Exploring Target Genes Involved in the Effect of Quercetin on the Response to Oxidative Stress in Caenorhabditis elegans
Abstract
1. Introduction
2. Materials and Methods
2.1. Standards and Reagents
2.2. Strains and Maintenance Conditions
2.3. Stress Assays
2.4. RT-qPCR Assays
2.5. Fluorescence Quantification and Visualization
2.6. Statistical Analysis
3. Results and Discussion
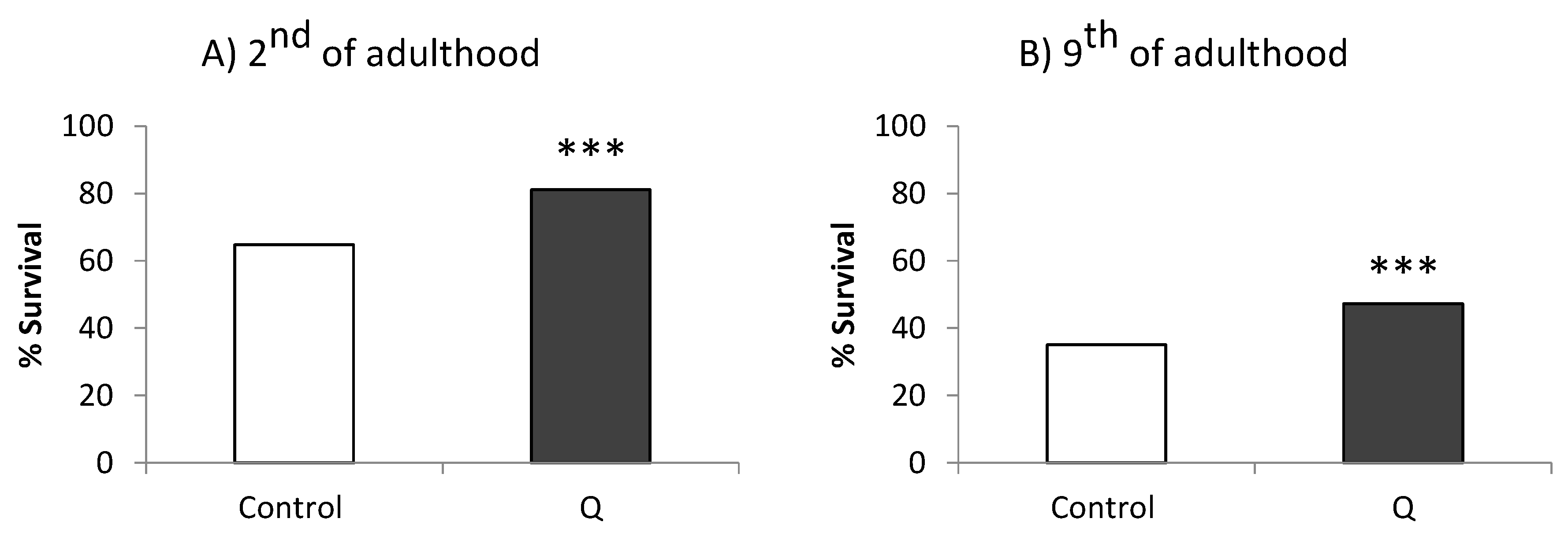
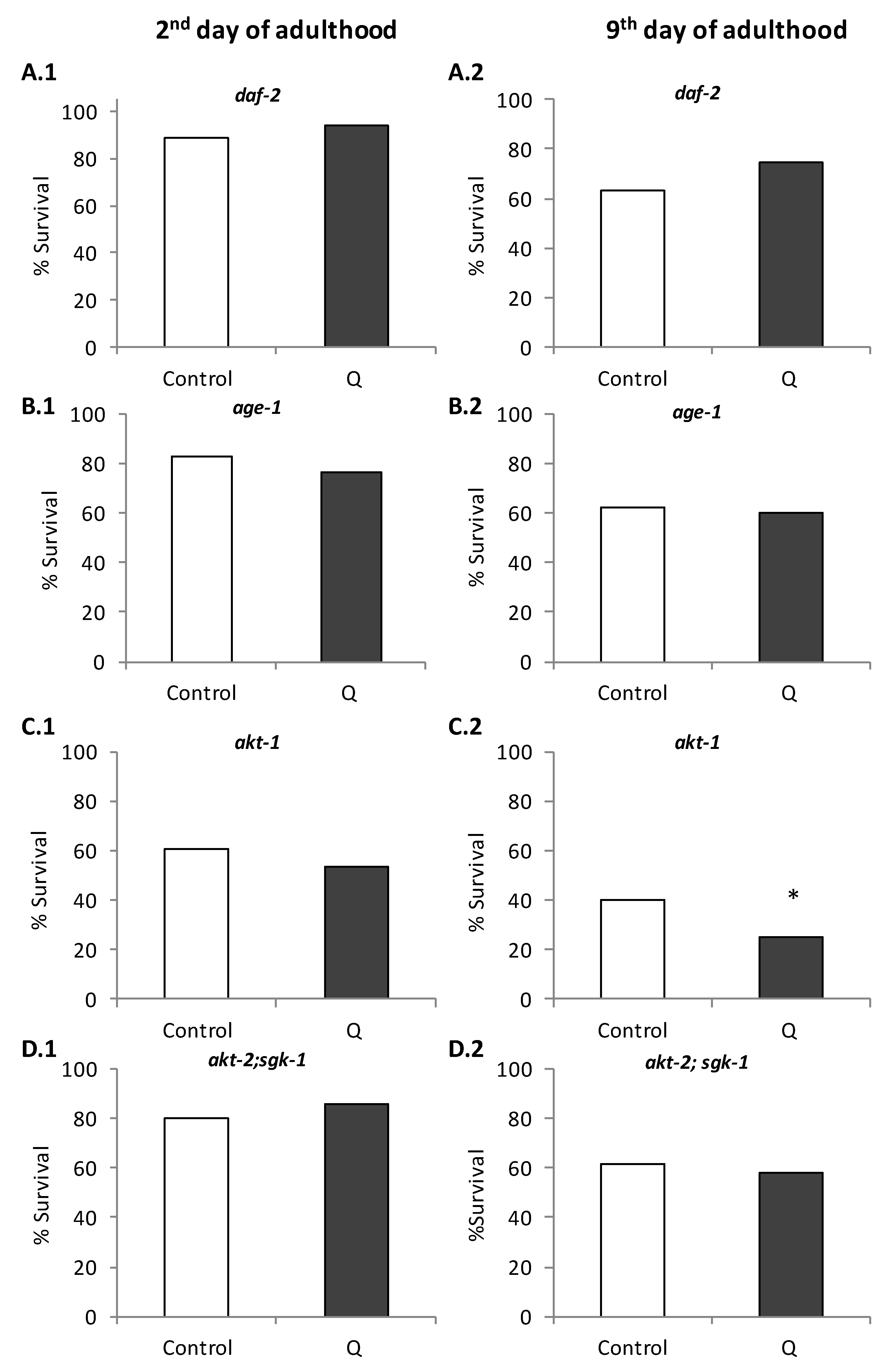
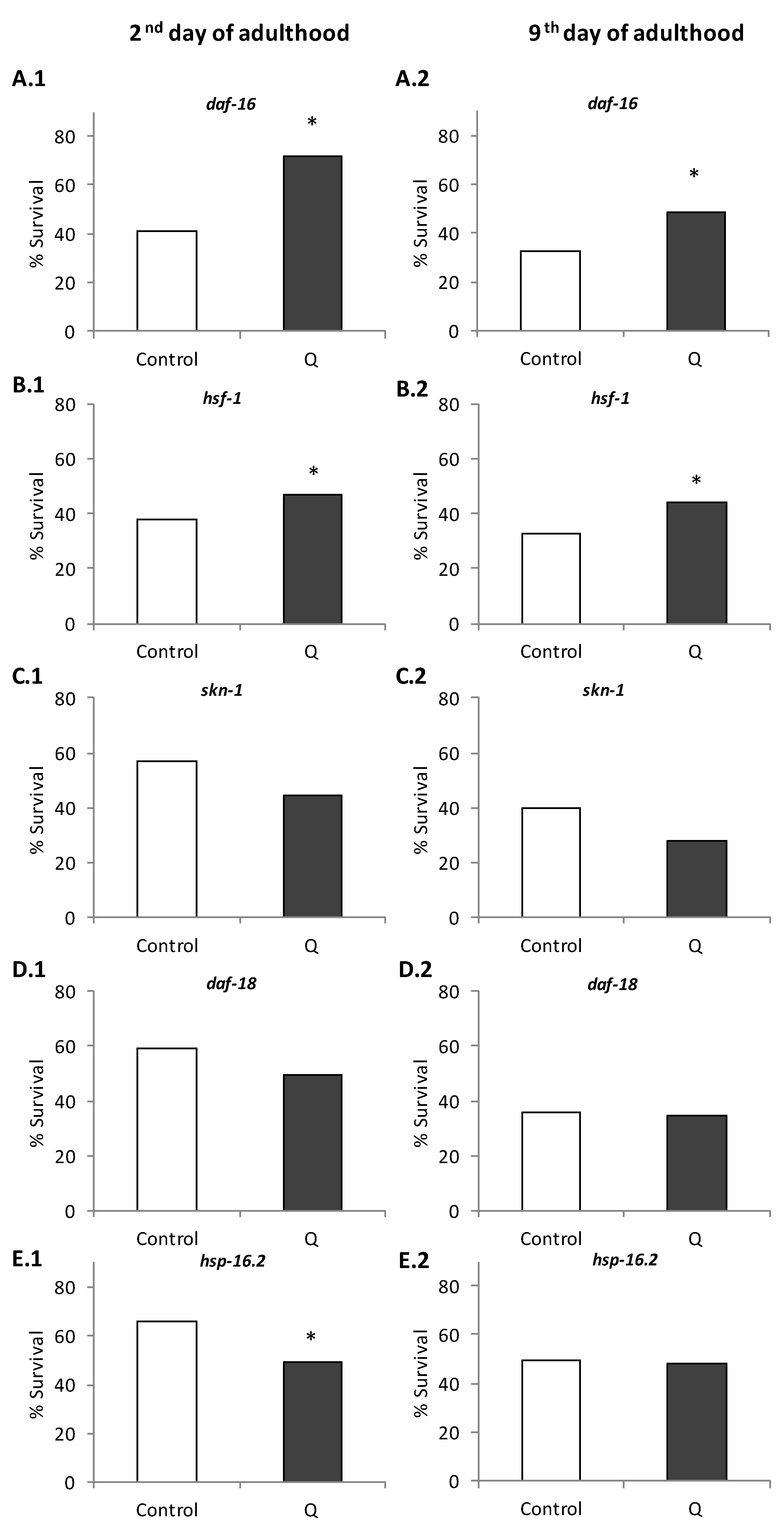
3.1. Assays in Wild Type and Mutant Worms
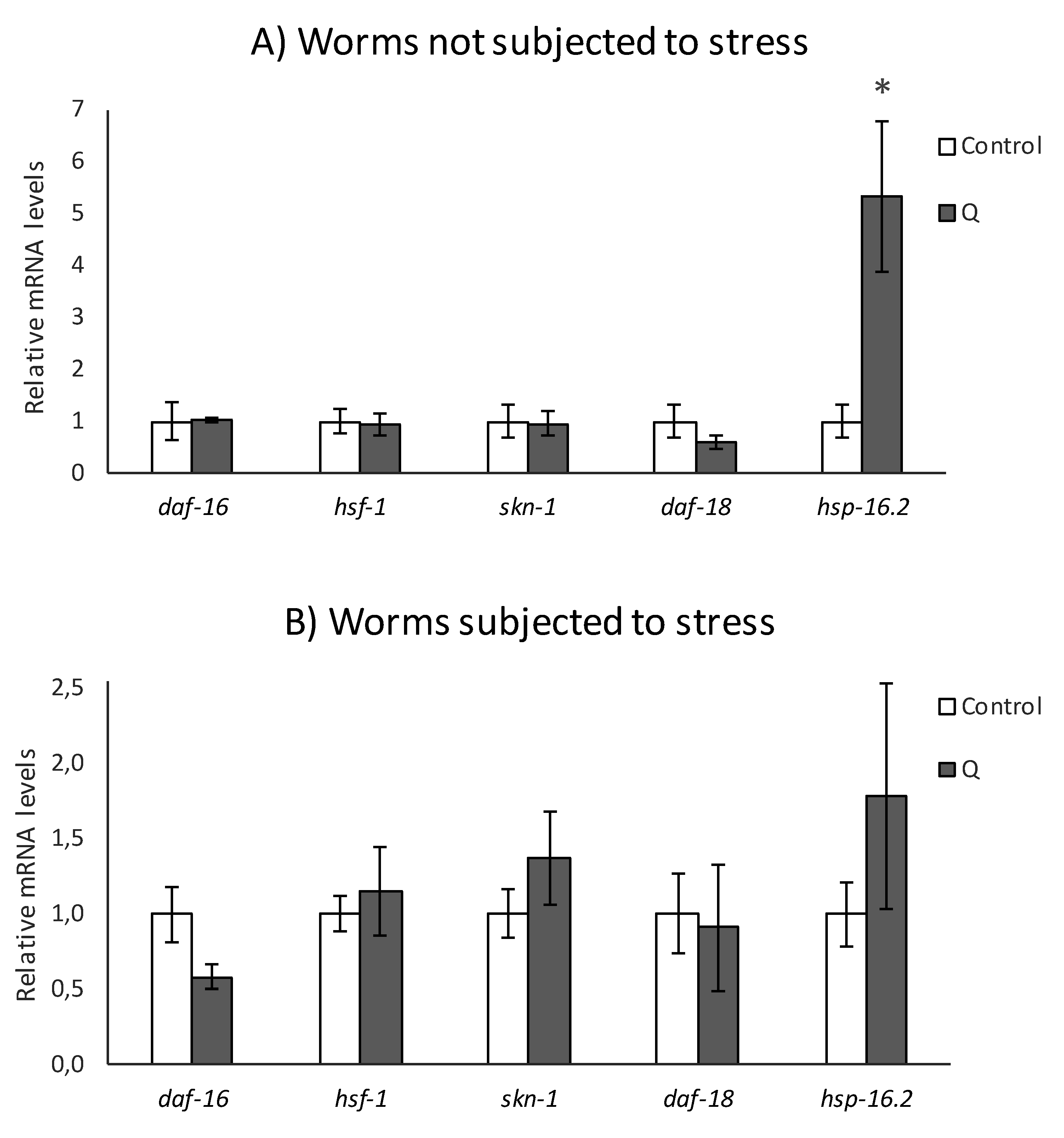
3.2. q-RT-PCR Analyses
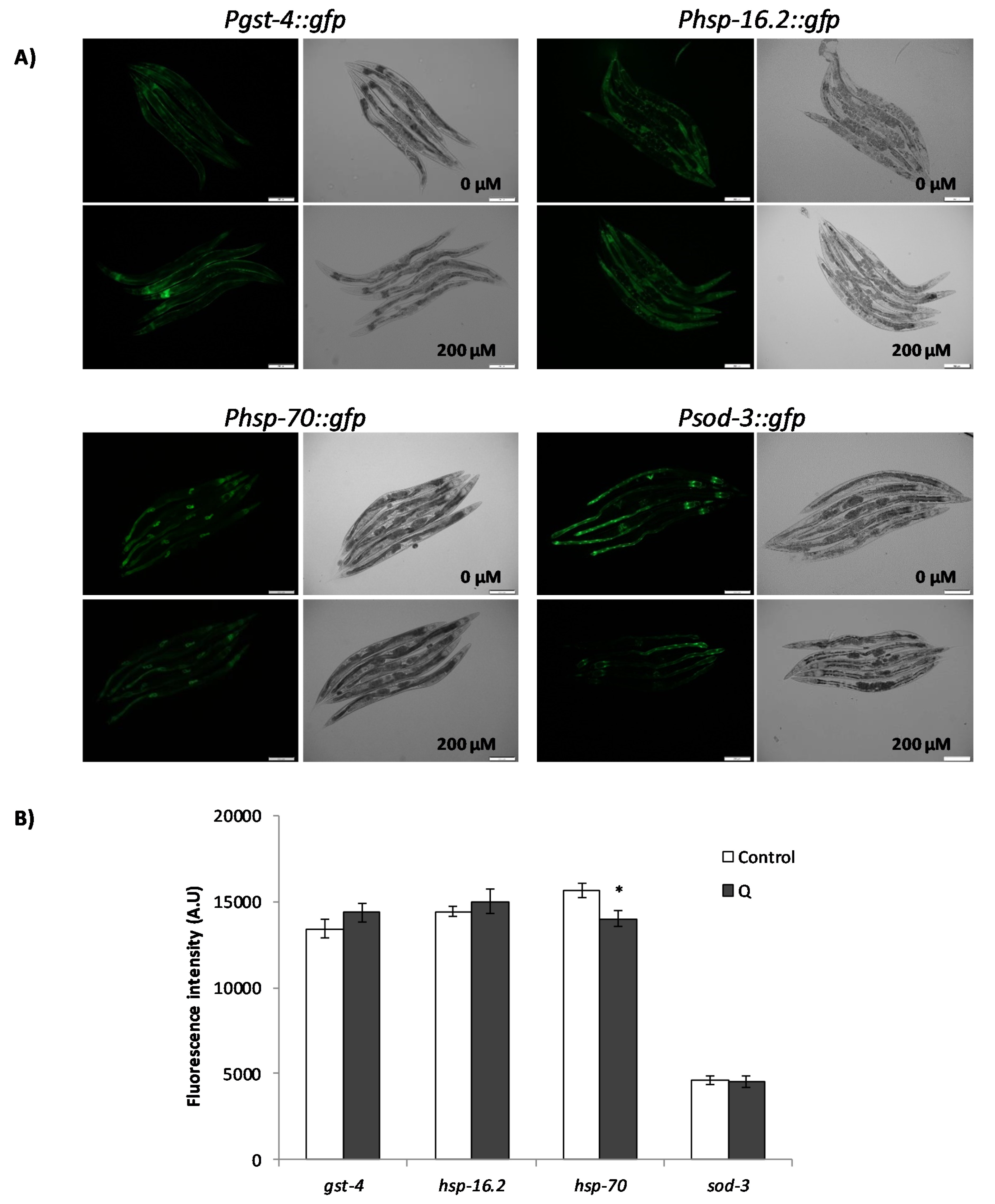
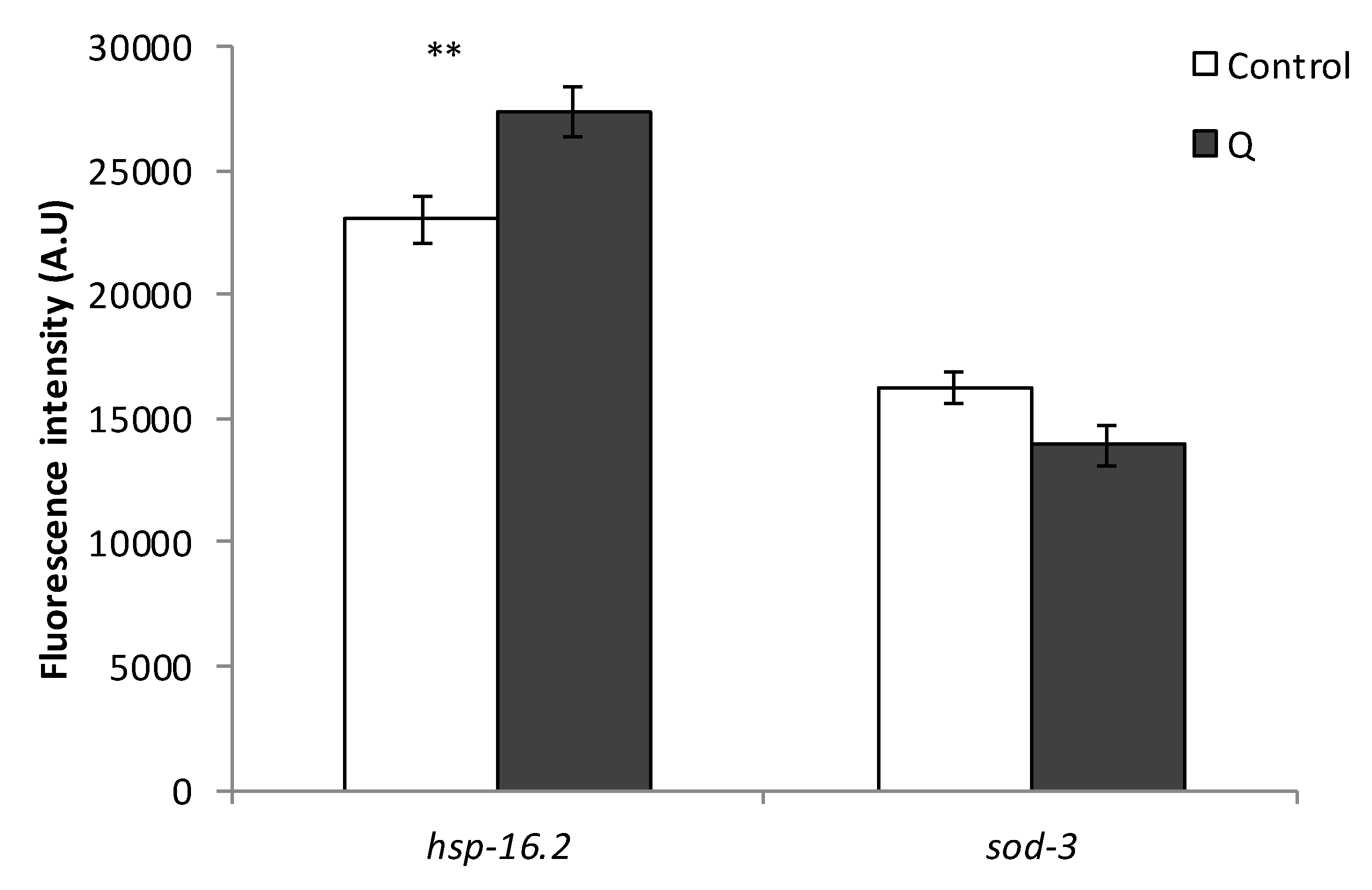
3.3. Assays with Fluorescent Reporters
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kaliora, A.C.; Dedoussis, G.V. Natural antioxidant compounds in risk factors for CVD. Pharm. Res. 2007, 56, 99–109. [Google Scholar] [CrossRef] [PubMed]
- Boots, A.W.; Haenen, G.R.; Bast, A. Health effects of quercetin: From antioxidant to nutraceutical. Eur. J. Pharm. 2008, 585, 325–337. [Google Scholar] [CrossRef] [PubMed]
- Darband, S.G.; Kaviani, M.; Yousefi, B.; Sadighparvar, S.; Pakdel, F.G.; Attari, J.A.; Mohebbi, I.; Naderi, S.; Majidinia, M. Quercetin: A functional dietary flavonoid with potential chemo-preventive properties in colorectal cancer. J. Cell. Physiol. 2018, 233, 6544–6560. [Google Scholar] [CrossRef] [PubMed]
- Cos, P.; Ying, L.; Calomme, M.; Hu, J.P.; Cimanga, K.; Van Poel, B.; Pieters, L.; Vlietinck, A.J.; Vanden Berghe, D. Structure-activity relationship and classification of flavonoids as inhibitors of xanthine oxidase and superoxide scavengers. J. Nat. Prod. 1998, 61, 71–76. [Google Scholar] [CrossRef] [PubMed]
- Middleton, E.; Kandaswami, C.; Theoharides, T.C. The effects of plant flavonoids on mammalian cells: Implications for inflammation, heart disease, and cancer. Pharm. Rev. 2000, 52, 673–751. [Google Scholar] [PubMed]
- González-Paramás, A.M.; Ayuda-Durán, B.; Martínez, S.; González-Manzano, S.; Santos-Buelga, D. The Mechanisms Behind the Biological Activity of Flavonoids. Curr. Med. Chem. 2018, 26, 1–14. [Google Scholar] [CrossRef]
- Kyriakakis, E.; Markaki, M.; Tavernarakis, N. Caenorhabditis elegans as a model for cancer research. Mol. Cell. Oncol. 2015, 2, e975027. [Google Scholar] [CrossRef]
- Surco-Laos, F.; Cabello, J.; Gómez-Orte, E.; González-Manzano, S.; González-Paramás, A.M.; Santos-Buelga, C.; Dueñas, M. Effects of O-methylated metabolites of quercetin on oxidative stress, thermotolerance, lifespan and bioavailability on Caenorhabditis elegans. Food Funct. 2011, 2, 445–456. [Google Scholar] [CrossRef]
- Kampkötter, A.; Timpel, C.; Zurawski, R.F.; Ruhl, S.; Chovolou, Y.; Proksch, P.; Wätjen, W. Increase of stress resistance and lifespan of Caenorhabditis elegans by quercetin. Comparative Biochemistry and Physiology. Comp. Biochem. Part B 2008, 149, 314–323. [Google Scholar] [CrossRef]
- Saul, N.; Pietsch, K.; Menzel, R.; Steinberg, C.E. Quercetin-mediated longevity in C. elegans: Is DAF-16 involved? Mech. Ageing Dev. 2008, 129, 611–613. [Google Scholar] [CrossRef]
- Pietsch, K.; Saul, N.; Menzel, R.; Stürzenbaum, S.R.; Steinberg, C.E. Quercetin mediated lifespan extension in Caenorhabditis elegans is modulated by age-1, daf-2, sek-1 and unc-43. Biogerontology 2009, 10, 565–578. [Google Scholar] [CrossRef] [PubMed]
- Xue, Y.L.; Ahiko, T.; Miyakawa, T.; Amino, H.; Hu, F.; Furihata, K.; Kita, K.; Shirasawa, T.; Sawano, Y.; Tanokura, M. Isolation and Caenorhabditis elegans lifespan assay of flavonoids from onion. J. Agric. Food Chem. 2011, 59, 5927–5934. [Google Scholar] [CrossRef]
- Grünz, G.; Haas, K.; Soukup, S.; Klingenspor, M.; Kulling, S.E.; Daniel, H.; Spanier, B. Structural features and bioavailability of four flavonoids and their implications for lifespan-extending and antioxidant actions in C. elegans. Mech. Ageing Dev. 2012, 133, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Kampkötter, A.; Nkwonkam, C.G.; Zurawski, R.F.; Timpel, C.; Chovolou, Y.; Wätjen, W.; Kahl, R. Investigations of protective effects of the flavonoids quercetin and rutin on stress resistance in the model organism Caenorhabditis elegans. Toxicology 2007, 234, 113–123. [Google Scholar] [CrossRef] [PubMed]
- Pietsch, K.; Saul, N.; Chakrabarti, S.; Stürzenbaum, S.R.; Menzel, R.; Steinberg, C.E. Hormetins, antioxidants and prooxidants: Defining quercetin-, caffeic acid- and rosmarinic acid-mediated life extension in C. elegans. Biogerontology 2011, 12, 329–347. [Google Scholar] [CrossRef]
- Dueñas, M.; Surco-Laos, F.; González-Manzano, S.; González-Paramás, A.M.; Gómez-Orte, E.; Cabello, J.; Santos-Buelga, C. Deglycosylation is a key step in biotransformation and lifespan effects of quercetin-3-O-glucoside in Caenorhabditis elegans. Pharmacol. Res. 2013, 76, 41–48. [Google Scholar] [CrossRef]
- Oh, S.W.; Mukhopadhyay, A.; Svrzikapa, N.; Jiang, F.; Davis, R.J.; Tissenbaum, H.A. JNK regulates lifespan in Caenorhabditis elegans by modulating nuclear translocation of forkhead transcription factor/DAF-16. Proc. Natl. Acad. Sci. USA 2005, 102, 4494–4499. [Google Scholar] [CrossRef]
- Troemel, E.R.; Chu, S.W.; Reinke, V.; Lee, S.S.; Ausubel, F.M.; Kim, D.H. p38 MAPK regulates expression of immune response genes and contributes to longevity in C. elegans. PLoS Genet. 2006, 2, e183. [Google Scholar] [CrossRef]
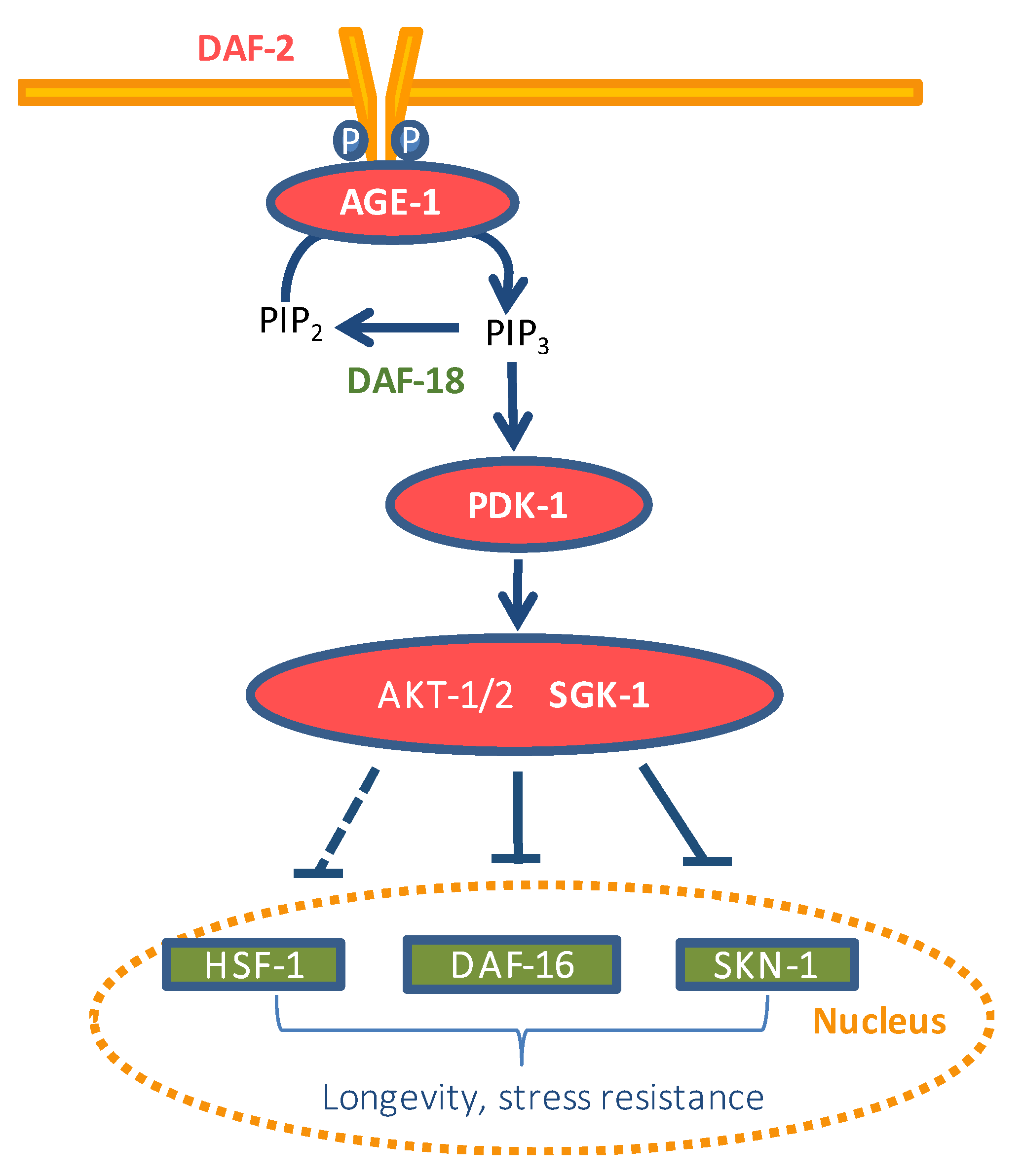
- Altintas, O.; Park, S.; Lee, S.J.V. The role of insulin/IGF-1 signaling in the longevity of model in vertebrates, C. Elegans D. Melanogaster. BMB Rep. 2016, 49, 81–92. [Google Scholar] [CrossRef]
- Lapierre, L.R.; Hansen, M. Lessons from C. elegans: Signaling pathways for longevity. Trends Endocrin. Met. 2012, 23, 637–644. [Google Scholar] [CrossRef]
- Kimura, K.D.; Tissenbaum, H.A.; Liu, Y.; Ruvkun, G. Daf-2, an insulin receptor-like gene that regulates longevity and diapause in Caenorhabditis elegans. Science 1997, 277, 942–946. [Google Scholar] [CrossRef] [PubMed]
- Ogg, S.; Paradis, S.; Gottlieb, S.; Patterson, G.I.; Lee, L.; Tissenbaum, H.A.; Ruvkun, G. The fork head transcription factor DAF-16 transduces insulin-like metabolic and longevity signals in C. elegans. Nature 1997, 389, 994–999. [Google Scholar] [CrossRef] [PubMed]
- Mohri-Shiomi, A.; Garsin, D.A. Insulin signaling and the heat shock response modulate protein homeostasis in the Caenorhabditis elegans intestine during infection. J. Biol. Chem. 2008, 283, 194–201. [Google Scholar] [CrossRef] [PubMed]
- Mansuri, M.L.; Parihar, P.; Solanki, I.; Parihar, M.S. Flavonoids in modulation of cell survival signaling pathways. Genes Nutr. 2014, 9, 400. [Google Scholar] [CrossRef]
- Zevian, S.C.; Yanowitz, J.L. Methodological considerations for heat shock of the nematode Caenorhabditis elegans. Methods 2014, 68, 450–457. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Kenyon, C.J. The genetics of ageing. Nature 2010, 464, 504–512. [Google Scholar] [CrossRef]
- Fitzenberger, E.; Deusing, D.J.; Marx, C.; Boll, M.; Lüersen, K.; Wenzel, U. The polyphenol quercetin protects the mev-1 mutant of Caenorhabditis elegans from glucose-induced reduction of survival under heat-stress depending on SIR-2.1, DAF-12, and proteasomal activity. Mol. Nutr. Food Res. 2014, 58, 984–994. [Google Scholar] [CrossRef]
- Tullet, J.M.; Hertweck, M.; An, J.H.; Baker, J.; Hwang, J.Y.; Liu, S.; Oliveira, R.P.; Baumeister, R.; Blackwell, T.K. Direct inhibition of the longevity-promoting factor SKN-1 by insulin-like signaling in C. elegans. Cell 2008, 132, 1025–1038. [Google Scholar] [CrossRef]
- Tullet, J.M.A.; Green, J.W.; Au, C.; Benedetto, A.; Thompson, M.A.; Clark, E.; Gilliat, A.F.; Young, A.; Schmeisser, K.; Gems, D. The SKN-1/Nrf2 transcription factor can protect against oxidative stress and increase lifespan in C. elegans by distinct mechanisms. Aging Cell 2017, 16, 1191–1194. [Google Scholar] [CrossRef]
- Ayuda-Durán, B.; González-Manzano, S.; Miranda-Vizuete, A.; Dueñas, M.; Santos-Buelga, C.; González-Paramás, A.M. Epicatechin modulates stress-resistance in C. elegans via insulin/IGF-1 signaling pathway. PLoS ONE 2019, 14, e0199483. [Google Scholar] [CrossRef] [PubMed]
- Paiva, F.A.; Bonomo, L.F.; Boasquivis, P.F.; de Paula, I.T.; Guerra, J.F.; Leal, W.M.; Silva, M.E.; Pedrosa, M.L.; Oliveira, R.P. Carqueja (Baccharis trimera) Protects against Oxidative Stress and β-Amyloid-Induced Toxicity in Caenorhabditis elegans. Oxid. Med. Cell. Longev. 2015, 740162. [Google Scholar] [CrossRef]
- Lund, J.; Tedesco, P.; Duke, K.; Wang, J.; Kim, S.K.; Johnson, T.E. Transcriptional profile of aging in C. elegans. Curr. Biol. 2002, 12, 1566–1573. [Google Scholar] [CrossRef]
- Walker, G.A.; White, T.M.; McColl, G.; Jenkins, N.L.; Babich, S.; Candido, E.P.; Johnson, T.E.; Lithgow, G.J. Heat shock protein accumulation is upregulated in a long-lived mutant of Caenorhabditis elegans. J. Gerontol. Ser. A 2001, 56, B281–B287. [Google Scholar] [CrossRef] [PubMed]
- Tullet, J.M. DAF-16 target identification in C. elegans: Past, present and future. Biogerontology 2015, 16, 221–234. [Google Scholar] [CrossRef] [PubMed]
- Kenyon, C.; Chang, J.; Gensch, E.; Rudner, A.; Tabtiang, R.A. C. elegans mutant that lives twice as long as wild type. Nature 1993, 366, 461–464. [Google Scholar] [CrossRef]
- Hekimi, S.; Burgess, J.; Bussière, F.; Meng, Y.; Bénard, C. Genetics of lifespan in C. elegans: Molecular diversity, physiological complexity, mechanistic simplicity. Trends Genet. 2001, 17, 712–718. [Google Scholar] [CrossRef]
- Uno, M.; Nishida, E. Lifespan-regulating genes in C. elegans. NPJ Aging Mech. Dis. 2016, 2, 16010. [Google Scholar] [CrossRef]
- Lin, K.; Hsin, H.; Libina, N.; Kenyon, C. Regulation of the Caenorhabditis elegans longevity protein DAF-16 by insulin/IGF-1 and germline signaling. Nat. Genet. 2001, 28, 139–145. [Google Scholar] [CrossRef]
- Hsu, A.; Coleen, T.; Kenyon, C. Regulation of aging and age-related disease by DAF-16 and heat-shock factor. Science 2003, 300, 1142–1145. [Google Scholar] [CrossRef]
- Tang, S.; Chen, H.; Cheng, Y.; Nasir, M.A.; Kemper, N.; Bao, E. The interactive association between heat shock factor 1 and heat shock proteins in primary myocardial cells subjected to heat stress. Int. J. Mol. Med. 2016, 37, 56–62. [Google Scholar] [CrossRef] [PubMed]
- Mertenskötter, A.; Keshet, A.; Gerke, P.; Paul, R.J. The p38 MAPK PMK-1 shows heat-induced nuclear translocation, supports chaperone expression, and affects the heat tolerance of Caenorhabditis elegans. Cell Stress Chaperones. 2013, 18, 293–306. [Google Scholar] [CrossRef] [PubMed]
- Pietsch, K.; Saul, N.; Swain, S.C.; Menzel, R.; Steinberg, C.E.; Stürzenbaum, S.R. Meta-Analysis of Global Transcriptomics Suggests that Conserved Genetic Pathways are Responsible for Quercetin and Tannic Acid Mediated Longevity in C. elegans. Front. Genet. 2012, 3, 48. [Google Scholar] [CrossRef] [PubMed]
- Bonomo, L.F.; Silva, D.N.; Boasquivis, P.F.; Paiva, F.A.; Guerra, J.F.; Martins, T.A.; de Jesus-Torres, Á.G.; de Paula, I.T.; Caneschi, W.L.; Jacolot, P.; et al. Açaí (Euterpe oleracea Mart.) modulates oxidative stress resistance in Caenorhabditis elegans by direct and indirect mechanisms. PLoS ONE 2014, 9, e89933. [Google Scholar] [CrossRef] [PubMed]

© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ayuda-Durán, B.; González-Manzano, S.; Miranda-Vizuete, A.; Sánchez-Hernández, E.; R. Romero, M.; Dueñas, M.; Santos-Buelga, C.; González-Paramás, A.M. Exploring Target Genes Involved in the Effect of Quercetin on the Response to Oxidative Stress in Caenorhabditis elegans. Antioxidants 2019, 8, 585. https://doi.org/10.3390/antiox8120585
Ayuda-Durán B, González-Manzano S, Miranda-Vizuete A, Sánchez-Hernández E, R. Romero M, Dueñas M, Santos-Buelga C, González-Paramás AM. Exploring Target Genes Involved in the Effect of Quercetin on the Response to Oxidative Stress in Caenorhabditis elegans. Antioxidants. 2019; 8(12):585. https://doi.org/10.3390/antiox8120585
Chicago/Turabian StyleAyuda-Durán, Begoña, Susana González-Manzano, Antonio Miranda-Vizuete, Eva Sánchez-Hernández, Marta R. Romero, Montserrat Dueñas, Celestino Santos-Buelga, and Ana M. González-Paramás. 2019. "Exploring Target Genes Involved in the Effect of Quercetin on the Response to Oxidative Stress in Caenorhabditis elegans" Antioxidants 8, no. 12: 585. https://doi.org/10.3390/antiox8120585
APA StyleAyuda-Durán, B., González-Manzano, S., Miranda-Vizuete, A., Sánchez-Hernández, E., R. Romero, M., Dueñas, M., Santos-Buelga, C., & González-Paramás, A. M. (2019). Exploring Target Genes Involved in the Effect of Quercetin on the Response to Oxidative Stress in Caenorhabditis elegans. Antioxidants, 8(12), 585. https://doi.org/10.3390/antiox8120585