Protein Oxidation Biomarkers and Myeloperoxidase Activation in Cerebrospinal Fluid in Childhood Bacterial Meningitis
Abstract
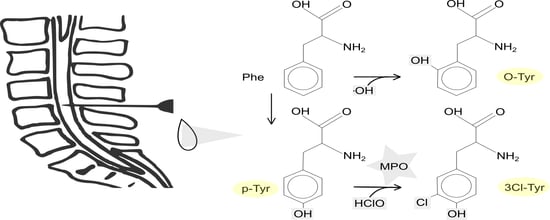
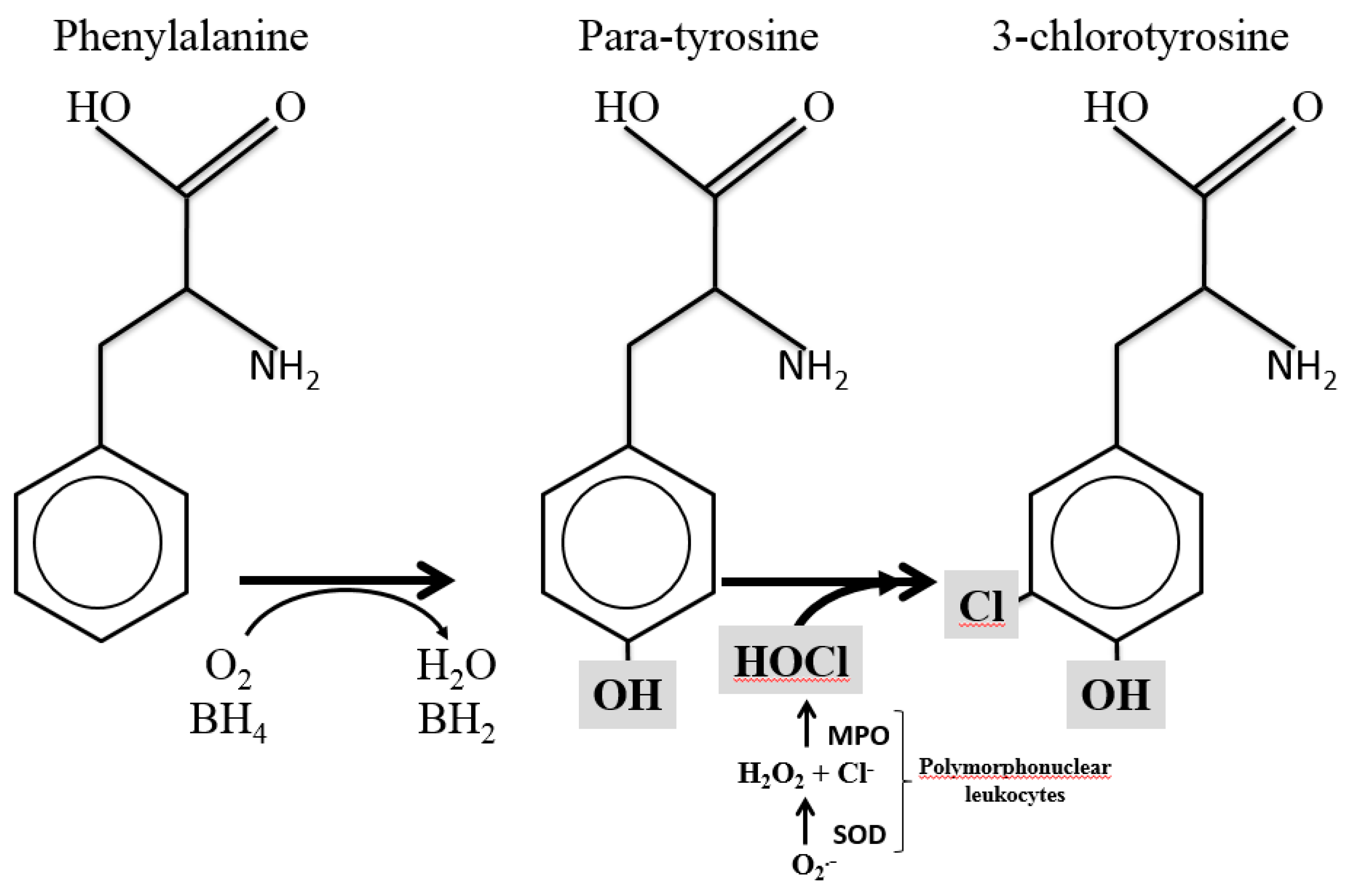
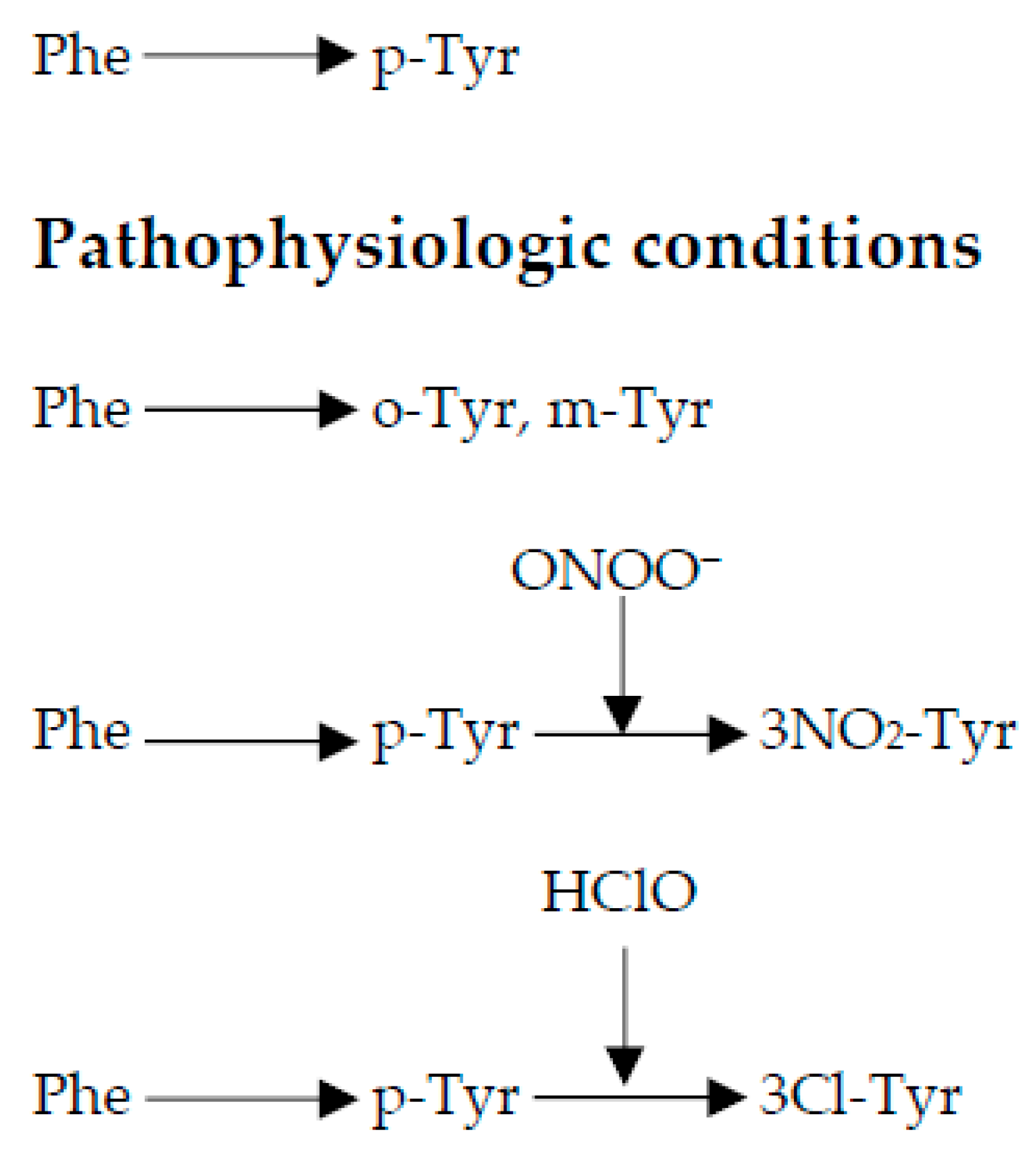
1. Introduction
2. Materials and Methods
2.1. Study Design and Patients
2.2. Standards and Reagents
2.3. Sample Preparation and Analysis Employing Liquid Chromatography Coupled to Tandem Mass Spectrometry (LC-MS/MS)
2.4. Statistical Analysis
2.5. Ethics
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- GBD 2015 Child Mortality Collaborators. Global, regional, national, and selected subnational levels of stillbirths, neonatal, infant, and under-5 mortality, 1980–2015: A systematic analysis for the Global Burden of Disease Study 2015. Lancet 2016, 388, 1725–1774. [Google Scholar] [CrossRef]
- Edmond, K.; Clark, A.; Korczak, V.S.; Sanderson, C.; Griffiths, U.K.; Rudan, I. Global and regional risk of disabling sequelae from bacterial meningitis: A systematic review and meta-analysis. Lancet Infect. Dis. 2010, 10, 317–328. [Google Scholar] [CrossRef]
- Gerber, J.; Nau, R. Mechanisms of injury in bacterial meningitis. Curr. Opin. Neurol. 2010, 23, 312–318. [Google Scholar] [CrossRef] [PubMed]
- Liechti, F.D.; Grandgirard, D.; Leib, S.L. Bacterial meningitis: Insights into pathogenesis and evaluation of new treatment options: A perspective from experimental studies. Future Microbiol. 2015, 10, 1195–1213. [Google Scholar] [CrossRef]
- Barichello, T.; Generoso, J.S.; Simoes, L.R.; Elias, S.G.; Quevedo, J. Role of oxidative stress in the pathophysiology of pneumococcal meningitis. Oxid. Med. Cell. Longev. 2013, 2013, 371465. [Google Scholar] [CrossRef]
- Winterbourn, C.C.; Kettle, A.J.; Hampton, M.B. Reactive oxygen species and neutrophil function. Annu. Rev. Biochem. 2016, 85, 765–792. [Google Scholar] [CrossRef] [PubMed]
- Barichello, T.; Generoso, J.S.; Collodel, A.; Moreira, A.P.; Almeida, S.M. Pathophysiology of acute meningitis caused by Streptococcus pneumoniae and adjunctive therapy approaches. Arq. Neuropsiquiatr. 2012, 70, 366–372. [Google Scholar] [CrossRef]
- Radi, R. Nitric oxide, oxidants, and protein tyrosine nitration. Proc. Natl. Acad. Sci. USA 2004, 101, 4003–4008. [Google Scholar] [CrossRef]
- Kato, Y. Neutrophil myeloperoxidase and its substrates: Formation of specific markers and reactive compounds during inflammation. J. Clin. Biochem. Nutr. 2016, 58, 99–104. [Google Scholar] [CrossRef]
- Srivastava, R.; Lohokare, R.; Prasad, R. Oxidative stress in children with bacterial meningitis. J. Trop. Pediatr. 2013, 59, 305–308. [Google Scholar] [CrossRef][Green Version]
- Dalle-Donne, I.; Rossi, R.; Colombo, R.; Giustarini, D.; Milzani, A. Biomarkers of oxidative damage in human disease. Clin. Chem. 2006, 52, 601–623. [Google Scholar] [CrossRef] [PubMed]
- Kuligowski, J.; Torres-Cuevas, I.; Quintas, G.; Rook, D.; van Goudoever, J.B.; Cubells, E.; Asensi, M.; Lliso, I.; Nunez, A.; Vento, M.; et al. Assessment of oxidative damage to proteins and DNA in urine of newborn infants by a validated UPLC-MS/MS approach. PLoS ONE 2014, 9, e93703. [Google Scholar] [CrossRef] [PubMed]
- Davies, M.J.; Fu, S.; Wang, H.; Dean, R.T. Stable markers of oxidant damage to proteins and their application in the study of human disease. Free Radic. Biol. Med. 1999, 27, 1151–1163. [Google Scholar] [CrossRef]
- Klein, M.; Koedel, U.; Pfister, H.W. Oxidative stress in pneumococcal meningitis: A future target for adjunctive therapy? Prog. Neurobiol. 2006, 80, 269–280. [Google Scholar] [CrossRef] [PubMed]
- Pelkonen, T.; Roine, I.; Cruzeiro, M.L.; Pitkaranta, A.; Kataja, M.; Peltola, H. Slow initial beta-lactam infusion and oral paracetamol to treat childhood bacterial meningitis: A randomised, controlled trial. Lancet Infect. Dis. 2011, 11, 613–621. [Google Scholar] [CrossRef]
- Chafer-Pericas, C.; Stefanovic, V.; Sanchez-Illana, A.; Escobar, J.; Cernada, M.; Cubells, E.; Nunez-Ramiro, A.; Andersson, S.; Vento, M.; Kuligowski, J. Novel biomarkers in amniotic fluid for early assessment of intraamniotic infection. Free Radic. Biol. Med. 2015, 89, 734–740. [Google Scholar] [CrossRef]
- Giridharan, V.V.; Simoes, L.R.; Dagostin, V.S.; Generoso, J.S.; Rezin, G.T.; Florentino, D.; Muniz, J.P.; Collodel, A.; Petronilho, F.; Quevedo, J.; et al. Temporal changes of oxidative stress markers in Escherichia coli K1-induced experimental meningitis in a neonatal rat model. Neurosci. Lett. 2017, 653, 288–295. [Google Scholar] [CrossRef] [PubMed]
- Tsukahara, H.; Haruta, T.; Todoroki, Y.; Hiraoka, M.; Noiri, E.; Maeda, M.; Mayumi, M. Oxidant and antioxidant activities in childhood meningitis. Life Sci. 2002, 71, 2797–2806. [Google Scholar] [CrossRef]
- Ray, G.; Aneja, S.; Jain, M.; Batra, S. Evaluation of free radical status in CSF in childhood meningitis. Ann. Trop. Paediatr. 2000, 20, 115–120. [Google Scholar] [CrossRef]
- Molnar, G.A.; Kun, S.; Selley, E.; Kertész, M.; Szélig, L.; Csontos, C.; Böddi, K.; Bogár, L.; Miseta, A.; Wittmann, I. Role of tyrosine isomers in acute and chronic diseases leading to oxidative stress—A review. Curr. Med. Chem. 2016, 23, 667–685. [Google Scholar] [CrossRef]
- Strasser, B.; Sperner-Unterweger, B.; Fuchs, D.; Gostner, J.M. Mechanisms of inflammation-associated depression: Immune influences on tryptophan and phenylalanine metabolisms. Curr. Top. Behav. Neurosci. 2017, 31, 95–115. [Google Scholar] [CrossRef] [PubMed]
- Mook-Kanamori, B.B.; Geldhoff, M.; van der Poll, T.; van de Beek, D. Pathogenesis and pathophysiology of pneumococcal meningitis. Clin. Microbiol. Rev. 2011, 24, 557–591. [Google Scholar] [CrossRef] [PubMed]
- Roine, I.; Peltola, H.; Fernandez, J.; Zavala, I.; Gonzalez Mata, A.; Gonzalez Ayala, S.; Arbo, A.; Bologna, R.; Mino, G.; Goyo, J.; et al. Influence of admission findings on death and neurological outcome from childhood bacterial meningitis. Clin. Infect. Dis. 2008, 46, 1248–1252. [Google Scholar] [CrossRef] [PubMed]
- Miric, D.; Katanic, R.; Kisic, B.; Zoric, L.; Miric, B.; Mitic, R.; Dragojevic, I. Oxidative stress and myeloperoxidase activity during bacterial meningitis: Effects of febrile episodes and the BBB permeability. Clin. Biochem. 2010, 43, 246–252. [Google Scholar] [CrossRef] [PubMed]
- Barichello, T.; Lemos, J.C.; Generoso, J.S.; Cipriano, A.L.; Milioli, G.L.; Marcelino, D.M.; Vuolo, F.; Petronilho, F.; Dal-Pizzol, F.; Vilela, M.C.; et al. Oxidative stress, cytokine/chemokine and disruption of blood-brain barrier in neonate rats after meningitis by Streptococcus agalactiae. Neurochem. Res. 2011, 36, 1922–1930. [Google Scholar] [CrossRef]
- Kastenbauer, S.; Koedel, U.; Becker, B.F.; Pfister, H.W. Oxidative stress in bacterial meningitis in humans. Neurology 2002, 58, 186–191. [Google Scholar] [CrossRef] [PubMed]
- Tsukahara, H.; Haruta, T.; Ono, N.; Kobata, R.; Fukumoto, Y.; Hiraoka, M.; Mayumi, M. Oxidative stress in childhood meningitis: Measurement of 8-hydroxy-2′-deoxyguanosine concentration in cerebrospinal fluid. Redox Rep. 2000, 5, 295–298. [Google Scholar] [CrossRef]
- Torres-Cuevas, I.; Kuligowski, J.; Carcel, M.; Cháfer-Pericás, C.; Asensi, M.; Solberg, R.; Cubells, E.; Nuñez, A.; Saugstad, O.D.; Vento, M.; et al. Protein-bound tyrosine oxidation, nitration and chlorination by-products assessed by ultraperformance liquid chromatography coupled to tandem mass spectrometry. Anal. Chim. Acta 2016, 913, 104–110. [Google Scholar] [CrossRef]
- Ipson, B.R.; Fisher, A.L. Roles of the tyrosine isomers meta-tyrosine and ortho-tyrosine in oxidative stress. Ageing Res. Rev. 2016, 27, 93–107. [Google Scholar] [CrossRef]
| Analyte | Retention Time ± s [min] | Calibration Range | R2 | y = a + bx | m/z Parent Ion | Cone [V] | Daughter Ion | Internal Standard | ||
|---|---|---|---|---|---|---|---|---|---|---|
| a [nm–1] | b [nM] | CE [eV] | m/z Quantification | |||||||
| Phenylalanine (Phe) | 2.32 ± 0.01 | 0.2–400 µM | 0.954 | 16.4 | 0.01 | 166.1 | 20 | 20 | 91.0 | Phe-D5 |
| Para-tyrosine (p-Tyr) | 1.01 ± 0.01 | 0.2–400 µM | 0.999 | 38.8 | 0.03 | 182.1 | 20 | 10 | 91.0 | p-Tyr-d2 |
| Ortho-tyrosine (o-Tyr) | 1.80 ± 0.01 | 1–2000 nM | 0.999 | –0.1 | 0.15 | p-Tyr-d2 | ||||
| 3-chlorotyrosine (3Cl-Tyr) | 1.90 ± 0.01 | 2–4000 nM | 0.999 | –2.1 | 1.68 | 216.0 | 20 | 15 | 170.0 | Phe-D5 |
| 3-nitrotyrosine (3NO2-Tyr) | 2.33 ± 0.01 | 1–2000 nM | 0.995 | 8.0 | 2.87 | 227.1 | 25 | 10 | 181.0 | Phe-D5 |
| 2-deoxyguanosine (2dG) | 1.45 ± 0.03 | 1–2000 nM | 0.999 | –0.3 | 0.41 | 268.0 | 25 | 15 | 152.0 | 2dG-13C15N |
| 8-oxo-2′-deoxyguanosine (8OHdG) | 2.04 ± 0.02 | 1–500 nM | 0.999 | 0.1 | 0.35 | 284.0 | 30 | 15 | 168.0 | 8OHdG-13C15N |
| Phenylalanine-d5 (Phe-D5) | 2.32 ± 0.01 | - | - | - | 171.5 | 30 | 20 | 125.0 | - | |
| p-Tyrosine-d2 (p-Tyr-D2) | 1.01 ± 0.02 | - | - | - | 184.1 | 20 | 10 | 138.1 | - | |
| 2-Deoxyguanosine-13C15N (2dG-13C15N) | 1.45 ± 0.02 | - | - | - | 271.0 | 15 | 10 | 155.0 | - | |
| 8-Oxo-2′-deoxyguanosine-13C15N (8OHdG-13C15N) | 2.04 ± 0.03 | - | - | - | 287.0 | 30 | 15 | 171.0 | - | |
| VARIABLE | BM (n = 79) |
|---|---|
| Age in months, median (IQR) | 12 (7–42) |
| Weight for age below—2SD | 19 (24%) |
| Duration of illness days, median (IQR) | 4 (3–7) |
| Previous antibiotics * | 30/74 (41%) |
| Glasgow coma score, median (IQR) | 11 (7–14) ᵃ |
| Another focus of infection | 19 (24%) |
| Cerebrospinal fluid | |
| Leukocyte count (×10⁶/L), median (IQR) | 1740 (353–3515) |
| Glucose concentration (mg/dL), median (IQR) | 17 (9–26) ᵇ |
| Blood | |
| CRP # on day 1 or 2 (mg/L), median ** (IQR) | 154 (81–161) ᶜ |
| Glucose (mg/dL), median (IQR) *** | 85 (62–111) ᵈ |
| Hemoglobin day 1 or 2 (g/dL), median (IQR) | 7.5 (6–9) e |
| Causative agent | |
| Streptococcus pneumoniae | 40/79 (51%) |
| Haemophilus influenzae type b | 24/79 (30%) |
| Neisseria meningitidis | 11/79 (14%) |
| Other bacteria | 4/79 (5%) |
| VARIABLE | BM, Luanda (n = 79) | Control, Helsinki (n = 10) | p Value | Ratio BM/Contol |
|---|---|---|---|---|
| Phenylalanine (Phe) | 88,346 (51,535−166,316) | 6558.0 (5249.76−8473) | < 0.0001 | 13.5 |
| Para-tyrosine (p-Tyr) | 64,214 (31,197−152,125) | 13,239 (10,096−17,677) | < 0.0001 | 4.9 |
| Ortho-tyrosine (o-Tyr) | 162.12 (65.23−2194.9) | 0.02 (0.020) | < 0.0001 | 8100 |
| 3-chlorotyrosine (3Cl-Tyr) | 423.34 (134.84−1311.95) | 4.155 (4.155) | < 0.0001 | 102 |
| 3-nitrotyrosine (3NO₂-Tyr) | 90.48 (59.37−135.47) | 2.745 (1.181−4.61) | < 0.0001 | 32.7 |
| 2′deoxiguanosine (2dG) | 303.57 (91.32−1329.69) | 0.768 (0.768−3.538) | < 0.0001 | 395 |
| 8-oxo-2′-deoxyguanosine (8OHdG) | 3.895 (3.895−17.778) | 0.043 (0.043) | < 0.0001 | 90.6 |
| Ratio 3Cl-Tyr/p-Tyr | 0.007 (0.003−0.022) | 3.531 × 10−4 (2.498 × 10−4−0.001) | < 0.0001 | 19.8 |
| Ratio o-Tyr/Phe | 0.002 (0.001−0.013) | 3.4995 × 10−6 (2.85 × 10−6−6.665 × 10−5) | < 0.0001 | 572 |
| Ratio 3NO₂-Tyr/p-Tyr | 0.001 (0.001−0.002) | 2.235 × 10−4(1088 × 10−4–4.444 × 10−4) | < 0.0001 | 4.5 |
| Ratio 8OHdG/2dG | 0.025 (0.005−0.063) | 0.056 (0.012−0.056) | 0.428 | 0.45 |
| Streptococcus pneumoniae | Haemophilus influenzae | Neisseria meningitidis | p Value | |
|---|---|---|---|---|
| VARIABLE | n = 40 | n = 24 | n = 11 | |
| Phenylalanine (Phe) | 84,788 (43,702−131,709) | 90,950 (58,433−172,710) | 166,203 (46,372−219,475) | 0.4454 |
| Para-tyrosine (p-Tyr) | 59,871 (39,143−135,736) | 53,641 (28,876−112,299) | 153,636 (30,053−226,175) | 0.4902 |
| Ortho-tyrosine (o-Tyr) | 155.77 (60.51−1973.3) | 109.175 (50.75−1212.4) | 687.97 (95.335−6987.8) | 0.3539 |
| 3-chlorotyrosine (3Cl-Tyr) | 719.72 (289.6−2654.7) | 317.17 (104.79−1251.7) | 246.17 (101.36−497.2) | 0.0568 |
| 3-nitrotyrosine (3NO₂-Tyr) | 77.26 (52.49−125.0) | 91.91 (79.130−158.63) | 107.28 (57.94−144.99) | 0.39 |
| 2′-deoxiguanosine (2dG) | 521.94 (104.37−6199.1) | 161.98 (93.29−536.1) | 84 (54.26−281.38) | 0.0213 |
| 8-oxo-2′-deoxyguanosine (8OHdG) | 4.103 (3.895−23.83) | 3.895 (3.895−8.655) | 3.895 (3.895−8.619) | 0.3452 |
| Ratio o-Tyr/Phe | 0.001 (0.001−0.011) | 0.001 (0.001−0.013) | 0.011 (0.002−0.033) | 0.2968 |
| Ratio 3Cl-Tyr/p-Tyr | 0.012 (0.005−0.028) | 0.004 (0.002−0.018) | 0.002 (0.002−0.033) | 0.0021 |
| Ratio 3NO₂-Tyr/p-Tyr | 0.001 (0.001−0.002) | 0.002 (0.001−0.003) | 0.001 (0.001−0.002) | 0.287 |
| Ratio 8OHdG/2dG | 0.02 (0.004−0.059) | 0.025 (0.011−0.058) | 0.046 (0.026−0.072) | 0.1647 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rugemalira, E.; Roine, I.; Kuligowski, J.; Sánchez-Illana, Á.; Piñeiro-Ramos, J.D.; Andersson, S.; Peltola, H.; Leite Cruzeiro, M.; Pelkonen, T.; Vento, M. Protein Oxidation Biomarkers and Myeloperoxidase Activation in Cerebrospinal Fluid in Childhood Bacterial Meningitis. Antioxidants 2019, 8, 441. https://doi.org/10.3390/antiox8100441
Rugemalira E, Roine I, Kuligowski J, Sánchez-Illana Á, Piñeiro-Ramos JD, Andersson S, Peltola H, Leite Cruzeiro M, Pelkonen T, Vento M. Protein Oxidation Biomarkers and Myeloperoxidase Activation in Cerebrospinal Fluid in Childhood Bacterial Meningitis. Antioxidants. 2019; 8(10):441. https://doi.org/10.3390/antiox8100441
Chicago/Turabian StyleRugemalira, Emilie, Irmeli Roine, Julia Kuligowski, Ángel Sánchez-Illana, José David Piñeiro-Ramos, Sture Andersson, Heikki Peltola, Manuel Leite Cruzeiro, Tuula Pelkonen, and Máximo Vento. 2019. "Protein Oxidation Biomarkers and Myeloperoxidase Activation in Cerebrospinal Fluid in Childhood Bacterial Meningitis" Antioxidants 8, no. 10: 441. https://doi.org/10.3390/antiox8100441
APA StyleRugemalira, E., Roine, I., Kuligowski, J., Sánchez-Illana, Á., Piñeiro-Ramos, J. D., Andersson, S., Peltola, H., Leite Cruzeiro, M., Pelkonen, T., & Vento, M. (2019). Protein Oxidation Biomarkers and Myeloperoxidase Activation in Cerebrospinal Fluid in Childhood Bacterial Meningitis. Antioxidants, 8(10), 441. https://doi.org/10.3390/antiox8100441