Rationale on the High Radical Scavenging Capacity of Betalains
Abstract
1. Introduction
2. Materials and Methods
2.1. General
2.2. Semisynthesis of Betalains
2.3. Radical Scavenging Capacity
2.4. Computational Methods
3. Results and Discussion
3.1. Semisynthesis and Electronic Properties of pBeets and mepBeets
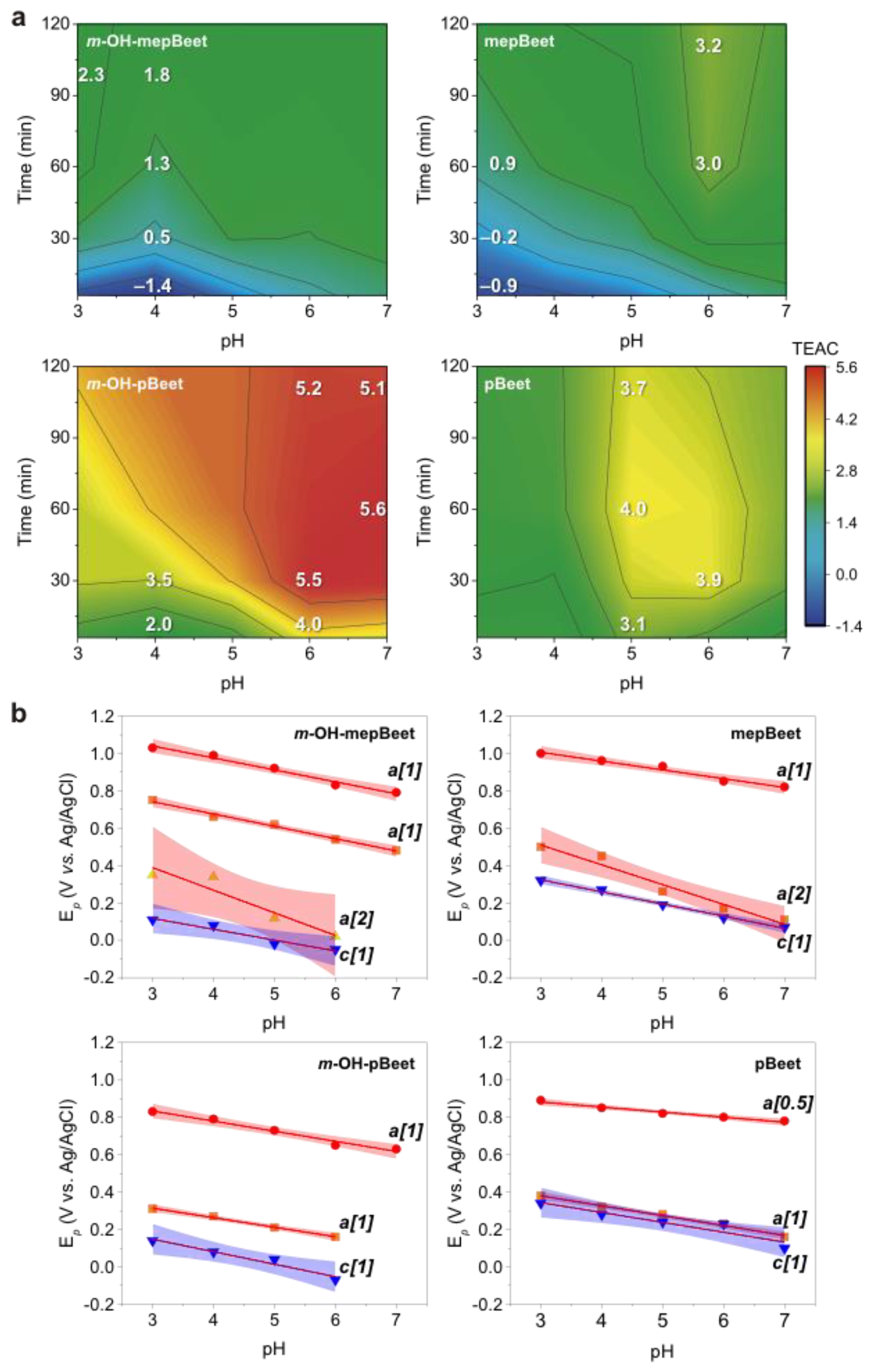
3.2. Radical Scavenging Capacity
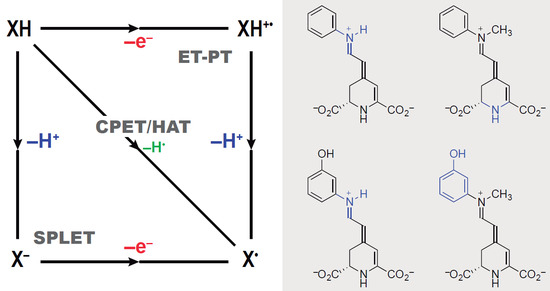
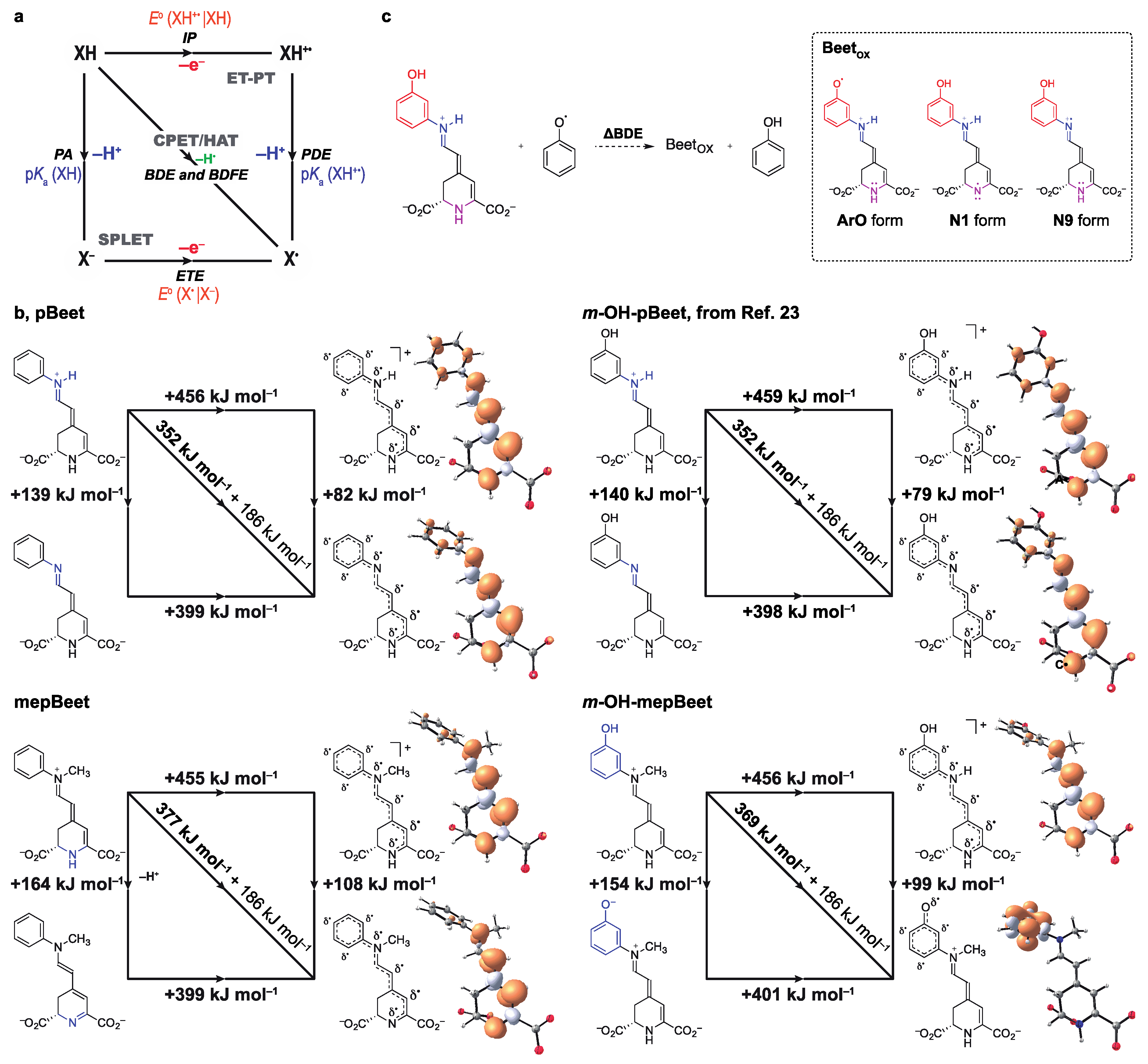
3.3. Mechanisms of Radical Scavenging by Betalains
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Koppenol, W.H.; Hider, R.H. Iron and redox cycling. Do’s and don’ts. Free Radic. Biol. Med. 2019, 133, 3–10. [Google Scholar] [CrossRef]
- Apel, K.; Hirt, H. Reactive oxygen species: Metabolism, oxidative stress, and signal transduction. Annu. Rev. Plant Biol. 2004, 55, 373–399. [Google Scholar] [CrossRef] [PubMed]
- Winterbourn, C.C. Reconciling the chemistry and biology of reactive oxygen species. Nat. Chem. Biol. 2008, 4, 278–286. [Google Scholar] [CrossRef] [PubMed]
- Sies, H. Oxidative stress: A concept in redox biology and medicine. Redox Biol. 2015, 4, 180–183. [Google Scholar] [CrossRef] [PubMed]
- Mittler, R. ROS Are Good. Trends Plant Sci. 2017, 22, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Herbert, V. The antioxidant supplement myth. Am. J. Clin. Nutr. 1994, 60, 157–158. [Google Scholar] [CrossRef]
- Prior, R.L.; Cao, G. Analysis of botanicals and dietary supplements for antioxidant capacity: A review. J. AOAC Int. 2000, 83, 950–956. [Google Scholar]
- Frei, B. Reactive oxygen species and antioxidant vitamins: Mechanisms of action. Am. J. Med. 1994, 97, S5–S13. [Google Scholar] [CrossRef]
- Salehi, B.; Martorell, M.; Arbiser, J.L.; Sureda, A.; Martins, N.; Maurya, P.K.; Sharifi-Rad, M.; Kumar, P.; Sharifi-Rad, J. Antioxidants: Positive or Negative Actors? Biomolecules 2018, 8, 124. [Google Scholar] [CrossRef]
- Rietjens, I.M.; Boersma, M.G.; Haan, L.; Spenkelink, B.; Awad, H.M.; Cnubben, N.H.; van Zanden, J.J.; Woude, H.; Alink, G.M.; Koeman, J.H. The pro-oxidant chemistry of the natural antioxidants vitamin C, vitamin E, carotenoids and flavonoids. Environ. Toxicol. Pharmacol. 2002, 11, 321–333. [Google Scholar] [CrossRef]
- Graham, H.N. Green tea composition, consumption, and polyphenol chemistry. Prev. Med. 1992, 21, 334–350. [Google Scholar] [CrossRef]
- Polturak, G.; Aharoni, A. “La vie en rose”: Biosynthesis, sources, and applications of betalain pigments. Mol. Plant 2018, 11, 7–22. [Google Scholar] [CrossRef] [PubMed]
- Davies, K.M. Swapping one red pigment for another. Nat. Genet. 2015, 47, 5–6. [Google Scholar] [CrossRef] [PubMed]
- Osbourn, A. Painting with betalains. Nat. Plants 2017, 3, 852–853. [Google Scholar] [CrossRef] [PubMed]
- Quina, F.H.; Bastos, E.L. Chemistry Inspired by the Colors of Fruits, Flowers and Wine. An. Acad. Bras. Cienc. 2018, 90, 681–695. [Google Scholar] [CrossRef] [PubMed]
- Esteves, L.C.; Pinheiro, A.C.; Pioli, R.M.; Penna, T.C.; Baader, W.J.; Correra, T.C.; Bastos, E.L. Revisiting the mechanism of hydrolysis of betanin. Photochem. Photobiol. 2018, 94, 853–864. [Google Scholar] [CrossRef]
- Treat, N.A.; Knorr, F.J.; McHale, J.L. Templated assembly of betanin chromophore on TiO2: Aggregation-enhanced light-harvesting and efficient electron injection in a natural dye-sensitized solar cell. J. Phys. Chem. C 2016, 120, 9122–9131. [Google Scholar] [CrossRef]
- Pavliuk, M.V.; Cieslak, A.M.; Abdellah, M.; Budinska, A.; Pullen, S.; Sokolowski, K.; Fernandes, D.L.A.; Szlachetko, J.; Bastos, E.L.; Ott, S.; et al. Hydrogen evolution with nanoengineered ZnO interfaces decorated using a beetroot extract and a hydrogenase mimic. Sustain. Energy Fuels 2017, 1, 69–73. [Google Scholar] [CrossRef]
- Pavliuk, M.V.; Fernandes, A.B.; Abdellah, M.; Fernandes, D.L.A.; Machado, C.O.; Rocha, I.; Hattori, Y.; Paun, C.; Bastos, E.L.; Sa, J. Nano-hybrid plasmonic photocatalyst for hydrogen production at 20% efficiency. Sci. Rep. 2017, 7, 8670. [Google Scholar] [CrossRef]
- Fernandes, D.L.A.; Paun, C.; Pavliuk, M.V.; Fernandes, A.B.; Bastos, E.L.; Sa, J. Green microfluidic synthesis of monodisperse silver nanoparticles via genetic algorithm optimization. RSC Adv. 2016, 6, 95693–95697. [Google Scholar] [CrossRef]
- Martinez, R.M.; Longhi-Balbinot, D.T.; Zarpelon, A.C.; Staurengo-Ferrari, L.; Baracat, M.M.; Georgetti, S.R.; Sassonia, R.C.; Verri, W.A.; Casagrande, R. Anti-inflammatory activity of betalain-rich dye of Beta vulgaris: Effect on edema, leukocyte recruitment, superoxide anion and cytokine production. Arch. Pharm. Res. 2015, 38, 494–504. [Google Scholar] [CrossRef] [PubMed]
- Esatbeyoglu, T.; Wagner, A.E.; Schini-Kerth, V.B.; Rimbach, G. Betanin—A food colorant with biological activity. Mol. Nutr. Food Res. 2015, 59, 36–47. [Google Scholar] [CrossRef] [PubMed]
- Parikh, I.; Hilpert, H.; Hermann, K.; Dreiding, A.S. Synthese von Betenamin und von Betalain-Modellsubstanzen. Helv. Chim. Acta 1986, 69, 1588–1596. [Google Scholar] [CrossRef]
- Khan, M.I. Plant betalains: Safety, antioxidant activity, clinical efficacy, and bioavailability. Compr. Rev. Food Sci. Food Saf. 2016, 15, 316–330. [Google Scholar] [CrossRef]
- Gliszczyńska-Świgło, A.; Szymusiak, H.; Malinowska, P. Betanin, the main pigment of red beet: Molecular origin of its exceptionally high free radical-scavenging activity. Food Addit. Contam. A 2006, 23, 1079–1087. [Google Scholar] [CrossRef]
- Albano, C.; Negro, C.; Tommasi, N.; Gerardi, C.; Mita, G.; Miceli, A.; De Bellis, L.; Blando, F. Betalains, Phenols and Antioxidant Capacity in Cactus Pear [Opuntia ficus-indica (L.) Mill.] Fruits from Apulia (South Italy) Genotypes. Antioxidants 2015, 4, 269–280. [Google Scholar] [CrossRef]
- Gonçalves, L.C.P.; Lopes, N.B.; Augusto, F.A.; Pioli, R.M.; Machado, C.O.; Freitas-Dörr, B.C.; Suffredini, H.B.; Bastos, E.L. Phenolic betalain as antioxidants: Meta means more. Pure Appl. Chem. 2019, in press. [Google Scholar] [CrossRef]
- Schliemann, W.; Kobayashi, N.; Strack, D. The decisive step in betaxanthin biosynthesis is a spontaneous reaction. Plant Physiol. 1999, 119, 1217–1232. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Dunning, T.H. Gaussian basis sets for use in correlated molecular calculations. I. The atoms boron through neon and hydrogen. J. Chem. Phys. 1989, 90, 1007–1023. [Google Scholar] [CrossRef]
- Zhao, Y.; Truhlar, D.G. The M06 suite of density functionals for main group thermochemistry, thermochemical kinetics, noncovalent interactions, excited states, and transition elements: Two new functionals and systematic testing of four M06-class functionals and 12 other functionals. Theor. Chem. Acc. 2008, 120, 215–241. [Google Scholar] [CrossRef]
- Dimić, D.; Milenković, D.; Dimitrić Marković, J.; Marković, Z. Antiradical activity of catecholamines and metabolites of dopamine: Theoretical and experimental study. PCCP 2017, 19, 12970–12980. [Google Scholar] [CrossRef] [PubMed]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09, Revision D.01; Gaussian, Inc.: Wallingford, CT, USA, 2009; citeulike-article-id:9096580. [Google Scholar]
- Marković, Z.; Đorović, J.; Petrović, Z.D.; Petrović, V.P.; Simijonović, D. Investigation of the antioxidant and radical scavenging activities of some phenolic Schiff bases with different free radicals. J. Mol. Model. 2015, 21, 293. [Google Scholar] [CrossRef] [PubMed]
- Szeląg, M.; Urbaniak, A.; Bluyssen Hans, A.R. A theoretical antioxidant pharmacophore for natural hydroxycinnamic acids. Open Chem. 2015, 13, 17–31. [Google Scholar] [CrossRef]
- Marković, Z.; Tošović, J.; Milenković, D.; Marković, S. Revisiting the solvation enthalpies and free energies of the proton and electron in various solvents. Comput. Theor. Chem. 2016, 1077, 11–17. [Google Scholar] [CrossRef]
- Petrović, Z.D.; Đorović, J.; Simijonović, D.; Petrović, V.P.; Marković, Z. Experimental and theoretical study of antioxidative properties of some salicylaldehyde and vanillic Schiff bases. RSC Adv. 2015, 5, 24094–24100. [Google Scholar] [CrossRef]
- Warren, J.J.; Tronic, T.A.; Mayer, J.M. Thermochemistry of proton-coupled electron transfer reagents and its implications. Chem. Rev. 2010, 110, 6961–7001. [Google Scholar] [CrossRef] [PubMed]
- Byrne, F.P.; Jin, S.; Paggiola, G.; Petchey, T.H.M.; Clark, J.H.; Farmer, T.J.; Hunt, A.J.; Robert McElroy, C.; Sherwood, J. Tools and techniques for solvent selection: Green solvent selection guides. Sustain. Chem. Proc. 2016, 4, 7–31. [Google Scholar] [CrossRef]
- Kimler, L.; Larson, R.A.; Messenger, L.; Moore, J.B.; Mabry, T.J. Betalamic acid, a new naturally occurring pigment. J. Chem. Soc. D Chem. Commun. 1971, 1329–1330. [Google Scholar] [CrossRef]
- Gandia-Herrero, F.; Escribano, J.; Garcia-Carmona, F. Structural implications on color, fluorescence, and antiradical activity in betalains. Planta 2010, 232, 449–460. [Google Scholar] [CrossRef]
- Gandia-Herrero, F.; Escribano, J.; Garcia-Carmona, F. Betaxanthins as pigments responsible for visible fluorescence in flowers. Planta 2005, 222, 586–593. [Google Scholar] [CrossRef] [PubMed]
- Bartoloni, F.H.; Goncalves, L.C.P.; Rodrigues, A.C.B.; Dorr, F.A.; Pinto, E.; Bastos, E.L. Photophysics and hydrolytic stability of betalains in aqueous trifluoroethanol. Monatsh. Chem. 2013, 144, 567–571. [Google Scholar] [CrossRef]
- Wendel, M.; Nizinski, S.; Tuwalska, D.; Starzak, K.; Szot, D.; Prukala, D.; Sikorski, M.; Wybraniec, S.; Burdzinski, G. Time-resolved spectroscopy of the singlet excited state of betanin in aqueous and alcoholic solutions. PCCP 2015, 17, 18152–18158. [Google Scholar] [CrossRef] [PubMed]
- Wendel, M.; Szot, D.; Starzak, K.; Tuwalska, D.; Gapinski, J.; Naskrecki, R.; Prukala, D.; Sikorski, M.; Wybraniec, S.; Burdzinski, G. Photophysical properties of betaxanthins: Vulgaxanthin I in aqueous and alcoholic solutions. J. Luminesc. 2015, 167, 289–295. [Google Scholar] [CrossRef]
- Alabugin, I.V.; Gilmore, K.M.; Peterson, P.W. Hyperconjugation. Wiley Interd. Rev. Comput. Mol. Sci. 2011, 1, 109–141. [Google Scholar] [CrossRef]
- Gonçalves, L.C.P.; Di Genova, B.M.; Dörr, F.A.; Pinto, E.; Bastos, E.L. Effect of dielectric microwave heating on color and antiradical capacity of betanin. J. Food Eng. 2013, 118, 49–55. [Google Scholar] [CrossRef]
- Gonçalves, L.C.P.; Da Silva, S.M.; DeRose, P.; Ando, R.A.; Bastos, E.L. Beetroot-pigment-derived colorimetric sensor for detection of calcium dipicolinate in bacterial spores. PLoS ONE 2013, 8, e73701. [Google Scholar] [CrossRef]
- Gonçalves, L.C.P.; Tonelli, R.R.; Bagnaresi, P.; Mortara, R.A.; Ferreira, A.G.; Bastos, E.L. A nature-inspired betalainic probe for live-cell imaging of Plasmodium-infected erythrocytes. PLoS ONE 2013, 8, e53874. [Google Scholar] [CrossRef]
- Nilsson, T. Studies into the pigments in beetroot (Beta vulgaris L. ssp. vulgaris var. rubra L.). Lantbr. Högsk. Annlr. 1970, 36, 179–219. [Google Scholar]
- Piattelli, M.; Minale, L.; Prota, G. Isolation, structure and absolute configuration of indicaxanthin. Tetrahedron 1964, 20, 2325–2329. [Google Scholar] [CrossRef]
- Karstens, T.; Kobs, K. Rhodamine-B and rhodamine-101 as reference substances for fluorescence quantum yield measurements. J. Phys. Chem. 1980, 84, 1871–1872. [Google Scholar] [CrossRef]
- Schaich, K.M.; Tian, X.; Xie, J. Hurdles and pitfalls in measuring antioxidant efficacy: A critical evaluation of ABTS, DPPH, and ORAC assays. J. Funct. Foods 2015, 14, 111–125. [Google Scholar] [CrossRef]
- Wybraniec, S.; Michalowski, T. New pathways of betanidin and betanin enzymatic oxidation. J. Agric. Food Chem. 2011, 59, 9612–9622. [Google Scholar] [CrossRef] [PubMed]
- Wybraniec, S.; Starzak, K.; Skopinska, A.; Nemzer, B.; Pietrzkowski, Z.; Michalowski, T. Studies on nonenzymatic oxidation mechanisms in neobetanin, betanin, and decarboxylated betanins. J. Agric. Food Chem. 2013, 61, 6465–6476. [Google Scholar] [CrossRef] [PubMed]
- Darcy, J.W.; Koronkiewicz, B.; Parada, G.A.; Mayer, J.M. A continuum of proton-coupled electron transfer reactivity. Acc. Chem. Res. 2018, 51, 2391–2399. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.; Ou, B.; Prior, R.L. The chemistry behind antioxidant capacity assays. J. Agric. Food Chem. 2005, 53, 1841–1856. [Google Scholar] [CrossRef]
- Tishchenko, O.; Truhlar, D.G.; Ceulemans, A.; Nguyen, M.T. A unified perspective on the hydrogen atom transfer and proton-coupled electron transfer mechanisms in terms of topographic features of the ground and excited potential energy surfaces as exemplified by the reaction between phenol and radicals. J. Am. Chem. Soc. 2008, 130, 7000–7010. [Google Scholar] [CrossRef] [PubMed]
- Weinberg, D.R.; Gagliardi, C.J.; Hull, J.F.; Murphy, C.F.; Kent, C.A.; Westlake, B.C.; Paul, A.; Ess, D.H.; McCafferty, D.G.; Meyer, T.J. Proton-coupled electron transfer. Chem. Rev. 2012, 112, 4016–4093. [Google Scholar] [CrossRef]
- Tian, X.; Schaich, K.M. Effects of molecular structure on kinetics and dynamics of the trolox equivalent antioxidant capacity assay with ABTS+•. J. Agric. Food Chem. 2013, 61, 5511–5519. [Google Scholar] [CrossRef]
- Bordwell, F.G.; Cheng, J.P.; Harrelson, J.A. Homolytic bond dissociation energies in solution from equilibrium acidity and electrochemical data. J. Am. Chem. Soc. 1988, 110, 1229–1231. [Google Scholar] [CrossRef]
- Reznik, H. Die pigmente der centrospermen als systematisches element. Planta 1957, 49, 406–434. [Google Scholar] [CrossRef]
- Gandia-Herrero, F.; Escribano, J.; Garcia-Carmona, F. Purification and antiradical properties of the structural unit of betalains. J. Nat. Prod. 2012, 75, 1030–1036. [Google Scholar] [CrossRef] [PubMed]
- Leopoldini, M.; Marino, T.; Russo, N.; Toscano, M. Antioxidant properties of phenolic compounds: H-atom versus electron transfer mechanism. J. Phys. Chem. A 2004, 108, 4916–4922. [Google Scholar] [CrossRef]
- Apak, R. Electron transfer-based antioxidant capacity assays and the cupric ion reducing antioxidant capacity (CUPRAC) assay. In Measurement of Antioxidant Activity & Capacity: Recent Trends and Applications; John Wiley & Sons Ltd: Chichester, UK, 2018; pp. 57–75. [Google Scholar]
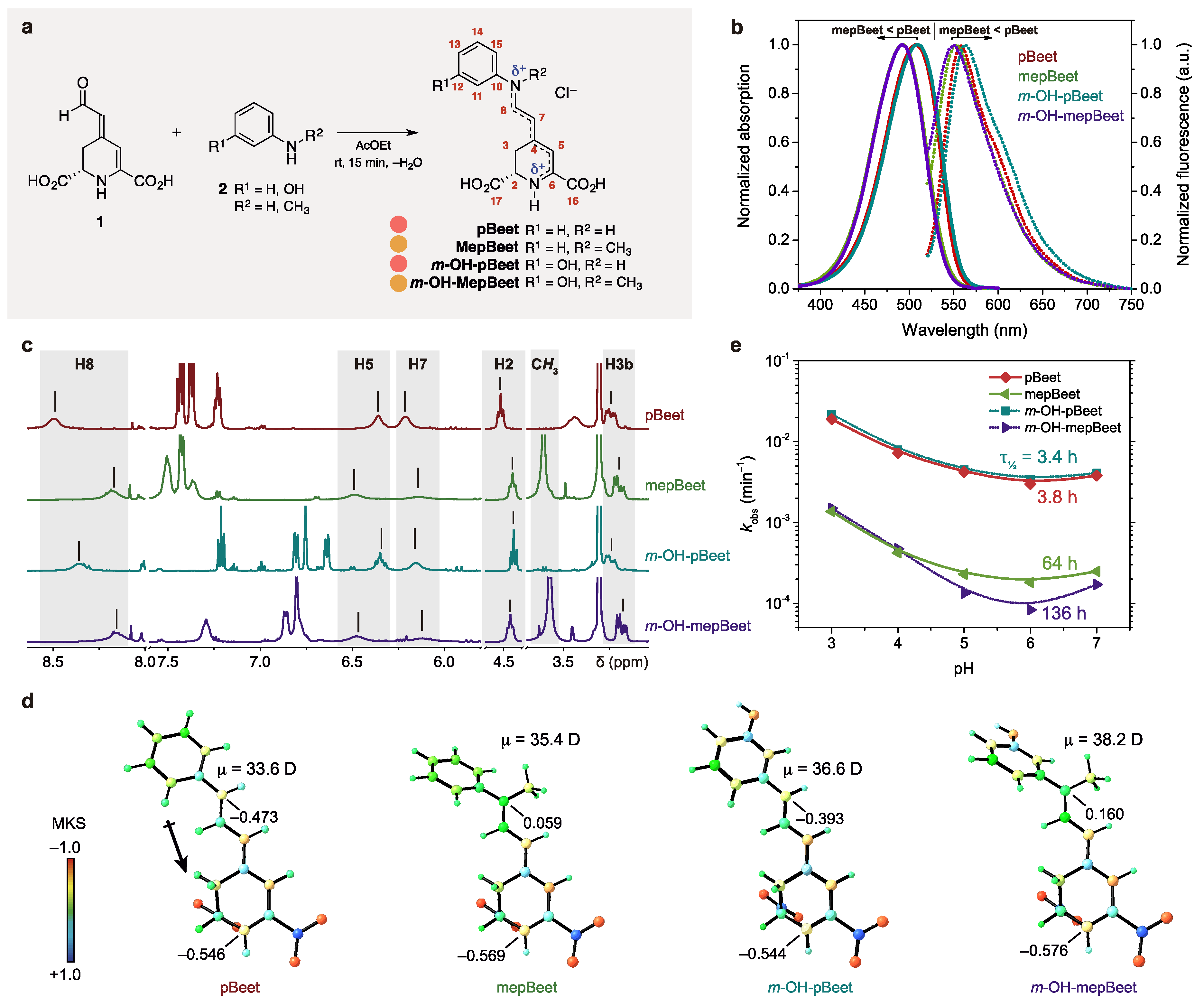
| Compound | λabs (nm) | ε b | λEM (nm) | Δλ (cm−1) | ΦFl (/ 10−3) c | ES (kJ mol−1) |
|---|---|---|---|---|---|---|
| pBeet | 508 | 61,300 | 558 | 1760 | 1.29 ± 0.04 | 220 |
| mepBeet | 492 | 60,600 | 553 | 2240 | 0.56 ± 0.04 | 230 |
| m-OH-pBeet | 508 | 64,000 | 563 | 1880 | 0.85 ± 0.03 | 220 |
| m-OH-mepBeet | 492 | 60,500 | 550 | 2140 | 0.39 ± 0.02 | 230 |
| Species b | ET-PT | HAT/PCET | SPLET | PhO•→PhOH | ||
|---|---|---|---|---|---|---|
| IP | PDE | BDE | PA | ETE | ΔBDEPhOH | |
| pBeet | 456 | |||||
| N9 | 82 | 352 | 139 | 399 | –11 | |
| N1 | 110 | 380 | 161 | 405 | 17 | |
| mepBeet | 455 | |||||
| N1 | 108 | 377 | 164 | 399 | 14 | |
| m-OH-pBeet c | 459 | |||||
| N9 | 79 | 352 | 140 | 398 | –12 | |
| ArO | 96 | 369 | 154 | 401 | 5 | |
| N1 | 109 | 382 | 162 | 406 | 18 | |
| m-OH-mepBeet | 456 | |||||
| ArO | 99 | 369 | 154 | 401 | 6 | |
| N1 | 108 | 378 | 164 | 400 | 15 | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nakashima, K.K.; Bastos, E.L. Rationale on the High Radical Scavenging Capacity of Betalains. Antioxidants 2019, 8, 222. https://doi.org/10.3390/antiox8070222
Nakashima KK, Bastos EL. Rationale on the High Radical Scavenging Capacity of Betalains. Antioxidants. 2019; 8(7):222. https://doi.org/10.3390/antiox8070222
Chicago/Turabian StyleNakashima, Karina K., and Erick L. Bastos. 2019. "Rationale on the High Radical Scavenging Capacity of Betalains" Antioxidants 8, no. 7: 222. https://doi.org/10.3390/antiox8070222
APA StyleNakashima, K. K., & Bastos, E. L. (2019). Rationale on the High Radical Scavenging Capacity of Betalains. Antioxidants, 8(7), 222. https://doi.org/10.3390/antiox8070222