A Comprehensive Insight on the Health Benefits and Phytoconstituents of Camellia sinensis and Recent Approaches for Its Quality Control
Abstract
1. Introduction
2. Health Benefits and Biological Activities of Green Tea (C. sinensis)
2.1. Antioxidant and Hepatoprotective Activity
2.2. Anticancer and Anti-Mutagenic Activity
2.3. Antimicrobial and Antiviral Activity
2.4. Anti-Schisosomiasis and Antiparasitic Activity
2.5. Cardioprotective Activity
2.6. Antidiabetic and Anti-Obesity Activity
2.7. Gastrointestinal Tract Problems Relieving Activity
2.8. Neuroprotective Activity
2.9. Anti-Inflammatory, Analgesic, Antipyretic, and Anti-Allergic Activity
2.10. Skeletomuscular System Relieving Activity
2.11. Miscellaneous Activity
3. Effect of Green Tea Administration on the Bioavailability of Other Drugs
4. Phytoconstituents of Green Tea (C. sinensis)
4.1. Polyphenols
4.2. Xanthine Bases/Purine Alkaloids
4.3. Triterpenoid Saponins
4.4. Amino Acids
4.5. Minerals and Trace Elements
4.6. Miscellaneous Compounds
5. Recent Approaches for Monitoring the Quality of Green Tea (C. sinensis)
5.1. Morphological Aspects
5.2. Physical Quality Parameters
5.2.1. Aroma and Flavor
5.2.2. Color, Grain Shape, and Size
5.3. Chemical Markers
5.3.1. Catechins
5.3.2. Caffeine
5.3.3. Minerals
5.3.4. Amino Acids
5.3.5. Carotene
5.4. Antioxidant Capacity and Determination of Antioxidant Constituents in Green Tea
5.5. Determination of Green Tea Contaminants
6. Current Trends in Green Tea in Researches and Future Recommendations
7. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Fathy, S.; Emam, M.; Agwa, S.A.; Zahra, F.A.; Youssef, F.; Sami, R. The antiproliferative effect of Origanum majorana on human hepatocarcinoma cell line: Suppression of NF-kB. Cell. Mol. Biol. 2016, 62, 80–84. [Google Scholar] [PubMed]
- Ashour, M.L.; Youssef, F.S.; Gad, H.A.; El-Readi, M.Z.; Bouzabata, A.; Abuzeid, R.M.; Sobeh, M.; Wink, M. Evidence for the anti-inflammatory activity of Bupleurum marginatum (Apiaceae) extracts using in vitro and in vivo experiments supported by virtual screening. J. Pharm. Pharmacol. 2018, 70, 952–963. [Google Scholar] [CrossRef] [PubMed]
- Janibekov, A.A.; Youssef, F.S.; Ashour, M.L.; Mamadalieva, N.Z. New flavonoid glycosides from two Astragalus species (Fabaceae) and validation of their antihyperglycaemic activity using molecular modelling and in vitro studies. Ind. Crop. Prod. 2018, 118, 142–148. [Google Scholar] [CrossRef]
- Thabet, A.A.; Youssef, F.S.; El-Shazly, M.; El-Beshbishy, H.A.; Singab, A.N.B. Validation of the antihyperglycaemic and hepatoprotective activity of the flavonoid rich fraction of Brachychiton rupestris using in vivo experimental models and molecular modelling. Food Chem. Toxicol. 2018, 114, 302–310. [Google Scholar] [CrossRef] [PubMed]
- Youssef, F.S.; Labib, R.M.; Eldahshan, O.A.; Singab, A.N. Synergistic hepatoprotective and antioxidant effect of Artichoke, Fig, Mulberry Herbal mixture on HepG2 Cells and their metabolic profiling Using NMR coupled with chemometrics. Chem. Biodivers. 2017, 14, e1700206. [Google Scholar] [CrossRef] [PubMed]
- Talaat, A.N.; Ebada, S.S.; Labib, R.M.; Esmat, A.; Youssef, F.S.; Singab, A.N.B. Verification of the anti-inflammatory activity of the polyphenolic-rich fraction of Araucaria bidwillii Hook. using phytohaemagglutinin-stimulated human peripheral blood mononuclear cells and virtual screening. J. Ethnopharmacol. 2018, 226, 44–47. [Google Scholar] [CrossRef]
- Couturier, F.J.; Colemont, L.J.; Fierens, H.; Verhoeven, V.M. Toxic hepatitis due to a food supplement: “Natural” is no synonym for “harmless”. Clin. Res. Hepatol. Gastroenterol. 2016, 40, e38–e43. [Google Scholar] [CrossRef]
- Aboulwafa, M.M.; Youssef, F.S.; Gad, H.A.; Sarker, S.D.; Nahar, L.; Al-Azizi, M.M.; Ashour, M.L. Authentication and discrimination of green tea samples using UV-Visible, FTIR and HPLC techniques coupled with chemometrics analysis. J. Pharm. Biomed. Anal. 2018, 164, 653–658. [Google Scholar] [CrossRef]
- Ferrara, L.; Montesano, D.; Senatore, A. The distribution of minerals and flavonoids in the tea plant (Camellia sinensis). Il Farmaco 2001, 56, 397–401. [Google Scholar] [CrossRef]
- Chen, Q.; Guo, Z.; Zhao, J. Identification of green tea’s (Camellia sinensis L.) quality level according to measurement of main catechins and caffeine contents by HPLC and support vector classification pattern recognition. J. Pharm. Biomed. Anal. 2008, 48, 1321–1325. [Google Scholar] [CrossRef]
- Graham, H.N. Green tea composition, consumption, and polyphenol chemistry. Prev. Med. 1992, 21, 334–350. [Google Scholar] [CrossRef]
- Rashidi, B.; Malekzadeh, M.; Goodarzi, M.; Masoudifar, A.; Mirzaei, H. Green tea and its anti-angiogenesis effects. Biomed. Pharmacother. 2017, 89, 949–956. [Google Scholar] [CrossRef] [PubMed]
- Jayashree, G.V.; Krupashree, K.; Rachitha, P.; Khanum, F. Patulin induced oxidative stress mediated apoptotic damage in mice, and its modulation by Green tea leaves (GTL). J. Clin. Exp. Hepatol. 2017, 7, 127–134. [Google Scholar] [CrossRef] [PubMed]
- Afzalpour, M.E.; Ghasemi, E.; Zarban, A. Effects of 10 weeks of high intensity interval training and green tea supplementation on serum levels of Sirtuin-1 and peroxisome proliferator-activated receptor gamma co-activator 1-alpha in overweight women. Sci. Sports 2017, 32, 82–90. [Google Scholar] [CrossRef]
- Nibir, Y.M.; Sumit, A.F.; Akhand, A.A.; Ahsan, N.; Hossain, M.S. Comparative assessment of total polyphenols, antioxidant and antimicrobial activity of different tea varieties of Bangladesh. Asian Pac. J. Trop. Biomed. 2017, 7, 352–357. [Google Scholar] [CrossRef]
- Levy, Y.; Narotzki, B.; Reznick, A.Z. Green tea, weight loss and physical activity. Clin. Nutr. 2017, 36, 315. [Google Scholar] [CrossRef] [PubMed]
- Samali, A.; Rukaiyatu, A.; Mustapha, K. Qualitative and quantitative evaluation of some herbal teas commonly consumed in Nigeria. Afr. J. Pharm. Pharmacol. 2012, 6, 384–388. [Google Scholar] [CrossRef]
- McCune, L.M.; Johns, T. Antioxidant activity in medicinal plants associated with the symptoms of diabetes mellitus used by the Indigenous Peoples of the North American boreal forest. J. Ethnopharmacol. 2002, 82, 197–205. [Google Scholar] [CrossRef]
- Barroso, M.F.; Ramalhosa, M.J.; Alves, R.C.; Dias, A.; Soares, C.M.D.; Oliva-Teles, M.T.; Delerue-Matos, C. Total antioxidant capacity of plant infusions: Assessment using electrochemical DNA-based biosensor and spectrophotometric methods. Food Cont. 2016, 68, 153–161. [Google Scholar] [CrossRef]
- Zhao, B.; Guo, Q.; Xin, W. Free radical scavenging by green tea polyphenols. Methods Enzymol. 2001, 335, 217. [Google Scholar]
- Sarkar, A.; Bhaduri, A. Black tea is a powerful chemopreventor of reactive oxygen and nitrogen species: Comparison with its individual catechin constituents and green tea. Biochem. Biophys. Res. Commun. 2001, 284, 173–178. [Google Scholar] [CrossRef] [PubMed]
- Nakagawa, T.; Yokozawa, T. Direct scavenging of nitric oxide and superoxide by green tea. Food Chem. Toxicol. 2002, 40, 1745–1750. [Google Scholar] [CrossRef]
- Cai, Y.-J.; Ma, L.-P.; Hou, L.-F.; Zhou, B.; Yang, L.; Liu, Z.-L. Antioxidant effects of green tea polyphenols on free radical initiated peroxidation of rat liver microsomes. Chem. Phys. Lipids 2002, 120, 109–117. [Google Scholar] [CrossRef]
- Khan, S.G.; Katiyar, S.K.; Agarwal, R.; Mukhtar, H. Enhancement of antioxidant and phase II enzymes by oral feeding of green tea polyphenols in drinking water to SKH-1 hairless mice: Possible role in cancer chemoprevention. Cancer Res. 1992, 52, 4050–4052. [Google Scholar] [PubMed]
- Wanasundara, U.N.; Shahidi, F. Antioxidant and pro-oxidant activity of green tea extracts in marine oils. Food Chem. 1998, 63, 335–342. [Google Scholar] [CrossRef]
- Rashidinejad, A.; Birch, E.J.; Hindmarsh, J.; Everett, D.W. Molecular interactions between green tea catechins and cheese fat studied by solid-state nuclear magnetic resonance spectroscopy. Food Chem. 2017, 215, 228–234. [Google Scholar] [CrossRef]
- Azimi, S.; Mansouri, Z.; Bakhtiari, S.; Tennant, M.; Kruger, E.; Rajabibazl, M.; Daraei, A. Does green tea consumption improve the salivary antioxidant status of smokers? Arch. Oral Biol. 2017, 78, 1–5. [Google Scholar] [CrossRef]
- Hasegawa, R.; Chujo, T.; Sai-Kato, K.; Umemura, T.; Tanimura, A.; Kurokawa, Y. Preventive effects of green tea against liver oxidative DNA damage and hepatotoxicity in rats treated with 2-nitropropane. Food Chem. Toxicol. 1995, 33, 961–970. [Google Scholar] [CrossRef]
- Bu-Abbas, A.; Clifford, M.; Walker, R.; Ioannides, C. Contribution of caffeine and flavanols in the induction of hepatic phase II activities by green tea. Food Chem. Toxicol. 1998, 36, 617–621. [Google Scholar] [CrossRef]
- Lee, S.F.; Liang, Y.C.; Lin, J.K. Inhibition of 1, 2, 4-benzenetriol-generated active oxygen species and induction of phase II enzymes by green tea polyphenols. Chem. Biol. Int. 1995, 98, 283–301. [Google Scholar] [CrossRef]
- Kim, E.; Lee, M.; Kim, S.S.; Kim, J.H.; Jeon, Y.K.; Kim, B.H.; Kim, I.J.; Kim, Y.K. Green tea but not coffee consumption is inversely associated with metabolic syndrome; An epidemiological study in Korean adults. Diabetes Res. Clin. Practice 2016, 120, S85. [Google Scholar] [CrossRef]
- Chen, J.; Wang, P.; Xia, Y.; Xu, M.; Pei, S. Genetic diversity and differentiation of Camellia sinensis L. (cultivated tea) and its wild relatives in Yunnan province of China, revealed by morphology, biochemistry and allozyme studies. Genet. Resour. Crop Evol. 2005, 52, 41–52. [Google Scholar] [CrossRef]
- Sobeh, M.; Mahmoud, M.F.; Petruk, G.; Rezq, S.; Ashour, M.L.; Youssef, F.S.; El-Shazly, A.M.; Monti, D.M.; Abdel-Naim, A.B.; Wink, M. Syzygium aqueum: A polyphenol-rich leaf extract exhibits antioxidant, hepatoprotective, pain-killing and anti-inflammatory activities in animal models. Front. Pharmacol. 2018, 9, 556. [Google Scholar] [CrossRef] [PubMed]
- Sobeh, M.; Youssef, F.S.; Esmat, A.; Petruk, G.; El-Khatib, A.H.; Monti, D.M.; Ashour, M.L.; Wink, M. High resolution UPLC-MS/MS profiling of polyphenolics in the methanol extract of Syzygium samarangense leaves and its hepatoprotective activity in rats with CCl4-induced hepatic damage. Food Chem. Toxicol. 2018, 113, 145–153. [Google Scholar] [CrossRef]
- Hamdaoui, M.H.; Snoussi, C.; Dhaouadi, K.; Fattouch, S.; Ducroc, R.; Le Gall, M.; Bado, A. Tea decoctions prevent body weight gain in rats fed high-fat diet; black tea being more efficient than green tea. J. Nutr. Intermed. Metab. 2016, 6, 33–40. [Google Scholar] [CrossRef]
- Casas-Grajales, S.; Muriel, P. Chapter 43—The Liver, Oxidative Stress, and Antioxidants. In Liver Pathophysiology; Academic Press: Boston, MA, USA, 2017; pp. 583–604. [Google Scholar]
- Li, J.; Sapper, T.N.; Mah, E.; Moller, M.V.; Kim, J.B.; Chitchumroonchokchai, C.; McDonald, J.D.; Bruno, R.S. Green tea extract treatment reduces NFκB activation in mice with diet-induced nonalcoholic steatohepatitis by lowering TNFR1 and TLR4 expression and ligand availability. J. Nutr. Biochem. 2017, 41, 34–41. [Google Scholar] [CrossRef]
- Reddyvari, H.; Govatati, S.; Matha, S.K.; Korla, S.V.; Malempati, S.; Pasupuleti, S.R.; Bhanoori, M.; Nallanchakravarthula, V. Therapeutic effect of green tea extract on alcohol induced hepatic mitochondrial DNA damage in albino wistar rats. J. Adv. Res. 2017, 8, 289–295. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, N.; Cheng, P.; Mukhtar, H. Cell cycle dysregulation by green tea polyphenol epigallocatechin-3-gallate. Biochem. Biophys. Res. Commun. 2000, 275, 328–334. [Google Scholar] [CrossRef]
- Wang, E.-J.; Barecki-Roach, M.; Johnson, W.W. Elevation of P-glycoprotein function by a catechin in green tea. Biochem. Biophys. Res. Commun. 2002, 297, 412–418. [Google Scholar] [CrossRef]
- Embola, C.; Sohn, O.; Fiala, E.; Weisburger, J. Induction of UDP-glucuronosyltransferase 1 (UDP-GT1) gene complex by green tea in male F344 rats. Food Chem. Toxicol. 2002, 40, 841–844. [Google Scholar] [CrossRef]
- Pan, C.-Y.; Kao, Y.-H.; Fox, A.P. Enhancement of inward Ca 2+ currents in bovine chromaffin cells by green tea polyphenol extracts. Neurochem. Int. 2002, 40, 131–137. [Google Scholar] [CrossRef]
- Jha, S.; Kanaujia, S.P.; Limaye, A.M. Direct inhibition of matrix metalloproteinase-2 (MMP-2) by (−)-epigallocatechin-3-gallate: A possible role for the fibronectin type II repeats. Gene 2016, 593, 126–130. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.P.; Schell, J.B.; Ho, C.-T.; Chen, K.Y. Green tea epigallocatechin gallate shows a pronounced growth inhibitory effect on cancerous cells but not on their normal counterparts. Cancer Lett. 1998, 129, 173–179. [Google Scholar] [CrossRef]
- Ahmad, N.; Adhami, V.M.; Gupta, S.; Cheng, P.; Mukhtar, H. Role of the retinoblastoma (PRB)–E2F/DP pathway in cancer chemopreventive effects of green tea polyphenol epigallocatechin-3-gallate. Arch. Biochem. Biophys. 2002, 398, 125–131. [Google Scholar] [CrossRef]
- Wang, X.; Tian, W. Green tea epigallocatechin gallate: A natural inhibitor of fatty-acid synthase. Biochem. Biophys. Res. Commun. 2001, 288, 1200–1206. [Google Scholar] [CrossRef]
- Morbidelli, L. Polyphenol-based nutraceuticals for the control of angiogenesis: Analysis of the critical issues for human use. Pharmacol. Res. 2016, 111, 384–393. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Cao, R.; Bråkenhielm, E. Antiangiogenic mechanisms of diet-derived polyphenols. J. Nutr. Biochem. 2002, 13, 380–390. [Google Scholar] [CrossRef]
- Wei, Y.; Chen, P.; Ling, T.; Wang, Y.; Dong, R.; Zhang, C.; Zhang, L.; Han, M.; Wang, D.; Wan, X.; et al. Certain (−)-epigallocatechin-3-gallate (EGCG) auto-oxidation products (EAOPs) retain the cytotoxic activities of EGCG. Food Chem. 2016, 204, 218–226. [Google Scholar] [CrossRef]
- Park, H.-R.; Hwang, D.; Suh, H.-J.; Yu, K.-W.; Kim, T.Y.; Shin, K.-S. Antitumor and antimetastatic activities of rhamnogalacturonan-II-type polysaccharide isolated from mature leaves of green tea via activation of macrophages and natural killer cells. Int. J. Biol. Macromol. 2017, 99, 179–186. [Google Scholar] [CrossRef]
- Khan, W.; Wang, Z.; Athar, M.; Bickers, D.; Mukhtar, H. Inhibition of the skin tumorigenicity of (k)-7P, 8wdihydroxy-90, IOa-epoxy-7, 8, 9, 10-tetrahydrobenzo [a] pyrene by tannic acid, green tea polyphenols and quercetin in Sencar mice. Cancer Lett. 1988, 42, 7–12. [Google Scholar] [CrossRef]
- Conney, A.H.; Wang, Z.-Y.; Huang, M.-T.; Ho, C.-T.; Yang, C.S. Inhibitory effect of green tea on tumorigenesis by chemicals and ultraviolet light. Prev. Med. 1992, 21, 361–369. [Google Scholar] [CrossRef]
- Katiyar, S.K.; Perez, A.; Mukhtar, H. Green tea polyphenol treatment to human skin prevents formation of ultraviolet light B-induced pyrimidine dimers in DNA. Clin. Cancer Res. 2000, 6, 3864–3869. [Google Scholar] [PubMed]
- Record, I.R.; Dreosti, I.E. Protection by black tea and green tea against UVB and UVA+ B induced skin cancer in hairless mice. Mut. Res. Fundam. Mol. Mech. Mutagenes. 1998, 422, 191–199. [Google Scholar] [CrossRef]
- Liao, B.; Ying, H.; Yu, C.; Fan, Z.; Zhang, W.; Shi, J.; Ying, H.; Ravichandran, N.; Xu, Y.; Yin, J.; et al. (−)-Epigallocatechin gallate (EGCG)-nanoethosomes as a transdermal delivery system for docetaxel to treat implanted human melanoma cell tumors in mice. Int. J. Pharm. 2016, 512, 22–31. [Google Scholar] [CrossRef] [PubMed]
- Uesato, S.; Kitagawa, Y.; Kamishimoto, M.; Kumagai, A.; Hori, H.; Nagasawa, H. Inhibition of green tea catechins against the growth of cancerous human colon and hepatic epithelial cells. Cancer Lett. 2001, 170, 41–44. [Google Scholar] [CrossRef]
- Yang, F.; Oz, H.S.; Barve, S.; De Villiers, W.J.; McClain, C.J.; Varilek, G.W. The green tea polyphenol (−)-epigallocatechin-3-gallate blocks nuclear factor-κB activation by inhibiting IκB kinase activity in the intestinal epithelial cell line IEC-6. Mol. Pharmacol. 2001, 60, 528–533. [Google Scholar] [PubMed]
- Arcone, R.; Palma, M.; Pagliara, V.; Graziani, G.; Masullo, M.; Nardone, G. Green tea polyphenols affect invasiveness of human gastric MKN-28 cells by inhibition of LPS or TNF-α induced Matrix Metalloproteinase-9/2. Biochim. Open 2016, 3, 56–63. [Google Scholar] [CrossRef]
- Zao, J.; Zhu, Q.; Cheng, S. The antagonistic action effects of green tea on micronuclei and apoptosis induced by 1, 2-dimethylhydrazine (1, 2 DMH) in colonic crypt cells of mice. Acta Nutr. Sin. 1992, 14, 251–259. [Google Scholar]
- Matsumoto, H.; Yamane, T.; Inagake, M.; Nakatani, H.; Iwata, Y.; Takahashi, T.; Nishimura, H.; Nishino, H.; Nakagawa, K.; Miyazawa, T. Inhibition of mucosal lipid hyperoxidation by green tea extract in 1, 2-dimethylhydrazine-induced rat colonic carcinogenesis. Cancer Lett. 1996, 104, 205–209. [Google Scholar] [CrossRef]
- Shin, C.M.; Lee, D.H.; Seo, A.Y.; Lee, H.J.; Kim, S.B.; Son, W.-C.; Kim, Y.K.; Lee, S.J.; Park, S.-H.; Kim, N.; et al. Green tea extracts for the prevention of metachronous colorectal polyps among patients who underwent endoscopic removal of colorectal adenomas: A randomized clinical trial. Clin. Nutr. 2017, 37, 452–458. [Google Scholar] [CrossRef]
- Xu, Y.; Ho, C.-T.; Amin, S.G.; Han, C.; Chung, F.-L. Inhibition of tobacco-specific nitrosamine-induced lung tumorigenesis in A/J mice by green tea and its major polyphenol as antioxidants. Cancer Res. 1992, 52, 3875–3879. [Google Scholar] [PubMed]
- Cao, J.; Xu, Y.; Chen, J.; Klaunig, J.E. Chemopreventive effects of green and black tea on pulmonary and hepatic carcinogenesis. Toxicol. Sci. 1996, 29, 244–250. [Google Scholar] [CrossRef]
- Zhu, J.; Jiang, Y.; Yang, X.; Wang, S.; Xie, C.; Li, X.; Li, Y.; Chen, Y.; Wang, X.; Meng, Y.; et al. Wnt/β-catenin pathway mediates (−)-Epigallocatechin-3-gallate (EGCG) inhibition of lung cancer stem cells. Biochem. Biophys. Res. Commun. 2017, 482, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Oya, Y.; Mondal, A.; Rawangkan, A.; Umsumarng, S.; Iida, K.; Watanabe, T.; Kanno, M.; Suzuki, K.; Li, Z.; Kagechika, H.; et al. Down-regulation of histone deacetylase 4, −5 and −6 as a mechanism of synergistic enhancement of apoptosis in human lung cancer cells treated with the combination of a synthetic retinoid, Am80 and green tea catechin. J. Nutr. Biochem. 2017, 42, 7–16. [Google Scholar] [CrossRef] [PubMed]
- Fujie, K.; Aoki, T.; Ito, Y.; Maeda, S. Sister-chromatid exchanges induced by trihalomethanes in rat erythroblastic cells and their suppression by crude catechin extracted from green tea. Mut. Res. Genet. Toxicol. 1993, 300, 241–246. [Google Scholar] [CrossRef]
- Otsuka, T.; Ogo, T.; Eto, T.; Asano, Y.; Suganuma, M.; Niho, Y. Growth inhibition of leukemic cells by (−)-epigallocatechin gallate, the main constituent of green tea. Life Sci. 1998, 63, 1397–1403. [Google Scholar] [CrossRef]
- Lung, H.L.; Ip, W.K.; Wong, C.K.; Mak, N.K.; Chen, Z.Y.; Leung, K.N. Anti-proliferative and differentiation-inducing activities of the green tea catechin epigallocatechin-3-gallate (EGCG) on the human eosinophilic leukemia EoL-1 cell line. Life Sci. 2002, 72, 257–268. [Google Scholar] [CrossRef]
- Cornwall, S.; Cull, G.; Joske, D.; Ghassemifar, R. Green tea polyphenol “epigallocatechin-3-gallate”, differentially induces apoptosis in CLL B-and T-Cells but not in healthy B-and T-Cells in a dose dependant manner. Leuk. Res. 2016, 51, 56–61. [Google Scholar] [CrossRef] [PubMed]
- Parodi, S.; Merlo, D.F.; Stagnaro, E. Coffee and tea consumption and risk of leukaemia in an adult population: A reanalysis of the Italian multicentre case-control study. Cancer Epidemiol. 2017, 47, 81–87. [Google Scholar] [CrossRef] [PubMed]
- Hirose, M.; Hoshiya, T.; Akagi, K.; Futakuchi, M.; Ito, N. Inhibition of mammary gland carcinogenesis by green tea catechins and other naturally occurring antioxidants in female Sprague-Dawley rats pretreated with 7, 12-dimethylbenz [a] anthracene. Cancer Lett. 1994, 83, 149–156. [Google Scholar] [CrossRef]
- Hirose, M.; Mizoguchi, Y.; Yaono, M.; Tanaka, H.; Yamaguchi, T.; Shirai, T. Effects of green tea catechins on the progression or late promotion stage of mammary gland carcinogenesis in female Sprague-Dawley rats pretreated with 7, 12-dimethylbenz (a) anthracene. Cancer Lett. 1997, 112, 141–147. [Google Scholar] [CrossRef]
- Tanaka, H.; Hirose, M.; Kawabe, M.; Sano, M.; Takesada, Y.; Hagiwara, A.; Shirai, T. Post-initiation inhibitory effects of green tea catechins on 7, 12-dimethylbenz [a] anthracene-induced mammary gland carcinogenesis in female Sprague–Dawley rats. Cancer Lett. 1997, 116, 47–52. [Google Scholar] [CrossRef]
- Li, W.; He, N.; Tian, L.; Shi, X.; Yang, X. Inhibitory effects of polyphenol-enriched extract from Ziyang tea against human breast cancer MCF-7 cells through reactive oxygen species-dependent mitochondria molecular mechanism. J. Food Drug Anal. 2016, 24, 527–538. [Google Scholar] [CrossRef] [PubMed]
- Leung, A.C.Y.; Cook, L.S.; Swenerton, K.; Gilks, B.; Gallagher, R.P.; Magliocco, A.; Steed, H.; Köbel, M.; Nation, J.; Brooks-Wilson, A.; et al. Tea, coffee, and caffeinated beverage consumption and risk of epithelial ovarian cancers. Cancer Epidemiol. 2016, 45, 119–125. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Ahmad, N.; Nieminen, A.-L.; Mukhtar, H. Growth inhibition, cell-cycle dysregulation, and induction of apoptosis by green tea constituent (−)-epigallocatechin-3-gallate in androgen-sensitive and androgen-insensitive human prostate carcinoma cells. Toxicol. Appl. Pharmacol. 2000, 164, 82–90. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.I.; Mukhtar, H. Gene expression profile in human prostate LNCaP cancer cells by (−) epigallocatechin-3-gallate. Cancer Lett. 2002, 182, 43–51. [Google Scholar] [CrossRef]
- Hiipakka, R.A.; Zhang, H.-Z.; Dai, W.; Dai, Q.; Liao, S. Structure–activity relationships for inhibition of human 5α-reductases by polyphenols. Biochem. Pharmacol. 2002, 63, 1165–1176. [Google Scholar] [CrossRef]
- Sawada, N. Risk and preventive factors for prostate cancer in Japan: The Japan public health center-based prospective (JPHC) study. J. Epidemiol. 2017, 27, 2–7. [Google Scholar] [CrossRef]
- Carvalho, M.; Jerónimo, C.; Valentão, P.; Andrade, P.B.; Silva, B.M. Green tea: A promising anticancer agent for renal cell carcinoma. Food Chem. 2010, 122, 49–54. [Google Scholar] [CrossRef]
- Annabi, B.; Lachambre, M.-P.; Bousquet-Gagnon, N.; Pagé, M.; Gingras, D.; Béliveau, R. Green tea polyphenol (−)-epigallocatechin 3-gallate inhibits MMP-2 secretion and MT1-MMP-driven migration in glioblastoma cells. Biochim. Biophys. Acta (BBA) Mol. Cell Res. 2002, 1542, 209–220. [Google Scholar] [CrossRef]
- Mazzio, E.A.; Bauer, D.; Mendonca, P.; Taka, E.; Soliman, K.F.A. Natural product HTP screening for attenuation of cytokine-induced neutrophil chemo attractants (CINCs) and NO2—in LPS/IFNγ activated glioma cells. J. Neuroimmunol. 2017, 302, 10–19. [Google Scholar] [CrossRef] [PubMed]
- Burkard, M.; Leischner, C.; Lauer, U.M.; Busch, C.; Venturelli, S.; Frank, J. Dietary flavonoids and modulation of natural killer cells: Implications in malignant and viral diseases. J. Nutr. Biochem. 2017, 46, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Edenharder, R.; Sager, J.W.; Glatt, H.; Muckel, E.; Platt, K.L. Protection by beverages, fruits, vegetables, herbs, and flavonoids against genotoxicity of 2-acetylaminofluorene and 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine (PhIP) in metabolically competent V79 cells. Mut. Res. Genet. Toxicol. Environ. Mut. 2002, 521, 57–72. [Google Scholar] [CrossRef]
- Kada, T.; Kaneko, K.; Matsuzaki, S.; Matsuzaki, T.; Hora, Y. Detection and chemical identification of natural bio-antimutagens: A case of the green tea factor. Mut. Res. Fundam. Mol. Mech. Mutagenes. 1985, 150, 127–132. [Google Scholar] [CrossRef]
- Wang, Z.Y.; Cheng, S.J.; Zhou, Z.C.; Athar, M.; Khan, W.A.; Bickers, D.R.; Mukhtar, H. Antimutagenic activity of green tea polyphenols. Mut. Res. Genet. Toxicol. 1989, 223, 273–285. [Google Scholar] [CrossRef]
- Bu-Abbas, A.; Clifford, M.; Walker, R.; Ioannides, C. Marked antimutagenic potential of aqueous green tea extracts: Mechanism of action. Mutagenesis 1994, 9, 325–331. [Google Scholar] [CrossRef]
- Kuroda, Y. Bio-antimutagenic activity of green tea catechins in cultured Chinese hamster V79 cells. Mut. Res. Environ. Mut. Relat. Subj. 1996, 361, 179–186. [Google Scholar] [CrossRef]
- Okai, Y.; Higashi-Okai, K. Potent suppressing activity of the non-polyphenolic fraction of green tea (Camellia sinensis) against genotoxin-induced umu C gene expression in Salmonella typhimurium (TA 1535/pSK 1002)–association with pheophytins a and b. Cancer Lett. 1997, 120, 117–123. [Google Scholar] [CrossRef]
- Gupta, S.; Saha, B.; Giri, A.K. Comparative antimutagenic and anticlastogenic effects of green tea and black tea: A review. Mut. Res. Rev. Mut. Res. 2002, 512, 37–65. [Google Scholar] [CrossRef]
- Ikigai, H.; Nakae, T.; Hara, Y.; Shimamura, T. Bactericidal catechins damage the lipid bilayer. Biochim. Biophys. Acta (BBA) Biomembr. 1993, 1147, 132–136. [Google Scholar] [CrossRef]
- Rasheed, A.; Haider, M. Antibacterial activity of Camellia sinensis extracts against dental caries. Arch. Pharm. Res. 1998, 21, 348–352. [Google Scholar] [CrossRef] [PubMed]
- Sugita-Konishi, Y.; Hara-Kudo, Y.; Amano, F.; Okubo, T.; Aoi, N.; Iwaki, M.; Kumagai, S. Epigallocatechin gallate and gallocatechin gallate in green tea catechins inhibit extracellular release of Vero toxin from enterohemorrhagic Escherichia coli O157: H7. Biochim. Biophys. Acta (BBA) Gen. Subj. 1999, 1472, 42–50. [Google Scholar] [CrossRef]
- Hui, X.; Liu, H.; Tian, F.-L.; Li, F.-F.; Li, H.; Gao, W.-Y. Inhibition of green tea and the catechins against 1-deoxy-d-xylulose 5-phosphate reductoisomerase, the key enzyme of the MEP terpenoid biosynthetic pathway. Fitoterapia 2016, 113, 80–84. [Google Scholar] [CrossRef]
- Dyer, P.D.R.; Kotha, A.K.; Gollings, A.S.; Shorter, S.A.; Shepherd, T.R.; Pettit, M.W.; Alexander, B.D.; Getti, G.T.M.; El-Daher, S.; Baillie, L.; et al. An in vitro evaluation of epigallocatechin gallate (eGCG) as a biocompatible inhibitor of ricin toxin. Biochim. Biophys. Acta (BBA) Gen. Subj. 2016, 1860, 1541–1550. [Google Scholar] [CrossRef] [PubMed]
- Rizk, M.A.; El-Sayed, S.A.E.-S.; AbouLaila, M.; Tuvshintulga, B.; Yokoyama, N.; Igarashi, I. Large-scale drug screening against Babesia divergens parasite using a fluorescence-based high-throughput screening assay. Vet. Parasitol. 2016, 227, 93–97. [Google Scholar] [CrossRef]
- Tsou, L.K.; Yount, J.S.; Hang, H.C. Epigallocatechin-3-gallate inhibits bacterial virulence and invasion of host cells. Bioorg. Med. Chem. 2017, 25, 2883–2887. [Google Scholar] [CrossRef]
- Piva, G.; Fracassetti, D.; Tirelli, A.; Mascheroni, E.; Musatti, A.; Inglese, P.; Piergiovanni, L.; Rollini, M. Evaluation of the antioxidant/antimicrobial performance of Posidonia oceanica in comparison with three commercial natural extracts and as a treatment on fresh-cut peaches (Prunus persica Batsch). Postharvest Biol. Technol. 2017, 124, 54–61. [Google Scholar] [CrossRef]
- Sakanaka, S.; Juneja, L.R.; Taniguchi, M. Antimicrobial effects of green tea polyphenols on thermophilic spore-forming bacteria. J. Biosci. Bioeng. 2000, 90, 81–85. [Google Scholar] [CrossRef]
- Yun, J.; Lee, D.G. Role of potassium channels in chlorogenic acid-induced apoptotic volume decrease and cell cycle arrest in Candida albicans. Biochim. Biophys. Acta (BBA) Gen. Subj. 2017, 1861, 585–592. [Google Scholar] [CrossRef]
- Carneiro, B.M.; Batista, M.N.; Braga, A.C.S.; Nogueira, M.L.; Rahal, P. The green tea molecule EGCG inhibits Zika virus entry. Virology 2016, 496, 215–218. [Google Scholar] [CrossRef]
- Hartjen, P.; Frerk, S.; Hauber, I.; Matzat, V.; Thomssen, A.; Holstermann, B.; Hohenberg, H.; Schulze, W.; zur Wiesch, J.S.; van Lunzen, J. Assessment of the range of the HIV-1 infectivity enhancing effect of individual human semen specimen and the range of inhibition by EGCG. AIDS Res. Ther. 2012, 9, 2. [Google Scholar] [CrossRef]
- Bin Dajem, S.M.; Shati, A.A.; Adly, M.A.; Ahmed, O.M.; Ibrahim, E.H.; Mostafa, O.M.S. Green tea (Camellia sinesis) ameliorates female Schistosoma mansoni-induced changes in the liver of Balb/C mice. Saudi J. Biol. Sci. 2011, 18, 361–368. [Google Scholar] [CrossRef] [PubMed]
- Wickramasinghe, S.; Yatawara, L.; Nagataki, M.; Agatsuma, T. Arginine kinase in Toxocara canis: Exon–intron organization, functional analysis of site-directed mutants and evaluation of putative enzyme inhibitors. Asian Pac. J. Trop. Med. 2016, 9, 995–1001. [Google Scholar] [CrossRef] [PubMed]
- Vinson, J.A.; Dabbagh, Y.A. Effect of green and black tea supplementation on lipids, lipid oxidation and fibrinogen in the hamster: Mechanisms for the epidemiological benefits of tea drinking. FEBS Lett. 1998, 433, 44–46. [Google Scholar] [CrossRef]
- Hartley, L.; Flowers, N.; Holmes, J.; Clarke, A.; Stranges, S.; Hooper, L.; Rees, K. Green and Black Tea for the Primary Prevention of Cardiovascular Disease; The Cochrane Library: Bethesda, MD, USA, 2013. [Google Scholar] [CrossRef]
- Wasilewski, R.; Ubara, E.O.; Klonizakis, M. Assessing the effects of a short-term green tea intervention in skin microvascular function and oxygen tension in older and younger adults. Microvasc. Res. 2016, 107, 65–71. [Google Scholar] [CrossRef]
- Yang, T.; Koo, M. Inhibitory effect of Chinese green tea on endothelial cell-induced LDL oxidation. Atherosclerosis 2000, 148, 67–73. [Google Scholar] [CrossRef]
- Song, D.U.; Do Jung, Y.; Chay, K.O.; Chung, M.A.; Lee, K.H.; Yang, S.Y.; Shin, B.A.; Ahn, B.W. Effect of drinking green tea on age-associated accumulation of Maillard-type fluorescence and carbonyl groups in rat aortic and skin collagen. Arch. Biochem. Biophys. 2002, 397, 424–429. [Google Scholar] [CrossRef]
- Pang, J.; Zhang, Z.; Zheng, T.-z.; Bassig, B.A.; Mao, C.; Liu, X.; Zhu, Y.; Shi, K.; Ge, J.; Yang, Y.-j. Green tea consumption and risk of cardiovascular and ischemic related diseases: A meta-analysis. Int. J. Cardiol. 2016, 202, 967–974. [Google Scholar] [CrossRef]
- Lustosa, B.B.; Polegato, B.; Minicucci, M.; Rafacho, B.; Santos, P.P.; Fernandes, A.A.; Okoshi, K.; Batista, D.; Modesto, P.; Gonçalves, A.; et al. Green tea (Cammellia sinensis) attenuates ventricular remodeling after experimental myocardial infarction. Int. J. Cardiol. 2016, 225, 147–153. [Google Scholar] [CrossRef]
- Bordoni, A.; Hrelia, S.; Angeloni, C.; Giordano, E.; Guarnieri, C.; Caldarera, C.M.; Biagi, P.L. Green tea protection of hypoxia/reoxygenation injury in cultured cardiac cells. J. Nutr. Biochem. 2002, 13, 103–111. [Google Scholar] [CrossRef]
- Cheng, T.O. All teas are not created equal: The Chinese green tea and cardiovascular health. Int. J. Cardiol. 2006, 108, 301–308. [Google Scholar] [CrossRef] [PubMed]
- Sun, T.-L.; Liu, Z.; Qi, Z.-J.; Huang, Y.-P.; Gao, X.-Q.; Zhang, Y.-Y. (−)-Epigallocatechin-3-gallate (EGCG) attenuates arsenic-induced cardiotoxicity in rats. Food Chem. Toxicol. 2016, 93, 102–110. [Google Scholar] [CrossRef] [PubMed]
- Oyama, J.; Shiraki, A.; Nishikido, T.; Maeda, T.; Komoda, H.; Shimizu, T.; Makino, N.; Node, K. EGCG, a green tea catechin, attenuates the progression of heart failure induced by the heart/muscle-specific deletion of MnSOD in mice. Int. J. Cardiol. 2017, 69, 417–427. [Google Scholar] [CrossRef] [PubMed]
- Ale-Agha, N.; Stahl, W.; Sies, H. (−)-Epicatechin effects in rat liver epithelial cells: Stimulation of gap junctional communication and counteraction of its loss due to the tumor promoter 12-O-tetradecanoylphorbol-13-acetate. Biochem. Pharmacol. 2002, 63, 2145–2149. [Google Scholar] [CrossRef]
- Dower, J.I.; Geleijnse, J.M.; Gijsbers, L.; Zock, P.L.; Kromhout, D.; Hollman, P.C. Effects of the pure flavonoids epicatechin and quercetin on vascular function and cardiometabolic health: A randomized, double-blind, placebo-controlled, crossover trial. Am. J. Clin. Nutr. 2015, 101, 914–921. [Google Scholar] [CrossRef]
- Keiichiro, M.; Mayumi, F.; Hara, Y. Effect of green tea catechins on plasma cholesterol level in cholesterol-fed rats. J. Nutr. Sci. Vitaminol. 1986, 32, 613–622. [Google Scholar]
- Yang, T.T.; Koo, M.W. Chinese green tea lowers cholesterol level through an increase in fecal lipid excretion. Life Sci. 1999, 66, 411–423. [Google Scholar] [CrossRef]
- Ali, M.; Afzal, M.; Gubler, C.; Burka, J.F. A potent thromboxane formation inhibitor in green tea leaves. Prostaglandins Leukot. Essent. Fat. Acids 1990, 40, 281–283. [Google Scholar] [CrossRef]
- Abe, I.; Seki, T.; Umehara, K.; Miyase, T.; Noguchi, H.; Sakakibara, J.; Ono, T. Green tea polyphenols: Novel and potent inhibitors of squalene epoxidase. Biochem. Biophys. Res. Commun. 2000, 268, 767–771. [Google Scholar] [CrossRef]
- Kasaoka, S.; Hase, K.; Morita, T.; Kiriyama, S. Green tea flavonoids inhibit the LDL oxidation in osteogenic disordered rats fed a marginal ascorbic acid in diet. J. Nutr. Biochem. 2002, 13, 96–102. [Google Scholar] [CrossRef]
- Yoshikawa, M.; Morikawa, T.; Yamamoto, K.; Kato, Y.; Nagatomo, A.; Matsuda, H. Floratheasaponins A−C, acylated oleanane-type triterpene oligoglycosides with anti-hyperlipidemic activities from flowers of the Tea plant (Camellia sinensis). J. Nat. Prod. 2005, 68, 1360–1365. [Google Scholar] [CrossRef] [PubMed]
- Abe, Y.; Umemura, S.; Sugimoto, K.-I.; Hirawa, N.; Kato, Y.; Yokoyama, N.; Yokoyama, T.; Iwai, J.; Ishii, M. Effect of green tea rich in γ-aminobutyric acid on blood pressure of Dahl salt-sensitive rats. Am. J. Hypertens. 1995, 8, 74–79. [Google Scholar] [CrossRef]
- Juneja, L.R.; Chu, D.-C.; Okubo, T.; Nagato, Y.; Yokogoshi, H. L-theanine—A unique amino acid of green tea and its relaxation effect in humans. Trends Food Sci. Technol. 1999, 10, 199–204. [Google Scholar] [CrossRef]
- Bertoldi, M.; Gonsalvi, M.; Voltattorni, C.B. Green tea polyphenols: Novel irreversible inhibitors of dopa decarboxylase. Biochem. Biophys. Res. Commun. 2001, 284, 90–93. [Google Scholar] [CrossRef] [PubMed]
- Yi, Q.-Y.; Li, H.-B.; Qi, J.; Yu, X.-J.; Huo, C.-J.; Li, X.; Bai, J.; Gao, H.-L.; Kou, B.; Liu, K.-L.; et al. Chronic infusion of epigallocatechin-3-O-gallate into the hypothalamic paraventricular nucleus attenuates hypertension and sympathoexcitation by restoring neurotransmitters and cytokines. Toxicol. Lett. 2016, 262, 105–113. [Google Scholar] [CrossRef] [PubMed]
- Antonello, M.; Montemurro, D.; Bolognesi, M.; Di Pascoli, M.; Piva, A.; Grego, F.; Sticchi, D.; Giuliani, L.; Garbisa, S.; Rossi, G.P. Prevention of hypertension, cardiovascular damage and endothelial dysfunction with green tea extracts. Am. J. Hypertens. 2007, 20, 1321–1328. [Google Scholar] [CrossRef]
- Ihm, S.-H.; Jang, S.-W.; Kim, O.-R.; Chang, K.; Oak, M.-H.; Lee, J.-O.; Lim, D.-Y.; Kim, J.-H. Decaffeinated green tea extract improves hypertension and insulin resistance in a rat model of metabolic syndrome. Atherosclerosis 2012, 224, 377–383. [Google Scholar] [CrossRef]
- Kang, W.-S.; Lim, I.-H.; Yuk, D.-Y.; Chung, K.-H.; Park, J.-B.; Yoo, H.-S.; Yun, Y.-P. Antithrombotic activities of green tea catechins and (−)-epigallocatechin gallate. Thromb. Res. 1999, 96, 229–237. [Google Scholar] [CrossRef]
- Lau, K.-M.; He, Z.-D.; Dong, H.; Fung, K.-P.; But, P.P.-H. Anti-oxidative, anti-inflammatory and hepato-protective effects of Ligustrum robustum. J. Ethnopharmacol. 2002, 83, 63–71. [Google Scholar] [CrossRef]
- Ohnishi, S.T.; Ohnishi, T.; Ogunmola, G.B. Green tea extract and aged garlic extract inhibit anion transport and sickle cell dehydration in vitro. Blood Cells Mol. Dis. 2001, 27, 148–157. [Google Scholar] [CrossRef]
- Peixoto, E.B.; Papadimitriou, A.; Teixeira, D.A.T.; Montemurro, C.; Duarte, D.A.; Silva, K.C.; Joazeiro, P.P.; Lopes de Faria, J.M.; Lopes de Faria, J.B. Reduced LRP6 expression and increase in the interaction of GSK3β with p53 contribute to podocyte apoptosis in diabetes mellitus and are prevented by green tea. J. Nutr. Biochem. 2015, 26, 416–430. [Google Scholar] [CrossRef] [PubMed]
- Sampath, C.; Rashid, M.R.; Sang, S.; Ahmedna, M. Green tea epigallocatechin 3-gallate alleviates hyperglycemia and reduces advanced glycation end products via nrf2 pathway in mice with high fat diet-induced obesity. Biomed. Pharmacother. 2017, 87, 73–81. [Google Scholar] [CrossRef]
- Pournourmohammadi, S.; Grimaldi, M.; Stridh, M.H.; Lavallard, V.; Waagepetersen, H.S.; Wollheim, C.B.; Maechler, P. Epigallocatechin-3-gallate (EGCG) activates AMPK through the inhibition of glutamate dehydrogenase in muscle and pancreatic ß-cells: A potential beneficial effect in the pre-diabetic state? Int. J. Biochem. Cell Biol. 2017, 88, 220–225. [Google Scholar] [CrossRef] [PubMed]
- Sabu, M.; Smitha, K.; Kuttan, R. Anti-diabetic activity of green tea polyphenols and their role in reducing oxidative stress in experimental diabetes. J. Ethnopharmacol. 2002, 83, 109–116. [Google Scholar] [PubMed]
- Yoshikawa, M.; Wang, T.; Sugimoto, S.; Nakamura, S.; Nagatomo, A.; Matsuda, H.; Harima, S. Functional saponins in tea flower (flower buds of Camellia sinensis): Gastroprotective and hypoglycemic effects of floratheasaponins and qualitative and quantitative analysis using HPLC. J. Pharm. Soc. Jpn. 2008, 128, 141–151. [Google Scholar] [CrossRef]
- Han, Q.; Yu, Q.-Y.; Shi, J.; Xiong, C.-Y.; Ling, Z.-J.; He, P.-M. Molecular characterization and hypoglycemic activity of a novel water-soluble polysaccharide from tea (Camellia sinensis) flower. Carbohydr. Polym. 2011, 86, 797–805. [Google Scholar] [CrossRef]
- Sun, L.; Warren, F.J.; Netzel, G.; Gidley, M.J. 3 or 3′-Galloyl substitution plays an important role in association of catechins and theaflavins with porcine pancreatic α-amylase: The kinetics of inhibition of α-amylase by tea polyphenols. J. Funct. Foods. 2016, 26, 144–156. [Google Scholar] [CrossRef]
- Juhel, C.; Armand, M.; Pafumi, Y.; Rosier, C.; Vandermander, J.; Lairon, D. Green tea extract (AR25®) inhibits lipolysis of triglycerides in gastric and duodenal medium in vitro. J. Nutr. Biochem. 2000, 11, 45–51. [Google Scholar] [CrossRef]
- Diepvens, K.; Kovacs, E.; Vogels, N.; Westerterp-Plantenga, M. Metabolic effects of green tea and of phases of weight loss. Physiol. Behav. 2006, 87, 185–191. [Google Scholar] [CrossRef]
- Janssens, P.L.H.R.; Hursel, R.; Westerterp-Plantenga, M.S. Nutraceuticals for body-weight management: The role of green tea catechins. Physiol. Behav. 2016, 162, 83–87. [Google Scholar] [CrossRef]
- Loftus, T.M.; Jaworsky, D.E.; Frehywot, G.L.; Townsend, C.A.; Ronnett, G.V.; Lane, M.D.; Kuhajda, F.P. Reduced food intake and body weight in mice treated with fatty acid synthase inhibitors. Science 2000, 288, 2379–2381. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Liu, D.; Zeng, R.; Xian, T.; Lu, Y.; Zeng, G.; Sun, Z.; Huang, B.; Huang, Q. Epigallocatechin-3-gallate inhibits adipogenesis through down-regulation of PPARγ and FAS expression mediated by PI3K-AKT signaling in 3T3-L1 cells. Eur. J. Pharmacol. 2017, 795, 134–142. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Chan, N.W.K.; Lau, C.W.; Yao, X.Q.; Chan, F.L.; Chen, Z.Y. Involvement of endothelium/nitric oxide in vasorelaxation induced by purified green tea (−) epicatechin. Biochim. Biophys Acta (BBA) Gen. Subj. 1999, 1427, 322–328. [Google Scholar] [CrossRef]
- Setozaki, S.; Minakata, K.; Masumoto, H.; Hirao, S.; Yamazaki, K.; Kuwahara, K.; Ikeda, T.; Sakata, R. Prevention of abdominal aortic aneurysm progression by oral administration of green tea polyphenol in a rat model. J. Vasc. Surg. 2016, 65, 1803–1812. [Google Scholar] [CrossRef]
- Shibata, K.; Moriyama, M.; Fukushima, T.; Kaetsu, A.; Miyazaki, M.; Une, H. Green tea consumption and chronic atrophic gastritis: A cross-sectional study in a green tea production village. J. Epidemiol. 2000, 10, 310–316. [Google Scholar] [CrossRef] [PubMed]
- Ahn, Y.-J.; Kawamura, T.; Kim, M.; Yamamoto, T.; Mitsuoka, T. Tea polyphenols: Selective growth inhibitors of Clostridium spp. Agric. Biol. Chem. 1991, 55, 1425–1426. [Google Scholar] [CrossRef]
- Jung, E.S.; Park, H.M.; Hyun, S.M.; Shon, J.-C.; Liu, K.-H.; Hwang, J.S.; Lee, C.H. Modulation of gut microbiome by green tea diet and its correlation with metabolic changes. Drug Metab. Pharmacokinet. 2017, 32, S62. [Google Scholar] [CrossRef]
- Hong, J.T.; Ryu, S.R.; Kim, H.J.; Lee, J.K.; Lee, S.H.; Kim, D.B.; Yun, Y.P.; Ryu, J.H.; Lee, B.M.; Kim, P.Y. Neuroprotective effect of green tea extract in experimental ischemia-reperfusion brain injury. Brain Res. Bull. 2000, 53, 743–749. [Google Scholar] [CrossRef]
- Guo, Q.; Zhao, B.; Li, M.; Shen, S.; Xin, W. Studies on protective mechanisms of four components of green tea polyphenols against lipid peroxidation in synaptosomes. Biochim. Biophys. Acta (BBA) Lipid Lipid Metab. 1996, 1304, 210–222. [Google Scholar] [CrossRef]
- Zhao, X.; Liu, F.; Jin, H.; Li, R.; Wang, Y.; Zhang, W.; Wang, H.; Chen, W. Involvement of PKCα and ERK1/2 signaling pathways in EGCG’s protection against stress-induced neural injuries in Wistar rats. Neuroscience 2017, 346, 226–237. [Google Scholar] [CrossRef]
- Suganuma, M.; Okabe, S.; Oniyama, M.; Tada, Y.; Ito, H.; Fujiki, H. Wide distribution of [3H](-)-epigallocatechin gallate, a cancer preventive tea polyphenol, in mouse tissue. Carcinogenesis 1998, 19, 1771–1776. [Google Scholar] [CrossRef] [PubMed]
- Nagai, K.; Jiang, M.H.; Hada, J.; Nagata, T.; Yajima, Y.; Yamamoto, S.; Nishizaki, T. (−)-Epigallocatechin gallate protects against NO stress-induced neuronal damage after ischemia by acting as an anti-oxidant. Brain Res. 2002, 956, 319–322. [Google Scholar] [CrossRef]
- Bai, Q.; Lyu, Z.; Yang, X.; Pan, Z.; Lou, J.; Dong, T. Epigallocatechin-3-gallate promotes angiogenesis via up-regulation of Nfr2 signaling pathway in a mouse model of ischemic stroke. Behav. Brain Res. 2017, 321, 79–86. [Google Scholar] [CrossRef] [PubMed]
- Ben, P.; Zhang, Z.; Zhu, Y.; Xiong, A.; Gao, Y.; Mu, J.; Yin, Z.; Luo, L. l-Theanine attenuates cadmium-induced neurotoxicity through the inhibition of oxidative damage and tau hyperphosphorylation. Neurotoxicology 2016, 57, 95–103. [Google Scholar] [CrossRef] [PubMed]
- Levites, Y.; Youdim, M.B.H.; Maor, G.; Mandel, S. Attenuation of 6-hydroxydopamine (6-OHDA)-induced nuclear factor-kappaB (NF-κB) activation and cell death by tea extracts in neuronal cultures1. Biochem. Pharmacol. 2002, 63, 21–29. [Google Scholar] [CrossRef]
- Weinreb, O.; Mandel, S.; Amit, T.; Youdim, M.B. Neurological mechanisms of green tea polyphenols in Alzheimer’s and Parkinson’s diseases. J. Nutr. Biochem. 2004, 15, 506–516. [Google Scholar] [CrossRef]
- Unno, K.; Takabayashi, F.; Kishido, T.; Oku, N. Suppressive effect of green tea catechins on morphologic and functional regression of the brain in aged mice with accelerated senescence (SAMP10). Exp. Gerontol. 2004, 39, 1027–1034. [Google Scholar] [CrossRef]
- McConnell, J.S.; McConnell, R.; Hossner, L. Ultraviolet spectra of acetic acid, glycine, and glyphosate. Proc. Ark. Acad. Sci. 1993, 47, 73–76. [Google Scholar]
- Choi, J.-Y.; Park, C.-S.; Kim, D.-J.; Cho, M.-H.; Jin, B.-K.; Pie, J.-E.; Chung, W.-G. Prevention of nitric oxide-mediated 1-methyl-4-phenyl-1, 2, 3, 6-tetrahydropyridine-induced Parkinson’s disease in mice by tea phenolic epigallocatechin 3-gallate. Neurotoxicology 2002, 23, 367–374. [Google Scholar] [CrossRef]
- Choi, Y.-T.; Jung, C.-H.; Lee, S.-R.; Bae, J.-H.; Baek, W.-K.; Suh, M.-H.; Park, J.; Park, C.-W.; Suh, S.-I. The green tea polyphenol (−)-epigallocatechin gallate attenuates β-amyloid-induced neurotoxicity in cultured hippocampal neurons. Life Sci. 2001, 70, 603–614. [Google Scholar] [CrossRef]
- Selkoe, D.J. Deciphering Alzheimer’s disease: The amyloid precursor protein yields new clues. Science 1990, 248, 1058–1060. [Google Scholar] [CrossRef] [PubMed]
- Jeon, S.-Y.; Bae, K.; Seong, Y.-H.; Song, K.-S. Green tea catechins as a BACE1 (β-Secretase) inhibitor. Bioorg. Med. Chem. Lett. 2003, 13, 3905–3908. [Google Scholar] [CrossRef] [PubMed]
- Geiser, R.J.; Chastain, S.E.; Moss, M.A. Regulation of Bace1 Mrna Expression in Alzheimer’S Disease by Green Tea Catechins and Black Tea Theaflavins. Biophys. J. 2017, 112, 362a. [Google Scholar] [CrossRef]
- Kaur, T.; Pathak, C.M.; Pandhi, P.; Khanduja, K.L. Effects of green tea extract on learning, memory, behavior and acetylcholinesterase activity in young and old male rats. Brain Cogn. 2008, 67, 25–30. [Google Scholar] [CrossRef] [PubMed]
- Molino, S.; Dossena, M.; Buonocore, D.; Ferrari, F.; Venturini, L.; Ricevuti, G.; Verri, M. Polyphenols in dementia: From molecular basis to clinical trials. Life Sci. 2016, 161, 69–77. [Google Scholar] [CrossRef] [PubMed]
- Pervin, M.; Unno, K.; Nakagawa, A.; Takahashi, Y.; Iguchi, K.; Yamamoto, H.; Hoshino, M.; Hara, A.; Takagaki, A.; Nanjo, F.; et al. Blood brain barrier permeability of (−)-epigallocatechin gallate, its proliferation-enhancing activity of human neuroblastoma SH-SY5Y cells, and its preventive effect on age-related cognitive dysfunction in mice. Biochem. Biophys. Rep. 2017, 9, 180–186. [Google Scholar] [CrossRef] [PubMed]
- Unno, K.; Hara, A.; Nakagawa, A.; Iguchi, K.; Ohshio, M.; Morita, A.; Nakamura, Y. Anti-stress effects of drinking green tea with lowered caffeine and enriched theanine, epigallocatechin and arginine on psychosocial stress induced adrenal hypertrophy in mice. Phytomedicine 2016, 23, 1365–1374. [Google Scholar] [CrossRef]
- Wang, Z.Y.; Khan, W.A.; Bickers, D.R.; Mukhtar, H. Protection against polycyclic aromatic hydrocarbon-induced skin tumor initiation in mice by green tea polyphenols. Carcinogenesis 1989, 10, 411–415. [Google Scholar] [CrossRef]
- Yang, F.; De Villiers, W.J.; McClain, C.J.; Varilek, G.W. Green tea polyphenols block endotoxin-induced tumor necrosis factor-production and lethality in a murine model. J. Nutr. 1998, 128, 2334–2340. [Google Scholar] [CrossRef]
- Chan, M.M.-Y.; Fong, D.; Ho, C.-T.; Huang, H.-I. Inhibition of inducible nitric oxide synthase gene expression and enzyme activity by epigallocatechin gallate, a natural product from green tea. Biochem. Pharmacol. 1997, 54, 1281–1286. [Google Scholar] [CrossRef]
- Higashi-Okai, K.; Otani, S.; Okai, Y. Potent suppressive activity of pheophytin a and b from the non-polyphenolic fraction of green tea (Camellia sinensis) against tumor promotion in mouse skin. Cancer Lett. 1998, 129, 223–228. [Google Scholar] [CrossRef]
- Ashraf, A.; Sadrneshin, S.; Mosavat, S.H.; Hashempur, M.H. Efficacy of the green tea (Camellia sinensis) tablet in knee osteoarthritis. Eur. J. Integr. Med. 2016, 8, 10–11. [Google Scholar] [CrossRef]
- Maeda, Y. Inhibitory effects of tea extracts on histamine release from mast cells. J. Jpn. Food Hyg. 1980, 30, 295–299. [Google Scholar] [CrossRef]
- Akagi, M.; Fukuishi, N.; Tomoko, K.; SAGESAKA, Y.M.; Akagi, R. Anti-allergic effect of tea-leaf saponin (TLS) from tea leaves (Camellia sinensis var. sinensis). Biol. Pharm. Bull. 1997, 20, 565–567. [Google Scholar] [CrossRef] [PubMed]
- Yoshikawa, M.; Nakamura, S.; Kato, Y.; Matsuhira, K.; Matsuda, H. Medicinal Flowers. XIV. 1) New acylated oleanane-type triterpene oligoglycosides with antiallergic activity from flower buds of chinese tea plant (Camellia sinensis). Chem. Pharm. Bull. 2007, 55, 598–605. [Google Scholar] [CrossRef] [PubMed]
- Pandit, C.; Anilakumar, K.R. Cold adaptive thermogenesis following consumption of certain pungent spice principles: A validation study. J. Therm. Biol. 2017, 64, 35–40. [Google Scholar] [CrossRef]
- Ng, H.L.H.; Premilovac, D.; Rattigan, S.; Richards, S.M.; Muniyappa, R.; Quon, M.J.; Keske, M.A. Acute vascular and metabolic actions of the green tea polyphenol epigallocatechin 3-gallate in rat skeletal muscle. J. Nutr. Biochem. 2017, 40, 23–31. [Google Scholar] [CrossRef]
- Kim, A.R.; Kim, K.M.; Byun, M.R.; Hwang, J.-H.; Park, J.I.; Oh, H.T.; Jeong, M.G.; Hwang, E.S.; Hong, J.-H. (−)-Epigallocatechin-3-gallate stimulates myogenic differentiation through TAZ activation. Biochem. Biophys. Res. Commun. 2017, 29, 378–384. [Google Scholar] [CrossRef]
- Takahashi, H.; Suzuki, Y.; Mohamed, J.S.; Gotoh, T.; Pereira, S.L.; Alway, S.E. Epigallocatechin-3-gallate increases autophagy signaling in resting and unloaded plantaris muscles but selectively suppresses autophagy protein abundance in reloaded muscles of aged rats. Exp. Gerontol. 2017, 92, 55–56. [Google Scholar] [CrossRef]
- Nakagawa, H.; Wachi, M.; Woo, J.-T.; Kato, M.; Kasai, S.; Takahashi, F.; Lee, I.-S.; Nagai, K. Fenton reaction is primarily involved in a mechanism of (−)-epigallocatechin-3-gallate to induce osteoclastic cell death. Biochem. Biophys. Res. Commun. 2002, 292, 94–101. [Google Scholar] [CrossRef]
- Chu, C.; Deng, J.; Xiang, L.; Wu, Y.; Wei, X.; Qu, Y.; Man, Y. Evaluation of epigallocatechin-3-gallate (EGCG) cross-linked collagen membranes and concerns on osteoblasts. Mater. Sci. Eng. C 2016, 67, 386–394. [Google Scholar] [CrossRef] [PubMed]
- Tomaszewska, E.; Dobrowolski, P.; Winiarska-Mieczan, A.; Kwiecień, M.; Tomczyk, A.; Muszyński, S.; Radzki, R. Alteration in bone geometric and mechanical properties, histomorphometrical parameters of trabecular bone, articular cartilage, and growth plate in adolescent rats after chronic co-exposure to cadmium and lead in the case of supplementation with green, black, red and white tea. Environ. Toxicol. Pharmacol. 2016, 46, 36–44. [Google Scholar] [PubMed]
- Kuroyanagi, G.; Tokuda, H.; Yamamoto, N.; Kainuma, S.; Fujita, K.; Ohguchi, R.; Kawabata, T.; Sakai, G.; Matsushima-Nishiwaki, R.; Harada, A.; et al. (−)-Epigallocatechin gallate synergistically potentiates prostaglandin E2-stimulated osteoprotegerin synthesis in osteoblasts. Prostaglandins Lipid Med. 2017, 128–129, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, S.; Rahman, A.; Hasnain, A.; Lalonde, M.; Goldberg, V.M.; Haqqi, T.M. Green tea polyphenol epigallocatechin-3-gallate inhibits the IL-1β-induced activity and expression of cyclooxygenase-2 and nitric oxide synthase-2 in human chondrocytes. Free Radic. Biol. Med. 2002, 33, 1097–1105. [Google Scholar] [CrossRef]
- Hashempur, M.H.; Sadrneshin, S.; Mosavat, S.H.; Ashraf, A. Green tea (Camellia sinensis) for patients with knee osteoarthritis: A randomized open-label active-controlled clinical trial. Clin. Nutr. 2016, 37, 85–90. [Google Scholar] [CrossRef] [PubMed]
- Horiba, N.; Maekawa, Y.; Ito, M.; Matsumoto, T.; Nakamura, H. A pilot study of Japanese green tea as a medicament: Antibacterial and bactericidal effects. J. Endod. 1991, 17, 122–124. [Google Scholar] [CrossRef]
- You, S. Study on feasibility of Chinese green tea polyphenols (CTP) for preventing dental caries. Chin. J. Stomatol. 1993, 28, 197–199. [Google Scholar]
- Kawarai, T.; Narisawa, N.; Yoneda, S.; Tsutsumi, Y.; Ishikawa, J.; Hoshino, Y.; Senpuku, H. Inhibition of Streptococcus mutans biofilm formation using extracts from Assam tea compared to green tea. Arch. Oral Biol. 2016, 68, 73–82. [Google Scholar] [CrossRef]
- Abdulbaqi, H.R.; Himratul-Aznita, W.H.; Baharuddin, N.A. Anti-plaque effect of a synergistic combination of green tea and Salvadora persica L. against primary colonizers of dental plaque. Arch. Oral Biol. 2016, 70, 117–124. [Google Scholar] [CrossRef]
- Kim-Park, W.K.; Allam, E.S.; Palasuk, J.; Kowolik, M.; Park, K.K.; Windsor, L.J. Green tea catechin inhibits the activity and neutrophil release of Matrix Metalloproteinase-9. J. Tradit. Complement. Med. 2016, 6, 343–346. [Google Scholar] [CrossRef]
- Morin, M.-P.; Grenier, D. Regulation of matrix metalloproteinase secretion by green tea catechins in a three-dimensional co-culture model of macrophages and gingival fibroblasts. Arch. Oral Biol. 2017, 75, 89–99. [Google Scholar] [CrossRef]
- Hu, S.; Zhang, X.; Chen, F.; Wang, M. Dietary polyphenols as photoprotective agents against UV radiation. J. Funct. Foods 2017, 30, 108–118. [Google Scholar] [CrossRef]
- Jongil, K.; Jaesung, H.; Younki, C.; Yongku, H.; Youngjin, J.; Yang, K.-H. Protective effects of green tea polyphenols on the ultraviolet-induced dermal extracellular damage. J. Dermatol. Sci. 1998, 16, 127. [Google Scholar]
- Katiyar, S.K.; Bergamo, B.M.; Vyalil, P.K.; Elmets, C.A. Green tea polyphenols: DNA photodamage and photoimmunology. J. Photochem. Photobiol. B Biol. 2001, 65, 109–114. [Google Scholar] [CrossRef]
- Elmets, C.A.; Singh, D.; Tubesing, K.; Matsui, M.; Katiyar, S.; Mukhtar, H. Cutaneous photoprotection from ultraviolet injury by green tea polyphenols. J. Am. Acad. Dermatol. 2001, 44, 425–432. [Google Scholar] [CrossRef] [PubMed]
- Katiyar, S.K.; Afaq, F.; Azizuddin, K.; Mukhtar, H. Inhibition of UVB-induced oxidative stress-mediated phosphorylation of mitogen-activated protein kinase signaling pathways in cultured human epidermal keratinocytes by green tea polyphenol (−)-epigallocatechin-3-gallate. Toxicol. Appl. Pharmacol. 2001, 176, 110–117. [Google Scholar] [CrossRef]
- Asadi, S.Y.; Parsaei, P.; Karimi, M.; Ezzati, S.; Zamiri, A.; Mohammadizadeh, F.; Rafieian-kopaei, M. Effect of green tea (Camellia sinensis) extract on healing process of surgical wounds in rat. Int. J. Surg. 2013, 11, 332–337. [Google Scholar] [CrossRef]
- Hong, Y.-H.; Jung, E.Y.; Noh, D.O.; Suh, H.J. Physiological effects of formulation containing tannase-converted green tea extract on skin care: Physical stability, collagenase, elastase, and tyrosinase activities. Int. Med. Res. 2014, 3, 25–33. [Google Scholar] [CrossRef]
- Lu, M.-J.; Chen, C. Enzymatic tannase treatment of green tea increases in vitro inhibitory activity against N-nitrosation of dimethylamine. Process Biochem. 2007, 42, 1285–1290. [Google Scholar] [CrossRef]
- An, T.-T.; Feng, S.; Zeng, C.-M. Oxidized epigallocatechin gallate inhibited lysozyme fibrillation more strongly than the native form. Redox Biol. 2017, 11, 315–321. [Google Scholar] [CrossRef]
- Sarkar, J.; Nandy, S.K.; Chowdhury, A.; Chakraborti, T.; Chakraborti, S. Inhibition of MMP-9 by green tea catechins and prediction of their interaction by molecular docking analysis. Biomed. Pharmacother. 2016, 84, 340–347. [Google Scholar] [CrossRef] [PubMed]
- Hwang, Y.P.; Jin, S.W.; Choi, J.H.; Choi, C.Y.; Kim, H.G.; Kim, S.J.; Kim, Y.; Lee, K.J.; Chung, Y.C.; Jeong, H.G. Inhibitory effects of l-theanine on airway inflammation in ovalbumin-induced allergic asthma. Food Chem. Toxicol. 2017, 99, 162–169. [Google Scholar] [CrossRef] [PubMed]
- Jówko, E.; Sacharuk, J.; Balasińska, B.; Ostaszewski, P.; Charmas, M.; Charmas, R. Green tea extract supplementation gives protection against exercise-induced oxidative damage in healthy men. Nutr. Res. 2011, 31, 813–821. [Google Scholar] [CrossRef] [PubMed]
- Mohamad Shalan, N.A.A.; Mustapha, N.M.; Mohamed, S. Morinda citrifolia leaf enhanced performance by improving angiogenesis, mitochondrial biogenesis, antioxidant, anti-inflammatory & stress responses. Food Chem. 2016, 212, 443–452. [Google Scholar] [PubMed]
- Sanguigni, V.; Manco, M.; Sorge, R.; Gnessi, L.; Francomano, D. Natural antioxidant ice cream acutely reduces oxidative stress and improves vascular function and physical performance in healthy individuals. Nutrition 2017, 33, 225–233. [Google Scholar] [CrossRef] [PubMed]
- Abdelrazek, H.M.A.; Helmy, S.A.; Elsayed, D.H.; Ebaid, H.M.; Mohamed, R.M. Ameliorating effects of green tea extract on cadmium induced reproductive injury in male Wistar rats with respect to androgen receptors and caspase-3. Reprod. Biol. 2016, 16, 300–308. [Google Scholar] [CrossRef]
- Zanchi, M.M.; Manfredini, V.; dos Santos Brum, D.; Vargas, L.M.; Spiazzi, C.C.; Soares, M.B.; Izaguirry, A.P.; Santos, F.W. Green tea infusion improves cyclophosphamide-induced damage on male mice reproductive system. Toxicol. Rep. 2015, 2, 252–260. [Google Scholar] [CrossRef] [PubMed]
- Roychoudhury, S.; Agarwal, A.; Virk, G.; Cho, C.-L. Potential role of green tea catechins in the management of oxidative stress-associated infertility. Reprod. BioMed. 2017, 34, 487–498. [Google Scholar] [CrossRef]
- Gadani, B.; Bucci, D.; Spinaci, M.; Tamanini, C.; Galeati, G. Resveratrol and Epigallocatechin-3-gallate addition to thawed boar sperm improves in vitro fertilization. Theriogenology 2017, 90, 88–93. [Google Scholar] [CrossRef]
- Satoh, K.; Sakamoto, Y.; Ogata, A.; Nagai, F.; Mikuriya, H.; Numazawa, M.; Yamada, K.; Aoki, N. Inhibition of aromatase activity by green tea extract catechins and their endocrinological effects of oral administration in rats. Food Chem. Toxicol. 2002, 40, 925–933. [Google Scholar] [CrossRef]
- Xue, K.X.; Wang, S.; Ma, G.J.; Zhou, P.; Wu, P.Q.; Zhang, R.F.; Xu, Z.; Chen, W.S.; Wang, Y.Q. Micronucleus formation in peripheral-blood lymphocytes from smokers and the influence of alcohol-and tea-drinking habits. Int. J. Cancer 1992, 50, 702–705. [Google Scholar] [CrossRef] [PubMed]
- Shalini, V.K.; Luthra, M.; Srinivas, L.; Rao, S.H.; Basti, S.; Reddy, M.; Balasubramanian, D. Oxidative damage to the eye lens caused by cigarette smoke and fuel smoke condensates. Ind. J. Biochem. Biophys. 1994, 31, 261–266. [Google Scholar]
- Chaudhury, S.; Roy, P.; Dasgupta, S. Green tea flavanols protect human γB-crystallin from oxidative photodamage. Biochimie 2017, 137, 46–55. [Google Scholar] [CrossRef] [PubMed]
- Alessio, H.M.; Hagerman, A.E.; Romanello, M.; Carando, S.; Threlkeld, M.S.; Rogers, J.; Dimitrova, Y.; Muhammed, S.; Wiley, R.L. Consumption of green tea protects rats from exercise-induced oxidative stress in kidney and liver. Nutr. Res. 2002, 22, 1177–1188. [Google Scholar] [CrossRef]
- Yokozawa, T.; Dong, E.; Oura, H. Proof that green tea tannin suppresses the increase in the blood methylguanidine level associated with renal failure. Exp. Toxicol. Pathol. 1997, 49, 117–122. [Google Scholar] [CrossRef]
- Delwing-Dal Magro, D.; Roecker, R.; Junges, G.M.; Rodrigues, A.F.; Delwing-de Lima, D.; da Cruz, J.G.P.; Wyse, A.T.S.; Pitz, H.S.; Zeni, A.L.B. Protective effect of green tea extract against proline-induced oxidative damage in the rat kidney. Biomed. Pharmacother. 2016, 83, 1422–1427. [Google Scholar] [CrossRef]
- Yoshikawa, M.; Sugimoto, S.; Kato, Y.; Nakamura, S.; Wang, T.; Yamashita, C.; Matsuda, H. Acylated oleanane-type triterpene saponins with acceleration of gastrointestinal transit and inhibitory effect on pancreatic lipase from flower buds of Chinese tea plant (Camellia sinensis). Chem. Biodivers. 2009, 6, 903–915. [Google Scholar] [CrossRef] [PubMed]
- Jodoin, J.; Demeule, M.; Béliveau, R. Inhibition of the multidrug resistance P-glycoprotein activity by green tea polyphenols. Biochim. Biophys. Acta (BBA) Mol. Cell Res. 2002, 1542, 149–159. [Google Scholar] [CrossRef]
- Chen, L.; Lee, M.-J.; Li, H.; Yang, C.S. Absorption, distribution, and elimination of Tea polyphenols in Rats. Drug Metab. Dispos. 1997, 25, 1045–1050. [Google Scholar]
- Bu-Abbas, A.; Clifford, M.; Walker, R.; Ioannides, C. Selective induction of rat hepatic CYP1 and CYP4 proteins and of peroxisomal proliferation by green tea. Carcinogenesis 1994, 15, 2575–2579. [Google Scholar] [CrossRef]
- Bu-Abbas, A.; Clifford, M.N.; Walker, R.; Ioannides, C. Modulation of hepatic cytochrome P450 activity and carcinogen bioactivation by black and decaffeinated black tea. Environ. Toxicol. Pharmacol. 1999, 7, 41–47. [Google Scholar] [CrossRef]
- Williams, S.N.; Shih, H.; Guenette, D.K.; Brackney, W.; Denison, M.S.; Pickwell, G.V.; Quattrochi, L.C. Comparative studies on the effects of green tea extracts and individual tea catechins on human CYP1A gene expression. Chem. Biol. Int. 2000, 128, 211–229. [Google Scholar] [CrossRef]
- Ikarashi, N.; Ogawa, S.; Hirobe, R.; Kon, R.; Kusunoki, Y.; Yamashita, M.; Mizukami, N.; Kaneko, M.; Wakui, N.; Machida, Y.; et al. Epigallocatechin gallate induces a hepatospecific decrease in the CYP3A expression level by altering intestinal flora. Eur. J. Pharm. Sci. 2017, 100, 211–218. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Yalcin, M.; Lin, Q.; Ardawi, M.-S.M.; Mousa, S.A. Self-assembly of green tea catechin derivatives in nanoparticles for oral lycopene delivery. J. Control. Release 2017, 248, 117–124. [Google Scholar] [CrossRef] [PubMed]
- Yao, L.H.; Jiang, Y.M.; Caffin, N.; D’Arcy, B.; Datta, N.; Liu, X.; Singanusong, R.; Xu, Y. Phenolic compounds in tea from Australian supermarkets. Food Chem. 2006, 96, 614–620. [Google Scholar] [CrossRef]
- Vinson, J.A.; Dabbagh, Y.A.; Serry, M.M.; Jang, J. Plant flavonoids, especially tea flavonols, are powerful antioxidants using an in vitro oxidation model for heart disease. J. Agric. Food Chem. 1995, 43, 2800–2802. [Google Scholar] [CrossRef]
- Millin, D.J.; Crispin, D.J.; Swaine, D. Nonvolatile components of black tea and their contribution to the character of the beverage. J. Agric. Food Chem. 1969, 17, 717–722. [Google Scholar] [CrossRef]
- Wang, Z.-Y.; Huang, M.-T.; Ferraro, T.; Wong, C.-Q.; Lou, Y.-R.; Reuhl, K.; Iatropoulos, M.; Yang, C.S.; Conney, A.H. Inhibitory effect of green tea in the drinking water on tumorigenesis by ultraviolet light and 12-O-tetradecanoylphorbol-13-acetate in the skin of SKH-1 mice. Cancer Res. 1992, 52, 1162–1170. [Google Scholar] [PubMed]
- McKay, D.L.; Blumberg, J.B. The role of tea in human health: An update. J. Am. Coll. Nutr. 2002, 21, 1–13. [Google Scholar] [CrossRef]
- Okuda, T.; Mori, K.; Hatano, T. Relationship of the structures of tannins to the binding activities with hemoglobin and methylene blue. Chem. Pharm. Bull. 1985, 33, 1424–1433. [Google Scholar] [CrossRef]
- Ricci, A.; Parpinello, G.P.; Palma, A.S.; Teslić, N.; Brilli, C.; Pizzi, A.; Versari, A. Analytical profiling of food-grade extracts from grape (Vitis vinifera sp.) seeds and skins, green tea (Camellia sinensis) leaves and Limousin oak (Quercus robur) heartwood using MALDI-TOF-MS, ICP-MS and spectrophotometric methods. J. Food Compos. Anal. 2017, 59, 95–104. [Google Scholar] [CrossRef]
- Bhagwat, S.; Haytowitz, D.B.; Holden, J.M. USDA Database for the Flavonoid Content of Selected Foods Release 3. US Department of Agriculture, ARS. 2011. Available online: http://www.nal.usda.gov/fnic/foodcomp/Data/Flav/flav.pdf (accessed on 5 October 2012).
- Lakenbrink, C.; Engelhardt, U.H.; Wray, V. Identification of two novel proanthocyanidins in green tea. J. Agric. Food Chem. 1999, 47, 4621–4624. [Google Scholar] [CrossRef] [PubMed]
- Nonaka, G.; Kawahara, O.; Nishioka, I. Tannins and related compounds. XV. A new class of dimeric flavan-3-ol gallates, theasinensins A and B, and proanthocyanidin gallates from green tea leaf. (1). Chem. Pharm. Bull. 1983, 31, 3906–3914. [Google Scholar] [CrossRef]
- Crozier, A.; Jaganath, I.B.; Clifford, M.N. Dietary phenolics: Chemistry, bioavailability and effects on health. Nat. Prod. Rep. 2009, 26, 1001–1043. [Google Scholar] [CrossRef] [PubMed]
- Sakamoto, Y. Flavones in Green Tea. Agric. Biol. Chem. 1967, 31, 1029–1034. [Google Scholar]
- Schmidt, M.; Schmitz, H.-J.; Baumgart, A.; Guedon, D.; Netsch, M.; Kreuter, M.-H.; Schmidlin, C.; Schrenk, D. Toxicity of green tea extracts and their constituents in rat hepatocytes in primary culture. Food Chem. Toxicol. 2005, 43, 307–314. [Google Scholar] [CrossRef] [PubMed]
- Man, S.; Gao, W.; Zhang, Y.; Huang, L.; Liu, C. Chemical study and medical application of saponins as anti-cancer agents. Fitoterapia 2010, 81, 703–714. [Google Scholar] [CrossRef]
- Horie, H.; Fukatsu, S.; Mukai, T.; Goto, T.; Kawanaka, M.; Shimohara, T. Quality evaluation on green tea. Sens. Actuators B Chem. 1993, 13, 451–454. [Google Scholar] [CrossRef]
- Sadzuka, Y.; Sugiyama, T.; Miyagishima, A.; Nozawa, Y.; Hirota, S. The effects of theanine, as a novel biochemical modulator, on the antitumor activity of adriamycin. Cancer Lett. 1996, 105, 203–209. [Google Scholar] [CrossRef]
- Cabrera, C.; Artacho, R.; Giménez, R. Beneficial effects of green tea—A review. J. Am. Coll. Nutr. 2006, 25, 79–99. [Google Scholar] [CrossRef]
- Ravichandran, R. Carotenoid composition, distribution and degradation to flavour volatiles during black tea manufacture and the effect of carotenoid supplementation on tea quality and aroma. Food Chem. 2002, 78, 23–28. [Google Scholar] [CrossRef]
- Zhu, Y.; Shao, C.-Y.; Lv, H.-P.; Zhang, Y.; Dai, W.-D.; Guo, L.; Tan, J.-F.; Peng, Q.-H.; Lin, Z. Enantiomeric and quantitative analysis of volatile terpenoids in different teas (Camellia sinensis). J. Chromatogr. A 2017, 1490, 177–190. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.J.; Park, S.Y.; Park, J.S.; Park, S.-K.; Jung, M.Y. Contents and compositions of policosanols in green tea (Camellia sinensis) leaves. Food Chem. 2016, 204, 94–101. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.; Li, N.; Zhao, M.; Yu, W.; Wu, J.-L. Metabolomic profiling delineate taste qualities of tea leaf pubescence. Food Res. Int. 2017, 94, 36–44. [Google Scholar] [CrossRef] [PubMed]
- Yan, L.-g.; Xi, J. Micro-mechanism analysis of ultrahigh pressure extraction from green tea leaves by numerical simulation. Sep. Purif. Technol. 2017, 180, 51–57. [Google Scholar] [CrossRef]
- Wunsch, D.C.; Hasselmo, M.E.; Venayagamoorthy, G.K. Advances in Neural Networks Research: IJCNN 2003; Elsevier: Amsterdam, The Netherlands, 2003. [Google Scholar]
- Goschnick, J.; Koronczi, I.; Frietsch, M.; Kiselev, I. Water pollution recognition with the electronic nose KAMINA. Sens. Actuators B Chem. 2005, 106, 182–186. [Google Scholar] [CrossRef]
- Horie, H.; Fukatsu, S.; Mukai, T.; Takeuchi, A.; Goto, T. Quality evaluation of medium class green tea. In Proceedings of the International Symposium on Tea Science, Shizuoka, Japan, 26–29 April 1991. [Google Scholar]
- Yu, H.; Wang, J. Discrimination of LongJing green-tea grade by electronic nose. Sens. Actuators B Chem. 2007, 122, 134–140. [Google Scholar] [CrossRef]
- Yu, H.; Wang, J.; Yao, C.; Zhang, H.; Yu, Y. Quality grade identification of green tea using E-nose by CA and ANN. LWT Food Sci. Technol. 2008, 41, 1268–1273. [Google Scholar] [CrossRef]
- Dutta, R.; Hines, E.; Gardner, J.; Kashwan, K.; Bhuyan, M. Tea quality prediction using a tin oxide-based electronic nose: An artificial intelligence approach. Sens. Actuators B Chem. 2003, 94, 228–237. [Google Scholar] [CrossRef]
- Chen, Q.; Zhao, J.; Chen, Z.; Lin, H.; Zhao, D.-A. Discrimination of green tea quality using the electronic nose technique and the human panel test, comparison of linear and nonlinear classification tools. Sens. Actuators B Chem. 2011, 159, 294–300. [Google Scholar] [CrossRef]
- Mirasoli, M.; Gotti, R.; Di Fusco, M.; Leoni, A.; Colliva, C.; Roda, A. Electronic nose and chiral-capillary electrophoresis in evaluation of the quality changes in commercial green tea leaves during a long-term storage. Talanta 2014, 129, 32–38. [Google Scholar] [CrossRef] [PubMed]
- Akella, G.; Henderson, S.; Drewnowski, A. Genetic Sensitivity to 6-N-Propylthiouracil (prop) and Sensory Acceptance of Soy Products and Japanese Green Tea. J. Am. Diet. Assoc. 1997, 97, A61. [Google Scholar] [CrossRef]
- Chen, Q.; Zhao, J.; Vittayapadung, S. Identification of the green tea grade level using electronic tongue and pattern recognition. Food Res. Int. 2008, 41, 500–504. [Google Scholar] [CrossRef]
- Xu, Y.-Q.; Zou, C.; Gao, Y.; Chen, J.-X.; Wang, F.; Chen, G.-S.; Yin, J.-F. Effect of the type of brewing water on the chemical composition, sensory quality and antioxidant capacity of Chinese teas. Food Chem. 2016, 236, 142–151. [Google Scholar] [CrossRef]
- Panigrahi, S.; Balasubramanian, S.; Gu, H.; Logue, C.; Marchello, M. Neural-network-integrated electronic nose system for identification of spoiled beef. LWT Food Sci. Technol. 2006, 39, 135–145. [Google Scholar] [CrossRef]
- Chen, Q.; Zhang, D.; Pan, W.; Ouyang, Q.; Li, H.; Urmila, K.; Zhao, J. Recent developments of green analytical techniques in analysis of tea’s quality and nutrition. Trends Food Sci. Technol. 2015, 43, 62–82. [Google Scholar] [CrossRef]
- Zhu, H.; Ye, Y.; He, H.; Dong, C. Evaluation of green tea sensory quality via process characteristics and image information. Food Bioprod. Process 2017, 102, 116–122. [Google Scholar] [CrossRef]
- Yin, J.; Xu, Y.; Yuan, H.; Yu, S.; Wei, K.; Chen, J.; Wang, F.; Wu, R. Dynamic change of main biochemical components of premium green tea fresh leaves during spreading. J. Tea Sci. 2009, 29, 102–110. [Google Scholar]
- Manasa, G.; Mascarenhas, R.J.; Satpati, A.K.; D’Souza, O.J.; Dhason, A. Facile preparation of poly(methylene blue) modified carbon paste electrode for the detection and quantification of catechin. Mater. Sci. Eng. C 2017, 73, 552–561. [Google Scholar] [CrossRef]
- Luypaert, J.; Zhang, M.; Massart, D. Feasibility study for the use of near infrared spectroscopy in the qualitative and quantitative analysis of green tea, Camellia sinensis (L.). Anal. Chim. Acta 2003, 478, 303–312. [Google Scholar] [CrossRef]
- Costa, L.M.; Gouveia, S.T.; Nobrega, J.A. Comparison of heating extraction procedures for Al, Ca, Mg, and Mn in tea samples. Anal. Sci. 2002, 18, 313–318. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Cáceres, P.L.; Martín, M.J.; Pablos, F.; González, A.G. Differentiation of tea (Camellia sinensis) varieties and their geographical origin according to their metal content. J. Agric. Food Chem. 2001, 49, 4775–4779. [Google Scholar] [CrossRef] [PubMed]
- Shu, W.; Zhang, Z.; Lan, C.; Wong, M. Fluoride and aluminium concentrations of tea plants and tea products from Sichuan Province, PR China. Chemosphere 2003, 52, 1475–1482. [Google Scholar] [CrossRef]
- Fung, K.F.; Zhang, Z.Q.; Wong, J.W.C.; Wong, M.H. Aluminium and fluoride concentrations of three tea varieties growing at lantau island, Hong Kong. Environ. Geochem. Health 2003, 25, 219–232. [Google Scholar] [CrossRef] [PubMed]
- Ashraf, W.; Mian, A.A. Levels of selected heavy metals in black tea varieties consumed in Saudi Arabia. Bull. Environ. Cont. Toxicol. 2008, 81, 101–104. [Google Scholar] [CrossRef]
- Pohl, P.; Szymczycha-Madeja, A.; Stelmach, E.; Welna, M. Multivariate data reduction and discrimination of black and green teas due to the physical fractionation pattern of selected metals determined in their infusions. Talanta 2016, 160, 314–324. [Google Scholar] [CrossRef] [PubMed]
- Schulzki, G.; Nüßlein, B.; Sievers, H. Transition rates of selected metals determined in various types of teas (Camellia sinensis L. Kuntze) and herbal/fruit infusions. Food Chem. 2017, 215, 22–30. [Google Scholar] [CrossRef]
- Nakagawa, M. Chemical components and taste of green tea. Jpn. Agric. Res. Quart. 1975, 9, 156–160. [Google Scholar]
- Mi, H.; Guan, M.; Liu, J.; Shan, H.; Fei, Q.; Huan, Y.; Feng, G. Conjugated polymer with carboxylate groups-Hg2 + system as a turn-on fluorescence probe for label-free detection of cysteine-containing compounds. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2017, 176, 168–173. [Google Scholar] [CrossRef]
- Van Nederkassel, A.; Daszykowski, M.; Massart, D.; Vander Heyden, Y. Prediction of total green tea antioxidant capacity from chromatograms by multivariate modeling. J. Chromatogr. A 2005, 1096, 177–186. [Google Scholar] [CrossRef]
- Morsy, M.; Khaled, M. Novel EPR characterization of the antioxidant activity of tea leaves. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2002, 58, 1271–1277. [Google Scholar] [CrossRef]
- Nissen, L.R.; Huynh-Ba, T.; Petersen, M.A.; Bertelsen, G.; Skibsted, L.H. Potential use of electron spin resonance spectroscopy for evaluating the oxidative status of potato flakes. Food Chem. 2002, 79, 387–394. [Google Scholar] [CrossRef]
- Arakawa, H.; Kanemitsu, M.; Tajima, N.; Maeda, M. Chemiluminescence assay for catechin based on generation of hydrogen peroxide in basic solution. Anal. Chim. Acta 2002, 472, 75–82. [Google Scholar] [CrossRef]
- Usha, S.P.; Shrivastav, A.M.; Gupta, B.D. Silver nanoparticle noduled ZnO nanowedge fetched novel FO-LMR based H2O2 biosensor: A twin regime sensor for in-vivo applications and H2O2 generation analysis from polyphenolic daily devouring beverages. Sens. Actuators B Chem. 2017, 241, 129–145. [Google Scholar] [CrossRef]
- Benvidi, A.; Nafar, M.T.; Jahanbani, S.; Tezerjani, M.D.; Rezaeinasab, M.; Dalirnasab, S. Developing an electrochemical sensor based on a carbon paste electrode modified with nano-composite of reduced graphene oxide and CuFe2O4 nanoparticles for determination of hydrogen peroxide. Mater. Sci. Eng. C 2017, 75, 1435–1447. [Google Scholar] [CrossRef] [PubMed]
- Pelillo, M.; Biguzzi, B.; Bendini, A.; Gallina Toschi, T.; Vanzini, M.; Lercker, G. Preliminary investigation into development of HPLC with UV and MS-electrospray detection for the analysis of tea catechins. Food Chem. 2002, 78, 369–374. [Google Scholar] [CrossRef]
- Naldi, M.; Fiori, J.; Gotti, R.; Périat, A.; Veuthey, J.-L.; Guillarme, D.; Andrisano, V. UHPLC determination of catechins for the quality control of green tea. J. Pharm. Biomed. Anal. 2014, 88, 307–314. [Google Scholar] [CrossRef]
- Tao, W.; Zhou, Z.; Zhao, B.; Wei, T. Simultaneous determination of eight catechins and four theaflavins in green, black and oolong tea using new HPLC–MS–MS method. J. Pharm. Biomed. Anal. 2016, 131, 140–145. [Google Scholar] [CrossRef]
- Kumar, A.S.; Shanmugam, R.; Nellaiappan, S.; Thangaraj, R. Tea quality assessment by analyzing key polyphenolic functional groups using flow injection analysis coupled with a dual electrochemical detector. Sens. Actuators B Chem. 2016, 227, 352–361. [Google Scholar] [CrossRef]
- Alessio, P.; Martin, C.S.; de Saja, J.A.; Rodriguez-Mendez, M.L. Mimetic biosensors composed by layer-by-layer films of phospholipid, phthalocyanine and silver nanoparticles to polyphenol detection. Sens. Actuators B Chem. 2016, 233, 654–666. [Google Scholar] [CrossRef]
- Vilian, A.T.E.; Puthiaraj, P.; Kwak, C.H.; Choe, S.R.; Huh, Y.S.; Ahn, W.-S.; Han, Y.-K. Electrochemical determination of quercetin based on porous aromatic frameworks supported Au nanoparticles. Electrochim. Acta 2016, 216, 181–187. [Google Scholar] [CrossRef]
- Chen, Q.; Zhao, J.; Lin, H. Study on discrimination of Roast green tea (Camellia sinensis L.) according to geographical origin by FT-NIR spectroscopy and supervised pattern recognition. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2009, 72, 845–850. [Google Scholar] [CrossRef] [PubMed]
- Alaerts, G.; Van Erps, J.; Pieters, S.; Dumarey, M.; Van Nederkassel, A.; Goodarzi, M.; Smeyers-Verbeke, J.; Vander Heyden, Y. Similarity analyses of chromatographic fingerprints as tools for identification and quality control of green tea. J. Chromatogr. B 2012, 910, 61–70. [Google Scholar] [CrossRef] [PubMed]
- Dumarey, M.; Van Nederkassel, A.; Deconinck, E.; Vander Heyden, Y. Exploration of linear multivariate calibration techniques to predict the total antioxidant capacity of green tea from chromatographic fingerprints. J. Chromatogr. A 2008, 1192, 81–88. [Google Scholar] [CrossRef]
- Chen, Q.; Zhao, J.; Guo, Z.; Wang, X. Determination of caffeine content and main catechins contents in green tea (Camellia sinensis L.) using taste sensor technique and multivariate calibration. J. Food Compos. Anal. 2010, 23, 353–358. [Google Scholar] [CrossRef]
- Iorgulescu, E.; Voicu, V.A.; Sârbu, C.; Tache, F.; Albu, F.; Medvedovici, A. Experimental variability and data pre-processing as factors affecting the discrimination power of some chemometric approaches (PCA, CA and a new algorithm based on linear regression) applied to (+/−)ESI/MS and RPLC/UV data: Application on green tea extracts. Talanta 2016, 155, 133–144. [Google Scholar]
- Jing, J.; Shi, Y.; Zhang, Q.; Wang, J.; Ruan, J. Prediction of Chinese green tea ranking by metabolite profiling using ultra-performance liquid chromatography–quadrupole time-of-flight mass spectrometry (UPLC–Q-TOF/MS). Food Chem. 2017, 221, 311–316. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Gao, J.; Huang, C.; Liu, W.; Tong, P.; Zhang, L. In situ hydrothermal growth of ZnO/g-C3N4 nanoflowers coated solid-phase microextraction fibers coupled with GC-MS for determination of pesticides residues. Anal. Chim. Acta 2016, 934, 122–131. [Google Scholar] [CrossRef]
- Qin, Y.; Zhang, J.; Zhang, Y.; Li, F.; Han, Y.; Zou, N.; Xu, H.; Qian, M.; Pan, C. Automated multi-plug filtration cleanup for liquid chromatographic–tandem mass spectrometric pesticide multi-residue analysis in representative crop commodities. J. Chromatogr. A 2016, 1462, 19–26. [Google Scholar] [CrossRef]
- Jiang, Z.; Li, H.; Cao, X.; Du, P.; Shao, H.; Jin, F.; Jin, M.; Wang, J. Determination of hymexazol in 26 foods of plant origin by modified QuEChERS method and liquid chromatography tandem-mass spectrometry. Food Chem. 2017, 228, 411–419. [Google Scholar] [CrossRef]
- Rolle, F.; Pennecchi, F.; Perini, S.; Sega, M. Metrological traceability of Polycyclic Aromatic Hydrocarbons (PAHs) measurements in green tea and mate. Measurement 2017, 98, 290–299. [Google Scholar] [CrossRef]
- Cebi, N.; Yilmaz, M.T.; Sagdic, O. A rapid ATR-FTIR spectroscopic method for detection of sibutramine adulteration in tea and coffee based on hierarchical cluster and principal component analyses. Food Chem. 2017, 229, 517–526. [Google Scholar] [CrossRef] [PubMed]
- Jinnarak, A.; Anantavichian, P.; Intanin, A.; Fungladda, S.; Choengchan, N.; Wilairat, P.; Nacapricha, D.; Teerasong, S. Sequential injection for determination of gamma-aminobutyric acid based on its effect on second order light scattering of silver nanoparticles. J. Food Compos. Anal. 2016, 51, 69–75. [Google Scholar] [CrossRef]
- Liu, L.; Fan, Y.; Fu, H.; Chen, F.; Ni, C.; Wang, J.; Yin, Q.; Mu, Q.; Yang, T.; She, Y. “Turn-off” fluorescent sensor for highly sensitive and specific simultaneous recognition of 29 famous green teas based on quantum dots combined with chemometrics. Anal. Chim. Acta 2017, 963, 119–128. [Google Scholar] [CrossRef] [PubMed]
- Jayabalan, R.; Malbaša, R.V.; Lončar, E.S.; Vitas, J.S.; Sathishkumar, M. A review on kombucha tea—Microbiology, composition, fermentation, beneficial effects, toxicity, and tea fungus. Compr. Rev. Food Sci. F 2014, 13, 538–550. [Google Scholar] [CrossRef]
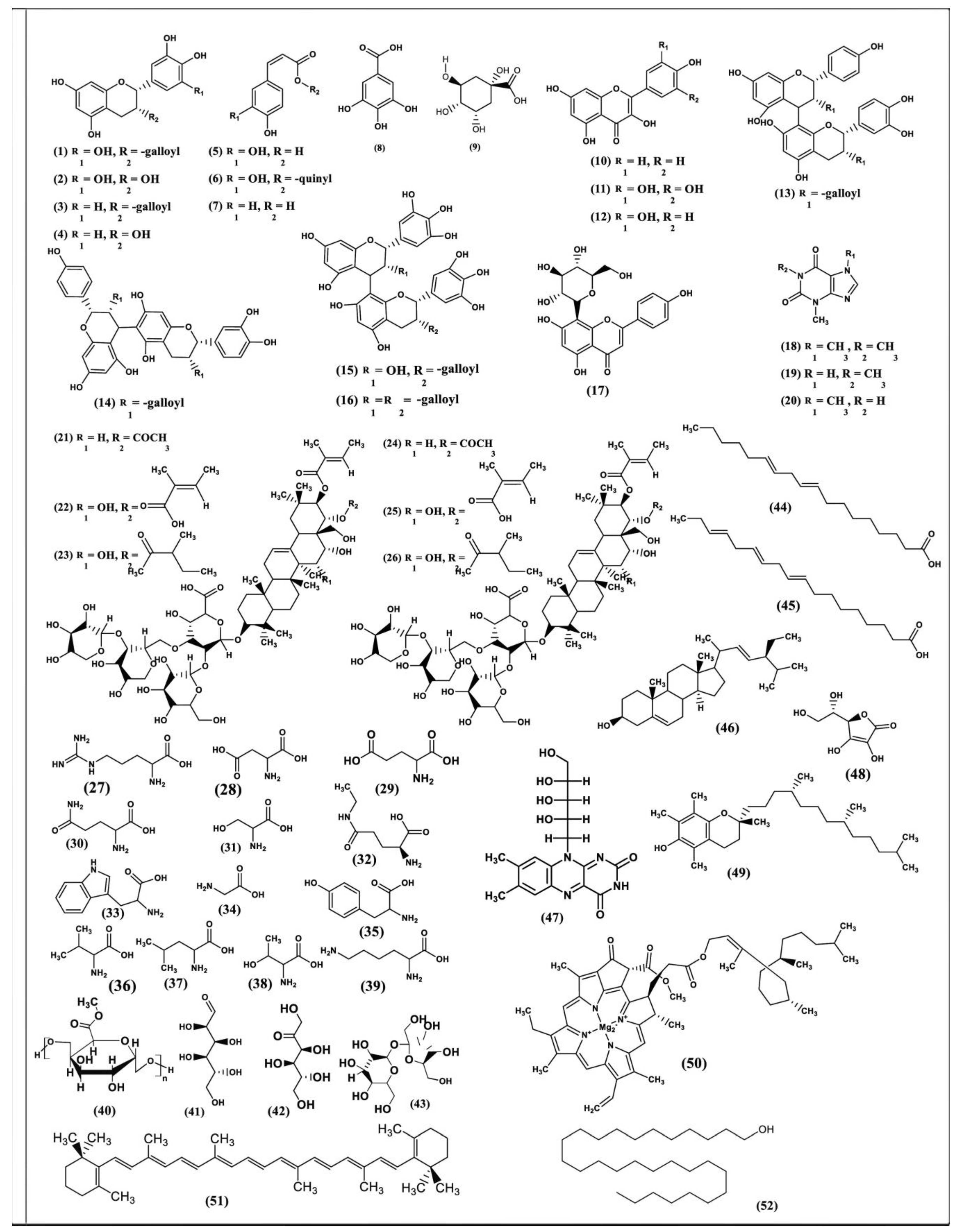
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aboulwafa, M.M.; Youssef, F.S.; Gad, H.A.; Altyar, A.E.; Al-Azizi, M.M.; Ashour, M.L. A Comprehensive Insight on the Health Benefits and Phytoconstituents of Camellia sinensis and Recent Approaches for Its Quality Control. Antioxidants 2019, 8, 455. https://doi.org/10.3390/antiox8100455
Aboulwafa MM, Youssef FS, Gad HA, Altyar AE, Al-Azizi MM, Ashour ML. A Comprehensive Insight on the Health Benefits and Phytoconstituents of Camellia sinensis and Recent Approaches for Its Quality Control. Antioxidants. 2019; 8(10):455. https://doi.org/10.3390/antiox8100455
Chicago/Turabian StyleAboulwafa, Maram M., Fadia S. Youssef, Haidy A. Gad, Ahmed E. Altyar, Mohamed M. Al-Azizi, and Mohamed L. Ashour. 2019. "A Comprehensive Insight on the Health Benefits and Phytoconstituents of Camellia sinensis and Recent Approaches for Its Quality Control" Antioxidants 8, no. 10: 455. https://doi.org/10.3390/antiox8100455
APA StyleAboulwafa, M. M., Youssef, F. S., Gad, H. A., Altyar, A. E., Al-Azizi, M. M., & Ashour, M. L. (2019). A Comprehensive Insight on the Health Benefits and Phytoconstituents of Camellia sinensis and Recent Approaches for Its Quality Control. Antioxidants, 8(10), 455. https://doi.org/10.3390/antiox8100455








