Nitrosative Stress in Retinal Pathologies: Review
Abstract
1. Introduction
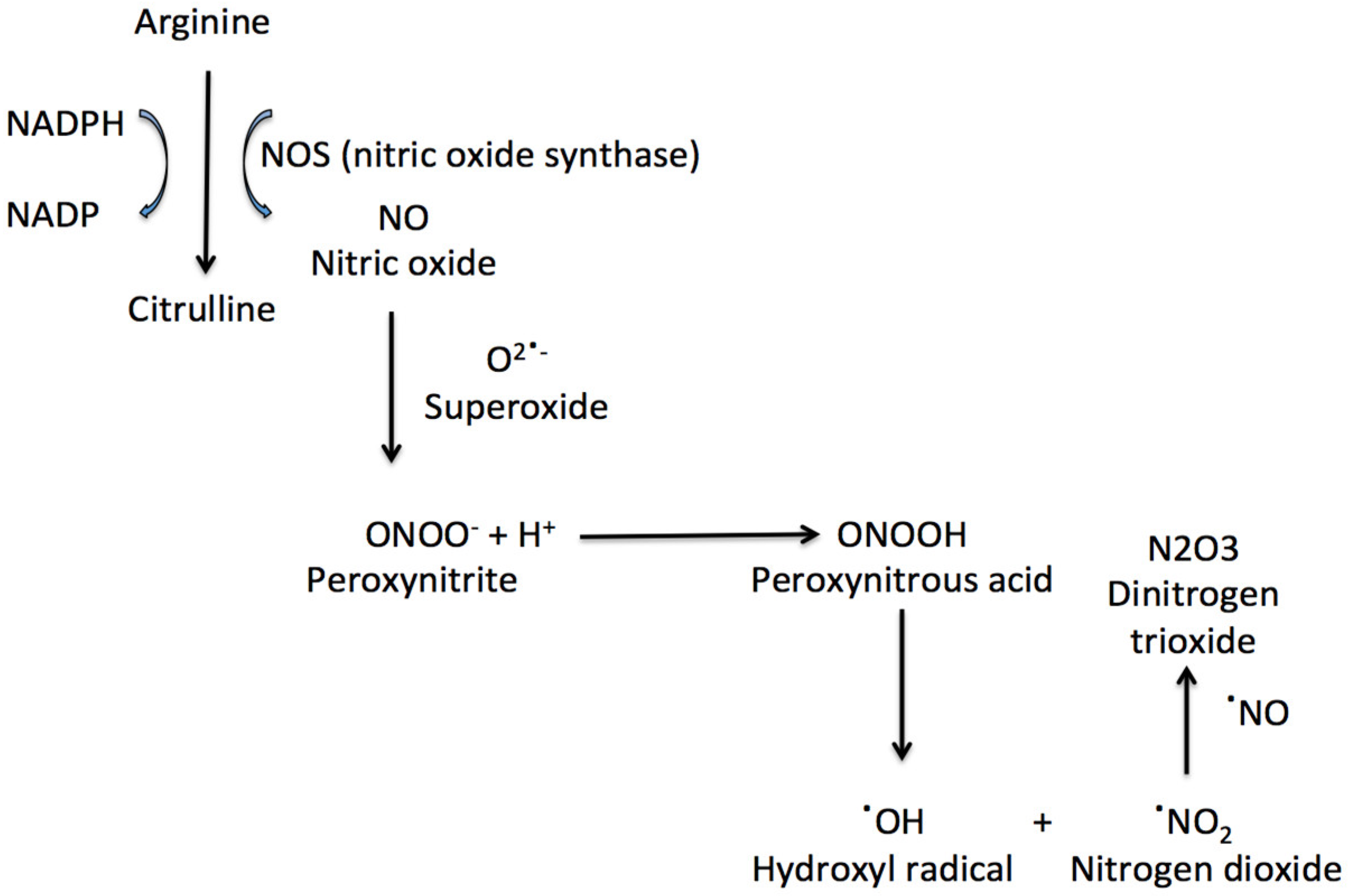
2. Nitric Oxide in the Retina
3. Diabetic Retinopathy
Can NO Inhibition Have a Role in Diabetic Retinopathy Therapy?
4. Retinitis Pigmentosa
Retinitis Pigmentosa Treatment with Nitric Oxide Synthase Inhibitors
5. Glaucoma
Nitric Oxide in Glaucoma Treatment
6. Age-Related Macular Degeneration
Treatment Perspectives of Age-Related Macular Degeneration with Molecules Related to Nitric Oxide Metabolism
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Bredt, D.S.; Snyder, S.H. Isolation of nitric oxide synthetase, a calmodulin-requiring enzyme. Proc. Natl. Acad. Sci. USA 1990, 87, 682–685. [Google Scholar] [CrossRef] [PubMed]
- Alderton, W.K.; Cooper, C.E.; Knowles, R.G. Nitric oxide synthases: Structure, function and inhibition. Biochem. J. 2001, 357, 593–615. [Google Scholar] [CrossRef] [PubMed]
- Vielma, A.H.; Retamal, M.A.; Schmachtenberg, O. Nitric oxide signaling in the retina: What have we learned in two decades? Brain Res. 2012, 1430, 112–125. [Google Scholar] [CrossRef] [PubMed]
- Förstermann, U.; Sessa, W.C. Nitric oxide synthases: Regulation and function. Eur. Heart J. 2012, 33, 829–837. [Google Scholar] [CrossRef] [PubMed]
- Knowles, R.G.; Moncada, S. Nitric oxide synthases in mammals. Biochem. J. 1994, 298, 249–258. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, I.M.; Ostwald, P.; Roth, S. Nitric oxide: A review of its role in retinal function and disease. Vision Res. 1996, 36, 2979–2994. [Google Scholar] [CrossRef]
- Cossenza, M.; Socodato, R.; Portugal, C.C.; Domith, I.C.; Gladulich, L.F.; Encarnação, T.G.; Calaza, K.C.; Mendonça, H.R.; Campello-Costa, P.; Paes-de-Carvalho, R. Nitric oxide in the nervous system: Biochemical, developmental, and neurobiological aspects. Vitam. Horm. 2014, 96, 79–125. [Google Scholar] [PubMed]
- Martínez-Ruiz, A.; Cadenas, S.; Lamas, S. Nitric oxide signaling: Classical, less classical, and nonclassical mechanisms. Free Radic. Biol. Med. 2011, 51, 17–29. [Google Scholar] [CrossRef] [PubMed]
- Knott, A.B.; Bossy-Wetzel, E. Nitric oxide in health and disease of the nervous system. Antioxid. Redox Signal. 2009, 11, 541–554. [Google Scholar] [CrossRef] [PubMed]
- Aslan, M.; Cort, A.; Yucel, I. Oxidative and nitrative stress markers in glaucoma. Free Radic. Biol. Med. 2008, 45, 367–376. [Google Scholar] [CrossRef] [PubMed]
- McBean, G.J.; López, M.G.; Wallner, F.K. Redox-based therapeutics in neurodegenerative disease. Br. J. Pharmacol. 2017, 174, 1750–1770. [Google Scholar] [CrossRef] [PubMed]
- Bradley, S.A.; Steinert, J.R. Nitric Oxide-Mediated Posttranslational Modifications: Impacts at the Synapse. Oxid. Med. Cell. Longev. 2016, 2016, 5681036. [Google Scholar] [CrossRef] [PubMed]
- Lima, M.G.; Maximino, C.; Matos-Oliveira, K.R.; Brasil, A.; Crespo-Lopez, M.E.; Batista-Ede, J.; Rocha, F.A.; Picanço-Diniz, D.L.; Herculano, A.M. Nitric oxide as a regulatory molecule in the processing of the visual stimulus. Nitric Oxide 2014, 36, 44–50. [Google Scholar] [CrossRef] [PubMed]
- Haverkamp, S.; Eldred, W.D. Localization of nNOS in photoreceptor, bipolar and horizontal cells in turtle and rat retinas. Neuroreport 1998, 9, 2231–2235. [Google Scholar] [CrossRef] [PubMed]
- Giove, T.J.; Deshpande, M.M.; Eldred, W.D. Identification of alternate transcripts of neuronal nitric oxide synthase in the mouse retina. J. Neurosci. Res. 2009, 87, 3134–3142. [Google Scholar] [CrossRef] [PubMed]
- Pang, J.J.; Gao, F.; Wu, S.M. Light responses and morphology of bNOS-immunoreactive neurons in the mouse retina. J. Comp. Neurol. 2010, 518, 2456–2474. [Google Scholar] [CrossRef] [PubMed]
- Blom, J.; Giove, T.; Deshpande, M.; Eldred, W.D. Characterization of nitric oxide signaling pathways in the mouse retina. J. Comp. Neurol. 2012, 520, 4204–4217. [Google Scholar] [CrossRef] [PubMed]
- Sennlaub, F.; Courtois, Y.; Goureau, O. Inducible nitric oxide synthase mediates the change from retinal to vitreal neovascularization in ischemic retinopathy. J. Clin. Investig. 2001, 107, 717–725. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Zhou, J.; Marshall, B.; Xin, H.; Atherton, S.S. Lack of iNOS facilitates MCMV spread in the retina. Investig. Ophthalmol Vis. Sci. 2007, 48, 285–292. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.P.; Li, Y.; Zhu, X.A.; Tso, M.O. Minocycline delayed photoreceptor death in rds mice through iNOS-dependent mechanism. Mol. Vis. 2007, 13, 1073–1082. [Google Scholar] [PubMed]
- McLeod, D.S.; Baba, T.; Bhutto, I.A.; Lutty, G.A. Co-expression of endothelial and neuronal nitric oxide synthases in the developing vasculatures of the human fetal eye. Graefe’s Arch. Clin. Exp. Ophthalmol. 2012, 250, 839–848. [Google Scholar] [CrossRef] [PubMed]
- Tekmen-Clark, M.; Gleason, E. Nitric oxide production and the expression of two nitric oxide synthases in the avian retina. Vis. Neurosci. 2013, 30, 91–103. [Google Scholar] [CrossRef] [PubMed]
- Cheon, E.W.; Park, C.H.; Kang, S.S.; Cho, G.J.; Yoo, J.M.; Song, J.K.; Choi, W.S. Change in endothelial nitric oxide synthase in the rat retina following transient ischemia. Neuroreport 2003, 14, 329–333. [Google Scholar] [CrossRef] [PubMed]
- Waldman, S.A.; Murad, F. Cyclic GMP synthesis and function. Pharmacol. Rev. 1987, 39, 163–196. [Google Scholar] [PubMed]
- Schmetterer, L.; Polak, K. Role of nitric oxide in the control of ocular blood flow. Prog. Retin. Eye Res. 2001, 20, 823–847. [Google Scholar] [CrossRef]
- Stringham, J.M.; Stringham, N.T. Nitric oxide and lutein: Function, performance, and protection of neural tissue. Foods 2015, 4, 678–689. [Google Scholar] [CrossRef] [PubMed]
- Mahajan, N.; Arora, P.; Sandhir, R. Perturbed biochemical pathways and associated oxidative stress lead to vascular dysfunctions in diabetic retinopathy. Oxid. Med. Cell. Longev. 2019, 2019, 8458472. [Google Scholar] [CrossRef] [PubMed]
- Rossino, M.G.; Casini, G. Nutraceuticals for the treatment of diabetic retinopathy. Nutrients 2019, 11, 771. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Verma, A.; Han, P.Y.; Nakagawa, T.; Johnson, R.J.; Grant, M.B.; Campbell-Thompson, M.; Jarajapu, Y.P.; Lei, B.; Hauswirth, W.W. Diabetic eNOS-knockout mice develop accelerated retinopathy. Investig. Ophthalmol. Vis. Sci. 2010, 51, 5240–5246. [Google Scholar] [CrossRef] [PubMed]
- Narayanan, S.P.; Rojas, M.; Suwanpradid, J.; Toque, H.A.; Caldwell, R.W.; Caldwell, R.B. Arginase in retinopathy. Prog. Retin. Eye Res. 2013, 36, 260–280. [Google Scholar] [CrossRef] [PubMed]
- Zheng, L.; Du, Y.; Miller, C.; Gubitosi-Klug, R.A.; Kern, T.S.; Ball, S.; Berkowitz, B.A. Critical role of inducible nitric oxide synthase in degeneration of retinal capillaries in mice with streptozotocin-induced diabetes. Diabetologia 2007, 50, 1987–1996. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Saxena, S.; Srivastav, K.; Shukla, R.K.; Mishra, N.; Meyer, C.H.; Kruzliak, P.; Khanna, V.K. Nitric oxide and oxidative stress is associated with severity of diabetic retinopathy and retinal structural alterations. Clin. Exp. Ophthalmol. 2015, 43, 429–436. [Google Scholar] [CrossRef] [PubMed]
- Kulaksızoglu, S.; Karalezli, A. Aqueous humour and serum levels of nitric oxide, malondialdehyde and total antioxidant status in patients with type 2 diabetes with proliferative diabetic retinopathy and nondiabetic senile cataracts. Can. J. Diabetes 2016, 40, 115–119. [Google Scholar] [CrossRef] [PubMed]
- Abu El-Asrar, A.M.; Meersschaert, A.; Dralands, L.; Missotten, L.; Geboes, K. Inducible nitric oxide synthase and vascular endothelial growth factor are colocalized in the retinas of human subjects with diabetes. Eye (London) 2004, 18, 306–313. [Google Scholar] [CrossRef] [PubMed]
- Kowluru, R.A.; Zhong, Q. Beyond AREDS: Is there a place for antioxidant therapy inthe prevention/treatment of eye disease? Investig. Ophthalmol. Vis. Sci. 2011, 52, 8665–8871. [Google Scholar] [CrossRef] [PubMed]
- Kowluru, R.A.; Kanwar, M.; Chan, P.S.; Zhang, J.P. Inhibition of retinopathy and retinal metabolic abnormalities in diabetic rats with AREDS-based micronutrients. Arch. Ophthalmol. 2008, 126, 1266–1272. [Google Scholar] [CrossRef] [PubMed]
- Arnal, E.; Miranda, M.; Johnsen-Soriano, S.; Alvarez-Nölting, R.; Díaz-Llopis, M.; Araiz, J.; Cervera, E.; Bosch-Morell, F.; Romero, F.J. Beneficial effect of docosahexanoic acid and lutein on retinal structural, metabolic, and functional abnormalities in diabetic rats. Curr. Eye Res. 2009, 34, 928–938. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Tang, L.; Chen, B. Oxidative stress: Implications for the development of diabetic retinopathy and antioxidant therapeutic perspectives. Oxid. Med. Cell. Longev. 2014, 2014, 752387. [Google Scholar] [CrossRef] [PubMed]
- Zheng, L.; Kern, T.S. Role of nitric oxide, superoxide, peroxynitrite and PARP in diabetic retinopathy. Front. Biosci. 2009, 14, 3974–3987. [Google Scholar] [CrossRef] [PubMed]
- Carr, B.C.; Emigh, C.E.; Bennett, L.D.; Pansick, A.D.; Birch, D.G.; Nguyen, C. Towards a treatment for diabetic retinopathy: Intravitreal toxicity and preclinical safety evaluation of inducible nitric oxide synthase nhibitors. Retina 2017, 37, 22–31. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hernández-Ramírez, E.; Sánchez-Chávez, G.; Estrella-Salazar, L.A.; Salceda, R. Nitrosative stress in the rat retina at the onset of streptozotocin-induced diabetes. Cell. Physiol. Biochem. 2017, 42, 2353–2363. [Google Scholar] [CrossRef] [PubMed]
- Othman, R.; Vaucher, E.; Couture, R. Bradykinin Type 1 Receptor–Inducible Nitric Oxide Synthase: A New Axis Implicated in Diabetic Retinopathy. Front. Pharmacol. 2019, 10, 300. [Google Scholar] [CrossRef] [PubMed]
- Bailey, A.; Pope, T.W.; Moore, S.A.; Campbell, C.L. The tragedy of TRIUMPH for nitric oxide synthesis inhibition in cardiogenic shock: Where do we go from here? Am. J. Cardiovasc. Drugs 2007, 7, 337–345. [Google Scholar] [CrossRef] [PubMed]
- Kanski, J.K.; Bowling, B. Clinical Ophthalmology: A Systematic Approach, 6th ed.; Elsevier: Philadelphia, PA, USA, 2007. [Google Scholar]
- Regillo, C.; Holekamp, N.; Johnson, M.W.; Kaiser, P.K.; Schubert, H.D.; Spaide, R.; Schmidt-Erfurth, U.M.; Bennett, P. Basic and Clinical Science Course: Section 12: Retina and Vitreous; American Academy of Ophthalmology: San Francisco, CA, USA, 2014. [Google Scholar]
- Jones, B.W.; Pfeiffer, R.L.; Ferrell, W.D.; Watt, C.B.; Marmor, M.; Marc, R.E. Retinal remodeling in human retinitis pigmentosa. Exp. Eye Res. 2016, 150, 149–165. [Google Scholar] [CrossRef] [PubMed]
- Hartong, D.T.; Berson, E.L.; Dryja, T.P. Retinitis pigmentosa. Lancet 2006, 368, 1795–1809. [Google Scholar] [CrossRef]
- Moreno, M.L.; Mérida, S.; Bosch-Morell, F.; Miranda, M.; Villar, V.M. Autophagy dysfunction and oxidative stress, two related mechanisms implicated in retinitis pigmentosa. Front. Physiol. 2018, 9, 1008. [Google Scholar] [CrossRef] [PubMed]
- Trachsel-Moncho, L.; Benlloch-Navarro, S.; Fernández-Carbonell, Á.; Ramírez-Lamelas, D.T.; Olivar, T.; Silvestre, D.; Poch, E.; Miranda, M. Oxidative stress and autophagy-related changes during retinal degeneration and development. Cell. Death Dis. 2018, 9, 812. [Google Scholar] [CrossRef] [PubMed]
- Ramírez-Lamelas, D.T.; Benlloch-Navarro, S.; López-Pedrajas, R.; Gimeno-Hernández, R.; Olivar, T.; Silvestre, D.; Miranda, M. Lipoic acid and progesterone alone or in combination ameliorate retinal degeneration in an experimental model of hereditary retinal degeneration. Front. Pharmacol. 2018, 9, 469. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Vallejo, V.; Benlloch-Navarro, S.; Trachsel-Moncho, L.; López-Pedrajas, R.; Almansa, I.; Romero, F.J.; Miranda, M. Alterations in glutamate cysteine ligase content in the retina of two retinitis pigmentosa animal models. Free Radic. Biol. Med. 2016, 96, 245–254. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Vallejo, V.; Benlloch-Navarro, S.; López-Pedrajas, R.; Romero, F.J.; Miranda, M. Neuroprotective actions of progesterone in an in vivo model of retinitis pigmentosa. Pharmacol. Res. 2015, 99, 276–288. [Google Scholar] [CrossRef] [PubMed]
- Yu, D.Y.; Cringle, S.; Valter, K.; Walsh, N.; Lee, D.; Stone, J. Photoreceptor death, trophic factor expression, retinal oxygen status, and photoreceptor function in the P23H rat. Investig. Ophthalmol. Vis. Sci. 2004, 45, 2013–2019. [Google Scholar] [CrossRef] [PubMed]
- Komeima, K.; Rogers, B.S.; Lu, L.; Campochiaro, P.A. Antioxidants reduce cone cell death in a model of retinitis pigmentosa. Proc. Natl. Acad. Sci. USA 2006, 103, 11300–11305. [Google Scholar] [CrossRef] [PubMed]
- Komeima, K.; Rogers, B.S.; Campochiaro, P.A. Antioxidants slow photoreceptor cell death in mouse models of retinitis pigmentosa. J. Cell. Physiol. 2007, 213, 809–815. [Google Scholar] [CrossRef] [PubMed]
- Benlloch-Navarro, S.; Trachsel-Moncho, L.; Fernández-Carbonell, Á.; Olivar, T.; Soria, J.M.; Almansa, I.; Miranda, M. Progesterone anti-inflammatory properties in hereditary retinal degeneration. J. Steroid Biochem. Mol. Biol. 2019, 189, 291–301. [Google Scholar] [CrossRef] [PubMed]
- Bowes, C.; Li, T.; Frankel, W.N.; Danciger, M.; Coffin, J.M.; Applebury, M.L.; Farber, D.B. Localization of a retroviral element within the rd gene coding for the beta subunit of cGMP phosphodiesterase. Proc. Natl. Acad. Sci. USA 1993, 90, 2955–2959. [Google Scholar] [CrossRef] [PubMed]
- Chang, B.; Hawes, N.L.; Pardue, M.T.; German, A.M.; Hurd, R.E.; Davisson, M.T.; Nusinowitz, S.; Rengarajan, K.; Boyd, A.P.; Sidney, S.S.; et al. Two mouse retinal degenerations caused by missense mutations in the beta-subunit of rod cGMP phosphodiesterase gene. Vision Res. 2007, 47, 624–633. [Google Scholar] [CrossRef] [PubMed]
- Loewen, C.J.; Moritz, O.L.; Molday, R.S. Molecular characterization of peripherin-2 and rom-1 mutants responsible for digenic retinitis pigmentosa. J. Biol. Chem. 2001, 276, 22388–22396. [Google Scholar] [CrossRef] [PubMed]
- Kanan, Y.; Khan, M.; Lorenc, V.E.; Long, D.; Chadha, R.; Sciamanna, J.; Green, K.; Campochiaro, P.A. Metipranolol promotes structure and function of retinal photoreceptors in the rd10 mouse model of human retinitis pigmentosa. J. Neurochem. 2019, 148, 307–318. [Google Scholar] [CrossRef] [PubMed]
- Komeima, K.; Usui, S.; Shen, J.; Rogers, B.S.; Campochiaro, P.A. Blockade of neuronal nitric oxide synthase reduces cone cell death in a model of retinitis pigmentosa. Free Radic. Biol. Med. 2008, 45, 905–912. [Google Scholar] [CrossRef] [PubMed]
- Tham, Y.C.; Li, X.; Wong, T.Y.; Quigley, H.A.; Aung, T.; Cheng, C.Y. Global prevalence of glaucoma and projections of glaucoma burden through 2040: A systematic review and meta-analysis. Ophthalmology 2014, 121, 2081–2090. [Google Scholar] [CrossRef] [PubMed]
- Bucolo, C.; Platania, C.B.M.; Drago, F.; Bonfiglio, V.; Reibaldi, M.; Avitabile, T.; Uva, M. Novel Therapeutics in Glaucoma Management. Curr. Neuropharmacol. 2018, 16, 978–992. [Google Scholar] [CrossRef] [PubMed]
- Dismuke, W.M.; Liang, J.; Overby, D.R.; Stamer, W.D. Concentration-related effects of nitric oxide and endothelin-1 on human trabecular meshwork cell contractility. Exp. Eye Res. 2014, 120, 28–35. [Google Scholar] [CrossRef] [PubMed]
- Grunwald, J.E.; DuPont, J.; Dreyer, E.B. Effect of chronic nitrate treatment on retinal vessel caliber in open-angle glaucoma. Am. J. Ophthalmol. 1997, 123, 753–758. [Google Scholar] [CrossRef]
- Doganay, S.; Evereklioglu, C.; Turkoz, Y.; Er, H. Decreased nitric oxide production in primary open-angle glaucoma. Eur. J. Ophthalmol. 2002, 12, 44–48. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.J.; Chiang, C.H.; Chow, J.C.; Lu, D.W. Aqueous humor nitric oxide levels differ in patients with different types of glaucoma. J. Ocul. Pharmacol. Ther. 2000, 16, 399–406. [Google Scholar] [CrossRef] [PubMed]
- Dreyer, E.B. A proposed role for excitotoxicity in glaucoma. J. Glaucoma 1998, 7, 62–67. [Google Scholar] [CrossRef] [PubMed]
- Nathanson, J.A.; McKee, M. Alterations of ocular nitric oxide synthase in human glaucoma. Investig. Ophthalmol. Vis. Sci. 1995, 36, 1774–1784. [Google Scholar]
- Kang, J.H.; Wiggs, J.L.; Rosner, B.A.; Hankinson, S.E.; Abdrabou, W.; Fan, B.J.; Haines, J.; Pasquale, L.R. Endothelial nitric oxide synthase gene variants and primary open-angle glaucoma: Interactions with sex and postmenopausal hormone use. Investig. Ophthalmol. Vis. Sci. 2010, 51, 971–979. [Google Scholar] [CrossRef] [PubMed]
- Pang, I.H.; Johnson, E.C.; Jia, L.; Cepurna, W.O.; Shepard, A.R.; Hellberg, M.R.; Clark, A.F.; Morrison, J.C. Evaluation of inducible nitric oxide synthase in glaucomatous optic neuropathy and pressure-induced optic nerve damage. Investig. Ophthalmol. Vis. Sci. 2005, 46, 1313–1321. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Toda, N.; Nakanishi-Toda, M. Nitric oxide: Ocular blood flow, glaucoma, and diabetic retinopathy. Prog. Retin. Eye Res. 2007, 26, 205–238. [Google Scholar] [CrossRef] [PubMed]
- Aliancy, J.; Stamer, W.D.; Wirostko, B. A review of nitric oxide for the treatment of glaucomatous disease. Ophthalmol. Ther. 2017, 6, 221–232. [Google Scholar] [CrossRef] [PubMed]
- Wong, W.L.; Su, X.; Li, X.; Cheung, C.M.; Klein, R.; Cheng, C.Y.; Wong, T.Y. Global prevalence of age-related macular degeneration and disease burden projection for 2020 and 2040: A systematic review and meta-analysis. Lancet Glob. Health 2014, 2, e106–e116. [Google Scholar] [CrossRef]
- Holekamp, N.M. Review of neovascular age-related macular degeneration treatment options. Am. J. Manag. Care 2019, 25, S172–S181. [Google Scholar] [PubMed]
- Handa, J.T.; Bowes, C.; Dick, A.D.; Gorin, M.B.; Miller, J.W.; Toth, C.A.; Ueffing, M.; Zarbin, M.; Farrer, L.A. A systems biology approach towards understanding and treating non-neovascular age-related macular degeneration. Nat. Commun. 2019, 10, 334–337. [Google Scholar] [CrossRef] [PubMed]
- Totan, Y.; Koca, C.; Erdurmuş, M.; Keskin, U.; Yiğitoğlu, R. Endothelin-1 and Nitric Oxide Levels in Exudative Age-Related Macular Degeneration. J. Ophthalmic Vis. Res. 2015, 10, 151–154. [Google Scholar] [PubMed]
- Friedman, E. A hemodynamic model of the pathogenesis of age-related macular degeneration. Am. J. Ophthalmol. 1997, 124, 677–682. [Google Scholar] [CrossRef]
- De Cillà, S.; Farruggio, S.; Vujosevic, S.; Raina, G.; Filippini, D.; Gatti, V.; Clemente, N.; Mary, D.; Vezzola, D.; Casini, G.; et al. Anti-vascular endothelial growth factors protect retinal pigment epithelium cells against oxidation by modulating nitric oxide release and autophagy. Cell. Physiol. Biochem. 2017, 42, 1725–1738. [Google Scholar] [CrossRef] [PubMed]
- Totan, Y.; Cekiç, O.; Borazan, M.; Uz, E.; Sögüt, S.; Akyol, O. Plasma malondialdehyde and nitric oxide levels in age related macular degeneration. Br. J. Ophthalmol. 2001, 85, 1426–1428. [Google Scholar] [CrossRef] [PubMed]
- Bhutto, I.A.; Baba, T.; Merges, C.; McLeod, D.S.; Lutty, G.A. Low nitric oxide synthases (NOSs) in eyes with age-related macular degeneration (AMD). Exp. Eye Res. 2010, 90, 155–167. [Google Scholar] [CrossRef] [PubMed]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cantó, A.; Olivar, T.; Romero, F.J.; Miranda, M. Nitrosative Stress in Retinal Pathologies: Review. Antioxidants 2019, 8, 543. https://doi.org/10.3390/antiox8110543
Cantó A, Olivar T, Romero FJ, Miranda M. Nitrosative Stress in Retinal Pathologies: Review. Antioxidants. 2019; 8(11):543. https://doi.org/10.3390/antiox8110543
Chicago/Turabian StyleCantó, Antolin, Teresa Olivar, Francisco Javier Romero, and María Miranda. 2019. "Nitrosative Stress in Retinal Pathologies: Review" Antioxidants 8, no. 11: 543. https://doi.org/10.3390/antiox8110543
APA StyleCantó, A., Olivar, T., Romero, F. J., & Miranda, M. (2019). Nitrosative Stress in Retinal Pathologies: Review. Antioxidants, 8(11), 543. https://doi.org/10.3390/antiox8110543





