Use of Sieving as a Valuable Technology to Produce Enriched Buckwheat Flours: A Preliminary Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample
2.2. Reagents and Chemicals
2.3. Protein, Ashes, and Total Starch Determination in Buckwheat Samples
2.3.1. Determination of Protein
2.3.2. Determination of Ashes Content
2.3.3. Determination of Total Starch
2.4. Extraction Methods
2.5. Determination of Free and Bound Phenolic Compounds by HPLC- MS
2.6. Statistical Analysis
3. Results and Discussion
3.1. Yield, and Protein, Starch, and Ashes Composition in Buckwheat Samples
3.2. Analytical Parameters of the Method Proposed
3.3. Identification of Phenolic Compounds in Buckwheat Fractions
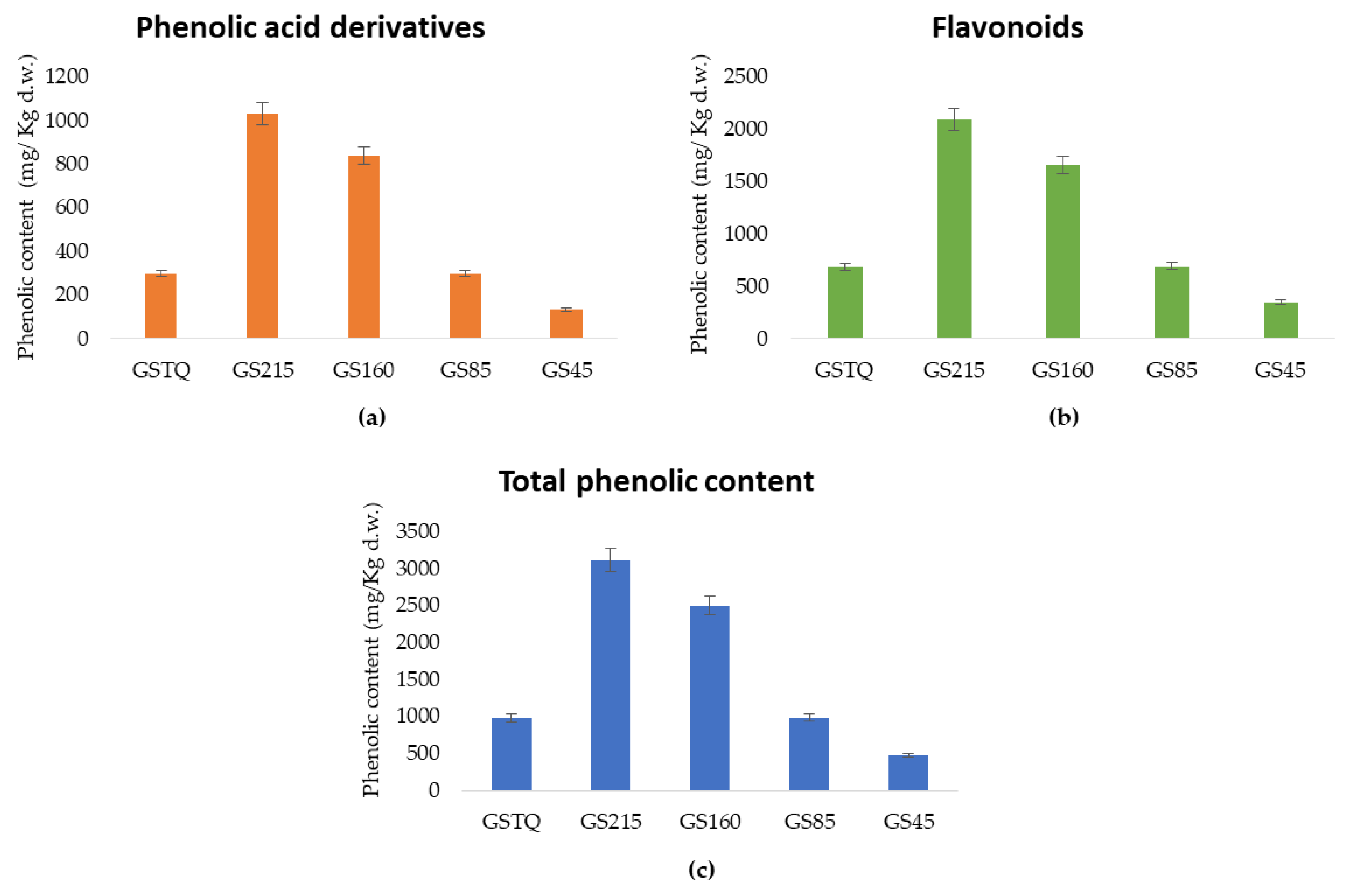
3.4. Quantification of Phenolic Compounds in Buckwheat Fractions
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bonafaccia, G.; Marocchini, M.; Kreft, I. Composition and technological properties of the flour and bran from common and tartary buckwheat. Food Chem. 2003, 80, 9–15. [Google Scholar] [CrossRef]
- Skrabanja, V.; Kreft, I.; Golob, T.; Modic, M.; Ikeda, S.; Ikeda, K.; Kreft, S.; Bonafaccia, G.; Knapp, M.; Kosmelj, K. Nutrient Content in Buckwheat Milling Fractions. Cereal Chem. 2004, 81, 172–176. [Google Scholar] [CrossRef]
- Steadman, K.J.; Burgoon, M.S.; Lewis, B.A.; Edwardson, S.E.; Obendorf, R.L. Minerals, phytic acid, tannin and rutin in buckwheat seed milling fractions. J. Sci. Food Agric. 2001, 81, 1094–1100. [Google Scholar] [CrossRef]
- Sedej, I.; Sakač, M.; Mandić, A.; Mišan, A.; Tumbas, V.; Čanadanović-Brunet, J. Buckwheat (Fagopyrum esculentum Moench) Grain and Fractions: Antioxidant Compounds and Activities. J. Food Sci. 2012, 77, C954–C959. [Google Scholar] [CrossRef] [PubMed]
- Van Hung, P.; Morita, N. Distribution of phenolic compounds in the graded flours milled from whole buckwheat grains and their antioxidant capacities. Food Chem. 2008, 109, 325–331. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, A.; Khalid, N.; Ahmad, A.; Abbasi, N.A.; Latif, M.S.Z.; Randhawa, M.A. Phytochemicals and biofunctional properties of buckwheat: A review. J. Agric. Sci. 2014, 152, 349–369. [Google Scholar] [CrossRef]
- Mikulajová, A.; Šedivá, D.; Hybenová, E.; Mošovská, S. Buckwheat cultivars—Phenolic compounds profiles and antioxidant properties. Acta Chim. Slovaca 2016, 9, 124–129. [Google Scholar] [CrossRef]
- Lee, C.C.; Shen, S.R.; Lai, Y.J.; Wu, S.C. Rutin and quercetin, bioactive compounds from tartary buckwheat, prevent liver inflammatory injury. Food Funct. 2013, 4, 794–802. [Google Scholar] [CrossRef]
- Inglett, G.E.; Chen, D.; Berhow, M.; Lee, S. Antioxidant activity of commercial buckwheat flours and their free and bound phenolic compositions. Food Chem. 2011, 125, 923–929. [Google Scholar] [CrossRef]
- Adom, K.K.; Liu, R.H. Antioxidant activity of grains. J. Agric. Food Chem. 2002, 50, 6182–6187. [Google Scholar] [CrossRef]
- Bobkov, S. Biochemical and Technological Properties of Buckwheat Grains. In Molecular Breeding and Nutritional Aspects of Buckwheat; Academic Press-Elsevier: Cambridge, MA, USA, 2016; ISBN 9780128036921. [Google Scholar]
- Steadman, K.J.; Burgoon, M.S.; Lewis, B.A.; Edwardson, S.E.; Obendorf, R.L. Buckwheat seed milling fractions: Description, macronutrient composition and dietary fibre. J. Cereal Sci. 2001, 33, 271–278. [Google Scholar] [CrossRef]
- Kalinová, J.P.; Vrchotováb, N.; Tříska, J. Phenolics levels in different parts of common buckwheat (Fagopyrum esculentum) achenes. J. Cereal Sci. 2019, 85, 243–248. [Google Scholar] [CrossRef]
- Vasanthan, T.; Temelli, F. Grain fractionation technologies for cereal beta-glucan concentration. Food Res. Int. 2008, 41, 876–881. [Google Scholar] [CrossRef]
- Kołodziejczyk, P.; Makowska, A.; Pospieszna, B.; Michniewicz, J.; Paschke, H. Chemical and nutritional characteristics of high-fibre rye milling fractions. Acta Sci. Pol. Technol. Aliment. 2018, 17, 149–157. [Google Scholar] [PubMed]
- Delcour, J.A.; Rouau, X.; Courtin, C.M.; Poutanen, K.; Ranieri, R. Technologies for enhanced exploitation of the health-promoting potential of cereals. Trends Food Sci. Technol. 2012, 25, 78–86. [Google Scholar] [CrossRef]
- Bolea, C.-A.; Vizireanu, C. Polyphenolic content and antioxidant properties of black rice flour. Food Technol. 2017, 41, 75–85. [Google Scholar]
- Renee, L.; Kubola, J.; Siriamornpun, S.; Herald, T.J.; Shi, Y. Wheat bran particle size influence on phytochemical extractability and antioxidant properties. Food Chem. 2014, 152, 483–490. [Google Scholar]
- Bressiani, J.; Oro, T.; Santetti, G.S.; Almeida, J.L.; Bertolin, T.E.; Gómez, M.; Gutkoski, L.C. Properties of whole grain wheat flour and performance in bakery products as a function of particle size. J. Cereal Sci. 2017, 75, 269–277. [Google Scholar] [CrossRef]
- ICC method 105/2. Standard Methods of the International Association for Cereal Science and Technology; International Association for Cereal Science and Technology Press: Vienna, Austria, 1995. [Google Scholar]
- ICC method 104/1. Standard Methods of the International Association for Cereal Science and Technology; International Association for Cereal Science and Technology Press: Vienna, Austria, 1995. [Google Scholar]
- Official Methods of Analysis of AOAC International 18th Edition; AOAC International Press: Gaithersburg, MD, USA, 2007.
- Verardo, V.; Arráez-Román, D.; Segura-Carretero, A.; Marconi, E.; Fernández-Gutiérrez, A.; Caboni, M.F. Determination of free and bound phenolic compounds in buckwheat spaghetti by RP-HPLC-ESI-TOF-MS: Effect of thermal processing from farm to fork. J. Agric. Food Chem. 2011, 59, 7700–7707. [Google Scholar] [CrossRef]
- Gómez-Caravaca, A.M.; Verardo, V.; Berardinelli, A.; Marconi, E.; Caboni, M.F. A chemometric approach to determine the phenolic compounds in different barley samples by two different stationary phases: A comparison between C18 and pentafluorophenyl core shell columns. J. Chromatogr. A 2014, 1355, 134–142. [Google Scholar] [CrossRef]
- Schutyser, M.A.I.; van der Goot, A.J. The potential of dry fractionation processes for sustainable plant protein production. Trends Food Sci. Technol. 2011, 22, 154–164. [Google Scholar] [CrossRef]
- Verardo, V.; Arráez-Román, D.; Segura-Carretero, A.; Marconi, E.; Fernández-Gutiérrez, A.; Caboni, M.F. Identification of buckwheat phenolic compounds by reverse phase high performance liquid chromatography e electrospray ionization-time of flight-mass spectrometry (RP-HPLC e ESI-TOF-MS). J. Cereal Sci. 2010, 52, 170–176. [Google Scholar] [CrossRef]
- Liu, Y.; Cai, C.; Yao, Y.; Xu, B. Alteration of phenolic profiles and antioxidant capacities of common buckwheat and tartary buckwheat produced in China upon thermal processing. J. Sci. Food Agric. 2019, 99, 5565–5576. [Google Scholar] [CrossRef] [PubMed]
| GSTQ | GS215 | GS160 | GS85 | GS45 | |
|---|---|---|---|---|---|
| Yield | 100 | 13.5 | 8.7 | 32.0 | 43.3 |
| Protein (N × 6.25) | 16.4 ± 0.04 | 35.2 ± 0.03 | 29.8 ± 0.04 | 11.3 ± 0.02 | 8.1 ± 0.06 |
| Ashes | 2.36 ± 0.003 | 6.05 ± 0.002 | 5.56 ± 0.001 | 1.51 ± 0.003 | 0.31 ± 0.002 |
| Total Starch | 72.6 ± 1.49 | 34.4 ± 1.10 | 43.3 ± 1.43 | 76.7 ± 2.00 | 81.2 ± 1.13 |
| Standards | Calibration Ranges (mg/L) | Calibration Curves (mg/g) | R2 | LOD (mg/L) | LOQ (mg/L) |
|---|---|---|---|---|---|
| Ferulic acid | LOQ-500 | y = 119572x + 16157 | 0.9985 | 0.0136 | 0.0452 |
| Catechin | LOQ-500 | y = 170925x + 8609.5 | 0.9994 | 0.0095 | 0.0316 |
| Quercetin | LOQ-500 | y = 402162x + 44862 | 0.9996 | 0.0040 | 0.0134 |
| Gallic acid | LOQ-500 | y = 123892x − 4971.6 | 0.9984 | 0.0131 | 0.0437 |
| Rutin | LOQ-500 | y = 199694x − 2067.2 | 1 | 0.0081 | 0.0271 |
| Peak | Retention Time | [M–H]− | Molecular Formula | Compound | Free | Bound | Ion Source Fragments |
|---|---|---|---|---|---|---|---|
| 1 | 2.07 | 315 | C13H15O9 | 2-Hydroxy-3-O-βD-glucopyranosyl benzoic acid | + | + | |
| 2 | 2.58 | 315 | C13H15O9 | Protocatechuic-4-O-glucoside acid | + | + | |
| 3 | 3.22 | 341 | C15H17O9 | Caffeic acid hexose | N.D. | + | 251 |
| 4 | 3.30 | 451 | C21H23O11 | Catechin-glucoside isomer A | + | + | 289 |
| 5 | 4.08 | 341 | C15H17O9 | Caffeic acid hexose | + | + | 179 |
| 6 | 4.17 | 289 | C15H13O6 | Catechin | + | + | |
| 7 | 4.40 | 487 | C21H27O13 | Swertiamacroside isomer A | + | + | 451 |
| 8 | 4.96 | 179 | C9H7O4 | Caffeic acid | + | + | |
| 9 | 5.49 | 289 | C15H13O6 | Epicatechin | + | + | |
| 10 | 6.25 | 561 | C30H25O11 | (Epi)Afzelchin-(Epi) catechin Isomer A | + | + | 543, 425, 289 |
| 11 | 6.26 | 197 | C9H9O5 | Syringic acid | N.D. | + | |
| 12 | 6.77 | 447 | C21H19O11 | Orientin | + | + | 357 |
| 13 | 6.96 | 447 | C21H19O11 | Isoorientin | + | N.D. | |
| 14 | 6.86 | 163 | C9H7O3 | p-Coumaric acid | N.D. | + | |
| 15 | 7 | 575 | C30H23O12 | Procyanidin A | N.D. | + | 289,285 |
| 16 | 7.46 | 317 | C15H9O8 | Myricetin | N.D. | + | |
| 17 | 7.76 | 431 | C21H19O10 | Vitexin | + | + | |
| 18 | 7.92 | 609 | C27H29O16 | Rutin | + | + | |
| 19 | 7.94 | 441 | C22H17O10 | Epicatechin gallate | + | + | 289, 169 |
| 20 | 7.96 | 833 | C45H37O16 | Epiafzelchin–epiafzelchin–epicatechin | + | N.D. | |
| 21 | 8.21 | 451 | C21H23O11 | Catechin-glucoside isomer B | N.D. | + | 289 |
| 22 | 8.23 | 487 | C21H27O13 | Swertiamacroside isomer B | + | + | 451 |
| 23 | 8.28 | 463 | C21H19O12 | Hyperin | + | N.D. | |
| 24 | 8.73 | 727 | C38H31O15 | Epiafzelchin–epicatechin-O-methylgallate | + | + | 461, 289 |
| 25 | 9.31 | 163 | C9H7O3 | p-Coumaric acid | N.D. | + | |
| 26 | 9.43 | 455 | C23H19O10 | (−)-Epicatechin-3-(3′’-O-methyl) gallate | + | + | 289, 183 |
| 27 | 9.47 | 561 | C30H25O11 | (Epi)afzelchin-(Epi) catechin Isomer B | + | N.D. | 543, 425, 289 |
| 28 | 9.9 | 757 | C39H33O16 | Procyanidin B2-dimethylgallate | + | N.D. | 289 |
| 29 | 10.71 | 741 | C39H33O15 | Epiafzelchin–epicatechin-O-Dimethylgallate | + | N.D. | 469, 319, 271 |
| 30 | 11.50 | 469 | C24H21O10 | Epicatechin-O-3,4-Dimethylgallate | + | + | 319, 271 |
| 31 | 12.35 | 463 | C21H19O12 | Isoquercitrin | + | + | |
| 32 | 12.56 | 301 | C15H10O7 | Quercetin | + | + |
| Phenolic Compound | GSTQ-Free | GS215-Free | GS160-Free | GS85-Free | GS45-Free |
|---|---|---|---|---|---|
| 2-Hydroxy-3-O-β D-glucopyranosyl benzoic acid | 42.71 ± 1.07c | 144.52 ± 1.88a | 128.46 ± 2.46b | 33.45 ± 1.79d | 18.73 ± 1.24e |
| Protocatechuic-4-O-glucoside acid | 65.56 ± 2.07c | 242.95 ± 2.41a | 203.55 ± 1.93b | 48.50 ± 1.24d | 27.16 ± 0.85e |
| Catechin-glucoside | 23.53 ± 0.33c | 45.91 ± 0.70a | 40.24 ± 0.58b | 22.81 ± 1.01c | 12.77 ± 0.46d |
| Caffeic acid hexose | 30.95 ± 0.74c | 107.51 ± 1.55a | 100.22 ± 0.92b | 23.28 ± 0.36d | 13.04 ± 0.19e |
| Catechin | 27.33 ± 0.12c | 72.30 ± 2.04a | 64.31 ± 1.36b | 21.95 ± 1.28d | 12.29 ± 0.21e |
| Swertiamacroside | 9.84 ± 0.16c | 15.79 ± 1.44a | 10.96 ± 1.02b | 8.25 ± 0.21d | 4.62 ± 0.11e |
| Caffeic Acid | 0.01 ± 0.001c | 0.06 ± 0.003a | 0.024 ± 0.001b | <LOQ | <LOQ |
| Epicatechin | 44.01 ± 1.48c | 118.75 ± 3.02a | 96.29 ± 2.64b | 43.50 ± 1.18c | 24.36 ± 0.45d |
| (Epi)Afzelchin-(epi) catechin isomer A | 20.06 ± 1.11c | 39.05 ± 0.81a | 31.44 ± 0.69b | 20.30 ± 1.77c | 11.37 ± 1.15d |
| Orientin | 1.58 ± 0.20c | 5.64 ± 0.39a | 3.12 ± 0.37b | 1.00 ± 0.09d | 0.56 ± 0.05e |
| Isorientin | 0.82 ± 0.14c | 3.17 ± 0.21a | 1.84 ± 0.11b | 0.65 ± 0.04d | 0.36 ± 0.01e |
| Vitexin | 2.02 ± 0.10c | 6.00 ± 0.26a | 4.11 ± 0.13b | 1.49 ± 0.05d | 0.83 ± 0.02e |
| Rutin | 87.33 ± 1.11c | 195.47 ± 3.62a | 175.70 ± 1.87b | 77.84 ± 0.94d | 43.59 ± 0.51e |
| Epicatechin-gallate | 7.22 ± 0.06c | 19.44 ± 0.82a | 14.81 ± 0.17b | 7.65 ± 0.12c | 4.28 ± 0.02d |
| Epiafzelchin–epiafzelchin–epicatechin | 8.01 ± 0.35c | 15.69 ± 0.29a | 11.64 ± 0.40b | 8.31 ± 0.03c | 4.66 ± 0.51d |
| Swertiamacroside | 10.17 ± 0.02c | 14.59 ± 0.09a | 12.76 ± 0.04b | 10.76 ± 0.37c | 6.02 ± 0.18d |
| Hyperin | 1.13 ± 0.01c | 3.72 ± 0.22a | 1.85 ± 0.08b | 0.72 ± 0.01d | 0.41 ± 0.002e |
| Epiafzelchin-epicatechin-O-methyl gallate | 28.73 ± 1.37c | 75.39 ± 2.60a | 62.88 ± 3.08b | 24.31 ± 1.09d | 13.61 ± 2.26e |
| (−)-Epicatechin-3-(3”-O-methyl) gallate | 15.18 ± 0.10c | 35.97 ± 3.58a | 28.43 ± 2.19b | 12.96 ± 1.43d | 7.26 ± 0.88e |
| (Epi)afzelchin-(epi) catechin isomer B | 9.95 ± 0.16c | 23.25 ± 1.66a | 19.29 ± 2.07b | 8.62 ± 0.59d | 4.83 ± 0.30e |
| Procyanidin B2-dimethylgallate | 21.06 ± 0.08c | 58.03 ± 2.01a | 50.67 ± 1.59b | 18.19 ± 1.27d | 10.18 ± 0.64e |
| Epiafzelchin–epicatechin-O-dimethylgallate | 93.83 ± 1.83c | 225.36 ± 4.12a | 186.37 ± 3.36b | 82.38 ± 2.60d | 46.14 ± 1.08e |
| Epicatechin-O-3,4-dimethylgallate | 39.10 ± 0.07c | 82.65 ± 1.31a | 74.51 ± 2.24b | 36.41 ± 1.03d | 20.39 ± 0.72e |
| Isoquercitrin | 0.46 ± 0.01d | 0.72 ± 0.02a | 0.63 ± 0.01b | 0.58 ± 0.04c | 0.33 ± 0.01e |
| Quercetin | 0.32 ± 0.01c | 1.68 ± 0.003a | 1.09 ± 0.01b | 0.18 ± 0.006d | 0.10 ± 0.001 |
| Total | 590.92 ± 13.25c | 1553.62 ± 32.16a | 1325.19 ± 18.14b | 514.10 ± 10.44d | 287.89 ± 7.91e |
| Flavonoids | 431.68 ± 20.86c | 1028.19 ± 19.88a | 869.22 ± 22.09b | 389.85 ± 15.48d | 218.32 ± 10.72e |
| Phenolic acid derivatives | 159.24 ± 6.48c | 525.42 ± 11.79a | 455.97 ± 13.93b | 124.24 ± 8.46d | 69.58 ± 7.11e |
| Phenolic Compound | GSTQ | GS215 | GS160 | GS-85 | GS45 |
|---|---|---|---|---|---|
| 2-hydroxy-3-O-β-D-glucopyranosyl benzoic acid | 6.34 ± 0.08d | 30.53 ± 1.22a | 26.42 ± 1.43b | 11.65 ± 0.04c | 5.24 ± 0.02e |
| Protocatechuic-4-O-glucoside acid | 4.26 ± 0.13d | 18.50 ± 1.05a | 15.77 ± 0.49b | 8.24 ± 0.36c | 3.20 ± 0.11e |
| Caffeic acid hexose | 0.51± 0.04e | 3.27 ± 0.05a | 2.89 ± 0.18b | 1.07 ± 0.06c | 0.80 ± 0.001d |
| Catechin-glucoside isomer a | 0.48 ± 0.01c | 2.03 ± 0.04a | 1.12 ± 0.01b | 0.50 ± 0.02c | 0.05 ± 0.0003d |
| Caffeic acid hexose | 20.33 ± 0.12d | 82.34 ± 2.10a | 56.26 ± 0.86b | 30.25 ± 1.15c | 10.10 ± 0.46e |
| Catechin | 77.79 ± 2.61c | 320.22 ± 3.09a | 241.04 ± 1.82b | 80.05 ± 1.94c | 36.05 ± 0.76d |
| Swertiamacroside | 38.30 ± 3.28c | 130.85 ± 1.28a | 88.47 ± 1.56b | 40.03 ± 2.46c | 12.06 ± 0.18d |
| Caffeic acid | 0.13 ± 0.001c | 1.02 ± 0.04a | 0.64 ± 0.02b | 0.13 ± 0.01c | 0.06 ± 0.001d |
| Epicatechin | 47.93 ± 0.09d | 202.64 ± 3.01a | 136.89 ± 2.74b | 50.05 ± 1.10c | 22.05 ± 2.63e |
| (Epi)afzelchin-(epi) catechin | 0.48 ± 0.03d | 3.52 ± 0.11a | 2.91 ± 0.004b | 1.05 ± 0.01c | 0.05 ± 0.002e |
| Syringic acid | 31.28 ± 0.90c | 100.73 ± 1.99a | 79.03 ± 0.69b | 31.26 ± 1.24c | 10.72 ± 0.57e |
| Orientin | 0.48 ± 0.02d | 3.15 ± 0.09a | 2.31 ± 0.003b | 0.96 ± 0.01c | 0.05 ± 0.003e |
| p-Coumaric acid | 2.11 ± 0.10d | 9.47 ± 0.11a | 6.42 ± 0.30b | 3.11 ± 0.14c | 1.10 ± 0.02e |
| Procyanidin A | 4.06 ± 0.08c | 11.88 ± 0.32a | 9.60 ± 0.24b | 4.04 ± 0.07c | 1.04 ± 0.04d |
| Myricetin | 0.05 ± 0.001c | 0.12 ± 0.01a | 0.09 ± 0.001b | 0.05 ± 0.0001c | 0.01 ± 0.002d |
| Vitexin | 3.10 ± 0.10d | 14.29 ± 0.46a | 11.08 ± 0.29b | 5.01 ± 0.06c | 2.01 ± 0.01e |
| Rutin | 40.09 ± 2.24d | 173.97 ± 2.08a | 127.24 ± 1.75b | 59.09 ± 0.28c | 27.09 ± 1.15e |
| Epicatechin gallate | 13.24 ± 0.69c | 50.94 ± 1.30a | 39.92 ± 0.84b | 12.07 ± 0.45c | 5.95 ± 0.28d |
| Catechin-glucoside isomer b | 18.04 ± 0.25d | 78.06 ± 0.92a | 70.34 ± 1.37b | 30.04 ± 0.66c | 10.03 ± 0.49e |
| Swertiamacroside | 30.04 ± 0.38d | 105.31 ± 1.56a | 89.39 ± 2.61b | 35.07 ± 0.81c | 14.05 ± 0.10e |
| Epiafzelchin–epicatechin-O-methylgallate | 8.05 ± 0.11d | 35.64 ± 0.86a | 26.18 ± 1.27b | 18.05 ± 0.78c | 8.05 ± 0.04d |
| p-Coumaric acid | 5.44 ± 0.44d | 22.47 ± 0.19a | 16.77 ± 1.06b | 13.55 ± 0.07c | 6.16 ± 0.86d |
| (−)-Epicatechin-3-(3’’-O-methyl) gallate | 14.22 ± 0.16c | 49.21 ± 0.88a | 28.58 ± 1.63b | 11.83 ± 0.23d | 6.42 ± 0.08d |
| Epicatechin-O-3,4-dimethylgallate | 1.31 ± 0.07d | 5.36 ± 0.20a | 3.89 ± 0.14b | 2.10 ± 0.11c | 0.94 ± 0.03e |
| Isoquercitrin | 4.10 ± 0.04d | 17.61 ± 0.17a | 13.62 ± 1.31b | 6.11 ± 0.08c | 3.10 ± 0.21e |
| Quercitrin | 20.10 ± 1.13c | 92.09 ± 3.44a | 77.05 ± 1.83b | 20.01 ± 2.06c | 7.05 ± 0.66d |
| Total | 392.23 ± 12.63d | 1565.22 ± 14.88a | 1173.92 ± 20.47b | 475.37 ± 9.12c | 193.41 ± 4.62e |
| Flavonoids | 253.51 ± 4.80d | 1060.73 ± 13.57a | 791.86 ± 11.08b | 301.01 ± 7.12c | 129.92 ± 6.61e |
| Phenolic acids | 138.72 ± 8.15d | 504.49 ± 5.76a | 382.06 ± 6.94b | 174.36 ± 10.15c | 63.50 ± 2.89e |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martín-García, B.; Pasini, F.; Verardo, V.; Gómez-Caravaca, A.M.; Marconi, E.; Caboni, M.F. Use of Sieving as a Valuable Technology to Produce Enriched Buckwheat Flours: A Preliminary Study. Antioxidants 2019, 8, 583. https://doi.org/10.3390/antiox8120583
Martín-García B, Pasini F, Verardo V, Gómez-Caravaca AM, Marconi E, Caboni MF. Use of Sieving as a Valuable Technology to Produce Enriched Buckwheat Flours: A Preliminary Study. Antioxidants. 2019; 8(12):583. https://doi.org/10.3390/antiox8120583
Chicago/Turabian StyleMartín-García, Beatriz, Federica Pasini, Vito Verardo, Ana María Gómez-Caravaca, Emanuele Marconi, and Maria Fiorenza Caboni. 2019. "Use of Sieving as a Valuable Technology to Produce Enriched Buckwheat Flours: A Preliminary Study" Antioxidants 8, no. 12: 583. https://doi.org/10.3390/antiox8120583
APA StyleMartín-García, B., Pasini, F., Verardo, V., Gómez-Caravaca, A. M., Marconi, E., & Caboni, M. F. (2019). Use of Sieving as a Valuable Technology to Produce Enriched Buckwheat Flours: A Preliminary Study. Antioxidants, 8(12), 583. https://doi.org/10.3390/antiox8120583