Antioxidants versus Food Antioxidant Additives and Food Preservatives
Abstract
1. Introduction
2. Preservatives Versus Antioxidants
3. Precautions with Preservatives and Antioxidant Food Additives
4. Antioxidants: Beneficial or Detrimental for Human Health
5. Future Prospects on Health Concerns Due to Food Additives
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Abdulmumeen, H.A.; Risikat, A.N.; Sururah, A.R. Food: Its preservatives, additives and applications. IJCBS 2012, 1, 36–47. [Google Scholar]
- Cömert, E.D.; Gökmen, V. Evolution of food antioxidants as a core topic of food science for a century. Food Res. Int. 2018, 105, 76–93. [Google Scholar] [CrossRef] [PubMed]
- Pressman, P.; Clemens, R.; Hayes, W.; Reddy, C. Food additive safety. Toxicol. Res. Appl. 2017, 1, 239784731772357. [Google Scholar] [CrossRef]
- Wedzicha, B. Preservatives, food uses. In Encyclopedia of Food Sciences and Nutrition; Elsevier Sciences B.V.: Amsterdam, The Netherlands, 2003; pp. 4776–4780. [Google Scholar]
- Wedzicha, B. Preservatives, classification and properties. In Encyclopedia of Food Sciences and Nutrition; Caballero, B., Trugo, L., Finglas, P., Eds.; Elsevier Science B.V.: Amsterdam, The Netherlands, 2003; pp. 4773–4776. [Google Scholar]
- Popoff, S.; Riddick, J.A.; Wirth, V.I.; Ough, L.D. Oxidation-reduction potentials. III. The mercuric-mercurous electrode. J. Am. Chem. Soc. 1931, 53, 1195–1206. [Google Scholar] [CrossRef]
- Conant, J.B. Reduction potentials of quinones III. The free energy of reduction referred to the gaseous state. J. Am. Chem. Soc. 1927, 49, 293–297. [Google Scholar] [CrossRef]
- Needham, J.; Needham, D.M. The Hydrogen-Ion Concentration and the Oxidation-Reduction Potential of the Cell-Interior: A Micro-Injection Study. Proc. R. Soc. B Biol. Sci. 1925, 98, 259–286. [Google Scholar] [CrossRef]
- Wang, D.; Huang, S.; Wang, C.; Yue, Y.; Zhang, Q. Computational prediction for oxidation and reduction potentials of organic molecules used in organic light-emitting diodes. Org. Electron. Phys. Mater. Appl. 2019, 64, 216–222. [Google Scholar] [CrossRef]
- Gould, G.W.; Russell, N.J. Sulfite. In Food Preservatives; Russell, N.J., Gould, G.W., Eds.; Springer: Boston, MA, USA, 2003; pp. 85–101. [Google Scholar]
- Wedzicha, B.L. Chemistry of Sulphur Dioxide in Foods; Elsevier Applied Science Publishers Ltd.: Essex, UK, 1984. [Google Scholar]
- Ough, C.S. Sulfur Dioxide and Sulfites. Antimicrob. Foods 1993, 137–190. [Google Scholar]
- Liu, W.; Hansen, J.N. Some chemical and physical properties of nisin, a small-protein antibiotic produced by Lactococcus lactis. Appl. Environ. Microbiol. 1990, 56, 2551–2558. [Google Scholar] [PubMed]
- Toxnet. Available online: https://toxnet.nlm.nih.gov/cgi-bin/sis/search/a?dbs+hsdb:@term+@DOCNO+1230 (accessed on 15 October 2019).
- Material Safety Data Sheet—Ethylene Glycol. Available online: https://fscimage.fishersci.com/msds/09400.htm (accessed on 15 October 2019).
- Grizzle, C.O. Effects of sodium azide on the blood pressure of rats. Stanf. Med. Bull. 1953, 11, 145–148. [Google Scholar]
- Black, M.M.; Zweifach, B.W.; Speer, F.D. Comparison of Hypotensive Action of Sodium Azide in Normotensive and Hypertensive Patients. Exp. Biol. Med. 1954, 85, 11–16. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, W.; Bronetto, J. Death from Liver Cirrhosis and Specific Alcoholic Beverage Consumption: An Ecological Study. Am. J. Public Health Nations Health 1962, 52, 1473–1482. [Google Scholar] [CrossRef] [PubMed]
- Pelletier, S. Wine consumption is not associated with a decreased risk of alcoholic cirrhosis in heavy drinkers. Alcohol Alcohol. 2002, 37, 618–621. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Fuentes, A.; Wirtz, S.; Vos, E.; Verhagen, H. Short Review of Sulphites as Food Additives. Eur. J. Nutr. Food Saf. 2015, 5, 113–120. [Google Scholar] [CrossRef] [PubMed]
- Lee, G.; Lim, H.S.; Yun, S.S.; Shin, J.-W.; Kim, M. Safety assessment of antioxidants and color fixatives for the Korean population using dietary intake monitoring. Food Addit. Contam. Part A 2019, 36, 876–883. [Google Scholar] [CrossRef] [PubMed]
- Kochen, J. Sulfur dioxide, a respiratory tract irritant, even if ingested. Pediatrics 1973, 52, 145–146. [Google Scholar] [PubMed]
- Bailey, M.E.; Fieger, E.A.; Novak, A.F. Phenol oxidase in shrimp and crab. J. Food Sci. 1960, 25, 565–572. [Google Scholar] [CrossRef]
- Bailey, M.E.; Fieger, E.A.; Novak, A.F. Physico-chemical properties of the enzymes involved in shrimp melanogenesis. J. Food Sci. 1960, 25, 557–564. [Google Scholar] [CrossRef]
- Otwell, W.S.; Iyengar, R.; McEvily, A.J. Inhibition of Shrimp Melanosis by 4-Hexylresorcinol. J. Aquat. Food Prod. Technol. 1992, 1, 53–65. [Google Scholar] [CrossRef]
- Bursać Kovačević, D.; Maras, M.; Barba, F.J.; Granato, D.; Roohinejad, S.; Mallikarjunan, K.; Montesano, D.; Lorenzo, J.M.; Putnik, P. Innovative technologies for the recovery of phytochemicals from Stevia rebaudiana Bertoni leaves: A review. Food Chem. 2018, 268, 513–521. [Google Scholar] [CrossRef] [PubMed]
- EFSA Panel on Food Additives and Nutrient Sources added to Food (ANS). Scientific Opinion on the re-evaluation of beetroot red (E 162) as a food additive. EFSA J. 2016, 13, 4318. [Google Scholar]
- Collman, J.P. Naturally Dangerous; University Science Books: Sausalito, CA, USA, 2001. [Google Scholar]
- Legends Website. Available online: www.snopes2.com (accessed on 15 October 2019).
- Antolovich, M.; Prenzler, P.D.; Patsalides, E.; McDonald, S.; Robards, K. Methods for testing antioxidant activity. Analyst 2002, 127, 183–198. [Google Scholar] [CrossRef] [PubMed]
- Lagendijk, J.; Ubbink, J.B.; Vermaak, W.J. Measurement of the ratio between the reduced and oxidized forms of coenzyme Q10 in human plasma as a possible marker of oxidative stress. J. Lipid Res. 1996, 37, 67–75. [Google Scholar] [PubMed]
- Kanďár, R. The ratio of oxidized and reduced forms of selected antioxidants as a possible marker of oxidative stress in humans. Biomed. Chromatogr. 2016, 30, 13–28. [Google Scholar] [CrossRef] [PubMed]
- Calabrese, V.; Santoro, A.; Monti, D.; Crupi, R.; Di Paola, R.; Latteri, S.; Cuzzocrea, S.; Zappia, M.; Giordano, J.; Calabrese, E.J.; et al. Aging and Parkinson’s Disease: Inflammaging, neuroinflammation and biological remodeling as key factors in pathogenesis. Free Radic. Biol. Med. 2018, 115, 80–91. [Google Scholar] [CrossRef] [PubMed]
- Franco, R.; Navarro, G.; Martínez-Pinilla, E. Hormetic and Mitochondria-Related Mechanisms of Antioxidant Action of Phytochemicals. Antioxidants 2019, 8, 373. [Google Scholar] [CrossRef] [PubMed]
- Franco, R.; Navarro, G.; Martínez-Pinilla, E. Antioxidant Defense Mechanisms in Erythrocytes and in the Central Nervous System. Antioxidants 2019, 8, 46. [Google Scholar] [CrossRef] [PubMed]
- Luzzatto, L.; Nannelli, C.; Notaro, R. Glucose-6-Phosphate Dehydrogenase Deficiency. Hematol. Oncol. Clin. N. Am. 2016, 30, 373–393. [Google Scholar] [CrossRef] [PubMed]
- Ho, H.; Cheng, M.; Chiu, D.T. Glucose-6-phosphate dehydrogenase—From oxidative stress to cellular functions and degenerative diseases. Redox Rep. 2007, 12, 109–118. [Google Scholar] [CrossRef] [PubMed]
- Vural, N.; Sardas, S. Biological activities of broad bean (Vicia faba L.) extracts cultivated in South Anatolia in favism sensitive subjects. Toxicology 1984, 31, 175–179. [Google Scholar] [CrossRef]
- Davidson, P.M.; Sofos, J.N.; Branen, A.L. Antimicrobials in Food, 3rd ed.; Taylor & Francis: Abingdon, UK, 2005. [Google Scholar]
- Calabrese, E.J. The threshold vs LNT showdown: Dose rate findings exposed flaws in the LNT model part The Russell-Muller debate. Environ. Res. 2017, 154, 435–451. [Google Scholar] [CrossRef] [PubMed]
- Rattan, S.I.S. Repeated mild heat shock delays ageing in cultured human skin fibroblasts. Biochem. Mol. Biol. Int. 1998, 45, 753–759. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Rattan, S.I.S. The Nature of Gerontogenes and Vitagenes: Antiaging Effects of Repeated Heat Shock on Human Fibroblasts. Ann. N. Y. Acad. Sci. 1998, 854, 54–60. [Google Scholar] [CrossRef] [PubMed]
- Noctor, G.; Foyer, C.H. Ascorbate and Glutathione: Keeping Active Oxygen Under Control. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1998, 49, 249–279. [Google Scholar] [CrossRef] [PubMed]
- Franco, R.; Martínez-Pinilla, E. Chemical rules on the assessment of antioxidant potential in food and food additives aimed at reducing oxidative stress and neurodegeneration. Food Chem. 2017, 235, 318–323. [Google Scholar] [CrossRef] [PubMed]
- Bánhegyi, G.; Csala, M.; Braun, L.; Garzó, T.; Mandl, J. Ascorbate synthesis-dependent glutathione consumption in mouse liver. FEBS Lett. 1996, 381, 39–41. [Google Scholar] [CrossRef]

| Antioxidants | |||||
| E300 | Ascorbic acid |  | E310 | Propyl gallate | 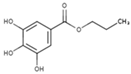 |
| E301 | Sodium ascorbate | 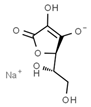 | E315 | Erythorbic acid | 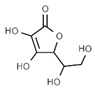 |
| E302 | Calcium ascorbate | 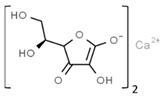 | E316 | Sodium erythorbate | 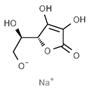 |
| E304 | Fatty acid esters of ascorbic acid |  | E319 | Tertiary-butyl hydroquinone (TBHQ) | 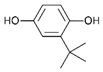 |
| E306 | Tocopherols | 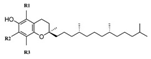 | E320 | Butylated hydroxyanisole (BHA) | 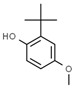 |
| E307 | Alpha-tocopherol | 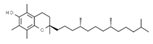 | E321 | Butylated hydroxytoluene (BHT) | 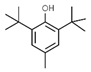 |
| E308 | Gamma-tocopherol | 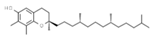 | E392 | Extracts of rosemary | 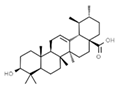 |
| E309 | Delta-tocopherol | 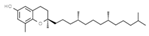 | E586 | 4-Hexylresorcinol | 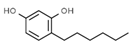 |
| Preservatives | |||||
| E200 | Sorbic acid | 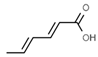 | E228 | Potassium hydrogen sulfite |  |
| E202 | Potassium sorbate | 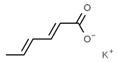 | E234 | Nisin a | 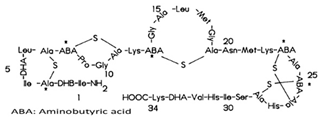 |
| E210 | Benzoic acid | 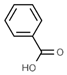 | E235 | Natamycin | 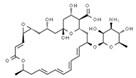 |
| E211 | Sodium benzoate | 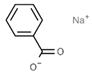 | E239 | Hexamethylene tetramine |  |
| E212 | Potassium benzoate | 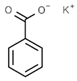 | E242 | Dimethyl dicarbonate | 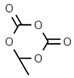 |
| E213 | Calcium benzoate | 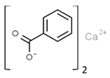 | E243 | Ethyl lauroyl arginate | 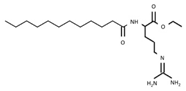 |
| E214 | Ethyl p-hydroxybenzoate | 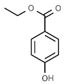 | E249 | Potassium nitrite | 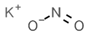 |
| E215 | Sodium ethyl p-hydroxybenzoate |  | E250 | Sodium nitrite | 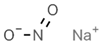 |
| E218 | Methyl p-hydroxybenzoate |  | E251 | Sodium nitrate |  |
| E219 | Sodium methyl p-hydroxybenzoate | 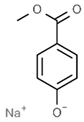 | E252 | Potassium nitrate | 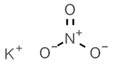 |
| E220 | Sulphur dioxide | 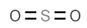 | E280 | Propionic acid | 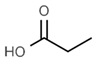 |
| E221 | Sodium sulfite | 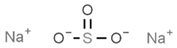 | E281 | Sodium propionate | 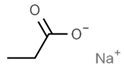 |
| E222 | Sodium hydrogen sulfite | 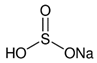 | E282 | Calcium propionate | 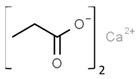 |
| E223 | Sodium metabisulfite | 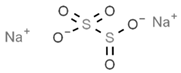 | E283 | Potassium propionate | 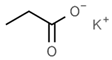 |
| E224 | Potassium metabisulfite | 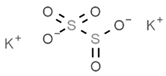 | E284 | Boric acid | 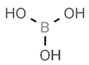 |
| E226 | Calcium sulfite | 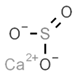 | E285 | Sodium tetraborate; borax | 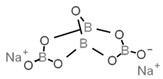 |
| E227 | Calcium hydrogen sulfite | 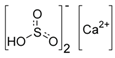 | E1105 | Lysozyme (N-acetylmuramide glycanhydrolase) | Enzyme de 14 kilodaltons |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Franco, R.; Navarro, G.; Martínez-Pinilla, E. Antioxidants versus Food Antioxidant Additives and Food Preservatives. Antioxidants 2019, 8, 542. https://doi.org/10.3390/antiox8110542
Franco R, Navarro G, Martínez-Pinilla E. Antioxidants versus Food Antioxidant Additives and Food Preservatives. Antioxidants. 2019; 8(11):542. https://doi.org/10.3390/antiox8110542
Chicago/Turabian StyleFranco, Rafael, Gemma Navarro, and Eva Martínez-Pinilla. 2019. "Antioxidants versus Food Antioxidant Additives and Food Preservatives" Antioxidants 8, no. 11: 542. https://doi.org/10.3390/antiox8110542
APA StyleFranco, R., Navarro, G., & Martínez-Pinilla, E. (2019). Antioxidants versus Food Antioxidant Additives and Food Preservatives. Antioxidants, 8(11), 542. https://doi.org/10.3390/antiox8110542






