Inhibitory Effects Induced by Vicia faba, Uncaria rhyncophylla, and Glycyrrhiza glabra Water Extracts on Oxidative Stress Biomarkers and Dopamine Turnover in HypoE22 Cells and Isolated Rat Striatum Challenged with 6-Hydroxydopamine
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Phytochemical Analysis
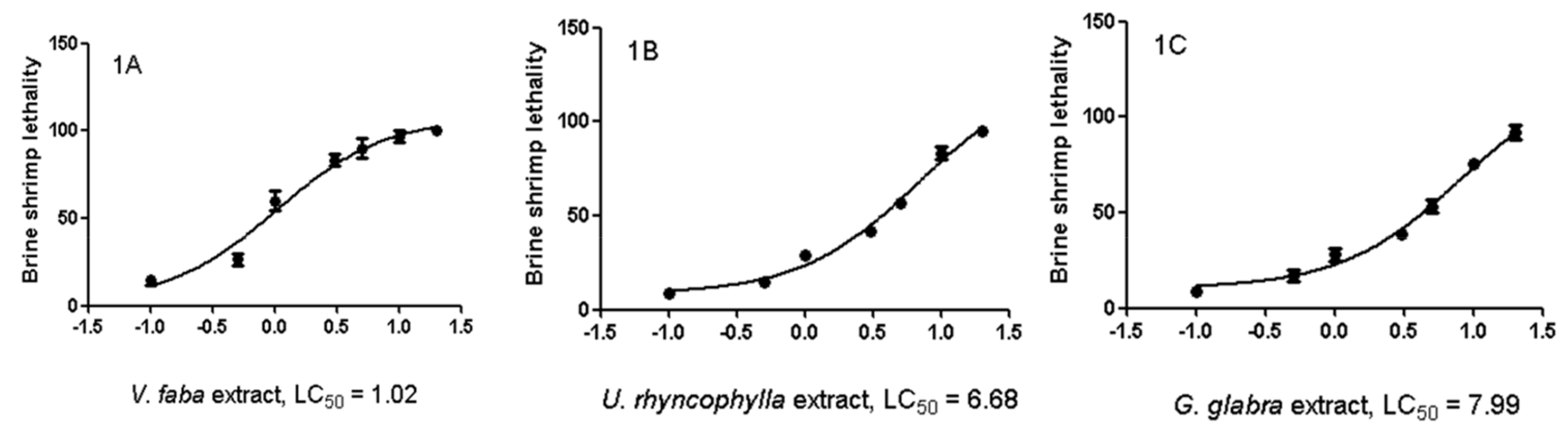
2.3. Artemia salina Lethality Test
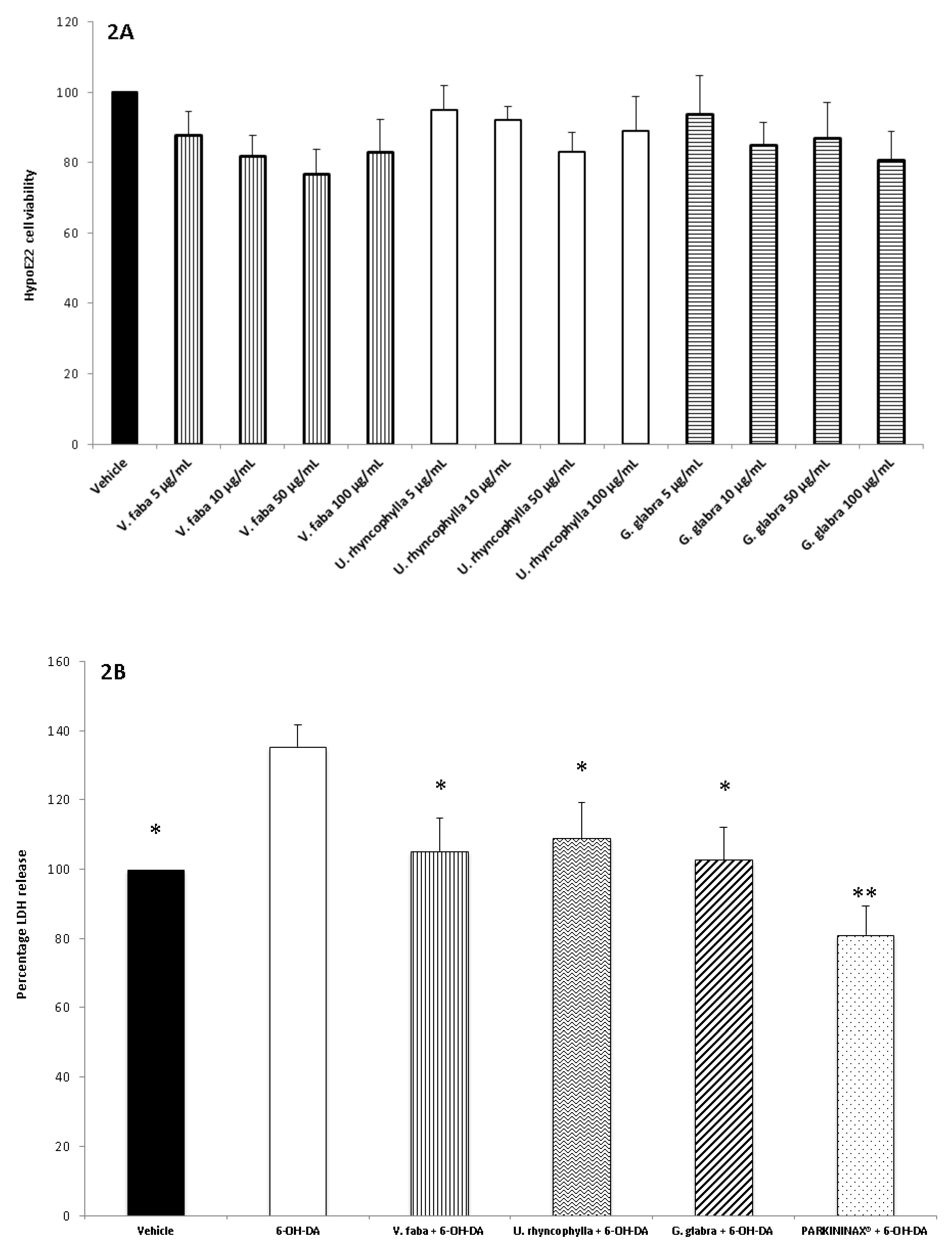
2.4. Cell Cultures and Viability Test
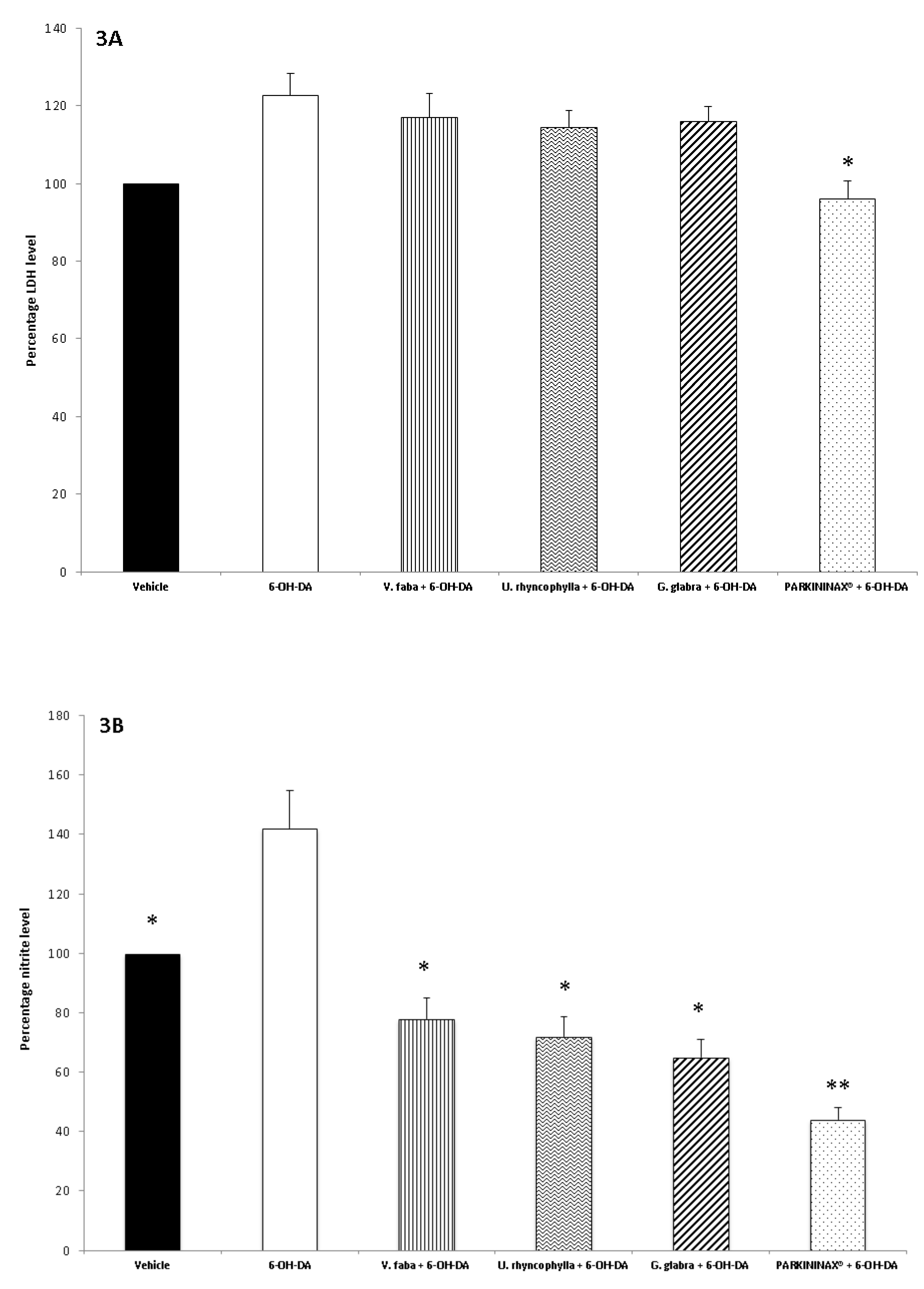
2.5. Ex Vivo Pharmacological Study
2.6. Striatum Levels of Nitrites
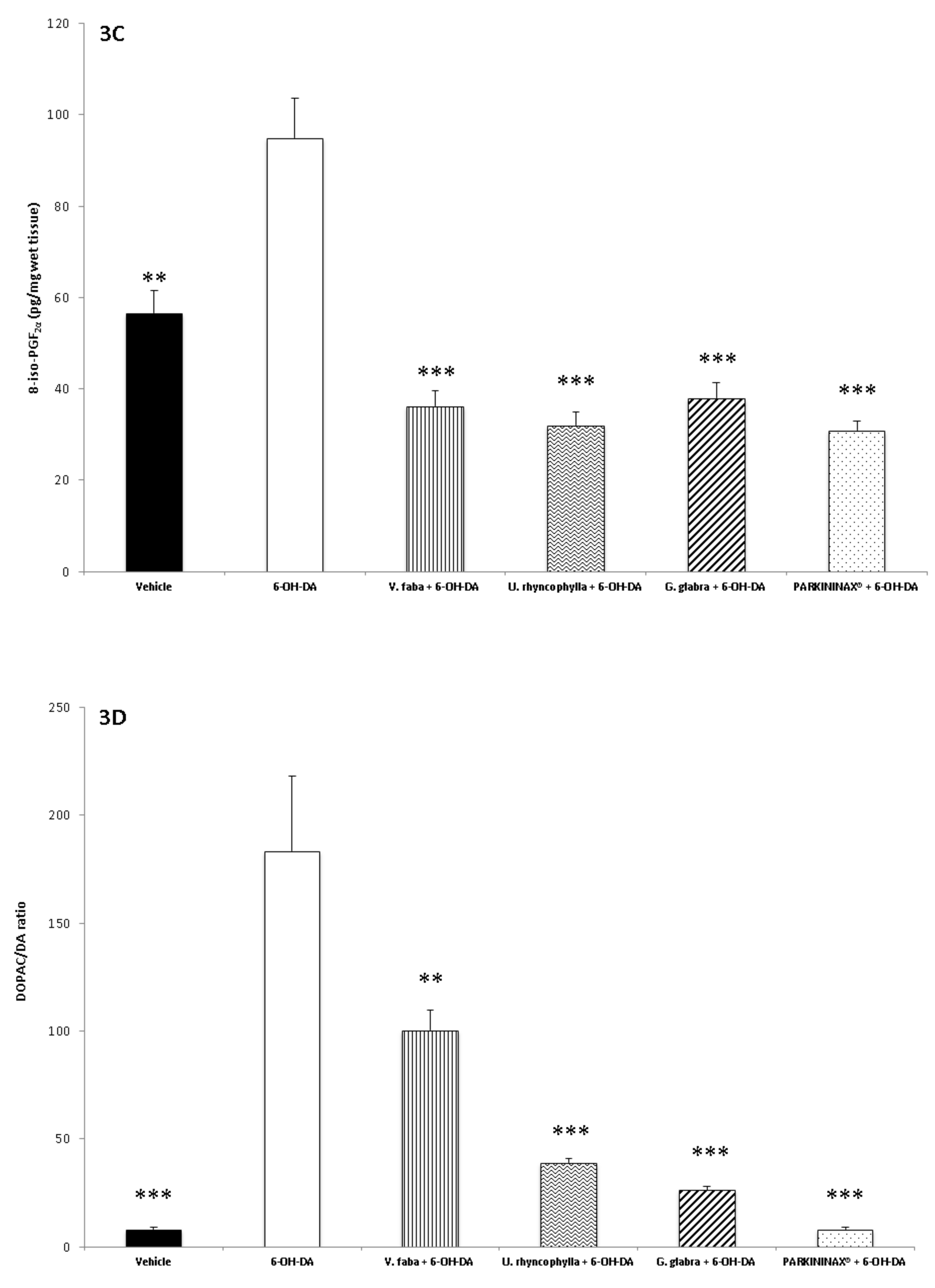
2.7. 8-iso-PGF2α Radioimmunoassay
2.8. Lactate Dehydrogenase Assay
2.9. Neurotransmitter Extraction and HPLC–EC Determination
2.10. Statistical Analysis
3. Results
3.1. Phytochemical Analysis
3.2. Pharmacological Studies
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Uttara, B.; Singh, A.V.; Zamboni, P.; Mahajan, R.T. Oxidative stress and neurodegenerative diseases: A review of upstream and downstream antioxidant therapeutic options. Curr. Neuropharmacol. 2009, 7, 65–74. [Google Scholar] [CrossRef] [PubMed]
- Jomova, K.; Vondrakova, D.; Lawson, M.; Valko, M. Metals, oxidative stress and neurodegenerative disorders. Mol. Cell Biochem. 2010, 345, 91–104. [Google Scholar] [CrossRef] [PubMed]
- Floyd, R.A.; Hensley, K. Oxidative stress in brain aging. Implications for therapeutics of neurodegenerative diseases. Neurobiol. Aging 2002, 23, 795–807. [Google Scholar] [CrossRef]
- Cobley, J.N.; Fiorello, M.L.; Bailey, D.M. 13 reasons why the brain is susceptible to oxidative stress. Redox Biol. 2018, 15, 490–503. [Google Scholar] [CrossRef] [PubMed]
- Serra, P.A.; Esposito, G.; Enrico, P.; Mura, M.A.; Migheli, R.; Delogu, M.R.; Miele, M.; Desole, M.S.; Grella, G.; Miele, E. Manganese increases l-DOPA auto-oxidation in the striatum of the freely moving rat: Potential implications to l-DOPA long-term therapy of Parkinson’s disease. Br. J. Pharmacol. 2000, 130, 937–945. [Google Scholar] [CrossRef]
- Srivastav, S.; Fatima, M.; Mondal, A.C. Important medicinal herbs in Parkinson’s disease pharmacotherapy. Biomed. Pharmacother. 2017, 92, 856–863. [Google Scholar] [CrossRef]
- Yuan, H.; Zhang, Z.W.; Liang, L.W.; Shen, Q.; Wang, X.D.; Ren, S.M.; Ma, H.J.; Jiao, S.J.; Liu, P. Treatment strategies for Parkinson’s disease. Neurosci. Bull. 2010, 26, 66–76. [Google Scholar] [CrossRef]
- LeWitt, P.A.; Fahn, S. Levodopa therapy for Parkinson disease: A look backward and forward. Neurology 2016, 5, S3–S12. [Google Scholar] [CrossRef]
- Joseph, J.A.; Shukitt-Hale, B.; Denisova, N.A.; Bielinski, D.; Martin, A.; McEwen, J.J.; Bickford, P.C. Reversals of age-related declines in neuronal signal transduction, cognitive, and motor behavioral deficits with bluberry, spinach, or strawberry dietary supplementation. J. Neurosci. 1999, 19, 8114–8121. [Google Scholar] [CrossRef]
- Ferrante, C.; Recinella, L.; Locatelli, M.; Guglielmi, P.; Secci, D.; Leporini, L.; Chiavaroli, A.; Leone, S.; Martinotti, S.; Brunetti, L.; et al. Protective Effects Induced by Microwave-Assisted Aqueous Harpagophytum Extract on Rat Cortex Synaptosomes Challenged with Amyloid β-Peptide. Phytother. Res. 2017, 31, 1257–1264. [Google Scholar] [CrossRef]
- Ramis, M.R.; Sarubbo, F.; Terrasa, J.L.; Moranta, D.; Aparicio, S.; Miralles, A.; Esteban, S. Chronic α-Tocopherol Increases Central Monoamines Synthesis and Improves Cognitive and Motor Abilities in Old Rats. Rejuvenation Res. 2016, 19, 159–171. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Wang, T.; Yue, F.; Li, X.; Wang, P.; Li, Y.; Chan, P.; Yu, S. Tea polyphenols alleviate motor impairments, dopaminergic neuronal injury, and cerebral α-synuclein aggregation in MPTP-intoxicated parkinsonian monkeys. Neuroscience 2015, 286, 383–392. [Google Scholar] [CrossRef] [PubMed]
- Tabatabaei-Malazy, O.; Larijani, B.; Abdollahi, M. Targeting metabolic disorders by natural products. J. Diabetes Metab. Disord. 2015, 14, 57. [Google Scholar] [CrossRef] [PubMed]
- Apaydin, H.; Ertan, S.; Ozekmekçi, S. Broad bean (Vicia faba)—A natural source of l-dopa—Prolongs on periods in patients with Parkinson’s disease who have on-off fluctuations. Mov. Disord. 2000, 15, 164–166. [Google Scholar] [CrossRef]
- Petramfar, P.; Hajari, F.; Yousefi, G.; Azadi, S.; Hamedi, A. Efficacy of oral administration of licorice as an adjunct therapy on improving the symptoms of patients with Parkinson’s disease, A randomized double blinded clinical trial. J. Ethnopharmacol. 2020, 247, 112226. [Google Scholar] [CrossRef]
- Shim, J.S.; Kim, H.G.; Ju, M.S.; Choi, J.G.; Jeong, S.Y.; Oh, M.S. Effects of the hook of Uncaria rhynchophylla on neurotoxicity in the 6-hydroxydopamine model of Parkinson’s disease. J. Ethnopharmacol. 2009, 126, 361–365. [Google Scholar] [CrossRef]
- Abnosi, M.H.; Yari, S. The toxic effect of gallic acid on biochemical factors, viability and proliferation of rat bone marrow mesenchymal stem cells was compensated by boric acid. J. Trace Elem. Med. Biol. 2018, 48, 246–253. [Google Scholar] [CrossRef]
- Serrano, J.; Cipak, A.; Boada, J.; Gonzalo, H.; Cacabelos, D.; Cassanye, A.; Pamplona, R.; Zarkovic, N.; Portero-Otin, M. Double-edged sword behaviour of gallic acid and its interaction with peroxidases in human microvascular endothelial cell culture (HMEC-1). Antioxidant and pro-oxidant effects. Acta Biochim. Pol. 2010, 57, 193–198. [Google Scholar] [CrossRef]
- Chandrasekhar, Y.; Phani Kumar, G.; Ramya, EM.; Anilakumar, K.R. Gallic Acid Protects 6-OHDA Induced Neurotoxicity by Attenuating Oxidative Stress in Human Dopaminergic Cell Line. Neurochem. Res. 2018, 43, 1150–1160. [Google Scholar] [CrossRef]
- Pervin, M.; Unno, K.; Ohishi, T.; Tanabe, H.; Miyoshi, N.; Nakamura, Y. Beneficial Effects of Green Tea Catechins on Neurodegenerative Diseases. Molecules 2018, 23, 1297. [Google Scholar] [CrossRef]
- Huang, N.; Zhang, Y.; Chen, M.; Jin, H.; Nie, J.; Luo, Y.; Zhou, S.; Shi, J.; Jin, F. Resveratrol delays 6-hydroxydopamine-induced apoptosis by activating the PI3K/Akt signaling pathway. Exp. Gerontol. 2019, 124, 110653. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.F.; Yu, X.L.; Ji, M.; Liu, S.Y.; Wu, X.L.; Wang, Y.J.; Liu, R.T. Resveratrol alleviates motor and cognitive deficits and neuropathology in the A53T α-synuclein mouse model of Parkinson’s disease. Food Funct. 2018, 9, 6414–6426. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Zhu, D.; Jiang, P.; Tang, X.; Lang, Q.; Yu, Q.; Zhang, S.; Che, Y.; Feng, X. Resveratrol synergizes with low doses of l-DOPA to improve MPTP-induced Parkinson disease in mice. Behav. Brain Res. 2019, 367, 10–18. [Google Scholar] [CrossRef] [PubMed]
- Menghini, L.; Leporini, L.; Vecchiotti, G.; Locatelli, M.; Carradori, S.; Ferrante, C.; Zengin, G.; Recinella, L.; Chiavaroli, A.; Leone, S.; et al. Crocus sativus L. stigmas and byproducts: Qualitative fingerprint, antioxidant potentials and enzyme inhibitory activities. Food Res. Int. 2018, 109, 91–98. [Google Scholar] [CrossRef]
- Rodríguez-Delgado, M.A.; Malovaná, S.; Pérez, J.P.; Borges, T.; García Montelongo, F.J. Separation of phenolic compounds by high-performance liquid chromatography with absorbance and fluorimetric detection. J. Chromatogr. A 2001, 912, 249–257. [Google Scholar] [CrossRef]
- Ferrante, C.; Recinella, L.; Ronci, M.; Menghini, L.; Brunetti, L.; Chiavaroli, A.; Leone, S.; Di Iorio, L.; Carradori, S.; Tirillini, B.; et al. Multiple pharmacognostic characterization on hemp commercial cultivars: Focus on inflorescence water extract activity. Food Chem. Toxicol. 2019, 125, 452–461. [Google Scholar] [CrossRef]
- Orlando, G.; Leone, S.; Ferrante, C.; Chiavaroli, A.; Mollica, A.; Stefanucci, A.; Macedonio, G.; Dimmito, M.P.; Leporini, L.; Menghini, L.; et al. Effects of Kisspeptin-10 on Hypothalamic Neuropeptides and Neurotransmitters Involved in Appetite Control. Molecules 2018, 23, 3071. [Google Scholar] [CrossRef]
- Menghini, L.; Leporini, L.; Scanu, N.; Pintore, G.; La Rovere, R.; Di Filippo, E.S.; Pietrangelo, T.; Fulle, S. Effect of phytochemical concentrations on biological activities of cranberry extracts. J. Biol. Regul. Homeost. Agents 2011, 25, 27–35. [Google Scholar]
- Chiavaroli, A.; Brunetti, L.; Orlando, G.; Recinella, L.; Ferrante, C.; Leone, S.; Di Michele, P.; Di Nisio, C.; Vacca, M. Resveratrol inhibits isoprostane production in young and aged rat brain. J. Biol. Regul. Homeost. Agents 2010, 24, 441–446. [Google Scholar]
- Ferrante, C.; Orlando, G.; Recinella, L.; Leone, S.; Chiavaroli, A.; Di Nisio, C.; Shohreh, R.; Manippa, F.; Ricciuti, A.; Vacca, M.; et al. Central apelin-13 administration modulates hypothalamic control of feeding. J. Biol. Regul. Homeost. Agents 2016, 30, 883–888. [Google Scholar]
- Ravipati, A.S.; Zhang, L.; Koyyalamudi, S.R.; Jeong, S.C.; Reddy, N.; Bartlett, J.; Smith, PT.; Shanmugam, K.; Münch, G.; Wu, M.J.; et al. Antioxidant and anti-inflammatory activities of selected Chinese medicinal plants and their relation with antioxidant content. BMC Complement. Altern. Med. 2012, 12, 173. [Google Scholar] [CrossRef] [PubMed]
- Karami, Z.; Emam-Djomeh, Z.; Mirzaee, HA.; Khomeiri, M.; Mahoonak, AS.; Aydani, E. Optimization of microwave assisted extraction (MAE) and soxhlet extraction of phenolic compound from licorice root. J. Food Sci. Technol. 2015, 52, 3242–3253. [Google Scholar] [CrossRef] [PubMed]
- Valente, I.M.; Cabrita, A.R.J.; Malushi, N.; Oliveira, H.M.; Papa, L.; Rodrigues, J.A.; Fonseca, A.J.M.; Maia, M.R.G. Unravelling the phytonutrients and antioxidant properties of European Vicia faba L. seeds. Food Res. Int. 2019, 116, 888–896. [Google Scholar] [CrossRef] [PubMed]
- Ramya, K.B.; Thaakur, S. Herbs containing l-Dopa: An update. Anc. Sci. Life 2007, 27, 50–55. [Google Scholar] [PubMed]
- Praticò, D. Alzheimer’s disease and oxygen radicals: New insights. Biochem. Pharmacol. 2002, 63, 563–567. [Google Scholar] [CrossRef]
- Plàtenik, J.; Stopka, P.; Vejrazka, M.; Stipek, S. Quinolinic acid-iron(ii) complexes: Slow autoxidation, but enhanced hydroxyl radical production in the Fenton reaction. Free Radic. Res. 2001, 34, 445–459. [Google Scholar] [CrossRef] [PubMed]
- Marnett, L.J. Lipid peroxidation-DNA damage by malondialdehyde. Mutat. Res. 1999, 424, 83–95. [Google Scholar] [CrossRef]
- Azizsoltani, A.; Piri, K.; Behzad, S.; Soleimani, M.; Nekouei, M.; Mahmoudi, Z.; Kazemi, A. Ethyl Acetate Extract of Licorice Root (Glycyrrhiza glabra) Enhances Proliferation and Osteogenic Differentiation of Human Bone Marrow Mesenchymal Stem Cells. Iran. J. Pharm. Res. 2018, 17, 1057–1067. [Google Scholar]
- Kannan, N.; Guruvayoorappan, C. Protective effect of Bauhinia tomentosa on acetic acid induced ulcerative colitis by regulating antioxidant and inflammatory mediators. Int. Immunopharmacol. 2013, 16, 57–66. [Google Scholar] [CrossRef]
- Hwang, I.K.; Lim, S.S.; Choi, K.H.; Yoo, K.Y.; Shin, H.K.; Kim, E.J.; Yoon-Park, J.H.; Kang, T.C.; Kim, Y.S.; Kwon, D.Y.; et al. Neuroprotective effects of roasted licorice, not raw form, on neuronal injury in gerbil hippocampus after transient forebrain ischemia. Acta Pharmacol. Sin. 2006, 27, 959–965. [Google Scholar] [CrossRef]
- Mollica, A.; Stefanucci, A.; Zengin, G.; Locatelli, M.; Macedonio, G.; Orlando, G.; Ferrante, C.; Menghini, L.; Recinella, L.; Leone, S.; et al. Polyphenolic composition, enzyme inhibitory effects ex-vivo and in-vivo studies on two Brassicaceae of north-central Italy. Biomed. Pharmacother. 2018, 107, 129–138. [Google Scholar] [CrossRef] [PubMed]
- Brunetti, L.; Menghini, L.; Orlando, G.; Recinella, L.; Leone, S.; Epifano, F.; Lazzarin, F.; Chiavaroli, A.; Ferrante, C.; Vacca, M. Antioxidant effects of garlic in young and aged rat brain in vitro. J. Med. Food 2009, 12, 1166–1169. [Google Scholar] [CrossRef] [PubMed]
- Brunetti, L.; Orlando, G.; Menghini, L.; Ferrante, C.; Chiavaroli, A.; Vacca, M. Ginkgo biloba leaf extract reverses amyloid beta-peptide-induced isoprostane production in rat brain in vitro. Planta Med. 2006, 72, 1296–1299. [Google Scholar] [CrossRef] [PubMed]
- Tsikas, D. Assessment of lipid peroxidation by measuring malondialdehyde (MDA) and relatives in biological samples: Analytical and biological challenges. Anal. Biochem. 2017, 524, 13–30. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Chen, X.; Wang, R.; Duan, H.; Wang, L.; Liang, L.; Nan, Y.; Liu, X.; Liu, A.; Jin, F. 3,5,4′-Tri-O-acetylresveratrol decreases seawater inhalation-induced acute lung injury by interfering with the NF-κB and i-NOS pathways. Int. J. Mol. Med. 2016, 37, 165–172. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.J.; Chang, C.K.; Liu, IM.; Chi, T.C.; Yu, H.J.; Cheng, J.T. Changes in endogenous monoamines in aged rats. Clin. Exp. Pharmacol. Physiol. 2001, 28, 285–289. [Google Scholar] [CrossRef] [PubMed]
- Hou, W.C.; Lin, R.D.; Chen, C.T.; Lee, M.H. Monoamine oxidase B (MAO-B) inhibition by active principles from Uncaria rhynchophylla. J. Ethnopharmacol. 2005, 100, 216–220. [Google Scholar] [CrossRef]
- Carrera, I.; Cacabelos, R. Current Drugs and Potential Future Neuroprotective Compounds for Parkinson’s Disease. Curr. Neuropharmacol. 2019, 17, 295–306. [Google Scholar] [CrossRef]
| Phenolic Compound | V. faba (mg/g dry Extract) | U. rhyncophylla (mg/g Dry Extract) | G. glabra (mg/g Dry Extract) |
|---|---|---|---|
| Gallic acid | 3.91 ± 0.35 | 30.80 ± 2.77 | 16.96 ± 0.68 |
| Catechin | 4.92 ± 0.59 | 6.11 ± 0.73 | 12.71 ± 0.99 |
| Epicatechin | n.d. | n.d. | 1.06 ± 0.05 |
| Resveratrol | 2.46 ± 0.25 | 25.25 ± 2.53 | 63.23 ± 7.14 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Orlando, G.; Chiavaroli, A.; Leone, S.; Brunetti, L.; Politi, M.; Menghini, L.; Recinella, L.; Ferrante, C. Inhibitory Effects Induced by Vicia faba, Uncaria rhyncophylla, and Glycyrrhiza glabra Water Extracts on Oxidative Stress Biomarkers and Dopamine Turnover in HypoE22 Cells and Isolated Rat Striatum Challenged with 6-Hydroxydopamine. Antioxidants 2019, 8, 602. https://doi.org/10.3390/antiox8120602
Orlando G, Chiavaroli A, Leone S, Brunetti L, Politi M, Menghini L, Recinella L, Ferrante C. Inhibitory Effects Induced by Vicia faba, Uncaria rhyncophylla, and Glycyrrhiza glabra Water Extracts on Oxidative Stress Biomarkers and Dopamine Turnover in HypoE22 Cells and Isolated Rat Striatum Challenged with 6-Hydroxydopamine. Antioxidants. 2019; 8(12):602. https://doi.org/10.3390/antiox8120602
Chicago/Turabian StyleOrlando, Giustino, Annalisa Chiavaroli, Sheila Leone, Luigi Brunetti, Matteo Politi, Luigi Menghini, Lucia Recinella, and Claudio Ferrante. 2019. "Inhibitory Effects Induced by Vicia faba, Uncaria rhyncophylla, and Glycyrrhiza glabra Water Extracts on Oxidative Stress Biomarkers and Dopamine Turnover in HypoE22 Cells and Isolated Rat Striatum Challenged with 6-Hydroxydopamine" Antioxidants 8, no. 12: 602. https://doi.org/10.3390/antiox8120602
APA StyleOrlando, G., Chiavaroli, A., Leone, S., Brunetti, L., Politi, M., Menghini, L., Recinella, L., & Ferrante, C. (2019). Inhibitory Effects Induced by Vicia faba, Uncaria rhyncophylla, and Glycyrrhiza glabra Water Extracts on Oxidative Stress Biomarkers and Dopamine Turnover in HypoE22 Cells and Isolated Rat Striatum Challenged with 6-Hydroxydopamine. Antioxidants, 8(12), 602. https://doi.org/10.3390/antiox8120602












