterization of 6-O-acyl-2-O-alpha-D-glucopyranosyl-L-ascorbic acids with a branched-acyl chain. Chem. Pharm. Bull. 2003, 51, 175–180. [Google Scholar] [CrossRef]
- Yamamoto, I.; Tai, A.; Fujinami, Y.; Sasaki, K.; Okazaki, S. Synthesis and characterization of a series of novel monoacylated ascorbic acid derivatives, 6-O-acyl-2-O-alpha-D-glucopyranosyl-L-ascorbic acids, as skin antioxidants. J. Med. Chem. 2002, 45, 462–468. [Google Scholar] [CrossRef]
- Takebayashi, J.; Kaji, H.; Ichiyama, K.; Makino, K.; Gohda, E.; Yamamoto, I.; Tai, A. Inhibition of free radical-induced erythrocyte hemolysis by 2-O-substituted ascorbic acid derivatives. Free Radic. Biol. Med. 2007, 43, 1156–1164. [Google Scholar] [CrossRef]
- Toyada-ono, Y.; Maeda, M.; Nakao, M.; Yoshimura, M.; Sugiura-tomimori, N.; Fukami, H.; Nishioka, H.; Miyashita, Y.; Kojo, S. A novel vitamin C analog, 2-O-(β-D-Glucopyranosyl)ascorbic acid: Examination of enzymatic synthesis and biological activity. J. Biosci. Bioeng. 2005, 99, 361–365. [Google Scholar] [CrossRef]
- Zhang, Z.; Liu, X.; Zhang, X.; Liu, J.; Hao, Y.; Yang, X.; Wang, Y. Comparative evaluation of the antioxidant effects of the natural vitamin C analog 2-O-β-D-glucopyranosyl-L-ascorbic acid isolated from Goji berry fruit. Arch. Pharm. Res. 2011, 34, 801–810. [Google Scholar] [CrossRef]
- Takebayashi, J.; Yagi, Y.; Ishii, R.; Abe, S.; Yamada, K.; Tai, A. Antioxidant Properties of 2-O -β-D-Glucopyranosyl-L-ascorbic Acid. Biosci. Biotechnol. Biochem. 2008, 72, 1558–1563. [Google Scholar] [CrossRef]
- Manfredini, S.; Vertuani, S.; Manfredi, B.; Rossoni, G.; Calviello, G.; Palozza, P. Novel antioxidant agents deriving from molecular combinations of vitamins C and E analogues: 3,4-Dihydroxy-5(R)-[2(R,S)-(6-hydroxy-2,5,7,8-tetramethyl-chroman-2(R,S)-yl-methyl)-[1,3]dioxolan-4(S)-yl]-5H-furan-2-one and 3-O-octadecyl derivatives. Bioorganic Med. Chem. 2000, 8, 2791–2801. [Google Scholar] [CrossRef]
- Cotelle, P.; Cotelle, N.; Teissier, E.; Vezin, H. Synthesis and antioxidant properties of a new lipophilic ascorbic acid analogue. Bioorganic Med. Chem. 2003, 11, 1087–1093. [Google Scholar] [CrossRef]
- Weber, V.; Coudert, P.; Rubat, C.; Duroux, E.; Vallée-Goyet, D.; Gardette, D.; Bria, M.; Albuisson, E.; Leal, F.; Gramain, J.C.; et al. Novel 4,5-diaryl-3-hydroxy-2(5H)-furanones as anti-oxidants and anti-inflammatory agents. Bioorg. Med. Chem. 2002, 10, 1647–1658. [Google Scholar] [CrossRef]
- Hoffer, L.J.; Levine, M.; Assouline, S.; Melnychuk, D.; Padayatty, S.J.; Rosadiuk, K.; Rousseau, C.; Robitaille, L.; Miller, W.H. Phase I clinical trial of i.v. ascorbic acid in advanced malignancy. Ann. Oncol. 2008, 19, 1969–1974. [Google Scholar] [CrossRef]
- Parrow, N.L.; Leshin, J.A.; Levine, M. Parenteral Ascorbate As a Cancer Therapeutic: A Reassessment Based on Pharmacokinetics. Antioxid. Redox Signal. 2013, 19, 2141–2156. [Google Scholar] [CrossRef]
- Miura, K.; Tai, A. 2-O-α-D-Glucopyranosyl-l-ascorbic acid as an antitumor agent for infusion therapy. Biochem. Biophys. Rep. 2017, 10, 232–236. [Google Scholar] [CrossRef]
- Miura, K.; Haraguchi, M.; Ito, H.; Tai, A. Potential antitumor activity of 2-O-α-D-glucopyranosyl-6-O-(2-pentylheptanoyl)-L-ascorbic acid. Int. J. Mol. Sci. 2018, 19, 535. [Google Scholar] [CrossRef]
- Zhang, Z.; Liu, X.; Wu, T.; Liu, J.; Zhang, X.; Yang, X.; Goodheart, M.J.; Engelhardt, J.F.; Wang, Y. Selective suppression of cervical cancer Hela cells by 2-O-β-d-glucopyranosyl-l-ascorbic acid isolated from the fruit of Lycium barbarum L. Cell Biol. Toxicol. 2011, 27, 107–121. [Google Scholar] [CrossRef]
- Cinatl, J.; Weber, B.; Rabenau, H.; Gumbel, H.O.; Chenot, J.F.; Scholz, M.; Encke, A.; Doerr, H.W. In vitro inhibition of human cytomegalovirus replication in human foreskin fibroblasts and endothelial cells by ascorbic acid 2-phosphate. Antivir. Res. 1995, 27, 405–418. [Google Scholar] [CrossRef]
- Roomi, M.; House, D.; Eckert-Maksić, M.; Maksić, Z.; Tsao, C. Growth suppression of malignant leukemia cell line in vitro by ascorbic acid (vitamin C) and its derivatives. Cancer Lett. 1998, 122, 93–99. [Google Scholar] [CrossRef]
- Osmak, M.; Eckert-Maksić, M.; Pavelić, K.; Maksić, Z.B.; Spavénti, R.; Beketić, L.; Kovacek, I.; Susković, B. 6-Deoxy-6-bromo-ascorbic acid inhibits growth of mouse melanoma cells. Res. Exp. Med. 1990, 190, 443–449. [Google Scholar] [CrossRef]
- Osmak, M.; Kovaček, I.; Ljubenkov, I.; Spaventi, R.; Eckert-Maksić, M. Ascorbic acid and 6-deoxy-6-chloro-ascorbic acid: Potential anticancer drugs. Neoplasma 1997, 44, 101–107. [Google Scholar]
- Grdiša, M.; Kralj, M.; Eckert-Maksić, M.; Maksić, Z.B.; Pavelić, K. 6-Amino-6-Deoxyascorbic Acid Induces Apoptosis in Human Tumor Cells. J. Cancer Res. Clin. Oncol. 1995, 121, 98–102. [Google Scholar] [CrossRef]
- Kralj, M.; Kojić-Prodić, B.; Banić, Z.; Grdiša, M.; Vela, V.; Šušković, B.; Pavelić, K. Synthesis, structural characterization and cytotoxic effect of 6-amino-6-deoxy-L-ascorbic acid derivates. Eur. J. Med. Chem. 1996, 31, 23–35. [Google Scholar] [CrossRef]
- Gazivoda, T.; Wittine, K.; Lovrić, I.; Makuc, D.; Plavec, J.; Cetina, M.; Mrvoš-Sermek, D.; Šuman, L.; Kralj, M.; Pavelić, K.; et al. Synthesis, structural studies, and cytostatic evaluation of 5,6-di-O-modified L-ascorbic acid derivatives. Carbohydr. Res. 2006, 341, 433–442. [Google Scholar] [CrossRef]
- Matsuda, S.; Shibayama, H.; Hisama, M.; Ohtsuki, M.; Iwaki, M. Inhibitory Effects of a Novel Ascorbic Derivative, Disodium Isostearyl 2-O-L-Ascorbyl Phosphate on Melanogenesis. Chem. Pharm. Bull. (Tokyo) 2008, 56, 292–297. [Google Scholar] [CrossRef] [Green Version]
- Shibayama, H.; Hisama, M.; Matsuda, S.; Ohtsuki, M.; Iwaki, M. Effect of a novel ascorbic derivative, disodium isostearyl 2-O-L-ascorbyl phosphate on human dermal fibroblasts: Increased collagen synthesis and inhibition of MMP-1. Biol. Pharm. Bull. 2008, 31, 563–568. [Google Scholar] [CrossRef]
- Bordignon, B.; Chiron, J.; Fontés, M. Ascorbic acid derivatives as a new class of antiproliferative molecules. Cancer Lett. 2013, 338, 317–327. [Google Scholar] [CrossRef]
- Ogawa, K.; Futakuchi, M.; Hirose, M.; Boonyaphiphat, P.; Mizoguchi, Y.; Miki, T.; Shirai, T. Stage and organ dependent effects of 1-O-hexyl-2,3,5-trimethylhydroquinone, ascorbic acid derivatives, N-heptadecane-8,10-dione and phenylethyl isothiocyanate in a rat multiorgan carcinogenesis model. Int. J. Cancer 1998, 76, 851–856. [Google Scholar] [CrossRef]
- Futakuchi, M.; Hirose, M.; Miki, T.; Tanaka, H.; Ozaki, M.; Shirai, T. Inhibition of DMBA-initiated rat mammary tumour development by 1-O-hexyl-2,3,5-trimethylhydroquinone, phenylethyl isothiocyanate, and novel synthetic ascorbic acid derivatives. Eur. J. Cancer Prev. 1998, 7, 153–159. [Google Scholar]
- Brinkevich, S.D.; Boreko, E.I.; Savinova, O.V.; Pavlova, N.I.; Shadyro, O.I. Radical-regulating and antiviral properties of ascorbic acid and its derivatives. Bioorg. Med. Chem. Lett. 2012, 22, 2424–2427. [Google Scholar] [CrossRef]
- Raić-Malić, S.; Hergold-Brundić, A.; Nagl, A.; Grdisa, M.; Pavelić, K.; De Clercq, E.; Mintas, M. Novel pyrimidine and purine derivatives of L-ascorbic acid: Synthesis and biological evaluation. J. Med. Chem. 1999, 42, 2673–2678. [Google Scholar] [CrossRef]
- Raić-Malić, S.; Svedruzić, D.; Gazivoda, T.; Marunović, A.; Hergold-Brundić, A.; Nagl, A.; Balzarini, J.; De Clercq, E.; Mintas, M. Synthesis and antitumor activities of novel pyrimidine derivatives of 2,3-O,O-dibenzyl-6-deoxy-L-ascorbic acid and 4,5-didehydro-5,6- dideoxy-L-ascorbic acid. J. Med. Chem. 2000, 43, 4806–4811. [Google Scholar] [CrossRef]
- Gazivoda, T.; Plevnik, M.; Plavec, J.; Kraljević, S.; Kralj, M.; Pavelić, K.; Balzarini, J.; De Clercq, E.; Mintas, M.; Raić-Malić, S. The novel pyrimidine and purine derivatives of l-ascorbic acid: Synthesis, one- and two-dimensional 1H and 13C NMR study, cytostatic and antiviral evaluation. Bioorg. Med. Chem. 2005, 13, 131–139. [Google Scholar] [CrossRef]
- Gazivoda, T.; Raić-Malić, S.; Marjanović, M.; Kralj, M.; Pavelić, K.; Balzarini, J.; De Clercq, E.; Mintas, M. The novel C-5 aryl, alkenyl, and alkynyl substituted uracil derivatives of l-ascorbic acid: Synthesis, cytostatic, and antiviral activity evaluations. Bioorg. Med. Chem. 2007, 15, 749–758. [Google Scholar] [CrossRef]
- Gazivoda, T.; Šokčević, M.; Kralj, M.; Šuman, L.; Pavelić, K.; De Clercq, E.; Andrei, G.; Snoeck, R.; Balzarini, J.; Mintas, M.; et al. Synthesis and antiviral and cytostatic evaluations of the new c-5 substituted pyrimidine and furo[2,3-d]pyrimidine 4′,5′-didehydro-L- ascorbic acid derivatives. J. Med. Chem. 2007, 50, 4105–4112. [Google Scholar] [CrossRef]
- Wittine, K.; Babić, M.S.; Košutić, M.; Cetina, M.; Rissanen, K.; Pavelić, S.K.; Paravić, A.T.; Sedić, M.; Pavelić, K.; Mintas, M. The new 5- or 6-azapyrimidine and cyanuric acid derivatives of l-ascorbic acid bearing the free C-5 hydroxy or C-4 amino group at the ethylenic spacer: CD-spectral absolute configuration determination and biological activity evaluations. Eur. J. Med. Chem. 2011, 46, 2770–2785. [Google Scholar] [CrossRef]
- Stipković Babić, M.; Makuc, D.; Plavec, J.; Martinović, T.; Kraljević Pavelić, S.; Pavelić, K.; Snoeck, R.; Andrei, G.; Schols, D.; Wittine, K.; et al. Novel halogenated 3-deazapurine, 7-deazapurine and alkylated 9-deazapurine derivatives of l -ascorbic or imino-l-ascorbic acid: Synthesis, antitumour and antiviral activity evaluations. Eur. J. Med. Chem. 2015, 102, 288–302. [Google Scholar] [CrossRef]
- Hakimelahi, G.H.; Mei, N.W.; Moosavi-Movahedi, A.A.; Davari, H.; Hakimelahi, S.; King, K.Y.; Hwu, J.R.; Wen, Y.S. Synthesis and biological evaluation of purine-containing butenolides. J. Med. Chem. 2001, 44, 1749–1757. [Google Scholar] [CrossRef]
- Hakimelahi, G.H.; Moosavi-Movahedi, A.A.; Sambaiah, T.; Zhu, J.L.; Ethiraj, K.S.; Pasdar, M.; Hakimelahi, S. Reactions of purines-containing butenolides with L-cysteine or N-acetyl-L-cysteine as model biological nucleophiles: A potent mechanism-based inhibitor of ribonucleotide reductase caused apoptosis in breast carcinoma MCF7 cells. Eur. J. Med. Chem. 2002, 37, 207–217. [Google Scholar] [CrossRef]
- Hakimelahi, G.H.; Ly, T.W.; Moosavi-Movahedi, A.A.; Jain, M.L.; Zakerinia, M.; Davari, H.; Mei, H.C.; Sambaiah, T.; Moshfegh, A.A.; Hakimelahi, S. Design, synthesis, and biological evaluation of novel nucleoside and nucleotide analogues as agents against DNA viruses and/or retroviruses. J. Med. Chem. 2001, 44, 3710–3720. [Google Scholar] [CrossRef]
- Wittine, K.; Stipković Babić, M.; Makuc, D.; Plavec, J.; Kraljević Pavelić, S.; Sedić, M.; Pavelić, K.; Leyssen, P.; Neyts, J.; Balzarini, J.; et al. Novel 1,2,4-triazole and imidazole derivatives of l-ascorbic and imino-ascorbic acid: Synthesis, anti-HCV and antitumor activity evaluations. Bioorg. Med. Chem. 2012, 20, 3675–3685. [Google Scholar] [CrossRef]
- Wang, H.; Xu, R.; Shi, Y.; Si, L.; Jiao, P.; Fan, Z.; Han, X.; Wu, X.; Zhou, X.; Yu, F.; et al. Design, synthesis and biological evaluation of novel l-ascorbic acid-conjugated pentacyclic triterpene derivatives as potential influenza virus entry inhibitors. Eur. J. Med. Chem. 2016, 110, 376–388. [Google Scholar] [CrossRef]
- Wei, M.X.; Feng, L.; Li, X.Q.; Zhou, X.Z.; Shao, Z.H. Synthesis of new chiral 2,5-disubstituted 1,3,4-thiadiazoles possessing γ-butenolide moiety and preliminary evaluation of in vitro anticancer activity. Eur. J. Med. Chem. 2009, 44, 3340–3344. [Google Scholar] [CrossRef]
- Li, X.; Li, X.-Q.; Liu, H.-M.; Zhou, X.-Z.; Shao, Z.-H. Synthesis and evaluation of antitumor activities of novel chiral 1,2,4-triazole Schiff bases bearing γ-butenolide moiety. Org. Med. Chem. Lett. 2012, 2, 26. [Google Scholar] [CrossRef]
- Wang, X.J.; Xu, H.W.; Guo, L.L.; Zheng, J.X.; Xu, B.; Guo, X.; Zheng, C.X.; Liu, H.M. Synthesis and in vitro antitumor activity of new butenolide-containing dithiocarbamates. Bioorg. Med. Chem. Lett. 2011, 21, 3074–3077. [Google Scholar] [CrossRef]
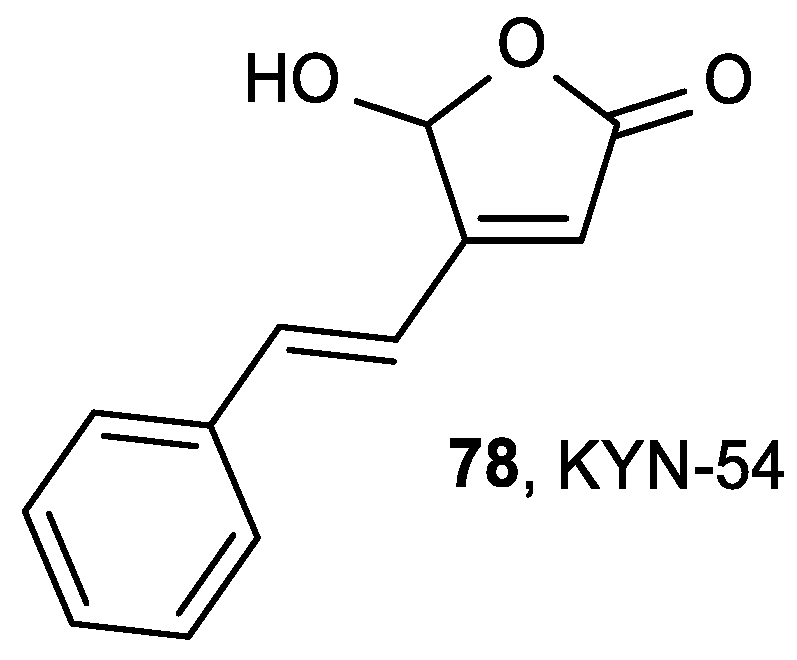
- Kawamori, T.; Tanaka, T.; Hirose, Y.; Satoh, K.; Hara, A.; Torihara, M.; Tamai, Y.; Yamahara, J.; Mori, H. Suppression of azoxymethane-induced colonic aberrant crypt foci by dietary exposure to a novel synthesized retinoidal butenolide, 5-hydroxy-4-(2-phenyl-(E)ethenyl)-2(5H)-furanone, in rats. Cancer Lett. 1995, 92, 159–165. [Google Scholar] [CrossRef]
- Mori, H.; Matsunaga, K.; Tanakamaru, Y.; Kawabata, K.; Yamada, Y.; Sugie, S.; Nishikawa, A. Effects of protocatechuic acid, S-methylmethanethiosulfonate or 5-hydroxy-4-(2-phenyl-(E)ethenyl)-2(5H)-furanone(KYN-54) on 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone-induced pulmonary carcinogenesis in mice. Cancer Lett. 1999, 135, 123–127. [Google Scholar] [CrossRef]
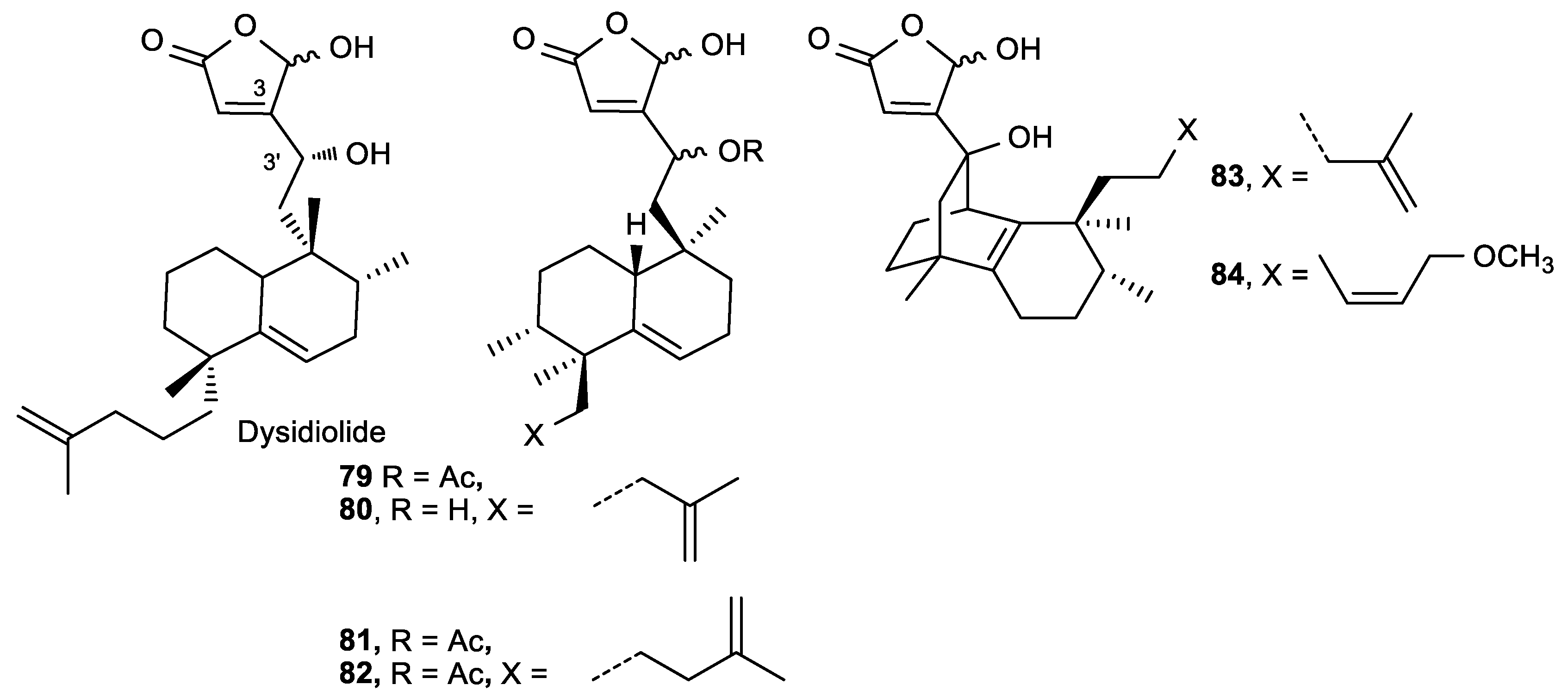
- Marcos, I.S.; Escola, M.A.; Moro, R.F.; Basabe, P.; Diez, D.; Sanz, F.; Mollinedo, F.; de la Iglesia-Vicente, J.; Sierra, B.G.; Urones, J.G. Synthesis of novel antitumoural analogues of dysidiolide from ent-halimic acid. Bioorg. Med. Chem. 2007, 15, 5719–5737. [Google Scholar] [CrossRef]
- Presley, C.C.; Rakotondraibe, L.H.; Brodie, P.J.; Callmander, M.W.; Randrianaivo, R.; Rasamison, V.E.; Rakotobe, E.; Kingston, D.G. A Synthetic Butenolide Diterpene is now a Natural Product Isolated from Metaporana sericosepala, a Plant from the Madagascar Dry Forest. Nat. Prod. Commun. 2015, 10, 1505–1507. [Google Scholar] [CrossRef]
- Alves, S.L.G.; Paixão, N.; Ferreira, L.G.R.; Santos, F.R.S.; Neves, L.D.R.; Oliveira, G.C.; Cortes, V.F.; Salomé, K.S.; Barison, A.; Santos, F.V.; et al. γ-Benzylidene digoxin derivatives synthesis and molecular modeling: Evaluation of anticancer and the Na, K-ATPase activity effect. Bioorg. Med. Chem. 2015, 23, 4397–4404. [Google Scholar] [CrossRef]
- Gao, H.; Guo, W.; Wang, Q.; Zhang, L.; Zhu, M.; Zhu, T.; Gu, Q.; Wang, W.; Li, D. Aspulvinones from a mangrove rhizosphere soil-derived fungus Aspergillus terreus Gwq-48 with anti-influenza A viral (H1N1) activity. Bioorg. Med. Chem. Lett. 2013, 23, 1776–1778. [Google Scholar] [CrossRef]

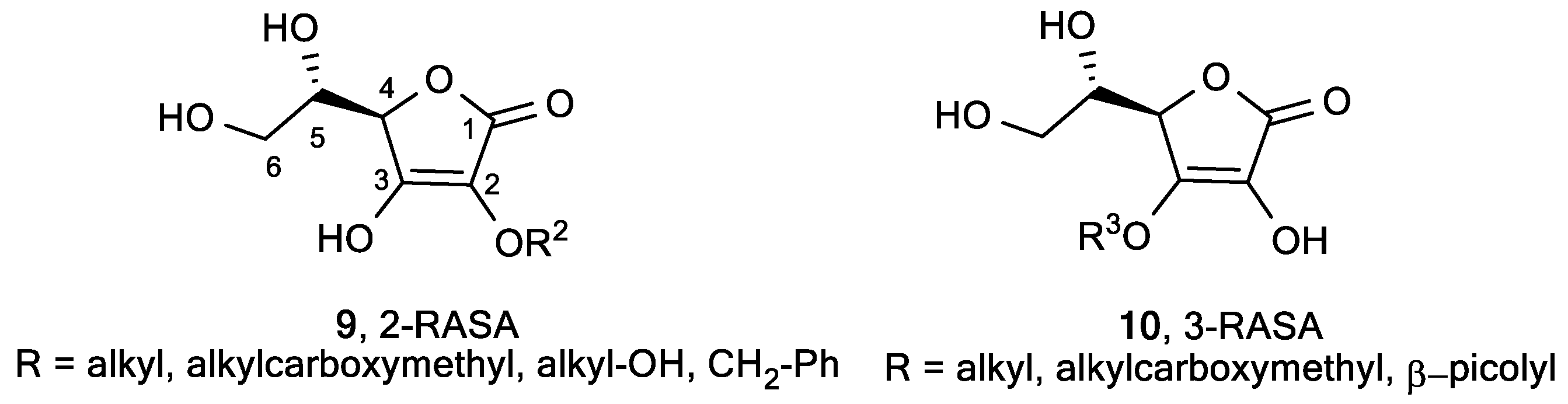
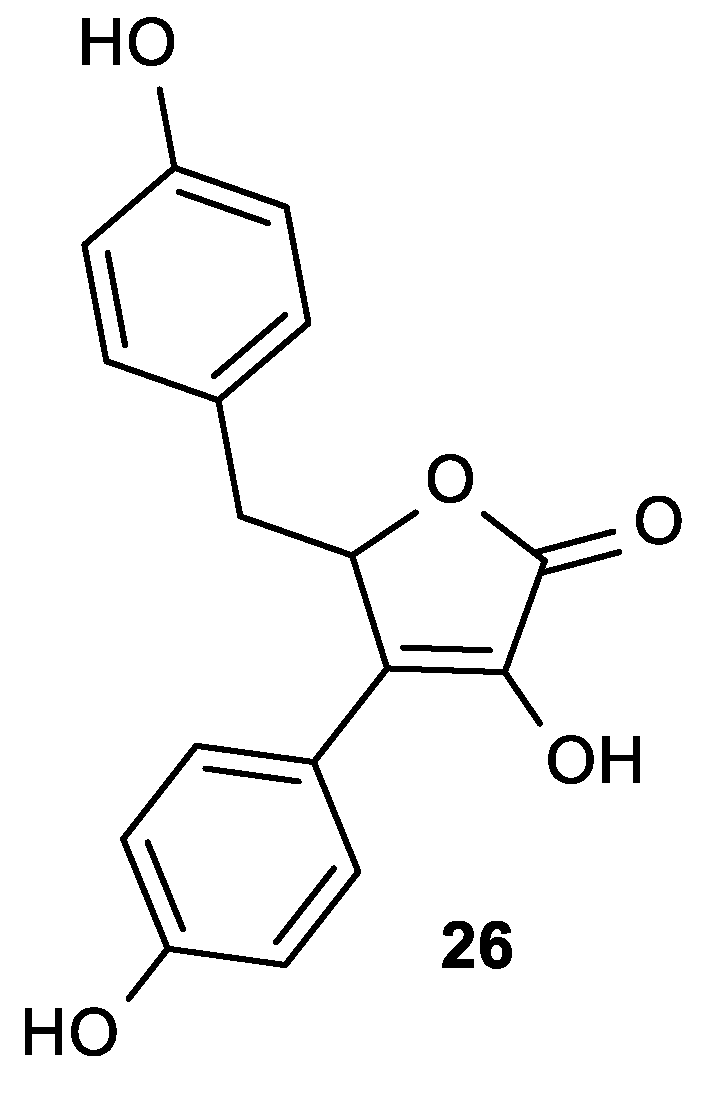
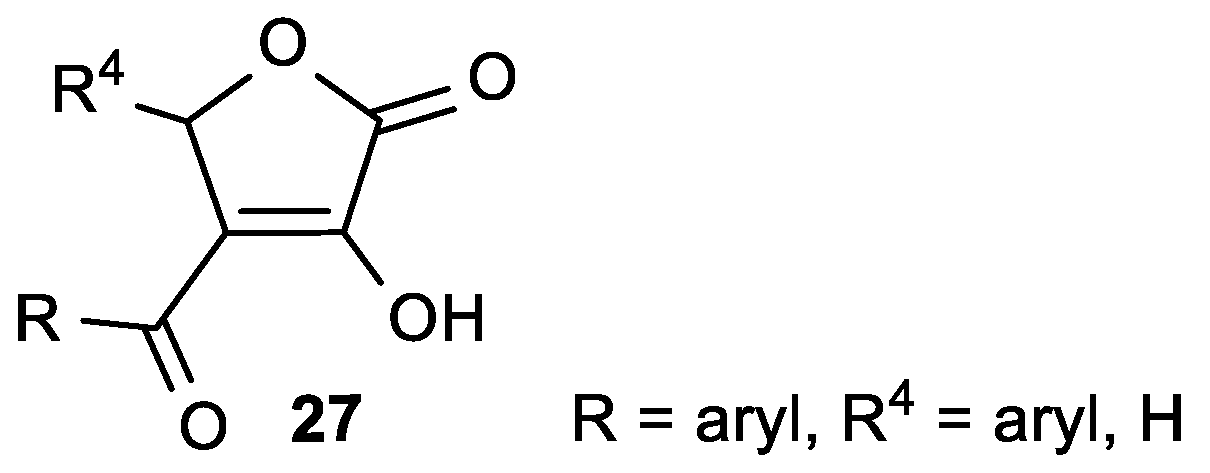
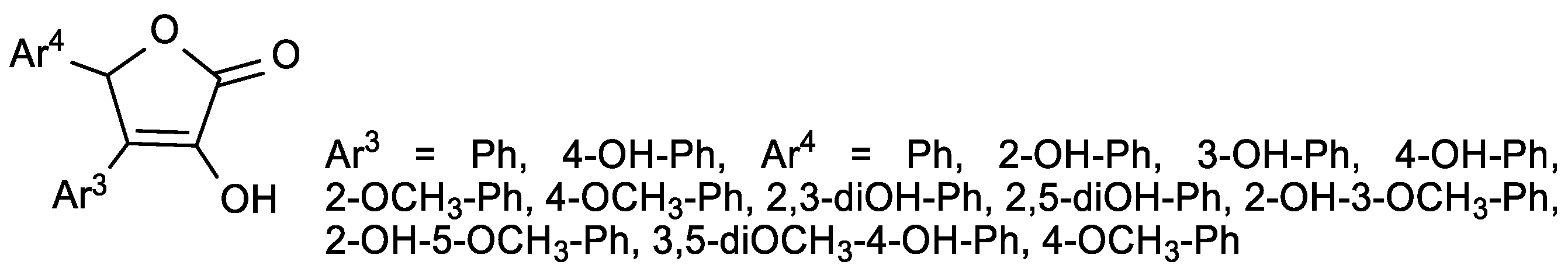
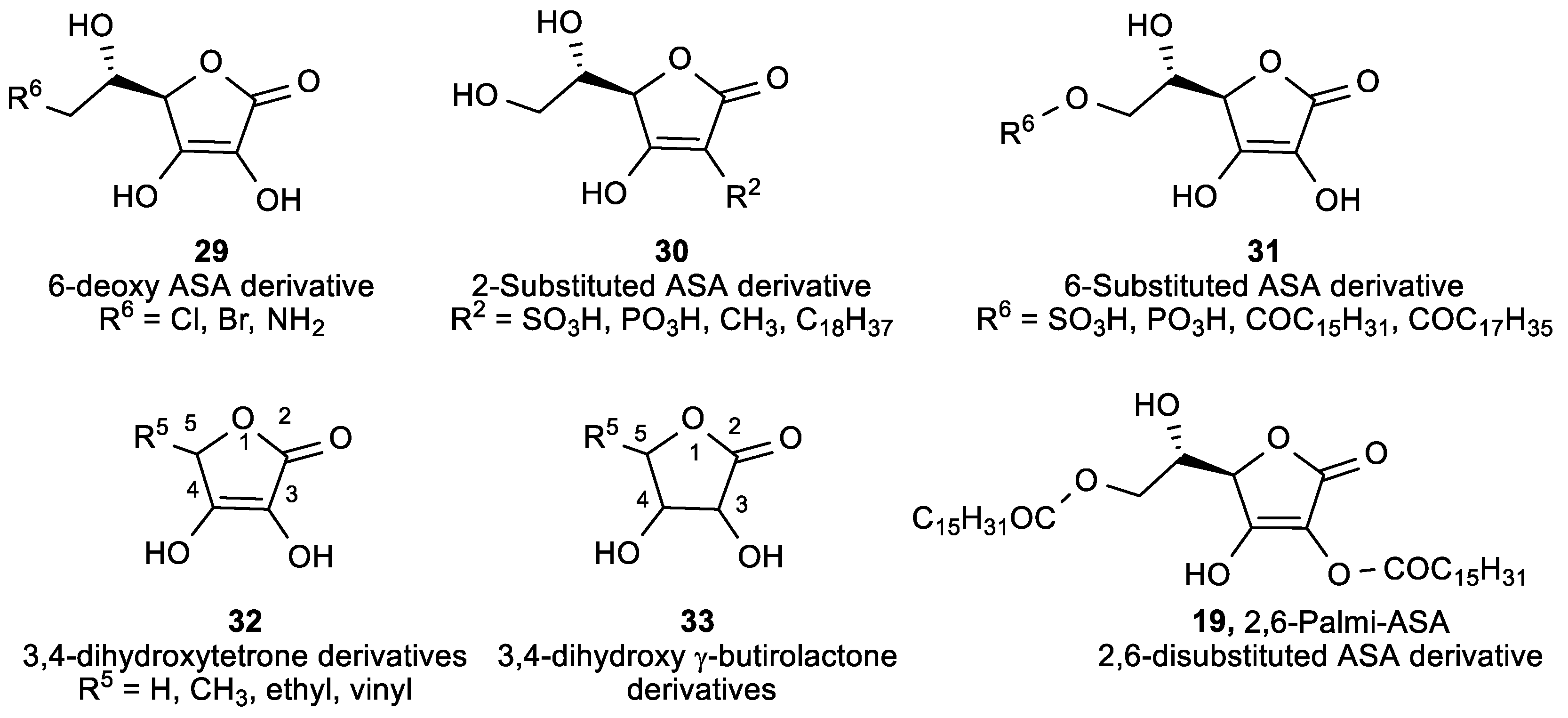
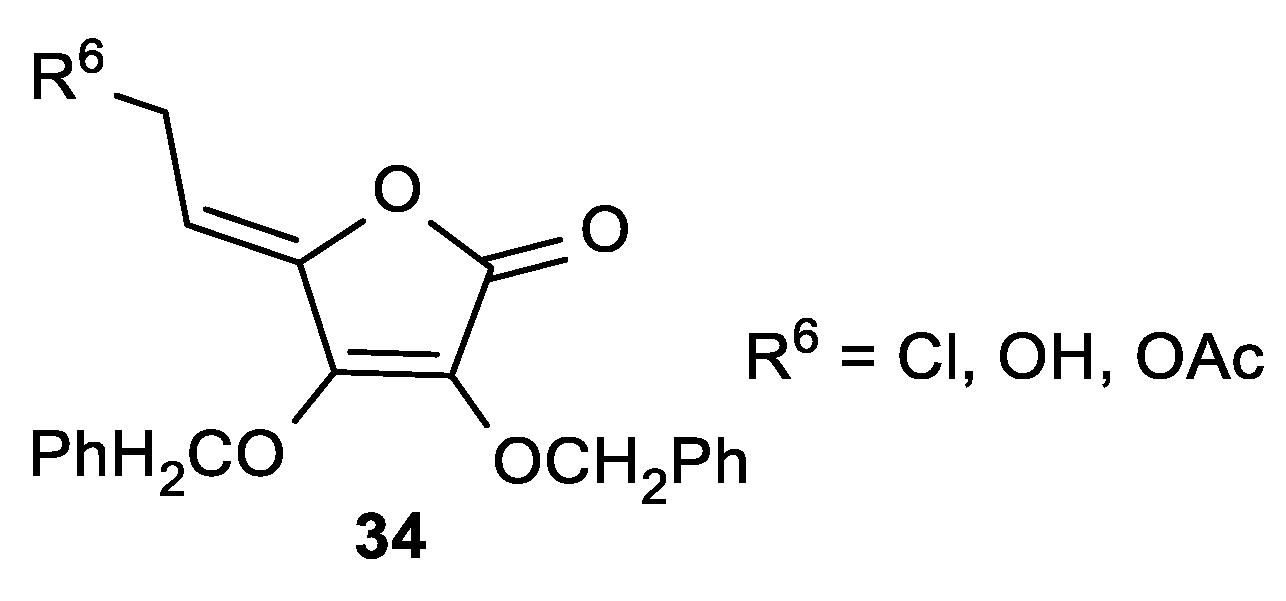
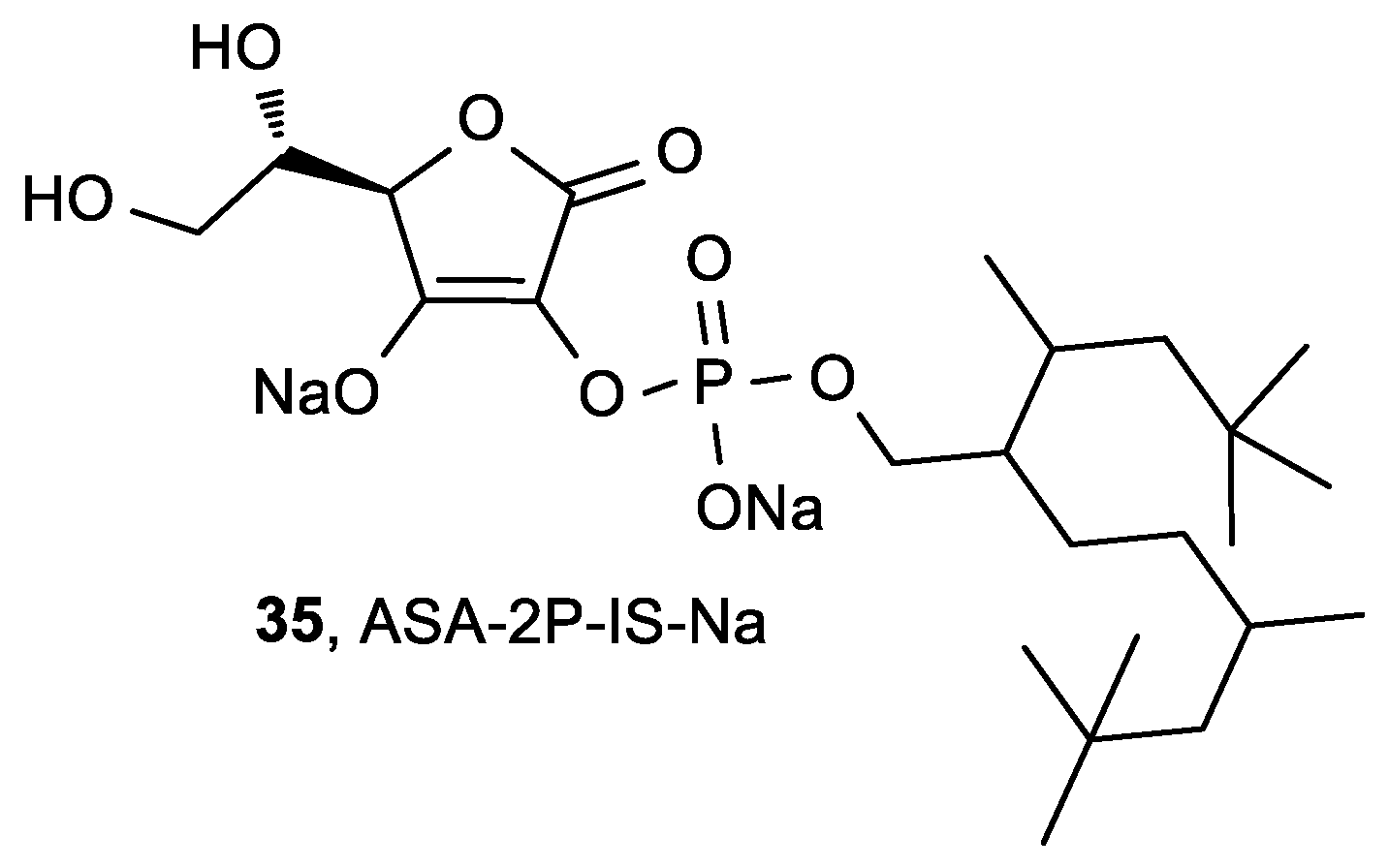
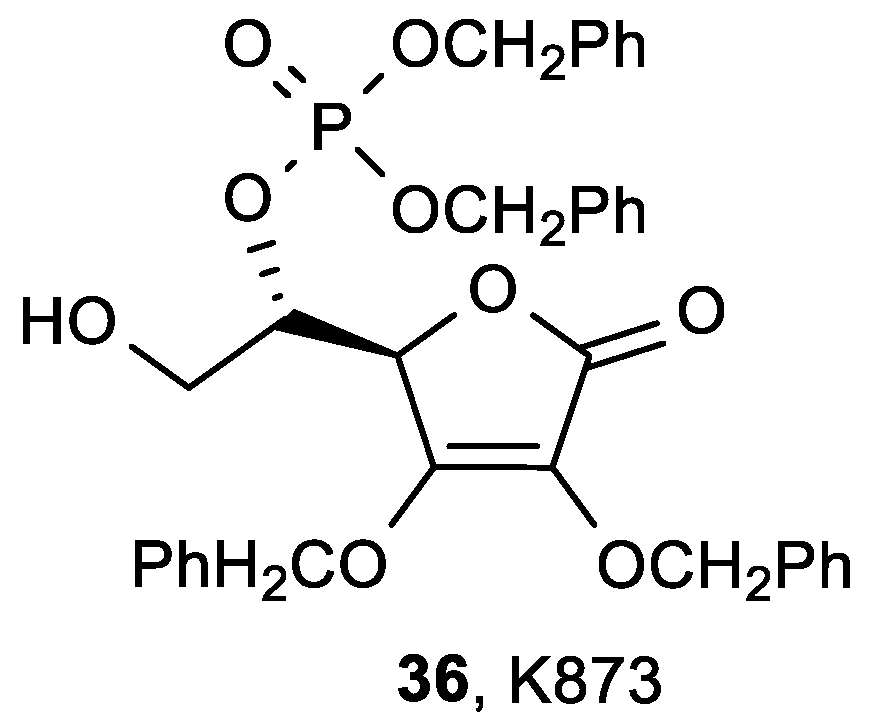
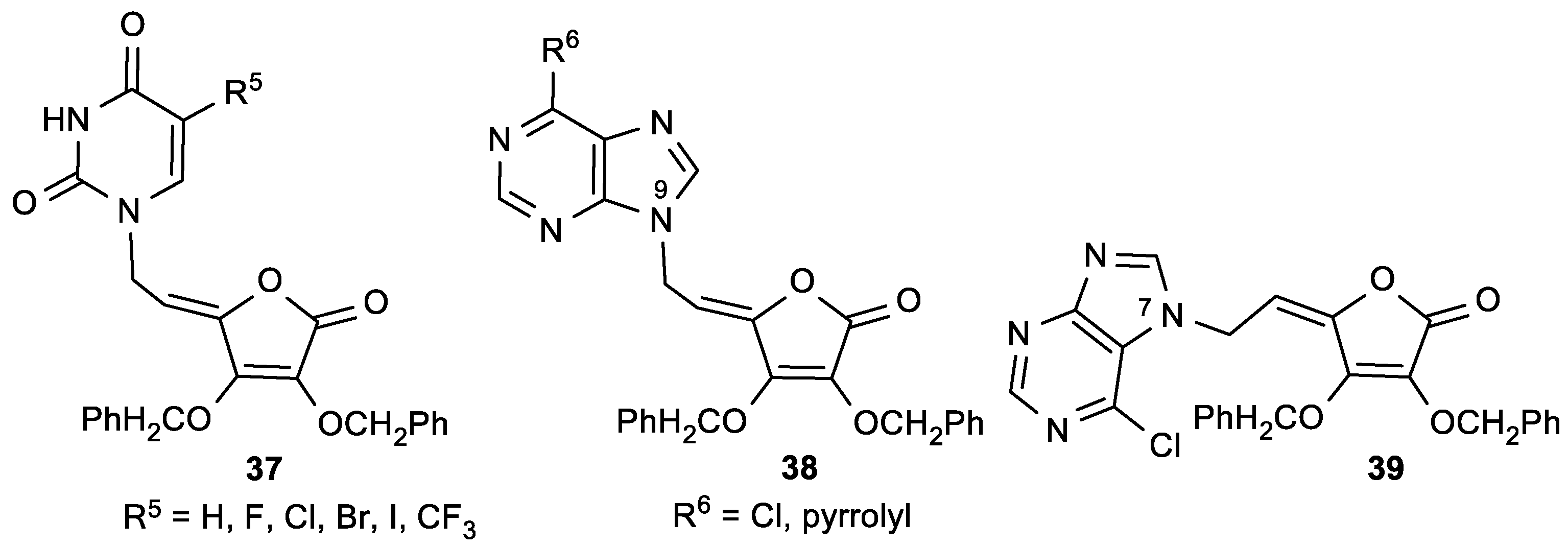
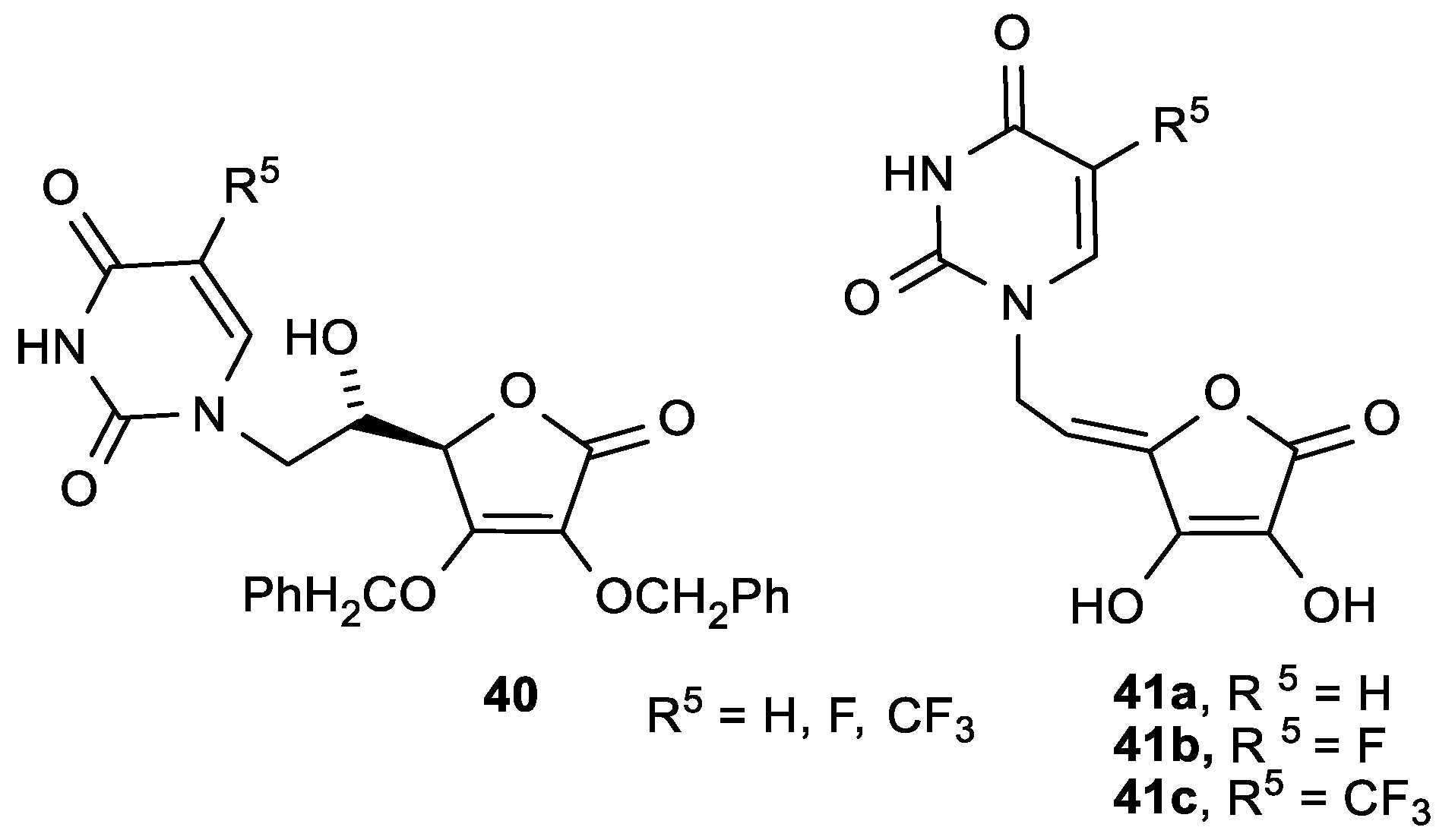

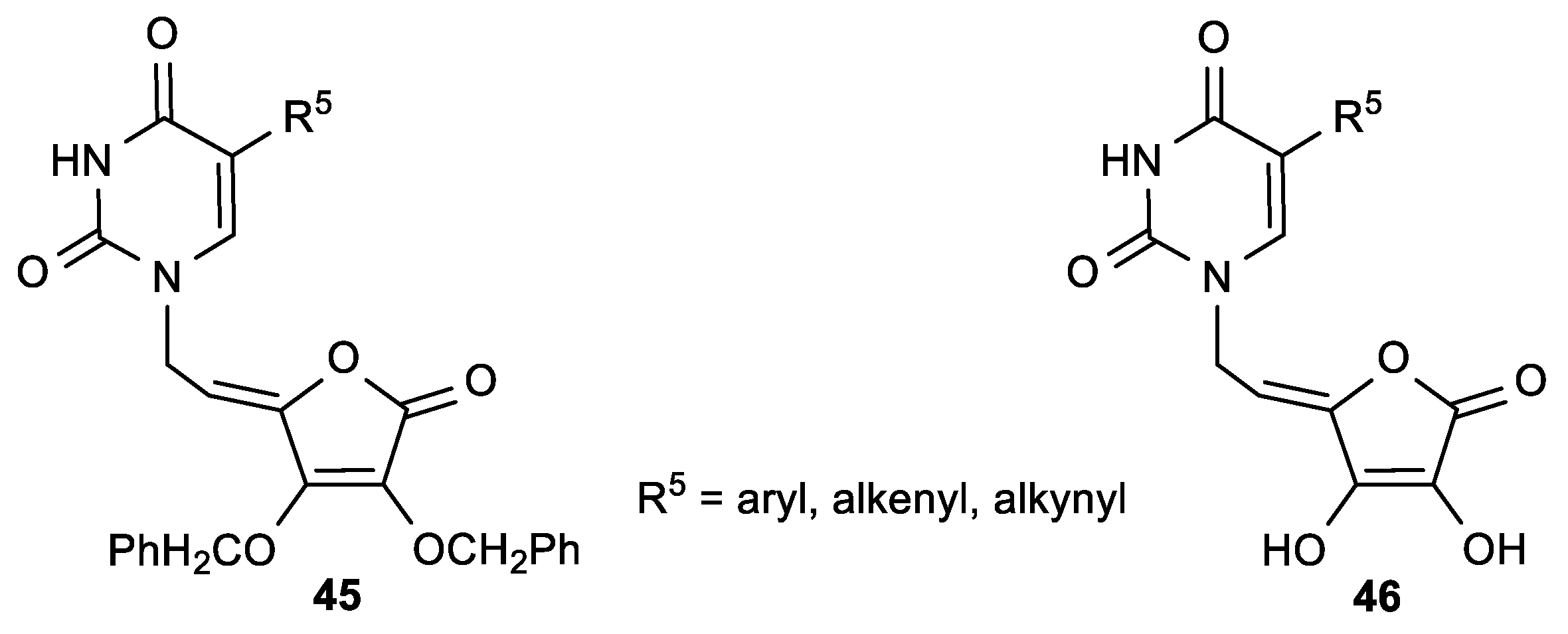
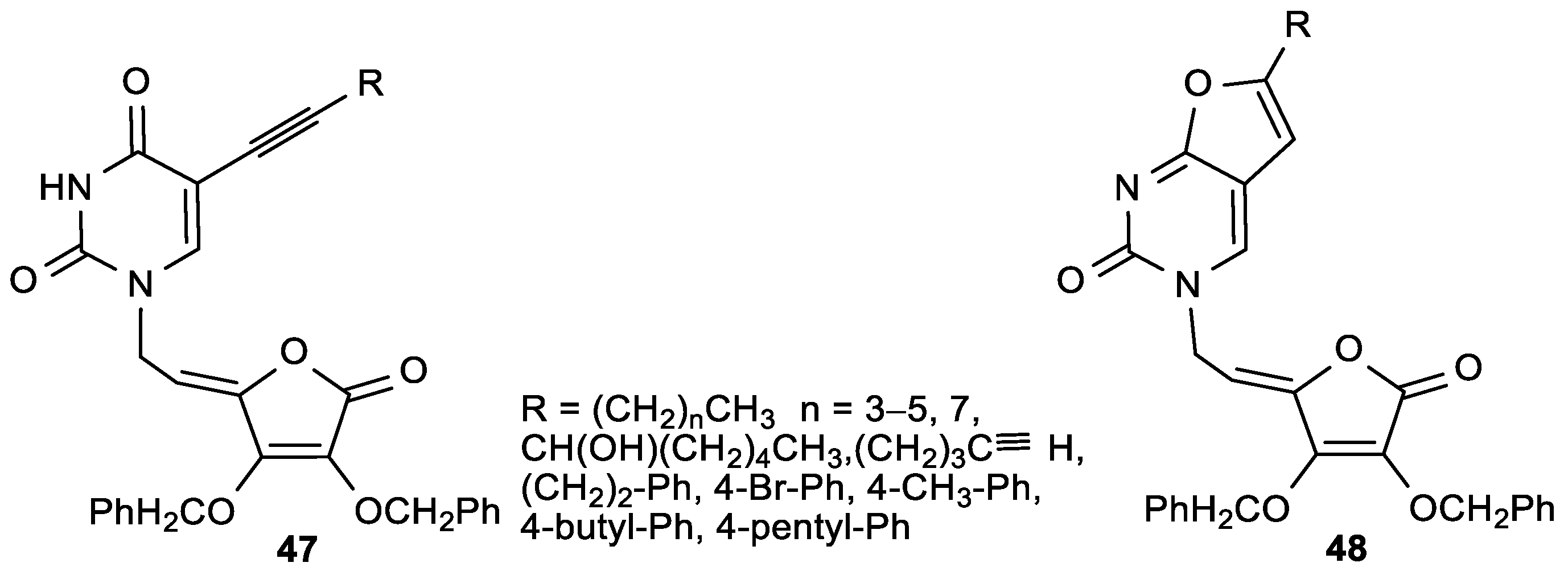
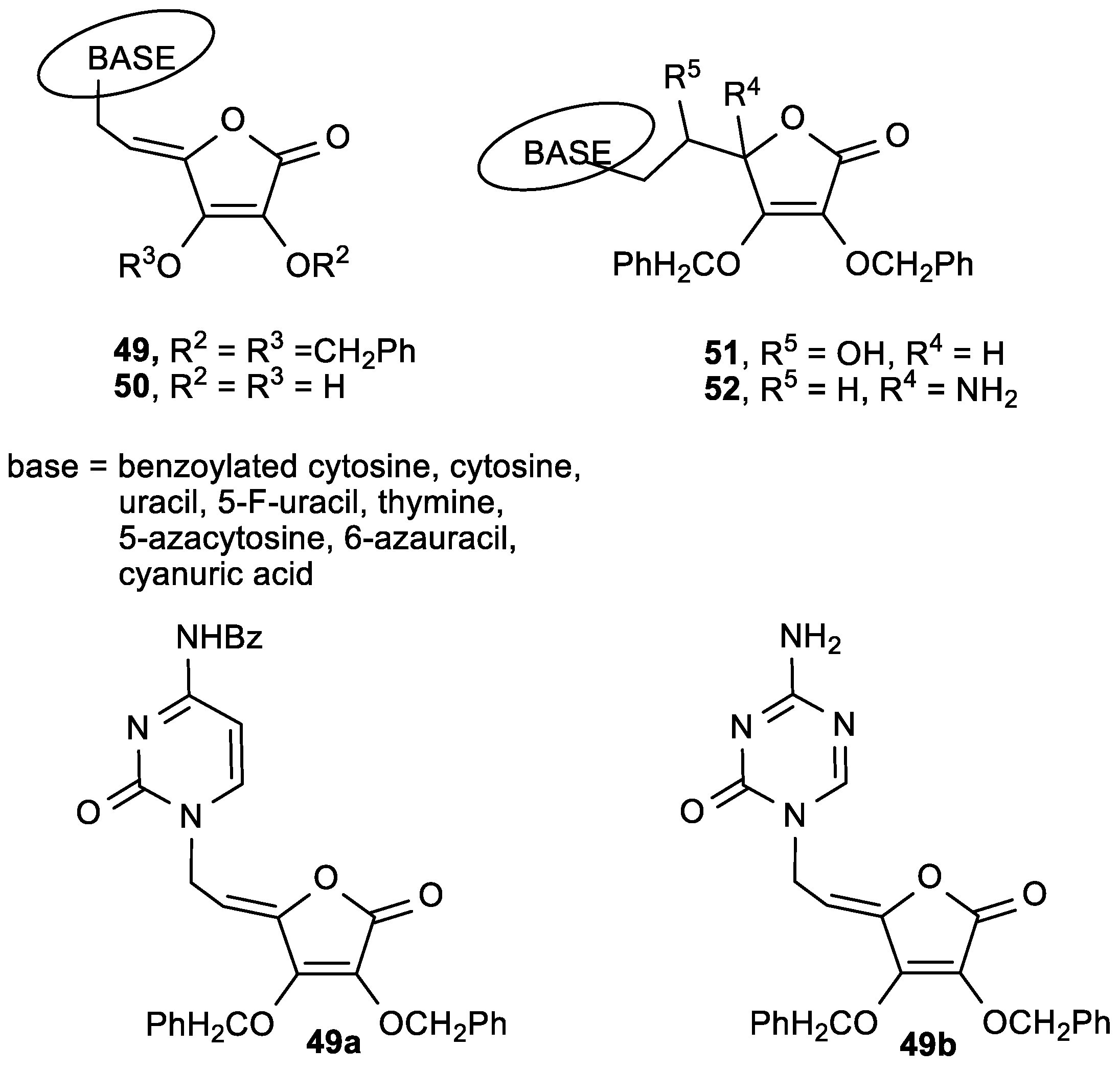

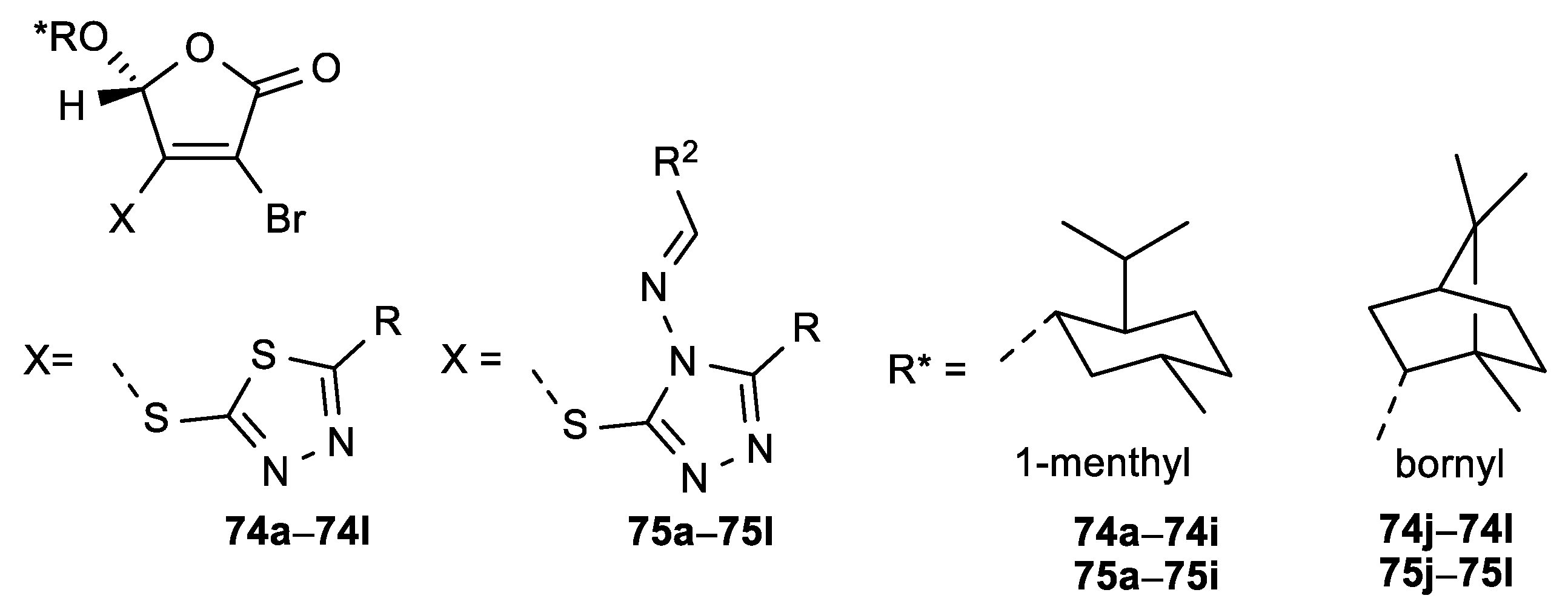
| Comp. | LO Inhibition | Scaveng. of DPPH (%) | Peak Potential (mV) c | |
|---|---|---|---|---|
| (%) a | IC50 b (μM) | |||
| 9a, R2 = (CH2)17CH3 | 88 | >90 | 240 | |
| 10a, R3 = (CH2)11CH3 | 88 | 3.1 | 61 | 300 |
| 10b, R3 = CH2CO(CH2)9CH3 | 88 | 3.3 | 39 | 380 |
| ASA | >90 | 210 | ||
| Comp. | Cytotoxicity | Radical Scaveng. Activity | |
|---|---|---|---|
| IC50 (×10−5 M) | EC50 (×10−5 M) | IC50/EC50 | |
| 11a, 6-Laur-ASA-3P | 8.8 | 7.6 | 1.16 |
| 11b, 6- Myri-ASA-3P | 5.3 | 6.5 | 0.82 |
| 11c, 6-Palmi-ASA-3P | 4.7 | 6.9 | 0.68 |
| 11d, 6-Stear-ASA-3P | 4.4 | 7.3 | 0.60 |
| Comp. | Scaveng. of DPPH |
|---|---|
| EC50 (μM) | |
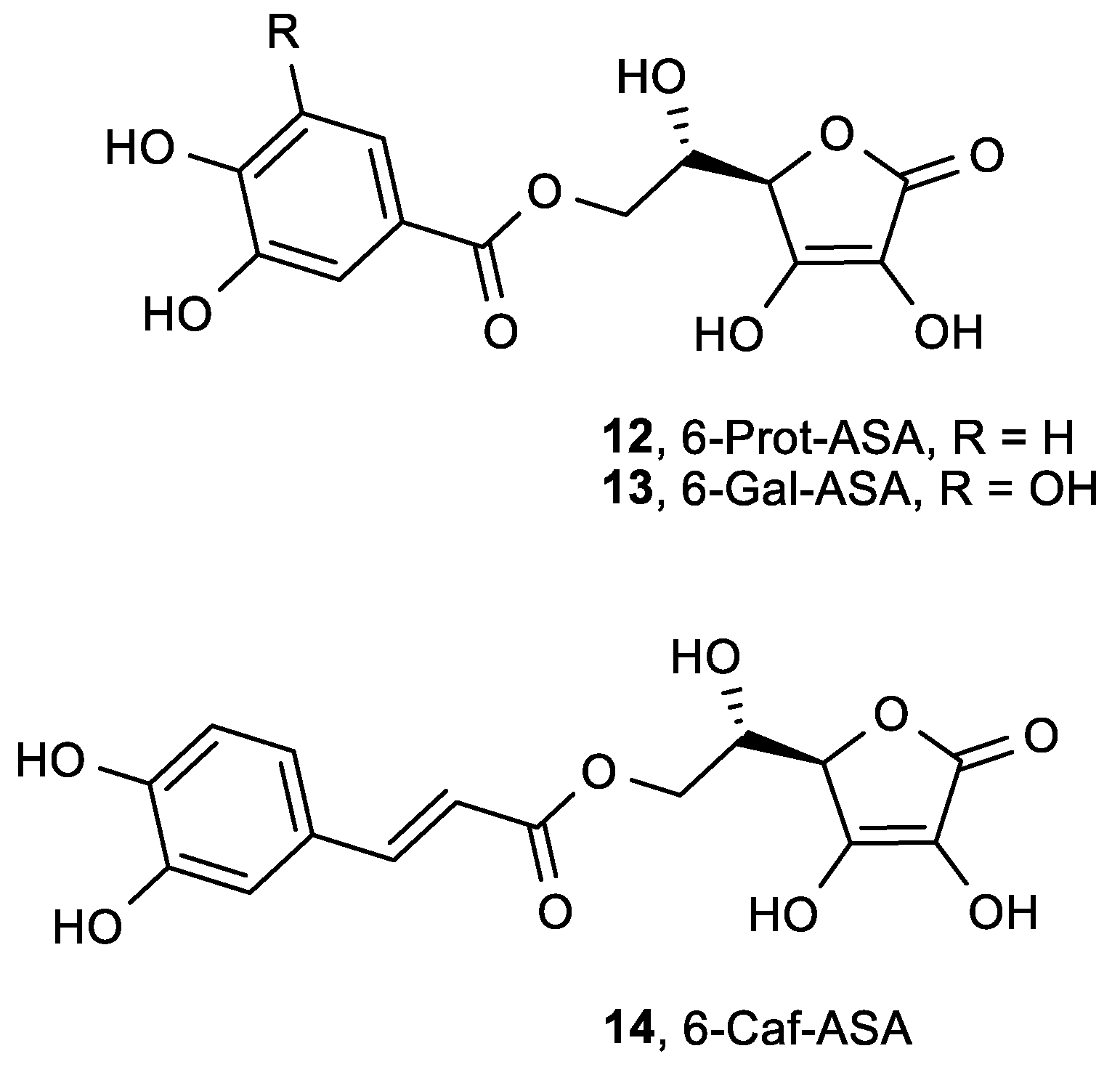
| 12, 6-Prot-ASA | 0.08 |
| 13, 6-Gal-ASA | 0.11 |
| 14, 6-Caf-ASA | 0.20 |
| ASA | 0.42 |
| Protocatechuic acid | 0.17 |
| Gallic acid | 0.10 |
| Caffeic acid | 0.17 |
| Comp. | Scaveng. of DPPH | |
|---|---|---|
| (%) | EC50 (×10−5 M) | |
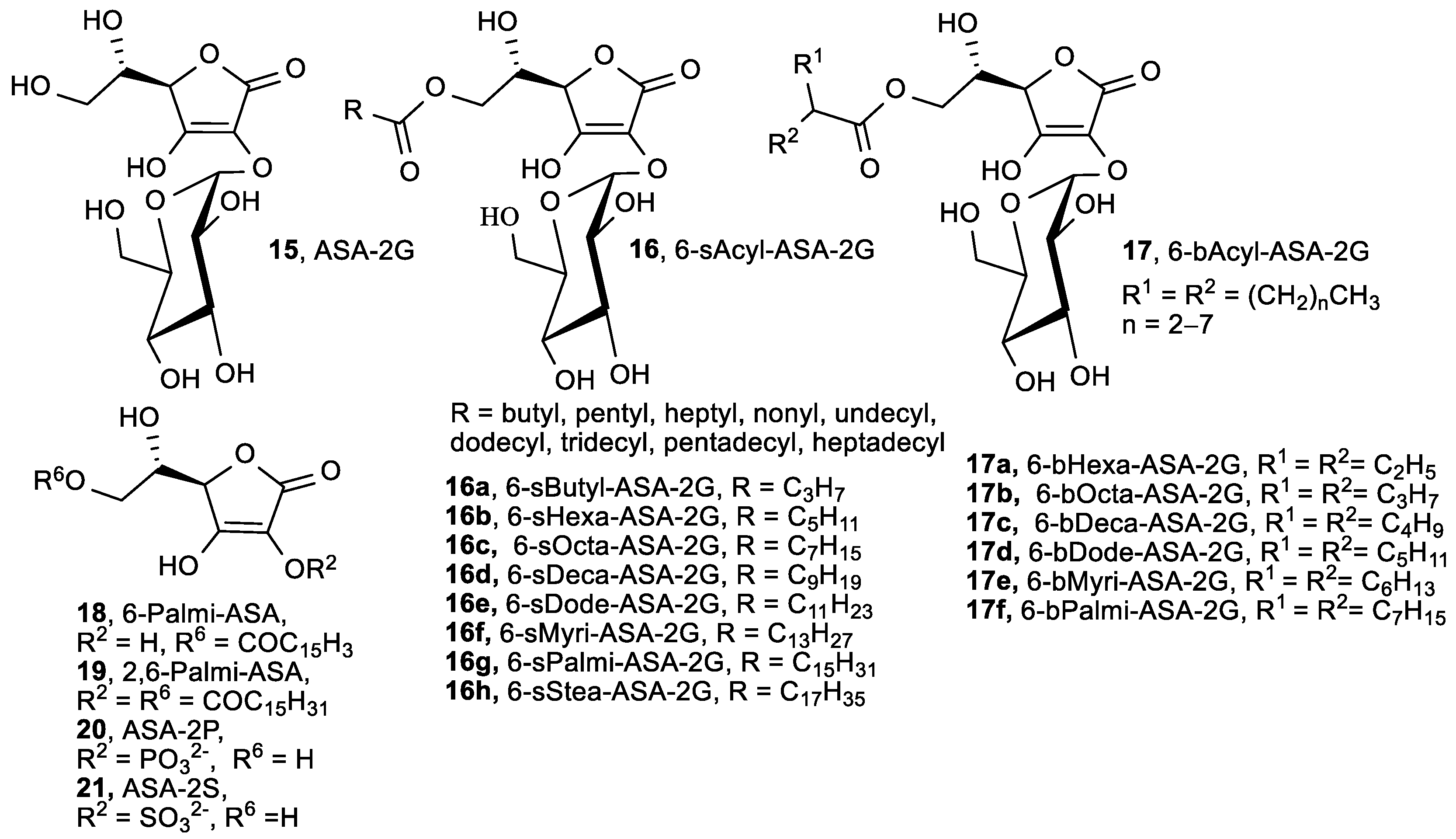
| 15, ASA-2G | 68.9 | 4.5 |
| 16, 6-sAcyl-ASA-2G | 69.2–77.5 | 4.1–5.9 |
| 17, 6-bAcyl-ASA-2G | 3.1–3.7 | |
| 18, 6-Palmi-ASA | 95.7 | 2.9 |
| 19, 2,6-Palmi-ASA | 23.7 | |
| 20, ASA-2P | 1.5 | |
| 21, ASA-2S | 0.0 | |
| ASA | 94.3 | 2.4 |
| α-Tocopherol | 90.9 | 1.9 |
| Comp. | LO a Inhibition IC50 (μM) |
|---|---|
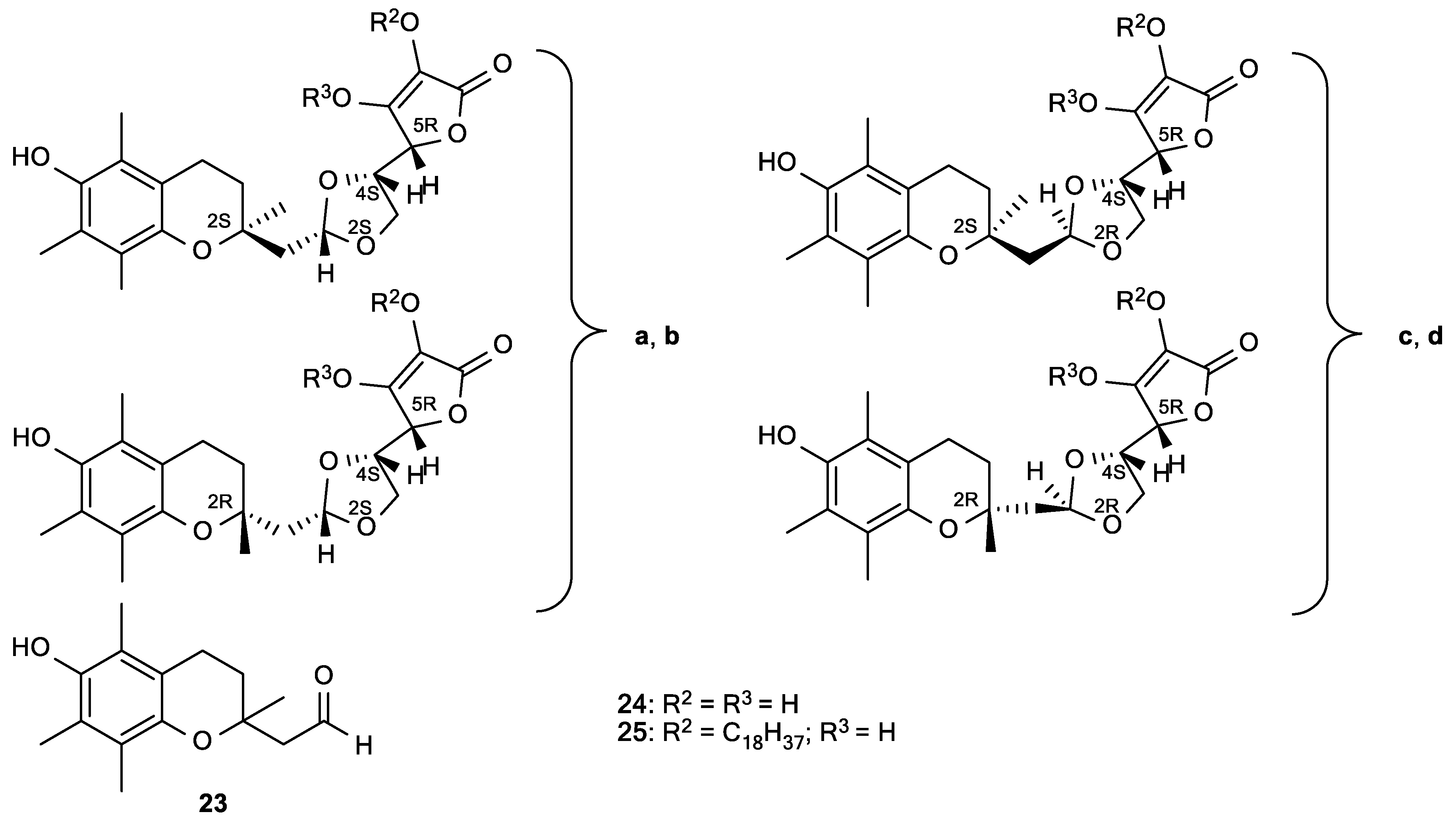
| 23 | 12 |
| 24a, 24b | 12 |
| 24c, 24d | 7 |
| ASA | 14 |
| α-tocopherol | 37 |
| Comp. | EC50/μM | LO a Inhib. | ||
|---|---|---|---|---|
| OH Inhib. | DPPH Inhib. | Cu2+ b/μM | AAPH b/μM | |
| 26 | 2 | 16 | 0.1 | 0.18 |
| ASA | 30 | 13 | 2.5 | - |
| Comp. | Scaveng. of DPPH (%) | Scaveng. of Superoxide Anion |
|---|---|---|
| 27a, R = 2-OCH3-Ph, R4 = H | 55.0 | 58% = IC50 = 3.63 × 10−3 M |
| 27b, R = 3-OH-Ph, R4 = H | 33.2 | 91% = IC50 = 1.45 × 10−3 M |
| 27c, R = 2-OH-Ph, R4 = H | 51.1 | NT a |
| 27d, R = 3-OH-Ph, R4 = 2-OH-Ph | 47.7 | 89% = IC50 = 1.35 × 10−3 M |
| ASA | 93.3 | 24% |
| Comp. | Scaveng. of DPPH IC50 (μM) | Scaveng. of Superoxide Anion IC50 (μM) | LO a Inhibit. IC50 (μM) |
|---|---|---|---|
| 28a, Ar3 = Ph, Ar4 = 2,3-diOH-Ph | 10.3 | 0.187 | 0.129 |
| ASA | 46.6 | 24 | Prooxidant |
| Vitamin E | 19.6 | NT b | NT b |
| Comp. | EC50 (µg/mL) P388D1 |
|---|---|
| Ascorbic acid and isomers | 3 |
| 18, 6-Palmi-ASA | 2 |
| 31a, ASA-6-stearate (6-Stear-ASA) | 2.5 |
| 29a, 6-Cl-ASA | 1 |
| 29b, 6-Br-ASA | 1 |
| 32a, 3,4-dihydroxytetrone, R5 = H | 3 |
| 32b, 3,4-dihydroxy-5-methyltetrone, R5 = CH3 | 2 |
| 32c, 3,4-dihydroxy-5-ethyltetrone, R5 = ethyl | 0.6 |
| 32d, 3,4-dihydroxy-5-vinylltetrone, R5 = vinyl | 0.35 |
| Comp. | IC50 (μM) | |||||
|---|---|---|---|---|---|---|
| HeLa | MCF-7 | MiaPaCa-2 | Hep2 | SW620 | WI38 | |
| 34a, R6 = Cl | 17 | 17 | 18 | 17 | 19 | 26 |
| IC50 (mM) | ||
|---|---|---|
| ASA | 36, K873 | |
| HuH7 | 1.0 | 0.1 |
| HT29 | 1.5 | 0.15 |
| Raji | 1.3 | 0.086 |
| CCL155 | 2.0 | 0.1 |
| Comp. | EC50 (μM) HSV-1 | MNTC a (μM) |
|---|---|---|
| 15 | 16.3 | 1183 |
| 16g | 4.5 | 86.81 |
| ASA | >2270 | 2270 |
| Comp. | IC50 (μM) | ||||
|---|---|---|---|---|---|
| L1210/0 | FM3A/0 | Molt4/C8 | CEM/0 | Hef522 | |
| 37a, R5 = H | 12.9 | 17.7 | 4.8 | 13.0 | 50 |
| 37b, R5 = F | 5.1 | 3.4 | 10.9 | 15.1 | 40 |
| 37c, R5 = Cl | 12.2 | 16.9 | 6.1 | 6.0 | 30 |
| 37d, R5 = Br | 7.5 | 17.1 | 3.9 | 3.5 | 30 |
| 37e, R5 = I | 7.5 | 22.6 | 3.9 | 3.3 | 40 |
| 37f, R5 = CF3 | 2.0 | 3.6 | 0.9 | 1.6 | 60 |
| 38a, R6 = Cl | 11.6 | 16.4 | 14.8 | 15.9 | 50 |
| 38b, R6 = pyrrolyl | 110 | >200 | 3.9 | 5.8 | >100 |
| 39 | 4.1 | 11.4 | 6.8 | 4.4 | 20 |
| Comp. | EC50 (μM) | CC50 (μM) | |||||
|---|---|---|---|---|---|---|---|
| TK+VZV | TK−VZV | CMV | |||||
| YS | OKA | 07/1 | YS/R | AD-169 | Davis | ||
| 37f, R5 = CF3 | 0.5 | 0.3 | 0.5 | 0.3 | >0.5 | 0.4 | 1 |
| 38b, R6 = pyrrolyl | 1.5 | 1.2 | 2.8 | 0.6 | >0.5 | >0.5 | 4 |
| 39 | >5 | 5.0 | >5 | 3.8 | >5 | >5 | 20 |
| ACV | 0.78 | 0.16 | 17 | 12 | NT a | NT a | >100 |
| BVDU | 0.005 | 0.001 | >50 | >50 | NT a | NT a | >100 |
| DHPG | NT a | NT a | NT a | NT a | 1 | 5 | >50 |
| HPMPC | NT a | NT a | NT a | NT a | 0.11 | 1 | NT a |
| IC50 (μg/mL) | ||||
|---|---|---|---|---|
| L1210/0 | FM3A/0 | Molt4/C8 | CEM/0 | |
| 41b, R5 = F | 1.4 | 0.78 | 31.8 | 20.9 |
| 5-FU | 0.04 | 0.02 | 2.9 | 1.2 |
| 5-FdUrd | 0.0003 | 0.0008 | 2.6 | 0.003 |
| Comp. | IC50 (μM) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| L1210/0 | Molt4/C8 | CEM/0 | HeLa | MCF-7 | MiaPaCa-2 | Hep-2 | SW 620 | WI 38 | |
| (Z)-41b, R5 = F | 5.2 | 118 | 77.4 | 50 | 59.3 | 58.1 | >100 | >100 | 42.6 |
| (Z)-41c, R5 = CF3 | >200 | >200 | >200 | 5.6 | 8.8 | 12.8 | 5.6 | 8.8 | 11.6 |
| 44a, R6 = Cl | 21.8 | 19.8 | 22.9 | 6.8 | 14.3 | 6.5 | 14.8 | 20 | 16.1 |
| 5-FU | 0.69 | 20 | 9.23 | 16 | 4.5 | 6.5 | 51 | 8.7 | 10 |
| ASA | >200 | >200 | >200 | >200 | >200 | >200 | >200 | >100 | >200 |
| DiBnASA | 199.4 | 143.2 | 151.7 | >100 | >200 | >100 | >200 | >100 | >100 |
| Comp. | IC50 (μM) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| L1210 | Molt4/C8 | CEM/0 | HeLa | MCF-7 | MiaPaCa-2 | Hep-2 | SW 620 | WI 38 | |
| 45a, R5 = propynyl | 0.47 | 0.78 | 0.77 | 0.2 | 0.2 | 0.5 | 0.7 | 0.3 | 0.2 |
| 45b, R5 = furyl | 6.8 | 6.6 | 7.0 | 2 | 2 | 1.6 | 2.2 | 2.4 | 1.8 |
| 45c, R5 = vinyl | 7.2 | 7.6 | 7.2 | 14.5 | 3.8 | 22 | 14.2 | 15.6 | 14.3 |
| 45d, R5 = ethynyl | 6.8 | 7.2 | 4.6 | 3.2 | 2.9 | 8.7 | 4.6 | 5 | 1.5 |
| 45e, R5 = isopentenyl | 8.2 | 8.3 | 7.7 | 2 | 4 | 5 | 2.6 | 4 | 2 |
| 46a, R5 = ethynyl-Ph | 8.1 | 8.2 | 7.6 | 2 | 1 | 3 | 3 | 4 | 1 |
| Comp. | EC50 (μM) | CC50(μM) Cytotoxicity | ||
|---|---|---|---|---|
| Sindbis Virus | Coxsackie Virus B4 | Vesicular Stomatitis Virus | ||
| 45a, R5 = propynyl | 1.6 | 1.6 | 1.6 | 8 |
| Ribavirin | 100 | >500 | 100 | >500 |
| Comp. | IC50 (μM) | |||||||
|---|---|---|---|---|---|---|---|---|
| L1210/0 | Molt4/C8 | CEM/0 | HeLa | MiaPaCa-2 | SW 620 | MCF-7 | H-460 | |
| 47a, R = hexyl | 6.8 | 3.0 | 2.0 | 12 | 3 | 4 | 4 | 2.4 |
| 47b, R = 4-Br-Ph | 10 | 15 | 7.6 | 8 | 13 | 7 | 21 | 11 |
| 47c, R = 4-CH3-Ph | 8.7 | 37 | 9.6 | 14 | 21 | 18 | 19 | 23 |
| 47d, R = 4-butyl-Ph | 8.2 | 9.3 | 8.3 | 4 | 16 | 7 | 17 | 12 |
| 47e, R = 4-pentyl-Ph | 8.0 | 6.9 | 6.6 | 3 | 10 | 6 | 9 | NT a |
| 48a, R = butyl | 4.5 | 9.0 | 7.7 | 17 | 15 | 16 | 16 | 16 |
| 48b, R = 4-Br-Ph | 9.5 | 9.6 | 8.3 | 20 | 14 | 20 | 13 | 16 |
| Comp. | EC50 (μM) | Cytotoxicity CC50 (μM) |
|---|---|---|
| CMV (Davis) | ||
| 47a, R = hexyl | 1.8 | 10 |
| 47c, R = 4-CH3-Ph | 3.8 | >20.4 |
| Ganciclovir | 2.6 | 262 |
| Cidofovir | 0.67 | 133 |
| Comp. | IC50 (μM) | |||||
|---|---|---|---|---|---|---|
| HeLa | MCF-7 | HepG2 | SW 620 | MiaPaCa-2 | WI 38 | |
| 49a | 7.20 | 2.03 | 1.98 | >100 | 24.24 | 8.49 |
| 49b | 5.91 | 3.49 | 2.72 | 5.62 | 0.92 | 4.67 |
| Comp. | IC50 (μM) | ||||||
|---|---|---|---|---|---|---|---|
| L1210/0 | CEM/0 | HeLa | MiaPaCa-2 | Hep-G2 | SW620 | 3T3 | |
| 57 | 4.7 | 4.1 | 12 | 33.9 | >100 | 47.1 | >100 |
| (Z, Z)-59 | 4.5 | 19 | 5.6 | 26.2 | >100 | 47.2 | >100 |
| 5-FU | NT a | NT a | 66.5 | 11.67 | 55.2 | 0.79 | 28.3 |
| Comp. | EC50 (μM) HCMV | CC50 (μM) | |
|---|---|---|---|
| AD-169 | Davis Line | HeLa | |
| 54a, R1 = R3 = F, R2 = Cl, R4 = CH2Ph | 8.94 | 8.94 | >100 |
| Ganciclovir | 6.12 | 4.72 | 328.5 |
| Comp. | IC50 (μM) | CC50 (μM) | IC50 (μM) | ||||
|---|---|---|---|---|---|---|---|
| L1210/0 | P388 | MCF-7 | Molt4/C8 | CEM/0 | Hef522 | TK-VZV (YS/R) | |
| 60a | 4.52 | 8.12 | 16.87 | 5.97 | 4.71 | 30.47 | 1.62 |
| 60b | 5.81 | 6.49 | 15.43 | 7.35 | 3.98 | 27.86 | 1.03 |
| 60c | 6.03 | 7.42 | 14.21 | 6.16 | 5.04 | 32.21 | 0.89 |
| 61 | 93.35 | 2.67 | >120 | 78.46 | 84.51 | 93.80 | 6.07 |
| 64c | 1.03 | 0.28 | 0.76 | 1.74 | 0.98 | 17.40 | - |
| ara-C | 0.17 | 0.14 | 1.03 | 0.65 | 0.78 | 2.14 × 10−2 | - |
| Acyclovir | 4.52 | - | - | - | - | - | 26.43 |
| Comp. | E. coli RDPR inhib. (%) a | |
|---|---|---|
| 100 μM | 1000 μM | |
| 60a | 59.47 | 9.94 |
| 60b | 58.96 | 11.07 |
| 60c | 55.34 | 10.20 |
| 63a | >99 | >99 |
| 64c | <1 | 0 |
| Comp. | LD50 (mg/kg) | IC50 (μg/mL) | |||
|---|---|---|---|---|---|
| HSV-1 | HIV-1 | HIV-2 | MSV | MT4 | |
| 66 | 710 | 1.4 | 1.0 | 0.93 | 265 |
| 67 | - | 6.0 | 7.1 | 0.02 | 14 |
| 68 | 675 | 4.9 | 4.2 | 13 | >300 |
| PMEA | - | 4.1 | 3.8 | 2.0 | 274 |
| PMEG | - | 16 | 18 | 0.19 | 16 |
| Comp. | IC50 (μM) | EC50 (μg/mL) | CC50 (μg/mL) | |
|---|---|---|---|---|
| CEM/0 | WI 38 | Hepatitis C | MT4 | |
| 71a | 10 | >100 | 13.3 | 70.5 |
| 71b | 7.3 | 73 | >100 | >100 |
| Doxorubicin | 0.39 | 0.04 | - | - |
| Comp. | EC50 (μM) | CC50 (μM) |
|---|---|---|
| A/WSN/33 | ||
| 72b | 8.7 | >200 |
| Oseltamivir | 12.5 | >200 |
| Comp. | IC50 (μM) | Comp. | IC50 (μM) |
|---|---|---|---|
| HeLa | HeLa | ||
| 74a, R = Ph | 3.0 | 75a, R = Ph, R2 = 4-Cl-Ph | 19.7 |
| 74b, R = 4-Cl-Ph | 2.2 | 75b, R = 4-OCH3-Ph, R2 = 4-Cl-Ph | 4.4 |
| 74c, R = 2-OH-Ph | 2.9 | 75c, R = Ph, R2 = 4-NO2-Ph | 11.6 |
| 74d, R = 4-OH-Ph | 2.6 | 75d, R = 4-OCH3-Ph, R2 = 4-NO2-Ph | 11.2 |
| 74e, R = 4-NO2-Ph | 0.9 | 75e, R = Ph, R2 = 2-furyl | 6.8 |
| 74f, R = 4-OCH3-Ph | 3.7 | 75f, R = 4-OCH3-Ph, R2 = 2-furyl | 5.1 |
| 74g, R = 2-furyl | 1.7 | 75g, R = CH3, R2 = 4-Cl-Ph | 8.2 |
| 74h, R = pyridine-4-yl | 1.3 | 75h, R = 4-OH-Ph, R2 = 4-OCH3-Ph | 7.1 |
| 74i, R = pyridine-3-yl | 5.6 | 75i, R = Ph, R2 = 4-OH-Ph | 3.7 |
| 74j, R = 2-furyl | 1.3 | 75j, R = Ph, R2 = 4-Cl-Ph | 4.5 |
| 74k, R = pyridine-4-yl | 3.0 | 75k, R = 4-OH-Ph, R2 = 4-OCH3-Ph | 6.2 |
| 74l, R = pyridine-3-yl | 3.4 | 75l, R = CH3, R2 = 2-OH-Ph | 1.8 |
| Cisplatin | 2.6 |
| Comp. | IC50 (μM) | Comp. | IC50 (μM) | ||
|---|---|---|---|---|---|
| HeLa | HeLa | ||||
| 76a, R = | (CH2CH3)2N- | 1.39 | 76f, R = |  | 2.43 |
| 76b, R = |  | 24.14 | 76g, R = |  | 5.44 |
| 76c, R = |  | 150.03 | 76h, R = |  | 15.08 |
| 76d, R = |  | 2.63 | 5-FU | 41.46 | |
| 76e, R = |  | 0.77 | |||
| Comp. | IC50 (μM) |
|---|---|
| HeLa, A-549, HT-29, HL-60 | |
| 79b, (3′R) | 3.3–11.1 |
| 80a, (3′S) | 3.2–4.8 |
| 80b, (3′R) | 3.3–5.4 |
| 81a, (3′S) | 2.6–3.5 |
| 81b, (3′R) | 2.9–3.5 |
| 82a, (3′S) | 3.0–3.8 |
| 82b, (3′R) | 2.3–3.8 |
| 83 | 0.3–3.1 |
| Comp. | IC50 (μM) | ||
|---|---|---|---|
| HeLa | RKO | WI-26 VA4 | |
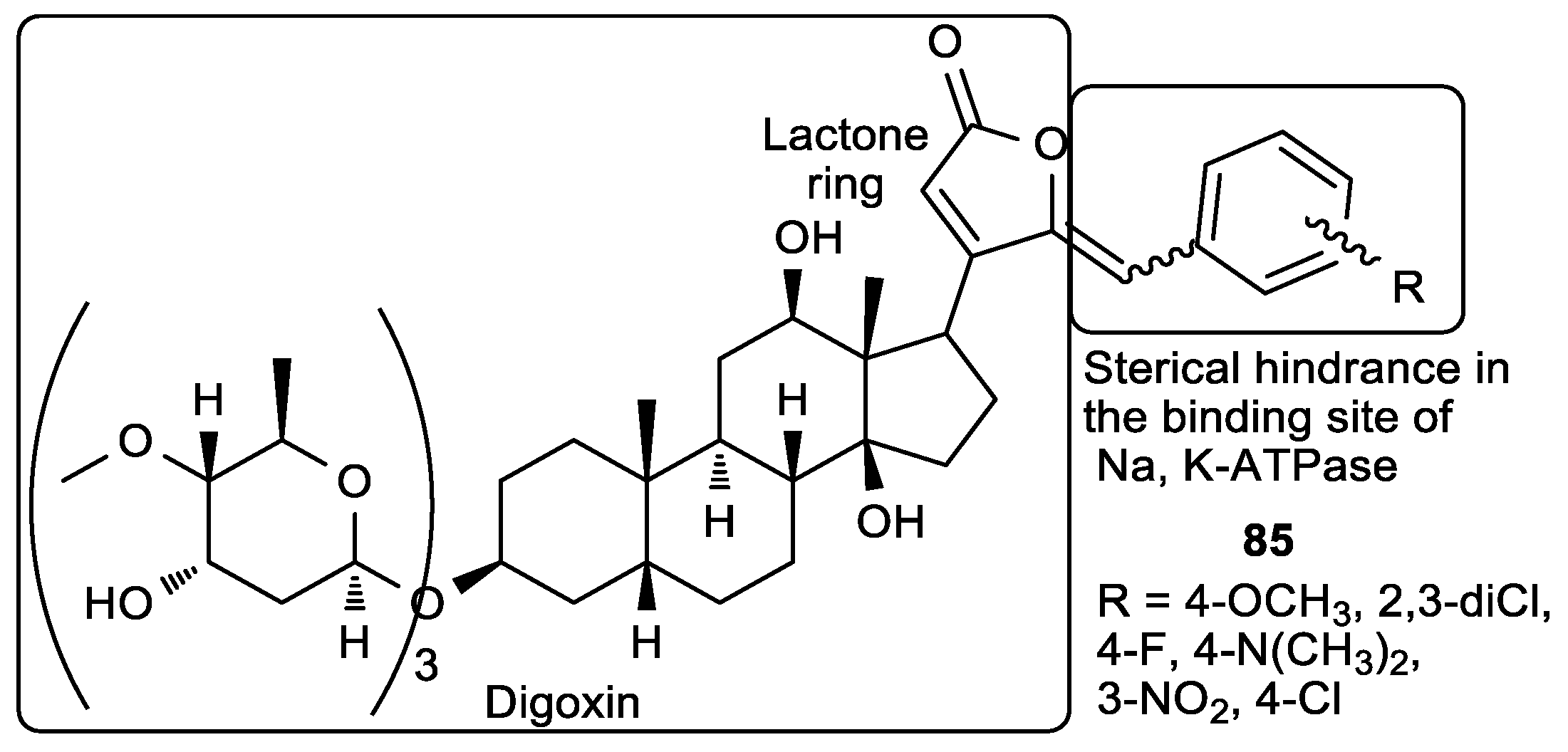
| (Z)-85a, R = 4-N(CH3)2 | 0.26 | 0.48 | 0.65 |
| Digoxin | 2.2 | 0.42 | NT a |
| Comp. | EC50 (μg/mL) | CC50 (μg/mL) |
|---|---|---|
| Influenza A (H1N1) | A549; MDCK | |
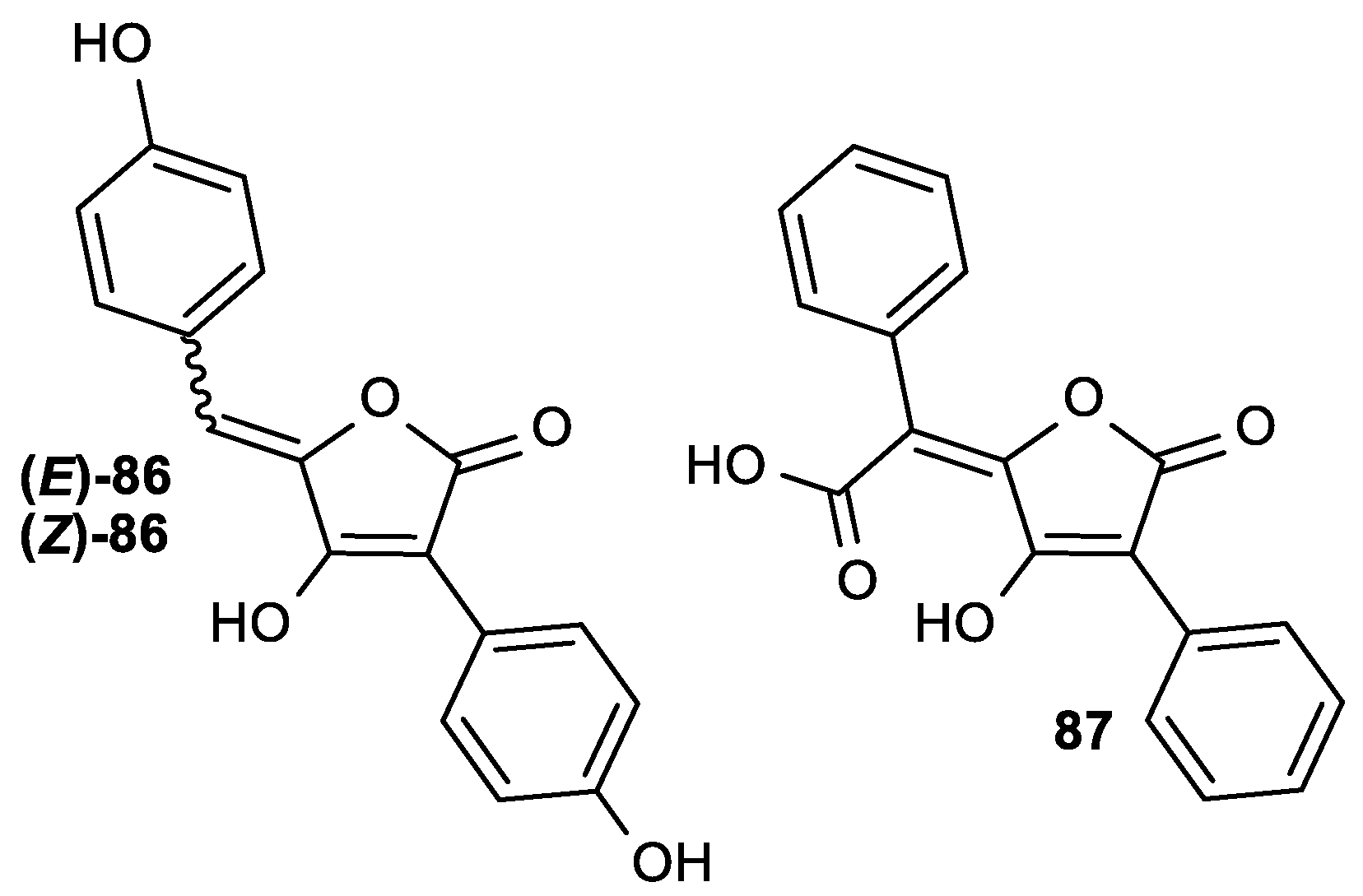
| (E)-86 | 32.3 | >250 |
| (Z)-86 | 56.9 | >250 |
| 87 | 29.1 | >250 |
| Ribavirin | 24.6 | |
| Zanamivir | 28.4 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Meščić Macan, A.; Gazivoda Kraljević, T.; Raić-Malić, S. Therapeutic Perspective of Vitamin C and Its Derivatives. Antioxidants 2019, 8, 247. https://doi.org/10.3390/antiox8080247
Meščić Macan A, Gazivoda Kraljević T, Raić-Malić S. Therapeutic Perspective of Vitamin C and Its Derivatives. Antioxidants. 2019; 8(8):247. https://doi.org/10.3390/antiox8080247
Chicago/Turabian StyleMeščić Macan, Andrijana, Tatjana Gazivoda Kraljević, and Silvana Raić-Malić. 2019. "Therapeutic Perspective of Vitamin C and Its Derivatives" Antioxidants 8, no. 8: 247. https://doi.org/10.3390/antiox8080247
APA StyleMeščić Macan, A., Gazivoda Kraljević, T., & Raić-Malić, S. (2019). Therapeutic Perspective of Vitamin C and Its Derivatives. Antioxidants, 8(8), 247. https://doi.org/10.3390/antiox8080247






