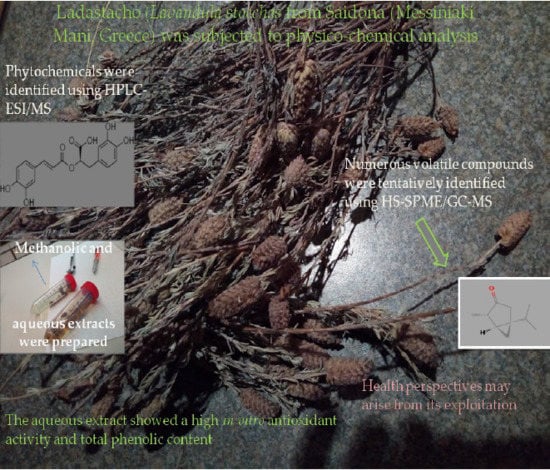
Physico-Chemical Parameters, Phenolic Profile, In Vitro Antioxidant Activity and Volatile Compounds of Ladastacho (Lavandula stoechas) from the Region of Saidona
Abstract
:1. Introduction
2. Materials and Methods
2.1. Collection of Lavandula stoechas and Preparation of Samples
2.2. Reagents and Solutions
2.3. Physicochemical Parameter Analysis
2.3.1. Determination of Acidity
2.3.2. Determination of pH
2.3.3. Determination of Electrical Conductivity, Salinity and Total Dissolved Solids
2.3.4. Extraction of Phenolic Compounds
2.3.5. Analysis of Lavandula stoechas Phenolic Compounds Using High-Performance Liquid Chromatography Electro Spray Ionization Mass Spectrometry (HPLC/ESI-MS)
2.3.6. Determination of in Vitro Antioxidant Activity
2.3.7. Preparation of the DPPH Free Radical Calibration Curve
2.3.8. Determination of in Vitro Antioxidant Activity of Lavandula stoechas Aqueous and Methanolic Extracts
2.3.9. Determination of Total Phenolic Content
3. Headspace Solid Phase Microextraction Coupled to Gas Chromatography/Mass Spectrometry (HS-SPME/GC-MS)
3.1. Headspace Extraction of Volatile Compounds
3.2. Gas Chromatography-Mass Spectrometry Unit and Analysis Conditions
3.3. Identification of Lavandula stoechas Volatile Compounds
3.4. Graph Preparation and Statistical Analysis
4. Results and Discussion
4.1. Physico-Chemical Parameter Analysis
4.2. Phenolic Profile: Qualitative and Quantitative Determinations
4.2.1. Qualitative Analysis
4.2.2. Quantitative Analysis: Total Phenolic Content
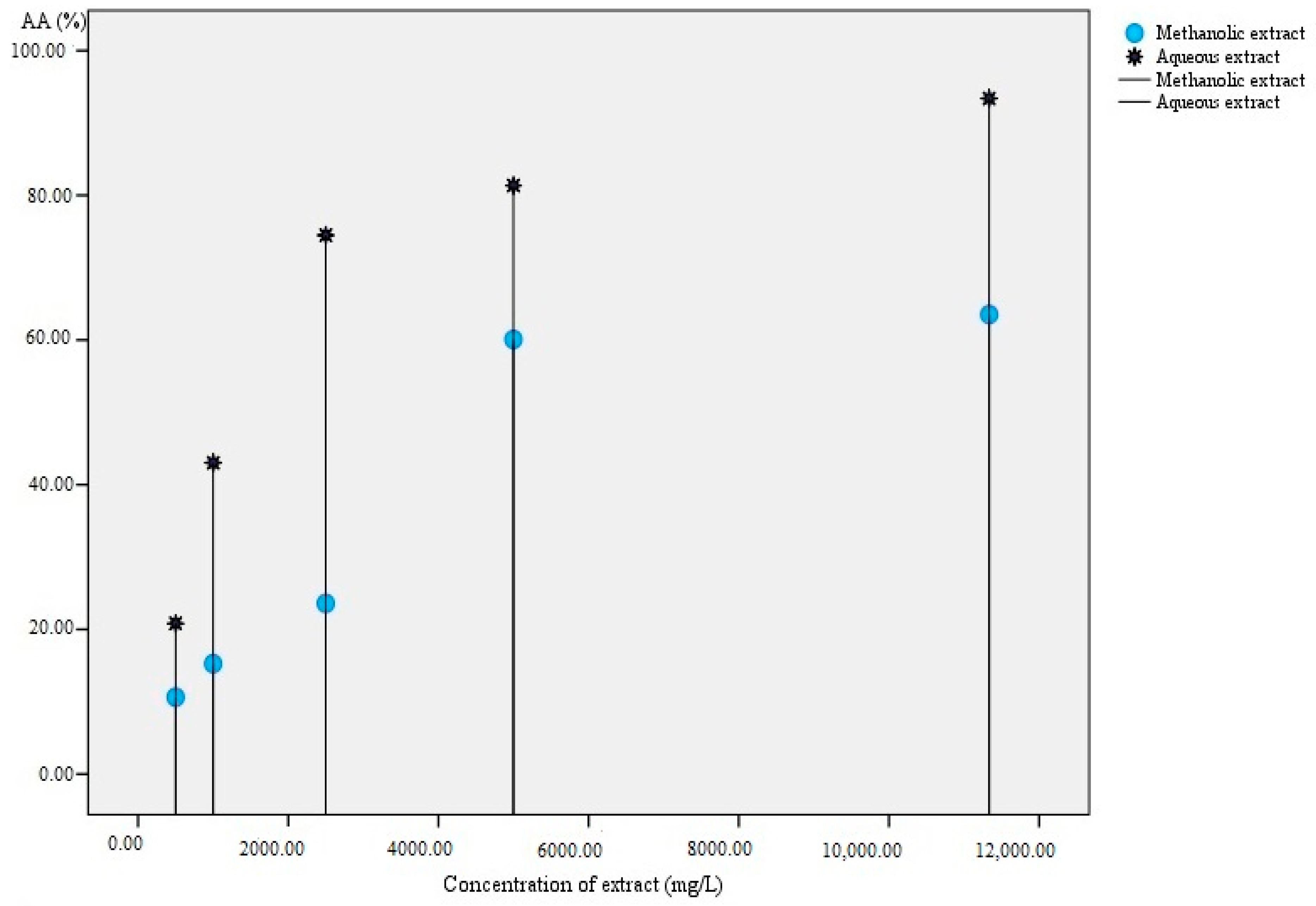
4.2.3. In Vitro Antioxidant Activity
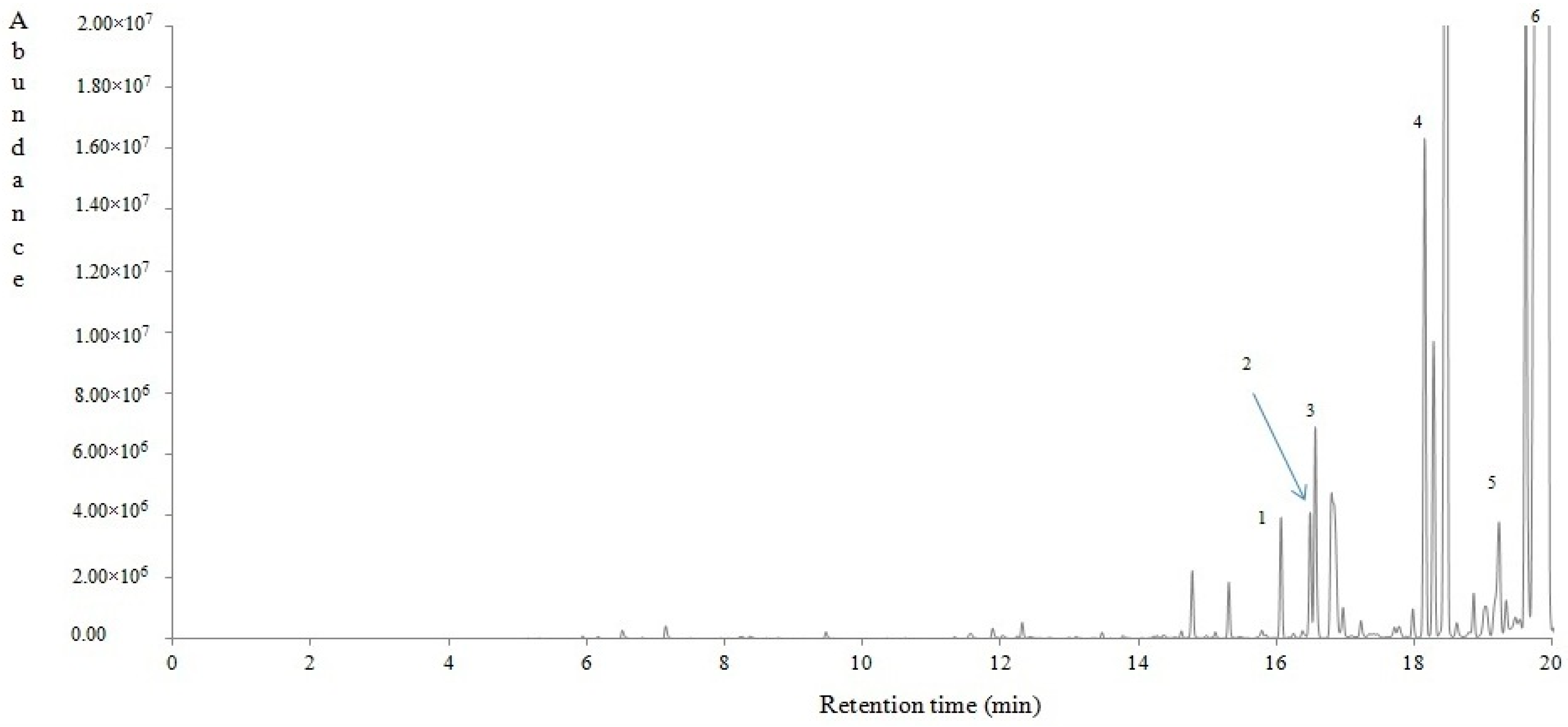
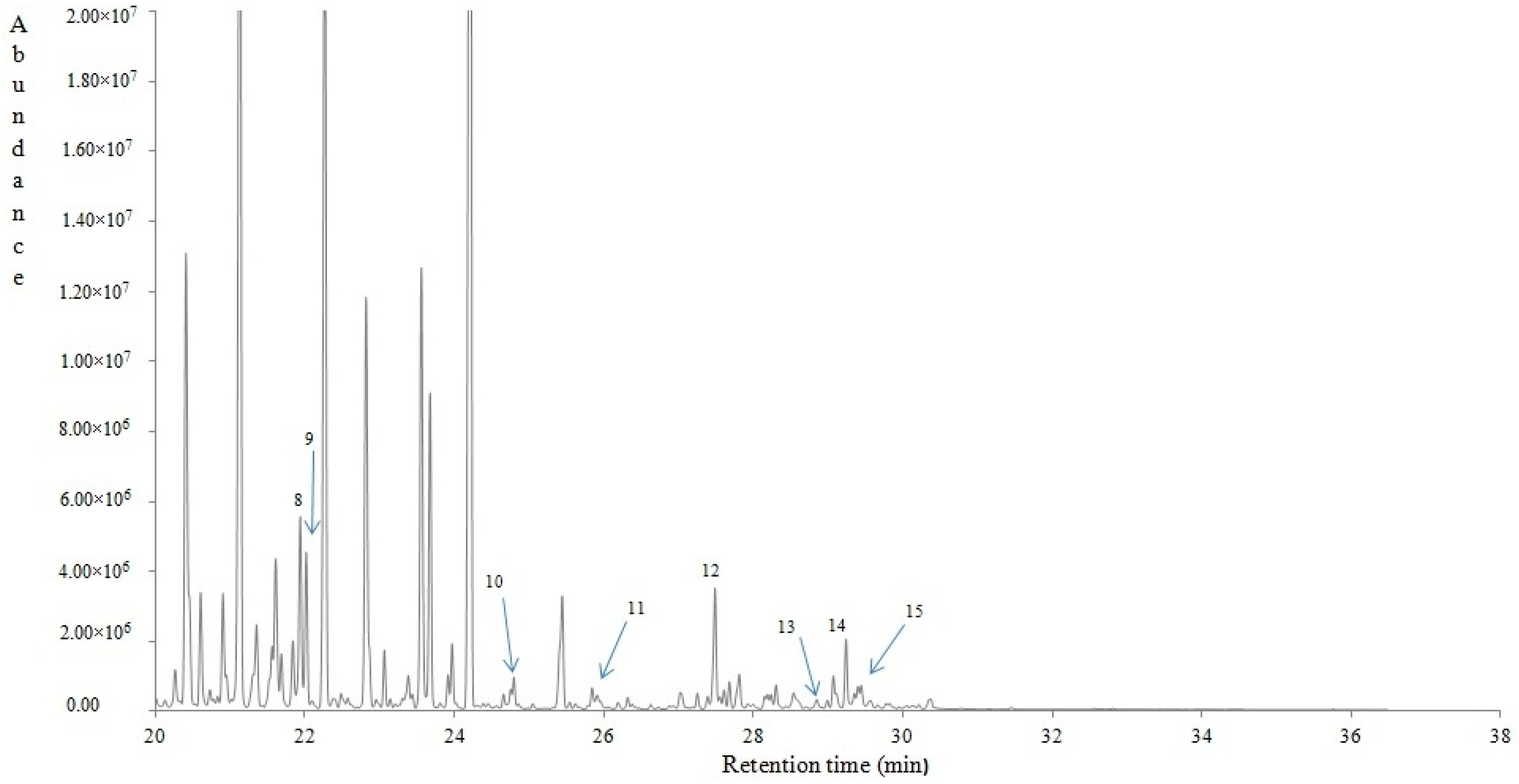
4.2.4. Volatile Compounds of Lavandula stoechas
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Outdoor Flowering Plants-Mona Lavender. Available online: www.hgtv.com (accessed on 19 October 2018).
- Plant Finder-Plectranthus Mona Lavender. Available online: www.missouribotanicalgarden.org (accessed on 19 October 2018).
- Grieve, M.A. Modern Herbal; Dover Publications, Inc.: New York, NY, USA, 1971; Volume II, ISBN 0-486-22799-5. [Google Scholar]
- Kathleen, N.B. The Sunset Western Garden Book, 7th ed.; Lane Magazine & Book Company: Los Angeles, CA, USA, 1975. [Google Scholar]
- Matt, E. Lavender; University of Kentucky Center for Crop Diversification: Lexington, KY, USA, 2017. [Google Scholar]
- Upson, T.; Andrews, S. The Genus Lavandula. Royal Botanic Gardens; Kew 2004; CRC Press: Boca Raton, FL, USA, 2004; ISBN 9780881926422. [Google Scholar]
- Lis-Balchin, M. Lavender: The Genus Lavandula; Taylor and Francis: Abingdon, UK, 2002; ISBN 9780203216521. [Google Scholar]
- Integrative Medicine Communications, Germany, American Botanical Council. Expanded Commission E Monograph: “Lavender Flower”. 2000. Available online: cms.herbalgram.org (accessed on 18 October 2018).
- British Broadcasting Corporation. Oils ‘Make Male Breasts Develop’. February 2007. Retrieved 2018-03-17. Available online: https://www.bbc.com/news/health-43429933 (accessed on 29 December 2018).
- British Broadcasting Corporation. More Evidence Essential Oils ‘Make Male Breasts Develop. 2018. Available online: https://www.realclearscience.com/2018/03/19/more_evidence_essential_oils_make_male_breasts_develop_280504.html (accessed on 19 March 2018).
- Lavender: Science and Safety, National Center for Complementary and Integrative Health. 2007. Available online: https://nccih.nih.gov/health/lavender/ataglance.htm (accessed on 5 November 2013).
- Cavanagh, H.M.A.; Wilkinson, J.M. “Lavender Essential Oil: A Review, Australian Infection Control; CSIRO Publishing: Clayton, Australia, 2005. [Google Scholar]
- Placzek, M.; Frömel, W.; Eberlein, B.; Gilbertz, K.P.; Przybilla, B. Evaluation of phototoxic properties of fragrances. Acta Derm. Venereol. 2007, 87, 312–316. [Google Scholar] [CrossRef] [PubMed]
- Murthy, H.N.; Manohar, S.H.; Naik, P.M.; Lee, E.J.; Paek, K.Y. Physicochemical characteristics and antioxidant activity of Lavandula bipinnata seed oil. Int. Food Res. J. 2014, 21, 1473–1476. [Google Scholar]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-Ciocalteu reagent. Meth. Enzymol. 1999, 299, 152–178. [Google Scholar]
- Karabagias, I.K.; Nikolaou, C.; Karabagias, V.K. Volatile fingerprints of common and rare honeys produced nn Greece: In search of PHVMs with implementation of the honey code. Eur. Food Res. Technol. 2018, in press. [Google Scholar] [CrossRef]
- Charrouf, Z.; Guillaume, D. Phenols and Polyphenols from Argania spinosa. Am. J. Food Technol. 2007, 2, 679–683. [Google Scholar] [CrossRef]
- Choudhary, M.I.; Naheed, N.; Abbaskhan, A.; Musharraf, S.G.; Hina, S.; Rahman, A.U. Phenolic and other constituents of fresh water fern Salvinia molesta. Phytochemistry 2008, 69, 1018–1023. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.S.; Kang, Y.S.; Jung, J.Y.; Lee, S.; Ohuchi, K.; Shin, K.H.; Kang, I.J.; Park, J.H.Y.; Shin, H.K.; Soon, S. Protein glycation inhibitors from the fruiting body of Phellinus linteus. Biol. Pharm. Bull. 2008, 31, 1968–1972. [Google Scholar] [CrossRef]
- Santos, S.O.; Freire, C.R.S.; Domingues, M.R.M.A.; Silvestre, A.J.D.; Pascoal, N.C. Characterization of phenolic components in polar extracts of Eucalyptus globulus labill. bark by high-performance liquid chromatography–mass spectrometry. J. Agric. Food Chem. 2011, 59, 9386–9393. [Google Scholar] [CrossRef]
- Quinde-Axtell, Z.; Baik, B.K. Phenolic compounds of barley grain and their implication in food product discoloration. J. Agric. Food Chem. 2006, 54, 9978–9984. [Google Scholar] [CrossRef]
- Olthof, M.R.; Hollman, P.C.; Katan, M.B. Chlorogenic acid and caffeic acid are absorbed in humans. J. Nutr. 2001, 131, 66–71. [Google Scholar] [CrossRef]
- Rajendra, N.P.; Karthikeyan, A.; Karthikeyan, S.; Reddy, B.V. Inhibitory effect of caffeic acid on cancer cell proliferation by oxidative mechanism in human HT-1080 fibrosarcoma cell line. Mol. Cell Biochem. 2011, 349, 11–19. [Google Scholar] [CrossRef]
- Hirose, M.; Takesada, Y.; Tanaka, H.; Tamano, S.; Kato, T.; Shirai, T. Carcinogenicity of antioxidants BHA, caffeic acid, sesamol, 4-methoxyphenol and catechol at low doses, either alone or in combination, and modulation of their effects in a rat medium-term multi-organ carcinogenesis model. Carcinogenesis 1998, 9, 207–212. [Google Scholar] [CrossRef]
- Clifford, M.N. Chlorogenic acids and other cinnamates. Nature, occurrence and dietary burden. J. Sci. Food Agric. 1999, 79, 362–372. [Google Scholar] [CrossRef]
- Petersen, M.; Abdullah, Y.; Benner, J.; Eberle, D.; Gehlen, K.; Hücherig, S.; Janiak, V.; Kim, K.H.; Sander, M.; Weitzel, C.; et al. Evolution of rosmarinic acid biosynthesis. Phytochemistry 2009, 70, 1663–1679. [Google Scholar] [CrossRef]
- Vogelsang, K.; Schneider, B.; Petersen, M. Production of rosmarinic acid and a new rosmarinic acid 3′-O-β-d-glucoside in suspension cultures of the hornwort Anthoceros agrestis Paton. Planta 2006, 223, 369–373. [Google Scholar] [CrossRef] [PubMed]
- Awad, R.; Muhammad, A.; Durst, T.; Trudeau, V.L.; Arnason, J.T. Bioassay-guided fractionation of lemon balm (Melissa officinalis L.) using an in vitro measure of GABA transaminase activity. Phytother. Res. 2009, 23, 1075–1081. [Google Scholar] [CrossRef]
- Lee, H.J.; Jeong, Y.I.; Lee, T.H.; Jung, I.D.; Lee, J.S.; Lee, C.M.; Kim, J.I.; Joo, H.; Lee, J.D.; Park, Y.M. Rosmarinic acid inhibits indoleamine 2,3-dioxygenase expression in murine dendritic cells. Biochem. Pharmacol. 2007, 73, 1412–1421. [Google Scholar] [CrossRef] [PubMed]
- Swarup, V.; Ghosh, J.; Ghosh, S.; Saxena, A.; Basu, A. Antiviral and anti-inflammatory effects of rosmarinic acid in an experimental murine model of Japanese encephalitis. Antimicrob. Agents Chemother. 2007, 51, 3367–3370. [Google Scholar] [CrossRef]
- Wang, J.; Xiong, X.; Feng, B. Cardiovascular effects of salvianolic acid B. Evid. Based Complement. Altern. Med. 2013, 2013, 16. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.H.; Liu, A.H.; Wu, H.L.; Westenbroek, C.; Song, Q.L.; Yu, H.M.; Gert, J.; Horst, T.; Li, X.J. Salvianolic acid B, an antioxidant from Salvia miltiorrhiza prevents Ab25–35-induced reduction in BPRP in PC12 cells. Biochem. Biophys. Res. Commun. 2006, 348, 593–599. [Google Scholar] [CrossRef]
- Zaabat, N.; Hay, A.E.; Michalet, S.; Darbour, N.; Bayet, C.; Skandrani, I.; Chekir-Ghedira, L.; Akkal, S.; Dijoux-Franca, M.G. Antioxidant and antigenotoxic properties of compounds isolated from Marrubium deserti de Noé. Food Chem. Toxicol. 2011, 49, 3328–3335. [Google Scholar] [CrossRef] [PubMed]
- Teslov, L.S.; Teslov, S.V. Cynaroside and luteolin from Campanula persicifolia and C. Rotundifolia. Chem. Nat. Comput. 1972, 8, 117. [Google Scholar] [CrossRef]
- Barberán, F.A.T.; Gil, M.I.; Tomás, F.; Ferreres, F.; Arques, A. Flavonoid aglycones and glycosides from Teucrium gnaphalodes. J. Nat. Prod. 1985, 48, 859–860. [Google Scholar] [CrossRef]
- Yuldashev, M.P. Cynaroside content of the plants Ferula varia and F. Foetida. Chem. Nat. Comput. 1997, 33, 597–598. [Google Scholar] [CrossRef]
- Hu, C.; Chun, Z.; Zhang, Y.; Kitts, D.D. Evaluation of antioxidant and prooxidant activities of bamboo Phyllostachys nigra Var. Henonis leaf extract in vitro. J. Agric. Food Chem. 2000, 48, 3170–3176. [Google Scholar] [CrossRef]
- Nüβlein, B.; Kreis, W. Purification and characterization of a cynaroside 7-O-β-d-glucosidase from Cynarae scolymi folium. Acta Hortic. 2005, 681, 413–420. [Google Scholar] [CrossRef]
- Lin, Y.P.; Chen, T.Y.; Tseng, H.W.; Lee, M.H.; Chen, S.T. Neural cell protective compounds isolated from Phoenix hanceana var. Formosana. Phytochemistry 2009, 70, 1173–1181. [Google Scholar] [CrossRef]
- Deligiannidou, G.E.; Kontogiorgis, C.; Hadjipavlou-Litina Dimitra, D.; Lazari, D.; Konstantinidis, T.; Papadopoulos, A. Antioxidant contribution of lavender (Lavandula angustifolia), sage (Salvia officinalis), tilia (Tilia tomentosa) and sideritis (Sideritis perfoliata) beverages prepared at home. SDRP J. Food Sci. Technol. 2018, 3, 360–377. [Google Scholar]
- Spiridon, I.; Colceru, S.; Anghel, N.; Teaca, C.A.; Bodirlau, R.; Armatu, A. Antioxidant capacity and total phenolic contents of oregano (Origanum vulgare), lavender (Lavandula angustifolia) and lemon balm (Melissa officinalis) from Romania. Nat. Prod. Res. 2011, 25, 1657–1661. [Google Scholar] [CrossRef]
- Lim, T.K. Edible Medicinal and Non-Medicinal Plants, Volume 12 Modified Stems, Roots, Bulbs; Springer International Publishing: New York, NY, USA; Zürich, Switzerland, 2016. [Google Scholar]
- Dewick, P.M. Medicinal Natural Products: A Biosynthetic Approach, 3rd ed.; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 2009; pp. 195–197. ISBN 978-0-470-74167-2. [Google Scholar]
- Umlauf, D.; Josef, J. Biosynthesis of the irregular monoterpene artemisia ketone, the sesquiterpene germacrene D and other isoprenoids in Tanacetum vulgare L. (Asteraceae). Phytochemistry 2004, 65, 2463–2470. [Google Scholar] [CrossRef]
- European Commission. Regulation (EC) No 1334/2008 of the European Parliament and Council of 16 December 2008. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX%3A32008R1334 (accessed on 26 March 2019).
- Opinion of the Scientific Committee on Food on Thujone Scientific Committee on Food. 2003. Available online: https://ec.europa.eu/food/sites/food/files/safety/docs/fs_food-improvement-agents_flavourings-out162.pdf (accessed on 28 October 2006).
- Laurie, C.D.; Matulka, R.A.; Burdock, G.A. Naturally Occurring Food Toxins. Toxins 2009, 2, 2289–2332. [Google Scholar] [CrossRef]
- Food and Drug Administration. FDA Regulation 21 CFR 172.510—Food Additives Permitted for Direct Addition to Food for Human Consumption. 2003. Available online: https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?CFRPart=172 (accessed on 28 October 2006).
- Department of the Treasury Alcohol and Tobacco Tax and Trade Bureau Industry Circular (2007-5) 17 October 2007, Retrieved May 5 2009. Available online: https://www.ttb.gov (accessed on 29 December 2018).
- Food and Drug Administration. Substances Generally Recognized as Safe. Archived 2005-11-30 at the Wayback Machine; 2003. Available online: https://www.fda.gov/food/ingredientspackaginglabeling/gras/ (accessed on 28 October 2006).
- Höld, K.M.; Sirisoma, N.S.; Iked, T.; Narahashi, T.; Casida, J.E. Alpha-thujone (the active component of absinthe): Gamma-aminobutyric acid type A receptor modulation and metabolic detoxification. Proc. Natl. Acad. Sci. USA 2000, 97, 3826–3831. [Google Scholar] [CrossRef] [PubMed]
- Goodman and Gilman, Pharmacological Basis of Therapeutics; Macmillan: Stuttgart, Germany, 1965; pp. 982–983.
- Marsden, W. The History of Sumatra Containing an Account. of the Government, Laws, Customs and Manners of the Native Inhabitants; Cambridge University Press: Cambridge, UK, 2005. [Google Scholar]
- Wickstrom, E. Poisons Information Monograph: Camphor; International Programme on Chemical Safety National Poison Center: Oslo, Norway, 1988. [Google Scholar]
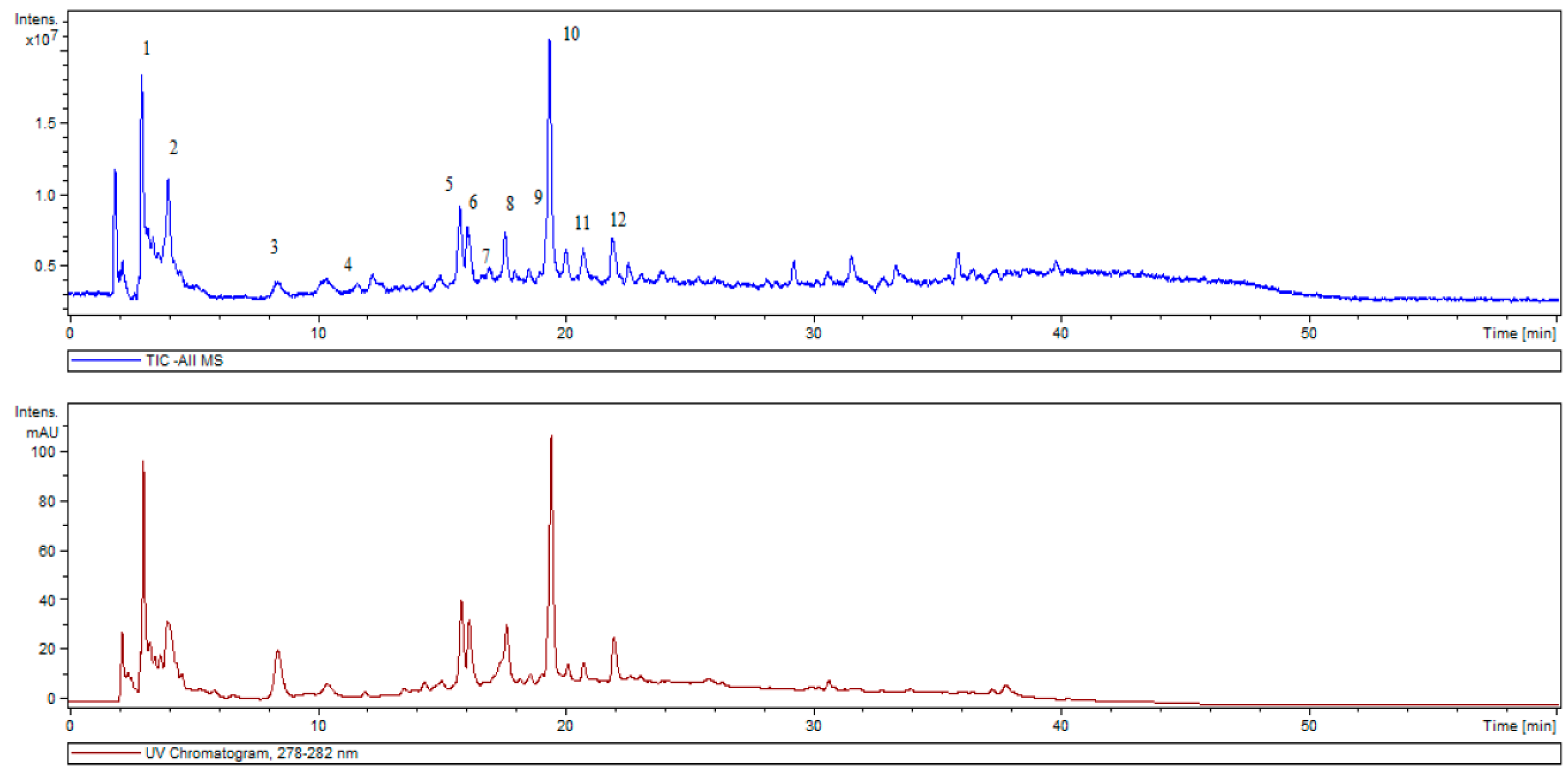
| Peak Number | RT (min) | [M−H]− (m/z) | Phytochemicals |
|---|---|---|---|
| 1 | 3.0 | 375(100), 573(46), 751(38) | 5-nonadecylresorcinol |
| 2 | 4.0 | 387(100), 607(30) | Unknown |
| 3 | 8.6 | 179(100), 135(25) | Caffeic acid |
| 4 | 11.1 | 473(100) | 6′′-O-Acetylgenistin |
| 5 | 15.8 | 463(100), 485(20) | Quercetin 3-O-glucoside |
| 6 | 16.1 | 461(100), 285(3) | Luteolin-O-glucuronide |
| 7 | 16.6 | 447(100) | Luteolin-O-glucoside |
| 8 | 17.7 | 359(100), 719(86) | Rosmarinic acid isomer |
| 9 | 19.2 | 445(100), 269(5) | Apigenin-O-glucuronide |
| 10 | 19.4 | 359(100), 719(30) | Rosmarinic acid |
| 11 | 20.7 | 549(100), 507(94) | Salvianolic acid derivative |
| 12 | 22.1 | 717(100), 519(43) | Salvianolic acid B (Lithospermic acid B) |
| RT (min) | KI | RTlit | Contribution (%) | Volatile compounds | Qualification | MOI |
|---|---|---|---|---|---|---|
| 6.51 | <800 | <800 | 0.06 | 3-Buten-2-one (Vinyl methyl ketone) | 91 | MS/KI |
| 7.14 | <800 | <800 | 0.08 | 3-Buten-2-ol, 2-methyl | 90 | MS/KI |
| 12.31 | <800 | <800 | 0.08 | Hexanal | 95 | MS/KI |
| 14.62 | 885 | 891 | 0.04 | 2-Heptanone | 94 | MS/KI |
| 15.31 | 913 | 915 | 0.28 | 3,3-Dimethylallyl acetate | 81 | MS/KI |
| 15.78 | 932 | - | 0.04 | 2,4,4-Trimethylcyclopentanone | 94 | MS/KI |
| 16.06 | 944 | 937 | 0.44 | (1S)-2,6,6-Trimethylbicyclo[3.1.1]hept-2-ene (apha-Pinene) | 96 | MS/KI |
| 16.48 | 962 | 958 | 0.48 | (−)-7,7-Dimethyl-2-methylenebicyclo[2.2.1]heptane (alpha-Fenchene) | 97 | MS/KI |
| 16.56 | 965 | 964 | 0.89 | 2,2-Dimethyl-3-methylidenebicyclo[2.2.1]heptane (Camphene) | 97 | MS/KI |
| 16.96 | 982 | 985 | 0.16 | 3-Octanone | 96 | MS/KI |
| 17.22 | 993 | 980 | 0.07 | 6,6-dimethyl-2-methylene-Bicyclo[3.1.1]heptane | 95 | MS/KI |
| 18.14 | 1034 | 1023 | 2.36 | 1-methyl-4-(1-methylethyl)-Benzene | 97 | MS/KI |
| 18.27 | 1039 | 1035 | 1.19 | 1-methyl-4-(prop-1-en-2-yl)Cyclohex-1-ene (dl-Limonene) | 99 | MS/KI |
| 18.45 | 1048 | 1044 | 9.77 | 1,3,3-trimethyl-2-oxabicyclo[2.2.2]Octane | 98 | MS/KI |
| 18.85 | 1066 | 1068 | 0.19 | 1-methyl-4-(1-methylethyl)-1,4-Cyclohexadiene | 97 | MS/KI |
| 19.20 | 1082 | 1080 | 1.05 | 2-Furanmethanol, 5-ethenyltetrahydro-.alpha.,.alpha.,5-trimethyl-, cis- (cis-Linalool oxide) | 91 | MS/KI |
| 19.31 | 1086 | 1096 | 0.19 | [(1S,4S)-4-methyl-1-propan-2-yl-4-bicyclo[3.1.0]hexanyl] acetate (trans-Sabinene hydrate) | 96 | MS/KI |
| 19.44 | 1092 | - | 0.28 | 3,4,4-trimethyl-2-Cyclohexen-1-one | 83 | MS/KI |
| 19.51 | 1096 | 1099 | 0.10 | 1-methyl-4-(1-methylethylidene)-Cyclohexene | 98 | MS/KI |
| 19.59 | 1099 | 1110 | 3.94 | 1-Octen-3-yl acetate | 90 | MS/KI |
| 19.86 | 1112 | 1117 | 32.14 | α: (1S,4R,5R)-4-methyl-1-(propan-2-yl)bicyclo[3.1.0]Hexan-3-one (alpha-Thujone) | 95 | MS/KI |
| 20.40 | 1139 | 1121 | 2.80 | (1R,3S,4S)-2,2,4-trimethylbicyclo[2.2.1]heptan-3-ol (alpha- Fenchyl alcohol) | 97 | MS/KI |
| 21.12 | 1174 | 1146 | 6.07 | 1,7,7-Trimethylbicyclo[2.2.1]Heptan-2-one (Camphor) | 97 | MS/KI |
| 21.34 | 1185 | 1164 | 0.66 | 6,6-dimethyl-4-methylidenebicyclo[3.1.1]Heptan-3-one (Pinocarvone) | 81 | MS/KI |
| 21.55 | 1195 | 1164 | 0.40 | endo-1,7,7-Trimethyl- bicyclo[2.2.1]Heptan-2-ol (Borneol) | 93 | MS/KI |
| 21.67 | 1201 | 1183 | 0.27 | 1-(3-methylphenyl)-Ethanone | 95 | MS/KI |
| 21.83 | 1209 | 1195 | 0.35 | .alpha.,.alpha.4-trimethyl-3-Cyclohexene-1-methanol (alpha-Terpineol) | 91 | MS/KI |
| 22.01 | 1219 | 1195 | 0.73 | 6,6-Dimethylbicyclo[3.1.1]hept-2-ene-2-carboxaldehyde (Myrtenal) | 97 | MS/KI |
| 22.26 | 1232 | 1223 | 3.91 | (2,2,4-trimethyl-3-bicyclo[2.2.1]Heptanyl) acetate (Fenchyl acetate) | 96 | MS/KI |
| 23.26 | 1284 | 1280 | 0.11 | 4-Isopropenyl-1-methyl-7-oxabicyclo[4.1.0]heptan-2-one (trans-Carvone oxide) | 88 | MS/KI |
| 23.55 | 1300 | 1285 | 2.04 | Bicyclo[2.2.1]heptan-2-ol, 1,7,7-trimethyl-, acetate, (1S-endo)- | 98 | MS/KI |
| 23.67 | 1306 | - | 1.61 | 1-methyl-4-(1-methylethylidene)-Cyclohexanol (γ-Terpineol) | 91 | MS/KI |
| 23.91 | 1320 | - | 0.15 | 1-methylene-4-(1-methylethenyl)-Cyclohexane (Pseudolimonene) | 85 | MS/KI |
| 23.96 | 1322 | - | 0.32 | 2,2-dimethyl-3-methylene- Bicyclo[2.2.1]heptane [Camphene, (1R,4S)-(+)-] | 91 | MS/KI |
| 24.20 | 1336 | 1332 | 7.75 | (6,6-Dimethylbicyclo[3.1.1]hept-2-en-2-yl)methyl acetate [(-)-Myrtenyl acetate] | 87 | MS/KI |
| 24.75 | 1367 | 1352 | 0.06 | 1H-Cyclopenta[1,3]cyclopropa[1,2]benzene, 3a,3b,4,5,6,7-hexahydro-3,7-dimethyl-4-(1-methylethyl)-, [3aS-(3aα,3bβ,4β,7α,7aS*)-(-)-] (alpha-Cubebene) | 99 | MS/KI |
| 24.79 | 1369 | 1369 | 0.09 | 2,6-Octadien-1-ol, 3,7-dimethyl-, acetate, (Z)- [cis-Geranyl acetate) | 91 | MS/KI |
| 25.44 | 1405 | 1378 | 0.61 | 1,2,4-Metheno-1H-indene, octahydro-1,7a-dimethyl-5-(1-methylethyl)-, [1S-(1.alpha.,2.alpha.,3a.beta.,4.alpha.,5.alpha.,7a.beta.,8S*)]-[(+)-Cyclosativene] | 94 | MS/KI |
| 25.84 | 1429 | 1396 | 0.07 | 1,4-Methano-1H-indene, octahydro-4-methyl-8-methylene-7-(1-methylethyl)-, [1S-(1.alpha.,3a.beta.,4.alpha.,7.alpha.,7a.beta.)]- [(+)-Sativene] | 99 | MS/KI |
| 27.25 | 1515 | 0.08 | 2,3-dihydro-1,3,3-trimethyl-2-methylene-1H-Indole | 80 | MS/KI | |
| 27.39 | 1523 | 1505 | 0.06 | Naphthalene, 1,2,4a,5,6,8a-hexahydro-4,7-dimethyl-1-(1-methylethyl)-, (1S,4aS,8aR)- (alpha-Muurolene) | 98 | MS/KI |
| 27.49 | 1530 | 1509 | 0.62 | Naphthalene, decahydro-4a-methyl-1-methylene-7-(1-methylethenyl)-, [4aR-(4a.alpha.,7.alpha.,8a.beta.)]-(beta-Selinene) | 99 | MS/KI |
| 27.55 | 1534 | 1505 | 0.05 | alpha-Eudesma-3,11-diene (alpha-Selinene) | 99 | MS/KI |
| 27.68 | 1542 | 1540 | 0.12 | Naphthalene, 1,2,3,5,6,8a-hexahydro-4,7-dimethyl-1-(1-methylethyl)-, (1S-cis)- | 99 | MS/KI |
| 28.30 | 1581 | 1525 | 0.13 | Naphthalene, 1,2,3,4,4a,7-hexahydro-1,6-dimethyl-4-(1-methylethyl)- | 87 | MS/KI |
| 28.85 | 1617 | - | 0.08 | 4aH-Cycloprop[e]azulen-4a-ol, decahydro-1,1,4,7-tetramethyl-, [1aR-(1a.alpha.,4.beta.,4a.beta.,7.alpha.,7a.beta.,7b.alpha.)]- (Palustrol) | 96 | MS/KI |
| 29.07 | 1631 | 1613 | 0.21 | (4,6)]dodecane,,12-trimethyl-9-methylene-, [1R-(1R*,4R*,6R*,10S*)]- (CAS); (-)-.beta.-Caryophyllene epoxide | 91 | MS/KI |
| 29.24 | 1643 | 1620 | 0.39 | 1H-Cycloprop[e]azulen-4-ol, decahydro-1,1,4,7-tetramethyl-, [1ar-(1a.alpha.,4.beta.,4a.beta.,7.alpha.,7a.beta.,7b.alpha.)]- (Viridiflorol) | 99 | MS/KI |
| 29.40 | 1653 | - | 0.11 | 1H-Cycloprop[e]azulene, decahydro-1,1,7-trimethyl-4-methylene-, [1aR-(1a.alpha.,4a.alpha.,7.alpha.,7a.beta.,7b.alpha.)]- [(+)-Aromadendrene] | 94 | MS/KI |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karabagias, I.K.; Karabagias, V.K.; Riganakos, K.A. Physico-Chemical Parameters, Phenolic Profile, In Vitro Antioxidant Activity and Volatile Compounds of Ladastacho (Lavandula stoechas) from the Region of Saidona. Antioxidants 2019, 8, 80. https://doi.org/10.3390/antiox8040080
Karabagias IK, Karabagias VK, Riganakos KA. Physico-Chemical Parameters, Phenolic Profile, In Vitro Antioxidant Activity and Volatile Compounds of Ladastacho (Lavandula stoechas) from the Region of Saidona. Antioxidants. 2019; 8(4):80. https://doi.org/10.3390/antiox8040080
Chicago/Turabian StyleKarabagias, Ioannis K., Vassilios K. Karabagias, and Kyriakos A. Riganakos. 2019. "Physico-Chemical Parameters, Phenolic Profile, In Vitro Antioxidant Activity and Volatile Compounds of Ladastacho (Lavandula stoechas) from the Region of Saidona" Antioxidants 8, no. 4: 80. https://doi.org/10.3390/antiox8040080
APA StyleKarabagias, I. K., Karabagias, V. K., & Riganakos, K. A. (2019). Physico-Chemical Parameters, Phenolic Profile, In Vitro Antioxidant Activity and Volatile Compounds of Ladastacho (Lavandula stoechas) from the Region of Saidona. Antioxidants, 8(4), 80. https://doi.org/10.3390/antiox8040080