Antioxidant Activities of Alkyl Substituted Pyrazine Derivatives of Chalcones—In Vitro and In Silico Study
Abstract
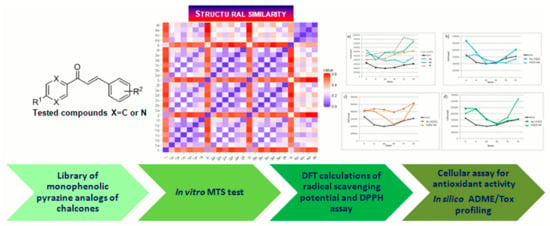
:1. Introduction
2. Materials and Methods
2.1. Studied Compounds
2.2. Cell Lines
2.3. Cytotoxic Activity
2.4. DPPH• Radical Scavenging Assay
2.5. Effects of Compounds on the Growth of THP-1 Cells Treated with H2O2
2.6. In Silico Methods
2.6.1. Predictions of Physicochemical and ADME/Tox Properties
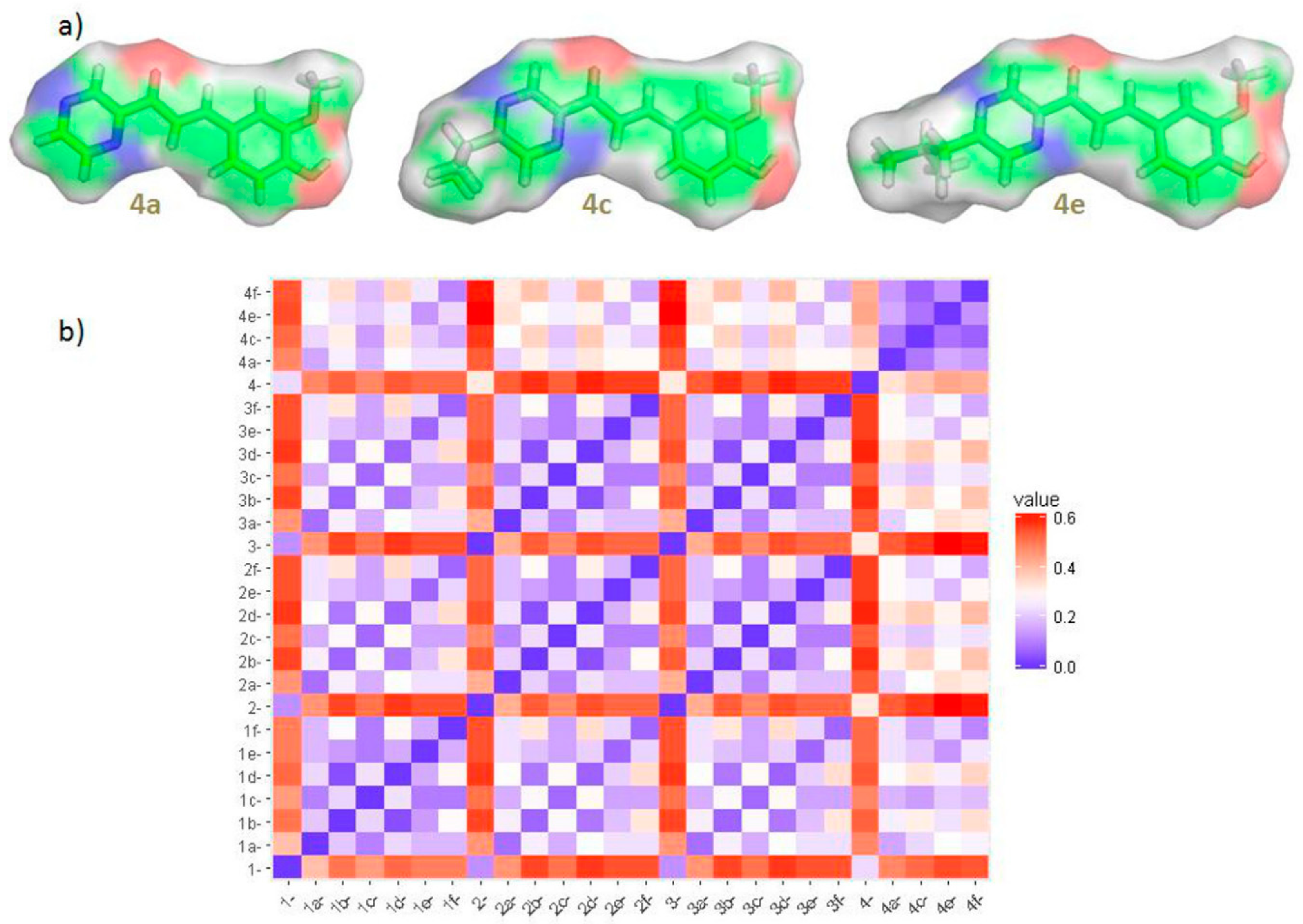
2.6.2. Molecular Similarity
2.6.3. DFT Calculations
3. Results and Discussion
3.1. In Vitro Cytotoxicity
3.2. In Vitro and in Silico Radical Scavenging Analysis
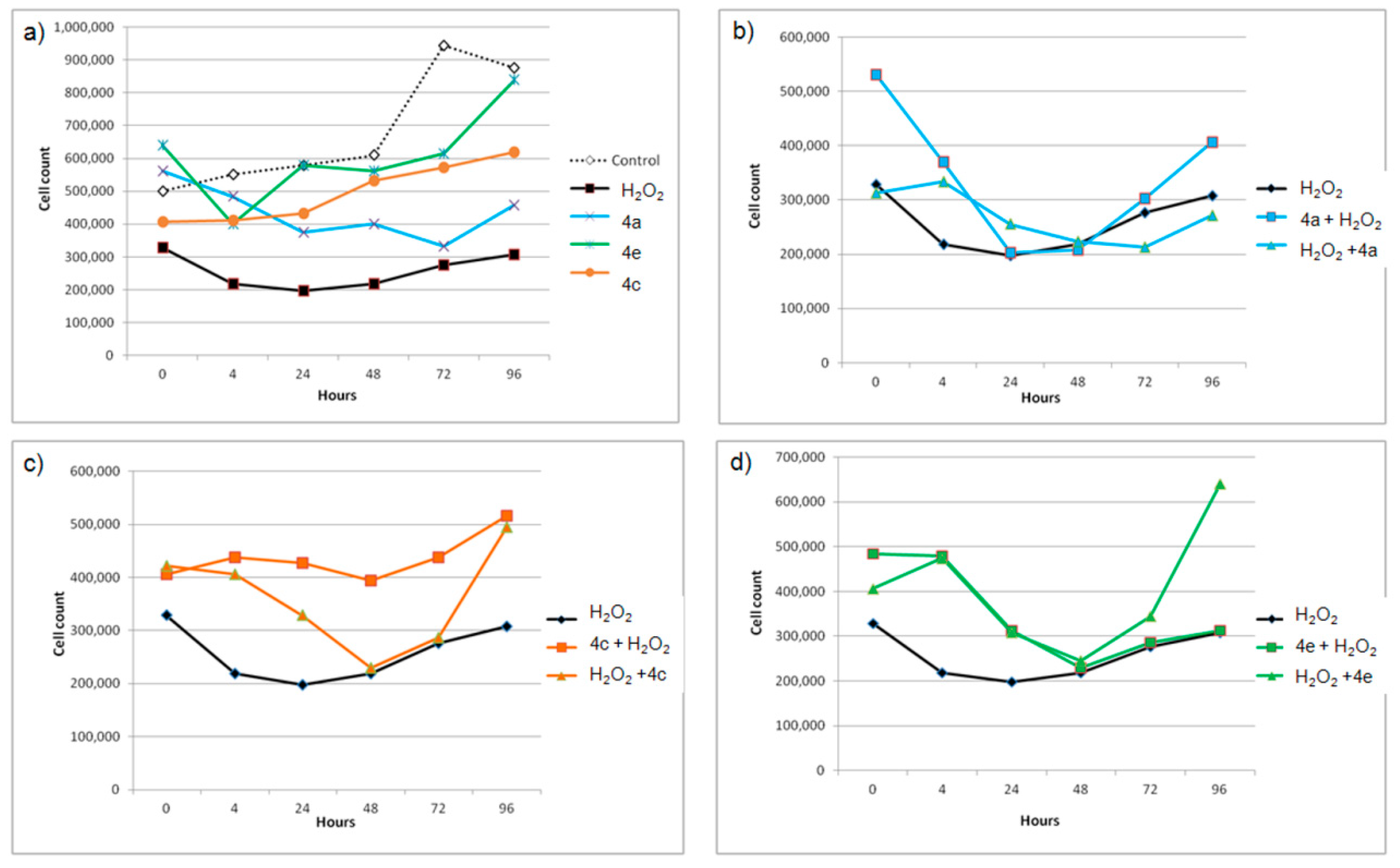
3.3. Effects of Selected Compounds on the Growth of THP-1 Cells Pre- or Post-Treated with H2O2
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sahu, N.K.; Balbhadra, S.S.; Choudhary, J.; Kohli, D.V. Exploring pharmacological significance of chalcone scaffold: A review. Curr. Med. Chem. 2012, 19, 209–225. [Google Scholar] [CrossRef] [PubMed]
- Gomes, M.N.; Muratov, E.N.; Pereira, M.; Peixoto, J.C.; Rosseto, L.P.; Cravo, P.V.L.; Andrade, C.H.; Neves, B.J. Chalcone Derivatives: Promising starting points for drug design. Molecules 2017, 22, 1210. [Google Scholar] [CrossRef] [PubMed]
- Ritter, M.; Martins, R.M.; Dias, D.; Pereira, C.M.P. Recent advances on the synthesis of chalcone with antimicrobial activities: A brief review. Lett. Org. Chem. 2014, 11, 498–508. [Google Scholar] [CrossRef]
- Mahapatra, D.K.; Bharti, S.K.; Asati, V. Chalcone derivatives: Anti-inflammatory potential and molecular targets perspectives. Curr. Top. Med. Chem. 2017, 17, 3146–3169. [Google Scholar] [CrossRef]
- Sharma, R.; Kumar, R.; Kodwani, R.; Kapoor, S.; Khar, A.; Bansal, R.; Khurana, S.; Singh, S.; Thomas, J.; Roy, B.; et al. A review on mechanism of anti-tumor activity of chalcones. Anticancer Agents Med. Chem. 2016, 16, 200–211. [Google Scholar] [CrossRef]
- Opletalová, V.; Hartl, J.; Patel, A.; Palát, K.; Buchta, V. Ring substituted 3-phenyl-1-(2-pyrazinyl)-2-propen-1-ones as potential photosynthesis-inhibiting, antifungal and antimycobacterial agents. Farmaco 2002, 57, 135–144. [Google Scholar] [CrossRef]
- Chlupáčová, M.; Opletalová, V.; Kuneš, J.; Silva, L.; Buchta, V.; Dušková, L.; Kráľová, K. Synthesis and biological evaluation of some ring-substituted (E)-3-aryl-1-pyrazin-2-ylprop-2-en-1-ones. Folia Pharm. Univ. Carol. 2005, 32, 31–43. [Google Scholar]
- Kucerova-Chlupacova, M.; Kunes, J.; Buchta, V.; Vejsova, M.; Opletalova, V. Novel pyrazine analogs of chalcone: Synthesis and evaluation of their antifungal and antimycobacterial activity. Molecules 2015, 20, 1104–1117. [Google Scholar] [CrossRef]
- Syam, S.; Abdelwahab, S.I.; Al-Mamary, M.A.; Mohan, S. Synthesis of chalcones with anticancer activities. Molecules 2012, 17, 6179–6195. [Google Scholar] [CrossRef]
- Karthikeyan, C.; Narayana Moorthy, N.S.H.; Ramasamy, S.; Vanam, U.; Manivannan, E.; Karunagaran, D.; Trivedi, P. Advances in chalcones with anticancer activity. Recent Pat. Anticancer Drug Discov. 2015, 10, 97–115. [Google Scholar] [CrossRef] [PubMed]
- Mahapatra, D.K.; Bharti, S.K.; Asati, V. Anti-cancer chalcone: Structural and molecular target perspectives. Eur. J. Med. Chem. 2015, 98, 69–114. [Google Scholar] [CrossRef] [PubMed]
- Katsori, A.M.; Hadjipavlou-Litina, D. Recent progress in therapeutic applications of chalcones. Expert Opin. Ther. Pat. 2011, 21, 1575–1596. [Google Scholar] [CrossRef] [PubMed]
- Forejtníková, H.; Lunerová, K.; Kubínová, R.; Jankovská, D.; Marek, R.; Kareš, R.; Suchý, V.; Vondráček, J.; Machala, M. Chemoprotective and toxic potentials of synthetic and natural chalcones and dihydrochalcones in vitro. Toxicology 2005, 208, 81–93. [Google Scholar] [CrossRef] [PubMed]
- Gerhäuser, C. Beer constituents as potential cancer chemopreventive agents. Eur. J. Med. Chem. 2005, 41, 1941–1954. [Google Scholar] [CrossRef] [PubMed]
- Batovska, D.I.; Todorova, I.T. Trends in utilization of the pharmacological potential of chalcones. Curr. Clin. Pharmacol. 2010, 5, 1–29. [Google Scholar] [CrossRef] [PubMed]
- Dimmock, J.R.; Elias, D.W.; Beazely, M.A.; Kandepu, N.M. Bioactivities of chalcones. Curr. Med. Chem. 1999, 6, 1125–1149. [Google Scholar] [PubMed]
- Sander, T.; Freyss, J.; von Korff, M.; Rufener, C. DataWarrior: An open-source program for chemistry aware data visualization and analysis. J. Chem. Inf. Model. 2015, 55, 460–473. [Google Scholar] [CrossRef] [PubMed]
- Machala, M.; Kubínová, R.; Hořavová, P.; Suchý, V. Chemoprotective potentials of homoisoflavonoids and chalcones of Dracaena cinnabari: Modulations of drug-metabolizing enzymes and antioxidant activity. Phytother. Res. 2001, 15, 114–118. [Google Scholar] [CrossRef] [PubMed]
- Kucerova-Chlupacova, M.; Dosedel, M.; Kunes, J.; Soltesova-Prnova, M.; Majekova, M.; Stefek, M. Chalcones and their pyrazine analogs: Synthesis, inhibition of aldose reductase, antioxidant activity, and molecular docking study. Mon. Chem. 2018, 149, 921–929. [Google Scholar] [CrossRef]
- Srivastava, S.K.; Ramana, K.V.; Bhatnagar, A. Role of aldose reductase and oxidative damage in diabetes and the consequent potential for therapeutic options. Endocr. Rev. 2005, 26, 380–392. [Google Scholar] [CrossRef]
- Go, M.L.; Wu, X.; Liu, X.L. Chalcones: An update on cytotoxic and chemopreventive properties. Curr. Med. Chem. 2005, 12, 481–499. [Google Scholar] [CrossRef]
- Rossi, M.; Caruso, F.; Crespi, E.J.; Pedersen, J.Z.; Nakano, G.; Duong, M.; McKee, C.; Lee, S.; Jiwrajka, M.; Caldwell, C.; et al. Probing antioxidant activity of 2’-hydroxychalcones: Crystal and molecular structures, in vitro antiproliferative studies and in vivo effects on glucose regulation. Biochimie 2013, 95, 1954–1963. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Z.J.; Kuo, S.C.; Chan, S.C.; Ko, F.N.; Teng, C.M. Antioxidant properties of butein isolated from Dalbergia odorifera. Biochim. Biphys. Acta 1998, 1392, 291–299. [Google Scholar] [CrossRef]
- Hatano, T.; Takagi, M.; Ito, H.; Yoshida, T. Phenolic constituents of liquorice. VII. A new chalcone with potent radical scavenging activity and accompanying phenolics from liquorice. Chem. Pharm. Bull. 1997, 45, 1485–1492. [Google Scholar] [CrossRef]
- Ni, L.; Meng, C.Q.; Sikorski, J. Recent advance in therapeutic chalcones. Expert Opin. Ther. Pat. 2004, 14, 1669–1691. [Google Scholar] [CrossRef]
- Rubelj, I.; Stepanić, V.; Jelić, D.; Škrobot Vidaček, N.; Ćukušić Kalajžić, A.; Ivanković, M.; Nujić, K.; Matijašić, M.; Verbanac, D. Tebrophen—An old polyphenol drug with anticancer potential. Molecules 2012, 17, 7864–7886. [Google Scholar] [CrossRef] [PubMed]
- Blois, M.S. Antioxidant determinations by the use of a stable free radical. Nature 1958, 181, 1199–1200. [Google Scholar] [CrossRef]
- Cesar, V.; Jozić, I.; Begović, L.; Vuković, T.; Mlinarić, S.; Lepeduš, H.; Borović Šunjić, S.; Žarković, N. Cell-type-specific modulation of hydrogen peroxide cytotoxicity and 4-hydroxynonenal binding to human cellular proteins in vitro by antioxidant Aloe vera extract. Antioxidants 2018, 7, 125. [Google Scholar] [CrossRef] [PubMed]
- Daina, A.; Michielin, O.; Zoete, V. SwissADME: A free web tool to evaluate pharmacokinetics, drug-likeness and medicinal chemistry friendliness of small molecules. Sci. Rep. 2017, 7, 42717. [Google Scholar] [CrossRef]
- The R Project for Statistical Computing. Available online: https://www.r-project.org/ (accessed on 28 January 2019).
- Durant, J.L.; Leland, B.A.; Henry, D.R.; Nourse, J.G. Reoptimization of MDL keys for use in drug discovery. J. Chem. Inf. Comput. Sci. 2002, 42, 1273–1280. [Google Scholar] [CrossRef]
- Stepanić, V.; Gall Trošelj, K.; Lučić, B.; Marković, Z.; Amić, D. Bond dissociation free energy as a general parameter for flavonoid radical scavenging activity. Food Chem. 2013, 141, 1562–1570. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Schultz, N.E.; Truhlar, D.G. Design of density functionals by combining the method of constraint satisfaction with parametrization for thermochemistry, thermochemical kinetics, and noncovalent interactions. J. Chem. Theory Comput. 2006, 2, 364–382. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.; et al. Gaussian 09 Revision D.01; Gaussian, Inc.: Wallingford, CT, USA, 2013. [Google Scholar]
- Marenich, A.V.; Cramer, C.J.; Truhlar, D.G. Universal solvation model based on solute electron density and a continuum model of the solvent defined by the bulk dielectric constant and atomic surface tensions. J. Phys. Chem. B 2009, 113, 6378–6396. [Google Scholar] [CrossRef]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Dinkova-Kostova, A.T.; Massiah, M.A.; Bozak, R.E.; Hicks, R.J.; Talalay, P. Potency of Michael reaction acceptors as inducers of enzymes that protect against carcinogenesis depends on their reactivity with sulfhydryl groups. Proc. Natl. Acad. Sci. USA 2001, 98, 3404–3409. [Google Scholar] [CrossRef] [PubMed]
- Ohkatsu, Y.; Satoh, T. Antioxidant and photo-antioxidant activities of chalcone derivatives. J. Jpn. Petrol. Inst. 2008, 51, 298–308. [Google Scholar] [CrossRef]
- Qian, A.P.; Shang, Y.J.; Teng, Q.F.; Chang, J.; Fan, G.J.; Wei, X.; Li, R.R.; Li, H.P.; Yao, X.J.; Dai, F.; et al. Hydroxychalxones as potent antioxidants: Structure-activity relationship analysis and mechanism considerations. Food Chem. 2011, 126, 214–248. [Google Scholar] [CrossRef]
- Cai, Y.Z.; Sun, M.; Xing, J.; Luo, Q.; Corke, H. Structure-RS activity relationship of phenolic compounds from traditional Chinese medicinal plants. Life Sci. 2006, 78, 2872–2888. [Google Scholar] [CrossRef]
- Nenadis, N.; Wang, L.F.; Tsimidou, M.Z.; Zhang, H.Y. Radical scavenging potential of phenolic compounds encountered in O. europaea products as indicated by calculation of bond dissociation enthalpy and ionization potential values. J. Agric. Food Chem. 2005, 53, 295–299. [Google Scholar] [CrossRef]
- Milkovic, L.; Vukovic, T.; Zarkovic, N.; Tatzber, F.; Bisenieks, E.; Kalme, Z.; Bruvere, I.; Ogle, Z.; Poikans, J.; Velena, A.; et al. Antioxidative 1,4-dihydropyridine derivatives modulate oxidative stress and growth of human osteoblast-like cells in vitro. Antioxidants 2018, 7, 123. [Google Scholar] [CrossRef]
- Kraljević Gazivoda, T.; Harej, A.; Sedić, M.; Pavelić Kraljević, S.; Stepanić, V.; Drenjančević, D.; Talapko, J.; Raić-Malić, S. Synthesis, in vitro anticancer and antibacterial activities and in silico studies of new 4-substituted 1,2,3-triazole-coumarin hybrids. Eur. J. Med. Chem. 2016, 124, 794–808. [Google Scholar] [CrossRef] [PubMed]
- De Freitas Silva, M.; Pruccoli, L.; Morroni, F.; Sita, G.; Seghetti, F.; Viegas, C.; Tarozzi, A. The Keap1/Nrf2-ARE pathway as a pharmacological target for chalcones. Molecules 2018, 23, 1803. [Google Scholar] [CrossRef] [PubMed]
- Lounsbury, N.; Mateo, G.; Jones, B.; Papaiahgari, S.; Thimmulappa, R.K.; Teijaro, C.; Gordon, J.; Korzekwa, K.; Ye, M.; Allaway, G.; et al. Heterocyclic chalcone activators of nuclear factor (erythroid-derived 2)-like 2 (Nrf2) with improved in vivo efficacy. Bioorg. Med. Chem. 2015, 23, 5352–5359. [Google Scholar] [CrossRef] [PubMed]
| Compound | X | R1 | R2 | HepG2 | THP-1 | DPPH● 1 | clogP 2 |
|---|---|---|---|---|---|---|---|
 | |||||||
| 1 | CH | H | 2-OH | >100 | 70 | >1000 | 2.96 |
| 1a | N | H | 2-OH | >100 | 40 | >1000 | 1.01 |
| 1b | N | propyl | 2-OH | >100 | >100 | 2.28 | |
| 1c | N | isopropyl | 2-OH | >100 | 96 | >1000 | 2.25 |
| 1d | N | butyl | 2-OH | 86 | 27 | >1000 | 2.73 |
| 1e | N | isobutyl | 2-OH | >100 | 94 | 2.50 | |
| 1f | N | tert- butyl | 2-OH | >100 | >100 | 2.65 | |
| 2 | CH | H | 3-OH | >100 | 51 | >1000 | 2.96 |
| 2a | N | H | 3-OH | 100 | 50 | >1000 | 1.01 |
| 2b | N | propyl | 3-OH | >100 | 48 | 2.28 | |
| 2c | N | isopropyl | 3-OH | 97 | 83 | >1000 | 2.25 |
| 2d | N | butyl | 3-OH | >100 | 67 | >1000 | 2.73 |
| 2e | N | isobutyl | 3-OH | >100 | >100 | 2.50 | |
| 2f | N | tert- butyl | 3-OH | >100 | >100 | 2.65 | |
| 3 | CH | H | 4-OH | >100 | >100 | >1000 | 2.96 |
| 3a | N | H | 4-OH | >100 | 97 | >1000 | 1.01 |
| 3b | N | propyl | 4-OH | >100 | 94 | 2.28 | |
| 3c | N | isopropyl | 4-OH | >100 | 78 | >1000 | 2.25 |
| 3d | N | butyl | 4-OH | >100 | 83 | >1000 | 2.73 |
| 3e | N | isobutyl | 4-OH | >100 | >100 | 2.50 | |
| 3f | N | tert- butyl | 4-OH | >100 | >100 | 2.65 | |
| 4 | CH | H | 3-OCH3, 4-OH | >100 | >100 | 2.89 | |
| 4a | N | H | 3-OCH3, 4-OH | >100 | >100 | 186 | 0.94 |
| 4c | N | isopropyl | 3-OCH3, 4-OH | >100 | >100 | 39 | 2.18 |
| 4e | N | isobutyl | 3-OCH3, 4-OH | >100 | >100 | 46 | 2.43 |
| 4f | N | tert- butyl | 3-OCH3, 4-OH | >100 | 36 | 2.58 | |
| Compound | BDEg | IPg | BDFEaq | IFEaq | pKa | ETFEaq |
|---|---|---|---|---|---|---|
| 1 | 84.9 | 188 | 85.7 | 107.8 | 10.9 | 77.5 |
| 1a | 85.7 | 192.5 | 82.9 | 106.9 | 8.4 | 78.1 |
| 2 | 88.5 | 191.2 | 86.5 | 108.9 | 13.2 | 75.2 |
| 2a | 88.5 | 192 | 87.5 | 108.7 | 13.4 | 75.9 |
| 3 | 85.2 | 184.6 | 83.9 | 103.5 | 11.1 | 77.5 |
| 3a | 85.4 | 188.4 | 84.4 | 102.8 | 10.7 | 76.6 |
| 4 | 86 | 177.5 | 84.7 | 99.3 | 12.8 | 73.9 |
| 4a | 85.8 | 178.4 | 84.8 | 99.2 | 12.0 | 75.2 |
| 4c | 85.7 | 176.4 | 84.2 | 97.8 | 11.8 | 74.8 |
| 4e | 85.9 | 176.4 | 86.4 | 99.2 | 13.3 | 74.9 |
| Apigenin (4′-OH) | 86.7 | 176.7 | 85.3 | 100.6 | 11.4 | 76.4 |
| Quercetin (4′-OH) | 79.3 | 176 | 80.2 | 98.8 | 8.9 | 74.8 |
| Vitamin C | 78.2 | 206.8 | 77.6 | 109 | 3.7 | 79.4 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stepanić, V.; Matijašić, M.; Horvat, T.; Verbanac, D.; Kučerová-Chlupáčová, M.; Saso, L.; Žarković, N. Antioxidant Activities of Alkyl Substituted Pyrazine Derivatives of Chalcones—In Vitro and In Silico Study. Antioxidants 2019, 8, 90. https://doi.org/10.3390/antiox8040090
Stepanić V, Matijašić M, Horvat T, Verbanac D, Kučerová-Chlupáčová M, Saso L, Žarković N. Antioxidant Activities of Alkyl Substituted Pyrazine Derivatives of Chalcones—In Vitro and In Silico Study. Antioxidants. 2019; 8(4):90. https://doi.org/10.3390/antiox8040090
Chicago/Turabian StyleStepanić, Višnja, Mario Matijašić, Tea Horvat, Donatella Verbanac, Marta Kučerová-Chlupáčová, Luciano Saso, and Neven Žarković. 2019. "Antioxidant Activities of Alkyl Substituted Pyrazine Derivatives of Chalcones—In Vitro and In Silico Study" Antioxidants 8, no. 4: 90. https://doi.org/10.3390/antiox8040090
APA StyleStepanić, V., Matijašić, M., Horvat, T., Verbanac, D., Kučerová-Chlupáčová, M., Saso, L., & Žarković, N. (2019). Antioxidant Activities of Alkyl Substituted Pyrazine Derivatives of Chalcones—In Vitro and In Silico Study. Antioxidants, 8(4), 90. https://doi.org/10.3390/antiox8040090