Functional Components, Antioxidant Activity and Hypoglycemic Ability Following Simulated Gastro-Intestinal Digestion of Pigments from Walnut Brown Shell and Green Husk
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents and Plant Materials
2.2. Preparation of Solid Pigment
2.3. In Vitro Gastro-Intestinal Digestion
2.4. Determination of Flavonoid
2.5. Determination of Phenol
2.6. Extraction of Conjugated Phenols
2.7. Estimation of the In Vitro Antioxidant Capacity
2.7.1. ABTS Radical Scavenging Capacity Assay (ABTS)
2.7.2. Ferric-Reducing Antioxidant Power Assay (FRAP)
2.7.3. Total Reducing Power (RP) Assay
2.7.4. DPPH Radical Scavenging Capacity Assay (DPPH)
2.7.5. OH Radical Scavenging Activity (OH)
2.8. Estimation of Hypoglycemic Ability
2.8.1. α-Amylase Inhibitory Activity
2.8.2. α-Glucosidase Inhibitory Activity
2.9. Statistical Analysis
3. Results and Discussion
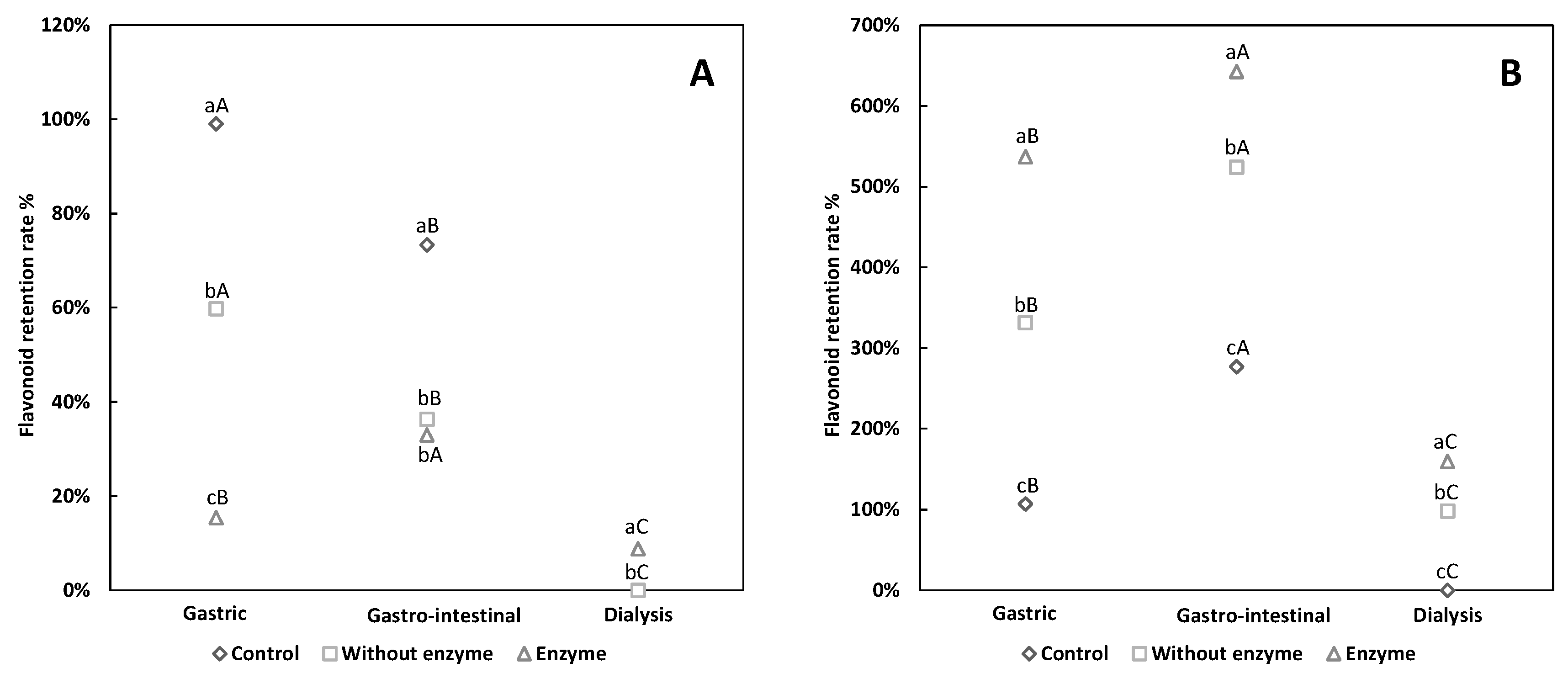
3.1. Comparative Analysis of Flavonoid Retention
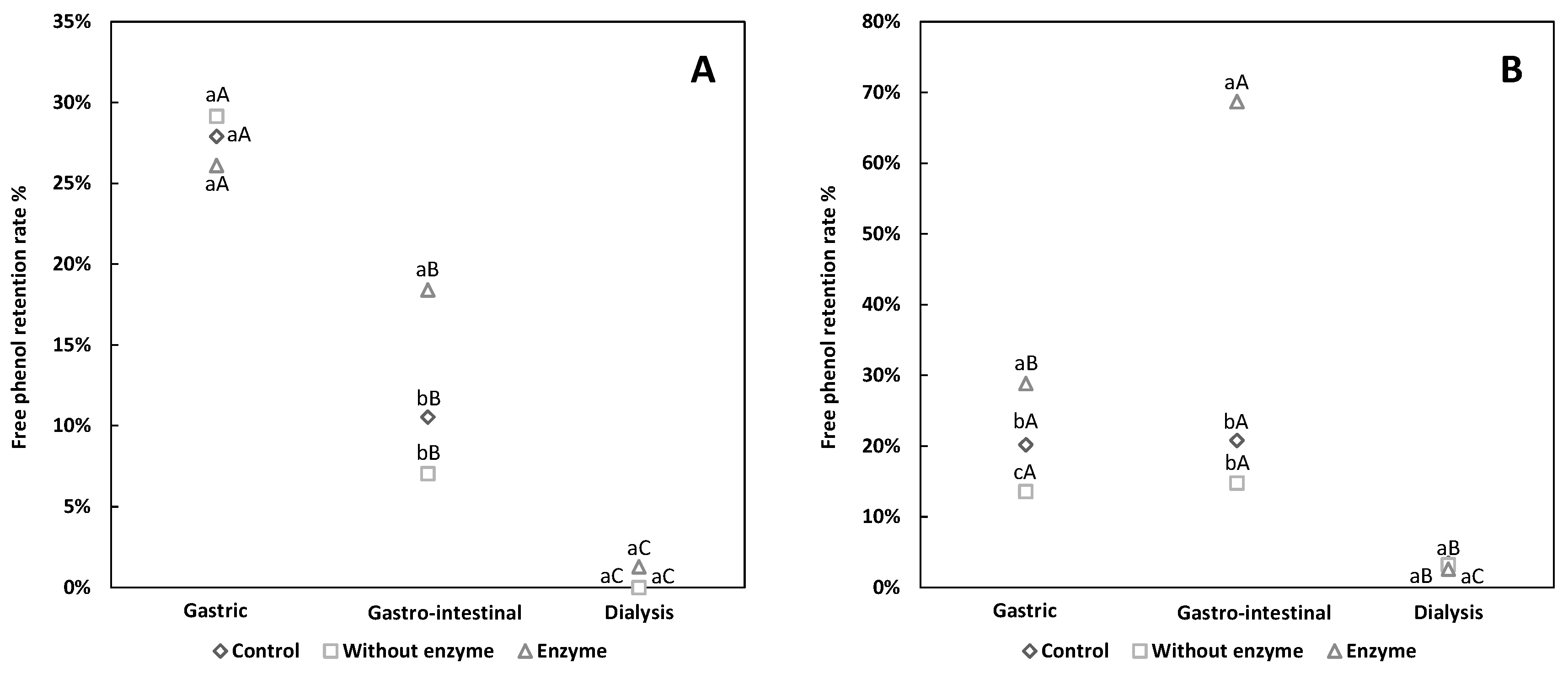
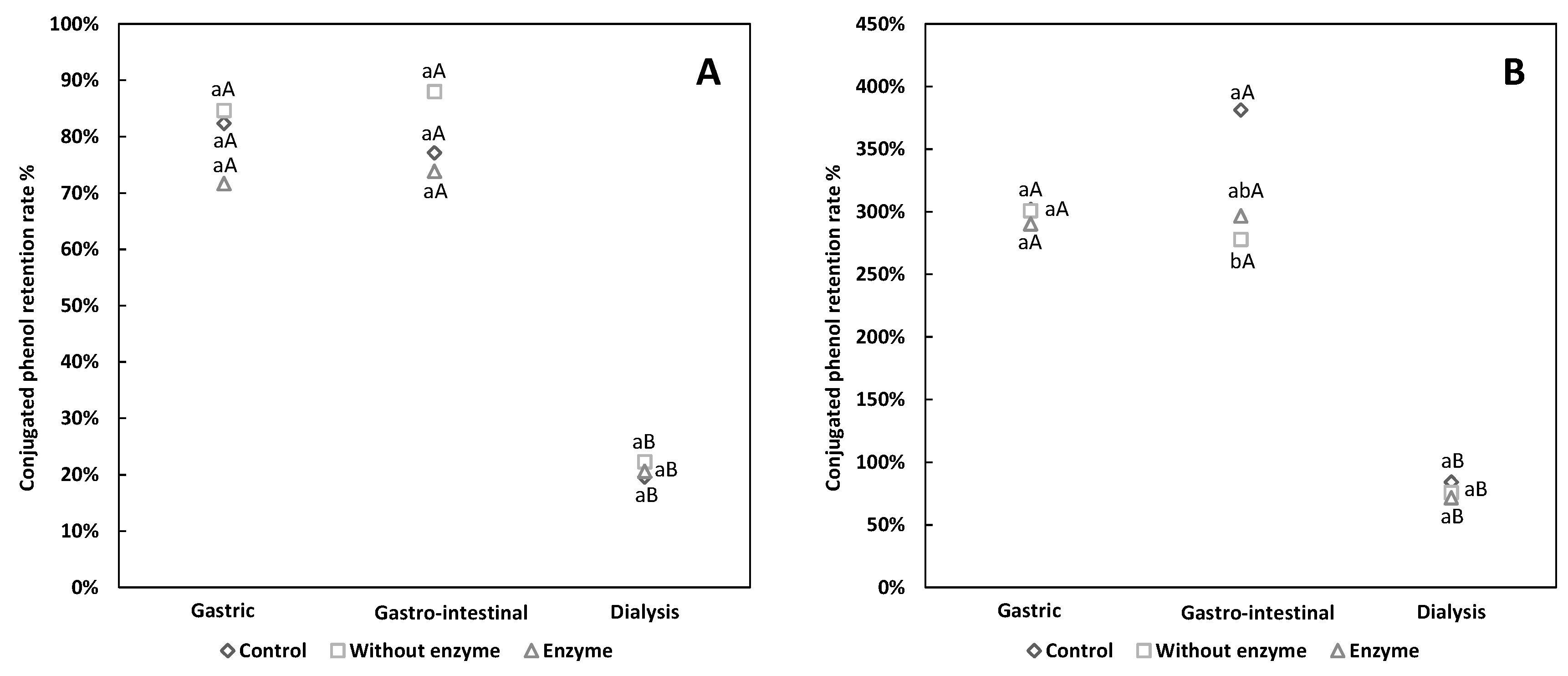
3.2. Comparative Analysis of Free and Conjugated Phenols Retention
3.3. Digestion Effect on the Antioxidant Ability of Pigments
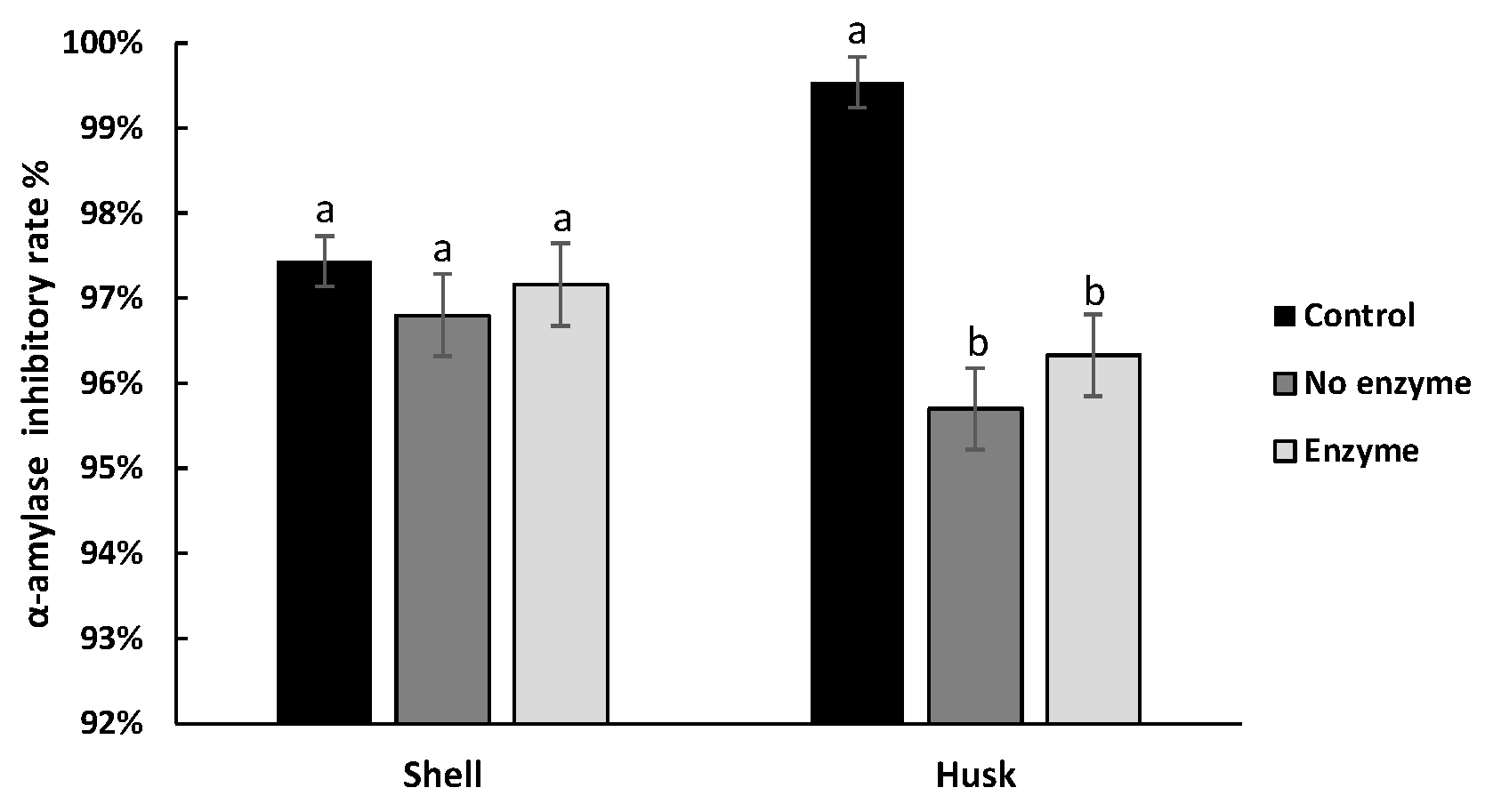
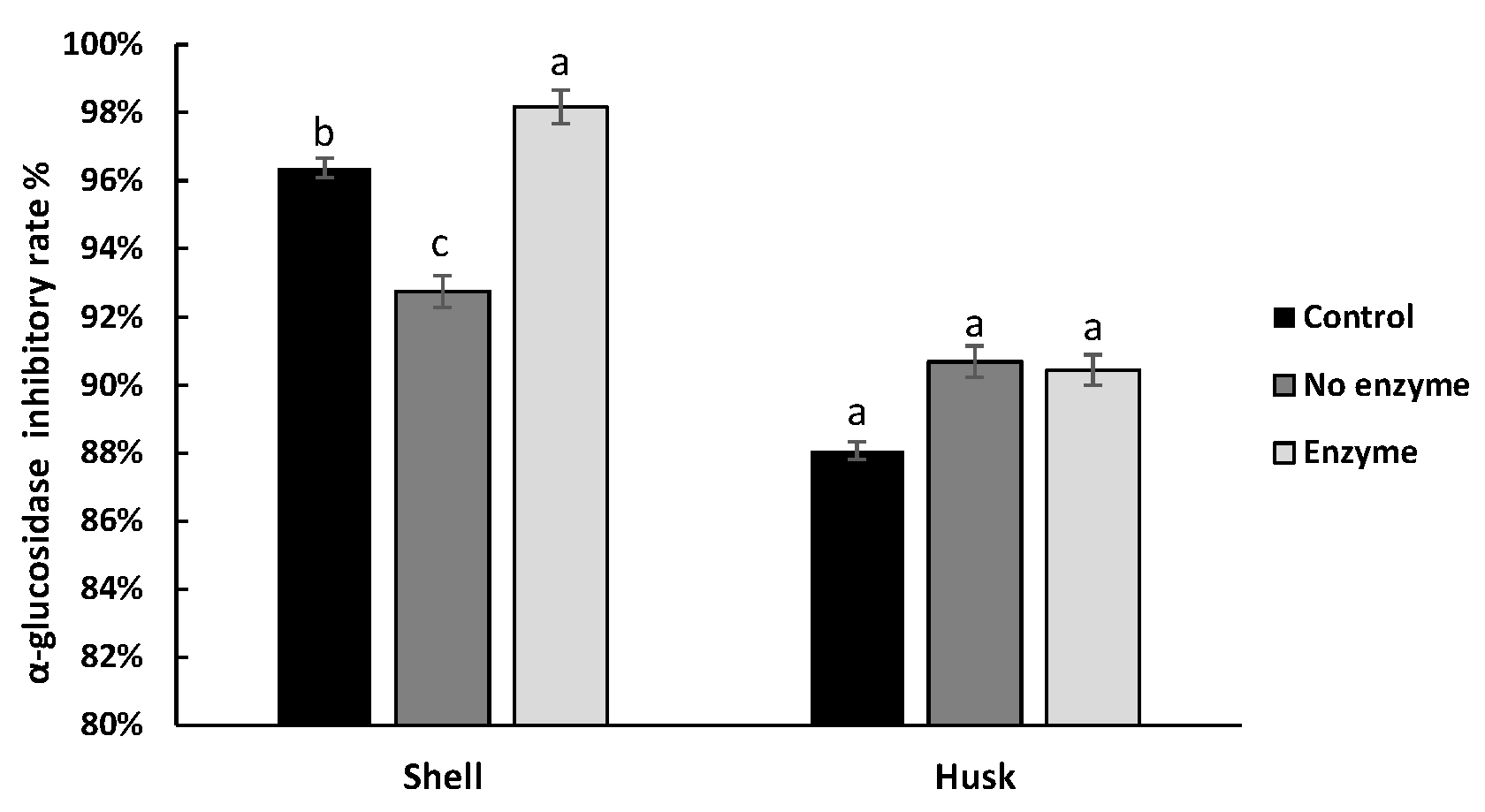
3.4. Effect of Digestion on the Hypoglycemic Ability of Pigments
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Parmar, R.S.; Singh, C. A comprehensive study of eco-friendly natural pigment and its applications. Biochem. Biophys. Rep. 2018, 13, 22–26. [Google Scholar] [CrossRef] [PubMed]
- Cervantes-Paz, B.; Yahia, E.M.; Ornelas-Paz, J.D.J.; Gardea-Béjar, A.A.; Ibarra-Junquera, V.; Pérez-Martínez, J.D. Effect of Heat Processing on the Profile of Pigments and Antioxidant Capacity of Green and Red Jalapeño Peppers. J. Agric. Food Chem. 2012, 60, 10822–10833. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Parrilla, E.; De La Rosa, L.A.; Amarowicz, R.; Shahidi, F. Protective effect of fresh and processed Jalapeño and Serrano peppers against food lipid and human LDL cholesterol oxidation. Food Chem. 2012, 133, 827–834. [Google Scholar] [CrossRef]
- Favre, G.; Hermosín-Gutiérrez, I.; Piccardo, D.; Gómez-Alonso, S.; González-Neves, G. Selectivity of pigments extraction from grapes and their partial retention in the pomace during red-winemaking. Food Chem. 2019, 277, 391–397. [Google Scholar] [CrossRef]
- Lingua, M.S.; Wunderlin, D.A.; Baroni, M.V. Effect of simulated digestion on the phenolic components of red grapes and their corresponding wines. J. Funct. Foods 2018, 44, 86–94. [Google Scholar] [CrossRef]
- Rabadan, A.; Gallardo-Guerrero, L.; Gandul-Rojas, B.; Álvarez-Ortí, M.; Pardo, J.E. Effect of roasting conditions on pigment composition and some quality parameters of pistachio oil. Food Chem. 2018, 264, 49–57. [Google Scholar] [CrossRef]
- Štampar, F.; Solar, A.; Hudina, M.; Veberic, R.; Colaric, M. Traditional walnut liqueur—Cocktail of phenolics. Food Chem. 2006, 95, 627–631. [Google Scholar] [CrossRef]
- Oliveira, I.; Sousa, A.; Ferreira, I.C.; Bento, A.A.; Estevinho, L.; Pereira, J.A.; Estevinho, M.L.M.F. Total phenols, antioxidant potential and antimicrobial activity of walnut (Juglans regia L.) green husks. Food Chem. Toxicol. 2008, 46, 2326–2331. [Google Scholar] [CrossRef]
- Shi, B.; Zhang, W.; Li, X.; Pan, X. Seasonal variations of phenolic profiles and antioxidant activity of walnut (Juglans sigillata Dode) green husks. Int. J. Food Prop. 2018, 20, S2635–S2646. [Google Scholar] [CrossRef]
- Rahmani, F.; Dehganiasl, M.; Heidari, R.; Rezaee, R.; Darvishzadeh, R. Genotype impact on antioxidant potential of hull and kernel in Persian walnut (Juglans regia L.). Int. Food Res. J. 2018, 25, 35–42. [Google Scholar]
- Wei, Q.; Ma, X.; Zhao, Z.; Zhang, S.; Liu, S. Antioxidant activities and chemical profiles of pyroligneous acids from walnut shell. J. Anal. Appl. Pyrolysis 2010, 88, 149–154. [Google Scholar] [CrossRef]
- Del Pino-García, R.; González-Sanjosé, M.L.; Rivero-Pérez, M.D.; García-Lomillo, J.; Muñiz, P. Total antioxidant capacity of new natural powdered seasonings after gastrointestinal and colonic digestion. Food Chem. 2016, 211, 707–714. [Google Scholar] [CrossRef] [PubMed]
- Lao, F.; Giusti, M.M. Extraction of purple corn (Zea mays L.) cob pigments and phenolic compounds using food-friendly solvents. J. Cereal Sci. 2018, 80, 87–93. [Google Scholar] [CrossRef]
- Luo, X.; Bai, R.; Zhen, D.; Yang, Z.; Huang, D.; Mao, H.; Li, X.; Zou, H.; Xiang, Y.; Liu, K.; et al. Response surface optimization of the enzyme-based ultrasound-assisted extraction of acorn tannins and their corrosion inhibition properties. Ind. Crops Prod. 2019, 129, 405–413. [Google Scholar] [CrossRef]
- Gunathilake, K.; Ranaweera, K.; Rupasinghe, H. Change of phenolics, carotenoids, and antioxidant capacity following simulated gastrointestinal digestion and dialysis of selected edible green leaves. Food Chem. 2018, 245, 371–379. [Google Scholar] [CrossRef]
- Tagliazucchi, D.; Verzelloni, E.; Bertolini, D.; Conte, A. In vitro bio-accessibility and antioxidant activity of grape polyphenols. Food Chem. 2010, 120, 599–606. [Google Scholar] [CrossRef]
- Li, X.; Lin, J.; Gao, Y.; Han, W.; Chen, D. Antioxidant activity and mechanism of Rhizoma Cimicifugae. Chem. Central J. 2012, 6, 140. [Google Scholar] [CrossRef]
- Ahmad, N.; Zuo, Y.; Lu, X.; Anwar, F.; Hameed, S. Characterization of free and conjugated phenolic compounds in fruits of selected wild plants. Food Chem. 2016, 190, 80–89. [Google Scholar] [CrossRef]
- Zhang, Q.; Tong, X.; Qi, B.; Wang, Z.; Li, Y.; Sui, X.; Jiang, L. Changes in antioxidant activity of Alcalase-hydrolyzed soybean hydrolysate under simulated gastrointestinal digestion and transepithelial transport. J. Funct. Foods 2018, 42, 298–305. [Google Scholar] [CrossRef]
- Zhang, J.; Hou, X.; Ahmad, H.; Zhang, H.; Zhang, L.; Wang, T. Assessment of free radicals scavenging activity of seven natural pigments and protective effects in AAPH-challenged chicken erythrocytes. Food Chem. 2014, 145, 57–65. [Google Scholar] [CrossRef]
- Liu, F.; Liu, W.; Tian, S. Artificial neural network optimization of Althaea rosea seeds polysaccharides and its antioxidant activity. Int. J. Boil. Macromol. 2014, 70, 100–107. [Google Scholar] [CrossRef] [PubMed]
- Cao, C.; Huang, Q.; Zhang, B.; Li, C.; Fu, X. Physicochemical characterization and in vitro hypoglycemic activities of polysaccharides from Sargassum pallidum by microwave-assisted aqueous two-phase extraction. Int. J. Boil. Macromol. 2018, 109, 357–368. [Google Scholar] [CrossRef] [PubMed]
- Chapdelaine, P.; Tremblay, R.R.; Dubé, J.Y. P-Nitrophenol-alpha-D-glucopyranoside as substrate for measurement of maltase activity in human semen. Clin. Chem. 1978, 24, 208–211. [Google Scholar] [PubMed]
- Huang, S.; Ma, Y.; Zhang, C.; Cai, S.; Pang, M. Bioaccessibility and antioxidant activity of phenolics in native and fermented Prinsepia utilis Royle seed during a simulated gastrointestinal digestion in vitro. J. Funct. Foods 2017, 37, 354–362. [Google Scholar] [CrossRef]
- Correa-Betanzo, J.; Allen-Vercoe, E.; McDonald, J.; Schroeter, K.; Corredig, M.; Paliyath, G. Stability and biological activity of wild blueberry (Vaccinium angustifolium) polyphenols during simulated in vitro gastrointestinal digestion. Food Chem. 2014, 165, 522–531. [Google Scholar] [CrossRef]
- Vallejo, F.; Gil-Izquierdo, A.; Pérez-Vicente, A.; Garcia-Viguera, C. In Vitro Gastrointestinal Digestion Study of Broccoli Inflorescence Phenolic Compounds, Glucosinolates, and Vitamin C. J. Agric. Food Chem. 2004, 52, 135–138. [Google Scholar] [CrossRef]
- Mosele, J.I.; Macià, A.; Romero, M.-P.; Motilva, M.-J. Stability and metabolism of Arbutus unedo bioactive compounds (phenolics and antioxidants) under in vitro digestion and colonic fermentation. Food Chem. 2016, 201, 120–130. [Google Scholar] [CrossRef]
- Li, Y.; Luo, X.; Wu, C.; Cao, S.; Zhou, Y.; Jie, B.; Cao, Y.; Meng, H.; Wu, G. Comparative Transcriptome Analysis of Genes Involved in Anthocyanin Biosynthesis in Red and Green Walnut (Juglans regia L.). Molecules 2017, 23, 25. [Google Scholar] [CrossRef]
- Manach, C.; Williamson, G.; Morand, C.; Scalbert, A.; Rémésy, C. Bioavailability and bioefficacy of polyphenols in humans. I. Review of 97 bioavailability studies. Am. J. Clin. Nutr. 2005, 81, 230S–242S. [Google Scholar] [CrossRef]
- Kahle, K.; Kempf, M.; Schreier, P.; Scheppach, W.; Schrenk, D.; Kautenburger, T.; Hecker, D.; Huemmer, W.; Ackermann, M.; Richling, E. Intestinal transit and systemic metabolism of apple polyphenols. Eur. J. Nutr. 2011, 50, 507–522. [Google Scholar] [CrossRef]
- Chiang, C.-J.; Kadouh, H.; Zhou, K. Phenolic compounds and antioxidant properties of gooseberry as affected by in vitro digestion. LWT 2013, 51, 417–422. [Google Scholar] [CrossRef]
- Sun, L.; Zhang, H.; Zhuang, Y. Preparation of Free, Soluble Conjugate, and Insoluble-Bound Phenolic Compounds from Peels of Rambutan (Nephelium lappaceum) and Evaluation of Antioxidant Activities in vitro. J. Food Sci. 2012, 77, 198–204. [Google Scholar] [CrossRef] [PubMed]
- Velderrain-Rodríguez, G.; Quirós-Sauceda, A.; Mercado-Mercado, G.; Ayala-Zavala, J.F.; Astiazarán-García, H.; Robles-Sánchez, R.M.; Wall-Medrano, A.; Sayago-Ayerdi, S.; González-Aguilar, G.A. Effect of dietary fiber on the bioaccessibility of phenolic compounds of mango, papaya and pineapple fruits by an in vitro digestion model. Food Sci. Technol. 2016, 36, 188–194. [Google Scholar] [CrossRef]
- Zheng, G.; Deng, J.; Wen, L.; You, L.; Zhao, Z.; Zhou, L. Release of phenolic compounds and antioxidant capacity of Chinese hawthorn “Crataegus pinnatifida” during in vitro digestion. J. Funct. Foods 2018, 40, 76–85. [Google Scholar] [CrossRef]
- Tang, X.-Z.; Dong, Y.-X.; Wei, S.-Q.; Zhang, X.-S.; Yin, Y.-P. Antioxidant Activity of Pigment Extracted from Green-Wheat-Bran. Agric. Sci. China 2010, 9, 825–832. [Google Scholar] [CrossRef]
- Heim, K.E.; Tagliaferro, A.R.; Bobilya, D.J. Flavonoid antioxidants: chemistry, metabolism and structure-activity relationships. J. Nutr. Biochem. 2002, 13, 572–584. [Google Scholar] [CrossRef]
- Bouayed, J.; Hoffmann, L.; Bohn, T. Total phenolics, flavonoids, anthocyanins and antioxidant activity following simulated gastro-intestinal digestion and dialysis of apple varieties: Bioaccessibility and potential uptake. Food Chem. 2011, 128, 14–21. [Google Scholar] [CrossRef]
- Kotásková, E.; Sumczynski, D.; Mlcek, J.; Valášek, P. Determination of free and bound phenolics using HPLC-DAD, antioxidant activity and in vitro digestibility of Eragrostis tef. J. Food Compos. Anal. 2016, 46, 15–21. [Google Scholar] [CrossRef]
- Kwon, G.S.; Forrest, M.L. Amphiphilic block copolymer micelles for nanoscale drug delivery. Drug Dev. Res. 2006, 67, 15–22. [Google Scholar] [CrossRef]
- Etxeberria, U.; De La Garza, A.L.; Campión, J.; Martínez, J.A.; I Milagro, F. Antidiabetic effects of natural plant extracts via inhibition of carbohydrate hydrolysis enzymes with emphasis on pancreatic alpha amylase. Expert Opin. Ther. Targets 2012, 16, 269–297. [Google Scholar] [CrossRef]
- Fernández-Agulló, A.; Pereira, E.; Freire, M.S.; Valentão, P.; Andrade, P.; González-Álvarez, J.; Pereira, J.A.; Andrade, P. Influence of solvent on the antioxidant and antimicrobial properties of walnut (Juglans regia L.) green husk extracts. Ind. Crops Prod. 2013, 42, 126–132. [Google Scholar] [CrossRef]
- Panwar, R.; Raghuwanshi, N.; Srivastava, A.K.; Sharma, A.K.; Pruthi, V. In-vivo sustained release of nanoencapsulated ferulic acid and its impact in induced diabetes. Mater. Sci. Eng. C 2018, 92, 381–392. [Google Scholar] [CrossRef] [PubMed]
- McDougall, G.J.; Stewart, D. The inhibitory effects of berry polyphenols on digestive enzymes. BioFactors 2005, 23, 189–195. [Google Scholar] [CrossRef] [PubMed]
- Qin, Y.; Wang, L.; Liu, Y.; Zhang, Q.; Li, Y.; Wu, Z. Release of phenolics compounds from Rubus idaeus L. dried fruits and seeds during simulated in vitro digestion and their bio-activities. J. Funct. Foods 2018, 46, 57–65. [Google Scholar] [CrossRef]
| Groups | Control | Without Enzyme | Enzyme |
|---|---|---|---|
| Walnut green husk pigment | |||
| Gastric | 1174.1 ± 14.7 a | 1192.4 ± 12.9 a | 102.8 ± 1.4 b |
| Gastro-intestinal | 1159.7 ± 34.0 a | 1191.1 ± 33.9 a | 449.8 ± 31.6 b |
| Dialysis | 349.8 ± 19.2 a | 46.2 ± 3.2 b | 44.9 ± 1.4 b |
| Walnut brown shell pigment | |||
| Gastric | 910.3 ± 49.5 a | 857.6 ± 52.7 a | 44.4 ± 3.6 b |
| Gastro-intestinal | 828.2 ± 22.9 b | 949.7 ± 49.1 a | 311.0 ± 18.5 c |
| Dialysis | 453.8 ± 34.0 a | 433.9 ± 9.4 a | 250.6 ± 10.3 b |
| Groups | Control | Without Enzyme | Enzyme |
|---|---|---|---|
| Walnut green husk pigment | |||
| Gastric | 71.9 ± 3.1 a | 38.9 ± 2.9 b | 68.9 ± 3.4 a |
| Gastro-intestinal | 50.6 ± 1.1 a | 39.5 ± 2.1 b | 31.5 ± 2.8 c |
| Dialysis | 58.3 ± 3.7 a | 43.5 ± 1.7 b | 42.7 ± 3.1 b |
| Walnut brown shell pigment | |||
| Gastric | 124.1 ± 10.4 a | 128.0 ± 7.7 a | 83.7 ± 0.6 b |
| Gastro-intestinal | 74.5 ± 3.4 a | 55.7 ± 1.6 b | 76.2 ± 1.7 a |
| Dialysis | 31.1 ± 2.4 a | 16.1 ± 0.4 b | 34.6 ± 3.2 a |
| Groups | Control | Without Enzyme | Enzyme |
|---|---|---|---|
| Walnut green husk pigment | |||
| Gastric | 48.33 ± 0.17 b | 44.93 ± 0.18 c | 53.03 ± 1.43 a |
| Gastro-intestinal | 38.65 ± 0.25 b | 39.74 ± 0.44 b | 50.29 ± 2.73 a |
| Dialysis | 29 ± 1.47 b | 30.67 ± 0.72 b | 35.44 ± 2.00 a |
| Walnut brown shell pigment | |||
| Gastric | 94.41 ± 1.46 a | 92.95 ± 0.76 a | 84.71 ± 1.57 b |
| Gastro-intestinal | 60.97 ± 2.00 b | 53.84 ± 0.89 c | 76.86 ± 1.86 a |
| Dialysis | 31.27 ± 0.36 b | 30.57 ± 1.12 b | 34.34 ± 0.30 a |
| Groups | Control | Without Enzyme | Enzyme |
|---|---|---|---|
| Walnut green husk pigment | |||
| Gastric | 838 ± 36 a | 944 ± 42 a | 920 ± 22 a |
| Gastro-intestinal | 988 ± 25 a | 908 ± 19 a | 982 ± 27 a |
| Dialysis | 867 ± 17 a | 642 ± 24 b | 555 ± 12 c |
| Walnut brown shell pigment | |||
| Gastric | 1047 ± 6 a | 938 ± 24 b | 996 ± 59 a,b |
| Gastro-intestinal | 970 ± 25 a | 910 ± 34 a | 813 ± 32 b |
| Dialysis | 991 ± 47 a | 997 ± 51 a | 997 ± 51 a |
| Groups | Control | No enzyme | Enzyme |
|---|---|---|---|
| Walnut green husk pigment | |||
| Gastric | 2016 ± 8 a | 2006 ± 8 a | 1992 ± 6 b |
| Gastro-intestinal | 1726 ± 5 b | 1865 ± 7 a | 1728 ± 1 b |
| Dialysis | 1897 ± 6 b | 2002 ± 8 a | 1894 ± 9 b |
| Walnut brown shell pigment | |||
| Gastric | 2032 ± 11 a | 2009 ± 6 b | 2015 ± 9 a,b |
| Gastro-intestinal | 1744 ± 4 b | 1773 ± 12 a | 1758 ± 9 a,b |
| Dialysis | 1840 ± 11 c | 1893 ± 2 b | 1933 ± 8 a |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, Y.; Li, S.; Zeng, F.; Qi, J.; Qin, W.; Tan, C.; Luo, Q.; Wu, D.; Zhang, Q.; Lin, D.; et al. Functional Components, Antioxidant Activity and Hypoglycemic Ability Following Simulated Gastro-Intestinal Digestion of Pigments from Walnut Brown Shell and Green Husk. Antioxidants 2019, 8, 573. https://doi.org/10.3390/antiox8120573
Sun Y, Li S, Zeng F, Qi J, Qin W, Tan C, Luo Q, Wu D, Zhang Q, Lin D, et al. Functional Components, Antioxidant Activity and Hypoglycemic Ability Following Simulated Gastro-Intestinal Digestion of Pigments from Walnut Brown Shell and Green Husk. Antioxidants. 2019; 8(12):573. https://doi.org/10.3390/antiox8120573
Chicago/Turabian StyleSun, Yanan, Shanshan Li, Fanhang Zeng, Jingyi Qi, Wen Qin, Cui Tan, Qingying Luo, Dingtao Wu, Qing Zhang, Derong Lin, and et al. 2019. "Functional Components, Antioxidant Activity and Hypoglycemic Ability Following Simulated Gastro-Intestinal Digestion of Pigments from Walnut Brown Shell and Green Husk" Antioxidants 8, no. 12: 573. https://doi.org/10.3390/antiox8120573
APA StyleSun, Y., Li, S., Zeng, F., Qi, J., Qin, W., Tan, C., Luo, Q., Wu, D., Zhang, Q., Lin, D., & Chen, H. (2019). Functional Components, Antioxidant Activity and Hypoglycemic Ability Following Simulated Gastro-Intestinal Digestion of Pigments from Walnut Brown Shell and Green Husk. Antioxidants, 8(12), 573. https://doi.org/10.3390/antiox8120573








