Antioxidants and Male Fertility: From Molecular Studies to Clinical Evidence
Abstract
:1. Introduction
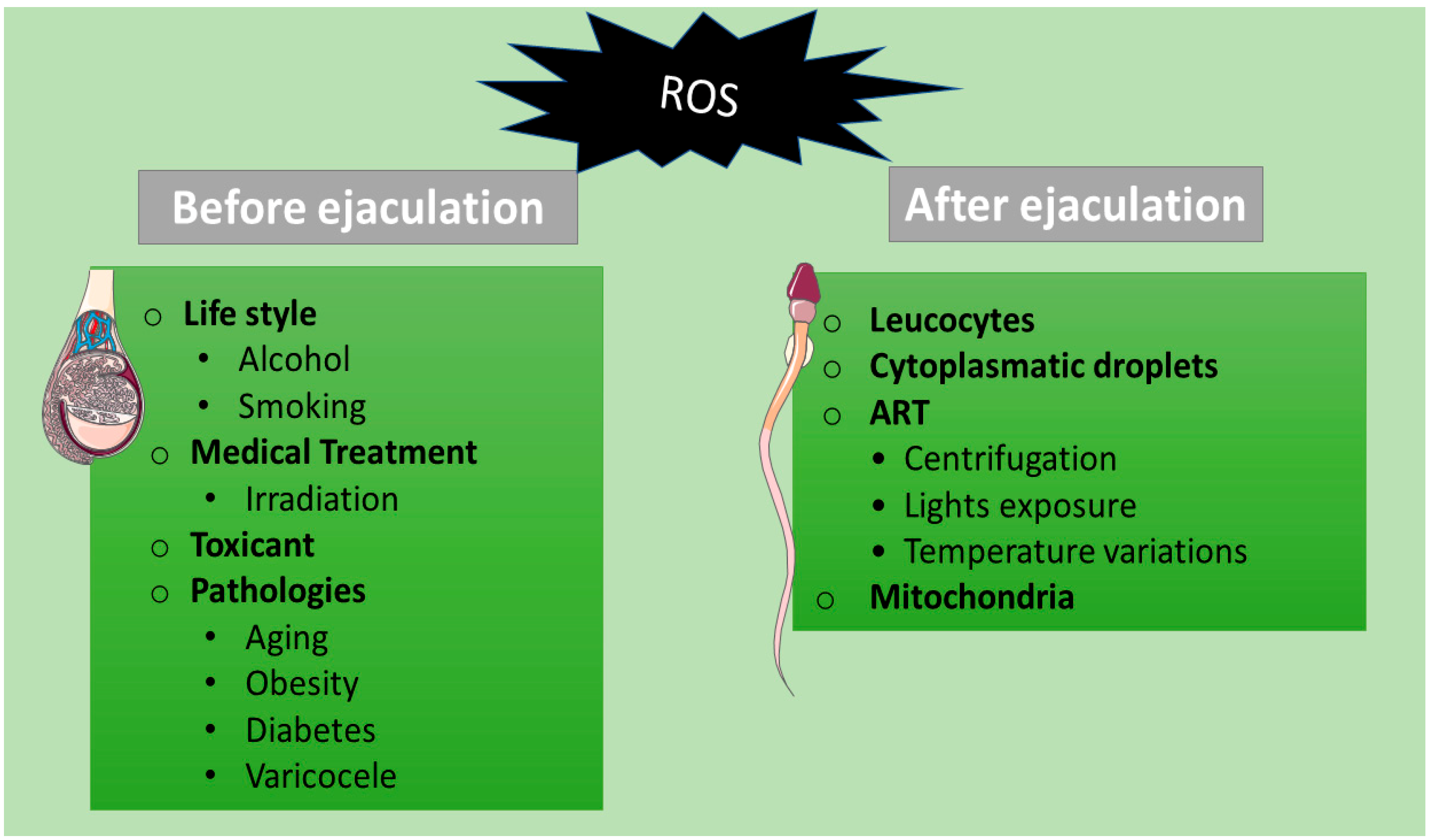
2. Sources of ROS in Spermatozoa
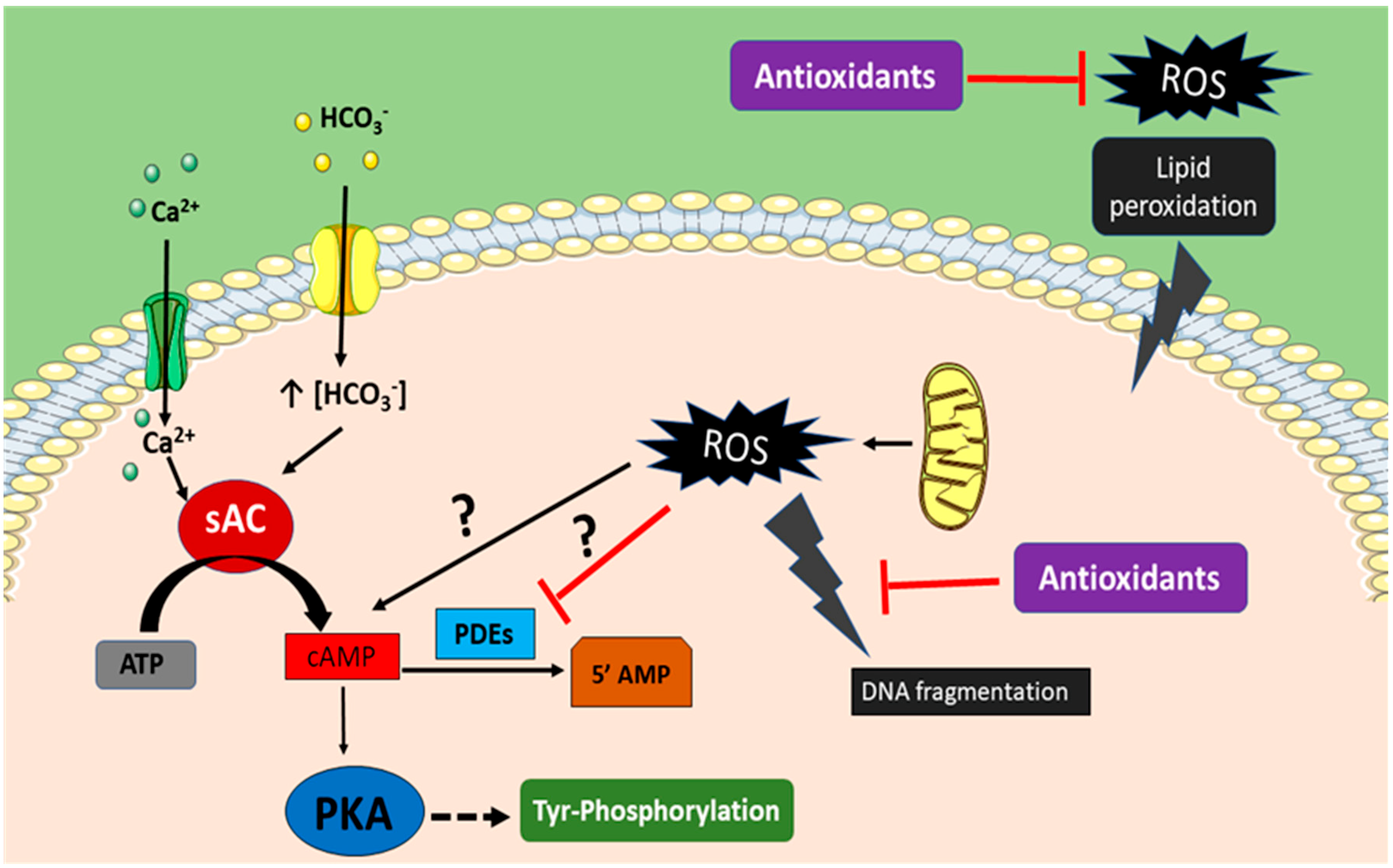
3. Bivalent Role of ROS on Sperm Function
4. Mechanism of ROS Defense in Spermatozoa
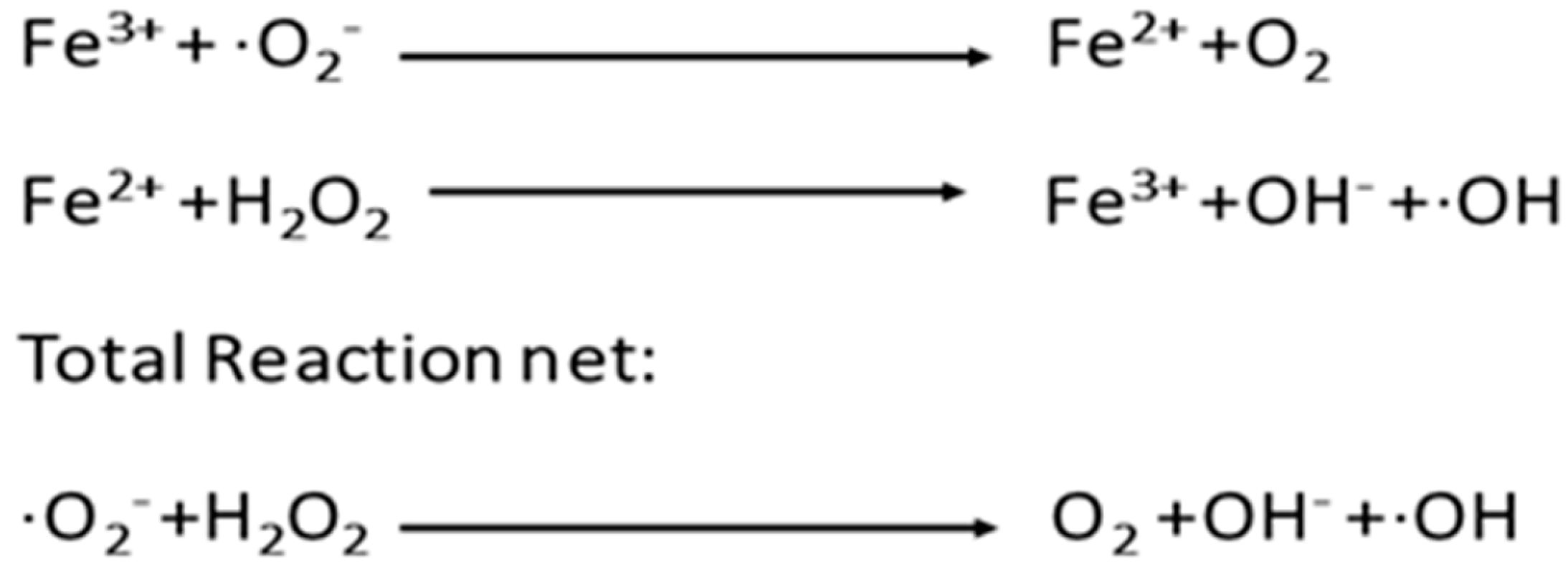
5. Lipid Peroxidation
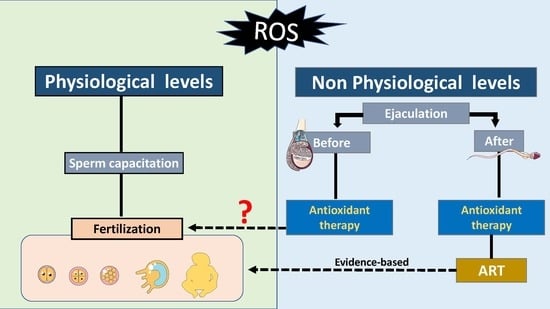
6. Effects of Oral Antioxidant Intake on Male Reproductive Outcome
6.1. Carnitines
6.2. Vitamins
6.3. Zinc
6.4. Natural Compounds—Traditional Medicine
7. Antioxidants as a Tool to Improve Male ART Outcomes
8. Antioxidants as a Therapy to Improve Reproduction Outcome
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Du Plessis, S.S.; Agarwal, A.; Mohanty, G.; van der Linde, M. Oxidative phosphorylation versus glycolysis: What fuel do spermatozoa use? Asian J. Androl. 2015, 17, 230–235. [Google Scholar] [CrossRef] [PubMed]
- Ankel-Simons, F.; Cummins, J.M. Misconceptions about mitochondria and mammalian fertilization: Implications for theories on human evolution. Proc. Natl. Acad. Sci. USA 1996, 93, 13859–13863. [Google Scholar] [CrossRef] [PubMed]
- Birben, E.; Sahiner, U.M.; Sackesen, C.; Erzurum, S.; Kalayci, O. Oxidative stress and antioxidant defense. World Allergy Organ. J. 2012, 5, 9–19. [Google Scholar] [CrossRef] [PubMed]
- Subramanian, V.; Ravichandran, A.; Thiagarajan, N.; Govindarajan, M.; Dhandayuthapani, S.; Suresh, S. Seminal reactive oxygen species and total antioxidant capacity: Correlations with sperm parameters and impact on male infertility. Clin. Exp. Reprod. Med. 2018, 45, 88–93. [Google Scholar] [CrossRef] [PubMed]
- Hammadeh, M.E.; Al Hasani, S.; Rosenbaum, P.; Schmidt, W.; Hammadeh, C.F. Reactive oxygen species, total antioxidant concentration of seminal plasma and their effect on sperm parameters and outcome of IVF/ICSI patients. Arch. Gynecol. Obstet. 2008, 277, 515–526. [Google Scholar] [CrossRef]
- Jurisicova, A.; Varmuza, S.; Casper, R.F. Programmed cell death and human embryo fragmentation. Mol. Hum. Reprod. 1996, 2, 93–98. [Google Scholar] [CrossRef]
- Agarwal, A.; Said, T.M.; Bedaiwy, M.A.; Banerjee, J.; Alvarez, J.G. Oxidative stress in an assisted reproductive techniques setting. Fertil. Steril. 2006, 86, 503–512. [Google Scholar] [CrossRef]
- Kumar, N.; Singh, A.K. Reactive oxygen species in seminal plasma as a cause of male infertility. J. Gynecol. Obstet. Hum. Reprod. 2018, 47, 565–572. [Google Scholar] [CrossRef]
- Aziz, N.; Saleh, R.A.; Sharma, R.K.; Lewis-Jones, I.; Esfandiari, N.; Thomas, A.J., Jr.; Agarwal, A. Novel association between sperm reactive oxygen species production, sperm morphological defects, and the sperm deformity index. Fertil. Steril. 2004, 81, 349–354. [Google Scholar] [CrossRef]
- Cooper, T.G. The epididymis, cytoplasmic droplets and male fertility. Asian J. Androl. 2011, 13, 130–138. [Google Scholar] [CrossRef]
- Gomez, E.; Buckingham, D.W.; Brindle, J.; Lanzafame, F.; Irvine, D.S.; Aitken, R.J. Development of an image analysis system to monitor the retention of residual cytoplasm by human spermatozoa: Correlation with biochemical markers of the cytoplasmic space, oxidative stress, and sperm function. J. Androl. 1996, 17, 276–287. [Google Scholar]
- Huszar, G.; Vigue, L. Correlation between the rate of lipid peroxidation and cellular maturity as measured by creatine kinase activity in human spermatozoa. J. Androl. 1994, 15, 71–77. [Google Scholar]
- Shekarriz, M.; DeWire, D.M.; Thomas, A.J., Jr.; Agarwal, A. A method of human semen centrifugation to minimize the iatrogenic sperm injuries caused by reactive oxygen species. Eur. Urol. 1995, 28, 31–35. [Google Scholar] [CrossRef]
- Whittington, K. Relative contribution of leukocytes and of spermatozoa to reactive oxygen species production in human sperm suspensions. Int. J. Androl. 1999, 22, 229–235. [Google Scholar] [CrossRef]
- Ford, W.C.; Whittington, K.; Williams, A.C. Reactive oxygen species in human sperm suspensions: Production by leukocytes and the generation of NADPH to protect sperm against their effects. Int. J. Androl. 1997, 20 (Suppl. 3), 44–49. [Google Scholar]
- Aitken, R.J.; Buckingham, D.W.; West, K.; Brindle, J. On the use of paramagnetic beads and ferrofluids to assess and eliminate the leukocytic contribution to oxygen radical generation by human sperm suspensions. Am. J. Reprod. Immunol. 1996, 35, 541–551. [Google Scholar] [CrossRef]
- Austin, C.R. Observations on the penetration of the sperm in the mammalian egg. Aust. J. Sci. Res. B 1951, 4, 581–596. [Google Scholar] [CrossRef]
- Chang, M.C. Fertilizing capacity of spermatozoa deposited into the fallopian tubes. Nature 1951, 168, 697–698. [Google Scholar] [CrossRef]
- Koppers, A.J.; De Iuliis, G.N.; Finnie, J.M.; McLaughlin, E.A.; Aitken, R.J. Significance of mitochondrial reactive oxygen species in the generation of oxidative stress in spermatozoa. J. Clin. Endocrinol. Metab. 2008, 93, 3199–3207. [Google Scholar] [CrossRef]
- Carrell, D.T.; Liu, L.; Peterson, C.M.; Jones, K.P.; Hatasaka, H.H.; Erickson, L.; Campbell, B. Sperm DNA fragmentation is increased in couples with unexplained recurrent pregnancy loss. Arch. Androl. 2003, 49, 49–55. [Google Scholar] [CrossRef]
- Lewis, S.E.; Aitken, R.J. DNA damage to spermatozoa has impacts on fertilization and pregnancy. Cell Tissue Res. 2005, 322, 33–41. [Google Scholar] [CrossRef]
- Ford, W.C. Regulation of sperm function by reactive oxygen species. Hum. Reprod. Update 2004, 10, 387–399. [Google Scholar] [CrossRef]
- Visconti, P.E.; Bailey, J.L.; Moore, G.D.; Pan, D.; Olds-Clarke, P.; Kopf, G.S. Capacitation of mouse spermatozoa. I. Correlation between the capacitation state and protein tyrosine phosphorylation. Development (Camb. Engl.) 1995, 121, 1129–1137. [Google Scholar]
- Visconti, P.E.; Moore, G.D.; Bailey, J.L.; Leclerc, P.; Connors, S.A.; Pan, D.; Olds-Clarke, P.; Kopf, G.S. Capacitation of mouse spermatozoa. II. Protein tyrosine phosphorylation and capacitation are regulated by a cAMP-dependent pathway. Development (Camb. Engl.) 1995, 121, 1139–1150. [Google Scholar]
- Raimondi, L.; Banchelli, G.; Sgromo, L.; Pirisino, R.; Ner, M.; Parini, A.; Cambon, C. Hydrogen peroxide generation by monoamine oxidases in rat white adipocytes: Role on cAMP production. Eur. J. Pharmacol. 2000, 395, 177–182. [Google Scholar] [CrossRef]
- Aitken, R.J.; Harkiss, D.; Knox, W.; Paterson, M.; Irvine, D.S. A novel signal transduction cascade in capacitating human spermatozoa characterised by a redox-regulated, cAMP-mediated induction of tyrosine phosphorylation. J. Cell Sci. 1998, 111 Pt 5, 645–656. [Google Scholar]
- Zhang, H.; Zheng, R.L. Promotion of human sperm capacitation by superoxide anion. Free Radic. Res. 1996, 24, 261–268. [Google Scholar] [CrossRef]
- Lewis, B.; Aitken, R.J. A redox-regulated tyrosine phosphorylation cascade in rat spermatozoa. J. Androl. 2001, 22, 611–622. [Google Scholar]
- Agarwal, A.; Virk, G.; Ong, C.; du Plessis, S.S. Effect of Oxidative Stress on Male Reproduction. World J. Men’s Health 2014, 32, 1–17. [Google Scholar] [CrossRef]
- O’Flaherty, C. Peroxiredoxin 6: The Protector of Male Fertility. Antioxid 2018, 7, 173. [Google Scholar] [CrossRef]
- Saleh, R.A.; Agarwal, A. Oxidative stress and male infertility: From research bench to clinical practice. J. Androl. 2002, 23, 737–752. [Google Scholar]
- Alvarez, J.G.; Storey, B.T. Differential incorporation of fatty acids into and peroxidative loss of fatty acids from phospholipids of human spermatozoa. Mol. Reprod. Dev. 1995, 42, 334–346. [Google Scholar] [CrossRef]
- Aitken, R.J.; Harkiss, D.; Buckingham, D.W. Analysis of lipid peroxidation mechanisms in human spermatozoa. Mol. Reprod. Dev. 1993, 35, 302–315. [Google Scholar] [CrossRef]
- Ayala, A.; Munoz, M.F.; Arguelles, S. Lipid peroxidation: Production, metabolism, and signaling mechanisms of malondialdehyde and 4-hydroxy-2-nonenal. Oxidative Med. Cell. Longev. 2014, 2014, 360438. [Google Scholar] [CrossRef]
- Mazzilli, F.; Rossi, T.; Sabatini, L.; Pulcinelli, F.M.; Rapone, S.; Dondero, F.; Gazzaniga, P.P. Human sperm cryopreservation and reactive oxygen species (ROS) production. Acta Eur. Fertil. 1995, 26, 145–148. [Google Scholar]
- Chatterjee, S.; Gagnon, C. Production of reactive oxygen species by spermatozoa undergoing cooling, freezing, and thawing. Mol. Reprod. Dev. 2001, 59, 451–458. [Google Scholar] [CrossRef]
- Kadirvel, G.; Kumar, S.; Kumaresan, A. Lipid peroxidation, mitochondrial membrane potential and DNA integrity of spermatozoa in relation to intracellular reactive oxygen species in liquid and frozen-thawed buffalo semen. Anim. Reprod. Sci. 2009, 114, 125–134. [Google Scholar] [CrossRef]
- Showell, M.G.; Mackenzie-Proctor, R.; Brown, J.; Yazdani, A.; Stankiewicz, M.T.; Hart, R.J. Antioxidants for male subfertility. Cochrane Database Syst. Rev. 2014, 12. [Google Scholar] [CrossRef]
- Comhaire, F.H.; Garem, Y.E.; Mahmoud, A.; Eertmans, F.; Schoonjans, F. Combined conventional/antioxidant “Astaxanthin” treatment for male infertility: A double blind, randomized trial. Asian J. Androl. 2005, 7, 257–262. [Google Scholar] [CrossRef]
- Vicari, E.; Calogero, A.E. Effects of treatment with carnitines in infertile patients with prostato-vesiculo-epididymitis. Hum. Reprod. (Oxf. Engl.) 2001, 16, 2338–2342. [Google Scholar] [CrossRef]
- Vicari, E.; La Vignera, S.; Calogero, A.E. Antioxidant treatment with carnitines is effective in infertile patients with prostatovesiculoepididymitis and elevated seminal leukocyte concentrations after treatment with nonsteroidal anti-inflammatory compounds. Fertil. Steril. 2002, 78, 1203–1208. [Google Scholar] [CrossRef]
- Balercia, G.; Regoli, F.; Armeni, T.; Koverech, A.; Mantero, F.; Boscaro, M. Placebo-controlled double-blind randomized trial on the use of L-carnitine, L-acetylcarnitine, or combined L-carnitine and L-acetylcarnitine in men with idiopathic asthenozoospermia. Fertil. Steril. 2005, 84, 662–671. [Google Scholar] [CrossRef] [PubMed]
- Busetto, G.M.; Agarwal, A.; Virmani, A.; Antonini, G.; Ragonesi, G.; Del Giudice, F.; Micic, S.; Gentile, V.; De Berardinis, E. Effect of metabolic and antioxidant supplementation on sperm parameters in oligo-astheno-teratozoospermia, with and without varicocele: A double-blind placebo-controlled study. Andrologia 2018, 50, e12927. [Google Scholar] [CrossRef] [PubMed]
- Bozhedomov, V.A.; Lipatova, N.A.; Bozhedomova, G.E.; Rokhlikov, I.M.; Shcherbakova, E.V.; Komarina, R.A. Using l- and acetyl-l-carnintines in combination with clomiphene citrate and antioxidant complex for treating idiopathic male infertility: A prospective randomized trial. Urologiia (Mosc. Russ. 1999) 2017, 3, 22–32. [Google Scholar] [CrossRef]
- Kizilay, F.; Altay, B. Evaluation of the effects of antioxidant treatment on sperm parameters and pregnancy rates in infertile patients after varicocelectomy: A randomized controlled trial. Int. J. Impot. Res. 2019, 1. [Google Scholar] [CrossRef]
- Kessopoulou, E.; Powers, H.J.; Sharma, K.K.; Pearson, M.J.; Russell, J.M.; Cooke, I.D.; Barratt, C.L. A double-blind randomized placebo cross-over controlled trial using the antioxidant vitamin E to treat reactive oxygen species associated male infertility. Fertil. Steril. 1995, 64, 825–831. [Google Scholar] [CrossRef]
- Suleiman, S.A.; Ali, M.E.; Zaki, Z.M.; el-Malik, E.M.; Nasr, M.A. Lipid peroxidation and human sperm motility: Protective role of vitamin E. J. Androl. 1996, 17, 530–537. [Google Scholar]
- Geva, E.; Bartoov, B.; Zabludovsky, N.; Lessing, J.B.; Lerner-Geva, L.; Amit, A. The effect of antioxidant treatment on human spermatozoa and fertilization rate in an in vitro fertilization program. Fertil. Steril. 1996, 66, 430–434. [Google Scholar] [CrossRef]
- Greco, E.; Romano, S.; Iacobelli, M.; Ferrero, S.; Baroni, E.; Minasi, M.G.; Ubaldi, F.; Rienzi, L.; Tesarik, J. ICSI in cases of sperm DNA damage: Beneficial effect of oral antioxidant treatment. Hum. Reprod. (Oxf. Engl.) 2005, 20, 2590–2594. [Google Scholar] [CrossRef]
- Moslemi, M.K.; Tavanbakhsh, S. Selenium-vitamin E supplementation in infertile men: Effects on semen parameters and pregnancy rate. Int. J. Gen. Med. 2011, 4, 99–104. [Google Scholar] [CrossRef]
- Tremellen, K.; Miari, G.; Froiland, D.; Thompson, J. A randomised control trial examining the effect of an antioxidant (Menevit) on pregnancy outcome during IVF-ICSI treatment. Aust. N. Z. J. Obstet. Gynaecol. 2007, 47, 216–221. [Google Scholar] [CrossRef] [PubMed]
- Tikkiwal, M.; Ajmera, R.L.; Mathur, N.K. Effect of zinc administration on seminal zinc and fertility of oligospermic males. Indian J. Physiol. Pharmacol. 1987, 31, 30–34. [Google Scholar] [PubMed]
- Omu, A.E.; Dashti, H.; Al-Othman, S. Treatment of asthenozoospermia with zinc sulphate: Andrological, immunological and obstetric outcome. Eur. J. Obstet. Gynecol. Reprod. Biol. 1998, 79, 179–184. [Google Scholar] [CrossRef]
- Kerner, J.; Hoppel, C. Genetic disorders of carnitine metabolism and their nutritional management. Annu. Rev. Nutr. 1998, 18, 179–206. [Google Scholar] [CrossRef] [PubMed]
- Jeulin, C.; Lewin, L.M. Role of free L-carnitine and acetyl-L-carnitine in post-gonadal maturation of mammalian spermatozoa. Hum. Reprod. Update 1996, 2, 87–102. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, A.; Said, T.M. Carnitines and male infertility. Reprod. Biomed. Online 2004, 8, 376–384. [Google Scholar] [CrossRef]
- Zhou, X.; Liu, F.; Zhai, S. Effect of L-carnitine and/or L-acetyl-carnitine in nutrition treatment for male infertility: A systematic review. Asia Pac. J. Clin. Nutr. 2007, 16 (Suppl. 1), 383–390. [Google Scholar]
- Gharagozloo, P.; Gutierrez-Adan, A.; Champroux, A.; Noblanc, A.; Kocer, A.; Calle, A.; Perez-Cerezales, S.; Pericuesta, E.; Polhemus, A.; Moazamian, A.; et al. A novel antioxidant formulation designed to treat male infertility associated with oxidative stress: Promising preclinical evidence from animal models. Hum. Reprod. (Oxf. Engl.) 2016, 31, 252–262. [Google Scholar] [CrossRef]
- Baker, H.W.; Edgar, D. Trials of antioxidants for male infertility. Aust. N. Z. J. Obstet. Gynaecol. 2008, 48, 125–126. [Google Scholar] [CrossRef]
- Ener, K.; Aldemir, M.; Isik, E.; Okulu, E.; Ozcan, M.F.; Ugurlu, M.; Tangal, S.; Ozayar, A. The impact of vitamin E supplementation on semen parameters and pregnancy rates after varicocelectomy: A randomised controlled study. Andrologia 2016, 48, 829–834. [Google Scholar] [CrossRef]
- Fallah, A.; Mohammad-Hasani, A.; Colagar, A.H. Zinc is an Essential Element for Male Fertility: A Review of Zn Roles in Men’s Health, Germination, Sperm Quality, and Fertilization. J. Reprod. Infertil. 2018, 19, 69–81. [Google Scholar]
- Zago, M.P.; Oteiza, P.I. The antioxidant properties of zinc: Interactions with iron and antioxidants. Free Radic. Biol. Med. 2001, 31, 266–274. [Google Scholar] [CrossRef]
- Martins, A.D.; Alves, M.G.; Bernardino, R.L.; Dias, T.R.; Silva, B.M.; Oliveira, P.F. Effect of white tea (Camellia sinensis (L.)) extract in the glycolytic profile of Sertoli cell. Eur. J. Nutr. 2014, 53, 1383–1391. [Google Scholar] [CrossRef]
- De Amicis, F.; Santoro, M.; Guido, C.; Russo, A.; Aquila, S. Epigallocatechin gallate affects survival and metabolism of human sperm. Mol. Nutr. Food Res. 2012, 56, 1655–1664. [Google Scholar] [CrossRef]
- Abshenas, J.; Babaei, H.; Zare, M.-H.; Allahbakhshi, A.; Sharififar, F. The effects of green tea (Camellia sinensis) extract on mouse semen quality after scrotal heat stress. Vet. Res. Forum 2011, 2, 242–247. [Google Scholar]
- Ding, J.; Wang, H.; Wu, Z.B.; Zhao, J.; Zhang, S.; Li, W. Protection of murine spermatogenesis against ionizing radiation-induced testicular injury by a green tea polyphenol. Biol. Reprod. 2015, 92, 1–13. [Google Scholar] [CrossRef]
- Dias, T.R.; Alves, M.G.; Tomas, G.D.; Socorro, S.; Silva, B.M.; Oliveira, P.F. White tea as a promising antioxidant medium additive for sperm storage at room temperature: A comparative study with green tea. J. Agric. Food Chem. 2014, 62, 608–617. [Google Scholar] [CrossRef]
- Oliveira, P.F.; Tomas, G.D.; Dias, T.R.; Martins, A.D.; Rato, L.; Alves, M.G.; Silva, B.M. White tea consumption restores sperm quality in prediabetic rats preventing testicular oxidative damage. Reprod. Biomed. Online 2015, 31, 544–556. [Google Scholar] [CrossRef]
- Rato, L.; Alves, M.G.; Dias, T.R.; Lopes, G.; Cavaco, J.E.; Socorro, S.; Oliveira, P.F. High-energy diets may induce a pre-diabetic state altering testicular glycolytic metabolic profile and male reproductive parameters. Andrology 2013, 1, 495–504. [Google Scholar] [CrossRef]
- Sharlip, I.D.; Jarow, J.P.; Belker, A.M.; Lipshultz, L.I.; Sigman, M.; Thomas, A.J.; Schlegel, P.N.; Howards, S.S.; Nehra, A.; Damewood, M.D.; et al. Best practice policies for male infertility. Fertil. Steril. 2002, 77, 873–882. [Google Scholar] [CrossRef]
- Jarow, J.P. Diagnostic approach to the infertile male patient. Endocrinol. Metab. Clin. N. Am. 2007, 36, 297–311. [Google Scholar] [CrossRef]
- MacLeod, J. The Role of Oxygen in the Metabolism and Motility of Human Spermatozoa. Am. J. Physiol.-Leg. Content 1943, 138, 512–518. [Google Scholar] [CrossRef]
- Di Santo, M.; Tarozzi, N.; Nadalini, M.; Borini, A. Human Sperm Cryopreservation: Update on Techniques, Effect on DNA Integrity, and Implications for ART. Adv. Urol. 2012, 2012, 854837. [Google Scholar] [CrossRef]
- Yeste, M. Sperm cryopreservation update: Cryodamage, markers, and factors affecting the sperm freezability in pigs. Theriogenology 2016, 85, 47–64. [Google Scholar] [CrossRef]
- Roca, J.; Gil, M.A.; Hernandez, M.; Parrilla, I.; Vazquez, J.M.; Martinez, E.A. Survival and fertility of boar spermatozoa after freeze-thawing in extender supplemented with butylated hydroxytoluene. J. Androl. 2004, 25, 397–405. [Google Scholar] [CrossRef]
- Trzcinska, M.; Bryla, M.; Gajda, B.; Gogol, P. Fertility of boar semen cryopreserved in extender supplemented with butylated hydroxytoluene. Theriogenology 2015, 83, 307–313. [Google Scholar] [CrossRef]
- Memon, A.A.; Wahid, H.; Rosnina, Y.; Goh, Y.M.; Ebrahimi, M.; Nadia, F.M. Effect of antioxidants on post thaw microscopic, oxidative stress parameter and fertility of Boer goat spermatozoa in Tris egg yolk glycerol extender. Anim. Reprod. Sci. 2012, 136, 55–60. [Google Scholar] [CrossRef]
- Yamaguchi, S.; Funahashi, H. Effect of the addition of beta-mercaptoethanol to a thawing solution supplemented with caffeine on the function of frozen-thawed boar sperm and on the fertility of sows after artificial insemination. Theriogenology 2012, 77, 926–932. [Google Scholar] [CrossRef]
- Lopez-Saucedo, J.; Paramio, M.T.; Fierro, R.; Izquierdo, D.; Catala, M.G.; Coloma, M.A.; Toledano-Diaz, A.; Lopez-Sebastian, A.; Santiago-Moreno, J. Sperm characteristics and heterologous in vitro fertilisation capacity of Iberian ibex (Capra pyrenaica) epididymal sperm, frozen in the presence of the enzymatic antioxidant catalase. Cryobiology 2014, 68, 389–394. [Google Scholar] [CrossRef]
- Sariozkan, S.; Bucak, M.N.; Tuncer, P.B.; Ulutas, P.A.; Bilgen, A. The influence of cysteine and taurine on microscopic-oxidative stress parameters and fertilizing ability of bull semen following cryopreservation. Cryobiology 2009, 58, 134–138. [Google Scholar] [CrossRef]
- Malo, C.; Gil, L.; Gonzalez, N.; Martinez, F.; Cano, R.; de Blas, I.; Espinosa, E. Anti-oxidant supplementation improves boar sperm characteristics and fertility after cryopreservation: Comparison between cysteine and rosemary (Rosmarinus officinalis). Cryobiology 2010, 61, 142–147. [Google Scholar] [CrossRef] [PubMed]
- Buyukleblebici, S.; Tuncer, P.B.; Bucak, M.N.; Eken, A.; Sariozkan, S.; Tasdemir, U.; Endirlik, B.U. Cryopreservation of bull sperm: Effects of extender supplemented with different cryoprotectants and antioxidants on sperm motility, antioxidant capacity and fertility results. Anim. Reprod. Sci. 2014, 150, 77–83. [Google Scholar] [CrossRef] [PubMed]
- Tuncer, P.B.; Buyukleblebici, S.; Eken, A.; Tasdemir, U.; Durmaz, E.; Buyukleblebici, O.; Coskun, E. Comparison of cryoprotective effects of lycopene and cysteamine in different cryoprotectants on bull semen and fertility results. Reprod. Domest. Anim. Zuchthyg. 2014, 49, 746–752. [Google Scholar] [CrossRef] [PubMed]
- Foote, R.H.; Brockett, C.C.; Kaproth, M.T. Motility and fertility of bull sperm in whole milk extender containing antioxidants. Anim. Reprod. Sci. 2002, 71, 13–23. [Google Scholar] [CrossRef]
- Succu, S.; Berlinguer, F.; Pasciu, V.; Satta, V.; Leoni, G.G.; Naitana, S. Melatonin protects ram spermatozoa from cryopreservation injuries in a dose-dependent manner. J. Pineal Res. 2011, 50, 310–318. [Google Scholar] [CrossRef] [PubMed]
- Bertoldo, M.J.; Guibert, E.; Tartarin, P.; Guillory, V.; Froment, P. Effect of metformin on the fertilizing ability of mouse spermatozoa. Cryobiology 2014, 68, 262–268. [Google Scholar] [CrossRef] [PubMed]
- Perez, L.; Arias, M.E.; Sanchez, R.; Felmer, R. N-acetyl-l-cysteine pre-treatment protects cryopreserved bovine spermatozoa from reactive oxygen species without compromising the in vitro developmental potential of intracytoplasmic sperm injection embryos. Andrologia 2015, 47, 1196–1201. [Google Scholar] [CrossRef]
- Truong, T.; Gardner, D.K. Antioxidants improve IVF outcome and subsequent embryo development in the mouse. Hum. Reprod. (Oxf. Engl.) 2017, 32, 2404–2413. [Google Scholar] [CrossRef]
- Gualtieri, R.; Barbato, V.; Fiorentino, I.; Braun, S.; Rizos, D.; Longobardi, S.; Talevi, R. Treatment with zinc, d-aspartate, and coenzyme Q10 protects bull sperm against damage and improves their ability to support embryo development. Theriogenology 2014, 82, 592–598. [Google Scholar] [CrossRef]
- Stipanuk, M.H.; Dominy, J.E., Jr.; Lee, J.I.; Coloso, R.M. Mammalian cysteine metabolism: New insights into regulation of cysteine metabolism. J. Nutr. 2006, 136, 1652s–1659s. [Google Scholar] [CrossRef]
- Fischer, B.; Bavister, B.D. Oxygen tension in the oviduct and uterus of rhesus monkeys, hamsters and rabbits. J. Reprod. Fertil. 1993, 99, 673–679. [Google Scholar] [CrossRef]
- Hardie, D.G.; Alessi, D.R. LKB1 and AMPK and the cancer-metabolism link-ten years after. BMC Biol. 2013, 11, 36. [Google Scholar] [CrossRef]
- Nguyen, T.M.; Seigneurin, F.; Froment, P.; Combarnous, Y.; Blesbois, E. The 5′-AMP-Activated Protein Kinase (AMPK) Is Involved in the Augmentation of Antioxidant Defenses in Cryopreserved Chicken Sperm. PLoS ONE 2015, 10, e0134420. [Google Scholar] [CrossRef]
- Hurtado de Llera, A.; Martin-Hidalgo, D.; Garcia-Marin, L.J.; Bragado, M.J. Metformin blocks mitochondrial membrane potential and inhibits sperm motility in fresh and refrigerated boar spermatozoa. Reprod. Domest. Anim. 2018, 53, 733–741. [Google Scholar] [CrossRef]
- Calle-Guisado, V.; Gonzalez-Fernandez, L.; Martin-Hidalgo, D.; Garcia-Marin, L.J.; Bragado, M.J. Metformin inhibits human spermatozoa motility and signalling pathways mediated by protein kinase A and tyrosine phosphorylation without affecting mitochondrial function. Reprod. Fertil. Dev. 2018, 31, 787–795. [Google Scholar] [CrossRef]
- Luboshitzky, R.; Shen-Orr, Z.; Herer, P. Seminal plasma melatonin and gonadal steroids concentrations in normal men. Arch. Androl. 2002, 48, 225–232. [Google Scholar] [CrossRef]
- Gonzalez-Arto, M.; Vicente-Carrillo, A.; Martinez-Pastor, F.; Fernandez-Alegre, E.; Roca, J.; Miro, J.; Rigau, T.; Rodriguez-Gil, J.E.; Perez-Pe, R.; Muino-Blanco, T.; et al. Melatonin receptors MT1 and MT2 are expressed in spermatozoa from several seasonal and nonseasonal breeder species. Theriogenology 2016, 86, 1958–1968. [Google Scholar] [CrossRef]
- Deng, S.L.; Sun, T.C.; Yu, K.; Wang, Z.P.; Zhang, B.L.; Zhang, Y.; Wang, X.X.; Lian, Z.X.; Liu, Y.X. Melatonin reduces oxidative damage and upregulates heat shock protein 90 expression in cryopreserved human semen. Free Radic. Biol. Med. 2017, 113, 347–354. [Google Scholar] [CrossRef]
- Martin-Hidalgo, D.; Baron, F.J.; Bragado, M.J.; Carmona, P.; Robina, A.; Garcia-Marin, L.J.; Gil, M.C. The effect of melatonin on the quality of extended boar semen after long-term storage at 17 degrees C. Theriogenology 2011, 75, 1550–1560. [Google Scholar] [CrossRef]
- Yamaguchi, S.; Funahashi, H.; Murakami, T. Improved fertility in gilts and sows after artificial insemination of frozen-thawed boar semen by supplementation of semen extender with caffeine and CaCl2. J. Reprod. Dev. 2009, 55, 645–649. [Google Scholar] [CrossRef]
- Donnelly, E.T.; McClure, N.; Lewis, S.E. Antioxidant supplementation in vitro does not improve human sperm motility. Fertil. Steril. 1999, 72, 484–495. [Google Scholar] [CrossRef]
- Menezo, Y.J.; Hazout, A.; Panteix, G.; Robert, F.; Rollet, J.; Cohen-Bacrie, P.; Chapuis, F.; Clement, P.; Benkhalifa, M. Antioxidants to reduce sperm DNA fragmentation: An unexpected adverse effect. Reprod. Biomed. Online 2007, 14, 418–421. [Google Scholar] [CrossRef]
- Menezo, Y.; Entezami, F.; Lichtblau, I.; Belloc, S.; Cohen, M.; Dale, B. Oxidative stress and fertility: Incorrect assumptions and ineffective solutions? Zygote (Camb. Engl.) 2014, 22, 80–90. [Google Scholar] [CrossRef]
- Klein, E.A.; Thompson, I.M., Jr.; Tangen, C.M.; Crowley, J.J.; Lucia, M.S.; Goodman, P.J.; Minasian, L.M.; Ford, L.G.; Parnes, H.L.; Gaziano, J.M.; et al. Vitamin E and the risk of prostate cancer: The Selenium and Vitamin E Cancer Prevention Trial (SELECT). JAMA 2011, 306, 1549–1556. [Google Scholar] [CrossRef]
- Miller, E.R., 3rd; Pastor-Barriuso, R.; Dalal, D.; Riemersma, R.A.; Appel, L.J.; Guallar, E. Meta-analysis: High-dosage vitamin E supplementation may increase all-cause mortality. Ann. Intern. Med. 2005, 142, 37–46. [Google Scholar] [CrossRef]
- Aruoma, O.I.; Halliwell, B.; Gajewski, E.; Dizdaroglu, M. Copper-ion-dependent damage to the bases in DNA in the presence of hydrogen peroxide. Biochem. J. 1991, 273 Pt 3, 601–604. [Google Scholar] [CrossRef]
| Antioxidant Type and Daily Dose | Period Intervention (months) | ART | Relevant Findings | Participants | Problem | Reference |
|---|---|---|---|---|---|---|
| Astaxantin (16 mg) | 3 | NI and IUI | ↑ Pregnancy rate 54.5% (5/11) vs. 10.5% (2/19) placebo group | 30 | Infertile | [39] |
| LC (1 g twice) LAC (0.5 g twice) | 3 | ↓ ROS levels ↑ Pregnancy (11.7%) in patients with abacterial-PVE with normal values of leucocytes It didn´t improve pregnancy (0%) in abacterial-PVE patients with high levels of leucocytes | 54 | PVE | [40] | |
| Nonsteroidal anti-inflammatory + carnitine (Carnitene, 2 g + Nicetile 1 g) Carnitine (Carnitene, 2 g + Nicetile 1 g) Nonsteroidal anti-inflammatory Nonsteroidal anti-inflammatory + carnitine (Carnitene, 2 g + Nicetile 1 g) | 2 + 2 4 4 4 | 23.1% pregnancy 0% pregnancy 6.2% pregnancy 3.8% pregnancy | 98 | PVE with ↑ levels of leucocytes | [41] | |
| LC (3 g), LAC (3 g), LC (2 g) + LAC (1 g) | 6 | NI | ↑ Total oxyradicals scavenging capacity of seminal fluid ↑ Sperm motility and concentration. Pregnancy rate was not modified | 60 | Asthenozoospermic | [42] |
| LC (1 mg), fumarate (725 mg), LAC (500 mg), Fructose (1000 mg), CoQ10 (20 mg), Vitamin C (90 mg), Zinc (10 mg), Folic acid (200 μg), Vitamin B12 (1.5 μg) | 6 | NI | ↑ Achieved pregnancy in treated men 22.2% (10/45) vs. 4.1% (2/49) non treated group | 104 | Oligo-and/or astheno-and/or teratozoospermia | [43] |
| LC fumarate (2 g), LAC (1 g) Clomiphene citrate (50 mg) and a complex of vitamins and microelements | 3–4 | NI | ↑ Sperm concentration No modification in pregnancy rates | 173 | Oligo- and/or asteno- and/or teratozoospermia | [44] |
| LC fumarate (1 g), Acetyl-L- carnitine HCl (0.5 g) Fructose (1 g), Citric acid (50 mg), Vitamin C (90 mg), Zinc (10 mg), Folic acid (200 µg), Selenium (50 µg), Coenzyme Q-10 (20 mg) Vitamin B12 (1.5 µg) | 6 | NI | ↑ Sperm concentration,% of sperm motile or progressive motility as well as sperm with normal morphology Treated men achieved 29% pregnancy versus 17.9% in the placebo group | 90 | After performed a varicocelectomy | [45] |
| Vitamin E (600 mg) | 3 | IVF | Improvement of zona pellucida binding test No effect on ROS levels No alteration on seminal plasma vitamin E levels | 30 | Infertile | [46] |
| Vitamin E (300 mg) | 3 | NI | 21% of men had improved sperm motility and achieved pregnancy where 81.8% of pregnancies finished with a live birth | 52 | Asthenospermic | [47] |
| Vitamin E (200 mg) | 1 | IVF | ↓ Sperm LPO ↑ Fertility rate: 19.3 ± 23.3 pre-treatment versus 29.1 ± 22.2 post-treatment | 15 | Normospermic infertile | [48] |
| Vitamin E (1 g) Vitmin D (1 g) | 2 | ICSI | 76.3% respond to the treatment with ↓DNA damage ↑ Pregnancy rate (6.9 vs. 49.3%) ↑ Implantation rate (2.2 vs. 19.2%) Equal embryo quality | 38 | Infertile men non responding to ICSI | [49] |
| Vitamin E (400 IU) Selenium (200 µg) | 3.5 | NI | 10.8% pregnancy | 690 | Infertile | [50] |
| Vitamin E (400 IU), Vitamin C (100 mg), Lycopene (6 mg), Zinc (25 mg), Selenium (26 μg), Folate (0.5 mg), Garlic (1000 mg) | 3 | IVF-ICSI | Doubled pregnancy rate (63.9 vs. 37.5%), Doubled implantation rate (46.2 vs. 24%) Doubled viable pregnancy rate (38.5 vs. 16%) | 60 | Infertile men with ↑ levels of DNA fragmentation and poor motility and membrane integrity | [51] |
| Zinc sulphate (220 mg) | 4 | NI | 21.4% (3/14) of patients achieved pregnancy Zinc levels were increased in seminal plasma | 14 | Human | [52] |
| Zinc sulphate (500 mg) | 3 | NI | Improved pregnancy (22.5%) vs. placebo (4.3%) Zinc levels were not modified on seminal plasma | 100 | Asthenozoospermic | [53] |
| Antioxidant Type and Dose | Administration | Procedure | Principal Results Found | Stress | Specie | Reference |
|---|---|---|---|---|---|---|
| BHT 0.4 mM | In vitro | IVF | ↑ Sperm survival ↓ Sperm MDA levels at the concentration ↑ Embryo develop 28.8% treated vs. 15.8% control | Cryopreservation | Boar | [75] |
| BHT 1 mM BHT | In vitro | IUI | ↑ Pregnancy rate (86.7 vs. 63.6%), ↑ nº of gilts farrowing (86.7 vs. 45.4%) ↑ nº of piglets born (10.8 ± 1.6 vs. 8.2 ± 2.2) | Cryopreservation | Boar | [76] |
| BHT (2 mM), Ascorbic acid (8.5 mg/mL), Cysteine (5 mM), Hypotaurine (10 mM) | In vitro | AI | ↓ Sperm LPO ↑Fertility: ascorbic acid (42.85%), BHT (35.71%), control (26.38%) | Cryopreservation | Goat | [77] |
| Caffeine (1.15 mM), β-mercaptoethanol (50 µM) | In vitro | AI | No effect on pregnancy rate ↑ Litter size in treated samples (10.0 ±1.0) vs. control (5.7 ± 1.5) | Cryopreservation | Boar | [78] |
| CAT (200 IU/mL) | In vitro | No differences on sperm parameters ↓ 2 pronucleus zygote (25.5% control vs. 13.2% treated)↓ Cleaved embryos: 7.6% treated vs. 16.7% control | Cryopreservation | Ram | [79] | |
| Carnitine, Folic acid, Lycopene, Selenium, Vitamin C, Vitamin E, Zinc | Oral | NI | Duplicate fertilization rate (73.7 vs. 35.2%) Halved fetus reabsorption (9 vs. 18%) | Gpx5 knockout (KO) + Scrotal heat stress (KO + HS) | Mouse | [58] |
| Cysteine (2 mM) | In vitro | IUI | ↑ SOD and CAT levels and = MDA levels ↑ Sperm total motility ↓ acrosome abnormalities Slight tendency to improve (p ˃ 0.05) non-return rate 74.54 (41/55) in comparison to control 57.14 (28/49) | Cryopreservation | Bull | [80] |
| Cysteine (10 mM), Rosemary extract (Rosmarinus officinalis). or a combination of both | In vitro | IVF | ↑% sperm motility and progressive motility ↓ Acrosome membrane damaged Rosemary yielded better cleave% without affects blastocysts | Cryopreservation | Boar | [81] |
| Cysteine (5 mM) Trehalose (25 mM) | In vitro | IUI | No improvement of antioxidants features No differences on non-return rate was found after IUI | Cryopreservation | Bull | [82] |
| Cysteamine (5 µM), Lycopene (500 µg/mL) | In vitro | IUI | No differences on non-returned rate | Cryopreservation | Bull | [83] |
| EGCG (50 mg/kg) | Intraperitoneal | Restore testicular function ↓ LPO and protein carbonyl levels ↑ Number of pups by littler | Ionizing radiation | Rat | [66] | |
| GSH (0.5 and 1.0 mM) GSH 0.5 mM + SOD 100 U/mL | In vitro | IUI | Equal nonreturn rates | Cryopreservation | Bull | [84] |
| Melatonin (1 mM) | In vitro | IVF | ↑ Sperm viability rates ↑% of total motile and progressive motile spermatozoa ↑ DNA integrity Faster first embryonic division | Cryopreservation | Ram | [85] |
| Metformin (50 to 5000 µM) | In vitro | IVF | Duplicate fertilization rate and embryo development | Cryopreservation | Mouse | [86] |
| NAC (1–10 mM) | In vitro | ICSI | Decrease ROS ICSI outcome wasn’t modified | Thawing + H2O2 | Bull | [87] |
| NAC (10 μM), LAC (10 μM), α-Lipoic Acid (5 μM) | In vitro | IVF | ↓ Embryo intracellular levels of H2O2 Accelerated embryo development and blastocysts ↑ TE and ICM cell numbers | Incubation under 20% O2 | Mouse | [88] |
| Taurine (2 mM) | In vitro | IUI | ↓ GSH and SOD levels but ↑ five-fold CAT levels ↑ MDA levels = nonreturn rates | Cryopreservation | Bull | [80] |
| Zinc chloride (10 µg/mL), d-aspartic acid (500 µg/mL) Coenzyme Q10 (40 µg/mL) | In vitro | IVF | ↑% of total spermatozoa motile and progressive motility ↓ Sperm and blastomeres DNA fragmentation ↑ 8-cells blastocyst: 51.4% treatment vs. 37.1% control | Cryopreservation | Bull | [89] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martin-Hidalgo, D.; Bragado, M.J.; Batista, A.R.; Oliveira, P.F.; Alves, M.G. Antioxidants and Male Fertility: From Molecular Studies to Clinical Evidence. Antioxidants 2019, 8, 89. https://doi.org/10.3390/antiox8040089
Martin-Hidalgo D, Bragado MJ, Batista AR, Oliveira PF, Alves MG. Antioxidants and Male Fertility: From Molecular Studies to Clinical Evidence. Antioxidants. 2019; 8(4):89. https://doi.org/10.3390/antiox8040089
Chicago/Turabian StyleMartin-Hidalgo, David, Maria Julia Bragado, Ana R. Batista, Pedro F. Oliveira, and Marco G. Alves. 2019. "Antioxidants and Male Fertility: From Molecular Studies to Clinical Evidence" Antioxidants 8, no. 4: 89. https://doi.org/10.3390/antiox8040089
APA StyleMartin-Hidalgo, D., Bragado, M. J., Batista, A. R., Oliveira, P. F., & Alves, M. G. (2019). Antioxidants and Male Fertility: From Molecular Studies to Clinical Evidence. Antioxidants, 8(4), 89. https://doi.org/10.3390/antiox8040089