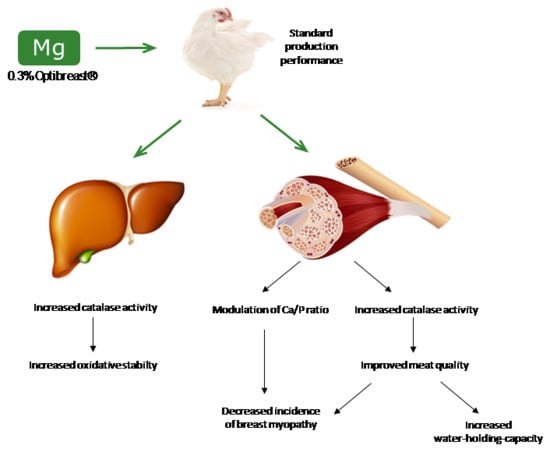
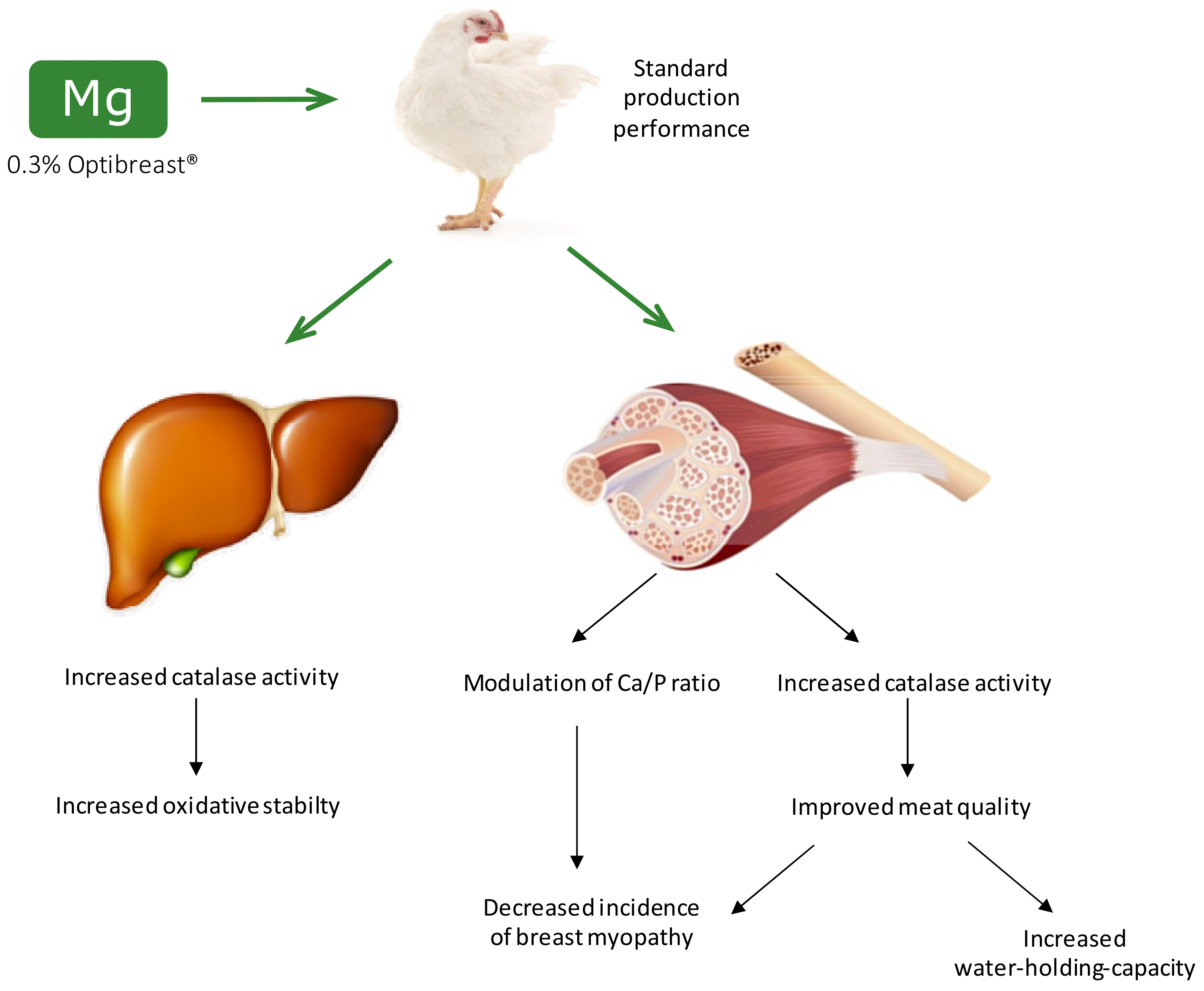
Benefits of Magnesium Supplementation to Broiler Subjected to Dietary and Heat Stress: Improved Redox Status, Breast Quality and Decreased Myopathy Incidence
Abstract
:1. Introduction
2. Material and Methods
2.1. Experimental Setting and Analyses at the Farm
2.2. Sampling and Assessment of Carcasses for Myopathies
2.3. Analysis of Biological Samples
2.3.1. Minerals
2.3.2. Oxidation Markers
2.3.3. Endogenous Antioxidant Enzymes
2.3.4. Quality Parameters in Chicken Breast
2.3.5. Statistical Analysis
3. Results and Discussion
3.1. Production Parameters in Broilers as Affected by Dietary Mg Supplementation
3.2. Mineral Composition of Tissues from Broilers as Affected by Dietary Mg Supplementation
3.3. Antioxidant Enzymes and Oxidation Markers in Tissues from Broilers as Affected by Dietary Mg Supplementation
3.4. Quality Parameters in Breast Muscles from Broilers as Affected by Dietary Mg Supplementation
3.5. Breast Myopathy Incidence in Broilers as Affected by Dietary Mg Supplementation
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kumar, A.; Roy, B.; Lakhani, G.P.; Jain, A. Evaluation of dried bread waste as feedstuff for growing crossbred pigs. Vet. World 2014, 7, 698–701. [Google Scholar] [CrossRef]
- Estevéz, M. Oxidative damage to poultry: From farm to fork. Poult. Sci. 2015, 94, 1368–1378. [Google Scholar] [CrossRef] [PubMed]
- Kohen, R.; Nyska, A. Oxidation of biological systems: Oxidative stress phenomena, antioxidants, redox reactions, and methods for their quantification. Toxicol. Pathol. 2002, 30, 620–650. [Google Scholar] [CrossRef] [PubMed]
- Min, B.; Nam, K.C.; Cordray, J.; Ahn, D.U. Endogenous factors affecting oxidative stability of beef loin, pork loin, and chicken breast and thigh meats. J. Food Sci. 2008, 73, C439–C446. [Google Scholar] [CrossRef] [PubMed]
- Min, B.; Ahn, D.U. Mechanism of lipid peroxidation in meat and meat products—A review. Food Sci. Biotechnol. 2005, 14, 152–163. [Google Scholar]
- Fellenberg, M.A.; Speisky, H. Antioxidants: Their effects on broiler oxidative stress and its meat oxidative stability. World Poult. Sci. J. 2006, 62, 53–70. [Google Scholar] [CrossRef]
- Shastak, Y.; Rodehutscord, M. A review of the role of magnesium in poultry nutrition. World Poult. Sci. J. 2015, 71, 125–138. [Google Scholar] [CrossRef]
- Petracci, M.; Soglia, F.; Madruga, M.; Ida, E.; Estévez, M. Wooden-Breast, White Striping, and Spaghetti Meat: Causes, consequences and consumer perception of emerging broiler meat abnormalities. Comp. Rev. Food Sci. Food Saf. 2019, 18, 565–583. [Google Scholar] [CrossRef]
- Abasht, B.; Mutryn, M.F.; Michalek, R.D.; Lee, W.R. Oxidative stress and metabolic perturbations in wooden breast disorder in chickens. PLoS ONE 2016, 11, e0153750. [Google Scholar] [CrossRef] [PubMed]
- Soglia, F.; Laghi, L.; Canonico, L.; Cavani, C.; Petracci, M. Functional property issues in broiler breast meat related to emerging muscle abnormalities. Food Res. Int. 2016, 89, 1071–1076. [Google Scholar] [CrossRef]
- Lee, S.R.; Britton, W.M. Magnesium-induced catharsis in chicks. J. Nutr. 1987, 117, 1907–1912. [Google Scholar] [CrossRef] [PubMed]
- FEDNA. Tablas FEDNA de Composición y Valor Nutritivo de Alimentos para la Fabricación de Piensos Compuestos, 2nd ed.; De Blas, J.C., Mateos, G.G., García-Rebollar, P., Eds.; Fundación Española para el Desarrollo de la Nutrición Animal: Madrid, Spain, 2010. [Google Scholar]
- Ganhão, R.; Morcuende, D.; Estévez, M. Protein oxidation in emulsified cooked burger patties with added fruit extracts: Influence on colour and texture deterioration during chill storage. Meat. Sci. 2010, 85, 402–409. [Google Scholar] [CrossRef] [PubMed]
- Armenteros, M.; Heinonen, M.; Ollilainen, V.; Toldrá, F.; Estévez, M. Analysis of protein carbonyls in meat products by using the DNPH-method, fluorescence spectroscopy and liquid chromatography-electrospray ionisation-mass spectrometry (LC-ESI-MS). Meat. Sci. 2009, 83, 104–112. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, R.H.; Ida, E.I.; Madruga, M.S.; Shimokomaki, M.; Estévez, M. Underlying connections between the redox system imbalance, protein oxidation and impaired quality traits in pale, soft and exudative (PSE) poultry meat. Food Chem. 2017, 215, 129–137. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.X.; Guo, Y.M.; Wang, Z. Effect of magnesium on reactive oxygen species production in the thigh muscles of broiler chickens. Br. Poult. Sci. 2007, 48, 84–89. [Google Scholar] [CrossRef] [PubMed]
- Stillmak, S.J.; Sunde, M.L. The use of high magnesium limestone in the diet of the laying hen. 2. Calcium and magnesium availability. Poult. Sci. 1971, 50, 564–572. [Google Scholar] [CrossRef]
- Afanasev, I.B.; Suslova, T.B.; Cheremisina, Z.P.; Abramova, N.E.; Korkina, L.G. Study of antioxidant properties of metal aspartates. Analyst 1995, 120, 859–862. [Google Scholar] [CrossRef] [PubMed]
- Fine, K.D.; Santa Ana, C.A.; Porter, J.L.; Fordtran, J.S. Intestinal absorption of magnesium from food and supplements. J. Clin. Investig. 1991, 88, 396–402. [Google Scholar] [CrossRef]
- Yang, Y.; Wu, Z.L.; Chen, Y.; Qiao, J.; Cao, M.Y.; Yuan, Y.M.; Nie, W.; Guo, Y.M. Magnesium deficiency enhances hydrogen peroxide production and oxidative damage in chick embryo hepatocyte in vitro. Biometals 2005, 18, 36–46. [Google Scholar] [CrossRef]
- Petracci, M.; Mudalal, S.; Soglia, F.; Cavani, C. Meat quality in fast-growing broiler chickens. World’s Poult. Sci. J. 2015, 71, 363–374. [Google Scholar] [CrossRef]
- Estévez, M.; Padilla, P.; Carvalho, L.; Martín, L.; Carrapiso, A.; Delgado, J. Malondialdehyde interferes with the formation and detection of primary carbonyls in oxidized proteins. Redox Biol. 2019, 26, 101277. [Google Scholar] [CrossRef] [PubMed]
- Utrera, M.; Estevez, M. Oxidation of myofibrillar proteins and impaired functionality: Underlying mechanisms of the carbonylation pathway. J. Agric. Food Chem. 2012, 60, 8002–8011. [Google Scholar] [CrossRef] [PubMed]
- Kuttappan, V.A.; Shivaprasad, H.L.; Shaw, D.P.; Valentine, B.A.; Hargis, B.M.; Clark, F.D.; McKee, S.R.; Owens, C.M. Pathological changes associated with white striping in broiler breast muscles. Poult. Sci. 2013, 92, 331–338. [Google Scholar] [CrossRef] [PubMed]
- Kuttappan, V.A.; Hargis, B.M.; Owens, C.M. White striping and woody breast myopathies in the modern poultry industry: A review. Poult. Sci. 2016, 95, 2724–2733. [Google Scholar] [CrossRef] [PubMed]
| Ingredients (%) | Starter 0–14 Days | Grower 14–28 Days | Finisher 28–42 Days |
|---|---|---|---|
| Corn | 30.95 | 36.98 | 40.99 |
| Soy flour 44% PB | 36.91 | 31.41 | 27.74 |
| Wheat | 25.00 | 25.00 | 25.00 |
| Soy oil | 2.71 | 2.64 | 2.60 |
| Calcium phosphate | 1.58 | 1.53 | 1.50 |
| Calcium bicarbonate | 1.42 | 1.17 | 1.01 |
| Vitamin premix 1 | 0.40 | 0.40 | 0.40 |
| DL-Methionine | 0.29 | 0.23 | 0.19 |
| L-Lysine HCL | 0.23 | 0.18 | 0.14 |
| Salt | 0.22 | 0.24 | 0.25 |
| Sodium bicarbonate | 0.21 | 0.19 | 0.18 |
| L-Threonine | 0.07 | 0.04 | 0.02 |
| Calculated analysis 2 (%) | |||
| Metabolizable energy, kcal/kg | 2900 | 2960 | 3000 |
| Dry matter | 88.39 | 88.23 | 88.12 |
| Starch | 34.83 | 38.61 | 41.14 |
| Total protein | 22.10 | 19.94 | 18.50 |
| Neutral detergent fiber | 9.22 | 9.09 | 9.01 |
| Ash | 6.11 | 5.55 | 5.18 |
| Ether extract | 4.93 | 4.97 | 5.00 |
| Total fiber | 3.21 | 3.07 | 2.98 |
| Lys total | 1.35 | 1.16 | 1.04 |
| Lys dig. | 1.19 | 1.02 | 0.91 |
| Calcium | 1.05 | 0.93 | 0.85 |
| Met+Cys total | 0.97 | 0.86 | 0.78 |
| Trh total | 0.88 | 0.77 | 0.70 |
| Met+Cys dig. | 0.87 | 0.76 | 0.69 |
| Thr dig. | 0.75 | 0.65 | 0.59 |
| Total phosphorous | 0.74 | 0.71 | 0.69 |
| Met total | 0.61 | 0.53 | 0.47 |
| Met dig. | 0.58 | 0.49 | 0.43 |
| Available phosphorous | 0.45 | 0.43 | 0.42 |
| Trp total | 0.27 | 0.24 | 0.22 |
| Trp dig. | 0.23 | 0.20 | 0.19 |
| Sodium | 0.16 | 0.16 | 0.16 |
| Magnesium 3 | 0.11 | 0.10 (0.38) | 0.12 (0.39) |
| α-tocopherol 4 | 40.3 | 52.3 (39.8) | 51.8 (46.9) |
| Starting Stage | DWG | DAFC | CI |
|---|---|---|---|
| Basal | 24.6 | 38.4 | 1.57 |
| Mg | 23.9 | 37.6 | 1.58 |
| SEM 1 (n = 9) | 0.44 | 0.92 | 0.05 |
| p-value | 0.15 | 0.63 | 0.28 |
| Growing Stage | 64.9 | 103.2 | 1.59 |
| Basal | 64.9 | 103.2 | 1.59 |
| Mg | 64.7 | 101.9 | 1.58 |
| SEM 1 (n = 9) | 0.75 | 0.89 | 0.02 |
| p-value | 0.72 | 0.30 | 0.48 |
| Finishing Stage | |||
| BASAL | 79.8 | 162.3 | 2.04 |
| Mg | 80.1 | 159.7 | 2.00 |
| SEM 1 (n = 9) | 1.26 | 1.55 | 0.02 |
| p-value | 0.85 | 0.41 | 0.58 |
| Tissue | Basal | Mg | SEM 1 | p-Value |
|---|---|---|---|---|
| Breast | ||||
| Ca2+ | 104.9 | 81.1 | 6.18 | <0.05 |
| Mg2+ | 251.2 | 249.8 | 6.90 | 0.48 |
| P | 256.4 | 259.0 | 5.90 | 0.12 |
| Blood | ||||
| Ca2+ | 197.9 | 147.7 | 13.02 | <0.05 |
| Mg2+ | 64.1 | 79.0 | 5.17 | <0.05 |
| P | 98.90 | 99.21 | 5.60 | 0.31 |
| Liver | ||||
| Ca2+ | 81.1 | 78.6 | 5.7 | 0.36 |
| Mg2+ | 91.4 | 112.3 | 6.8 | <0.05 |
| P | 288.5 | 299.6 | 6.5 | 0.38 |
| Tissue | Basal | Mg | SEM 1 | p-Value |
|---|---|---|---|---|
| Breast | ||||
| CAT | 0.51 | 0.94 | 0.06 | <0.05 |
| SOD | 14.53 | 14.47 | 0.05 | 0.640 |
| TBARS | 0.05 | 0.05 | 0.01 | 0.621 |
| Protein carbonyls | 0.70 | 0.72 | 0.02 | 0.503 |
| Plasma | ||||
| CAT | 1.29 | 3.70 | 0.46 | <0.05 |
| SOD | 19.23 | 19.23 | 0.16 | 0.265 |
| TBARS 2 | 3.64 | 3.65 | 0.31 | 0.656 |
| Protein carbonyls | 11.27 | 9.75 | 0.16 | <0.05 |
| Liver | ||||
| CAT | 6.26 | 9.20 | 0.17 | <0.05 |
| SOD | 16.07 | 16.17 | 0.78 | 0.546 |
| TBARS | 0.15 | 0.15 | 0.01 | 0.478 |
| Protein carbonyls | 5.06 | 3.24 | 0.06 | <0.05 |
| Parameter | Basal | Mg | SEM 1 | p-Value |
|---|---|---|---|---|
| pH 45 min | 6.26 | 6.29 | 0.24 | 0.65 |
| pH final | 5.76 | 5.76 | 0.08 | 0.54 |
| Redness | 3.81 | 4.06 | 0.99 | 0.32 |
| Lightness | 51.76 | 53.89 | 1.67 | <0.05 |
| Yellowness | 2.18 | 1.34 | 0.48 | <0.05 |
| WHC | 88.67 | 94.37 | 2.23 | <0.05 |
| Myopathy | Basal | Mg | p-Value |
|---|---|---|---|
| WB#1-NORMAL | 12 | 22 | <0.05 |
| WB#2-MILD | 16 | 8 | <0.05 |
| WB#3-SEVERE | 4 | 2 | <0.1 |
| WS#1-NORMAL | 11 | 20 | <0.05 |
| WS#2-MILD | 17 | 10 | <0.05 |
| WS#3-SEVERE | 4 | 2 | <0.1 |
| Parameter | Normal | WB | WS | SEM 1 | p-Value |
|---|---|---|---|---|---|
| Moisture 2 | 74.14 a | 74.21 a | 71.14 b | 0.25 | <0.05 |
| Lipid 2 | 1.64 b | 1.74 b | 2.18 a | 0.08 | <0.05 |
| Protein 2 | 24.19 | 23.93 | 23.52 | 0.31 | 0.12 |
| pH 45 min | 6.28 | 6.23 | 6.17 | 0.08 | 0.61 |
| pH final | 5.73 | 5.75 | 5.76 | 0.02 | 0.75 |
| Redness | 4.09 | 4.63 | 4.86 | 0.33 | 0.36 |
| Lightness | 53.29 a | 51.48 b | 51.93 b | 0.92 | <0.05 |
| Yellowness | 1.99 | 1.82 | 1.97 | 0.54 | 0.34 |
| WHC 2 | 93.08 a | 88.49 b | 87.56 b | 0.86 | <0.05 |
| MDA 3 | 0.047 | 0.040 | 0.057 | 0.01 | 0.21 |
| Protein carbonyls 4 | 0.66 b | 1.18 a | 0.78 ab | 0.02 | <0.05 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Estevez, M.; Petracci, M. Benefits of Magnesium Supplementation to Broiler Subjected to Dietary and Heat Stress: Improved Redox Status, Breast Quality and Decreased Myopathy Incidence. Antioxidants 2019, 8, 456. https://doi.org/10.3390/antiox8100456
Estevez M, Petracci M. Benefits of Magnesium Supplementation to Broiler Subjected to Dietary and Heat Stress: Improved Redox Status, Breast Quality and Decreased Myopathy Incidence. Antioxidants. 2019; 8(10):456. https://doi.org/10.3390/antiox8100456
Chicago/Turabian StyleEstevez, Mario, and Massimiliano Petracci. 2019. "Benefits of Magnesium Supplementation to Broiler Subjected to Dietary and Heat Stress: Improved Redox Status, Breast Quality and Decreased Myopathy Incidence" Antioxidants 8, no. 10: 456. https://doi.org/10.3390/antiox8100456
APA StyleEstevez, M., & Petracci, M. (2019). Benefits of Magnesium Supplementation to Broiler Subjected to Dietary and Heat Stress: Improved Redox Status, Breast Quality and Decreased Myopathy Incidence. Antioxidants, 8(10), 456. https://doi.org/10.3390/antiox8100456